Abstract
Since its initial identification as one of the genes most highly upregulated upon T-cell activation, osteopontin (or Eta-1, as it was designated then) has been demonstrated to have many roles in the regulation of the immune response on multiple levels. It contributes to the development of immune-mediated and inflammatory diseases, and it regulates the host response to infection. In some cases, the mechanisms of these effects have been elucidated, while other mechanistic functions of the protein remain obscure. The protein itself makes these analyses complex, since it binds to a series of different integrins, and in addition to its classically secreted form, an intracellular form of osteopontin has been identified, which participates in several aspects of immune regulation. In this review, we focus on the role of osteopontin in a series of immune-related diseases, particularly those where significant advances have been made in recent years: multiple sclerosis, rheumatoid arthritis, lupus and related diseases, Sjögren’s disease, colitis, and 1 area of inflammatory pathology, alcoholic and nonalcoholic liver diseases. A recurring theme in these diseases is a link between osteopontin and pathogenic T cells, particularly T helper 17 cells, where osteopontin produced by dendritic cells supports IL-17 expression, contributing to pathology. In addition, a role for osteopontin in B-cell differentiation is becoming clear. In general, osteopontin contributes to pathology in these diseases, but there are examples where it has a protective role; deciphering the mechanisms underlying these differences and the specific receptors for osteopontin will be a research challenge for the future. Aside from its newly discovered role in the development of Sjögren’s disease, the role of osteopontin in inflammatory conditions in the oral cavity is still poorly understood. Elucidation of this role will be of interest.
Keywords: immunity, Th17, B cells, autoimmunity, integrins, OPN
Introduction/Historical Perspective
As a secreted, phosphorylated integrin-binding protein, osteopontin (OPN) is an atypical immune regulator. Nevertheless, its role in modulating the immune response has been definitively established over the last 3 decades. One of the earliest descriptions of OPN was as Eta-1, found by Cantor’s group to be one of the most upregulated genes early after T-cell activation (reviewed in Uede 2011). Subsequently, OPN was shown to regulate T-cell development, enhancing differentiation along the T helper (Th) 1 pathway and suppressing creation of Th2 cells (Ashkar et al. 2000). Later, OPN was shown to support Th17 differentiation (Murugaiyan et al. 2008). OPN was independently identified as a major noncollagenous protein of bone and as a protein overexpressed in cancer and associated with metastasis. The OPN promoter has numerous transcription factor–binding sites, and OPN gene expression is often highly upregulated in various diseases and pathologies. The heterogeneous roles of OPN are still poorly understood and are likely in part related to its ability to support cell migration as an integrin-binding protein, but its role in cellular signaling has been firmly established. The recent identification of an intracellular form of OPN (iOPN) that mediates aspects of intracellular signaling introduced new dimensions to the biology of this protein. Here, we focus on recent findings on the role of OPN in immune-mediated diseases, with emphasis on the molecular mechanisms of OPN effects, as summarized in the Table. Since immune-mediated diseases typically result from a misdirected immune response, OPN frequently contributes to pathology in these situations. But it must be kept in mind that OPN has an important protective role in enhancing the immune response in the case of various infections. For details on earlier work, please see several recent reviews (Inoue and Shinohara 2011; Konno et al. 2011; Rittling 2011; Uede 2011).
Table.
Role of OPN in Immune-related Diseases.
| Disease: Mechanism | Species: H/M | Effect of OPN: Cell Types Involved | Reference |
|---|---|---|---|
| Inflammatory bowel disease | |||
| • Loss of OPN in CD103– DCs resulted in disease amelioration. Transgenic overexpression of OPN in CD103– DCs rendered them highly pathogenic. | M | Pathogenic, CD103– DCs (mesenteric lymph nodes) | Kourepini et al. (2014) |
| • OPN haplotype significantly associated with CD. | H | ??? | Glas et al. (2011) |
| • Bovine milk OPN in drinking water suppressed DSS-induced colitis in part by restoring macrophage activity and TGFβ1 release, which promotes tissue repair. | M | Protective, DSS-induced acute colitis | da Silva et al. (2009) |
| Hepatic diseases | |||
| • In human alcoholic liver disease, OPN expression correlated with hepatic inflammation, TGFβ expression, and neutrophil accumulation. | H | Potentially pathogenic, hepatic cells, neutrophils | Patouraux et al. (2012) |
| • OPN activated hepatic stellate cells and induced collagen I, leading to liver fibrosis via integrin avβ3 engagement and activation of the PI3K/pAkt/NF-κB signaling pathway. | M | Pathogenic, hepatic stellate cells | Urtasun et al. (2012) |
| • LPS-induced OPN expression in human hepatic stellate cells; alcohol-fed OPN-deficient mice were protected from neutrophil infiltration and displayed reduced expression of inflammatory cytokines. | H, M, in vitro and in vivo | Pathogenic, hepatic stellate cells, neutrophils | Morales-Ibanez et al. (2013) |
| • Increased neutrophil infiltration in OPN-/- mice in alcoholic hepatitis and alcoholic liver disease experimental models. | M | Protective, neutrophils | Lazaro et al. (2015) |
| Multiple sclerosis | |||
| • OPN caused disease relapse in mice and promoted the survival of activated T cells. | M, H, in vitro and in vivo | Pathogenic role, T cells; DCs | Hur et al. (2007) |
| • OPN expression increased in peripheral and central nervous system DCs in EAE mice. Anti-OPN antibody improved EAE severity by reducing IL-17 production. Increased OPN in DCs and OPN receptors on T cells in patients with multiple sclerosis. | M, in vivo, H, in vitro | Pathogenic, DCs | Murugaiyan et al. (2008) |
| Emphysema | |||
| • Lack of OPN delayed disease pathologies with reduced IL-17 and MMP-12 expression. | M, in vivo | Pathogenic, DCs | Shan et al. (2012) |
| Lupus | |||
| • Transgenic expression of OPN in B cells expanded B-cell subsets with high immunoglobulin/autoantibody levels. | M, in vivo | Pathogenic, B-cell development | Iizuka et al. (1998) |
| • iOPN regulated B-cell development through BCL6 stabilization in T follicular helper cells. | M, in vivo | Pathogenic, B cell | Leavenworth et al. (2015) |
| Psoriasis | |||
| • Lack of OPN prevents the imiquimod-induced expansion of B-cell population associated with skin inflammation; OPN induced IL-17 expression in inflammatory T cells. | M, in vivo | Pathogenic, B cell | Frenzel et al. (2015) |
| Sjögren’s syndrome | |||
| • Transgenic mice expressing OPN spontaneously developed Sjögren’s disease, characterized by lymphocytic infiltration into salivary tissue and higher autoantibody levels. | M | Pathogenic, B cell | Husain-Krautter et al. (2015) |
| Rheumatoid arthritis | |||
| • Interaction between thrombin-cleaved OPN and α9β1 integrin promoted arthritis. | M, in vivo | Pathogenic | Kon et al. (2014) |
| Allergy and asthma | |||
| • OPN enhanced or suppressed IgE production in mouse models. OPN is overexpressed in asthma. | M, H | Pathogenic/protective | Konno et al. (2011) |
As described in the text, immune-related diseases associated with OPN are listed, together with information about the role of OPN, and whether the protein has a pathogenic or protective effect. See text for abbreviations.
CD, Crohn’s disease; DC, dendritic cell; DSS, dextran sulfate sodium; EAE, experimental autoimmune encephalomyelitis; H, human; iOPN, intracellular osteopontin; M, murine; OPN, osteopontin.
OPN Structure/Receptors
OPN is a member of a class of proteins called intrinsically disordered proteins (Kurzbach et al. 2013). Several studies confirmed the original observation (Fisher et al. 2001) that OPN has little or no detectable tertiary structure by NMR. OPN is heavily posttranslationally modified, with as many as 36 phosphorylation sites (Christensen et al. 2005), although the extent of phosphorylation varies with the source of the protein (Christensen et al. 2007). It is also a very acidic protein: the pI of the unmodified protein is 4.37, lowering to 3.35 when fully phosphorylated, as in milk OPN.
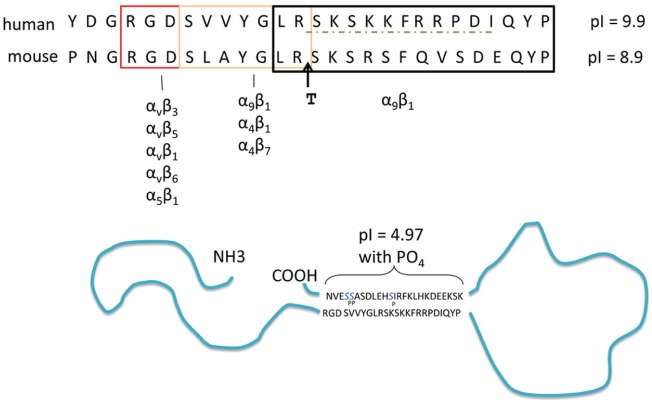
OPN binds to a series of integrins through 2 adjacent sequences (Fig. 1). One, containing a central RGD, binds with high affinity to αv-containing integrins, most famously αvβ3. The adjacent SVVYGLR sequence binds α9β1, as well as α4β1 and α4β7, but in the case of the α4β1 integrin, OPN can bind to only the highest activation state of the integrin (Hui et al. 2015). Recently, a third adjacent conserved sequence has been shown to mediate effects of OPN: LRSKSRSFQVSDEQY in mouse or RSKSKKFRR in human. LRSK . . . QY binds to α9β1 integrin and participates in development of some forms of arthritis (Kon et al. 2014), while RSKSKKFRR has chemotactic activity for human dendritic cells (DCs), although the receptor for this protein has not been identified (Shao et al. 2014). A direct or perhaps indirect interaction of OPN with some forms of CD44 has been reported, but the specific binding sequences in OPN remain elusive (Uede 2011).
Figure 1.
Potential intermolecular interactions within osteopontin (OPN). Top: sequence of the integrin-binding sequences of human and mouse OPN; the specific integrins interacting with different sequences (boxed) are indicated. T indicates the thrombin cleavage site; the dashed line indicates the sequence involved in migration of dendritic cells. The isoelectric point of this region from human and mouse OPN is indicated. Bottom: possible 3-dimensional structure of OPN, showing interaction between the acidic C-terminal peptide and the basic integrin-binding domain.
In recent years, evidence has been accumulating for some degree of secondary structure in OPN, despite its unstructured nature. An interaction between the N- and C-terminal ends of OPN has been detected in NMR studies (Yamaguchi et al. 2010). A biological effect of this interaction may be responsible for the recent observation that modification of the extreme C-terminal end of OPN, which is highly conserved across species, by addition of a his-tag or phosphorylation can block the ability of OPN to support binding to αvβ3 integrin, as detected by adhesion assays (Christensen et al. 2012). Cleavage of OPN by thrombin, physically separating the RGD sequence from the C-terminus, eliminates the inhibitory effect. This result is consistent with a low-affinity interaction between the C-terminus and the region surrounding the RGD, with this interaction blocking access to the RGD sequence. Kazanecki et al. (2007) suggested that short segments of β-sheet formed in these regions may support this interaction. It is also of interest that the sequence containing the identified cell-binding regions is the only region of the molecule with a basic pI (Fig. 1). Thus, a combination of β-sheet and electrostatic interactions may form the basis of this interaction. That thrombin cleavage prevents the C-terminal blocking effect demonstrates that close proximity of the 2 sequences is required and that the affinity of the interaction is not sufficient to support binding between 2 separate molecules. This view is supported by high-resolution structural studies showing that OPN undergoes cooperative folding and unfolding events (Kurzbach et al. 2013).
OPN in Multiple Sclerosis
OPN was identified as 1 of the disease-specific transcripts in brain tissue from patients with multiple sclerosis (Chabas et al. 2001). Multiple sclerosis is regarded as an autoimmune disease caused by infiltration of reactive T cells into brain lesions, and it cycles through period of disease and remission. Chabas et al. (2001) showed that in experimental autoimmune encephalitis (a mouse model for multiple sclerosis), the disease is less severe in mice lacking OPN, thereby establishing its pathogenic role in this disease, particularly in the prevention of remission. Mechanistic studies demonstrated that OPN administration can cause disease recurrence in mice that are in remission, and they linked OPN-induced signaling pathways to survival of pathogenic T cells in vitro and in vivo (Hur et al. 2007) and to production of IL-17 by CD4+ T cells (Murugaiyan et al. 2008). These results suggest that OPN contributes to the progression of multiple sclerosis in animal models by amplifying the T-cell response through multiple mechanisms. More recent effort has focused on the role of OPN in human disease and the correlation of OPN expression with symptoms during treatment (reviewed in Harris and Sadiq 2009). While OPN levels are reduced following treatment, 1 study found no specific association between OPN levels and disease activity (Kivisakk et al. 2014). Since OPN is upregulated in many pathologic conditions, a lack of specificity may limit the use of OPN as a biomarker in this and other diseases.
An effect of OPN on Th17 cells was also recently demonstrated in emphysema (Shan et al. 2012). OPN was found to be one of the most highly upregulated genes in DCs from lungs of emphysema patients as compared with healthy controls. In a mouse model of cigarette smoke–induced emphysema, development of disease was reduced in OPN-deficient mice, in parallel with reduced IL-17, IFNγ, and MMP-12 expression. Antigen-presenting cells from wild-type but not OPN-deficient mice with emphysema induced IL-17 expression in normal T cells. These results can explain the observations that emphysema patients express high levels of IL-17A and IFNγ in lung tissue: expression of these cytokines is upregulated through antigen-presenting cell–expressed OPN interacting with T cells, resulting in upregulation of IFNγ and IL-17. These cytokines were shown in other experiments to contribute to MMP-12 expression and development of emphysema in the mouse model. These results are consistent with the observations in mouse models of multiple sclerosis, where DC expression of OPN induces IL-17 expression in T cells, thus contributing to disease.
Systemic Lupus Erythematosus and B Cell–related Diseases (Sjögren’s)
A role of OPN in systemic lupus erythematosus (SLE) has been proposed on the basis of expression and functional studies. SLE is an autoimmune disease that affects numerous organs, originating in a deregulation of B and T lymphocytes, resulting in production of autoantibodies, most notoriously against double-stranded DNA (Rekvig 2015). Several molecular mechanisms resulting in B-cell dysregulation have been identified, with a central theme being dysregulated signaling in follicular B cells and altered immune cell reactivity to self-antigens (Han et al. 2015). B cells migrate from the bone marrow to specialized structures in peripheral lymph nodes known as follicles. After antigenic stimulation, follicles are converted to germinal centers, where specialized T follicular helper (Tfh) cells interact with B cells to stimulate antibody gene rearrangement and differentiation into plasma and memory cells. A second T-cell subset derived from regulatory T cells, Tfr (T follicular regulatory), has more recently been shown to regulate B-cell differentiation, and defects in these cells have been suggested as contributing to autoimmunity (reviewed in Sage and Sharpe 2015).
The association of OPN with SLE in humans is reflected in increased expression in patients with SLE. In addition, polymorphisms in the OPN gene that may affect expression levels have been identified in several studies in SLE patients, but there is a need for more extensive studies to validate these results (reviewed in Kaleta 2014). At a mechanistic level, there are demonstrated roles for OPN in B-cell development. An early description of OPN described its role in promoting immunoglobulin production in B-cell preparations (Lampe et al. 1991) and noted strongly increased OPN expression in T cells in the MRL/lpr mouse model of SLE. This observation was later confirmed in vivo, where transgenic expression of OPN in B cells resulted in expansion of B-cell subsets and increased immunoglobulin levels (Iizuka et al. 1998). Most compelling, these transgenic OPN-overexpressing mice developed anti-DNA antibodies, demonstrating a direct link between OPN overexpression and SLE-type immune dysregulation.
OPN has been implicated in other diseases with B-cell involvement. In a mouse model of psoriasis, OPN deficiency prevented the expansion of the B-cell population associated with skin inflammation (Frenzel et al. 2015). Of considerable interest is the recent report that transgenic mice expressing OPN in B cells spontaneously develop Sjögren’s disease, characterized by lymphocytic infiltration into salivary tissue, increased autoantibody levels, and reduced saliva production (Husain-Krautter et al. 2015). Augmented B-cell survival and proliferation were observed in this model, but a role of the increased OPN levels on T-cell function is also a possible mechanism. Together these observations support a role for OPN in promoting B-cell differentiation, resulting in increased antibody production. While this effect of OPN may be protective in the case of various infections, it appears to be pathologic in autoimmune disorders where autoantibody production results in pathology.
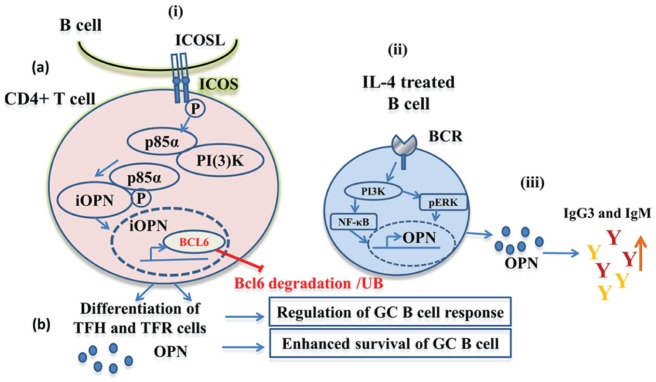
A novel mechanistic role for iOPN in development of B cells in germinal centers was demonstrated by data from Leavenworth et al. (2015), using a new mouse model that expresses only iOPN. In these mice, the endogenous OPN gene was replaced by a mutated allele, in which sequences downstream of the ATG were removed, eliminating the signal sequence and fusing the ATG to the first codon of the secreted form of OPN. The mutant allele also contains flox sites flanking a stop codon upstream of the ATG, so these mice are effectively OPN deficient. Upon Cre expression, the stop codon is removed, leading to expression of OPN lacking a signal sequence in tissues where Cre is expressed. This mouse strain was used to show that iOPN is required for high-affinity antibody production and generation of B cells in response to systemic antigen. In OPN-deficient animals, antibody production and B-cell numbers in germinal centers were severely reduced, while these features were restored in iOPN-expressing mice. B-cell differentiation in lymph node germinal centers in the absence of OPN was due to deficient Tfh cells that provide help for B-cell development in germinal centers, as well as Tfr cells. iOPN supported Tfh- and Tfr-cell differentiation and survival through a mechanism linking ICOS ligand binding to stabilization of the transcriptional regulator BCL6. In this scenario, iOPN binds to the 85-kD subunit of PI3K, which is released following ICOS stimulation, and the complex relocates to the nucleus, where OPN binds BCL-6, preventing its ubiquitination and degradation (Fig. 2). These results may explain the effect of OPN on B cell–associated diseases, since increased expression of iOPN would enhance Tfh-cell numbers, increasing B-cell differentiation and antibody production. Since iOPN and secreted OPN are encoded by a single mRNA, it is likely that they are regulated in parallel and that increased serum OPN would reflect increased iOPN expression in T and other cells. B-cell dysregulation in follicular B cells is thought to underlie the development of SLE (Han et al. 2015), so this mechanism may underlie some pathology associated with this disease.
Figure 2.
Schematic of osteopontin (OPN)–mediated regulation of B-cell response. (i) Role of intracellular form of OPN (iOPN) in B-cell development. (a) Activation of ICOS on CD4+ T cells by ICOS-L on antigen-presenting cells or B cells stimulates p85α release and complex formation with iOPN. This complex enters into the nucleus where iOPN interacts with BCL6, protecting it from ubiquitination, driving differentiation into Tfh cells and Tfr cells (Leavenworth et al. 2015). (b) Tfh cells release OPN, enhancing survival and sustained germinal center (GC) B-cell response to antigens. (ii) Potential role of B cell–derived OPN in humoral immunity. In severe infections, allergic, and other disorders, IL-4 activates B-cell signaling through BCR and PI3K, NF-κB, and pERK, which induces OPN expression and secretion (Rothstein and Guo 2009). (iii) Since OPN is a polyclonal B-cell activator, it can enhance B-cell immunoglobulin production, particularly the IgM and IgG3 antibodies (Iizuka et al. 1998).
Rheumatoid Arthritis
An autoimmune disorder affecting primarily women >40 y old, rheumatoid arthritis (RA) arises from accumulation of immune cells in the synovial joint spaces, resulting in pain and reduced range of motion. The role of OPN in this disorder was initially suggested when OPN-deficient mice were protected against development of joint pathology in a mouse model of RA. Later work implicated the SLAYGLR (in mouse OPN) α9 integrin-binding sequence of OPN in this protection, and the thrombin-cleaved form of OPN was found to be present at higher levels in RA patients than in healthy controls. Antibodies specific for the SLAYGLR/SVVYGLR sequence provided protection in both mouse and nonhuman primate models of RA (reviewed in Uede 2011). Recently, a sequence just C-terminal to the SLAYGLR has also been shown to mediate OPN effects on RA development in mice through the α9 integrin (Kon et al. 2014). The mechanism of the effects of OPN in this disease include recruitment of inflammatory cells and production of pathogenic cytokines in synovial fibroblasts and macrophages, mediated by the α9β1 integrin. More recently, however, antihuman OPN SVVYGLR antibodies failed to provide significant clinical improvement in RA patients in a clinical trial, although this was a phase 1 study with a relatively small number of patients (Boumans et al. 2012). Since the association of OPN serum levels and RA disease severity is controversial (Iwadate et al. 2014; Ji et al. 2014), this result suggests that there may be variability in the role of OPN in individual patients with RA. In support of this idea, a recent large study implicates certain OPN gene polymorphisms as associated with RA, identifying OPN as a potential susceptibility gene for this disease (Gazal et al. 2015).
OPN in Liver Damage and Injury
There has been considerable interest recently in the role of OPN in inflammatory diseases of the liver. Alcoholic liver disease (ALD), including alcoholic hepatitis (AH), is a clinical condition that manifests from fatty liver to hepatic inflammation, necrosis, progressive fibrosis, cirrhosis, and hepatocellular carcinoma (Gao and Bataller 2011). Similarly, liver dysfunction associated with nonalcoholic fatty liver disease is associated with liver fibrosis, cirrhosis, and cancer (Syn et al. 2012). ALD and comorbidities are associated with infiltration of inflammatory cells, including Kupffer cells, monocytes, macrophages, neutrophils, and lymphocytes to liver, resulting in liver damage. Several studies suggest that hepatic inflammation is mediated partially through expression of OPN by various immune and nonimmune cell types. In a rat model of AH, OPN expression correlated with neutrophil infiltration, especially in female rats, and blocking OPN with a specific antibody reduced the neutrophil accumulation (Ramaiah and Rittling 2007). This is just 1 example of several studies where OPN was shown to exert proinflammatory effects through integrin interactions in liver inflammation, enhancing neutrophil accumulation (reviewed in Ramaiah and Rittling 2007).
These results are supported by recent studies in human liver diseases. OPN appeared as 1 of the most highly upregulated genes in transcriptome analysis of patients with AH (Morales-Ibanez et al. 2013), and OPN expression correlated with hepatic inflammation, TGFβ expression, and neutrophil accumulation in ALD (Patouraux et al. 2012). OPN in vitro activates hepatic stellate cells and initiates the increase in collagen I leading to liver fibrosis, via integrin αvβ3 engagement and activation of the PI3K/pAkt/NF-κB signaling pathway (Urtasun et al. 2012). LPS induced OPN expression in human hepatic stellate cells, and alcohol-fed OPN-deficient mice were protected from neutrophil infiltration and displayed reduced expression of inflammatory cytokines (Morales-Ibanez et al. 2013), altogether supporting a pathogenic role of OPN in AH. Alcohol consumption has been suggested to lead to intestinal dysbiosis and increased intestinal permeability (leaky gut) that results in translocation of bacteria-derived LPS from the gut to the liver (Hartmann et al. 2015). This mechanism likely explains the observation that orally administered OPN from bovine milk can suppress the development of AH in a mouse model, through increasing gut epithelial integrity and reducing neutrophil accumulation in the liver (Ge et al. 2013).
OPN action in alcohol-induced liver injury is suggested to be mediated by hepatic stellate cell signaling and by supporting cell migration and activation of fibrinolysis, extracellular matrix, and fibrogenic pathways (Seth et al. 2014). However, contrasting results have also been reported, where OPN was shown to have a protective role in AH and experimental ALD, with increased neutrophil infiltration in OPN– mice (Lazaro et al. 2015). The reason for these different results is not immediately apparent but may be related to the specifics of the disease induction model and, possibly, the sex and strain of the mice used. Elucidation of the mechanisms underlying these different results will be of interest and will lead to increased understanding of the role of OPN in these diseases.
OPN in Inflammatory Bowel Diseases
Inflammatory bowel diseases (IBDs) are immune-mediated diseases typically resulting from abnormal mucosal T-cell response to commensal bacteria in intestine. IBD manifests as 2 main pathologies, including ulcerative colitis (UC) and Crohn’s disease (CD), which involve chronic intestinal inflammation, mucosal damage, and epithelial barrier dysfunction. In CD, mucosal T cells exhibit a predominant Th1 phenotype, whereas UC presents with a Th2 phenotype (Strober et al. 2007).
As in other pathologies, OPN has been found to be upregulated in association with these diseases, suggesting a mechanistic link. Early work demonstrated OPN expression in macrophages and fibroblasts (higher in UC than CD; Masuda et al. 2005), as well as in epithelial cells and plasma cells (Sato et al. 2005). Recently, elevated plasma levels were shown to be correlated with disease activity (Komine-Aizawa et al. 2015). However, reduced expression of OPN in UC and CD as compared with normal controls was found in a cohort of Chinese patients (Tang et al. 2014), although this difference may be explained by a focus on expression in epithelial rather than inflammatory cells. Interestingly, a haplotype consisting of 8 single-nucleotide polymorphisms in the OPN gene was found to exhibit significant associations with CD susceptibility (Glas et al. 2011), further supporting an association of OPN with this disease. Since Th1 skewing of effector T cells underlies the development of IBD and since OPN is associated with Th1- and Th17-mediated immune response in CD (Gordon and MacDonald 2005), a role of OPN in promoting these pathologies seems likely.
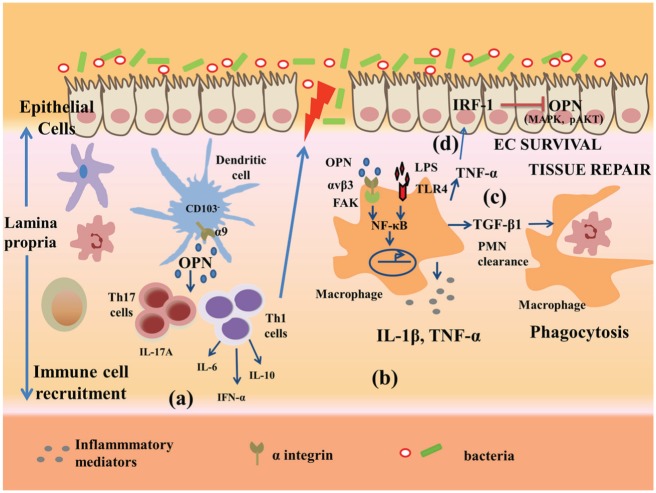
Elucidation of the precise role of OPN through the use of mouse models of colitis supports a role for OPN in the development of disease. OPN deficiency suppresses pathology in dextran sulfate sodium (DSS)– and trinitrobenzenesulphonic acid (TNBS)–induced colitis (Zhong et al. 2006; Oz et al. 2012). Kourepini and colleagues (2014) recently described a mechanistic basis for this effect. They showed that a subset of DCs lacking CD103 expression (CD103– DCs) residing in mesenteric lymph nodes expressed high levels of OPN during TNBS- as well as DSS-induced colitis. Loss of OPN in CD103– DCs resulted in disease amelioration, whereas transgenic overexpression of OPN in the same cells rendered them highly pathogenic. Systemic neutralization of OPN was therapeutic in these colitis models, confirming that secreted OPN is the important form of the molecule in this disease. Furthermore, disrupting the interaction of the OPN domain SLAYGLR with integrin α9 suppressed the inflammatory potential and pathogenicity (Kourepini et al. 2014). A new approach to colitis therapy involves administration of mesenchymal stem cells, which can ameliorate colitis by reducing the number of infiltrating effector T cells in the colon: expression of OPN in mesenchymal stem cells due to deletion of the transcription factor Aire suppresses this effect (Parekkadan et al. 2012). These results are consistent with a role for OPN expressed by CD103– DCs or other cells, in enhancing Th1- and Th17-cell development in colitis, thereby contributing to pathology (Fig. 3).
Figure 3.
Potential role of osteopontin (OPN) in mucosal intestinal immunity. In experimental murine models of colitis, OPN expressed by epithelial cells and intestinal immune cells supports the infiltration of immune cells. (a) Intestinal CD103– dendritic cells express high levels of OPN, which promotes T helper–cell responses (Th1 and Th17) and cytokine release, leading to increased disease pathogenicity (Kourepini et al. 2014). (b) Activated OPN during colitis or following LPS treatment in macrophages activates 1) αvβ3 integrin, 2) signaling via FAK phosphorylation, and 3) transcription factor NF-kB, which produces inflammatory mediators, including IL-1β and TNF-α (Aziz et al. 2009). (c) OPN in lumen induces macrophage release of TGFβ1, which promotes neutrophil clearance following tissue repair (da Silva et al. 2009). (d) TNF-α-induced inflammation upregulates IRF-1, which inhibits OPN-induced phosphorylation of ERK, P38, and AKT in epithelial cells and aggravates injury to intestinal epithelial cells (Tang et al. 2014).
Interestingly, previous work showed a protective effect of OPN in DSS-induced colitis (da Silva et al. 2006) and that treatment of colitic mice with bovine milk OPN in drinking water improved intestinal damage in DSS-induced acute colitis, in part by restoring macrophage activity and TGFb1 release, which promotes tissue repair (da Silva et al. 2009). Heilmann and coworkers (2009) introduced the concept of a dual role of OPN in experimental colonic inflammation, since OPN was found to worsen colitis during acute exposure of DSS, while providing a protective function in chronic DSS treatment. An alternative explanation for these conflicting results may rest in different effects of OPN on epithelial integrity (protective) versus T cell–mediated tissue damage (pathologic). In support of this idea, TNFα induces the transcription factor IRF-1 in intestinal epithelial cells in vitro, and IRF-1 was shown to suppress OPN expression. OPN blocks apoptosis via AKT, P38, and ERK signaling in these cells, so a reduction in OPN expression led to increased apoptosis (Tang et al. 2014). This protective effect of OPN on intestinal epithelial cells in vitro is in agreement with the effect of orally administered OPN in supporting intestinal integrity, which ameliorated intestinal inflammation in addition to hepatic injury (Ge et al. 2013). In addition, OPN binds to multiple integrins, so it is possible that its interactions with different integrins may mediate distinct phenotypic changes. Additional experiments are needed to evaluate these possibilities.
Conclusions
The past few years have led to an expanded understanding of the role of OPN in immunologic reactions, first established with the discovery of Eta-1. Novel insights into the mechanisms of action of OPN have been revealed, including the recurring theme of OPN’s contribution to the development of pathogenic T-cell populations, especially Th17 cells. Of interest, several studies demonstrated that OPN expression by DCs enhances T-cell differentiation into Th17 cells. Several outstanding questions are revealed by these results, however. Specifically, what is the role of OPN expression in different cell types? Since OPN is itself upregulated in activated T cells, how does this contribute to the development of pathologic T-cell populations? Can autocrine expression of OPN in T cells stimulate their own differentiation? Or is the expression of OPN in the context of the DC–T cell interaction important? Similarly, what is the relative role of secreted and intracellular OPN in B-cell development? iOPN in T cells is suggested to support B-cell differentiation in germinal centers, but overexpression of OPN in B cells themselves leads to B-cell dysfunction and autoimmunity. Tissue or cell type–specific deletion of OPN could be used to resolve these questions, so new mouse models based on conditional knockouts or Crispr-Cas-mediated deletion are needed. These models might also help to address conflicting reports of the effect of OPN that have been obtained in several model systems, as in colitis and AH. In addition, the more widespread use and availability of mutant forms of OPN lacking 1 or more integrin-binding sites would be useful for resolving issues about specific OPN-integrin interactions required for its different effects. The idea that OPN can have multiple effects in different cell types, with distinct outcomes for disease phenotypes, is supported by several studies. Therefore, given the complexity of the protein and its varied receptors, it will be important in future work to elucidate the specific OPN-receptor interactions involved in various responses as well as the proximal signaling pathways. While downstream signaling pathways are frequently described, elucidation of the membrane proximal events associated with OPN action will help to determine which integrin interactions are most relevant in specific cell types. It is clear that OPN plays a key role in the development of several aspects of the immune response, but detailed mechanistic understanding of these varied roles will be required for the development of therapeutic approaches to suppress its pathologic effects in human diseases.
Author Contributions
S.R. Rittling, R. Singh, contributed to conception and design, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by the National Institute of Dental and Craniofacial Diseases of the National Institutes of Health (R01DE22380).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. 2000. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 287(5454):860–864. [DOI] [PubMed] [Google Scholar]
- Aziz M, Ishihara S, Mishima Y, Oshima N, Moriyama I, Yuki T, Kadowaki Y, Rumi MA, Amano Y, Kinoshita Y. 2009. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent {alpha}v{beta}3 integrin signaling. J Immunol. 182(11):7222–7232. [DOI] [PubMed] [Google Scholar]
- Boumans MJ, Houbiers JG, Verschueren P, Ishikura H, Westhovens R, Brouwer E, Rojkovich B, Kelly S, den Adel M, Isaacs J, et al. 2012. Safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of the monoclonal antibody ASK8007 blocking osteopontin in patients with rheumatoid arthritis: a randomised, placebo controlled, proof-of-concept study. Ann Rheum Dis. 71(2):180–185. [DOI] [PubMed] [Google Scholar]
- Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, et al. 2001. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 294(5547):1731–1735. [DOI] [PubMed] [Google Scholar]
- Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT, Sorensen ES. 2007. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem. 282(27):19463–19472. [DOI] [PubMed] [Google Scholar]
- Christensen B, Klaning E, Nielsen MS, Andersen MH, Sørensen ES. 2012. C-terminal modification of osteopontin inhibits interaction with the alphaVbeta3-integrin. J Biol Chem. 287(6):3788–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B, Nielsen MS, Haselmann KF, Petersen TE, Sorensen ES. 2005. Post-translationally modified residues of native human osteopontin are located in clusters: identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem J. 390(Pt 1): 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva AP, Ellen RP, Sørensen ES, Goldberg HA, Zohar R, Sodek J. 2009. Osteopontin attenuation of dextran sulfate sodium-induced colitis in mice. Lab Invest. 89(10):1169–1181. [DOI] [PubMed] [Google Scholar]
- da Silva AP, Pollett A, Rittling SR, Denhardt DT, Sodek J, Zohar R. 2006. Exacerbated tissue destruction in DSS-induced acute colitis of OPN-null mice is associated with downregulation of TNF-alpha expression and non-programmed cell death. J Cell Physiol. 208(3):629–639. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. 2001. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 280(2):460–465. [DOI] [PubMed] [Google Scholar]
- Frenzel DF, Borkner L, Scheurmann J, Singh K, Scharffetter-Kochanek K, Weiss JM. 2015. Osteopontin deficiency affects imiquimod-induced psoriasis-like murine skin inflammation and lymphocyte distribution in skin, draining lymph nodes and spleen. Exp Dermatol. 24(4):305–307. [DOI] [PubMed] [Google Scholar]
- Gao B, Bataller R. 2011. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 141(5):1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazal S, Sacre K, Allanore Y, Teruel M, Goodall AH; The CARDIOGENICS Consortium, Tohma S, Alfredsson L, Okada Y, Xie G, Constantin A, et al. 2015. Identification of secreted phosphoprotein 1 gene as a new rheumatoid arthritis susceptibility gene. Ann Rheum Dis. 74(3):e19. [DOI] [PubMed] [Google Scholar]
- Ge X, Lu Y, Leung TM, Sorensen ES, Nieto N. 2013. Milk osteopontin, a nutritional approach to prevent alcohol-induced liver injury. Am J Physiol Gastrointest Liver Physiol. 304(10):G929–G939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas J, Seiderer J, Bayrle C, Wetzke M, Fries C, Tillack C, Olszak T, Beigel F, Steib C, Friedrich M, et al. 2011. The role of osteopontin (OPN/SPP1) haplotypes in the susceptibility to Crohn’s disease. PLoS One. 6(12):e29309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JN, MacDonald TT. 2005. Osteopontin: a new addition to the constellation of cytokines which drive T helper cell type 1 responses in Crohn’s disease. Gut. 54(9):1213–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Zhuang H, Shumyak S, Yang L, Reeves WH. 2015. Mechanisms of autoantibody production in systemic lupus erythematosus. Front Immunol. 6:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris VK, Sadiq SA. 2009. Disease biomarkers in multiple sclerosis: potential for use in therapeutic decision making. Mol Diagn Ther. 13(4):225–244. [DOI] [PubMed] [Google Scholar]
- Hartmann P, Seebauer CT, Schnabl B. 2015. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 39(5):763–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann K, Hoffmann U, Witte E, Loddenkemper C, Sina C, Schreiber S, Hayford C, Holzlohner P, Wolk K, Tchatchou E, et al. 2009. Osteopontin as two-sided mediator of intestinal inflammation. J Cell Mol Med. 13(6):1162–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui T, Sorensen ES, Rittling SR. 2015. Osteopontin binding to the alpha 4 integrin requires highest affinity integrin conformation, but is independent of post-translational modifications of osteopontin. Matrix Biol. 41:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. 2007. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat.Immunol. 8(1):74–83. [DOI] [PubMed] [Google Scholar]
- Husain-Krautter S, Kramer JM, Li W, Guo B, Rothstein TL. 2015. The osteopontin transgenic mouse is a new model for Sjögren’s syndrome. Clin Immunol. 157(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka J, Katagiri Y, Tada N, Murakami M, Ikeda T, Sato M, Hirokawa K, Okada S, Hatano M, Tokuhisa T, et al. 1998. Introduction of an osteopontin gene confers the increase in B1 cell population and the production of anti-DNA autoantibodies. Lab Invest. 78(12):1523–1533. [PubMed] [Google Scholar]
- Inoue M, Shinohara ML. 2011. Intracellular osteopontin (iOPN) and immunity. Immunol Res. 49(1–3):160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwadate H, Kobayashi H, Kanno T, Asano T, Saito R, Sato S, Suzuki E, Watanabe H, Ohira H. 2014. Plasma osteopontin is correlated with bone resorption markers in rheumatoid arthritis patients. Int J Rheum Dis. 17(1):50–56. [DOI] [PubMed] [Google Scholar]
- Ji HI, Lee SH, Song R, Yang HI, Lee YA, Hong SJ, Kim S, Park YB, Lee SK, Yoo MC, et al. 2014. Serum level of osteopontin as an inflammatory marker does not indicate disease activity or responsiveness to therapeutic treatments in patients with rheumatoid arthritis. Clin Rheumatol. 33(3):397–402. [DOI] [PubMed] [Google Scholar]
- Kaleta B. 2014. Role of osteopontin in systemic lupus erythematosus. Arch Immunol Ther Exp (Warsz). 62(6):475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanecki CC, Uzwiak DJ, Denhardt DT. 2007. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 102(4):912–924. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Healy BC, Francois K, Gandhi R, Gholipour T, Egorova S, Sevdalinova V, Quintana F, Chitnis T, Weiner HL, et al. 2014. Evaluation of circulating osteopontin levels in an unselected cohort of patients with multiple sclerosis: relevance for biomarker development. Mult Scler. 20(4):438–444. [DOI] [PubMed] [Google Scholar]
- Komine-Aizawa S, Masuda H, Mazaki T, Shiono M, Hayakawa S, Takayama T. 2015. Plasma osteopontin predicts inflammatory bowel disease activities. Int Surg. 100(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon S, Nakayama Y, Matsumoto N, Ito K, Kanayama M, Kimura C, Kouro H, Ashitomi D, Matsuda T, Uede T. 2014. A novel cryptic binding motif, LRSKSRSFQVSDEQY, in the C-terminal fragment of MMP-3/7-cleaved osteopontin as a novel ligand for alpha9beta1 integrin is involved in the anti-type II collagen antibody-induced arthritis. PLoS One. 9(12):e116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno S, Kurokawa M, Uede T, Nishimura M, Huang SK. 2011. Role of osteopontin, a multifunctional protein, in allergy and asthma. Clin Exp Allergy. 41(10):1360–1366. [DOI] [PubMed] [Google Scholar]
- Kourepini E, Aggelakopoulou M, Alissafi T, Paschalidis N, Simoes DC, Panoutsakopoulou V. 2014. Osteopontin expression by CD103– dendritic cells drives intestinal inflammation. Proc Natl Acad Sci U S A. 111(9):E856–E865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzbach D, Platzer G, Schwarz TC, Henen MA, Konrat R, Hinderberger D. 2013. Cooperative unfolding of compact conformations of the intrinsically disordered protein osteopontin. Biochemistry. 52(31):5167–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe MA, Patarca R, Iregui MV, Cantor H. 1991. Polyclonal B-cell activation by the Eta-1 cytokine and the development of autoimmune disease. J Immunol. 147:2902–2906. [PubMed] [Google Scholar]
- Lazaro R, Wu R, Lee S, Zhu NL, Chen CL, French SW, Xu J, Machida K, Tsukamoto H. 2015. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology. 61(1):129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavenworth JW, Verbinnen B, Yin J, Huang H, Cantor H. 2015. A p85alpha-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat Immunol. 16(1):96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Takahashi Y, Asai S, Hemmi A, Takayama T. 2005. Osteopontin expression in ulcerative colitis is distinctly different from that in Crohn’s disease and diverticulitis. J Gastroenterol. 40(4):409–413. [DOI] [PubMed] [Google Scholar]
- Morales-Ibanez O, Dominguez M, Ki SH, Marcos M, Chaves JF, Nguyen-Khac E, Houchi H, Affo S, Sancho-Bru P, Altamirano J, et al. 2013. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology. 58(5):1742–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G, Mittal A, Weiner HL. 2008. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. 181(11):7480–7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz HS, Zhong J, de Villiers WJ. 2012. Osteopontin ablation attenuates progression of colitis in TNBS model. Dig Dis Sci. 57(6):1554–1561. [DOI] [PubMed] [Google Scholar]
- Parekkadan B, Fletcher AL, Li M, Tjota MY, Bellemare-Pelletier A, Milwid JM, Lee JW, Yarmush ML, Turley SJ. 2012. Aire controls mesenchymal stem cell-mediated suppression in chronic colitis. Mol Ther. 20(1):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patouraux S, Bonnafous S, Voican CS, Anty R, Saint-Paul MC, Rosenthal-Allieri MA, Agostini H, Njike M, Barri-Ova N, Naveau S, et al. 2012. The osteopontin level in liver, adipose tissue and serum is correlated with fibrosis in patients with alcoholic liver disease. PLoS One. 7(4):e35612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah SK, Rittling S. 2007. Pathophysiological role of osteopontin in hepatic inflammation, toxicity and cancer. Toxicol Sci. 103(1):4–13. [DOI] [PubMed] [Google Scholar]
- Rekvig OP. 2015. The anti-DNA antibody: origin and impact, dogmas and controversies. Nat Rev Rheumatol. 11(9):530–540. [DOI] [PubMed] [Google Scholar]
- Rittling SR. 2011. Osteopontin in macrophage function. Expert Rev Mol Med. 13:e15. [DOI] [PubMed] [Google Scholar]
- Rothstein TL, Guo B. 2009. Receptor crosstalk: reprogramming B cell receptor signalling to an alternate pathway results in expression and secretion of the autoimmunity-associated cytokine, osteopontin. J Intern Med. 265(6):632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage PT, Sharpe AH. 2015. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 36(7):410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Nakai T, Tamura N, Okamoto S, Matsuoka K, Sakuraba A, Fukushima T, Uede T, Hibi T. 2005. Osteopontin/Eta-1 upregulated in Crohn’s disease regulates the Th1 immune response. Gut. 54(9):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth D, Duly A, Kuo PC, McCaughan GW, Haber PS. 2014. Osteopontin is an important mediator of alcoholic liver disease via hepatic stellate cell activation. World J Gastroenterol. 20(36):13088–13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, Seryshev A, Zhang Y, Hilsenbeck S, Chang SH, Dong C, et al. 2012. Cigarette smoke induction of osteopontin (SPP1) mediates T(H)17 inflammation in human and experimental emphysema. Sci Transl Med. 4(117):117ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Morser J, Leung LL. 2014. Thrombin cleavage of osteopontin disrupts a pro-chemotactic sequence for dendritic cells, which is compensated by the release of its pro-chemotactic C-terminal fragment. J Biol Chem. 289(39):27146–27158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W, Fuss I, Mannon P. 2007. The fundamental basis of inflammatory bowel disease. J Clin Invest. 117(3):514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, Xie G, Philips G, Chan IS, Karaca GF, et al. 2012. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 61(9):1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Yang G, Zhang S, Wu C, Chen M. 2014. Opposite effects of interferon regulatory factor 1 and osteopontin on the apoptosis of epithelial cells induced by TNF-alpha in inflammatory bowel disease. Inflamm Bowel Dis. 20(11):1950–1961. [DOI] [PubMed] [Google Scholar]
- Uede T. 2011. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol Int. 61(5):265–280. [DOI] [PubMed] [Google Scholar]
- Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, Ge X, Fiel MI, Nieto N. 2012. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology. 55(2):594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Hanashima S, Yagi H, Takahashi Y, Sasakawa H, Kurimoto E, Iguchi T, Kon S, Uede T, Kato K. 2010. NMR characterization of intramolecular interaction of osteopontin, an intrinsically disordered protein with cryptic integrin-binding motifs. Biochem Biophys Res Commun. 393(3):487–491. [DOI] [PubMed] [Google Scholar]
- Zhong J, Eckhardt ER, Oz HS, Bruemmer D, de Villiers WJ. 2006. Osteopontin deficiency protects mice from Dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 12(8):790–796. [DOI] [PubMed] [Google Scholar]