The art of lineage tracing was pioneered in the 19th century by Charles O. Whitman and his colleagues, who were motivated by the realization that cells did not arise through spontaneous generation but rather came from preexisting cells (Conklin 1905). These discoveries subsequently led to the demonstration that the ultimate fates of individual cells were often distinct with each cell, giving rise to cells that had specific roles in development. Lineage tracing has since become an established and essential tool for studying embryonic development, adult tissue homeostasis, stem cell properties, and the mechanisms underlying tissue repair and regeneration. More recently, live cell imaging has been combined with lineage tracing to provide unprecedented real-time insights into progenitor cell origin and fate. In this issue, Jing et al. (2015) use cell lineage–tracing methods to reevaluate the current dogma concerning bone formation and reveal an unexpected fate for hypertrophic chondrocytes in mandibular condylar cartilage.
It is well established that bone formation occurs by 2 distinct processes known as intramembranous and endochondral ossification. During intramembranous ossification (or dermal bone formation, as it is often called), mesenchymal cells differentiate directly into osteoblasts. Osteoblasts are the cells that synthesize bone and thus form the major cellular component of bone. In contrast, during endochondral ossification, mesenchymal progenitor cells differentiate into chondrocytes and generate a cartilage template or scaffold, which is then replaced by bone. The majority of bone throughout the body, including the axial and appendicular skeleton, is formed via endochondral ossification, whereas most of the craniofacial skeleton is formed via intramembranous ossification. Interestingly, the distinction from the mode of cranial versus trunk bone formation is also reflected in the origin of the mesenchymal progenitors. In the head, mesenchymal precursors are derived from cranial neural crest cells, whereas in the trunk, mesenchymal progenitors originate in the sclerotome of the somites and in the lateral plate mesoderm.
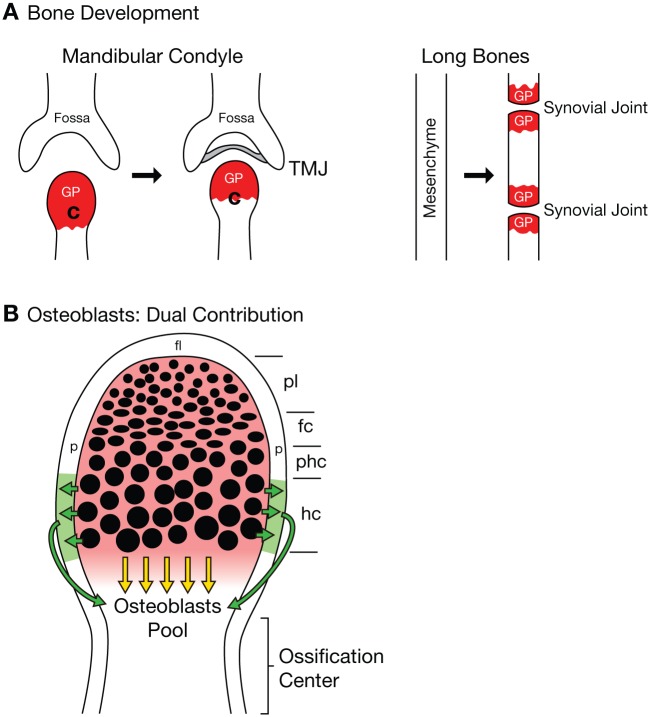
Much of our understanding of endochondral bone development has come from studies of long bones in the appendicular skeleton, particularly the tibia. The growth plate, which is also known as the epiphyseal plate or physis, is the area of tissue growth near the ends of the long bones, where chondrocytes are produced and undergo maturation. The growth plate consists of 5 well-defined zones: reserve, proliferation (proliferating and flattened chondrocytes), maturation and hypertrophy, calcification, and ossification (Kronenberg 2003). In the reserve zone, chondrocytes are small and round and express Sox9. During proliferation, chondrocytes form columns and express the transcription factors Sox5, Sox6, and Sox9, as well as collagen II and aggrecan, which are the major components of the immature chondrocyte extracellular matrix. In the prehypertrophic and hypertrophic zones, chondrocytes enlarge and become more distant from one another. In concert with these size changes, the expression of Sox5, Sox6, and Sox9 is downregulated, while parathyroid hormone 1 receptor and Indian hedgehog are upregulated, and the extracellular matrix of hypertrophic chondrocytes becomes enriched with collagen X (ColX). Late hypertrophic chondrocytes, which represent the final state of chondrocyte maturation, downregulate ColX and upregulate vascular endothelial growth factor A, metalloproteinase 13, and osteopontin, which are responsible for recruiting endothelial cells, as well as the osteoclast and osteoblast precursors that initiate the formation and remodeling of bone (reviewed in Kozhemyakina et al. 2015). Similarly, in the cranial skeleton, the mandibular condyle is an important growth site in the mandible, with similarities to the growth plate of the long bones, displaying 4 distinct zones: a fibrous cell layer; a progenitor cell layer with high expression of Sox9; a zone of flattened chondrocytes that express Sox5, Sox6, and Sox9; and a zone of hypertrophic chondrocytes that are abundant in ColX (Fig. B; Sarnat 1966; Silbermann and Frommer 1972; Purcell et al. 2009).
Figure.
Growth plate in the mandibular condyle. (A) Comparison of bone development in the mandible and in the long bones. In the mandible, the growth plate (GP) is located at the superior end of the condyle (C), which is where the condyle grows and extends toward the glenoid fossa to form the temporomandibular joint (TMJ), with a joint disc between them (gray). In the long bones, the continuous mesenchyme forms the joints by a process called segmentation. Each cartilaginous segment extends bidirectionally toward the joints, thus generating 2 GPs, 1 at each end. (B) Osteoblasts develop from 2 sources: 1) by contribution from the perichondrium that is adjacent to the hypertrophic chondrocytes (in green), which receive their signal to produce osteoblasts (green arrows); 2) by direct transdifferentiation of the mature hypertrophic chondrocytes (bone derived from cartilage, yellow arrows; bone, green; and cartilage, red; Jing et al. 2015). fc, flatten chondrocytes; fl, fibrous layer; hc, hypertrophic chondrocytes; p, perichondrium; pl, progenitor cell layer; phc, prehypertrophic chondrocytes.
It is generally thought that during endochondral ossification, hypertrophic chondrocytes—at their terminal state of chondrocyte maturation—undergo cell death and are subsequently replaced by bone. However, whether hypertrophic chondrocytes truly undergo apoptosis or transdifferentiate into osteoblasts to form bone has been a contentious issue for decades (reviewed by Shapiro et al. 2005). Recently, the application of CRE-LOX techniques for lineage tracing, in combination with analyses of gene function in mice, has convincingly shown in vivo that not all hypertrophic chondrocytes die; instead, they can transdifferentiate directly into osteoblasts (G. Yang et al. 2014; L. Yang et al. 2014; Zhou et al. 2014; Park et al. 2015).
Jing et al. (2015) provide the first in vivo evidence that mandibular chondrocytes directly transform into bone cells during development of the mandible. Using 1) triple transgenic mice containing collagen I (ColI) carrying a GFP reporter (green) to follow bone progenitors (osteoblasts); 2) an inducible CRE allele under the control of aggrecan or ColX to mark chondrocytes and hypertrophic chondrocytes, respectively; and 3) Rosa 26-tomato (red) to trace chondrocytes and hypertrophic chondrocyte lineages, Jing et al. reveal that most osteoblasts in the growth plate derive from the chondroblast lineage. Based on the number and location of yellow cells (bone cells derived from chondrogenic progenitors) in different zones of the growth plate, it is clear that hypertrophic chondrocytes contribute to the majority of the bone cells in the subchondral region of the mandibular condyle. Thus, not all hypertrophic chondrocytes undergo cell death. Furthermore, the authors could estimate the proportion of osteoblasts that were chondrocyte derived and the location of initial ossification in the mandibular condylar cartilage.
Consistent with this observation during bone formation in the head, the direct transformation of cartilage into bone has recently been reported for long bones in the trunk skeleton (G. Yang et al. 2014; L. Yang et al. 2014; Zhou et al. 2014). However, within the appendicular skeleton, long bones develop from continuous mesenchyme. As the mesenchyme condenses, this initiates a process of segmentation that facilitates joint formation. The bones continue growing bidirectionally toward the joints, creating a growth plate at each end. In contrast, in the cranial skeleton, the mandibular condyle grows toward the glenoid fossa of the temporal bone, thereby generating the temporomandibular joint between them (Fig. A). Thus, the mandibular condyle presents only 1 growth plate, as opposed to the long bones, which have 1 in each epiphysis. Despite the different modes of bone formation in the head and trunk, as well as the distinct origins of their respective chondroprogenitors, the capacity for hypertrophic chondrocytes to differentiate into osteoblasts remains the same throughout the body.
The growth plate is of particular clinical significance, as it is often the primary site for fractures in young individuals. Although bone has a great capacity for repair, there are many clinical circumstances in which regeneration fails to occur. In these cases, auto- or allografts of bone are necessary to promote bone regeneration through direct osteogenesis. However, this common approach often results in osteonecrosis and limited integration with the host tissue. Recently, a pioneering study using cartilage grafts instead of bone grafts in long bone fracture repair demonstrated a considerably higher success rate of bone regeneration (Bahney et al. 2014). These results suggest that the mechanisms governing bone repair and regeneration are similar to the mechanisms that regulate normal embryonic bone development. Likewise, the mandible is also often in need of extensive repair due to malformation or fracture (Inman et al. 2013; Trainor and Andrews 2013), and current surgical procedures are quite invasive and not always fully corrective. Therefore, the work from Jing et al. (2015) provides new information that could advance less invasive cranial bone regeneration and repair.
In the future, it will be interesting to explore the molecular mechanisms governing the transdifferentiation of hypertrophic chondrocytes into osteoblasts and to determine whether all osteoblasts have the same molecular roles. As a result, the lessons learned from developmental biology could provide clues to novel strategies for bone repair and regeneration.
Author Contributions
P. Purcell, P.A. Trainor, contributed to data interpretation, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We are deeply grateful to Longzhi Tan (PhD candidate, Harvard University) for drawing the Figure.
Footnotes
Research in the Trainor laboratory is supported by the Stowers Institute for Medical Research and the National Institute for Dental and Craniofacial Research (DE 016082).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bahney CS, Hu DP, Taylor AJ, Ferro F, Britz HM, Hallgrimsson B, Johnstone B, Miclau T, Marcucio RS. 2014. Stem cell–derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res. 29(5):1269–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin EG. 1905. The organization and cell-lineage of the ascidian egg. J Acad Natl Sci Phila. 13:1–119. [Google Scholar]
- Inman KE, Purcell P, Kume T, Trainor PA. 2013. Interaction between Foxc1 and Fgf8 during mammalian jaw patterning and in the pathogenesis of syngnathia. PLoS Genet. 9(12):e1003949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J, de Crombrugghe B, Hinton RJ, Feng JQ. 2015. Chondrocytes directly transform into bone cells in mandibular condyle growth. J Dent Res. 94(12):1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhemyakina E, Lassar AB, Zelzer E. 2015. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 142(5):817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg HM. 2003. Developmental regulation of the growth plate. Nature. 423(6937):332–336. [DOI] [PubMed] [Google Scholar]
- Park J, Gebhardt M, Golovchenko S, Perez-Branguli F, Hattori T, Hartmann C, Zhou X, deCrombrugghe B, Stock M, Schneider H, et al. 2015. Dual pathways to endochondral osteoblasts: a novel chondrocyte-derived osteoprogenitor cell identified in hypertrophic cartilage. Biol Open. 4(5):608–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell P, Joo BW, Hu JK, Tran PV, Calicchio ML, O’Connell DJ, Maas RL, Tabin CJ. 2009. Temporomandibular joint formation requires two distinct hedgehog-dependent steps. Proc Natl Acad Sci U S A. 106(43):18297–18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat BG. 1966. Developmental facial abnormalities and the temporomandibular joint. Dent Clin North Am. 1966:587–600. [PubMed] [Google Scholar]
- Shapiro IM, Adams CS, Freeman T, Srinivas V. 2005. Fate of the hypertrophic chondrocyte: microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res C Embryo Today. 75(4):330–339. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Frommer J. 1972. The nature of endochondral ossification in the mandibular condyle of the mouse. Anat Rec. 172(4):659–667. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Andrews BT. 2013. Facial dysostoses: etiology, pathogenesis and management. Am J Med Genet C Semin Med Genet. 163C(4):283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Zhu L, Hou N, Lan Y, Wu XM, Zhou B, Teng Y, Yang X. 2014. Osteogenic fate of hypertrophic chondrocytes. Cell Res. 24(10):1266–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. 2014. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 111(33):12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. 2014. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 10(12):e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]