Abstract
Recent brain imaging studies suggested that both the frontal and temporal cortices are important candidate areas for mediating the symptoms of internet addiction. We hypothesized that deficits of prefrontal and temporal cortical function in patients with on-line game addiction (PGA) would be reflected in decreased levels of N-acetyl aspartate (NAA) and cytosolic, choline containing compound (Cho). Seventy three young PGA and 38 age and sex matched healthy control subjects were recruited in the study. Structural MR and 1H MRS data were acquired using a 3.0 T MRI scanner. Voxels were sequentially placed in right frontal cortex and right medial temporal cortices. In the right frontal cortex, the levels of NAA in PGA were lower than those in healthy controls. In the medial temporal cortex, the levels of Cho in PGA participants were lower than those observed in healthy controls. The Young Internet Addiction Scale (YIAS) scores and perseverative responses in PGA were negatively correlated with the level of NAA in right frontal cortex. The Beck Depressive Inventory (BDI) scores in the PGA cohort were negatively correlated with Cho levels in the right temporal lobe. To the best of our knowledge, this is the first MRS study of individuals with on-line game addiction. Although, the subjects with on-line game addiction in the current study were free from psychiatric co-morbidity, patients with on-line game addiction appear to share characteristics with ADHD and MDD in terms of neurochemical changes in frontal and temporal cortices.
Keywords: N-acetyl aspartate, Choline containing compound, Frontal cortex, Medial temporal cortex
1. Introduction
1.1. Brain studies of internet addiction: the roles of the frontal and temporal cortices
Recent brain imaging studies have suggested that both frontal and temporal cortices are important candidate areas for mediating the symptoms of internet addiction. Weng et al. (2013) reported gray matter atrophy in right frontal cortex and supplementary motor areas in patients with on-line game addiction. Ko et al. (2009) reported that in response to game scenes, the right orbito-frontal cortex, bilateral anterior cingulate and medial frontal cortex, right dorsolateral prefrontal cortex, and right caudate nucleus were more activated in patients with on-line game addiction, compared to a control group, In response to game cues, Sun et al. (2012) reported that dorsolateral prefrontal cortex, bilateral temporal cortex, cerebellum, right inferior parietal lobule, right cuneus, right hippocampus, parahippocampal gyrus, left caudate nucleus were activated to a greater extent in patients with on-line game addiction, compared to healthy controls.
1.2. Internet addiction and the symptoms of ADHD and/or MDD
Recently, there have been debates as to whether problematic internet use constitutes a set of pathological symptoms which meet criteria for an independent disorder (addiction), or instead represent symptoms of other psychopathologies (Aboujaoude et al., 2006). First, DSM-V defines this condition not as ‘internet addiction’ but as ‘internet gaming disorder’ due to lack of standard definition, prevalence, formal treatment, and natural history of cases. Second, the narrow ranges of onset age and prevalence age are different from those observed in chemical addiction (Haagsma et al., 2012; Peltzer and Phaswana-Mafuya, 2013). In addition, high rates of comorbidity, particularly with ADHD and MDD have been reported in many studies (Cho et al., 2008; Ha et al., 2007, 2006; Park et al., 2013; Yoo et al., 2004). In addition, several studies have reported that the clinical characteristics of internet addiction are similar to those observed in patients with ADHD or MDD. Excessive internet use or on-line game play has been associated with inattention, hyperactivity, and oppositional behaviors, which are also typical symptoms in patients with ADHD (Chan and Rabinowitz, 2006; Yen et al., 2007). Kraut et al. (1998) noted that clinical symptoms associated with internet addiction included depression, loneliness, and disrupted social communication which can be frequently observed in patients with MDD. Moreover, the severity of co-morbid conditions was positively correlated with the extent of internet addiction (Han et al., 2009; Han et al., 2012b).
1.3. Metabolic changes in patients with ADHD or MDD
To the best of our knowledge, no magnetic resonance spectroscopy (MRS) studies in patients with on-line game addiction have been reported. However, based on findings in individuals with common co-morbid disorders, we anticipated that patients with on-line game addiction would have similar deficits to those which have been observed in patients with ADHD or MDD. In patients with ADHD and patients with MDD, neurochemical changes in frontal and temporal cortices, respectively, have been reported. In a review of sixteen magnetic resonance spectroscopy studies of ADHD, Perlov et al. (2009) summarized the changes in N-acetyl-aspartate (NAA) and cytosolic, choline containing compounds (Cho) that have been identified in frontal areas. In particular, decreased levels of NAA in right frontal cortex have been reported, compared to healthy controls (Arcos-Burgos et al., 2012; Tafazoli et al., 2013). In a review of 18 MRS articles on pediatric depression, Kondo et al. (2011) reported decreased levels of Cho, Glutamate, and NAA in the temporal cortex. MacMaster et al. (2008) and Kusumakar et al. (2001) have reported decreased medial temporal cortex (amygdala) Cho-creatine ratios in adolescents with MDD.
1.4. Hypothesis
Based on the view that internet gaming disorder is potentially consistent with the symptoms of ADHD and/or MDD, the present study assessed the right frontal and medial temporal cortices in patients with on-line game addiction using MRS. Those two regions are candidate areas for metabolic changes in ADHD and/or MDD. We hypothesized that decreased levels of NAA, Glutamate and Cho would be observed in patients with on-line game addiction, similar to findings observed in patients with ADHD or MDD. In addition, we also hypothesized that subjects with on-line game addiction would demonstrate poor performance on Wisconsin Card Sorting Test (WCST), consistent with the dysfunction of the prefrontal cortex. Importantly, the patients with on-line game addiction in this study were free from psychiatric co-morbidity.
2. Methods
2.1. Subjects
One hundred ninety three adolescents and adults with problematic on-line game play who visited the On-line Game Clinic Center from June 2011–June 2012 were screened in the current study. Through advertisements posted at Chung Ang University Medical Center, 38 age and sex matched healthy control subjects agreed to participate in the study. All subjects were screened with the Structured Clinical Interview for DSM-IV (Ha et al., 2006; Preuss et al., 2010). In addition, all subjects were assessed with questionnaires regarding their pattern of on-line game play. Further, the Young Internet Addiction Scale (Young, 1996), the Beck Depressive Inventory (Beck et al., 1961), and the Beck Anxiety Inventory (Beck et al., 1988) were completed. Parents and main care takers completed Dupaul’s ADHD scale-Korean version (K-ARS) for patients. Internal consistency of the K-ARS has been reported to range from 0.77 to 0.89 (So et al., 2002). For assessment of executive function, including set shifting, working memory, and inhibitory control process, a computerized version of the Wisconsin Card Sorting Test (CNT4.0, Maxmedica Inc) was administered to all subjects (Lee et al., 2002). The reliability of the computerized version-WCST is reported as Cronbach’s α = 0.783 (Lee et al., 2002). Set shifting as represented by perseverative errors is thought to be associated with prefrontal cortex function (Pedersen et al., 2012).
The criteria for on-line game addiction in the current study were similar to those employed in other previous studies (Han et al., 2010; Kim et al., 2012; Ko et al., 2009); (1) excessive on-line game play time (more than 4 h per/day/30 h per week), (2) Young Internet Addiction Scale scores >50, (3) irritable, anxious, and aggressive behavior when stopping on-line game play, and (4) impaired behaviors or distress, economic problems, and maladaptive patterns of regular life due to on-line game play. Maladaptive patterns of regular life included disrupted diurnal rhythms (sleeping during the day and gaming at night, irregular meals, and failure to maintain personal hygiene), school truancy, and loss of job. Exclusion criteria included (1) participants with a history or current episode of other axis I psychiatric diseases including major depressive disorder and ADHD, (2) participants taking psychiatric medications for internet addiction, (3) IQ < 80, (4) substance abuse history, and (5) participants with neurological or medical disorders, and (6) participant with claustrophobia. Ultimately, 73 patients with online game addiction were included in the study. Fifty one patients with ADHD, 34 patients with major depression, 9 patients with dual diagnosis ADHD and major depression, 7 patients with schizophrenia, 5 patients with obsessive compulsive disorder, 4 patients with alcohol dependence, 4 patients with autism spectrum disorders, and 2 patients with mental retardation were excluded. Four patients could not be scanned due to claustrophobia. The Chung Ang University Hospital Institutional Review Board approved the research protocol for this study. Written informed consent was provided by patients over 18 years of age. In adolescents under 18 years old, the written informed consent was provided by parents and adolescents provided written informed assent.
2.2. MRS processing
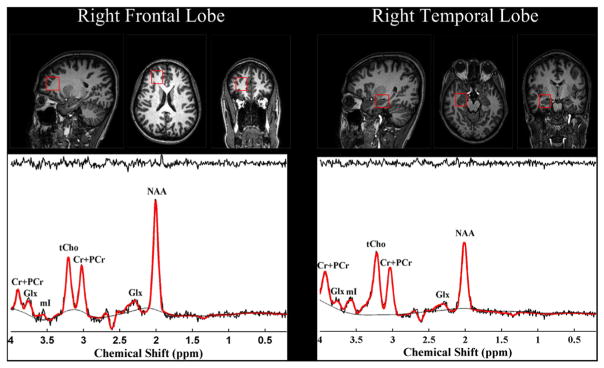
Structural MR and 1H MRS data were acquired using a 3.0 T Philips Achieva 3.0 T TX MRI scanner (Philips, Eindhoven, the Netherlands) and an 8 channel Sense head coil. All 1H spectra were acquired using a Point RESolved Spectroscopy (PRESS) localized single voxel pulse sequence. Voxels were sequentially placed in right frontal cortex and right medial temporal cortex, respectively, as shown in Fig. 1. The medial temporal lobe voxel was prescribed to cover the hippocampal formation. The anterior border in the coronal plane/layer was at the level of the dens. The posterior border was the output of the middle cerebellar peduncle from the pons. The prefrontal VOI was placed anterior to the right corpus callosum using the plane of the inferior frontal gyrus as the bottom border. Spectroscopic data from cubic volumes of 2.5 × 2.5 × 2.5 cm3 were obtained with TR/TE 2000/144 ms, receiver bandwidth 2 kHz, 128 averages, vector size 1024. To facilitate voxel placement, high resolution T1 weighted images were acquired using a three dimensional T1-weighted TEF (turbo echo field) pulse sequence with the following imaging parameters: TR/TE = 8.134/3.725 ms; Flip angle = 8°; Field of View = 220 × 220 × 180 mm3; 256 × 256 × 180 matrix size; 0.8594 × 0.8594 × 1 mm3 spatial resolution; Bandwidth = 192 Hz/pixel. All 1H MRS data were fit using the commercially available Linear Combination (LC) Model software (Provencher, 1993) in conjunction with a simulated basis set. Metabolite signal is normalized to unsuppressed water signal. The derived metabolite concentrations were expressed in institutional unit, which is defined in LC Model manual (www.s-provencher.com/pub/LCModel/manual/manual.pdf). The LC Model fit analysis window was set to cover the 0.2–4.0 ppm chemical shift region. Fig. 1 demonstrates a representative MRS spectrum with baseline spectrum, fitted spectrum and residue noise spectrum. Metabolites fit with Cramer Rao lower bounds <20% were included in the final analysis. Brain tissue segmentation was performed using the BET (Smith, 2002) and FAST (Zhang et al., 2001) tools provided with the freely available FMRIB software library (Smith et al., 2004). Home-built Matlab functions were used to extract 3D volumes corresponding to the spectroscopic voxels and to compute the gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) tissue content inside the voxel for each subject. The WM fraction was calculated as the ratio to total brain matter, i.e. 100×WM/(GM + WM + CSF).
Fig. 1.
Voxel position and MRS spectra in right frontal and medial temporal lobe. NAA: N-acetyl-aspartate, Glx: Glutamine + Glutamate; Cr: Total Creatine; Cho: Total Choline; mI: myo-Inositol.
2.3. Statistical analysis
Mean age, years of education, YIAS scores, IQ, and voxel tissue composition in each VOI were compared between two groups using independent t tests. ANCOVA was performed for each metabolite (NAA, Glx, Cr, Cho, mI) with the level of the metabolite as the dependent factor, age as co-variate, and diagnosis as between-subjects factors. To correct for multiple comparisons (two ROIs with five metabolites), α was set at p ≤ 0.005 (0.05/10).
3. Results
3.1. Demographic characteristics
There were no significant differences in age, sex ratio, or full-scale IQ between patients with on-line game addiction (PGA) and healthy control subjects. The YIAS scores (t = 16.1, p < 0.01) and game playing time (t = 36.3, p < 0.01) in PGA were higher than those in healthy controls. The BDI scores in PGA subjects were higher than those in healthy controls (t = 3.1, p < 0.01). There were no significant differences in the K-ARS scores (t = 0.11, p = 0.90) and BAI scores (t = 1.5, p = 0.14) between two groups. The perseverative responses (F = 3.5, p = 0.02) and perseverative errors (F = 3.4, p = 0.02) in the PGA participants were higher than those in healthy comparison subjects (Table 1).
Table 1.
Demographic data.
| PGA (73) | Healthy controls (38) | Statistics, post hoc | |
|---|---|---|---|
| Age | 20.6 ± 5.1 | 22.0 ± 5.2 | t = 1.3, p = 0.18 |
| Sex (male/female) | 68/5 | 35/3 | χ2 = 0.04, p = 0.84 |
| Education (years) | 13.6 ± 5.1 | 15.0 ± 5.2 | t = 1.4, p = 0.18 |
| IQ | 104.9 ± 13.0 | 103.8 ± 11.8 | t = 0.39, p = 0.76 |
| YIAS | 59.1 ± 6.8 | 33.8 ± 9.4 | t = 16.1, p < 0.01* |
| Game time (hours/week) | 40.5 ± 6.3 | 1.5 ± 2.6 | t = 36.3, p < 0.01* |
| BDI | 11.9 ± 9.3 | 6.8 ± 5.3 | t = 3.1, p < 0.01* |
| K-ARS | 8.9 ± 5.9 | 8.8 ± 5.3 | t = 0.11, p = 0.90 |
| BAI | 6.7 ± 6.9 | 4.5 ± 4.6 | t = 1.5, p = 0.14 |
| WCST | |||
| PR | 15.5 ± 8.6 | 11.4 ± 4.4 | F = 2.8, p < 0.01* |
| PE | 10.5 ± 5.8 | 7.7 ± 2.6 | F = 2.8, p < 0.01* |
PGA: patients with on-line game addiction, BDI: Beck Depression Inventory, BAI: Beck Anxiety Inventory, K-ARS: Dupaul’s ADHD scale-Korean version, WCST: Wis-consin Card Sorting Test, PR: perseverative response, PE: perseverative error.
Statistically significant.
3.2. MRS voxel tissue composition and metabolite levels in right frontal cortex and medial temporal cortex
There were no significant differences in the mean volume percentage of gray matter (frontal; temporal, F = 1.09, p = 0.36; F = 0.93, p = 0.43), white matter (F = 1.31, p = 0.27; F = 1.01, p = 0.39), or CSF (F = 1.36, p = 0.26; F = 0.83, p = 0.48) of the MRS voxels in right frontal cortex and medial temporal cortex between the two groups. In the right frontal cortex, the levels of NAA in PGA were lower than those in healthy controls (F = 21.8, p < 0.001). There were no significant differences in other metabolites within the right frontal cortex between the two groups (Table 2). In the medial temporal cortex, the levels of Cho in PGA participants were lower than those in healthy controls (F = 8.52, p < 0.0049). There were no significant differences in other metabolites within the right temporal lobe voxel between two groups (Table 3).
Table 2.
Tissue composition of MRS voxels and metabolites in right frontal cortex.
| Pure GA (73) | Healthy controls (38) | Statistics, post hoc | |
|---|---|---|---|
| Gray matter (vol %) | 30.0 ± 5.3 | 31.3 ± 3.8 | F = 1.09, p = 0.36 |
| White matter (vol %) | 61.0 ± 6.7 | 60.7 ± 6.3 | F = 1.31, p = 0.27 |
| CSF (vol %) | 8.9 ± 2.5 | 8.0 ± 2.7 | F = 1.36, p = 0.26 |
| NAA | 21.9 ± 4.1 | 25.2 ± 2.8 | F = 21.8, p < 0.001*, |
| Cho | 3.8 ± 1.1 | 3.7 ± 1.2 | F = 0.07, p = 0.94 |
| Cr + PCr | 11.7 ± 2.5 | 12.2 ± 1.7 | F = 0.66, p = 0.42 |
| mI | 17.8 ± 10.1 | 18.0 ± 9.4 | F = 0.01, p = 0.98 |
| Glx | 7.8 ± 3.1 | 7.4 ± 2.9 | F = 0.03, p = 0.87 |
ANCOA, covariate: age, NAA: N-acetyl-aspartate, Cr + PCr: creatine + phosphocreatine, Cho: choline compounds, mI: myo-inositol, and Glx: glutamate, glutamine.
Statistically significant.
Table 3.
Tissue composition of MRS voxels and metabolites in right medial temporal cortex.
| Pure GA (P) | Healthy controls (38) | Statistics, post hoc | |
|---|---|---|---|
| Gray matter (vol %) | 49.0 ± 4.6 | 49.6 ± 4.2 | F = 0.93, p = 0.43 |
| White matter (vol %) | 42.6 ± 5.2 | 41.3 ± 3.9 | F = 1.01, p = 0.39 |
| CSF (vol %) | 8.4 ± 2.5 | 9.1 ± 2.2 | F = 0.83, p = 0.48 |
| NAA | 14.3 ± 2.2 | 14.3 ± 2.5 | F = 1.22, p = 0.27 |
| Cho | 2.9 ± 1.2 | 3.6 ± 1.0 | F = 8.52, p = 0.0049* |
| Cr + PCr | 10.3 ± 2.3 | 10.7 ± 1.4 | F = 1.14, p = 0.29 |
| mI | 8.5 ± 3.9 | 9.4 ± 4.1 | F = 1.73, p = 0.19 |
| Glx | 6.3 ± 1.9 | 4.7 ± 1.4 | F = 1.38, p = 0.26 |
ANCOA, covariate: age, NAA: N-acetyl-aspartate, Cr + PCr: creatine + phosphocreatine, Cho: choline compounds, mI: myo-inositol, and Glx: glutamate, glutamine.
3.3. Correlations YIAS scores and metabolites
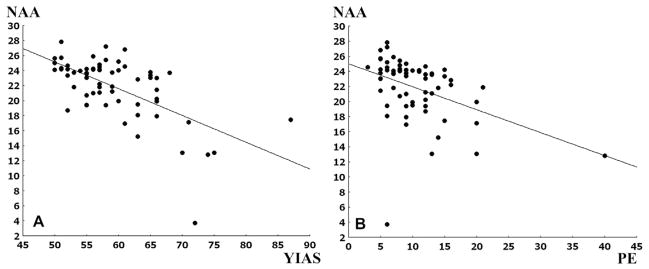
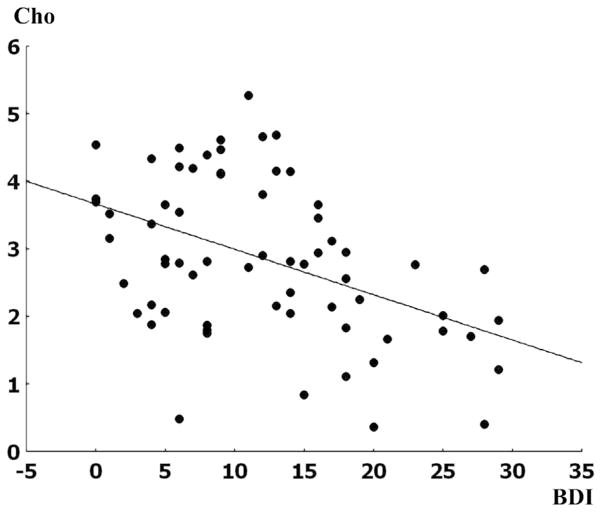
The YIAS scores in PGA were negatively correlated (r = −0.54, p < 0.01) with the level of NAA in right frontal cortex (Fig. 2). Perseverative responses (r = −0.50, p < 0.01) and perseverative errors (r = −0.49, p < 0.01) were negatively correlated with the level of NAA in right frontal cortex in the PGA subjects (Fig. 2). After removing the extreme PE value in Fig. 2, the negative correlation between PE and NAA remains significant (r = −0.58, p < 0.01). The BDI scores in the PGA cohort were negatively correlated with the level of Cho in right temporal lobe (r = −0.44, p < 0.01) (Fig. 3). There were no significant correlations between K-ARS scores and the levels of NAA or Cho.
Fig. 2.
The correlation between YIAS scores and perseverative responses and the levels of NAA in the right frontal cortex of PGA subjects A. The correlation between YIAS scores and the level of NAA in the right frontal cortex in patients with online game addiction (PGA) Pearson correlation, r = −0.62, p < 0.01, B: The correlation between perseverative error (PE) and the level of NAA in right frontal cortex in PGA, Pearson correlation, r = −0.42, p < 0.01, when two extreme PE values were removed, the correlation is r = −0.58, p < 0.01, NAA: N-acetyl-aspartate, YIAS: Young Internet Addiction Scale Score.
Figure 3.
The correlation between BDI and Cho levels in the right temporal lobe in PGA subjects Pearson correlation, r = −0.44, p < 0.01, BDI: Beck Depressive Inventory scores, PGA: patients with online game addiction, Cho: choline containing compounds.
3.4. Discussion
The present results indicate that patients with on-line game addiction had decreased levels of NAA in right frontal cortex and decreased levels of Cho in the right temporal cortex. Moreover, the levels of NAA in right frontal cortex in PGA subjects were negatively correlated with the severity of on-line game addiction and perseverative errors on the WCST.
3.5. Decreased levels of NAA within right frontal cortex in PGA
We observed decreased levels of NAA in the right frontal lobe voxels in PGA subjects, compared to healthy control subjects. In addition, the present results also showed higher rates of perseverative responses and perseverative errors in PGA subjects than those in healthy control subjects. NAA is synthesized in mitochondria and levels may be sensitive to changes in energy production (Patel and Clark, 1979; Stork and Renshaw, 2005). Clinical studies have reported that characteristics of internet addiction include inattention, impulse control difficulties, and executive dysfunction which may be related to frontal cortex dysfunction (Chan and Rabinowitz, 2006; Karim and Chaudhri, 2012; Romano et al., 2013). Several fMRI studies have also suggested possible deficits of right frontal cortex function in patients with internet addiction (Ko et al., 2009; Park et al., 2010). In addition, worse performance in terms of perseverative errors and the number of completed categories in WCST have also been reported in patients with on-line game addiction (Han et al., 2012a). The worse performance in WCST is thought to reflect a deficit in executive function in patients with ADHD (Reeve and Schandler, 2001). Decreased executive function in both the PGA and ADHD groups may be associated with impairment of impulse control (Vitale et al., 2011) which would make them vulnerable to excessive on-line game play. Based on the findings of decreased NAA and increased perseverative errors, we suggest that patients with online game addiction may have right frontal cortical dysfunction that is independent of a diagnosis of either ADHD or MDD.
3.6. Decreased level of Cho within the right temporal cortex voxel in PGA subjects
The present study noted decreased Cho levels in the right temporal cortex in PGA participants. Alterations of Cho in MRS are thought to arise from changes in membrane synthesis and composition (Govindaraju et al., 2000; Moore and Galloway, 2002). Decreased Cho may also reflect loss of neuronal integrity (Govindaraju et al., 2000; Moore and Galloway, 2002). In a long-term follow up MRS study of 12 depressed patients, Obergriesser et al. (2003) reported that increased levels of Cho in hippocampus were associated with improvement of depressive symptoms after ECT treatment.
Interestingly, the present results noted that the BDI scores in PGA subjects were negatively correlated with the levels of Cho although none of the study subjects met criteria for MDD. Recent studies have generally suggested a correlation between internet addiction and MDD (Dalbudak et al., 2013; Han et al., 2012b; Lee et al., 2008). Dalbudak et al. (2013) reported that the rates of alexithymia and depression were higher in university students with a high risk of internet addiction. Lee et al. (2008) suggested that adolescents with excessive internet use had depression-like characteristics in terms of the short allelic variant of the serotonin transporter gene and harm avoidance temperaments. Block et al. (2009) reported that increases in NAA and Cho in hippocampus in response to antidepressant medications (escitalopram and nortriptyline) might represent neurorestorative effects. Based on the findings of decreased choline and the correlation between BDI scores and choline level, we suggest that patients with on-line game addiction may have medial temporal cortex dysfunction that is independent of either ADHD or MDD.
3.7. Common pathology between on-line game addiction, ADHD, and MDD
Putting together the findings from the frontal and temporal cortices in the present study, patients with on-line game addiction, although they were free from clinical co-morbidities, shared deficits similar to those reported in individuals with ADHD or MDD. Given these findings, we suggest that possible co-morbid psychiatric diseases should be fully considered during the process of diagnosis for on-line game addiction. Moreover, on-line game addiction may represent a diagnostically subthreshold form of ADHD or MDD. Second, treatment for ADHD or MDD may affect the course of on-line game addiction. For example, eight weeks methylphenidate treatment was noted to improve inattentive scores and the severity of internet addiction in children with problematic internet use and ADHD (Han et al., 2009). Similarly, eight-weeks of bupropion treatment improved not only depressive symptoms and but also the severity of on-line game addiction in a cohort of depressed PGA (Han et al., 2012b). Individuals with problematic online gaming might benefit from treatment with these (or similar) agents even in the absence of a clinical diagnosis of ADHD or MDD.
There are several limitations to the present study. As there are no established diagnostic criteria, we used empirical criteria to define on-line game addiction subjects as others have done. The MRI voxels (gray matter vs white matter = 30% vs 60%) sampled in the frontal lobe did not contain similar amounts of gray and white matter compared with the temporal lobe voxel (gray matter vs white matter = 50% vs 40%). Thus, it is difficult to determine whether our results reflect changes in gray matter, white matter or both.
To the best of our knowledge, this is the first MRS study of online game addiction. Patients with on-line game addiction may have frontal and temporal deficits. On-line game addiction may share characteristics with ADHD and MDD in terms of the deficits in frontal and temporal cortices.
Acknowledgments
Role of funding sources
Funding for this study was provided by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120013).; Ministry of Health & Welfare, Republic of Korea had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
This work was supported by Korean Game Culture Foundation (2012–2013) and a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120013).
Footnotes
Contributors
Authors Doug Hyun Han and Perry F. Renshaw designed the study and wrote the protocol. Authors Young Sik Lee and Xianfeng Shi have participated in the analysis and interpretation of the work. All authors contributed to and have approved the final manuscript.
Conflicts of interest and financial disclosure
All authors have no conflict of financial interests in relation to the work described.
References
- Aboujaoude E, Koran LM, Gamel N, Large MD, Serpe RT. Potential markers for problematic internet use: a telephone survey of 2,513 adults. CNS Spectr. 2006;11:750–5. doi: 10.1017/s1092852900014875. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Londono AC, Pineda DA, Lopera F, Palacio JD, Arbelaez A, et al. Analysis of brain metabolism by proton magnetic resonance spectroscopy (1H-MRS) in attention-deficit/hyperactivity disorder suggests a generalized differential ontogenic pattern from controls. Atten Defic Hyperact Disord. 2012;4:205–12. doi: 10.1007/s12402-012-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Block W, Traber F, von Widdern O, Metten M, Schild H, Maier W, et al. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12:415–22. doi: 10.1017/S1461145708009516. [DOI] [PubMed] [Google Scholar]
- Chan PA, Rabinowitz T. A cross-sectional analysis of video games and attention deficit hyperactivity disorder symptoms in adolescents. Ann Gen Psychiatry. 2006;5:16. doi: 10.1186/1744-859X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SC, Kim JW, Kim BN, Lee JH, Kim EH. Biogenetic temperament and character profiles and attention deficit hyperactivity disorder symptoms in Korean adolescents with problematic Internet use. Cyberpsychol, Behav Soc Netw. 2008;11:735–7. doi: 10.1089/cpb.2007.0285. [DOI] [PubMed] [Google Scholar]
- Dalbudak E, Evren C, Aldemir S, Coskun KS, Ugurlu H, Yildirim FG. Relationship of internet addiction severity with depression, anxiety, and alexithymia, temperament and character in university students. Cyberpsychol Behav Soc Netw. 2013;16:272–8. doi: 10.1089/cyber.2012.0390. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Ha JH, Kim SY, Bae SC, Bae S, Kim H, Sim M. Depression and Internet addiction in adolescents. Psychopathology. 2007;40:424–30. doi: 10.1159/000107426. [DOI] [PubMed] [Google Scholar]
- Ha JH, Yoo HJ, Cho IH, Chin B, Shin D, Kim JH. Psychiatric comorbidity assessed in Korean children and adolescents who screen positive for Internet addiction. J Clin Psychiatry. 2006;67:821–6. doi: 10.4088/jcp.v67n0517. [DOI] [PubMed] [Google Scholar]
- Haagsma MC, Pieterse ME, Peters O. The prevalence of problematic video gamers in the Netherlands. Cyberpsychol Behav Soc Netw. 2012;15:162–8. doi: 10.1089/cyber.2011.0248. [DOI] [PubMed] [Google Scholar]
- Han DH, Hwang JW, Renshaw PF. Bupropion sustained release treatment decreases craving for video games and cue-induced brain activity in patients with Internet video game addiction. Exp Clin Psychopharmacol. 2010;18:297–304. doi: 10.1037/a0020023. [DOI] [PubMed] [Google Scholar]
- Han DH, Lee YS, Na C, Ahn JY, Chung US, Daniels MA, et al. The effect of methyl-phenidate on Internet video game play in children with attention-deficit/ hyperactivity disorder. Compr Psychiatry. 2009;50:251–6. doi: 10.1016/j.comppsych.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Han DH, Lyoo IK, Renshaw PF. Differential regional gray matter volumes in patients with on-line game addiction and professional gamers. J Psychiatr Res. 2012a;46:507–15. doi: 10.1016/j.jpsychires.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Lyoo IK, Renshaw PF. Bupropion in the treatment of problematic online game play in patients with major depressive disorder. J Psychopharmacol. 2012b;26:689–96. doi: 10.1177/0269881111400647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim R, Chaudhri P. Behavioral addictions: an overview. J Psychoact Drugs. 2012;44:5–17. doi: 10.1080/02791072.2012.662859. [DOI] [PubMed] [Google Scholar]
- Kim SM, Han DH, Lee YS, Renshaw PF. Combined cognitive behavioral therapy and bupropion for the treatment of problematic on-line game play in adolescents with major depressive disorder. Comput Hum Behav. 2012;28:1954–9. [Google Scholar]
- Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, et al. Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res. 2009;43:739–47. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Hellem TL, Sung YH, Kim N, Jeong EK, Delmastro KK, et al. Review: magnetic resonance spectroscopy studies of pediatric major depressive disorder. Depress Res Treat. 2011;2011:650450. doi: 10.1155/2011/650450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut R, Patterson M, Lundmark V, Kiesler S, Mukopadhyay T, Scherlis W. Internet paradox. A social technology that reduces social involvement and psychological well-being? Am Psychol. 1998;53:1017–31. doi: 10.1037//0003-066x.53.9.1017. [DOI] [PubMed] [Google Scholar]
- Kusumakar V, MacMaster FP, Gates L, Sparkes SJ, Khan SC. Left medial temporal cytosolic choline in early onset depression. Can J Psychiatry. 2001;46:959–64. doi: 10.1177/070674370104601009. [DOI] [PubMed] [Google Scholar]
- Lee JA, Shin DJ, Lee CU, Lee MS. Executive function in psychiatric patients groups through Wisconsin Card Sorting Test Computer Version (WCST) J Korean Neuropsychiatr Assoc. 2002;41:322–34. [Google Scholar]
- Lee YS, Han DH, Yang KC, Daniels MA, Na C, Kee BS, et al. Depression like characteristics of 5HTTLPR polymorphism and temperament in excessive internet users. J Affect Disord. 2008;109:165–9. doi: 10.1016/j.jad.2007.10.020. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Moore GJ, Russell A, Mirza Y, Taormina SP, Buhagiar C, et al. Medial temporal N-acetyl-aspartate in pediatric major depression. Psychiatry Res. 2008;164:86–9. doi: 10.1016/j.pscychresns.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GJ, Galloway MP. Magnetic resonance spectroscopy: neurochemistry and treatment effects in affective disorders. Psychopharmacol Bull. 2002;36:5–23. [PubMed] [Google Scholar]
- Obergriesser T, Ende G, Braus DF, Henn FA. Long-term follow-up of magnetic resonance-detectable choline signal changes in the hippocampus of patients treated with electroconvulsive therapy. J Clin Psychiatry. 2003;64:775–80. doi: 10.4088/jcp.v64n0706. [DOI] [PubMed] [Google Scholar]
- Park HS, Kim SH, Bang SA, Yoon EJ, Cho SS, Kim SE. Altered regional cerebral glucose metabolism in internet game overusers: a 18F-fluorodeoxyglucose positron emission tomography study. CNS Spectr. 2010;15:159–66. doi: 10.1017/s1092852900027437. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee YS, Kim BN, Jeong JH, Han DH. The factors for the aggression in patients with on-line game addiction: behavioral inhibition/activation system and co-morbid disease. J Korean Neuropsychiatr Assoc. 2013;52:84–90. [Google Scholar]
- Patel TB, Clark JB. Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J. 1979;184:539–46. doi: 10.1042/bj1840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A, Wilmsmeier A, Wiedl KH, Bauer J, Kueppers K, Koelkebeck K, et al. Anterior cingulate cortex activation is related to learning potential on the WCST in schizophrenia patients. Brain Cogn. 2012;79:245–51. doi: 10.1016/j.bandc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Peltzer K, Phaswana-Mafuya N. Problem drinking and associated factors in older adults in South Africa. Afr J Psychiatry. 2013;16:104–9. doi: 10.4314/ajpsy.v16i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlov E, Philipsen A, Matthies S, Drieling T, Maier S, Bubl E, et al. Spectroscopic findings in attention-deficit/hyperactivity disorder: review and meta-analysis. World J Biol Psychiatry. 2009;10:355–65. doi: 10.1080/15622970802176032. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Watzke AB, Zimmermann J, Wong JW, Schmidt CO. Cannabis withdrawal severity and short-term course among cannabis-dependent adolescent and young adult inpatients. Drug Alcohol Depend. 2010;106:133–41. doi: 10.1016/j.drugalcdep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Reeve WV, Schandler SL. Frontal lobe functioning in adolescents with attention deficit hyperactivity disorder. Adolescence. 2001;36:749–65. [PubMed] [Google Scholar]
- Romano M, Osborne LA, Truzoli R, Reed P. Differential psychological impact of internet exposure on Internet addicts. PLoS One. 2013;8:e55162. doi: 10.1371/journal.pone.0055162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- So YK, Noh JS, Kim YS, Ko SG, Koh YJ. The reliability and validity of Korean Parent and Teacher ADHD Rating Scale. J Korean Neuropsychiatr Assoc. 2002;41:283–9. [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–19. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ying H, Seetohul RM, Xuemei W, Ya Z, Qian L, et al. Brain fMRI study of crave induced by cue pictures in online game addicts (male adolescents) Behav Brain Res. 2012;233:563–76. doi: 10.1016/j.bbr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Tafazoli S, O’Neill J, Bejjani A, Ly R, Salamon N, McCracken JT, et al. 1H MRSI of middle frontal gyrus in pediatric ADHD. J Psychiatr Res. 2013;47:505–12. doi: 10.1016/j.jpsychires.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale C, Santangelo G, Trojano L, Verde F, Rocco M, Grossi D, et al. Comparative neuropsychological profile of pathological gambling, hypersexuality, and compulsive eating in Parkinson’s disease. Mov Disord. 2011;26:830–6. doi: 10.1002/mds.23567. [DOI] [PubMed] [Google Scholar]
- Weng CB, Qian RB, Fu XM, Lin B, Han XP, Niu CS, et al. Gray matter and white matter abnormalities in online game addiction. Eur J Radiol. 2013;82:1308–12. doi: 10.1016/j.ejrad.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Yen JY, Ko CH, Yen CF, Wu HY, Yang MJ. The comorbid psychiatric symptoms of Internet addiction: attention deficit and hyperactivity disorder (ADHD), depression, social phobia, and hostility. J Adolesc Health. 2007;41:93–8. doi: 10.1016/j.jadohealth.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Cho SC, Ha J, Yune SK, Kim SJ, Hwang J, et al. Attention deficit hyperactivity symptoms and internet addiction. Psychiatry Clin Neurosci. 2004;58:487–94. doi: 10.1111/j.1440-1819.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- Young KS. Psychology of computer use: XL. Addictive use of the Internet: a case that breaks the stereotype. Psychol Rep. 1996;79:899–902. doi: 10.2466/pr0.1996.79.3.899. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]