Abstract
In a previous report, we demonstrated the inverse association of high serum 8-isoprostane levels, a marker for oxidative stress, with decreased serum IgG antibodies to oral bacteria. The association between increased serum IgG with increased plaque and periodontitis (increased probing depths) was attenuated by high systemic oxidative stress. Other investigations have reported a role for systemic oxidative stress as a stimulus of hepatic C-reactive protein (CRP) response. These observations led us to hypothesize that the reported relationship of periodontitis to elevated serum CRP, a systemic inflammatory marker, may be modified by oxidative stress and that the levels of serum antibodies to oral bacteria might be an intermediary explanatory variable linking the association of systemic oxidative stress, periodontal disease, and levels of CRP. This hypothesis was explored as a secondary analysis of the Dental ARIC (Atherosclerosis Risk in Communities) study using serum levels of CRP, serum IgG levels to 16 oral organisms, serum levels of 8-isoprostane, and periodontal status. The findings indicate periodontitis is associated with high CRP in the presence of elevated oxidative stress that serves to suppress the IgG response. Only within the highest 8-isoprostane quartile was periodontitis (pocket depth) associated with increased serum CRP levels (P = 0.0003). Increased serum IgG antibody levels to oral bacteria were associated with lowered serum CRP levels. Thus, systemic oxidative stress, which has been demonstrated to be associated with increased levels of CRP in other studies, appears to be associated with the suppression of bacterial-specific IgG levels, which in the presence of periodontal disease can result in an enhanced systemic CRP response. Conversely, individuals with increased serum IgG antibodies to plaque bacteria exhibit lowered serum CRP levels. These 2 factors, oxidative stress and the serum IgG response, appear to function in opposing directions to modify serum levels of CRP and the association with periodontitis.
Keywords: biomarkers, biostatistics, bacteria, host pathogen interaction, acute phase reaction, immunity
Introduction
Periodontitis has been associated with increased serum C-reactive protein (CRP) levels, a marker for systemic inflammation (Ebersole et al. 1997; Slade et al. 2003), leading to the hypothesis that periodontitis might increase serum mediators of systemic inflammation and risk of systemic inflammatory diseases (Noack et al. 2001). Mechanistically, bacteremia, the entry of plaque microbial toxins/molecules into the circulation, and/or release of cytokines from the inflamed periodontium could induce systemic inflammatory mediators (Offenbacher et al. 1999), thereby prompting expression and release of acute phase reactants (e.g., CRP) from the liver and other tissues. Systemic inflammation has been implicated as having a possible etiologic role in cardiovascular disease (Libby 2002), preterm birth (Romero et al. 2006), and other systemic diseases. Therapeutic interventions that improve periodontal health have been reported to decrease serum CRP (Ebersole et al. 1997; D’Aiuto et al. 2004; Koromantzos et al. 2012). Others, however, have not shown decreased CRP following periodontal therapy (Ioannidou et al. 2006). Moreover, intervention trials have not reproducibly demonstrated that treatment of periodontitis improves clinical outcomes in cardiovascular disease (Offenbacher, Beck, Moss, et al. 2009; Tonetti 2009) or preterm birth (Offenbacher, Beck, Jared, et al. 2009) patient populations. Clearly, mechanistic insights are needed to further explain the association of periodontitis with systemic inflammatory conditions.
Subjects differ in genetics, medical history, physiologic status, and behavior and can differ in immune and inflammatory response to infectious challenge (Barreiro and Quintana-Murci 2010) and in response to therapy (Kalow 1997). Identifying subject differences that shape systemic responses to periodontitis will be important to determining if subject subgroups differ in risk of systemic consequences from periodontitis and its treatment.
Previously, we reported (Singer et al. 2009) that physiologic increases in serum 8-isoprostane levels, a stable lipid marker of oxidative stress, are associated with decreased serum IgG antibody levels against 17 oral bacteria, including periodontal pathogens. Quartiles of increased serum 8-isoprotane were associated with significant decreases in all 17 serum IgG antibodies. Of particular interest, only subjects in the lowest quartile of serum 8-isoprostane concentration had increased serum IgG antibody levels in the presence of elevated (top quartile) plaque (or plaque [percent sites with Plaque Index ≥1] plus pocket depth [top quartile number of pockets ≥5 mm]). Thus, physiologic differences in a lipid marker of systemic oxidative stress identified subject subset(s) with decreased/increased serum IgG antibody responses to oral bacteria and periodontitis.
Several studies have reported the association between elevated serum CRP levels and increased systemic 8-isoprostane levels (Handelman et al. 2001; Cottone et al. 2007; Dohi et al. 2007). In humans, the CRP gene promoter sequence contains a nuclear factor (NF)–κB binding site (Voleti and Agrawal 2005), which responds to oxidative stress to enhance expression (Schreck et al. 1992). The systemic levels of oxidative stress represent a balance of oxidative exposures, including bacterial, traumatic, and ischemic challenges to tissues in the milieu of dietary influenced levels of lipid and water-soluble antioxidants. In the present investigation, we conducted a secondary analysis to examine whether the association between periodontal disease and elevated CRP was associated with physiologic differences in oxidative stress (serum 8-isoprostane levels) and whether this association was modified by the levels of serum IgG antibodies to oral bacteria. We hypothesized that high oxidative stress would be associated with low IgG levels to oral bacteria, which in the presence of periodontal disease would have the potential to result in greater hepatic exposure to oral organisms and further increase serum CRP levels. Stated another way, we hypothesized high IgG antibody responses (possibly blocking invasion) might be expected to decrease the ability of oral bacteria to induce systemic inflammatory responses.
Materials and Methods
Subjects
The Atherosclerosis Risk in Communities (ARIC) study (Atherosclerosis Risk in Communities Investigators 1989) is a prospective investigation supported by the National Heart, Lung, and Blood Institute (NHLBI) of community dwellers to characterize the natural history of atherosclerosis and cardiovascular disease in 4 US communities (Jackson, Mississippi; Washington County, Maryland; suburban Minneapolis, Minnesota; and Forsyth County, North Carolina). The Jackson cohort was composed entirely of African Americans. A sample of 15,792 community-dwelling residents aged 45 to 64 y at baseline took part in an evaluation of cardiovascular risk factors and their sequelae. The Dental ARIC, an ancillary study funded by the National Institute of Dental and Craniofacial Research (NIDCR), was conducted during ARIC visit 4 (1996 to 1999) and was cross-sectional in design. The Dental ARIC study consisted of an oral examination, collection of serum, and interviews among a diverse population of 6,793 middle-aged adults. It includes measures of a serum marker of oxidative stress (8-isoprostane), measures of levels of a panel of serum antibodies to oral bacteria, and clinical measures of total oral microbial load (i.e., plaque) and extent of periodontal disease. There were no exclusion criteria except for total edentulism and need for antibiotic prophylaxis for the dental examination. Data were collected on factors known to contribute to oxidative stress (e.g., smoking and diabetes). The protocols for this clinical investigation were reviewed and approved by the respective institutions’ institutional review boards.
Exposure Variables
Probing depth (PD) was assessed on 6 sites for all teeth. Plaque was measured using the Plaque Index (Löe 1967). The ARIC periodontal examiners were calibrated to a standard examiner, range for weighted κs was 0.86 to 0.94. Intraclass coefficients ranged from 0.87 to 0.95. Percent agreement within 1 mm ranged from 88.9% to 94.9%. We chose to focus our analysis using probing depths as a measure of periodontal status and plaque as a measure of microbial exposure. Among the clinical signs, PD was the single best predictor of serum IgG antibody levels. To create quartiles of extent scores, participants were ranked on the percentage of sites with PD ≥5 mm. Participants in the top quartile of number of sites with PD ≥5 mm were considered “high.” Participants in the remaining 3 quartiles of PD ≥5 mm extent were considered “low.”
Serum 8-Isoprostane
Direct 8-isoprostane PGF2α (d-8-iso PGF2α) is a stable end product of both specific inflammatory enzymatic pathways as well as nonspecific mechanisms and reflects total lipid peroxidation, representing an excellent in vivo marker for oxidative stress (Sies 2000). The d-8-iso PGF2α assay was conducted on serum samples of ARIC subjects as previously described (Singer et al. 2009). Participants were ranked in quartiles based on their serum concentration of d-8-iso PGF2α.
Outcome Variables
The main outcome variables were serum CRP levels and serum IgG antibody levels to 16 selected periodontal organisms: Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens, Tannerella forsythia, Treponema denticola, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, Campylobacter rectus, Micromonas micros, Veillonella parvula, Capnocytophaga ochracea, Selenomonas noxia, Actinomyces viscosus, Streptococcus intermedius, Streptococcus sanguis, and Streptococcus oralis. Serum samples collected as part of the ARIC examination were divided into aliquots at each examination site, frozen at −80°C, transported on dry ice to our laboratory, and stored in aliquots at −80°C. Samples were assayed for serum CRP levels using the high-sensitivity method described previously (Slade et al. 2003). Samples were assayed for IgG antibody levels directed against the 16 periodontal organisms using the checkerboard immunoblotting technique described by Sakellari et al. (1997).
Periodontal organisms selected were representatives from clusters of organisms associated with periodontal disease and health by Socransky et al. (1998). Summation scores for total serum IgG antibody level titers were made by summing IgG concentrations for each organism assayed.
Covariates
Participants were defined as never smokers, former smokers, or current smokers by interview. The former and current categories were further divided into light or heavy smokers, with light smokers reporting more than 0 but fewer than 20 pack-years of smoking and heavy smokers reporting 20 or more pack-years of smoking. This scheme resulted in a 5-level categorization of smoking that takes into account both temporality and magnitude of smoking status as an exposure. Education was divided into basic (less than 12 years), intermediate (12 to 16 years), or advanced (17 to 21 years) and was included to control for socioeconomic status. Age in years at visit 4 was included, and a variable representing race/ethnicity (African American or white) and ARIC center was designed to control for the ethnic, regional, and examiner differences in the ARIC cohort. Persons who were not African American or white and the few African Americans in the Maryland and Minnesota centers were excluded from analysis due to small numbers.
Participants fasted for 12 hours before the clinical examination, and blood was collected for plasma lipids including high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides, and serum glucose. Diabetes mellitus was defined as fasting serum glucose ≥126 mg/dL (200 mg/dL if nonfasting), pharmacological treatment for diabetes, or being told by a doctor one has diabetes or sugar in the blood (self-reported).
Statistical Analysis
Statistical analyses and data management were performed using SAS (SAS Institute, Cary, NC, USA), statistical significance was set at P ≤ 0.05, and the unit of analysis was the person. Frequency distributions, means, empirical distribution functions, and standard errors were determined to describe the data. When distributions were skewed, log transformations were applied. Bivariate relationships were investigated using t tests for continuous variables, as well as Cochran Mantel-Haenszel χ2 statistics and odds ratios with 95% confidence intervals (CIs) for differences between categorical variables. Multivariable modeling was performed using SAS Proc GLM to calculate least squares means adjusting for other study variables. Potential confounders were specified a priori, based on the literature as being associated with either exposure or outcomes. We explored effects of infections other than periodontal disease (e.g., sinusitis, bronchitis, kidney disease, and pneumonia) and potential modifiers of oxidative stress (e.g., arthritis) on serum 8-isoprostane and CRP levels. We have only included in the analyses effect modifiers or confounders that influence the association by 5% or more, whether or not they were significant main effects.
Multivariate models were developed for serum CRP levels for each of the 16 oral biofilm IgG antibodies and 4 clusters of IgG antibodies that represent total biofilm IgG, total red IgG, total orange IgG, and total other IgG (Singer et al. 2009). The IgG clusters (i.e., other, orange, and red) were directed against microbial species associated with health, gingivitis, and periodontitis, respectively (Sakellari et al. 1997). The models included demographic, behavioral, and medical variables identified in the χ2 models for serum levels of CRP, as well as plaque and pocket depth, and an interaction term for pocket depth and serum 8-isoprostane. The models compared serum CRP concentrations corresponding to quartiles of each of the 16 serum IgG antibodies and IgG antibody clusters. Models included all subjects with complete validated data sets for the indicated variables. Subjects with PD examinations + validated CRP, IgG, and 8-isoprostane bioassays totaled 4,567. The STROBE checklist was completed and its guidelines followed.
Results
Bivariate Associations for 8-Isoprostane, CRP, and Total Oral Biofilm IgG Antibody
To determine the factors related to serum concentrations of 8-isoprostane, CRP, and total IgG antibody against the oral biofilm microorganisms, bivariate analyses were conducted (Table 1) for relevant clinical variables. Serum concentrations of 8-isoprostane above the median were associated with age, race/field center, smoking, serum triglycerides, and pocket depth. While there was a relationship between smoking history and serum 8-isoprostane concentrations (Table 1), it was evident that current smokers had lower 8-isoprostane concentrations than former smokers, heavy smokers had lower 8-isoprostane levels than light smokers, and current heavy smokers had lower concentrations than nonsmokers. Mean serum CRP levels were associated with sex, race/field center, education, income, smoking, body mass index (BMI), diabetes, hypertension, serum LDL, triglycerides, pocket depth, plaque, and serum 8-isoprostane. As reported previously (Singer et al. 2009), mean total biofilm IgG antibody was related to sex, race/field center, smoking, BMI, hypertension, pocket depth, plaque, serum HDL, triglycerides, and serum 8-isoprostane.
Table 1.
Bivariate Analyses for Study Variables and Serum 8-Isoprostane, Total Oral Biofilm IgG Antibody, and CRP.
| Log Median 8-Isoprostane |
LS Mean CRP |
Mean Log Total Biofilm IgG Antibody |
||||||
|---|---|---|---|---|---|---|---|---|
| Group | N | Log Median 8-Isoprostane (Interquartile Range) | P Value | LS Mean CRP (SE) | P Value | Mean Log Tot Biofilm IgG Ab (SE) | P Value | |
| Age | <65 y | 3,039 | 3.66 (1.23–5.75) | 6.66 (0.22) | 2.82 (0.01) | |||
| 65+ y | 1,678 | 3.93 (2.45–6.00) | 0.0060 | 6.68 (0.29) | 0.9701 | 2.83 (0.01) | 0.7782 | |
| Sex | Female | 2,643 | 3.83 (1.23–6.00) | 8.06 (0.26) | 2.81 (0.01) | |||
| Male | 2,074 | 3.67 (1.23–5.75) | 0.0710 | 4.89 (0.22) | <0.0001 | 2.85 (0.01) | 0.0073 | |
| Race/field | Mississippi | 689 | 3.14 (2.56–4.42) | 8.29 (0.05) | 2.96 (0.02) | |||
| center | North Carolina, AAs | 117 | 3.79 (1.23–5.38) | 10.61 (1.10) | 3.01 (0.05) | |||
| North Carolina, whites | 1,338 | 3.59 (2.62–5.67) | 6.11 (0.33) | 2.81 (0.01) | ||||
| Maryland | 1,339 | 3.48 (1.23–5.59) | 5.98 (0.33) | 2.77 (0.01) | ||||
| Minnesota | 1,234 | 4.71 (2.78–6.00) | <0.0001 | 6.74 (0.34) | <0.0001 | 2.81 (0.01) | <0.0001 | |
| Years of education | <11 y | 631 | 3.50 (2.15–.30) | 8.22 (0.47) | 2.85 (0.02) | |||
| 12–16 y | 2,065 | 3.78 (1.23–6.00) | 6.82 (0.26) | 2.81 (0.01) | ||||
| >16 y | 2,017 | 3.83 (1.58–6.00) | 0.0476 | 6.03 (0.27) | 0.0002 | 2.83 (0.01) | 0.1400 | |
| Income ($) | <25K | 1,112 | 3.72 (2.11–5.73) | 7.70 (0.36) | 2.83 (0.02) | |||
| 25K to <50K | 1,669 | 3.82 (1.23–5.81) | 7.02 (0.29) | 2.83 (0.01) | ||||
| 50K+ | 1,759 | 3.79 (1.23–6.00) | 0.6532 | 5.72 (0.28) | <0.0001 | 2.81 (0.01) | 0.2801 | |
| Smoking | Never | 2,216 | 3.74 (1.23–5.77) | 8.29 (0.25) | 2.83 (0.01) | |||
| Former light | 997 | 3.99 (2.18–6.00) | 10.61 (0.38) | 2.83 (0.02) | ||||
| Former heavy | 787 | 3.86 (2.52–6.00) | 6.11 (0.42) | 2.85 (0.02) | ||||
| Current light | 110 | 3.82 (2.69–6.00) | 5.98 (1.13) | 2.83 (0.05) | ||||
| Current heavy | 421 | 3.57 (1.23–5.69) | 0.0461 | 6.74 (0.58) | 0.0123 | 2.74 (0.03) | 0.0114 | |
| BMI, kg/m2 | <25 | 1,256 | 3.59 (1.23–5.71) | 4.66 (0.33) | 2.79 (0.01) | |||
| 25 to <30 | 1,918 | 3.76 (1.72–6.00) | 5.92 (0.27) | 2.82 (0.01) | ||||
| 30 to <35 | 1,017 | 3.88 (2.06–6.00) | 7.77 (0.37) | 2.86 (0.02) | ||||
| 35+ | 518 | 3.74 (2.61–5.73) | 0.1528 | 12.05 (0.51) | <0.0001 | 2.86 (0.02) | 0.0028 | |
| Diabetes | No | 4,038 | 3.74 (1.23–5.98) | 6.30 (0.18) | 2.82 (0.01) | |||
| Yes | 666 | 3.82 (2.54–5.86) | 0.4516 | 8.88 (0.54) | <0.0001 | 2.84 (0.02) | 0.2526 | |
| Hypertension | No | 3,108 | 3.73 (1.23–5.81) | 5.86 (0.20) | 2.81 (0.01) | |||
| Yes | 1,585 | 3.80 (2.27–6.00) | 0.5102 | 8.18 (0.34) | <0.0001 | 2.86 (0.01) | 0.0020 | |
| LDL | Low | 2,897 | 3.78 (1.23–6.00) | 6.99 (0.23) | 2.83 (0.01) | |||
| High | 1,818 | 3.72 (1.23–5.79) | 0.4087 | 6.15 (0.26) | 0.0187 | 2.81 (0.01) | 0.2168 | |
| HDL | Low | 3,882 | 3.73 (1.23–5.75) | 6.77 (0.20) | 2.82 (0.01) | |||
| High | 835 | 3.94 (2.57–6.00) | 0.2344 | 6.20 (0.36) | 0.2165 | 2.86 (0.02) | 0.0232 | |
| Triglycerides | Low | 2,715 | 3.50 (1.23–5.41) | 6.04 (0.22) | 2.84 (0.01) | |||
| High | 2,002 | 4.16 (2.66–6.00) | <0.0001 | 7.52 (0.28) | <0.0001 | 2.80 (0.01) | 0.0047 | |
| Pocket depth | Low PD (extent 5 mm PD, Q1–Q3) | 3,521 | 3.67 (1.23–6.00) | 6.29 (0.18) | 2.81 (0.01) | |||
| High PD (extent 5 mm PD, Q4) | 1,149 | 3.88 (1.84–5.74) | 0.0382 | 7.70 (0.43) | 0.0005 | 2.87 (0.01) | 0.0003 | |
| Plaque | Low plaque (extent PI, Q1–Q3) | 3,346 | 3.81 (1.23–6.00) | 6.28 (0.20) | 2.81 (0.01) | |||
| High plaque (extent PI, Q4) | 1,118 | 3.66 (2.55–5.24) | 0.0973 | 7.87 (0.42) | 0.0001 | 2.90 (0.02) | <0.0001 | |
| Serum 8- isoprostane quartiles | 1 | NA | NA | 4.46 (0.34) | 2.94 (0.01) | |||
| 2 | NA | NA | 5.83 (0.35) | 2.89 (0.01) | ||||
| 3 | NA | NA | 7.95 (0.34) | 2.77 (0.01) | ||||
| 4 | NA | NA | 8.44 (0.34) | <0.0001 | 2.69 (0.02) | <0.0001 | ||
AAs, African Americans; BMI, body mass index; CRP, C-reactive protein; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; LS, least squares; NA, not applicable; PD, pocket depth; PI, Plaque Index; Q1, quartile 1, Q2, quartile 2; Q3, quartile 3; Q4, quartile 4; SE, standard error.
Serum 8-Isoprotane and Serum CRP Levels
In multivariate models (Table 2, left column) increasing quartiles of 8-isoprostane were associated with significantly increased mean serum CRP levels. Multivariate models were then constructed comparing CRP levels between quartiles of increasing serum 8-isoprostane in subsets of subjects having “high” or “low” levels of periodontal pocket depth (Table 2, 2 right columns). Quartiles of increasing serum 8-isoprostane concentration were associated with significant increases in serum CRP levels in subjects with either “high” or “low” pocket depth. “High” pocket depth was associated with increased serum CRP levels only among subjects within the top quartile of serum 8-isoprostane level.
Table 2.
Adjusted Mean CRP Levels by Quartiles of Serum 8-Isoprostane and by Extent of PD of ≥5 mm.
| Mean CRP (SE)a |
Mean CRP (SE)b |
||||
|---|---|---|---|---|---|
| Quartiles of Serum 8-Isoprostane | Overall | P Value vs Quartile 1 | Extent 5 mm PD in Bottom 3 Quartiles | Extent 5 mm PD in Top Quartile | Pr > |t|c |
| 1 | 4.74 (0.35) | Referent | 4.58 (0.40) | 5.27 (0.74) | 0.41 |
| 2 | 5.76 (0.35) | 0.04 | 5.77 (0.40)* | 5.64 (0.76) | 0.88 |
| 3 | 7.71 (0.35) | <0.0001 | 7.39 (0.41)*,** | 8.82 (0.68)*,** | 0.08 |
| 4 | 8.35 (0.36) | <0.0001 | 7.62 (0.40)*,** | 10.67 (0.74)*,**,† | 0.0003 |
CRP, C-reactive protein; PD, pocket depth; SE, standard error.
Adjusted for race/center, sex, body mass index (BMI), age, smoking, diabetes, hypertension, education, income, pocket depth, plaque, and serum low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides (n = 4,231).
Adjusted for race/center, sex, BMI, age, smoking, diabetes, hypertension, education, income, plaque, LDL, HDL, and triglycerides (n = 4,231). Significantly different (P < 0.05) from indicated quartile (i.e., quartile 1 = *, quartile 2 = **, or quartile 3 = †) of 8-isoprostane.
Probability of difference between mean CRP for subsets of extent 5 mm PD in bottom 3 quartiles vs. extent 5 mm PD in top quartile at indicated quartile of serum 8-isoprostane.
Interaction of Serum IgG Antibody Levels with Serum CRP
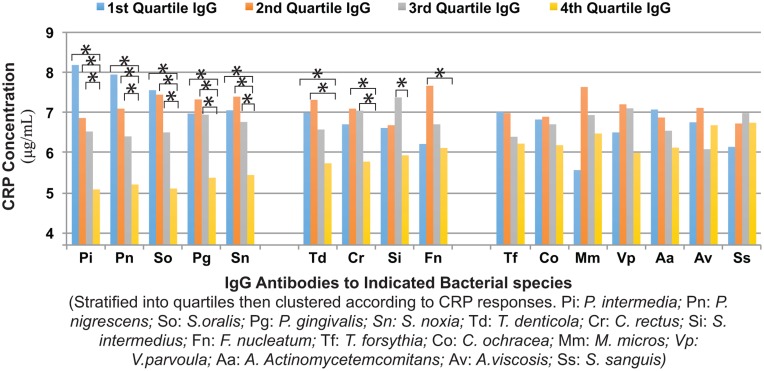
There were significant differences in serum CRP concentrations between multivariate models for quartiles of decreasing levels of 16 serum IgG antibodies and the IgG antibody clusters. Quartiles of individual IgG antibodies clustered into 3 groups (Fig. 1). In the first group (IgGs: Pi, Pn, So, Pg, and Sn), IgG quartiles Q1, Q2, and Q3 had significantly greater CRP levels than did Q4, the quartile with the highest concentration of IgG antibody. In the second group (IgGs: Td, Cr, Si, and Fn), at least 1 IgG quartile (Q1, Q2, or Q3) had higher CRP than did Q4. In the third group (IgGs: Tf, Co, Mm, Vp, Aa, Av, and Ss), no IgG quartile had a CRP level greater than in Q4. Quartiles of decreasing levels of total Biofilm, red, orange, and other IgG antibodies (Table 3) were associated with increased serum CRP levels. The differences between CRP concentrations in quartiles 3 and 4 were significant for the total and red IgG clusters but not for the orange and other IgG clusters.
Figure 1.
Associations between serum IgG antibodies and serum C-reactive protein (CRP) levels. IgG antibodies against each of 16 bacterial species are shown stratified into quartiles (Q1–Q4) of IgG concentration versus serum CRP concentrations (µg/mL). Organism titers are grouped according to response and significance. Three distinct antibody response patterns are shown (Pi, Pn, So, Pg, and Sn; Td, Cr, Si, and Fn; Tf, Co, Mm, Vp, Aa, Av, and Ss). All models were adjusted for race/center, age, sex, body mass index, income, education, smoking, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, pocket depth, and plaque comparing the lowest titer (Q4) to Q1 to Q3, with * indicating significant difference (P ≤ 0.01). n = 4,226 for all except Aa, Pg, Pn, and Pi (n = 4,225); Td (n = 4,224); Av (n = 4,216); and Tf (n = 4,103).
Table 3.
Adjusted Mean Serum CRP Levels According to Quartiles of Serum IgG Antibody vs. Clusters of Oral Bacteria.a
| Mean (SE) Serum CRP Concentration |
|||||||
|---|---|---|---|---|---|---|---|
| Quartiles of IgG Antibodies |
P Value for Indicated Comparison of Serum IgG Antibody Quartiles |
||||||
| Antibody Clusters | 1 | 2 | 3 | 4 | Q1–Q4 | Q2–Q4 | Q3–Q4 |
| Total biofilm IgG | 7.58 (0.37) | 7.47 (0.36) | 6.32 (0.36) | 5.28 (0.36) | <0.0001 | <0.0001 | 0.0415 |
| Total red IgG | 7.50 (0.36) | 6.71 (0.36) | 6.99 (0.36) | 5.42 (0.36) | <0.0001 | 0.0124 | 0.0021 |
| Total orange IgG | 7.71 (0.36) | 7.34 (0.36) | 6.07 (0.35) | 5.52 (0.35) | <0.0001 | 0.0003 | 0.2653 |
| Total other IgG | 7.48 (0.36) | 7.02 (0.36) | 6.27 (0.35) | 5.85 (0.35) | 0.0012 | 0.019 | 0.3918 |
CRP, C-reactive protein; SE, standard error.
Adjusted for race/center, age, sex, body mass index, income, education, smoking, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, pocket depth, and plaque (n = 4,103).
Discussion
Findings reported here support our hypothesis that increased oxidative stress modifies the association between periodontitis and serum CRP. The association between periodontitis and CRP appears only in the presence of high oxidative stress and is concomitantly associated with a suppression of serum IgG. Also, increased serum IgG antibodies to oral bacteria are associated with decreased serum CRP concentrations. Thus, physiologic differences in serum 8-isoprostane levels appear to modify systemic acute phase and serum IgG antibody responses and their association(s) with periodontitis. This suggests that oxidative stress level might modify the intrinsic risk from systemic exposure to the oral microflora by suppressing serum IgG.
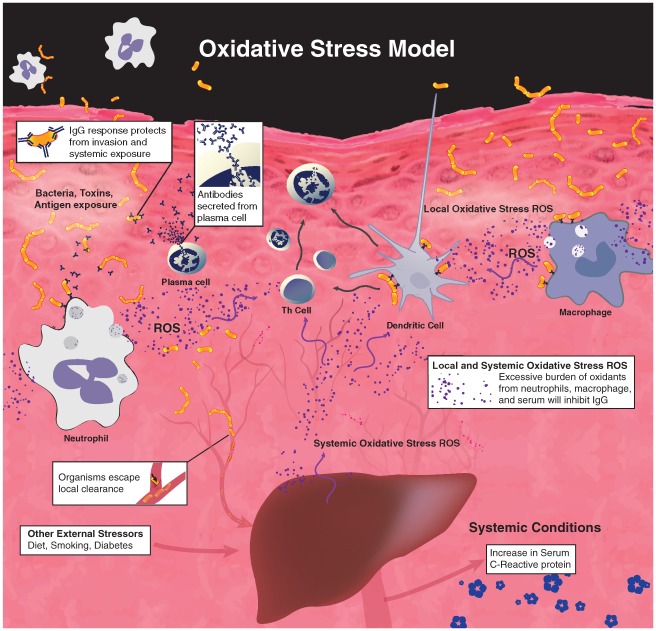
The findings in this report suggest a mechanistic model that appears in Figure 2. Specifically, oxidative stress shifts Th cell and antigen-presenting cell responses such that Th-1 cell and plasma cell functions likely become impaired (Singer et al. 2009), thereby decreasing IgG antibody responses against oral bacteria. Decreased serum-neutralizing IgG antibodies to oral bacteria enable plaque microbial toxins/molecules to enter the circulation and/or increase release of cytokines from the inflamed periodontium. Either outcome could induce systemic inflammatory mediators that prompt expression and release of acute phase reactants (e.g., CRP) from the liver and other tissues.
Figure 2.
Oxidative stress suppresses IgG response to pathogenic bacteria. Reactive oxygen species (ROS) are produced by activated phagocytes as their defense against pathogens. External stressors such as diet, smoking, and diabetes increase reactive oxygen species as well. Excessive burden of these oxidants will attenuate IgG synthesis, leading to compromised local clearance of pathogens. The increase of serum C-reactive protein shows correlation with periodontitis only when there is such overburden of ROS.
Although data presented here are cross-sectional in nature and only demonstrate associations, other investigations support this model. For example, Qin et al. (2014) demonstrate a series of oral bacteria genera (e.g., Streptococcus and Prevotella species) become prominent in the human gut microbiome in association with liver cirrhosis. Liver cirrhosis is associated with elevated serum CRP (Tilg et al. 1992) and 8-isoprostane (Aboutwerat et al. 2003) levels. Our finding (Fig. 1) that IgG antibodies to Streptococcus and Prevotella species are among the most potent in modifying serum CRP levels indicates a parallel between these findings and suggests hepatic function/dysfunction may be connected to levels of specific oral bacteria, IgG antibodies against those bacteria, and serum 8-isoprostane.
In cross-sectional studies, increased serum concentrations of 8-isoprostane have been correlated with increased levels of serum CRP in patients with end-stage renal disease (Lim et al. 2002), stroke patients (Sánchez-Moreno et al. 2004), and a healthy adult population (Dohi et al. 2007). Our findings indicate physiologic increases in serum 8-isoprostane concentrations relate to significant increases in serum CRP levels in a representative adult population. It is possible that lipid-based oxidative stress facilitates signaling pathways initiated by serum cytokine mediators (e.g., interleukin [IL]–6, IL-1, or tumor necrosis factor–α) induced by systemic exposure of the oral biofilm microflora (and/or other systemic inflammatory stimuli). In vitro studies show CRP expression is enhanced in the presence of elevated NF-κB expression (Voleti and Agrawal 2005; Bode et al. 2012), and NF-κB–mediated gene expression is increased by oxidative stress in hepatocytes (Gong et al. 2001) and other cell types (Li and Karin 1999).
Our multivariate models (Table 3) demonstrate that high levels of the total, other, orange, and red IgG antibody clusters (and 9 of 16 IgG serum antibodies) are associated with significantly lower serum CRP levels. This suggests that perhaps serum IgG antibodies to oral bacteria prevent signaling processes leading to expression of CRP (Fig. 2). The finding supports the concept that IgG antibodies to periodontal pathogens have the strongest effects on CRP levels, although modulating effects on CRP levels are not exclusive to those antibodies (Table 3 and Fig. 1). Periodontal pathogens can advance periodontal pathology, break down periodontal barrier functions, invade the periodontium, and lead to bacteremia. Our findings, however, indicate other oral bacteria (e.g., So and Sn) and IgG antibodies against them also are important modifiers of serum CRP responses. Perhaps periodontal pathology prompted by periodontal pathogens enables systemic exposure of other oral bacteria potent in prompting CRP responses. CRP is named for its interaction with Streptococcus antigens (Pepys and Hirschfield 2003), and a connection of Streptococcus species with liver function/dysfunction is evident from other investigations (Qin et al. 2014). Our findings also are consistent with the concept (Kinane et al. 2015) that subject populations differ in the ability to contain periodontal inflammation to a localized response as opposed to a systemic response.
This investigation is limited by our use of secondary analyses of data that are cross-sectional in nature. However, this is a large population-based data set that includes measures of oxidative stress, serum IgG antibodies, and CRP using methods that are well established and informative.
Together, these findings suggest systemic oxidative stress (at the lipid level) and serum IgG antibody levels to oral bacteria are important modifiers of systemic acute phase responses to the oral microflora and periodontitis (Fig. 2). The effects of systemic oxidative stress and serum IgG antibodies function in opposing directions in modifying serum CRP levels and indeed have an inverse relationship between each other. In studies not shown here, we found ARIC subjects with serum 8-isoprostane levels above the median and having elevated (>median) IgG antibodies to Pi and Pn had decreased (P < 0.05) serum CRP, while for subjects with serum 8-isoprostane below the median, having increased IgG antibodies to Pi, So, and Sn was associated with decreased CRP. This suggests oxidative stress also is related to qualitative changes in the profile of IgG antibodies modifying serum CRP levels. Defining quantitative and qualitative effects of oxidative stress on IgG antibody responses to oral bacteria and their modification of CRP responses will be a rich area for research to define mechanistic pathways connecting periodontitis to systemic inflammation, inflammatory diseases, and perhaps clinical outcomes.
Our findings may help to explain differences in the literature regarding the response of serum CRP levels to periodontal therapy (Ioannidou et al. 2006). That is, physiologic differences in serum levels of 8-isoprostane in a community-based population were associated with significant differences in baseline serum CRP, serum IgG antibodies to oral bacteria, and the association of CRP with periodontitis. Without accounting for these population variables (or key variables in our multivariate models), demonstrating an impact of periodontal therapy on serum CRP levels could prove difficult.
Increased serum levels of 8-isoprostane have been associated with clinical outcomes in atherosclerosis (Mueller et al. 2004) and preterm birth clinical trials (Parra et al. 2005). The findings presented here suggest serum levels of 8-isoprostane may be a useful variable for the identification of patient populations wherein periodontitis would be a risk factor for increased systemic inflammation and consequent clinical outcomes. Clinical trials focused on the role of periodontitis in systemic inflammation, and systemic diseases may benefit from controlling for this variable and/or using it to identify subpopulations wherein periodontitis presents the greatest risk for systemic disease.
Dietary levels of antioxidants and flavonoids have been related to decreased serum CRP levels in various patient populations (Devaraj and Jialal 2000; Prasad 2006; Chun et al. 2008; di Giuseppe et al. 2008; Block et al. 2009). Together with our findings, this suggests intervention strategies targeting oxidative signaling pathways may modify systemic immune and inflammatory responses to periodontitis and merit further exploration.
Author Contributions
R.E. Singer, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; K. Moss, J.D. Beck, S. Offenbacher, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; S.J. Kim, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
The ARIC study is supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC- 55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01- HC-55022, and R01-DE-11551. This study was also funded by National Institutes of Health training grant T90 DE021986 (to S.J.K.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aboutwerat A, Pemberton PW, Smith A, Burrows PC, McMahon RF, Jain SK, Warnes TW. 2003. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta. 1637(2):142–150. [DOI] [PubMed] [Google Scholar]
- Atherosclerosis Risk in Communities Investigators. 1989. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 129(4):687–702. [PubMed] [Google Scholar]
- Barreiro LB, Quintana-Murci L. 2010. From evolutionary genetics to human immunology: how selection shapes host defence genes. Nat Rev Genet. 11(1):17–30. [DOI] [PubMed] [Google Scholar]
- Block G, Jensen CD, Dalvi TB, Norkus EP, Hudes M, Crawford PB, Holland N, Fung EB, Schumacher L, Harmatz P. 2009. Vitamin C treatment reduces elevated C-reactive protein. Free Radic Biol Med. 46(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F. 2012. Hepatic acute phase proteins—regulation by IL-6– and IL-1–type cytokines involving STAT3 and its crosstalk with NF-kappaB–dependent signaling. Eur J Cell Biol. 91(6–7):496–505. [DOI] [PubMed] [Google Scholar]
- Chun OK, Chung SJ, Claycombe KJ, Song WO. 2008. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 138(4):753–760. [DOI] [PubMed] [Google Scholar]
- Cottone S, Mule G, Nardi E, Vadala A, Lorito MC, Guarneri M, Arsena R, Palermo A, Cerasola G. 2007. C-reactive protein and intercellular adhesion molecule-1 are stronger predictors of oxidant stress than blood pressure in established hypertension. J Hypertens. 25(2):423–428. [DOI] [PubMed] [Google Scholar]
- D’Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, Tonetti MS. 2004. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 83(2):156–160. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Jialal I. 2000. Alpha tocopherol supplementation decreases serum C-reactive protein and monocyte interleukin-6 levels in normal volunteers and type 2 diabetic patients. Free Radic Biol Med. 29(8):790–792. [DOI] [PubMed] [Google Scholar]
- di Giuseppe R, Di Castelnuovo A, Centritto F, Zito F, De Curtis A, Costanzo S, Vohnout B, Sieri S, Krogh V, Donati MB, et al. 2008. Regular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy Italian population. J Nutr. 138(10):1939–1945. [DOI] [PubMed] [Google Scholar]
- Dohi Y, Takase H, Sato K, Ueda R. 2007. Association among C-reactive protein, oxidative stress, and traditional risk factors in healthy Japanese subjects. Int J Cardiol. 115(1):63–66. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Machen RL, Steffen MJ, Willmann DE. 1997. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 107(2):347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Waris G, Tanveer R, Siddiqui A. 2001. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF–kappa B. Proc Natl Acad Sci U S A. 98(17):9599–9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelman GJ, Walter MF, Adhikarla R, Gross J, Dallal GE, Levin NW, Blumberg JB. 2001. Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int. 59(5):1960–1966. [DOI] [PubMed] [Google Scholar]
- Ioannidou E, Malekzadeh T, Dongari-Bagtzoglou A. 2006. Effect of periodontal treatment on serum C-reactive protein levels: a systematic review and meta-analysis. J Periodontol. 77(10):1635–1642. [DOI] [PubMed] [Google Scholar]
- Kalow W. 1997. Pharmacogenetics in biological perspective. Pharmacol Rev. 49(4):369–379. [PubMed] [Google Scholar]
- Kinane DF, Zhang P, Benakanakere M, Singleton J, Biesbrock A, Nonnenmacher C, He T. 2015. Experimental gingivitis, bacteremia and systemic biomarkers: a randomized clinical trial. J Periodontal Res. 50(6):864–869. [DOI] [PubMed] [Google Scholar]
- Koromantzos PA, Makrilakis K, Dereka X, Offenbacher S, Katsilambros N, Vrotsos IA, Madianos PN. 2012. Effect of non-surgical periodontal therapy on C-reactive protein, oxidative stress, and matrix metalloproteinase (MMP)-9 and MMP-2 levels in patients with type 2 diabetes: a randomized controlled study. J Periodontol. 83(1):3–10. [DOI] [PubMed] [Google Scholar]
- Li N, Karin M. 1999. Is NF-kappaB the sensor of oxidative stress? FASEB J. 13(10):1137–1143. [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. 2002. Nature. 420(6917):868–874. [DOI] [PubMed] [Google Scholar]
- Lim PS, Chang YM, Thien LM, Wang NP, Yang CC, Chen TT, Hsu WM. 2002. 8-Iso-prostaglandin F2alpha as a useful clinical biomarker of oxidative stress in ESRD patients. Blood Purif. 20(6):537–542. [DOI] [PubMed] [Google Scholar]
- Löe H. 1967. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 38(6, Suppl):610–616. [DOI] [PubMed] [Google Scholar]
- Mueller T, Dieplinger B, Gegenhuber A, Haidinger D, Schmid N, Roth N, Ebner F, Landl M, Poelz W, Haltmayer M. 2004. Serum total 8-iso-prostaglandin F2alpha: a new and independent predictor of peripheral arterial disease. J Vasc Surg. 40(4):768–773. [DOI] [PubMed] [Google Scholar]
- Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. 2001. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 72(9):1221–1227. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Beck JD, Jared HL, Mauriello SM, Mendoza LC, Couper DJ, Stewart DD, Murtha AP, Cochran DL, Dudley DJ, et al. ; Maternal Oral Therapy to Reduce Obstetric Risk (MOTOR) Investigators. 2009. Effects of periodontal therapy on rate of preterm delivery: a randomized controlled trial. Obstet Gynecol. 114(3):551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Beck JD, Moss K, Mendoza L, Paquette DW, Barrow DA, Couper DJ, Stewart DD, Falkner KL, Graham SP, et al. 2009. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 80(2):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Madianos PN, Champagne CM, Southerland JH, Paquette DW, Williams RC, Slade G, Beck JD. 1999. Periodontitis-atherosclerosis syndrome: an expanded model of pathogenesis. J Periodontal Res. 34(7):346–352. [DOI] [PubMed] [Google Scholar]
- Parra M, Rodrigo R, Barja P, Bosco C, Fernandez V, Munoz H, Soto-Chacon E. 2005. Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am J Obstet Gynecol. 193(4):1486–1491. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. 2003. C-reactive protein: a critical update. J Clin Invest. 111(12):1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. 2006. C-reactive protein (CRP)–lowering agents. Cardiovasc Drug Rev. 24(1):33–50. [DOI] [PubMed] [Google Scholar]
- Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, et al. 2014. Alterations of the human gut microbiome in liver cirrhosis. Nature. 513(7516):59–64. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. 2006. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 11(5):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakellari D, Socransky SS, Dibart S, Eftimiadi C, Taubman MA. 1997. Estimation of serum antibody to subgingival species using checkerboard immunoblotting. Oral Microbiol Immunol. 12(5):303–310. [DOI] [PubMed] [Google Scholar]
- Sánchez-Moreno C, Dashe JF, Scott T, Thaler D, Folstein MF, Martin A. 2004. Decreased levels of plasma vitamin C and increased concentrations of inflammatory and oxidative stress markers after stroke. Stroke. 35(1):163–168. [DOI] [PubMed] [Google Scholar]
- Schreck R, Albermann K, Baeuerle PA. 1992. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 17(4):221–237. [DOI] [PubMed] [Google Scholar]
- Sies H. 2000. What is oxidative stress? In: Keaney JF Jr., editor. Oxidative stress and vascular disease. New York (NY): Springer; p. 1–8. [Google Scholar]
- Singer RE, Moss K, Beck JD, Offenbacher S. 2009. Association of systemic oxidative stress with suppressed serum IgG to commensal oral biofilm and modulation by periodontal infection. Antioxid Redox Signal. 11(12):2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. 2003. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med. 163(10):1172–1179. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol. 25(2):134–144. [DOI] [PubMed] [Google Scholar]
- Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G, Huber C. 1992. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 103(1):264–274. [DOI] [PubMed] [Google Scholar]
- Tonetti MS. 2009. Periodontitis and risk for atherosclerosis: an update on intervention trials. J Clin Periodontol. 36(Suppl 10):15–19. [DOI] [PubMed] [Google Scholar]
- Voleti B, Agrawal A. 2005. Regulation of basal and induced expression of C-reactive protein through an overlapping element for OCT-1 and NF-kappaB on the proximal promoter. J Immunol. 175(5):3386–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]