Abstract
Neural interfacing devices are an artificial mechanism for restoring or supplementing the function of the nervous system lost as a result of injury or disease. Conducting polymers (CPs) are gaining significant attention due to their capacity to meet the performance criteria of a number of neuronal therapies including recording and stimulating neural activity, the regeneration of neural tissue and the delivery of bioactive molecules for mediating device-tissue interactions. CPs form a flexible platform technology that enables the development of tailored materials for a range of neuronal diagnostic and treatment therapies. In this review the application of CPs for neural prostheses and other neural interfacing devices are discussed, with a specific focus on neural recording, neural stimulation, neural regeneration, and therapeutic drug delivery.
Keywords: conducting polymers, neural interfaces, neural recording, neural stimulation
1. Introduction
Recording and stimulation of excitable tissue activity has been integral to medical diagnostics and treatment of disease since the development of the first echocardiogram (ECG) in 1887 by Augustus D. Waller of St Mary’s Medical School, London.[1] While initial attempts to record the activity of electroactive tissues within humans required submersing the patient’s hands and a foot in a bucket of saline, many more refined options have since been developed. In particular implantable neural interfaces including neuroprosthetic devices have yielded fine control over both recording from neural cells and activation of neural bundles [2–8] to replace function, bypass damaged tissue or encourage regrowth of injured nerves.[9–11] The first commercial electrically active devices such as cardiac pacemakers and cochlear implants used metallic electrodes to interface with neural tissue, a trend that continues into the present day. Most stimulating or recording devices on the market use platinum or stainless steel electrodes to affect or analyze a neural response. These devices, which now include vision prostheses, brain-machine interfaces, urinary pacemakers and neuromuscular interfaces for restoring limb function or driving artificial limbs, communicate with biological systems through implantable metallic electrodes by transducing electronic current to and from ionic current.[12] While these materials have been the standard for best practice for many decades, the performance of devices which use metals for neural interfacing has reached a plateau. [6, 8]
While metals used for neural interfacing have been selected due to their ‘inert’ material properties, it is these same characteristics, which prevent adequate interaction with surrounding tissues, resulting in suboptimal in vivo performance. Metals are more than 4 orders of magnitude stiffer than neural tissue [13] and present an inorganic and largely unfeatured surface morphology which discourages tissue interaction.[7, 14] The format of metallic electrode arrays has been modified across several decades in an attempt to produce more flexible devices that move with tissue and minimize inflammatory reactions.[15, 16] However, despite some success in producing flexible metallic arrays, the strain mismatch and lack of compatible focal adhesion sites for cells, continues to incite a foreign body response, which generates scar tissue (i.e. gliosis) at the neural interface. Ultimately this prevents the target tissue from contacting the electrodes. Gliosis is characterized by presence of both reactive astrocytes and activated microglia at the site of implantation that ultimately creates a non-conductive sheath around the electrode, preventing further electrical communication between the implanted electrode and neurons (Figure 1).[17–20] Consequently, signal quality of recordings are reduced and the stimuli required to activate tissue increases over time.[21, 22] It has been proposed that bionic devices with increased signal resolution (for example, better sound perception for cochlear implant recipients) can’t be achieved with conventional metals and hence new materials are a necessity to improve device performance.[23–25] While a number of metallic modifications have been explored including iridium based coatings (fractal iridium, iridium oxide and activated iridium oxide) and titanium nitride coatings, [26, 27] these materials are still relatively stiff and are not as easily biofunctionalized as polymer based coating technologies. The past decade has explored a range of more complex materials, which have been designed to improve tissue interactions and create more effective long-term neural interfaces.
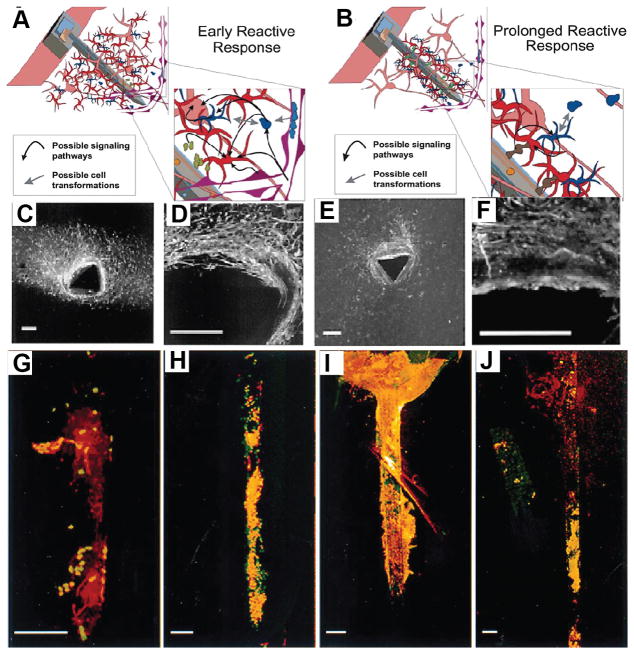
Figure 1.
Schematics showing cellular responses during early (A) and sustained (B) reactive responses observed following neural device insertion. The early response (A) is characterized by a large region containing reactive astrocytes and microglia around inserted devices. The sustained response (B) is characterized by a compact sheath of cells around insertion sites. Inserts depict potential cell–cell interactions and signaling path ways. Neurons (pink), astrocytes (red), monocyte derived cells including microglia (blue), and vasculature (purple) are depicted. (C), (D) GFAP immunohistochemistry (Marker for astrocytes) of tissues slices from brains and (E), (F) ED1 immunohistochemistry (marker for microglia) of tissues slices due to the implantation of neural electrodes.. GFAP-immunohistochemistry (red) and nuclear staining (green) of devices removed from brains at 1 day (G) and 1 (H), 6 (I), and 12 (J) weeks post insertion. All scale bars = 100 μm. Reproduced with permission.[17] Copyright 2003.
Organic materials, including conductive polymers,[7, 28, 29] carbon nanotubes, [30, 31] hydrogels including protein based materials and tissue engineered constructs containing cells,[16] have been popular choices for developing next-generation neural interfaces, with application not only in stimulating and recording devices but also tissue engineering approaches for the regeneration of nerves and cardiac tissues. While there is a wide range of material options for improving neuroprosthetic device and nerve guide performance, a comprehensive review by Aregueta-Robles et al. [32] details this wider field of technologies. The more narrow focus of this current review is the class of polymers termed conjugated or conductive polymers (CPs), which gained eminence in the biomedical field throughout the 1990s, as a result of seminal research conducted by Wallace et al. [33] and Barisci et al. [34] in biosensors. In the subsequent decade CPs, in particular electropolymerized CPs have shown significant promise as medical electrode coatings. These polymers can provide fast charge transfer while retaining the flexibility in synthesis typical of most polymers, enabling materials to be tailored with specific mechanical, chemical and biological properties.[35–38] CPs have been used to coat metallic electrodes and demonstrated superior performance over metals for delivering charge at the neuroprosthetic interface [3, 7, 14] and higher quality recordings in diagnostics and monitoring. [39–43] They have also had a significant role in the development of constructs for neural regeneration, both at bionic device interfaces and for nerve guidance [9, 44–47]. In all of these applications, the ability for CPs to be modified with biomolecules has enabled them to also provide drug delivery functionality [41, 47–50]. This review highlights the variety of CPs used in neural interfacing and the benefits, which can be achieved through control of CP material properties. The different ways in which CPs and their composites have been applied in vivo is also discussed with future directions towards their use beyond research and into clinical applications.
2. Conductive Polymer Approaches
Organic electronics have provided new technologies, which can meet the needs of being more tissue compatible through proving a softer interface, while improving communication between tissues and devices.[37, 38] Of particular interest are electropolymerized CPs that transfer charge by both ionic and electronic mechanisms.[51] A number of composite materials have been developed which improve control of physico-chemical properties of electrodeposited CPs, imparting tailored mechanical, electrical and biological performance. Hydrogels, elastomers and biological polymers such as proteins have been popular choices in creating CP composites,[13, 23, 49, 50, 52] but challenges remain in developing long-term neural interfaces.
2.1. Conducting Polymers
Conducting polymers have the alternating single bonds and double bonds (π bonds), which when fabricated with efficient doping mechanisms create a continuous but unstable pathway along the polymer backbone.[53, 54] Overlapping carbon orbitals along the polymer backbone lead to delocalization of electrons and the existence of mobile charge carriers (dopants) moving along the π bonded carbon atoms result in electrical conductivity of the CPs (Figure 2A).[55] The doping process is reversible and the doping level controls the electrical conductivity of CPs over the full range from insulators to metals. CPs can be synthesized by oxidative chemical or electrochemical polymerization and doped using negatively charged counter ions during polymerization process to maintain their charge neutrality (Figure 2B).[56, 57] While chemical polymerization is more cost effective method for mass production of CPs, the resulting polymer suffers from poor electrical conductivity because of less control over the doping level.[57, 58] In addition, the use of non-biocompatible oxidants such as ferric chloride limits their biomedical applications. Electrochemical polymerization is preferable, especially if a thin layer of polymer with controlled thickness is required as a coating for conductive metallic electrodes. This process can be initiated by applying electrical voltage/current via a cathode to the monomer solution and polymer can be formed on the surface of an anode. The polymers are deposited in an oxidized and high conductivity state containing negatively charged counter ions (dopants) incorporated into the polymer film from the electrolyte solution during the polymerization process.[57, 58] The electrical conductivity,[58] wettability,[59] color [60] and volume [61, 62] of CPs can be altered by manipulating the electrochemical state of the material through reduction-oxidation (redox) reactions that vary the doping level of the polymer (Figure 2C). In addition, the physical and electrical properties of the resultant CP strongly depend on the electropolymerization conditions including pH of monomer solution, temperature, applied voltage or current and dopant choice. The main advantage of electrochemical fabrication of CPs is the control over the thickness and conductivity of the polymer film in a simple one step process. [57, 58] As a result, this method is by far the most popular for neural interfacing applications, and despite some recent promising applications of chemically synthesized CPs (including the commercially available PEDOT:PSS),[63, 64] this review focuses primarily on electrodeposited CPs.
Figure 2.
(A) Chemical structures of various conducting polymers PPy, PEDOT, PT, PANI. (B) Polymerization of PEDOT and doped with anion (dopant) A. (C) Oxidation and reduction behavior of conducting polymers are dependent on dopant size. Small anion A is able to migrate in and out of the PPy matrix to balance the charge across the backbone. Large anion B is immobilized within the PPy matrix and hence relies on smaller cation X. to migrate into the polymer from the surrounding electrolyte and maintain charge balance across the polymer. Reproduced with permission.[50] Copyright 2009.
CPs have been widely used for biomedical applications, [37, 65, 66] in particular, for neural prosthetics and interfaces [4, 7, 23, 39, 41, 49, 67–76] because: (1) drugs and biomolecules can be trapped within the polymer backbone or reservoirs, and be precisely released during re-doping and de-doping process (redox reaction);[49, 68, 77–81] (2) CPs can be functionalized by addition of proteins to improve biocompatibility and promote specific cellular responses;[82, 83] (3) CPs have both ionic and electronic conductivities thus facilitating efficient charge transduction from ions to electrons.[51, 57, 58] Among CPs, poly(pyrrole) (PPy), poly(aniline) (PANI), polythiophene (PTh) and its derivatives including poly(3,4-ethylene dioxythiophene) (PEDOT) have been applied to neural interfacing electrodes. These CP variants have been chosen due to their reported biocompatibility and excellent electrical conductivity.[41, 84, 85] PANI has high environmental stability and ease of charge transport properties and has been used for biomedical applications.[86–88] However, the investigation of PANI for neural interfaces has generated relatively less interest in comparison to PPy and PEDOT, presumably due to its reported poor cell adhesion and growth properties.[89] PPy is the most thoroughly investigated CP for neural applications [73, 90–93] due to the superior solubility of monomer in the water, excellent mechanical actuation, flexible method of preparation, and cytocompatibility.[66, 94, 95] In contrast, PEDOT is relatively new [65] but has a high electrical conductivity[60] and outstanding chemical stability[60, 96] coupled with evidence of in vivo biocompatibility,[3] which has led to PEDOT dominating the research field of CPs for neural interfacing over the past 10 years.
There are several dopants which have been commonly explored for biomedical applications, including poly(styrene sulfonate) (PSS), paratoluene sulfonate (pTS) and a range of perchlorate ions (ClO4−) While other dopant species have been explored, including the use of peptides and carbon nanotubes, the sulfonate series have been shown to be the most robust and biocompatible synthetic dopant species.[14, 97] It has also been shown that varying the dopant species can have significant impact on CP surface morphology, electrochemical stability, biocompatibility and mechanical modulus. As a general rule, smaller dopants, such as pTS, produce rougher and hence more electroactive films, but are softer than larger dopants, such as the polymeric PSS.[14, 97, 98]
The choice of dopant and CP monomer are the two critical factors that impact on performance of these materials in situ. It is desirable that the CP has high charge transfer capacity in an aqueous environment, to function as an electrode. However, rough CPs fabricated from smaller dopant anions (such as PEDOT/pTS) tend to suffer from friable mechanics that prevent them from being employed in implantable devices.[14, 97] Smoother CPs (such as PEDOT/PSS or PPy based films) have improved biocompatibility due to more cohesive film properties, but suffer from reduced charge transfer capacity and can delaminate with repeat electrical cycling. Ultimately, a trade-off between mechanical, electrical and biological properties must be made, with no ideal combination dominating the field.[97, 98] This tradeoff has driven the development of CP composites, which use additional polymer systems in an attempt to produce a CP based biomaterial with optimal mechanical and electrical properties.
2.2. Conductive Composites
While CPs meet most of the critical criteria for neural interfacing materials, their full potential, measured in vitro, has not been translated to the in vivo environment. There are two key issues that prevent homogenous CPs from performing well in the biological environment: (i) their mechanical properties, and (ii) the persistence of scar tissue encapsulation. Conventional electrodeposited CPs are stiff and friable, with typical elastic moduli of the order of 1 to 8 GPa [99, 100] and when applied as a coating, material loss has been shown to exceed 15 % in ASTM tape tests.[13, 49] Additionally, CPs have been reported to delaminate under electrical stimulation.[3, 67] These mechanical issues have limited the application of electrodeposited CPs as standalone materials, but have driven the development of new electroactive hybrid or composite materials with softer, more robust mechanics. These include blends of CPs and hydrogels,[5, 13, 101–103] CPs and elastomers,[104] carbon nanotube composites [31, 90, 105] and CP nanotubes with gel-like cores.[67, 69]
Research conducted by Green et al. [13] has demonstrated that hybrid or composite conductive hydrogels allow modulation of CP mechanical properties yielding materials with an average moduli of 2 MPa while maintaining similar electrical performance as their CP only counterparts (Figure 3A–F).[13] In similar studies by Kim et al. [5, 106] hydrogel coatings on microelectrode probes were found to increase the charge storage capacity and decrease electrode impedance compared to conventional CP coatings. It was proposed that the electrodeposition of CP within a hydrogel, which can swell in the aqueous environment, enables charge transfer to occur through a significantly greater surface area, created by the volume of the CP-hydrogel overlying the electrode. Brittle properties of electrodeposited CPs are also addressed as these polymer composites exhibit a high degree of matrix interpenetration, such that the hydrogel effectively encapsulates the friable CP preventing material loss.[13, 101] This is a particularly important property for neural interfacing, where particulates can cause a frustrated immune reaction [18, 107], increasing scar tissue and limiting the efficacy of the device or treatment.
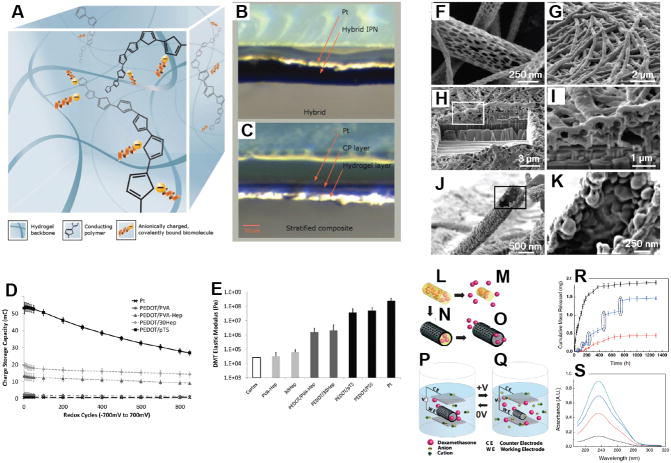
Figure 3.
(A) Schematic of ideal hybrid configuration and photo comparison of hybrid material created from using a bound dopant (B), compared to stratified composite produced from using a free dopant (C), both material samples are hydrated. (D) Charge storage capacity of hybrids calculated from CV performed versus Ag/AgCl at 120mV s−1 from −700 to 700mV over 850 cycles. (E) Elastic moduli (calculated from DMT model) under hydrated conditions, compared to Pt, homogeneous CPs and neural tissue. Reproduced with permission.[13] Copyright 2012. (F) Diameters of the PLGA fibers were distributed over the range 40–500 nm with the majority being between 100–200 nm. (G) Electropolymerized PEDOT nanotubes on the electrode site of an acute neural probe tip after removing the PLGA core fibers. (H) A section of (G) cut with a focused ion beam showing the silicon substrate layer and PEDOT nanoscale fiber coating. (I) Higher-magnification image of (H) showing the PEDOT nanotubes crossing each other. (J) A single PEDOT nanotube which was polymerized around a PLGA nanoscale fiber, followed by dissolution of the PLGA core fiber. This image shows the external texture at the surface of the nanotube. (K) Higher-magnification image of a single PEDOT nanotube demonstrating the textured morphology that has been directly replicated from the external surface of the electrospun PLGA fiber templates. The average wall thickness of PEDOT nanotubes varied from 50–100 nm, with the nanotube diameters ranging from 100 to 600 nm. Schematic illustration of the controlled release of dexamethasone: (L) dexamethasone-loaded electrospun PLGA, (M) hydrolytic degradation of PLGA fibers leading to release of the drug, and (N) electrochemical deposition of PEDOT around the dexamethasone-loaded electrospun PLGA fiber slows down the release of dexamethasone. (O) PEDOT nanotubes in a neutral electrical condition. (P, Q) External electrical stimulation controls the release of dexamethasone from the PEDOT nanotubes due to contraction or expansion of the PEDOT. By applying a positive voltage, electrons are injected into the chains and positive charges in the polymer chains are compensated. To maintain overall charge neutrality, counterions are expelled towards the solution and the nanotubes contract. This shrinkage causes the drugs to come out of the ends of tubes. (R) Cumulative mass release of dexamethasone from: PLGA nanoscale fibers (black squares), PEDOT-coated PLGA nanoscale fibers (red circles) without electrical stimulation, and PEDOT-coated PLGA nanoscale fibers with electrical stimulation of 1 V applied at the five specific times indicated by the circled data points (blue triangles). (S) UV absorption of dexamethasone- loaded PEDOT nanotubes after 16 h (black), 87 h (red), 160 h (blue), and 730 h (green). The UV spectra of dexamethasone have peaks at a wavelength of 237 nm. Data are shown with a ± standard deviation (n = 15 for each case). Reproduced with permission.[68] Copyright 2006.
An alternate approach for improving on homogenous CPs is to create composite materials by modifying the CP fabrication format. Typically, research has focused on coating planar electrodes with thin films of CP, but new promising approaches have yielded methods for creating three-dimensional CP constructs. CP nanotubes were developed by Abidian et al. [68, 69] by electrodepositing PEDOT on electrospun degradable polymer constructs. These CP nanotubes have been shown to not only increase electrical performance through increased surface area, but also enable the actuation of drug delivery through nanofluidic channels (Figure 3G–S). An alternate approach for producing deformable CPs has been described by Shenoy et al. [108] and Wang et al. [109], where polyurethane foams were impregnated with PPy. These structures not only had improved elasticity and a porous morphology, but conductivity could be controlled through loading of the CP component. However, in these studies the materials were designed for use in biosensors and the maximum material conductivity was in the semi-conductor range (<1 × 10−3 S/cm) with charge transfer rates being well below the range required for medical electrodes.
While it has been shown that the electrical properties of CPs can be preserved when they are combined with hydrogels,[13, 52, 71, 101, 110] there is still interest in further developing the mechanical performance of CP based materials. Researchers have been exploring fully flexible bioelectronics, which do not rely on metallic substrates, and as a result produce neural interfacing devices without metal tracks or electrodes.[104, 111] Hydrogels soften CPs and prevent friable material loss, but ultimately they remain relatively brittle materials which can delaminate and crack, breaking the electrical connection.[111] Sasaki et al. [104] have further developed the integration of elastomers with CPs in an attempt to develop fully flexible, elastic electrode arrays. In these studies PEDOT was grown through polyurethane to produce highly flexible tracks, which retained conductivity during stretching. While the array produced from this material was able to power a diode, the conductivity was found to be substantially lower than that of conventional metals.
Another benefit of these complex polymer composites is the significant potential for supporting active agent binding or release.[68, 71, 112, 113] Where CPs can be functionalized through incorporation of proteins or peptides as dopants or inclusions,[41, 47–50, 114] the bioactivity is limited due to the small available volume for these molecules within the dense and constricted CP matrix. Hydrogels, elastomers and other organic polymers can be synthesized to incorporate substantially greater volumes of bioactive molecules that can be bound,[115, 116] passively released[71] or released in a controlled manner through degradation or electrochemical switching of the polymer component.[117–119] It is clear that CP composites have yielded a range of flexible and multifunctional materials than can be tailored to specific tissue interfacing applications. However, their long-term in vivo performance is relatively unknown, with only a few acute studies reported in the literature.[5, 39]
3. Neural Applications
In the biomedical research arena the focus of CP development has mostly been for neuroprosthetic electrodes, including brain machine interfaces,[5, 68] retinal prostheses,[120, 121] and cochlear implants.[77, 81, 112] The former is considered a recording application where the latter two are primarily stimulating implants. As the field has expanded and polymer technologies enable more flexible synthesis options, CPs have found application in neural regeneration therapies such as nerve guides. In all of these areas the ability incorporate bioactive molecules within the polymer matrix has led to studies that demonstrate the drug delivery efficacy of CPs, predominantly in combination with either electrode or nerve guide therapies.
3.1. Recording Devices
Much of the literature on CP performance in vivo has focused on recording electrodes for brain-machine interfaces, research or diagnostic devices. Since the size of metal electrodes is often greater than the size of individual cells, researchers are limited to sampling from a few neurons out of the billions of neurons within an organ.[122–124] As a result, the study of how large networks of neurons interact is a significant challenge. Electrodeposited CPs have been proposed for this application to enable the fabrication of smaller electrodes which are sensitive enough to detect action potentials (i.e. spikes) from individual cells. An additional benefit of reducing electrode size is the subsequent ability to miniaturize the array, limiting the damage to neural cells during implantation. Implantation trauma is thought to create a “kill zone” of around 100 μm around an implant,[18–20] which further inhibits the ability to record neural activity within the immediate vicinity of the device.
Several studies have been undertaken to assess the effectiveness of CPs as recording electrodes, as summarized in Table 1. Due to the limited time frame for which most of these studies have been conducted, the long-term efficacy of CPs for recording is still largely unknown. In the sub-chronic (studies performed for longer than 1 day) and chronic studies ( > 30 days) the foreign body reaction was observed to reduce the number of active recording channels, as the scar tissue effectively “walls off” the electrodes regardless of their coating type.[4, 39, 122] This can be in as little as one-week post-implantation, but is thought to stabilize around two weeks post-implantation.[4, 39] It is important to note that in all of these studies stiff probe arrays were used to enable penetration of the cortical tissue. These micromachined silicon constructs are likely to cause a frustrated immune response, as they do not move freely with the pulsatile motion of the cortex. As a result the softer CP is less likely to improve the tissue interface, which is largely dominated by the mechanical mismatch of the array.[5, 125]
Table 1.
Summary of in vivo performance of CP coated recording electrodes.
| In vivo model | Conductive Polymer | Implant Period | Summary of study findings | Ref |
|---|---|---|---|---|
| Rat motor cortex | PEDOT doped with tetraethylammonium perchlorate | 6 weeks | Average impedance of the PEDOT coated electrodes was ½ that of the uncoated metallic controls throughout the test period, but both electrode types experience significant increases in impedance over the first week. The PEDOT coated electrodes recorded 17% more quality units than the uncoated controls. | [4] |
| Rat motor cortex | PEDOT doped with tetraethylammonium perchlorate | 8 days | PEDOT coating reduced impedance and improved SNR by a factor of 2, but ultimately functionality was lost, presumably due to encapsulation. | [122] |
| Guinea pig auditory cortex | PEDOT/PSS in alginate | 2 hours | Hydrogel swelling was found to push neurons away from recording sites with no improvement over control electrodes. | [5] |
| Guinea pig cerebellum | PPy doped with silk like polymer with fibronectin (SLPF) | < 1 day | Neuron activity was recorded on PPy coated sites but no analysis compared to controls was applied. | [41] |
| Rat inferior colliculus | PPy/pTS & PEDOT/pTS | < 1 day | PEDOT performed better than PPy and also the uncoated control, with a higher SNR and detection of a greater number of spikes. | [40] |
| Rat barrel cortex | PEDOT nanotubes with PLLA cores | 7 weeks | PEDOT nanotubes maintained a stable impedance at roughly ½ the magnitude of the control electrodes after impedance stabilized at roughly 15 days post implantation. More electrode sites experienced an SNR >4 for the PEDOT nanotubes than the controls. | [39] |
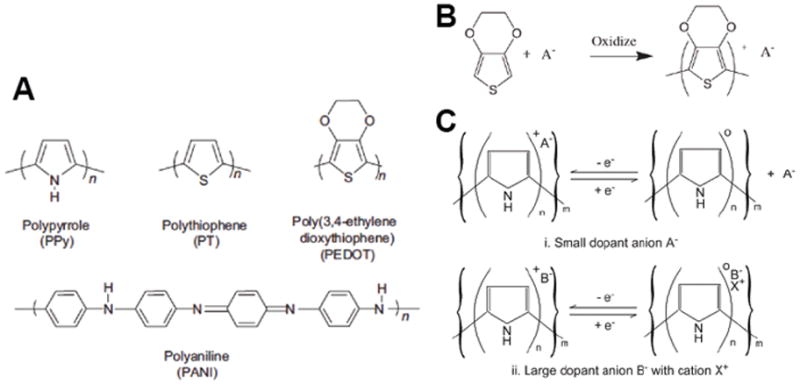
In recording electrodes the two most important metrics are the signal-to-noise ratio (SNR) and the number of cell events or units detected. In most in vivo studies the CP coated electrodes did provide improvement in both SNR and unit detection.[4, 39, 122] Most studies reported an average halving in the electrode impedance, which translated to higher SNRs and spike counts. However, this effect was not sustained in many of the reported studies. It was found that particular materials, such as hydrogels were not effective in imparting improvements to the neural interface.[5] While composites of CPs and hydrogels have shown significant promise in attenuating the mechanical difference between stiff metal probes and soft neural tissues, the swelling properties of the hydrogel component can cause displacement of the tissues (Figure 4). In the study reported by Kim et al. [5] this property increased SNR and reduced unit activity recordings.
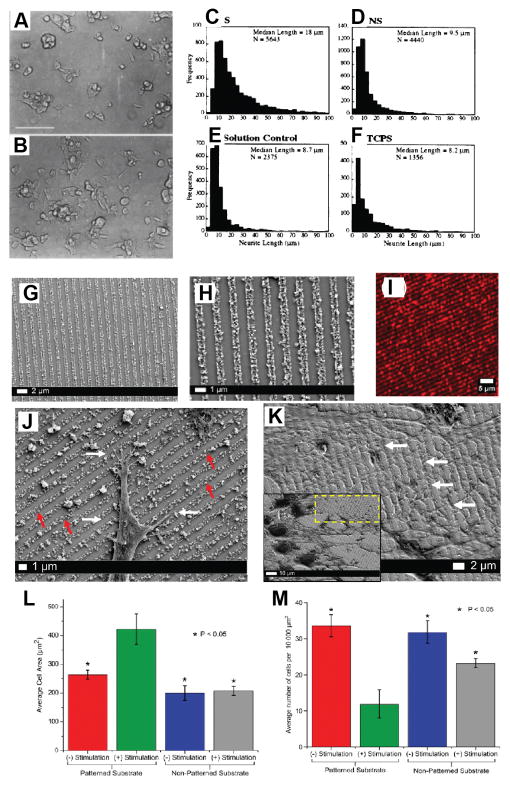
Figure 4.
(A) Procedure for hydrogel coating the neural electrodes. Optical images of reswelling of the hydrogel-coated electrodes in an agar (1%)-phantom matrix as a function of time. HG coatings in the agar matrix made of (B) PBS and (C). deionized water. (C) Plot of reswelling of hydrogel coatings in the PBS agar. The initial thickness of hydrogel coatings was 50 lm as determined by optical microscopy during the dipcoating process. (E) The average percentages of clearly detectable units as a function of the thickness of hydrogel-coated electrodes in the auditory cortex with a 200 ms noise burst. The HG thickness indicates the initial thickness of hydrogel coatings before drying. The inset shows a representative recorded signal with a SNR of 5.0. (*) Significance between control (bare electrode) and hydrogel-coated electrodes. Reproduced with permission.[5] Copyright 2010
A more recent approach pioneered by Richarson-Burns et al. [70] was direct electrodeposition of CPs around cells and tissues. This in situ electrodeposition resulted in the growth of disperse CP clouds along the extracellular matrix.[126] The concept behind this approach was that CP electrodes could be formed which penetrated beyond the glial scar to maintain connection with neural tissue over chronic time frames. Subsequent studies demonstrated that this technique could be used in living animals to produce PEDOT/PSS electrodes within the cortex,[127] but that impedance benefits were not maintained past acute wound healing periods of 3 – 4 weeks. Additionally, the presence of the CP beyond the initial glial scar resulted in the migration of inflammatory cells to the extents of the polymer cloud – effectively increasing the area of scarring.
It is clear that CPs can provide some benefit in recording electrodes, however, the potential advantages demonstrated in vitro are not reflected in chronically implanted devices. Additionally, there are only a small number of studies that have sought to establish the performance of CPs applied to chronically implanted electrodes. This has led to difficulties in establishing standard metrics across different papers. It is recommended that future studies look to establish consistent metrics that will enable comparison of different approaches across studies, and ultimately present an understanding of the benefits and limitations of CP based electrode coatings. At present, it can be observed that CPs provide some improvement over metal electrode impedance across sub-chronic time frames, but this benefit it diminished as scar tissue encapsulation isolates the device, regardless of material composition. Despite this, some improvement in SNR and ability to record cell activity is maintained.
3.2. Stimulating Devices
Similar to recording electrodes, the major driving force for applying CPs to neurostimulating electrodes is the need to reduce electrode size without compromising the safety and efficacy of the devices.[8] In stimulating devices, smaller electrode sites are desirable as they facilitate the development of more densely packed arrays. It is generally understood that the use of smaller electrodes on arrays, which consequently accommodate larger numbers of stimulation sites, will enable improved resolution in the biological response for implant recipients. This could include an improvement in sound perception for cochlear implant recipients or the ability to read and discern facial expressions for visual prosthesis users. It has often been proposed than an optimal neural interface will enable communication with individual cells,[128] necessitating electrode technologies which can operate on the micro and nano-scales.
Historically, stimulating electrodes used in commercial devices have been fabricated from platinum, but this metal has significant limitations in the amount of charge that can be safely injected. When these charge injection limits are exceeded chemical reactions occur at the interface of platinum electrodes, which produce changes in the local pH and the generation of gases.[129] Ultimately this can result in tissue death and the dissolution of electrodes.[6] Therefore, the major limiting factor in reducing the size of metallic electrodes is their ability to safely inject charge through a reduced surface area. Due to the high roughness of electrodeposited CPs, these coating technologies have substantially higher injection limits.[23, 130] Reports suggest that PEDOT has a charge injection limit ranging from 10 – 30 times greater than platinum, depending on the dopant chemistry and thickness of the coating.[7, 14] However, the translation of this significant improvement in charge transfer from in vitro tests to the in vivo environment has not yet been realized. Table 2 presents a summary of the few papers that have assessed in vivo CP performance for stimulating neuroprostheses.
Table 2.
Summary of in vivo neurostimulation with CP coated electrodes
| In vivo model | Conductive Polymer | Implant Period | Summary of study findings | Ref |
|---|---|---|---|---|
| Feline suprachoroidal space | PEDOT/pTS | 3 days | PEDOT was found to retain and impedance of around ½ that of platinum. | [7] |
| Rat cortex | PEDOT/PSS | 16 days | No significant difference in impedance after 6 days in vivo. At 2 weeks the residual voltage for PEDOT electrodes was around 70% smaller than the PtIr uncoated control electrodes. | [3] |
| Guinea pig cochlea | PPy/pTS | 2 weeks | In vivo electrical performance was not monitored, but NGF delivered from within the PPy coating in combination with electrical stimulation was found to improve implant performance (hearing perception in deafened guinea pigs). | [77] |
There are several issues that need to be addressed to yield a thorough understanding of the role and benefits of CPs in stimulating electrode applications. These include commensurate methods for measuring injection limit in both the in vitro and in vivo environment, the need to improve both electrochemical and optical assessment of CPs in situ, and finally an understanding of the role of protein interactions with roughened electrodes on charge transfer mechanisms. Each of these issues is not only important to the assessment of CP neural interfaces, but to electrode interfaces in general. To date there are limited assessment techniques that enable accurate assessment of electrode performance in the in vivo environment. The major challenges lie in the spatial constraints, the complex biological milieu and mechanical mismatch of tissues and implants.
Ultimately, to assess electrical performance at a neural interface a three-electrode arrangement is required which enables a reference electrode to be placed within close proximity of the electrode under test. In an in vitro environment a saturated calomel (SCL) or silver/silver chloride (Ag/AgCl) reference electrode is placed directly above the test electrode, ideally within a few molecular layers of the electrode surface.[131] In this arrangement the reference electrode can be used to detect small changes in the chemical environment at the test electrode surface when applying a current or voltage between a test and counter electrode. It is this 3-electrode system which forms the basis of cyclic voltammetry, impedance spectroscopy and charge injection limit measurements.[6] However, when translated to the in vivo environment there is typically no space for placement of a reference electrode. Several attempts in the literature have been made to ascertain in vivo electrochemical measurements,[8, 26] but there is no consistent and accepted method for producing an accurate understanding of the electrode chemistry in this environment.
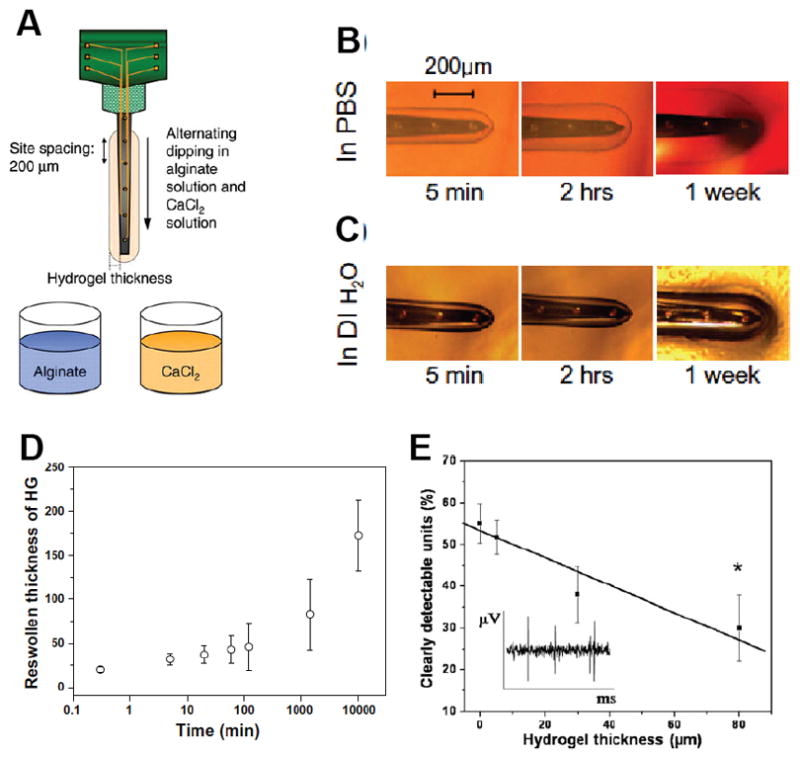
The additional confounding factor is that in vitro electrical measurements are conventionally conducted in physiological saline. This fluid represents the ionic content of body fluid but does not contain any of the natural proteins that adhere and interact with electrodes upon implantation. It has been shown that protein interactions have a more significant impact on the electrical performance of organic CPs compared to inorganic metals.[7] This differing protein interaction then creates a disconnect in the comparative relationship between in vitro and in vivo data when assessing CPs relative to state-of-the-art metals. In a study by Green et al. [7] it was shown that CP coatings can reduce the interfacial voltage of a platinum electrode by 85% when tested in physiological saline, but in vivo the reduction in interfacial voltage is limited to 45% (Figure 5). To accurately assess the performance of CPs in their application to stimulating neuroprosthetics there remains a significant need for methods development and translational approaches to produce in vitro data that relates to in vivo performance.
Figure 5.
Laser micromachined arrays used in studies: (A) nine-electrode array used for in vitro studies with 7 PEDOT/pTS-coated sites (dark) and two bare Pt sites (light); (B) 24-electrode array in hexagonal (hex) configuration used for in vivo studies with 12 bare Pt (light electrode sites) and 12 PEDOT/pTS-coated electrodes (dark electrodes). SEM of (C) bare Pt electrodes, (D) coated with PEDOT/pTS at 200Å~ magnification and (E) PEDOT/pTS at 1000Å~ magnification. (F) Average maximum voltage drop across electrodes, measured in PBS with small biphasic pulse (100 μA, 100 μs) versus large, low impedance Pt counter. Each data point represents the mean voltage drop across 50 individual electrode sites and error bars represent one standard deviation (n = 50). (G) Average voltage drop across PEDOT electrodes compared to Pt electrodes during a 36 h acute study. Each data point represents the mean voltage of 12 electrodes with error bars showing one standard deviation (n = 12). Reproduced with permission.[7] Copyright 2013
In the few studies that have been conducted in vivo it was clear that electrical stimulation of CPs could impact on their mechanical integrity. Studies by Venkatraman et al. [3] reported that delamination of PEDOT/PSS coatings from wires in rat cortex may have resulted in a loss of electrode performance. In vitro studies by Green et al. [14] systematically tested different CP electrode coatings by varying the dopant type of PEDOT and subjecting them to high frequencies of biphasic stimulation. It was shown that PEDOT/PSS is very brittle and is prone to failure by cracking and delamination, where PEDOT doped with the smaller anion pTS (PEDOT/pTS) was significantly more robust under continued cycles of electrical stimulation at clinically relevant levels. Mechanical characterization by Baek et al. [132] confirmed that PEDOT/PSS was stiffer than other PEDOT variants fabricated with smaller more mobile dopants. However, assessment of coating integrity in the in vivo environment is quite difficult. In a typical implant study, the electrode array is explanted from the tissue prior to examination and tissue histology. This is because histological sectioning cannot easily be performed with the metallic components of the implant remaining in the tissue. However, by necessity this means that the coatings experience substantial shear forces, which can result in CP delamination as a result of array removal from the tissue rather than electrically induced cracking and delamination. A robust CP coating for a stimulating electrode must remain laminated to the underlying metallic electrode under implant/explant conditions, polymer ageing and electrical stimulation in a biological environment. Studies have shown that the most effective method is to use mechanical interlocking or rather roughening the surface of the metallic electrode prior to electrodeposition of the CP.[14] This technique can more than double the useable lifetime of a CP coated electrode under stimulation, enabling delivery of more than 1.5 billion pulses at clinically extreme levels.
While CPs have been explored extensively for electrode coatings, there remains a number of challenges in achieving a robust and consistent performance for stimulating neuroprosthetics. It is plausible that CPs can impart substantial improvements in electrical performance, but characterization of the neural interface must be more thoroughly understood before effective conclusions can be made. As with recording electrodes it is proposed that CP coated electrodes will still be subjected to scar tissue encapsulation, but the presence of the CP may enable more charge to be safely delivered at this interface to bypass the scar tissue to retain activation of the target neurons.
3.3. Neural Regeneration
Axonal guidance is crucial for wiring of the nervous system. Axons navigate along specific pathways towards their synaptic targets using the attractive and repulsive biophysical and biochemical guidance signals in their extracellular environments.[133–135] Despite significant research progress on axonal regeneration, functional regeneration of axon remains a challenge due to the complex cellular environment that dominates at post-injury tissue sites. CPs have been utilized as substrate materials for promoting axonal outgrowth using electrical stimulation,[45] topographic contact guidance,[67, 136, 137] growth factor delivery,[80, 81] and biomolecule doping and entrapment.[47, 138]
Electrical stimulation through CPs has been employed to promote axonal regeneration.[92, 139, 140] Schmidt et al. electrodeposited PPy doped with PSS on indium tin oxide (ITO) substrates and examined the effect of electrical stimulation through PPy films on neurite outgrowth. PC-12 cells (a clonal modal of neural cells) were grown on PPy then subjected to an electrical potential of 100 mV for 2 h (Figure 6A and 6B). The results showed a significant increase in neurite length (18.14 μm) compared with control substrates that were not subjected to electrical stimulation (9.5 μm,). This represented a 90% increase in neurite length that was significantly higher than the 20–40% increase in neurite length reported for primary neurons on piezoelectric materials.[141] The effect of electrical stimulation passed through the solution and not through the PPy films has also been examined. It was demonstrated that neurite length distributions across those samples were indistinguishable from unstimulated controls (Figure 6C–F). This suggested that neurite length enhancements were the result of electronic conduction through the PPy and not from ionic conduction thorough solution.[45] In a study by Kotwal et al. the effect of protein adsorption on PPy/PSS films was evaluated during electrical stimulation.[139] It was shown that electrical stimulation of 10 μA for 2h increased the adsorption of fibronectin, a serum protein, to the PPy films deposited on gold-coated substrates. PC-12 cells cultured on PPy film that had been previously adsorbed with fibronectin during electrical stimulation expressed longer neurite than unstimulated substrates. These results suggested that increased cell adhesive protein adsorption on electrically stimulated CPs can promote enhanced neurite outgrowth.[139]
Figure 6.
PC-12 cell differentiation on PPy without (A) and with (B) application of an electric potential. PC-12 cells were grown on PPy for 24 h in the presence of NGF, then exposed to electrical stimulation (100 mV) across the polymer film, S (B). Images were acquired 24 h after stimulation. Cells grown for 48 h but not subjected to electrical stimulation, NS, are shown for comparison (A). Bar= 100 μm. Neurite length histograms. Shown are histograms of neurite lengths for cells on PPy with (S, stimulated) (C) and without (NS, unstimulated) (D) potential applied through PPy, on PPy with potential applied through the solution (E), and on tissue culture polystyrene (TCPS) (F). Reproduced with permission.[45] Copyright 1999. Characterization of the multilayered PEDOT:PSS conducting nanoparticles and their assembly as linear conduits. (G, H) SEM micrographs of the nanoparticle patterns at a magnification of 25kX (G) and 11kX (H) indicating the formation of tightly packed and highly ordered nanoparticle arrays. (I) Confocal fluorescence image of the RhB functionalized PEDOT:PSS nanoparticle arrays at 20X magnification. (J) High magnification (12kX magnification) SEM images demonstrating specific and preferential interactions of neurites (white arrows) with the PEDOT:PSS linear conduits (red arrows). (K) High-magnification SEM image (magnification 8kX) indicating the formation of extensive dendritic networks (white arrows) guided by the PEDOT:PSS arrays. Inset: The corresponding low-magnification image of the area (yellow box) analyzed (magnification 3kX). (L) Significant increase in the average cell area is observed 72 h after exogenous electrical stimulation on the nanoparticle platform in comparison to unstimulated and non-patterned controls. (M) Corresponding decrease in PC12 cell proliferation observed 72 h after exogenous electrical stimulation on the nanoparticle platform in comparison to unstimulated and non-patterned controls. Reproduced with permission.[136] Copyright 2015.
Micro and nano-structured CPs can provide topographic contact guidance for neurons to enhance neurite outgrowth.[67, 92, 136] Linear arrays of PEDOT:PSS nanoparticles have been electrostatically assembled on patterned ITO substrates using polydimethylsiloxane (PDMS) stamping. In this study, PEDOT:PSS nanoparticles with an average size 200 nm were used, which imparted the relatively low electrical conductivity of 2.5 × 10−12 S cm−1. [136] PC12 cells were cultured on the nanoparticles and subjected to an electrical stimulation (monophasic pulsed current at a frequency of 250 Hz with 2 ms pulse width and 1mA amplitude for 2 h), over a 72 h period. It was shown that exogenous electrical stimulation induced dendritic sprouting of the PC12 cells, which was guided by the PEDOT:PSS linear conduits. Significant increase in the average cell area was observed 72 h after exogenous electrical stimulation on the nanoparticle platform in comparison to non-stimulated and non-patterned control samples (Figure 6G–M).[136] Similarly, Lee et al. fabricated PPy coated poly(lactide-co-glycolide) (PLGA) nanofibers to examine the combined effect of topographical features of nanofibers and electrical stimulation on axonal regeneration.[137] PPy was chemically polymerized on random and aligned electrospun PLGA nanofibers (Figure 7A–D). PPy-coated nanofibers supported improved growth of PC-12 cells and hippocampal neurons compared to non-coated PLGA nanofibers (Figure 7E and F). Electrical stimulation studies revealed that PC-12 cells stimulated with a potential of 10 mV cm−1 on PPy-PLGA scaffolds exhibited about 40–50% longer neurites and 40–90% more neurite formation than non-stimulated cells on PPy-PLGA scaffolds. The effect of aligned and random scaffolds on neurite length was also compared. Stimulated cells on aligned PPy-PLGA scaffolds had longer neurites and more neurite cells than stimulated cells on random PPy-PLGA scaffolds. (Figure 7G) These results suggest that the combined effect of topographical features and electrical stimulation could enhance neurite extension and thus might be utilized for neural tissue regeneration.[137]
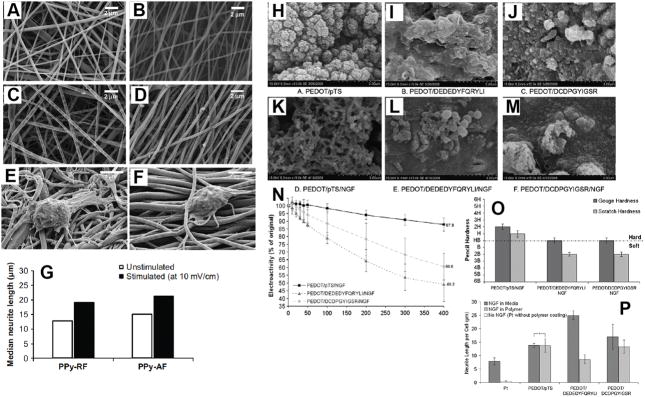
Figure 7.
Scanning electron micrographs of electrospun PLGA nanofibers, (A) and (B), and their PPy-coated fibers, (C) and (D). (A) Randomly oriented nanofibers (RF), (B) aligned nanofibers (AF), (C) PPy-coated randomly oriented fibers (PPy–RF), and (D) PPy-coated aligned fibers (PPy–AF). SEM images of PC12 cells cultured on (E) PPy–RF and (F) PPy–AF for 2 days. (G) Median neurite lengths PC12 cells when unstimulated and when electrically stimulated (10 mV/cm) on random (PPy–RF) and aligned (PPy–AF) PPy–PLGA fibers. At least 300 neurites were analyzed from four substrates for each condition. Reproduced with permission.[137] Copyright 2009. SEM at 15,000_ magnification of NGF entrapped in PEDOT films compared with control films produced without NGF modification of the electrolyte (H) PEDOT/pTS, (I) PEDOT/DEDEDYFQRYLI, (J) PEDOT/DCDPGYIGSR, (K) PEDOT/pTS/NGF, (L) PEDOT/DEDEDYFQRYLI/NGF, (M) PEDOT/DCDPGYIGSR/NGF. (N) Electrochemical activity loss for NGF-loaded PEDOT films over 400 cycles of redox. Loss of electroactivity is plotted as a percentage of the original activity measured at cycle 1, normalising the polymers to a common baseline. The percentage activity remaining after 400 cycles is labeled. Error bars represent the standard error of the mean (n = 3). (O) Effect of NGF loading on ASTM hardness of PEDOT-based polymers. Gouge hardness is representative of a minor disruption to the polymer surface and scratch hardness depicts the level at which no polymer was removed from the Pt substrate. Error bars represent the standard error of the mean (n = 6). (P) Neurite outgrowth of PC12 cells on laminin peptide-doped PEDOT with NGF incorporation at 96 h post-plating. Neurite outgrowth is represented as the neurite length per adhered cell, calculated by normalising the total neurite length to the cell density. The standard error of the mean is given (n = 3). *No significant difference. Reproduced with permission.[49] Copyright 2010
CPs have been doped with biomolecules such negatively charged fragments of laminin and fibronectin to promote neurite outgrowth. Green et al. used anionically modified laminin peptide DEDEDYFQRYLI and DCDPGYIGSR as dopants for electrochemically polymerized PEDOT coatings on platinum (Pt) electrodes.[50] The electrochemical activity and cellular response of peptide-doped PEDOT was compared to the synthetically doped PEDOT/pTS. Cyclic voltammetry (cycling voltage from −0.8 V to 0.6 V with a scan rate 120 mV s−1) for 400 continuous cycles revealed that electroactivity of all samples degraded steadily over the first 100 cycles but electroactivity of PEDOT/pTS tended to plateau in the subsequent 300 cycles. Both PEDOT/DEDEDYFQRYLI and PEDOT/DCDPGYIGSR exhibited greater electroactivity loss compared to PEDOT/pTS, however, the overall loss of electrical activity was not greatly reduced by the incorporation of laminin peptides. PC-12 cell culture demonstrated the influence of the YFQRYLI ligand in promoting neurite outgrowth. PEDOT/DEDEDYFQRYLI increased the neurite length per cell compared to both PEDOT/pTS control and the DCDPGYIGSR-doped PEDOT. Also it was observed that PEDOT/DCDPGYIGSR samples had consistently higher cell density than DEDEDYFQRYLI, and a higher density when laminin coated than PEDOT/pTS.[50] In another study, Poole-Warren and co-workers examined the effect of co-incorporation of two biomolecules on the properties of PEDOT films. NGF was entrapped within the PEDOT film doped with DEDEDYFQRYLI, DCDPGYIGSR, and pTS during electrodeposition (Figure 7H–M).[49] NGF entrapment within the PEDOT was found to generate a softer interface than PEDOT without NGF entrapment. However, results revealed that the use of DEDEDYFQRYLI and DCDPGYIGSR dopant combined with NGF entrapment produced a PEDOT film with diminished electrical performance (Figure 7N) and mechanical stability (Figure 7O). Entrapped NGF within the PEDOT film was still biologically active and PEDOT/pTS/NGF samples produced neurite lengths comparable to controls where NGF was directly added to the cell culture medium. The peptide-doped, NGF-loaded films (PEDOT/DEDEDYFQRYLI/NGF and PEDOT/DCDPGYIGSR/NGF) were unable to replicate the results seen in the controls (Figure 7P).[49]
Thompson et al. investigated the effect of releasing two neurotrophins simultaneously on neuronal survival and elongation. Neurotrophin-3 (NT-3) and brain-derived neurotrophic factor (BDNF) were incorporated within pTS-doped PPy during electropolymerization (PPy/pTS/NT-3, PPy/pTS/BDNF, and PPy/pTS/BDNF/NT-3) and were released from electrically stimulated (biphasic ±1 mA current pulses with 100 μs pulse width, 20 μs open-circuit interphase gap and 3.78 ms short-circuit phase between pulses at 250 Hz) and unstimulated samples.[81] The mechanism of neurotrophin release is still not fully understood. Small ionic species can be delivered from conducting polymer film during de-doping and re-doping process (redox reaction), while larger molecules such as neurotrophins are much bigger than small ionic molecules, so the diffusion of larger molecules out of the CP structure is expected to be limited. [36, 142] Despite this, the release of BDNF and NT-3 was achieved from both unstimulated and stimulated PPy films. The growth factor release from stimulated electrodes was much higher than that from unstimulated PPy, demonstrating that redox processes, including de-doping ion flux, actuation based or related to solvent flux through the polymer film, resulted in increased release of the neurotrophins. The release of BDNF from stimulated PPy/pTS/BDNF/NT-3 films was 40 ± 20 ng cm−2 while similarly stimulated films containing BDNF only (PPy/pTS/BDNF) were shown to release 21.8± 0.7 ng cm−2 of BDNF. Neural processes extending from a cochlear explant cultured on PPy samples was observed to have significantly increased in length when electrical stimulation was applied across all polymers, with the exception of PPy/pTS and PPy/pTS/BDNF. The combination of multiple growth factors and electrical stimulation, specifically the PPy/pTS/BDNF/NT-3 films, significantly increased the neurite outgrowth compared with films in which only one neurotrophin was incorporated (PPy/pTS/BDNF and PPy/pTS/NT-3).[81]
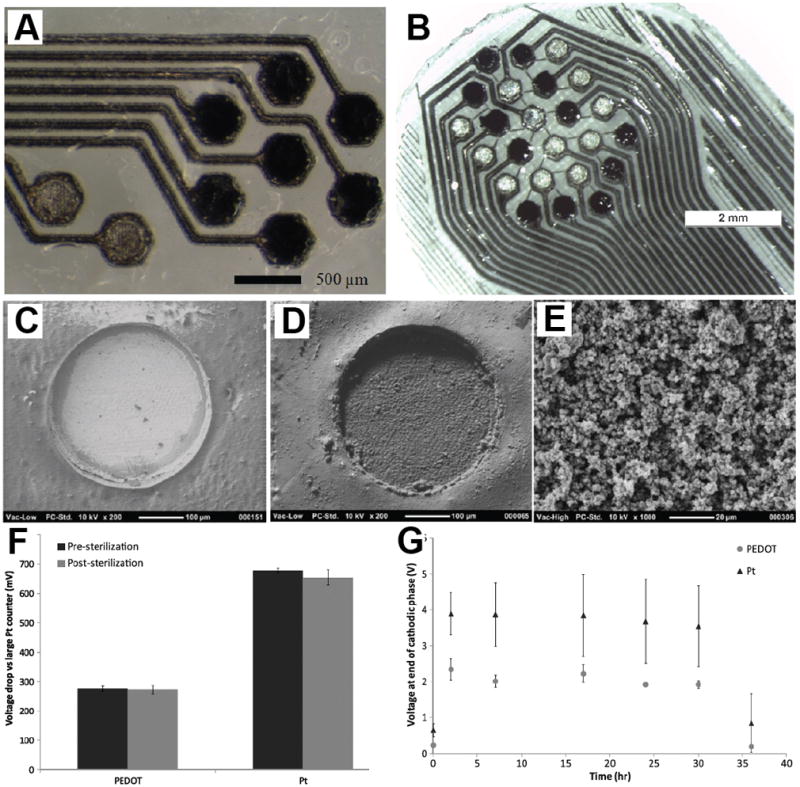
Recently Abidian et al. investigated the effect of PEDOT on in vivo axonal regeneration.[110] PEDOT was electrochemically polymerized inside the lumen of a tubular agarose gel to create a fully coated-PEDOT agarose (FPEDOTA) conduit and a partially coated-PEDOT agarose (PPEDOTA) conduit (Figure 8A–T). These conduits were assessed as a tissue bridge using a nerve gap model in rats, and compared to autograft controls. Specific sample types were FPEDOTA, PPEDOTA and plain hydrogel (PA). Conduits were implanted across 10 mm peroneal nerve gaps and were evaluated after 12 weeks postoperatively (Figure 8U–X). Results including extensor digitorum longus muscle contractile force measurements (Figure 8Y), a muscle innervated by the peroneal nerve, and nerve histomorphometry revealed that hybrid conducting polymer–hydrogel conduits (FPEDOTA and PPEDOTA) supported superior neural regeneration as compared to the plain hydrogel conduits (PA). This was assumed to be due to the mechanical refinement of the hydrogel conduit through electrodeposition of PEDOT, which prevented the lumen from collapsing following the inevitable hydrogel swelling in an aqueous environment.[13] PPEDOTA conduits demonstrated slightly better performance presumably due to better diffusion of biomolecules into and within the lumen of the conduits.
Figure 8.
Schematic illustration of fabrication process and optical micrographs of FPEDOTA and PPEDOTA conduits. FPEDOTA: A) PS template fiber (gray color in cross section (a)). B) Sputter coating the PS fiber with thin layer of AuPd (yellow color in cross section (b)). C) Dipping into the agarose solution (blue color in cross section (c)). D) Formation of agarose layer (blue color in cross section (d)). E) Electropolymerization of PEDOT on the surface of the AuPd-coated PS fibers (black color in cross section (e)). F) Dissolving the PS fiber (white color in cross section (f)). G) Electropolymerization of PEDOT on the surface of AuPd within the inner lumen (black color in cross section (g)). PPEDOTA: H) PS template fiber (gray color in cross section (h,i)). I) Wrapping of masking tape in spiral fashion around the PS fiber. J) Sputter coating the PS fiber with thin layer of AuPd (yellow color in cross section (j,k)). K) Removal of masking tape. L) Dipping into the agarose solution (blue color in cross section (l)). M) Formation of agarose layer (blue color in cross section (m). N) Electropolymerization of PEDOT on the surface of the AuPd-coated PS fibers (black color in cross section (n)). O) Dissolving the PS fiber (white color in cross section (o)). P) Electropolymerization of PEDOT on the surface of AuPd within the inner lumen (black color in cross section (p)). Q–R) Optical micrographs of FPEDOTA conduits with different magnifications. Red arrows show growth of PEDOT within the agarose hydrogel (R). S–T) Optical micrographs of PPEDOTA conduits with different magnify cations before electrodeposition PEDOT on the surface of AuPd within the inner lumen. Optical micrographs of implanted conduits, and EDL maximal specific muscle force. (U,V) Optical images of implanted FPEDOTA conduits in 10 mm peroneal nerve gap in rats. (W,X) Optical images of implanted PA conduits in 10 mm peroneal nerve gap in rats. (Y) Bar graph maximal specific muscle force. Column height represents the mean while error bars reflect the standard deviation of the mean (n = 5). The arrows show the location of stents. Optical micrographs of peripheral nerves at midgraft. (1, 2) Autograft. (3, 4) PA. (5, 6) FPEDOTA. (7, 8) PPEDOTA. Scale bars are 50 μm. Reproduced with permission.[110] Copyright 2012
Neural regeneration effected through CP technologies at device interfaces has not yet been realized. While several researchers have demonstrated that it is possible to incorporate biomolecules, electrical or topographical cues that improve cell growth and guide regeneration, the in vitro studies have not been reliably recapitulated in vivo. Furthermore, the addition of biomolecules to CPs has a significant impact on the material properties, reducing electroactivity and mechanical stability. While CP composites provide a pathway to addressing these limitations, the translation of in vitro findings to the implant environment has not been adequately explored. Future studies should focus on the use of additional polymer components for providing mechanical strength to CPs and a matrix for the delivery of therapeutics, as detailed in the following section.
3.4. Therapeutic Drug Delivery
Drug delivery has been explored extensively for neural applications of CP based materials.[68, 79–81, 143–146] One of the key proponents for utilizing polymer electrodes as a replacement for metallic electrodes is that they cannot only deliver more charge but also provide a platform for introducing therapeutic molecules. Ideally, these CP based electrodes have the capacity to deliver a range of factors which can support neural cell regeneration, encourage target cell integration with the electrode surface (to reduce the charge required to activate a physiological response) and also provide a range of molecules which mediate the tissue reaction to the implanted device, including anti-inflammatories and antibiotics. However, the greatest challenge in engineering such a bioactive electrode material is the limited space or volume in which these biomolecules can be incorporated. There are three main approaches to incorporating bioactive agents within CPs and CP composites: (i) as a dopant or covalently bound molecule which is immobilized within the polymer matrix;[50, 138] (ii) as a dopant which is mobile and can be electrochemically delivered to the tissues;[147, 148] or (iii) as an entrapped molecule which is either passively or actively driven out of the polymer and into the tissues.[80, 81] The choice is largely dictated by the specific biofunctional requirements of the drug.
Factors that do not need to be engulfed by the cell to have an effect need to be presented at the polymer surface, and as such can be incorporated in a manner that renders them accessible, but immobile. Commonly, these bioactive molecules have included cell attachment factors to encourage neural cell integration with the polymer. Laminin peptides [50, 138] and RGD sequences,[41] have been incorporated within CPs and CP composites to encourage cell attachment. These factors can be easily added as a dopant of the CP, or chemically bound to the surface after fabrication. Ultimately, these approaches have been successful in demonstrating in vitro attachment of neurons where the cells are plated directly on the material. However, a study by Cui et al. demonstrated that this effect was not maintained in vivo.[41] It was expected that inflammatory reactions at the implant site resulted in glial cells populating the tissue and dominating interactions with the device. The “kill zone” surrounding an implant has been previously described by Polikov et al. and specifies that neurons within the first 100 – 200 μm of the implant interface die as a result of the implantation and subsequent inflammatory reactions.[18] As a consequence it is not clear whether there is any benefit to incorporating factors into materials that are targeted at neural cell attachment. In fact, studies have shown that when these bulky molecules are used to dope homogenous CPs they cause a significant reduction in the mechanical and electrical properties. New approaches for incorporating these molecules within composite materials that address these CP limitations have shown similar success in vitro but have not been assessed in vivo.
Drugs that require uptake by cells must be incorporated within the polymer matrix, and then mobilized at a later stage to be delivered to tissues. Typically these mobile drugs would include growth factors and anti-inflammatories. Of these two groups, only anti-inflammatory molecules such as dexamethasone phosphate (DP) have significant charge and can be incorporated as dopants.[149] A major advantage of incorporating mobile drugs as dopants is that they can be delivered by electrochemical switching of the potential across the material.[36, 83, 149] To both incorporate a drug as a dopant and then deliver it in a controlled manner, the molecule must also be relatively small, to enable it to move through the polymer matrix. Both DP and valproic acid (VA) have been used to dope CPs and investigated as anti-inflammatory molecules.[150, 151] When the charged drug is delivered by application of an electrical potential, it is important to realize that the CP component of the material may become undoped and lose electroactivity. However, it is proposed that both in vitro and in vivo, the presence of anions such as Cl− within the electrolyte solution (or body fluid) can flux into the polymer matrix and dope the CP, effectively replacing the delivered drug. While drugs can be effectively incorporated as dopants and delivered by electrical stimulation, it has been shown that the inclusion of therapeutic levels of drugs for extended time periods is a challenge. This is not only a problem for doping drugs but also those that are simply entrapped within polymer-coated electrodes and devices.
Drugs that do not have a significant charge or are bulky molecules must be entrapped within the CP or other composite polymer matrix.[25] Typically growth factors are delivered by this method as they are not strongly charged and are often more than 10kDa in size, which impacts significantly on material properties. However, to increase the amount of drug incorporated within the material anti-inflammatories have also been incorporated via entrapment within composite CPs. Delivery of entrapped molecules can be provided by either degradable polymer elements, usually not the CP component, or active mechanical actuation of the material system, a technique which can be mediated by the CP component.[68] Both of these techniques provide a degree of control over drug delivery, enabling different release profiles to be attained. Abidian et al. showed one of the most successful demonstrations of this concept, where dexamethasone was entrapped within biodegradable cores of PEDOT nanotubes.[68] This provided therapeutic levels of the drug which were delivered for a sustained period of up to 54 days, with the amount of DP being controlled by actuation of the PEDOT. In this system the PEDOT outer shell effectively pumped the drug out of the hydrogel core (Figure 3L–S). It is expected that either growth factors or anti-inflammatories will need to be supplied to tissues for more than 14 days, exceeding the neuronal wound-healing phase. While there have been some promising in vitro results, they are yet to be reflected in vivo. The few studies that have looked at providing neurotrophins in vivo,[77] have found that the amount of growth factor required to regenerate nerves at the device interface is substantially more than the amount that can be incorporated within an electrode coating.
Ultimately, drug delivery via CPs at the neural interface remains a challenge, with two key areas creating the major hurdles: (i) incorporation of sustained therapeutic levels; and (ii) imparting a biological effect that overcomes the dominant scar tissue reaction in vivo. The most prevalent approach within the field at present is the use of complementary polymer systems in combination with CPs to increase the reservoir in which drugs can be incorporated, while providing a more complex milieu of factors. Multiple bioactive drugs are likely to be needed to mitigate not only acute inflammatory responses but also prevent sustained frustration of neuronal healing that generates scar tissue across the chronic implant lifetime.
4. Conclusion
The ultimate goal of a neural prosthesis is to create a functional link between the outside world and the nervous system via recording from or stimulation of the nervous system, to assist patients with sensory and motor neural disabilities. The quality and long-term performance of neural devices ultimately rests on the quality of electrode materials that enable a functional and long lasting interface. Neural microelectrodes use conventional metallic materials that are not intrinsically compatible and do not integrate with neural tissue, due to the mechanically stiff and chemically inorganic interface. These metals also suffer from high impedance and low charge injection capacity, which is essential for sustained neural recording and stimulation.
CPs have been investigated as an alternate electrode material with the potential to provide a more reliable electrode-tissue interface as they can moderate the mechanical mismatch, have both ionic and electronic conductivity, and can be functionalized or decorated with biomolecules to enhance electrode-tissue integration. It has been shown that CPs can enhance the electrical performance of neural recording and stimulation by decreasing the impedance and increasing the charge transfer density. However, the major improvements reported from in vitro studies are significantly diminished in the in vivo environment as scar tissue reactions remain dominant. Drugs and biomolecules can be incorporated within the CP structure and precisely released at the site of implantation to suppress the inflammation or promote neuronal processes toward the electrodes. In addition, CPs can be utilized to enhance axonal regeneration by providing physical, chemical, and electrical cues to guide axon growth. While these advantages make them a popular choice for improving the device-tissue interface, the addition of biomolecules often comes at a cost, with the need for trade-offs in electrical and mechanical performance.
Despite significant advances in the application of CPs for neural interfaces, development of seamless integration and biologically integrated CP materials for neural interface technologies remains a challenge. New approaches of developing composite systems where CPs are combined with alternate polymers, such as hydrogels and elastomers, are providing options for improving the long-term performance of CP based coatings. It is expected that future directions for neural interfacing CP technologies will involve more complex polymer combinations, which allow for tailored control of mechanical, electrical and biological properties.
Acknowledgments
We would like to acknowledge support from National Institute of Health R01 NS087224
Biographies
Mohammad Reza Abidian Received his Ph.D. in Biomedical Engineering from University of Michigan in 2007. He completed a postdoctoral training in the Center for Neural Communication Technology at the University of Michigan. Since 2010 Abidian is an Assistant Professor in the Departments of Biomedical Engineering, Materials Science & Engineering, and Chemical Engineering. His current research interests include: Multifunctional organic-inorganic hybrid nanobiomaterials for smart targeted drug delivery to brain tumors and conducting polymers for axonal regeneration and neurochemical detection.
Rylie Green received her PhD (Biomedical Engineering) in 2009 from the University of New South Wales under the supervision of Scientia Prof Nigel Lovell and Prof Laura Poole-Warren. Her doctoral research focused on developing bioactive conducting polymers as coatings for neuroprosthetic electrodes. During her postdoctoral studies, Dr Green investigated the in situ polymerization of conducting polymer electrodes directly within the cortical tissue, as a visiting scholar with the Martin Research Group, led by Prof David Martin. Dr Green is currently a research fellow at UNSW and is investigating the application of electrode coating technologies to the developmental bionic eye device as a member of the ARC funded special initiative, Bionic Vision Australia. Additionally, in collaboration with Cochlear Ltd., she is investigating her patented hybrid conducting polymer hydrogel electrode concept. Dr Green’s research interests encompass conducting polymers, hybrid polymer systems, tissue engineering, neural interfaces, neuroprosthetic devices and drug delivery.
Contributor Information
Dr. Rylie Green, Email: r.green@unsw.edu.au, Graduate School of Biomedical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia
Prof. Mohammad Reza Abidian, Email: mabidian@psu.edu, Biomedical Engineering Department, Materials Science & Engineering Department, Chemical Engineering Department, Materials Research Institute, Huck Institutes of the Life Sciences, Pennsylvania State University, University Park, PA 16802 (USA)
References
- 1.Besterman R Creese. Waller--pioneer of electrocardiography. Br Heart J. 1979;42:61–64. doi: 10.1136/hrt.42.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wark HAC, Sharma R, Mathews KS, Fernandez E, Yoo J, Christensen B, Tresco P, Rieth L, Solzbacher F, Normann RA, Tathireddy P. A new high-density (25 electrodes/mm 2 ) penetrating microelectrode array for recording and stimulating sub-millimeter neuroanatomical structures. Journal of Neural Engineering. 2013;10:045003. doi: 10.1088/1741-2560/10/4/045003. [DOI] [PubMed] [Google Scholar]
- 3.Venkatraman S, Hendricks J, King ZA, Sereno AJ, Richardson-Burns S, Martin D, Carmena JM. In Vitro and In Vivo Evaluation of PEDOT Microelectrodes for Neural Stimulation and Recording. Neural Systems and Rehabilitation Engineering, IEEE Transactions on. 2011;19:307–316. doi: 10.1109/TNSRE.2011.2109399. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig KA, Uram JD, Yang J, Martin DC. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with poly(3,4-ethylenedioxythiophene) (PEDOT) film. J Neural Eng. 2006;3:59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- 5.Kim D-H, Wiler JA, Anderson DJ, Kipke DR, Martin DC. Conducting polymers on hydrogel-coated neural electrode provide sensitive neural recordings in auditory cortex. Acta Biomaterialia. 2010;6:57–62. doi: 10.1016/j.actbio.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Cogan SF. Neural Stimulation and Recording Electrodes. Ann Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 7.Green RA, Matteucci PB, Hassarati RT, Giraud B, Dodds CWD, Chen S, Byrnes-Preston PJ, Suaning GJ, Poole-Warren LA, Lovell NH. Performance of conducting polymer electrodes for stimulating neuroprosthetics. Journal of Neural Engineering. 2013;10:016009. doi: 10.1088/1741-2560/10/1/016009. [DOI] [PubMed] [Google Scholar]
- 8.Green RA, Matteucci PB, Dodds CWD, Palmer J, Dueck WF, Hassarati RT, Byrnes-Preston PJ, Lovell NH, Suaning GJ. Laser patterning of platinum electrodes for safe neurostimulation. Journal of Neural Engineering. 2014;11:056017. doi: 10.1088/1741-2560/11/5/056017. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt CE, Leach JB. NEURAL TISSUE ENGINEERING: Strategies for Repair and Regeneration. Annual Review of Biomedical Engineering. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 10.Runge MB, Dadsetan M, Baltrusaitis J, Ruesink T, Lu L, Windebank AJ, Yaszemski MJ. Development of Electrically Conductive Oligo(polyethylene glycol) Fumarate-Polypyrrole Hydrogels for Nerve Regeneration. Biomacromolecules. 2010;11:2845–2853. doi: 10.1021/bm100526a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgens RB. Electrically mediated regeneration and guidance of adult mammalian spinal axons into polymeric channels. Neuroscience. 1999;91:251–264. doi: 10.1016/s0306-4522(98)00584-3. [DOI] [PubMed] [Google Scholar]
- 12.Wise KD, Anderson DJ, Hetke JF, Kipke DR, Najafi K. Wireless implantable microsystems: High-density electronic interfaces to the nervous system. Proceedings of the Ieee. 2004;92:76–97. [Google Scholar]
- 13.Green RA, Hassarati RT, Goding J, Baek S, Lovell NH, Martens PJ, Poole-Warren LA. Conductive Hydrogels: Mechanically Robust Hybrids for Use as Biomaterials. Macromol Biosci. 2012;12 doi: 10.1002/mabi.201100490. [DOI] [PubMed] [Google Scholar]
- 14.Green RA, Hassarati RT, Bouchinet L, Lee CS, Cheong GLM, Yu JF, Dodds CW, Suaning GJ, Poole-Warren LA, Lovell NH. Substrate dependent stability of conducting polymer coatings on medical electrodes. Biomaterials. 2012;33:5875–5886. doi: 10.1016/j.biomaterials.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. Journal of the Peripheral Nervous System. 2005;10:229–258. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- 16.Mehdi J, John LS, Christoph W, Jeffrey RC. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. Journal of Neural Engineering. 2015;12:011001. doi: 10.1088/1741-2560/12/1/011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W. Brain responses to micro-machined silicon devices. Brain Research. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- 18.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. Journal of Neuroscience Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Experimental Neurology. 2005;195:115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Biran R, Martin DC, Tresco PA. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. Journal of Biomedical Materials Research Part A. 2007;82A:169–178. doi: 10.1002/jbm.a.31138. [DOI] [PubMed] [Google Scholar]
- 21.McCreery DB, Yuen TG, Bullara LA. Chronic microstimulation in the feline ventral cochlear nucleus: physiologic and histologic effects. Hearing Research. 2000;149:223–238. doi: 10.1016/s0378-5955(00)00190-8. [DOI] [PubMed] [Google Scholar]
- 22.McCreery DB, Yuen TG, Agnew WF, Bullara LA. A characterization of the effects on neuronal excitability due to prolonged microstimulation with chronically implanted microelectrodes. Biomedical Engineering, IEEE Transactions on. 1997;44:931–939. doi: 10.1109/10.634645. [DOI] [PubMed] [Google Scholar]
- 23.Abidian MR, Martin DC. Multifunctional Nanobiomaterials for Neural Interfaces. Advanced Functional Materials. 2009;19:573–585. [Google Scholar]
- 24.Hetling JR, Baig-Silva MS. Neural prostheses for vision: designing a functional interface with retinal neurons. Neurol Res. 2004;26:21–34. doi: 10.1179/016164104773026499. [DOI] [PubMed] [Google Scholar]
- 25.Green RA, Lovell NH, Wallace GG, Poole-Warren LA. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials. 2008;29:3393–3399. doi: 10.1016/j.biomaterials.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 26.Cogan SF, Plante TD, Ehrlich J, Shah HA, Chen J, Rizzo JF. In vivo Electrochemical Characterization of Activated Iridium Oxide Stimulation Electrodes Implanted Sub-Retinally in Rabbit. Invest Ophthalmol Vis Sci. 2005;46:1515. [Google Scholar]
- 27.Weiland JD, Anderson DJ, Humayun MS. In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes. Biomedical Engineering, IEEE Transactions on. 2002;49:1574–1579. doi: 10.1109/TBME.2002.805487. [DOI] [PubMed] [Google Scholar]
- 28.Stauffer WR, Cui XT. Polypyrrole doped with 2 peptide sequences from laminin. Biomaterials. 2006;27:2405–2413. doi: 10.1016/j.biomaterials.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Cui X, Martin DC. Fuzzy gold electrodes for lowering impedance and improving adhesion with electrodeposited conducting polymer films. Sensors and Actuators A: Physical. 2003;103:384–394. [Google Scholar]
- 30.Wang J, Dai JH, Yarlagadda T. Carbon nanotube-conducting-polymer composite nanowires. Langmuir. 2005;21:9–12. doi: 10.1021/la0475977. [DOI] [PubMed] [Google Scholar]
- 31.Sahoo NG, Jung YC, So HH, Cho JW. Polypyrrole coated carbon nanotubes: Synthesis, characterization, and enhanced electrical properties. Synthetic Metals. 2007;157:374–379. [Google Scholar]
- 32.Aregueta-Robles UA, Woolley AJ, Poole-Warren LA, Lovell NH, Green RA. Organic electrode coatings for next-generation neural interfaces. Frontiers in Neuroengineering. 2014;7 doi: 10.3389/fneng.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace GG, Smyth M, Zhao H. Conducting electroactive polymer-based biosensors. TrAC Trends in Analytical Chemistry. 1999;18:245–251. [Google Scholar]
- 34.Barisci JN, Hughes D, Minett A, Wallace GG. Characterisation and analytical use of a polypyrrole electrode containing anti-human serum albumin. Analytica Chimica Acta. 1998;371:39–48. [Google Scholar]
- 35.Poole-Warren L, Martens P, Green R. Biosynthetic Polymers for Medical Applications. Elsevier; 2015. [Google Scholar]
- 36.Smela E. Conjugated polymer actuators for biomedical applications. Advanced Materials. 2003;15:481–494. [Google Scholar]
- 37.Berggren M, Richter-Dahlfors A. Organic bioelectronics. Advanced Materials. 2007;19:3201–3213. [Google Scholar]
- 38.Rivnay J, Owens RM, Malliaras GG. The Rise of Organic Bioelectronics. Chemistry of Materials. 2014;26:679–685. [Google Scholar]
- 39.Abidian MR, Ludwig KA, Marzullo TC, Martin DC, Kipke DR. Interfacing Conducting Polymer Nanotubes with the Central Nervous System: Chronic Neural Recording using Poly(3,4-ethylenedioxythiophene) Nanotubes. Advanced Materials. 2009;21:3764–3770. doi: 10.1002/adma.200900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander RH, Simeon JM, Jun C, Robert MIK, Gordon GW, Antonio GP. Conducting polymer coated neural recording electrodes. Journal of Neural Engineering. 2013;10:016004. doi: 10.1088/1741-2560/10/1/016004. [DOI] [PubMed] [Google Scholar]
- 41.Cui X, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, Martin DC. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J Biomed Mater Res. 2001;56:261–272. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 42.Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsaki G. NeuroGrid: recording action potentials from the surface of the brain. Nature Neuroscience. 2015;18:310–315. doi: 10.1038/nn.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leleux P, Badier JM, Rivnay J, Benar C, Herve T, Chauvel P, Malliaras GG. Conducting Polymer Electrodes for Electroencephalography. Advanced Healthcare Materials. 2014;3:490–493. doi: 10.1002/adhm.201300311. [DOI] [PubMed] [Google Scholar]
- 44.Asplund M. Degradable conjugated conducting polymers and nerve guidance. In: Poole-Warren LA, Martens PJ, Green RA, editors. Biosynthetic Polymers for Medical Applications. Elsevier; 2015. [Google Scholar]
- 45.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8948–8953. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivers TJ, Hudson TW, Schmidt CE. Synthesis of a Novel Biodegradable Electrically Conducting Polymer for Biomedical Applications. Advanced Functional Materials. 2002;12:33–37. [Google Scholar]
- 47.Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: Bioactive electrically conducting polymer for enhanced neurite extension. J Biom Mater Res Part A. 2007;81A:135–149. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asplund M, von Holst H, Inganäs O. Composite biomolecule/PEDOT materials for neural electrodes. Biointerphases. 2008;3:83–93. doi: 10.1116/1.2998407. [DOI] [PubMed] [Google Scholar]
- 49.Green RA, Lovell NH, Poole-Warren LA. Impact of co-incorporating laminin peptide dopants and neurotrophic growth factors on conducting polymer properties. Acta Biomaterialia. 2010;6:63–71. doi: 10.1016/j.actbio.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 50.Green RA, Lovell NH, Poole-Warren LA. Cell attachment functionality of bioactive conducting polymers for neural interfaces. Biomaterials. 2009;30:3637–3644. doi: 10.1016/j.biomaterials.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 51.Deslouis C, ElMoustafid T, Musiani MM, Tribollet B. Mixed ionic-electronic conduction of a conducting polymer film. Ac impedance study of polypyrrole. Electrochimica Acta. 1996;41:1343–1349. [Google Scholar]
- 52.Kim D-H, Abidian M, Martin DC. Conducting polymers grown in hydrogel scaffolds coated on neural prosthetic devices. Journal of Biomedical Materials Research - Part A. 2004;71:577–585. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- 53.Bredas JL, Street GB. Polarons, bipolarons, and solitons in conducting polymers. Accounts of Chemical Research. 1985;18:309–315. [Google Scholar]
- 54.Shirakawa H. The Discovery of Polyacetylene Film: The Dawning of an Era of Conducting Polymers (Nobel Lecture) Angewandte Chemie International Edition. 2001;40:2574–2580. doi: 10.1002/1521-3773(20010716)40:14<2574::AID-ANIE2574>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 55.Roncali J. CONJUGATED POLY(THIOPHENES) - SYNTHESIS, FUNCTIONALIZATION, AND APPLICATIONS. Chemical Reviews. 1992;92:711–738. [Google Scholar]
- 56.Heeger AJ. Nobel Lecture: Semiconducting and metallic polymers: The fourth generation of polymeric materials. Reviews of Modern Physics. 2001;73:681–700. doi: 10.1002/1521-3773(20010716)40:14<2591::AID-ANIE2591>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 57.Heeger AJ. Semiconducting and metallic polymers: The fourth generation of polymeric materials (Nobel lecture) Angewandte Chemie-International Edition. 2001;40:2591–2611. doi: 10.1002/1521-3773(20010716)40:14<2591::AID-ANIE2591>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 58.MacDiarmid AG. “Synthetic metals”: A novel role for organic polymers (Nobel lecture) Angewandte Chemie-International Edition. 2001;40:2581–2590. doi: 10.1002/1521-3773(20010716)40:14<2581::AID-ANIE2581>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 59.Xu LB, Chen W, Mulchandani A, Yan YS. Reversible conversion of conducting polymer films from superhydrophobic to superhydrophilic. Angewandte Chemie-International Edition. 2005;44:6009–6012. doi: 10.1002/anie.200500868. [DOI] [PubMed] [Google Scholar]
- 60.Groenendaal BL, Jonas F, Freitag D, Pielartzik H, Reynolds JR. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Advanced Materials. 2000;12:481–494. [Google Scholar]
- 61.Baker CO, Shedd B, Innis PC, Whitten PG, Spinks GM, Wallace GG, Kaner RB. Monolithic actuators from flash-welded polyaniline nanofibers. Advanced Materials. 2008;20:155–+. [Google Scholar]
- 62.Smela E, Gadegaard N. Surprising volume change in PPy(DBS): An atomic force microscopy study. Advanced Materials. 1999;11:953–+. [Google Scholar]
- 63.Liao C, Zhang M, Yao MY, Hua T, Li L, Yan F. Flexible Organic Electronics in Biology: Materials and Devices. Advanced Materials. 2014 doi: 10.1002/adma.201402625. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 64.Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsaki G. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci. 2015;18:310–315. doi: 10.1038/nn.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S. Applications of conducting polymers and their issues in biomedical engineering. Journal of the Royal Society Interface. 2010;7:S559–S579. doi: 10.1098/rsif.2010.0120.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guimard NK, Gomez N, Schmidt CE. Conducting polymers in biomedical engineering. Progress in Polymer Science. 2007;32:876–921. [Google Scholar]
- 67.Abidian MR, Corey JM, Kipke DR, Martin DC. Conducting-Polymer Nanotubes Improve Electrical Properties, Mechanical Adhesion, Neural Attachment, and Neurite Outgrowth of Neural Electrodes. Small. 2010;6:421–429. doi: 10.1002/smll.200901868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abidian MR, Kim DH, Martin DC. Conducting-Polymer Nanotubes for Controlled Drug Release. Advanced Materials. 2006;18:405–409. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abidian MR, Martin DC. Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes. Biomaterials. 2008;29:1273–1283. doi: 10.1016/j.biomaterials.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richardson-Burns SM, Hendricks JL, Foster B, Povlich LK, Kim D-H, Martin DC. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials. 2007;28:1539–1552. doi: 10.1016/j.biomaterials.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheong GLM, Lim KS, Jakubowicz A, Martens PJ, Poole-Warren LA, Green RA. Conductive hydrogels with tailored bioactivity for implantable electrode coatings. Acta biomaterialia. 2013 doi: 10.1016/j.actbio.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 72.Harris AR, Morgan SJ, Chen J, Kapsa RMI, Wallace GG, Paolini AG. Conducting polymer coated neural recording electrodes. Journal of Neural Engineering. 2013;10 doi: 10.1088/1741-2560/10/1/016004. [DOI] [PubMed] [Google Scholar]
- 73.Liu X, Yue ZL, Higgins MJ, Wallace GG. Conducting polymers with immobilised fibrillar collagen for enhanced neural interfacing. Biomaterials. 2011;32:7309–7317. doi: 10.1016/j.biomaterials.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 74.Xiao YH, Cui XY, Hancock JM, Bouguettaya MB, Reynolds JR, Martin DC. Electrochemical polymerization of poly(hydroxymethylated-3,4-ethylenedioxythiophene) (PEDOT-MeOH) on multichannel neural probes. Sensors and Actuators B-Chemical. 2004;99:437–443. [Google Scholar]
- 75.Xiao YH, Cui XY, Martin DC. Electrochemical polymerization and properties of PEDOT/S-EDOT on neural microelectrode arrays. Journal of Electroanalytical Chemistry. 2004;573:43–48. [Google Scholar]
- 76.Xiao YH, Martin DC, Cui XY, Shenai M. Surface modification of neural probes with conducting polymer poly(hydroxymethylated-3,4-ethylenedioxythiophene) and its biocompatibility. Applied Biochemistry and Biotechnology. 2006;128:117–129. doi: 10.1385/abab:128:2:117. [DOI] [PubMed] [Google Scholar]
- 77.Richardson RT, Wise AK, Thompson BC, Flynn BO, Atkinson PJ, Fretwell NJ, Fallon JB, Wallace GG, Shepherd RK, Clark GM, O’Leary SJ. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials. 2009;30:2614–2624. doi: 10.1016/j.biomaterials.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Isaksson J, Kjall P, Nilsson D, Robinson ND, Berggren M, Richter-Dahlfors A. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nature Materials. 2007;6:673–679. doi: 10.1038/nmat1963. [DOI] [PubMed] [Google Scholar]
- 79.Simon DT, Kurup S, Larsson KC, Hori R, Tybrandt K, Goiny M, Jager EH, Berggren M, Canlon B, Richter-Dahlfors A. Organic electronics for precise delivery of neurotransmitters to modulate mammalian sensory function. Nature Materials. 2009;8:742–746. doi: 10.1038/nmat2494. [DOI] [PubMed] [Google Scholar]
- 80.Thompson BC, Moulton SE, Ding J, Richardson R, Cameron A, O’Leary S, Wallace GG, Clark GM. Optimising the incorporation and release of a neurotrophic factor using conducting polypyrrole. Journal of Controlled Release. 2006;116:285–294. doi: 10.1016/j.jconrel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Thompson BC, Richardson RT, Moulton SE, Evans AJ, O’Leary S, Clark GM, Wallace GG. Conducting polymers, dual neurotrophins and pulsed electrical stimulation - Dramatic effects on neurite outgrowth. Journal of Controlled Release. 2010;141:161–167. doi: 10.1016/j.jconrel.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 82.De Giglio E, Sabbatini L, Colucci S, Zambonin G. Synthesis, analytical characterization, and osteoblast adhesion properties on RGD-grafted polypyrrole coatings on titanium substrates. Journal of Biomaterials Science-Polymer Edition. 2000;11:1073–1083. doi: 10.1163/156856200743580. [DOI] [PubMed] [Google Scholar]
- 83.George PM, LaVan DA, Burdick JA, Chen CY, Liang E, Langer R. Electrically controlled drug delivery from biotin-doped conductive polypyrrole. Advanced Materials. 2006;18:577–+. [Google Scholar]
- 84.Cui X, Wiler J, Dzaman M, Altschuler RA, Martin DC. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials. 2003;24:777–787. doi: 10.1016/s0142-9612(02)00415-5. [DOI] [PubMed] [Google Scholar]
- 85.Wang Z, Liu S, Wu P, Cai C. Detection of glucose based on direct electron transfer reaction of glucose oxidase immobilized on highly ordered polyaniline nanotubes. Analytical Chemistry. 2009;81:1638–1645. doi: 10.1021/ac802421h. [DOI] [PubMed] [Google Scholar]
- 86.Mattioli-Belmonte M, Giavaresi G, Biagini G, Virgili L, Giacomini M, Fini M, Giantomassi F, Natali D, Torricelli P, Giardino R. Tailoring biomaterial compatibility: In vivo tissue response versus in vitro cell behavior. International Journal of Artificial Organs. 2003;26:1077–1085. doi: 10.1177/039139880302601205. [DOI] [PubMed] [Google Scholar]
- 87.Wang H-j, Ji L-w, Li D-f, Wang J-y. Characterization of nanostructure and cell compatibility of polyaniline films with different dopant acids. Journal of Physical Chemistry B. 2008;112:2671–2677. doi: 10.1021/jp0750957. [DOI] [PubMed] [Google Scholar]
- 88.Li MY, Guo Y, Wei Y, MacDiarmid AG, Lelkes PI. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials. 2006;27:2705–2715. doi: 10.1016/j.biomaterials.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 89.Bidez PR, Li SX, MacDiarmid AG, Venancio EC, Wei Y, Lelkes PI. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. Journal of Biomaterials Science-Polymer Edition. 2006;17:199–212. doi: 10.1163/156856206774879180. [DOI] [PubMed] [Google Scholar]
- 90.Green RA, Williams CM, Lovell NH, Poole-Warren LA. Novel neural interface for implant electrodes: improving electroactivity of polypyrrole through MWNT incorporation. Journal of Materials Science: Materials in Medicine. 2008;19:1625–1629. doi: 10.1007/s10856-008-3376-7. [DOI] [PubMed] [Google Scholar]
- 91.Molino PJ, Yue ZL, Zhang BB, Tibbens A, Liu X, Kapsa RMI, Higgins MJ, Wallace GG. Influence of Biodopants on PEDOT Biomaterial Polymers: Using QCM-D to Characterize Polymer Interactions with Proteins and Living Cells. Advanced Materials Interfaces. 2014;1 [Google Scholar]
- 92.Gomez N, Lee JY, Nickels JD, Schmidt CE. Micropatterned polypyrrole: A combination of electrical and topographical characteristics for the stimulation of cells. Advanced Functional Materials. 2007;17:1645–1653. doi: 10.1002/adfm.200600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang XD, Gu XS, Yuan CW, Chen SJ, Zhang PY, Zhang TY, Yao J, Chen F, Chen G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. Journal of Biomedical Materials Research Part A. 2004;68A:411–422. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 94.Gelmi A, Higgins MJ, Wallace GG. Physical surface and electromechanical properties of doped polypyrrole biomaterials. Biomaterials. 2010;31:1974–1983. doi: 10.1016/j.biomaterials.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 95.Ateh DD, Navsaria HA, Vadgama P. Polypyrrole-based conducting polymers and interactions with biological tissues. Journal of the Royal Society Interface. 2006;3:741–752. doi: 10.1098/rsif.2006.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heywang G, Jonas F. POLY(ALKYLENEDIOXYTHIOPHENE)S - NEW, VERY STABLE CONDUCTING POLYMERS. Advanced Materials. 1992;4:116–118. [Google Scholar]
- 97.Baek S, Green RA, Poole-Warren LA. The biological and electrical trade-offs related to the thickness of conducting polymers for neural applications. Acta biomaterialia. 2014;10:3048–3058. doi: 10.1016/j.actbio.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 98.King ZA, Shaw CM, Spanninga SA, Martin DC. Structural, chemical and electrochemical characterization of poly(3,4-Ethylenedioxythiophene) (PEDOT) prepared with various counter-ions and heat treatments. Polymer. 2011;52:1302–1308. doi: 10.1016/j.polymer.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang XS, Feng XQ. Effects of thickness on mechanical properties of conducting polythiophene films. Journal of Materials Science Letters. 2002;21:715–717. [Google Scholar]
- 100.Wang XS, Tang HP, Li XD, Hua X. Investigations on the Mechanical Properties of Conducting Polymer Coating-Substrate Structures and Their Influencing Factors. International Journal of Molecular Sciences. 2009;10:5257–5284. doi: 10.3390/ijms10125257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hassarati RT, Dueck WF, Tasche C, Carter PM, Poole-Warren LA, Green RA. Improving Cochlear Implant Properties Through Conductive Hydrogel Coatings. Neural Systems and Rehabilitation Engineering, IEEE Transactions on. 2014;22:411–418. doi: 10.1109/TNSRE.2014.2304559. [DOI] [PubMed] [Google Scholar]
- 102.Kim DH, Abidan M, Martin DC. Conducting polymers grown in hydrogel scaffolds coated on neural prosthetic devices. J Biomed Mater Res. 2004;71:577–585. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- 103.Patton AJ, Green RA, Poole-Warren LA. Mediating conducting polymer growth within hydrogels by controlling nucleation. APL Materials. 2015;3 [Google Scholar]
- 104.Sasaki M, Karikkineth BC, Nagamine K, Kaji H, Torimitsu K, Nishizawa M. Highly Conductive Stretchable and Biocompatible Electrode–Hydrogel Hybrids for Advanced Tissue Engineering. Advanced Healthcare Materials. 2014 doi: 10.1002/adhm.201400209. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 105.Cui XL, Engelhard MH, Lin YH. Preparation, characterization and anion exchange properties of polypyrrole/carbon nanotube nanocomposites. Journal of Nanoscience and Nanotechnology. 2006;6:547–553. doi: 10.1166/jnn.2006.122. [DOI] [PubMed] [Google Scholar]
- 106.Kim DH, Abidan M, Martin DC. Synthesis and Characterization of Conducting Polymers Grown in Hydrogels for Neural Applications. Mat Res Soc Symp Proc. 2004;1:F5.5.1–5.5.6. [Google Scholar]
- 107.Gällentoft L, Pettersson LME, Danielsen N, Schouenborg J, Prinz CN, Linsmeier CE. Size-dependent long-term tissue response to biostable nanowires in the brain. Biomaterials. 2015;42:172–183. doi: 10.1016/j.biomaterials.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 108.Shenoy SL, Kaya P, Erkey C, Weiss RA. Synthesis of conductive elastomeric foams by an in situ polymerization of pyrrole using supercritical carbon dioxide and ethanol cosolvents. Synthetic Metals. 2001;123:509–514. [Google Scholar]
- 109.Wang Y, Sotzing GA, Weiss RA. Conductive Polymer Foams as Sensors for Volatile Amines. Chemistry of Materials. 2003;15:375–377. [Google Scholar]
- 110.Abidian MR, Daneshvar ED, Egeland BM, Kipke DR, Cederna PS, Urbanchek MG. Hybrid Conducting Polymer–Hydrogel Conduits for Axonal Growth and Neural Tissue Engineering. Advanced Healthcare Materials. 2012;1:762–767. doi: 10.1002/adhm.201200182. [DOI] [PubMed] [Google Scholar]
- 111.Sekine S, Ido Y, Miyake T, Nagamine K, Nishizawa M. Conducting Polymer Electrodes Printed on Hydrogel. Journal of the American Chemical Society. 2010;132:13174–13175. doi: 10.1021/ja1062357. [DOI] [PubMed] [Google Scholar]
- 112.Chikar JA, Hendricks JL, Richardson-Burns SM, Raphael Y, Pfingst BE, Martin DC. The use of a dual PEDOT and RGD-functionalized alginate hydrogel coating to provide sustained drug delivery and improved cochlear implant function. Biomaterials. 2012;33:1982–1990. doi: 10.1016/j.biomaterials.2011.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barthus RC, Lira LM, Torresi SICd. Conducting polymer- hydrogel blends for electrochemically controlled drug release devices. Journal of the Brazilian Chemical Society. 2008;19:630–636. [Google Scholar]
- 114.Kim DH, Richardson-Burns SM, Hendricks JL, Sequera C, Martin DC. Effect of Immobilized Nerve Growth Factor on Conductive Polymers: Electrical Properties and Cellular Response. Adv Funct Mater. 2006;17:1–8. [Google Scholar]
- 115.Lim KS, Kundu J, Reeves A, Poole-Warren LA, Kundu SC, Martens PJ. The Influence of Silkworm Species on Cellular Interactions with Novel PVA/Silk Sericin Hydrogels. Macromolecular Bioscience. 2012;12:322–332. doi: 10.1002/mabi.201100292. [DOI] [PubMed] [Google Scholar]
- 116.Lim KS, Alves MH, Poole-Warren LA, Martens PJ. Covalent incorporation of non-chemically modified gelatin into degradable PVA-tyramine hydrogels. Biomaterials. 2013;34:7097–7105. doi: 10.1016/j.biomaterials.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Tsai T-S, Pillay V, Choonara YE, du Toit LC, Modi G, Naidoo D, Kumar P. A Polyvinyl Alcohol-Polyaniline Based Electro-Conductive Hydrogel for Controlled Stimuli-Actuable Release of Indomethacin. Polymers. 2011;3:150–172. [Google Scholar]
- 118.Lira LM, Córdoba de Torresi SI. Conducting polymer-hydrogel composites for electrochemical release devices: Synthesis and characterization of semi-interpenetrating polyaniline-polyacrylamide networks. Electrochemistry Communications. 2005;7:717–723. [Google Scholar]
- 119.Lira LM, Barthus RC, Córdoba de Torresi SI. Conducting polymers and hydrogels for electrochemically controlled drug release devices. 210th meeting of the Electrochemical Society; Cancun, Mexico. 2006. [Google Scholar]
- 120.Green RA, Suaning GJ, Poole-Warren LA, Lovell NH Ieee. Bioactive Conducting Polymers for Neural Interfaces Application to Vision Prosthesis. 2009 4th International Ieee/Embs Conference on Neural Engineering; 2009. pp. 60–63. [Google Scholar]
- 121.Jeong J, Lee SW, Min K, Eom K, Bae SH, Kim SJ Ieee. Eye-Surface Conformable Telemetric Structure for Polymer-based Retinal Prosthesis. 2011 Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc) 2011:1097–1100. doi: 10.1109/IEMBS.2011.6090256. [DOI] [PubMed] [Google Scholar]
- 122.Kip AL, Nicholas BL, Mike DJ, Sarah MR-B, Jeffrey LH, Daryl RK. Poly(3,4-ethylenedioxythiophene) (PEDOT) polymer coatings facilitate smaller neural recording electrodes. Journal of Neural Engineering. 2011;8:014001. doi: 10.1088/1741-2560/8/1/014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Buzsaki G. Large-scale recording of neuronal ensembles. Nature Neuroscience. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 124.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 125.Woolley AJ, Desai HA, Gaire J, Ready AL, Otto KJ. Intact Histological Characterization of Brain-implanted Microdevices and Surrounding Tissue. Journal of Visualized Experiments: JoVE. 2013:50126. doi: 10.3791/50126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ouyang L, Green R, Feldman KE, Martin DC. Direct local polymerization of poly(3,4-ethylene dioxythiophene in rat cortex. In: Schouenborg J, Garwicz M, Danielsen N, editors. Progress in Brain Research. Elsevier; 2011. [DOI] [PubMed] [Google Scholar]
- 127.Liangqi O, Crystal LS, Chin-chen K, Amy LG, David CM. In vivo polymerization of poly(3,4-ethylenedioxythiophene) in the living rat hippocampus does not cause a significant loss of performance in a delayed alternation task. Journal of Neural Engineering. 2014;11:026005. doi: 10.1088/1741-2560/11/2/026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rutten WLC. SELECTIVE ELECTRICAL INTERFACES WITH THE NERVOUS SYSTEM. Annual Review of Biomedical Engineering. 2002;4:407–452. doi: 10.1146/annurev.bioeng.4.020702.153427. [DOI] [PubMed] [Google Scholar]
- 129.Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. Journal of Neuroscience Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 130.Zhou DD, Cui XT, Hines A, Greenberg RJ. Conducting Polymers in Neural Stimulation Applications. In: Zhou DD, Greenbaum E, editors. Implantable Neural Prostheses 2: Techniques and Engineering Approaches. 2010. pp. 217–252. [Google Scholar]
- 131.Cogan SF, Troyk PR, Ehrlich J, Plante TD. In vitro comparison of the charge-injection limits of activated iridium oxide (AIROF) and platinum-iridium microelectrodes. Biomedical Engineering, IEEE Transactions on. 2005;52:1612–1614. doi: 10.1109/TBME.2005.851503. [DOI] [PubMed] [Google Scholar]
- 132.Baek S, Green RA, Poole-Warren LA. Effects of dopants on the biomechanical properties of conducting polymer films on platinum electrodes. Journal of Biomedical Materials Research Part A. 2013 doi: 10.1002/jbm.a.34945. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 133.Baier H, Bonhoeffer F. AXON GUIDANCE BY GRADIENTS OF A TARGET-DERIVED COMPONENT. Science. 1992;255:472–475. doi: 10.1126/science.1734526. [DOI] [PubMed] [Google Scholar]
- 134.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 135.TessierLavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 136.Ho D, Zou J, Chen X, Munshi A, Smith NM, Agarwal V, Hodgetts SI, Plant GW, Bakker AJ, Harvey AR, Luzinov I, Iyer KS. Hierarchical Patterning of Multifunctional Conducting Polymer Nanoparticles as a Bionic Platform for Topographic Contact Guidance. Acs Nano. 2015;9:1767–1774. doi: 10.1021/nn506607x. [DOI] [PubMed] [Google Scholar]
- 137.Lee JY, Bashur CA, Goldstein AS, Schmidt CE. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–4335. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Green RA, Lovell NH, Poole-Warren LA. Impact of co-incorporating laminin peptide dopants and neurotrophic growth factors on conducting polymer properties. Acta Biomaterialia. 2010;6:63–71. doi: 10.1016/j.actbio.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 139.Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22:1055–1064. doi: 10.1016/s0142-9612(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 140.Park K-H, Jo EA, Na K. Heparin/Polypyrrole (PPy) composite on gold-coated matrix for the neurite outgrowth of PC 12 cells by electrical stimulation. Biotechnology and Bioprocess Engineering. 2007;12:463–469. [Google Scholar]
- 141.Valentini RF, Sabatini AM, Dario P, Aebischer P. POLYMER ELECTRET GUIDANCE CHANNELS ENHANCE PERIPHERAL-NERVE REGENERATION IN MICE. Brain Research. 1989;480:300–304. doi: 10.1016/0006-8993(89)90196-0. [DOI] [PubMed] [Google Scholar]
- 142.Jager EWH, Smela E, Inganas O. Microfabricating conjugated polymer actuators. Science. 2000;290:1540–1545. doi: 10.1126/science.290.5496.1540. [DOI] [PubMed] [Google Scholar]
- 143.Pernaut JM, Reynolds JR. Use of conducting electroactive polymers for drug delivery and sensing of bioactive molecules. A redox chemistry approach. Journal of Physical Chemistry B. 2000;104:4080–4090. [Google Scholar]
- 144.Su D, Di F, Xing J, Che JF, Xiao YH. Application of Conducting Polymers in Controlled Drug Delivery System. Progress in Chemistry. 2014;26:1962–1976. [Google Scholar]
- 145.Svirskis D, Travas-Sejdic J, Rodgers A, Garg S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. Journal of Controlled Release. 2010;146:6–15. doi: 10.1016/j.jconrel.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 146.Xiao YH, Ye XX, He L, Che JF. New carbon nanotube-conducting polymer composite electrodes for drug delivery applications. Polymer International. 2012;61:190–196. [Google Scholar]
- 147.Massoumi B, Entezami A. Electrochemically controlled binding and release of dexamethasone from conducting polymer bilayer films. Journal of Bioactive and Compatible Polymers. 2002;17:51–62. [Google Scholar]
- 148.Wadhwa R, Lagenaur CF, Cui XT. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. Journal of Controlled Release. 2006;110:531–541. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 149.Stevenson G, Moulton SE, Innis PC, Wallace GG. Polyterthiophene as an electrostimulated controlled drug release material of therapeutic levels of dexamethasone. Synth Met. 2010;160:1107–1114. [Google Scholar]
- 150.Goding JA, Gilmour AD, Martens PJ, Poole-Warren LA, Green RA. Small bioactive molecules as dual functional co-dopants for conducting polymers. Journal of Materials Chemistry B. 2015 doi: 10.1039/c5tb00384a. [DOI] [PubMed] [Google Scholar]
- 151.Stevenson G, Moulton SE, Innis PC, Wallace GG. Polyterthiophene as an electrostimulated controlled drug release material of therapeutic levels of dexamethasone. Synthetic Metals. 2010;160:1107–1114. [Google Scholar]