Abstract
Background
Gold nanoparticles are emerging as promising agents for cancer therapy and are being investigated as drug carriers, photothermal agents, contrast agents and radiosensitisers.
Objective
The aim of this study is to understand characteristics of secondary electrons generated from interaction of gold nanoparticles GNPs with x-rays as a function of nanoparticle size and beam energy and thereby further understanding of GNP-enhanced radiotherapy.
Methods
Effective range, defection angle, dose deposition, energy, and interaction processes of electrons produced from the interaction of x-rays with a GNP were calculated by Monte Carlo simulations. The MCNPX code was used to simulate and track electrons generated from 30 and 50 nm diameter GNP when it is irradiated with a cobalt-60 and 6MV photon and electron beam in water.
Results
When a GNP was present, depending on beam types used, secondary electron production increased by 10- to 2000-fold compared to absence of a GNP.
Conclusion
GNPs with larger diameters also contributed to more doses.
Keywords: MCNPX Code, Megavoltage Radiotherapy, Gold Nanoparticle, Dose Enhancement
Introduction
Nanoparticles have been extensively investigated in diagnostic radiology and radiation therapy. Radio sensitization observed by several authors point out gold nanoparticles (GNP) as the best candidates to be used for flagships of Radionanotherapy. It is well known that dose enhancement can be achieved by introducing a material of high atomic number into a medium such as water when irradiated by kVp x-rays[1, 2]. This effect, which is mainly due to large increase interaction cross-section of photoelectric effect with decreasing energy range, has been extensively studied for cancer radiotherapy[3-8]. Various groups have investigated the possibility of using injected gold nanoparticle (GNPs) for dose enhancement in radiotherapy[9-12]. The dosimetric suitability of GNP application in radiation therapy is one relevant aspect that can justify its applicability in clinical environments.
Increased electron generation by interaction of x-ray photons with GNPs is the main cause of dose increase in GNP-enhanced radiotherapy. Therefore, to understand the role of GNP in enhancing dose, we investigated through Monte Carlo (MC) simulations when nanoparticles were replaced with water. We also investigated the proportion of energy that is self-absorbed by GNP compared to what escapes from nanoparticles. The aim of this study is to understand the characteristics of secondary electrons generated from the interaction of gold nanoparticles GNPs with x-rays as a function of nanoparticle size and beam energy and thereby further understanding of GNP-enhanced radiotherapy. Dose deposition, energy and interaction processes of electrons produced from the interaction of x-rays with a GNP were calculated by Monte Carlo simulations. MCNPX code was used to simulate electrons generated from 30 and 50 nm diameters GNP when it is irradiated with a cobalt-60 and 6MV photon in water.
Materials and Methods
Monte Carlo Simulation Using MCNPX
MC simulations of a GNP irradiated by x-rays in MV energy ranges were carried out using MCNPX code. MCNPX is an MC code that provides a set of physics processes for transport and interactions of particles with matter. Generally, photon and electron transport quantities such as energy deposition were determined by codes based on condensed history. In this study, we used MCNPX code, which gives support for interactions in heterogeneous medium. MCNPX includes routines that allow for the precise definition of complex particle transport volumes and analysis of results. Release 2.4 was used. It is recognized that there is difficulty on this scale to verify the calculated results from microdosimetry experimentally. Nevertheless, it is worthwile for studies such as this one to exist in order to gain physical insights into the phenomenon of radiation dose enhancement due to GNPs. Our findings aim to further the understanding of results from published biological studies regarding GNP-enhanced radiotherapy.
Simulation Geometry and Setup
Simulation geometry is shown in Figure 1. A spherical GNP, either 30 or 50 nm in diameter, was centered inside a cubic volume consisting of water.
Figure 1.
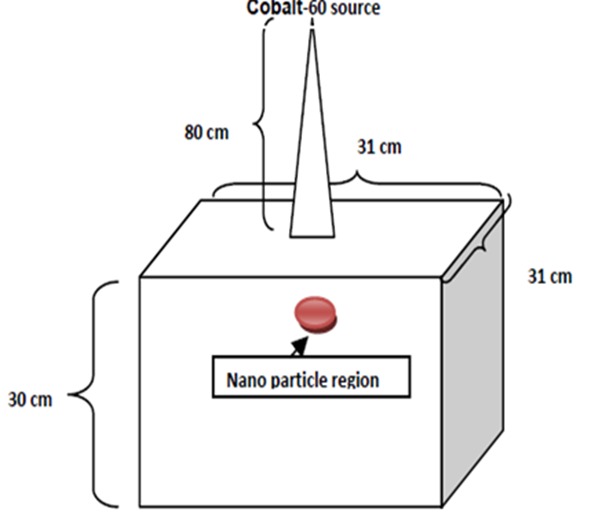
Schematic Diagrams of Monte Carlo Simulation Geometry
Nanoparticle was irradiated by 2 polyenergetic photon sources, namely; cobalt-60 and 6 MV. They are shown in Figure 2, normalized to a maximum value of 1000.
Figure 2.
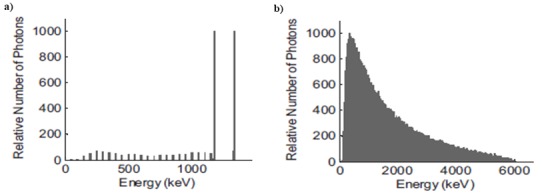
Energy Spectra of Beams Used to Irradiate Nanoparticles for Monte Carlo Simulation. Beams are(a) Cobalt-60 (b) 6 MV.
All photon paths from the source were parallel to the central axis. The number of primary photons used in each simulation was 250×106. Calculation was performed in a single run without splitting histories. The production threshold, or the energy below which no secondary particle are generated, was set to the minimum of 250ev. Error metric were based on the square root of the number of entries per histogram bin. Simulation time ranged from 20 to 65 h depending on the energy of photon beam. To calculate absorbed dose and energy, deposition of each electron step was recorded. Dose deposited within GNP was adjusted for the density of gold.
Results
Figure 3 shows radial dose distribution of a GNP irradiated by photon beams of cobalt-60 and 6 MV, normalized by the maximum dose of each plot. Data show the fall-off of absorbed dose from the center of GNP, excluding the volume within nanoparticles.
Figure 3.
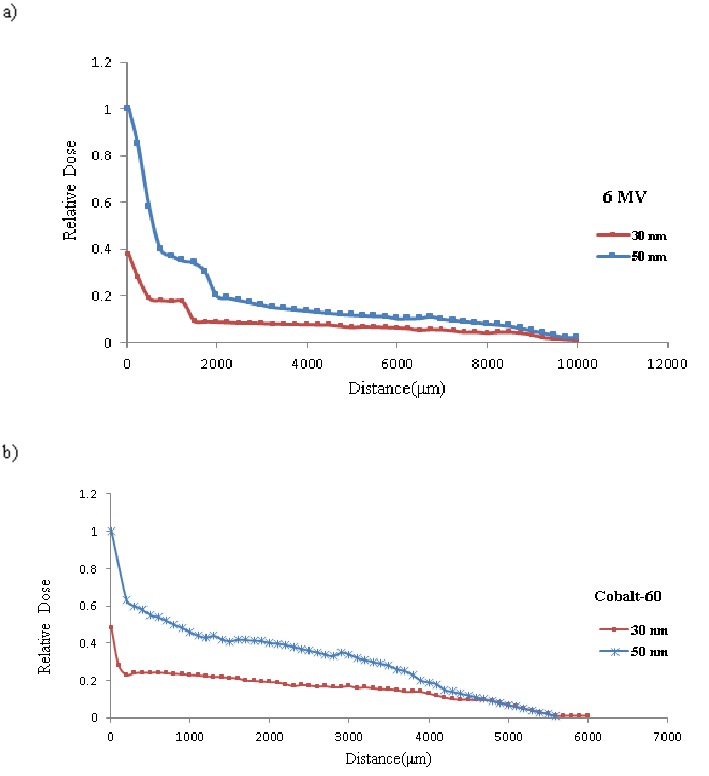
Absorb Dose in Water as Function of Distance away from the Center of Gold Nanoparticle. Data for Beams (a) 6 MV and (b) Cobalt-60.
The mean energy of electrons as it is injected from the GNP, as well as total relative dose, are summarized in Table 1 for different beams and nanoparticle sizes. Results for water substituted into the GNP volume are also shown for comparison. Note that in this table, a greater uncertainty is associated with the values corresponding to simulations higher energy beams and where GNP is replaced with water due to lower interaction probabilities.
Table 1.
Results are compared to the case when gold is absent (substituted by water). The calculated dose in this table excludes deposition within nanoparticles volume.
| Features | Nanoparticle diameter(nm) | Cobalt-60 | 6 MV | ||
|---|---|---|---|---|---|
| Gold | Water | Gold | Water | ||
| Mean electron Energy(Kev) | 30 | 420 ±10 | 680 ± 60 | 370 ± 9 | 1200 ± 100 |
| 50 | 454± 9 | 720 ± 40 | 386 ± 7 | 1150 ± 70 | |
| Total relative dose outside nanoparticles | 30 | 4.31 ± 0.01×10-2 | 1.36 ± 0.01×10-2 | 3.00 ± 0.01×10-3 | 1.17 ± 0.01×10-3 |
| 50 | 1.005 ± 0.002×10-1 | 2.93 ±0.01×10-2 | 1.088 ± 0.002×10-2 | 1.50 ±0.01×10-3 | |
Conclusions
We have carried out MC simulations to investigate the radial dose distribution, energy, and interaction processes of electrons produced by photon beams’ interaction with a nanoparticle as a function of beam energy and nanoparticle size. The purpose of this study is to gain mechanistic insights to GNP interaction with x-rays in order to account for the dose enhancement observed in gold nanoparticle-enhanced radiotherapy. MCNPX code was used to simulate electron emission from 30 and 50 nm diameter nanoparticles irradiated with parallel photon beams of cobalt-60 and 6 MV.
Conflict of Interest: None
References
- 1.Spiers FW. The influence of energy absorption and electron range on dosage in irradiated bone. Br J Radiol. 1949;22(261):521–33. doi: 10.1259/0007-1285-22-261-521. doi: 10.1259/0007-1285-22-261-521. PubMed PMID: 18147720. [DOI] [PubMed] [Google Scholar]
- 2.Matsudaira H, Ueno AM, Furuno I. Iodine contrast medium sensitizes cultured mammalian cells to X rays but not to gamma rays. Radiat Res. 1980;84(1):144–8. PubMed PMID: 7454977. [PubMed] [Google Scholar]
- 3.Regulla DF, Hieber LB, Seidenbusch M. Physical and biological interface dose effects in tissue due to X-ray-induced release of secondary radiation from metallic gold surfaces. Radiat Res. 1998;150(1):92–100. PubMed PMID: 9650606. [PubMed] [Google Scholar]
- 4.Nath R, Bongiorni P, Rockwell S. Iododeoxyuridine radiosensitization by low- and high-energy photons for brachytherapy dose rates. Radiat Res. 1990;124(3):249–58. PubMed PMID: 2263725. [PubMed] [Google Scholar]
- 5.Castillo MH, Button TM, Doerr R, Homs MI, Pruett CW, Pearce JI. Effects of radiotherapy on mandibular reconstruction plates. Am J Surg. 1988;156(4):261–3. doi: 10.1016/s0002-9610(88)80287-3. PubMed PMID: 3177746. [DOI] [PubMed] [Google Scholar]
- 6.Santos Mello R, Callisen H, Winter J, Kagan AR, Norman A. Radiation dose enhancement in tumors with iodine. Med Phys. 1983;10(1):75–8. doi: 10.1118/1.595378. PubMed PMID: 6843516. [DOI] [PubMed] [Google Scholar]
- 7.Norman A, Ingram M, Skillen RG, Freshwater DB, Iwamoto KS, Solberg T. X-ray phototherapy for canine brain masses. Radiat Oncol Investig. 1997;5(1):8–14. doi: 10.1002/(SICI)1520-6823(1997)5:1<8::AID-ROI2>3.0.CO;2-1. doi: 10.1002/(sici)1520-6823(1997)5:1<8::aid-roi2>3.0.co;2-1. PubMed PMID: 9303051. [DOI] [PubMed] [Google Scholar]
- 8.Rose JH, Norman A, Ingram M, Aoki C, Solberg T, Mesa A. First radiotherapy of human metastatic brain tumors delivered by a computerized tomography scanner (CTRx) Int J Radiat Oncol Biol Phys. 1999;45(5):1127–32. doi: 10.1016/s0360-3016(99)00347-8. PubMed PMID: 10613304. [DOI] [PubMed] [Google Scholar]
- 9.Monroe J, Hainfeld J, Sohn J, Wessels B. Initial Monte Carlo analysis of the dosimetric effects of gold nanoparticles radiosensitizers. Med Phys. 2005;32:2022–3. doi: 10.1118/1.1998043. [Google Scholar]
- 10.Cho S, Vassiliev O, Jang S, Krishnan S. Modifications of mega voltage photon beams for gold nanoparticle-aided radiation therapy (GNRT): A Monte Carlo study. Med Phys. 2006;33:2121. [Google Scholar]
- 11.Cho S, Vassiliev O, Krishnan S. Microscopic estimation of tumor dose enhancement during gold nanoparticle-aided radiation therapy (GNRT) using diagnostic energy range x-rays. Med Phys. 2007;34:2468–9. [Google Scholar]
- 12.Kulkarni R, Sramek B, Lisle A, Boote E, Caldwell C, Katti K. WE-D-M100J-01: Enhancement of Cell Killing by X-Ray Irradiation in the Presence of Gold Nanoparticles. Med Phys. 2007;34: 2602. [Google Scholar]


