Abstract
Bone formation is dependent on the differentiation of osteoblasts from mesenchymal stem cells (MSCs). In addition to serving as progenitors, MSCs reduce inflammation and produce factors that stimulate tissue formation. Upon injury, MSCs migrate to the periodontium, where they contribute to regeneration. We examined the effect of clopidogrel and aspirin on MSCs following induction of periodontitis in rats by placement of ligatures. We showed that after the removal of ligatures, which induces resolution of periodontal inflammation, clopidogrel had a significant effect on reducing the inflammatory infiltrate. It also increased the number of osteoblasts and MSCs. Mechanistically, the latter was linked to increased proliferation of MSCs in vivo and in vitro. When given prior to inducing periodontitis, clopidogrel had little effect on MSC or osteoblasts numbers. Applying aspirin before or after induction of periodontitis did not have a significant effect on the parameters measured. These results suggest that clopidogrel may have a positive effect on MSCs in conditions where a reparative process has been initiated.
Keywords: periodontal diseases, inflammation, aspirin, gingiva, osteoblast, regeneration
Introduction
Periodontitis is stimulated by bacteria that induces inflammation, which leads to bone resorption and is one of the most common osteolytic diseases (Graves et al. 2011). The stimulation of an inflammatory response and osteoclast-mediated bone resorption is an important component of periodontal bone loss (Bartold et al. 2010). Following bone resorption, new bone formation occurs due to the activity of osteoblasts (Sims and Walsh 2012). The formation of new bone reflects the production of osteoid, which is dependent on the differentiation of osteoblasts from mesenchymal stem cells (MSCs; Knight and Hankenson 2013). MSCs play important roles in tissue formation by reducing inflammation, producing factors that stimulate tissue formation, and serving as progenitor cells (Q. Wang et al. 2012; Zhang et al. 2012).
MSCs migrate to sites in the periodontium, where they become activated and produce an array of bioactive mediators with various biological functions (Pignolo and Kassem 2011). These cells can self-renew and differentiate into various types to cells that are needed for periodontal regeneration (Navabazam et al. 2013). Although MSCs perform an important function by reducing inflammation, they are affected by inflammation. Proinflammatory cytokines interferon γ and tumor necrosis factor α (TNFα) synergistically impair self-renewal and differentiation of MSCs (Q. Wang et al. 2012; L. Wang et al. 2013). TNFα inhibits MSC differentiation into osteoblasts and induces apoptosis of MSC, as well as negatively affecting mature osteoblasts (Osta et al. 2014). Inhibition of interferon γ and TNFα improve the capacity of MSCs to regenerate bone (Liu et al. 2011). Inflammation inhibits pathways induced by bone morphogenetic protein (BMP) or Wnt proteins that are needed to induce osteoblastic differentiation of MSCs (Pacios et al. 2012; Chang et al. 2013; Tang et al. 2013). TNFα and interleukin 17 have been shown to impair differentiation of MSCs to osteoblasts (Chang et al. 2013). Inhibition of inflammation enhances the capacity of MSCs to regenerate craniofacial bone (Liu et al. 2011; Chang et al. 2013). Thus, factors that affect inflammation are likely to be important in periodontal regeneration, in part, because of their effect on MSCs.
The ligature-induced periodontitis in the rat has many of the characteristics seen with human periodontal disease (Graves et al. 2008; Nassar et al. 2009). Removal of the pathogenic stimulus represented by the ligatures initiates resolution of inflammation and triggers a repair process (Liu et al. 2006; Coimbra et al. 2011; Pacios et al. 2012). We previously demonstrated that clopidogrel helps resolve inflammation in this periodontal model (Coimbra et al. 2011). Clopidogrel is used clinically to prevent platelet degranulation by binding to P2Y12 receptor expressed on the platelet membranes, which inhibits the endogenous ligand adenosine diphosphate (Bennett 2001; Gao et al. 2009). In the majority of in vivo studies, clopidogrel also inhibits inflammation (Evangelista et al. 2005; Ayral et al. 2007) and has been shown to inhibit the progression of atherosclerotic lesions by reducing levels of inflammatory factors (Li et al. 2007).
On the basis of these considerations, we examined the effect of clopidogrel on MSCs during repair of the periodontium initiated by removal of ligatures following an episode of periodontal bone loss. In results presented here, the number of osteoblasts were assessed by histologic analysis, and the number of MSCs were quantified by immunohistochemistry using antibody to CD271 and by immunofluorescence using an antibody to CD146. During resolution of inflammation, clopidogrel increased the number of MSCs and osteoblasts while reducing the number of inflammatory cells. Mechanistically, this was linked to increased proliferation of MSCs in vivo and in vitro.
Material and Methods
Animals
Sixty male adult Holtzman rats (Rattus norvergicus albinus) weighing 250 to 300 g were housed under similar conditions in cages with access to food and water ad libitum. All experimental protocols were approved by the institution’s Ethics Committee for the Use of Experimental Animals and performed in accordance with the guidelines of the Brazilian National Council for the Control of Animal Experimentation.
In Vivo Experimental Design
Animals were anesthetized with ketamine and xylazine chloride (Francotar and Virbaxil, respectively, both from Virbac do Brasil Ind. Com. Ltda, São Paulo, Brazil); then, a 3.0 cotton thread was tied at the cervical portion of the lower right and left first molars in a subgingival position (Holzhausen et al. 2002). Ten days after placement, ligatures were removed, eliminating the inflammatory stimulus and allowing resolution of inflammation over the subsequent 3 d. During these 3 d immediately after ligature removal, animals were treated with daily doses of aspirin (30 mg/kg), clopidogrel (75 mg/kg), or vehicle alone (0.9% saline) by gavage. The doses of clopidogrel or aspirin have been shown to cause effective inhibition of platelet aggregation and thrombus formation in rats (Ma et al. 2001; Coimbra et al. 2011). Age-matched rats that did not receive ligatures—the no-periodontitis groups—were treated with aspirin, clopidogrel, or vehicle alone for 3 d prior to euthanasia (drug treatment–only rats).
Histologic Analysis
At the end of the experimental period, animals were euthanized, and hemimandibles were fixed in 4% paraformaldehyde at 4 °C for 48 h, followed by decalcification in 4.13% ethylenediaminetetraacetic acid (EDTA) solution (pH 7.2; Sigma-Aldrich, São Paulo, Brazil). Serial paraffin sections (5 µm) were obtained from buccal-lingual aspects of the left first molars and stained with hematoxylin and eosin or used for immunohistochemistry or immunofluorescence. The region of interest was the buccal and lingual bone and adjacent periodontal tissue associated with the first mandibular molars. Osteoblasts were morphologically defined as cuboidal cells lining the bone surface in areas of bone remodeling and counted at 200× magnification by an experienced examiner unaware of the experimental groups. The percentage of MSCs in gingival connective tissue from the top of the alveolar bone crest to the cementoenamel junction was assessed with antibody specific for CD-271 (1:10,000 dilution; Abcam, Cambridge, MA, USA) and for CD-146 (1:1,500 dilution; Millipore, Billerica, MA, USA) or matched control antibody. CD-271 is a marker for MSCs (Alvarez-Viejo 2013). Primary antibody was detected by avidin–biotin–horseradish peroxidase complex using a biotinylated secondary antibody (Abcam). To enhance the signal-to-noise ratio, citrate (pH 6) antigen retrieval was used along with tyramide signal amplification that enhances the chromogenic signal (PerkinElmer, Waltham, MA, USA). Sections were examined at 600× magnification.
CD271-, Ki-67-, and CD45-immunopositive cells were detected with specific antibody to each by double immunofluorescence. The analysis involved colocalization of CD271 and Ki-67 to assess proliferating MSCs and colocalization of CD271 and CD45 to count CD271+CD45– cells. For double immunofluorescence, residual biotin sites and peroxidase activity associated with the first primary antibody—CD271 (1:10,000; Abcam), Ki-67 (1:20; Vector Laboratories, Burlingame, CA, USA), and CD45 (1:100; Abcam)—were blocked with avidin/biotin blocking reagent and 3% hydrogen peroxide (Vector Laboratories). A second primary antibody was then detected by the same method described above using avidin-fluorescein (Vector Laboratories). Nuclei were visualized with DAPI counterstain. We examined the connective tissue in the buccal and lingual gingiva of the mandibular first molars for CD271-positive cells. In some experiments, MSC proliferation was measured by colocalization of CD271 and the proliferation marker Ki-67 using specific antibodies to each. It established whether CD271-positive cells were of hematopoietic origin by determining if CD271 cells were also positive for the leukocyte marker CD45. These experiments used specific CD271 antibody that was detected with secondary antibody and Texas Red fluoroprobe and CD45 antibody or Ki-67 antibody that was detected with a species-specific secondary antibody and fluorescein-labeled fluoroprobe. In each case, sections were also subjected to DAPI nuclear stain. Matched control antibodies were used as negative controls in each experiment. Sections were examined at 600× magnification and 5 fields per section, and colocalization of Texas red and fluorescein-labeled cells was assessed using the software NIS–Elements AR (Nikon, Melville, NY, USA).
In Vitro Proliferation Assay
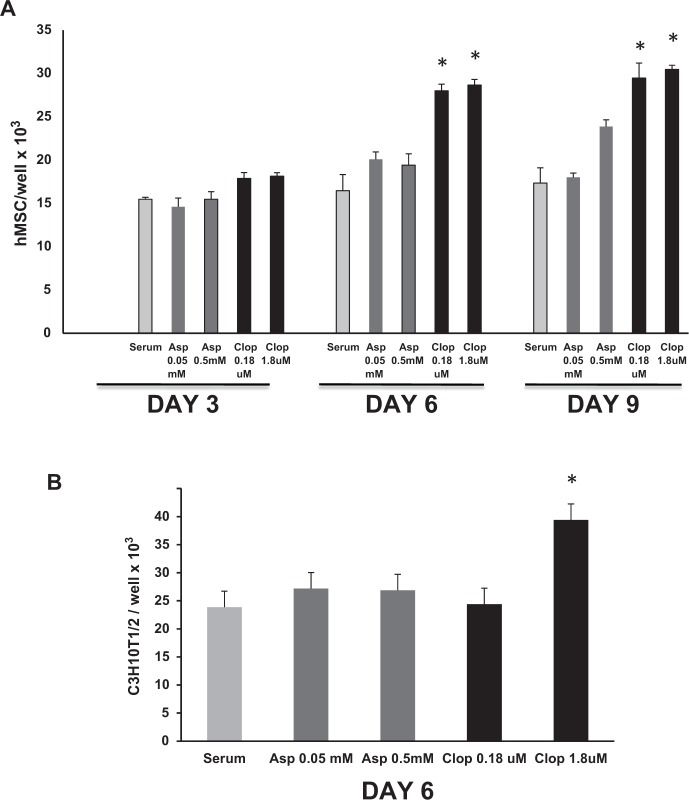
Human bone marrow–derived MSCs obtained as previously described (L. Wang et al. 2013) or murine mesenchymal stem cells (C3H10T1/2) were cultured in alpha-MEM media (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 1% penicillin/streptomycin. For proliferation assays, 8 × 103 cells were seeded in each well of 8-well chamber slides. All experiments were performed in triplicate and repeated 3 times independently. Cells were incubated in 10% fetal bovine serum in culture media with or without clopidogrel (0.18 and 1.8 µM) or aspirin (0.05 and 0.5 mM) with doses based on the IC50 and was below the level of toxicity. Cells were fixed and visualized with DAPI mounting medium (Vector Laboratories) to count the number of cells in 5 random fields per well at 600× magnification.
Statistical Analysis
All data were expressed as mean ± SEM. Differences among the groups were analyzed by analysis of variance, followed by Tukey’s test for multiple comparisons. P values <5% were considered statistically significant.
Results
Clopidogrel Increases the Number of Osteoblasts in Areas of Bone Remodeling
The number of osteoblasts was measured as cuboidal bone cells lining bone in areas of remodeling. There were no differences among the groups without periodontitis. In the periodontitis groups with resolving inflammation, clopidogrel treatment stimulated a 37% increase in the number of osteoblasts (P < 0.05) compared with vehicle alone. In contrast, treatment with aspirin did not affect osteoblast numbers (Fig. 1A).
Figure 1.

Clopidogrel (clop) increases the number of osteoblasts and mesenchymal stem cells (CD271+ and CD146+) during resolution of inflammation. Serial paraffin sections were obtained from buccal-lingual aspects from animals treated with saline solution, aspirin (asp), and clop at baseline and during resolution of inflammation. Osteoblast number was counted as cuboidal bone–lining cells in areas of bone remodeling in hematoxylin and eosin–stained sections, and mesenchymal stem cells were identified by immunohistochemistry and immunofluorescence with antibody specific for CD271 and CD146. (A) Number of osteoblasts per unit length in areas of bone remodeling. (B) Percentage of mesenchymal stem cells (CD271+) from the connective tissue. (C) Percentage of mesenchymal stem cells (CD146+) from the connective tissue. Results are from 10 animals/group. Values are expressed as mean ± SEM. *P < 0.05.
Clopidogrel Increases the Number of MSCs
Immunohistochemistry was performed using antibody to CD271, a marker of MSCs in rat connective tissue (Liu et al. 2014). There was no immunostaining with matched control IgG (data not shown). Without induction of periodontitis, clopidogrel or aspirin did not affect MSC numbers compared with treatment with vehicle alone (P > 0.05). However, in rats with periodontitis, administration of clopidogrel caused a 46% and 40% increase in CD271- and CD146-positive cells, respectively, compared with NaCl-treated animals during resolution of periodontal inflammation (P < 0.05). Aspirin had no effect under these conditions (P > 0.05; Fig. 1B, C; Appendix Fig.).
Clopidogrel, Not Aspirin, Increases the Number of Proliferating MSCs In Vivo
Proliferation was measured by immunofluorescence using a Ki-67 specific antibody, a marker of proliferation (Padial-Molina et al. 2013). Clopidogrel caused a 33% increase in the number of proliferating cells in the gingival connective tissue of animals without induction of periodontitis (P < 0.05). In rats that had periodontitis, there was a 45% increase in the overall level of cell proliferation stimulated by clopidogrel versus vehicle treatment (P < 0.05). Treatment with aspirin did not affect the number of proliferating cells in vivo (P > 0.05; Fig. 2A). The effect on MSC proliferation was measured by double immunofluorescence assay using CD271- and Ki-67-specific antibodies (Fig. 2B, C). Clopidogrel caused a 67% increase in the number of proliferating CD271+ MSCs versus those treated with vehicle alone (Figs. 3B, C, 4). Treatment with aspirin had no effect on MSC proliferation in any group (Fig. 2B, C).
Figure 2.
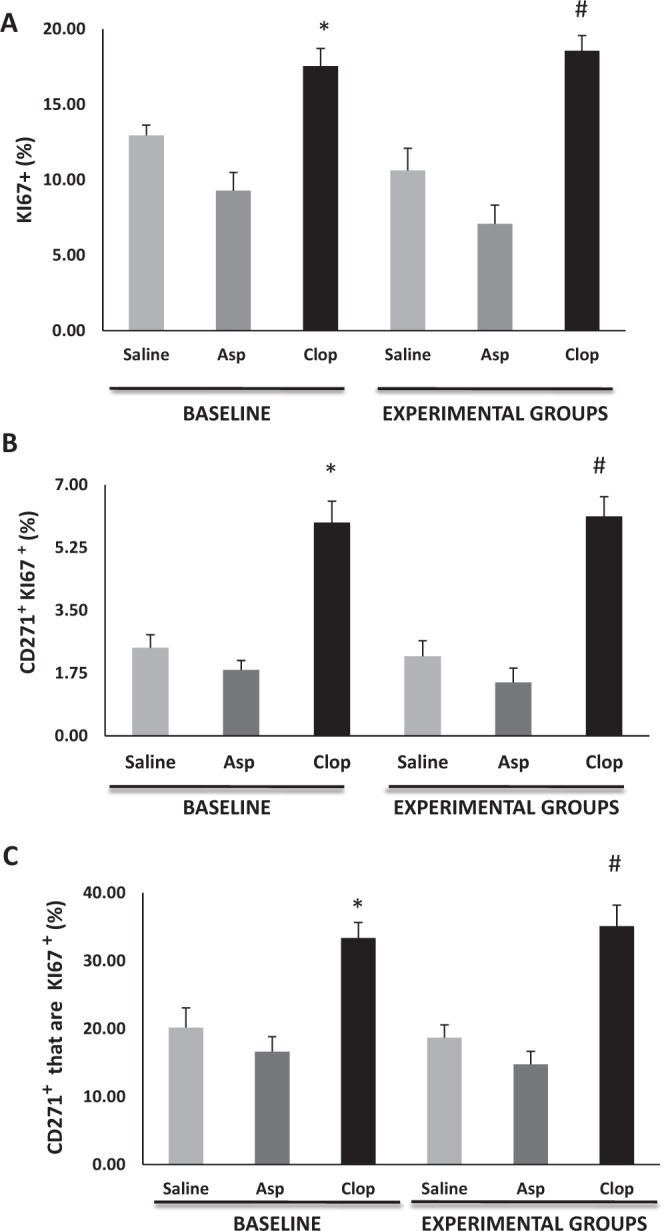
Clopidogrel (clop) increases proliferation of mesenchymal stem cells (MSCs). Serial paraffin sections were obtained from buccal-lingual aspects from animals treated with saline solution, aspirin (asp), and clop. The effect on MSC proliferation was measured in the connective tissue by double immunofluorescence assay using CD271- and Ki-67-specific antibodies. (A) Percentage of cells in proliferation (Ki-67+). (B) The percent total cells that are double positive for CD271 and Ki67. (C) The percent CD271+ cells that are Ki67+. Results are from 10 animals/group. Values are expressed as mean ± SEM. Significant difference (P < 0.05) among groups at *baseline and #experimental groups.
Figure 3.

Clopidogrel (clop) reduces the inflammatory infiltrate during resolution of inflammation. Double immunofluorescence assay using an specific antibody to CD271 and CD45 was performed to rule out the possibility that hematopoietic stem cells were included in the analysis of mesenchymal stem cells. (A) Percentage of CD45+ cells. (B) Percentage of mesenchymal stem cells that are also CD45+. (C) The percentage CD271-positive cells that are CD271/CD45 double positive. Results are from 10 animals/group. Values are expressed as mean ± SEM. *P < 0.001. #P < 0.05. asp, aspirin.
Figure 4.
Clopidogrel (clop) increases proliferation of human primary mesenchymal stem cells (MSCs) and murine MSCs C3H10T1/2. In vitro experiments were carried out to examine the direct effect of clop and aspirin (asp) on proliferation of human (A) and murine MSCs C3H10T1/2 (B). Five fields per well were examined in triplicate at 600×. Values are expressed as mean ± SEM. *P < 0.001.
Clopidogrel Reduces the Inflammatory Infiltrate in the Gingiva
Inflammatory cells were measured by immunofluorescence using a specific antibody to CD45, a panleukocyte marker (Suades et al. 2014). Clopidogrel had no significant effect on the number of CD45+ cells in rats that did not have periodontitis. In rats that had periodontitis induced, clopidogrel treatment during the resolution phase caused a 60% decrease in the number of leukocytes compared with saline-treated animals (P < 0.05; Fig. 3A). Double immunofluorescence was performed to rule out the possibility that the CD271 cells were hematopoietic since MSCs are CD45 negative (Dominici et al. 2006). A very small percentage of CD271 cells were double immunopositive for CD271 and CD45 as expected, but the vast majority of CD271-positive cells were not hematopoietic (Fig. 3B, C).
Clopidogrel Increases Proliferation of MSCs In Vitro
In vitro experiments were carried out to examine the direct effect of clopidogrel and aspirin on proliferation of primary human MSCs and C3H10T1/2 cells, a murine MSC line (Ko et al. 2015). Clopidogrel increased the number of MSCs. After 6 d of stimulation, clopidogrel increased the number of human MSCs by 56%, and after 9 d, the number was increased 67% versus matched control cells (P < 0.05; Fig. 4A). Aspirin had no effect (P > 0.05). Similarly, clopidogrel significantly increased the number of murine MSC, but aspirin had no effect (P < 0.05; Fig. 4B).
Discussion
In the present study, we evaluated the effects of aspirin and clopidogrel on MSC during the initial period of resolution of periodontal inflammation following induction of periodontitis. This period was chosen for examination based on our recent report showing that periodontal repair can be clearly observed during the 3-d period immediately subsequent to the removal of disease-inducing ligatures (Coimbra et al. 2014). Clopidogrel increased the number of osteoblasts and the number of MSCs during this period. However, there were no significant changes induced by clopidogrel in the absence of inducing periodontitis. Aspirin, a known analgesic, had no effect on osteoblast counts or on the number of MSCs. However, there is no evidence that the analgesic effect of aspirin had an effect such as increased inflammation caused by greater occlusal trauma, since no changes in the periodontium were observed consistent with increased occlusal trauma.
We found that clopidogrel increased the number of CD271+ MSCs. Few, if any, of the CD271+ cells in the periodontium were also CD45+. The fact that MSCs were CD45– is consistent with other reports (Dominici et al. 2006) indicating that CD271+ cells in the periodontal tissue were not hematopoietic.
Clopidogrel enhanced proliferation of MSCs in vivo and in vitro, but aspirin did not have this effect. Clopidogrel, a P2Y12 receptor agonist, increases cell proliferation (Ki-67+) in diabetic mice during wound healing but has not been linked to proliferation of MSCs (Scherer et al. 2011). The pathway through which clopidogrel enhances MSC proliferation is unknown. However, our results suggest that there may be 2 distinct mechanisms. In vitro results indicate that clopidogrel directly increases MSC cell number, even when incubated in culture media supplemented with 10% fetal bovine serum that strongly induces proliferation. In vivo results show that clopidogrel reduces the presence of leukocytes in the gingiva, indicative of reduced proliferation.
We previously demonstrated that aspirin and clopidogrel decrease formation of an inflammatory infiltrate induced by experimental periodontitis but that the effect of clopidogrel is more pronounced than aspirin (Coimbra et al. 2011). Clopidogrel also improves the repair process that occurs following cessation of experimental periodontitis and reduces osteoclast formation and bone loss more effectively than aspirin (Coimbra et al. 2014). Thus, data presented here in vitro and in vivo are consistent in showing that clopidogrel enhances MSC proliferation and increases MSC numbers and are consistent with previous publications indicating that the repair process is enhanced by clopidogrel (Coimbra et al. 2014). Taken together, these studies indicate that clopidogrel is more effective than aspirin in resolving inflammation and promoting repair in the periodontium. The resolution of inflammation and the induction of repair processes are important in reestablishing homeostasis following an episode of periodontal bone loss and peritoneal injury (Hasturk et al. 2007; Pacios et al. 2012; Q. Wang et al. 2012).
In summary, we showed that after the removal of the pathologic stimulus in the early/transition phase between disease-associated inflammation and repair, clopidogrel has a significant effect in increasing osteoblast numbers, reducing the inflammatory infiltrate, increasing the percentage of proliferating cells, and increasing the number of proliferating MSCs and the percentage total cells that were MSCs. In contrast, clopidogrel had little effect on MSC numbers or osteoblasts numbers in cells that did not have a prior stimulus of periodontitis. These results suggest that clopidogrel may have a positive effect in conditions where reparative processes predominate. In addition, the results are unlikely to be simply an effect on platelet degranulation since aspirin alone had little effect. Instead, our data suggest that clopidogrel has an effect on MSCs that involve a direct effect on these cells and also an indirect effect mediated by a change in the microenvironment, specifically a decrease in inflammation. Administration of clopidogrel may be helpful in the development of new approaches to improve cell-based regenerative procedures in the periodontium and elsewhere.
Author Contributions
L.S. Coimbra, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J.P. Steffens, S. Alsadun, contributed to data acquisition and analysis, drafted and critically revised the manuscript; M. Laino Albiero, contributed to data acquisition and analysis, critically revised the manuscript; C. Rossa Jr, R.J. Pignolo, contributed to data interpretation, critically revised the manuscript; L.C. Spolidorio, D.T. Graves, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study has been supported by the State of São Paulo Research Foundation (FAPESP) grant number 2010-10715/9, National Council for Scientific and Technological Development (CNPq), and grants from the National Institute of Dental and Craniofacial Research (R01DE17732, R01AG028873, and R01DE019108).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, Ferrero-Gutierrez A, Fernandez-Rodriguez MA, Gala J, Otero-Hernandez J. 2013. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplant Proc. 45(1):434–439. [DOI] [PubMed] [Google Scholar]
- Ayral Y, Rauch U, Goldin-Lang P, Stellbaum C, Deiner C, Schwimmbeck PL, Schultheiss HP, Pels K. 2007. Prolonged application of clopidogrel reduces inflammation after percutaneous coronary intervention in the porcine model. Cardiovasc Revasc Med. 8(3):183–188. [DOI] [PubMed] [Google Scholar]
- Bartold PM, Cantley MD, Haynes DR. 2010. Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 2000. 53:55–69. [DOI] [PubMed] [Google Scholar]
- Bennett JS. 2001. Novel platelet inhibitors. Annu Rev Med. 52:161–184. [DOI] [PubMed] [Google Scholar]
- Chang JC, Fujita S, Tonami H, Kato K, Iwata H, Hsu SH. 2013. Cell orientation and regulation of cell-cell communication in human mesenchymal stem cells on different patterns of electrospun fibers. Biomed Mater. 8(5):055002. [DOI] [PubMed] [Google Scholar]
- Coimbra LS, Rossa C, Jr, Guimarães MR, Gerlach RF, Muscará MN, Spolidorio DM, Herrera BS, Spolidorio LC. 2011. Influence of antiplatelet drugs in the pathogenesis of experimental periodontitis and periodontal repair in rats. J Periodontol. 82(5):767–777. [DOI] [PubMed] [Google Scholar]
- Coimbra LS, Steffens JP, Rossa C, Jr, Graves DT, Spolidorio LC. 2014. Clopidogrel enhances periodontal repair in rats through decreased inflammation. J Clin Periodontol. 41(3):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 8(4):315–317. [DOI] [PubMed] [Google Scholar]
- Evangelista V, Manarini S, Dell’Elba G, Martelli N, Napoleone E, Di Santo A, Lorenzet PS. 2005. Clopidogrel inhibits platelet-leukocyte adhesion and platelet-dependent leukocyte activation. Thromb Haemost. 94(3):568–577. [PubMed] [Google Scholar]
- Gao C, Boylan B, Bougie D, Gill JC, Birenbaum J, Newman DK, Aster RH, Newman PJ. 2009. Eptifibatide-induced thrombocytopenia and thrombosis in humans require FcgammaRIIa and the integrin beta3 cytoplasmic domain. J Clin Invest. 119(3):504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. 2008. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 35(2):89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Li J, Cochran DL. 2011. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res. 90(2):143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. 2007. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 179(10):7021–7029. [DOI] [PubMed] [Google Scholar]
- Holzhausen M, Rossa Júnior C, Marcantonio Júnior E, Nassar PO, Spolidório DM, Spolidório LC. 2002. Effect of selective cyclooxygenase-2 inhibition on the development of ligature-induced periodontitis in rats. J Periodontol. 73(9):1030–1036. [DOI] [PubMed] [Google Scholar]
- Knight MN, Hankenson KD. 2013. Mesenchymal stem cells in bone regeneration. Adv Wound Care (New Rochelle). 2(6):306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko KI, Coimbra LS, Tian C, Alblowi J, Kayal RA, Einhorn TA, Gerstenfeld LC, Pignolo RJ, Graves DT. 2015. Diabetes reduces mesenchymal stem cells in fracture healing through a TNFα-mediated mechanism. Diabetologia. 58(3):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang Y, Ren H, Zhang Y, Zhu X. 2007. Effect of clopidogrel on the inflammatory progression of early atherosclerosis in rabbits model. Atherosclerosis. 194(2):348–356. [DOI] [PubMed] [Google Scholar]
- Liu R, Bal HS, Desta T, Krothapalli N, Alyassi M, Luan Q, Graves DT. 2006. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res. 85(6):510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. 2011. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 17(12):1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yin Z, Zhang R, Yan K, Chen L, Chen F, Huang W, Lv B, Sun C, Jiang X. 2014. MSCs inhibit bone marrow-derived DC maturation and function through the release of TSG-6. Biochem Biophys Res Commun. 450(4):1409–1415. [DOI] [PubMed] [Google Scholar]
- Ma L, Elliott SN, Cirino G, Buret A, Ignarro LJ, Wallace JL. 2001. Platelets modulate gastric ulcer healing: role of endostatin and vascular endothelial growth factor release. Proc Natl Acad Sci. 98(11):6470–6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar CA, Nassar PO, Andia DC, Guimarães MR, Spolidorio LC. 2009. Effects of long-term FK 506 therapy on the alveolar bone and cementum of rats. Transplant Proc. 41(5):1871–1874. [DOI] [PubMed] [Google Scholar]
- Navabazam AR, Sadeghian Nodoshan F, Sheikhha MH, Miresmaeili SM, Soleimani M, Fesahat F. 2013. Characterization of mesenchymal stem cells from human dental pulp, periapical follicle and periodontal ligament. Iran J Reprod Med. 11(3):235–242. [PMC free article] [PubMed] [Google Scholar]
- Osta B, Benedetti G, Miossec P. 2014. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol. 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, Petrov S, Alawi F, Graves DT. 2012. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 26(4):1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padial-Molina M, Volk SL, Rios HF. 2013. Periostin increases migration and proliferation of human periodontal ligament fibroblasts challenged by tumor necrosis factor-α and Porphyromonas gingivalis lipopolysaccharides. J Periodontal Res. 49(3):405–414. [DOI] [PubMed] [Google Scholar]
- Pignolo RJ, Kassem M. 2011. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 26(8):1685–1693. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Pietramaggiori G, Matthews JC, Gennaoui A, Demcheva M, Fischer TH, Valeri CR, Orgill DP. 2011. Poly-N-acetyl glucosamine fibers induce angiogenesis in ADP inhibitor-treated diabetic mice. J Trauma. 71(2 Suppl 1):S183–S186. [DOI] [PubMed] [Google Scholar]
- Sims NA, Walsh NC. 2012. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 10(2):109–117. [DOI] [PubMed] [Google Scholar]
- Suades R, Padró T, Alonso R, López-Miranda J, Mata P, Badimon L. 2014. Circulating CD45+/CD3+ lymphocyte-derived microparticles map lipid-rich atherosclerotic plaques in familial hypercholesterolaemia patients. Thromb Haemost. 111(1):111–121. [DOI] [PubMed] [Google Scholar]
- Tang Y, Xie H, Chen J, Geng L, Chen H, Li X, Hou Y, Lu L, Shi S, Zeng X, et al. 2013. Activated NF-κB in bone marrow mesenchymal stem cells from systemic lupus erythematosus patients inhibits osteogenic differentiation through downregulating Smad signaling. Stem Cells Dev. 22(4):668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brennan TA, Russell E, Kim JH, Egan KP, Chen Q, Israelite C, Schultz DC, Johnson FB, Pignolo RJ. 2013. R-Spondin 1 promotes vibration-induced bone formation in mouse models of osteoporosis. J Mol Med. 91(12):1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhao Y, Liu Y, Akiyama K, Chen C, Qu C, Jin Y, Shi S. 2013. IFN-γ and TNF-α synergistically induce mesenchymal stem cell impairment and tumorigenesis via NFκB signaling. Stem Cells. 31(7):1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Li Q, Zhang L, Lin H, Hu J, Li D, Shi S, Cui S, Zhou J, Ji J, et al. 2012. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS One. 7(8):e43768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li X, Luo J, Zhang L, Ma L, Lv Z, Xue L. 2012. The allogeneic umbilical cord mesenchymal stem cells regulate the function of T helper 17 cells from patients with rheumatoid arthritis in an in vitro co-culture system. BMC Musculoskelet Disord. 13:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Lu Y, Luan Y, Zhao JJ. 2012. Effect of bone marrow mesenchymal stem cells on experimental pulmonary arterial hypertension. Exp Ther Med. 4(5):839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.