Abstract
Atypical teratoid/rhabdoid tumors (ATRT) are rare, highly malignant, embryonal CNS tumors with a poor prognosis. Therapy relies on highly toxic chemotherapy and radiotherapy. To improve outcomes and decrease morbidity, more targeted therapy is required. Gene expression analysis revealed elevated expression of multiple kinases in ATRT tissues. Aurora Kinase A was one of the candidate kinases. The objective of this study was to evaluate the impact of Aurora Kinase A inhibition in ATRT cell lines. Our analysis revealed that inhibition of Aurora Kinase A induces cell death in ATRT cells and the small molecule inhibitor MLN 8237 sensitizes these cells to radiation. Furthermore, inhibition of Aurora Kinase A resulted in decreased activity of pro-proliferative signaling pathways. These data indicate that inhibition of Aurora Kinase A is a promising small molecule target for ATRT therapy.
Keywords: Atypical teratoid rhabdoid tumor, ATRT, Aurora Kinase A
Introduction
Atypical teratoid/rhabdoid tumors (ATRT) are rare, highly malignant, embryonal CNS tumors that most commonly affect young children. They historically carry a poor prognosis with median survival time less than 11 months [1–3]. The two main prognostic factors that affect overall outcome are the extent of tumor resection and early radiation therapy (RT) [2]. More recent studies indicate that patients benefit from intensified multimodal therapies, including high-dose chemotherapy with autologous stem cell rescue [4] and dose intensified sarcoma therapy [5]. Despite an increase in overall survival with these approaches, therapy-related toxicity frequently remains a critical problem in this young age group.
ATRT is frequently associated with a mutation or deletion of the hSNF5/INI1/SMARCB1 gene found on chromosome 22q [6, 7]. SMARCB1 is a key component of the chromatin-remodeling complex, and can function as a tumor suppressor. Its absence has been shown to be associated with cell cycle progression [8]. A small subset of ATRT patients exhibits germline mutations in the SMARCB1 gene and thus is prone to the rhabdoid tumor syndrome. While investigations into the molecular mechanisms of this tumor have shed light on the biological behavior, there has been little progress in developing novel therapeutics.
Protein kinases are likely therapeutic targets as many malignancies display abnormal activation of these molecules [9]. We have recently shown that Aurora Kinase A is a potential target in both medulloblastoma and high-grade glioblastoma [10, 11]. Aurora Kinase A plays a critical role in mitosis by regulating centrosome duplication, bipolar spindle formation, alignment of chromosomes on the mitotic spindle, and the mitotic checkpoint [12]. In addition to CNS tumors Aurora Kinase A has been implicated in many types of cancer including breast, colon, ovarian, liver, non-small cell lung, and pancreatic cancers [13–18]. Its role in ATRT is less well studied. Recent evidence suggests that the SMARCB1 gene suppresses Aurora Kinase A expression in non-CNS rhabdoid tumor cells and is required for tumor cell survival [19]. In the present study we show that Aurora Kinase A is a promising target for therapy in ATRT and that the small molecule drug MLN 8237 is a potent radio sensitizer of ATRT cells.
Methods
Cell lines and primary tumor samples
The BT12 and BT16 ATRT cell lines were purchased from American Type Cell Culture (Rockville, MD). Cell lines were cultured in DMEM (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA).
Primary patient samples were obtained from The Children’s Hospital, Denver, and were conducted in accordance with local and federal human research protection guidelines and institutional review board regulations. Informed consent was obtained for all specimens collected. Normal brain tissue was collected from autopsy and purchased from Ambion (Austin, TX) and Clonetech Laboratories, Inc. (Mountain View, CA).
Gene expression microarray analysis
Sixteen patient tumor samples comprising the first cohort were evaluated for gene expression using Affymetrix U133 Plus 2.0 GeneChip microarrays as previously described [20]. Briefly, samples were collected at the time of surgery and snap-frozen in liquid nitrogen. Ribonucleic acid was extracted from each sample using an RNeasy kit (Qiagen, Valencia, CA) and hybridized to HG-U133 Plus 2.0 Gene-Chips (Affymetrix, Santa Clara, CA) according to the manufacturer’s instructions. Microarray data from the samples was background-corrected and normalized using the gcRMA algorithm. One probe set per gene, based on highest overall expression level across samples, was selected for use in subsequent analyses. Differential expression of genes was determined using a Student’s t test [20].
Transfection of RNAi vectors
A siRNA targeting Aurora Kinase A mRNA and a non-targeting siRNA were purchased from Ambion. SiRNA were transfected into ATRT cell lines using the siPORT NeoFX Transfection Agent (Ambion). A final concentration of 5 nM of siRNA for a 6-well plate was transfected into the ATRT cell lines. The manufacturer’s suggested protocol for a reverse transfection was used with the siRNA.
Quantitative real-time polymerase chain reaction
Ribonucleic acid was isolated 48 h after transfection using a Qiagen RNeasy kit (Valencia, CA). Gene expression primers and probes for Aurora Kinase A and GAPDH (Hs99999905_m1) were purchased from Applied Biosystems (Carlsbad, CA). Assays were performed in triplicate according to the manufacturer’s recommendations. GAP-DH was used as an endogenous control and the gene expression relative quantity was calculated using the ΔΔCt method. Gene expression assays were performed on an ABI StepOnePlus Real-Time PCR system.
Small molecule inhibitor MLN 8237
The small molecule AURORA KINASE A inhibitor MLN 8237 was purchased from Axon Medchem (Groninberg, Netherlands). The drug was reconstituted in dimethyl sulfoxide (DMSO) and aliquots were stored in a desiccator at −20°C. An equivalent amount of DMSO for the highest concentration of drug was used for each experiment as a vector control.
Cell proliferation and apoptosis assays
Cell proliferation was assayed by MTS [3-(4,5-dimethyl-thiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay using CellTiter 96 AQueous One Solution (Promega, Madison, WI). Seventy-two hours after transfection with siRNA, 20 microliters of MTS reagent was added to the wells. For drug treatment, cells were plated for 24 h before adding MLN 8237 and 30 microliters of MTS reagent were added to the wells 72 h after the addition of the drug. Plates were read using a BioTek Synergy 2 plate reader (Winooski, VT) every hour for 4 h after the addition of the MTS reagent. Experiments were done in triplicate and background absorbance was subtracted from all wells before analysis.
For the colony formation assay, cells were transfected with siRNA for 48 h and then plated at 500 cells per well of a 6-well plate in triplicate. For drug treatment, cells were plated in triplicate 24 h before addition of MLN 8237. Cells were then treated with drug for 24 h and then allowed to grow in normal culture medium. After seven days of growth, the medium was aspirated, the wells were washed with PBS, and the colonies were stained with 0.5% crystal violet/25% methanol solution. The number of colonies per well was counted using a dissecting microscope with a threshold of 50 cells necessary to constitute a colony.
Apoptosis was assessed 72 h after siRNA transfection or after 24 h of MLN 8237 treatment followed by 24 h in normal culture medium. ATRT cell lines were seeded in triplicate into 12-well plates for 24 h. Cells were transfected with Aurora Kinase A siRNA or control siRNA and media (inclusive of de-adherent cells) and adherent cells were collected, centrifuged, and counted by ViaCount assay on a Guava EasyCyte flow cytometer (Millipore). For each transfection 30,000 cells per cell line were incubated with Nexin reagent (Annexin V-PE and 7-AAD, Millipore) for 20 min. Cell fluorescence was measured and analyzed on the Guava EasyCyte flow cytometer. Numerical differences in apoptotic fractions were analyzed with Graph-Pad Prism software.
Western blotting
Western blotting was performed per standard methods. Briefly, BT-12 and BT-16 cells were treated with MLN 8237 for 24, and 48 h. At each timepoint, cells were collected, washed with PBS, resuspended in chilled RIPA buffer and Protease Inhibitor Cocktail (Roche), incubated on ice for 15 min, and centrifuged for 30 min. Protein quantification was performed via the Bradford assay. Incubation of protein lysates with Laemmli sample buffer (Bio-Rad) was performed at 95°C for 5 min. Samples were then subjected to SDS-PAGE (15% Tris–HCl precast gel, Bio-Rad) and transferred onto a PVDF membrane. Immunoblotting was performed via the Snap I.D. system (Millipore) with primary Aurora Kinase A [p21Waf1/Cip1 (BD Biosciences), actin (Chemicon)] and HRP-conjugated secondary (Jackson Laboratories) antibodies. Chemiluminescence was elicited with SuperSignal West pico and femto substrates (Fermo Scientific) and then exposed to film.
Combination of MLN 8237 and ionizing radiation
Five hundred cells per well of a 6-well plate were plated in triplicate for 24 h before addition of MLN 8237. Cells were exposed to drug for 24 h, and then drug-containing medium was aspirated and normal culture medium was added. Cells were then immediately irradiated. After 8 days of additional growth, wells were stained as described above with crystal violet solution. Survival curves were generated after normalizing for the amount of MLN 8237-induced death. Non-linear regressions were calculated for each line. The radiation dose intersecting the nonlinear regression for a 10% (SF0.1) and 50% (SF0.5) surviving fraction was calculated for each drug dose. The sensitizer enhancement ratio (SER) was then calculated as follows:
The SER allows for direct comparison of the effect of the putative sensitizing agent relative to control treated cells.
Luciferase analysis
Pathway analysis was performed using the Cignal Finder 10 Pathway Reporter Arrays (SA Biosciences) per the manufacturers’ instructions. Cells were seeded into 96 well plates containing luciferase reporters to common cancer pathways along with surefect transfection reagent. 12 h later cells were treated with MLN 8237 (IC30) or DMSO control and cells incubated for a further 24 h. Luciferase activity was measured using the Dual Luciferase Assay system (Promega) on a Glomax multi luminometer (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity and the ratio calculated. Data were analyzed with GraphPad Prism software.
Statistical analysis
Statistical significance was determined using a Student’s t test. Error bars represent the standard error of the mean (n ≥ 3) unless otherwise specified. Graph Pad Prism 5 was used to calculate IC50 values and to compute the nonlinear regression equations.
Results
Aurora Kinase A is over expressed in ATRT
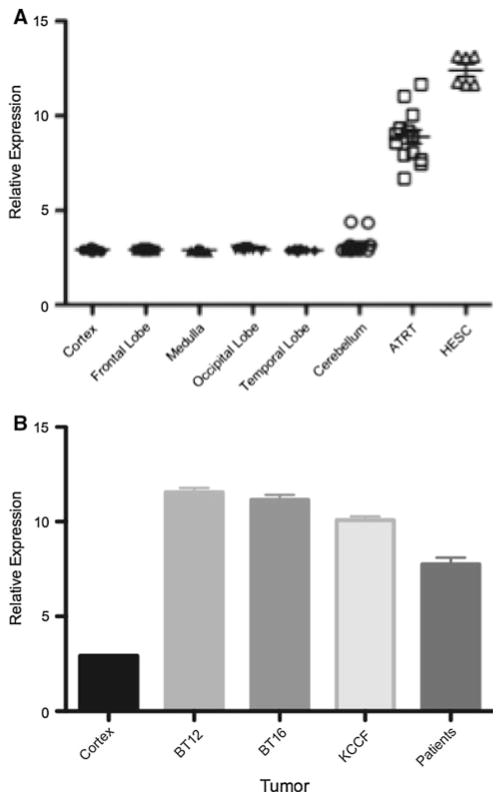
We initially hypothesized that kinases involved in cell cycle regulation would be likely candidates as novel therapeutic targets in ATRT. To begin to address this question, we first examined expression of cell cycle-regulated kinases in a cohort of ATRT patients we had previously studied [21]. We found that expression of Aurora Kinase A mRNA was altered in all, of our patient samples when compared to normal brain (Fig. 1a). Interestingly Aurora Kinase A is also expressed at similarly high levels in human embryonic stem cells (HESC) as shown in Fig. 1a. There was no clear correlation between the high Aurora Kinase A-expressing samples and age, gender or outcomes. These data indicate that Aurora Kinase may be associated with the oncogenic process in general and is not specific to a particular molecular subgroup of ATRT. We next evaluated expression of Aurora Kinase A mRNA in a panel of well-characterized ATRT cell lines. Consistent with our patient tissue data, all ATRT cell lines tested expressed Aurora Kinase A mRNA at significantly higher levels (10- to 20-fold higher, P < 0.01) compared to normal brain (Fig. 1b).
Fig. 1.
Expression level of Aurora kinase A mRNA in ATRT. a Aurora kinase A mRNA levels by microarray in 14 primary ATRT patient samples compared to normal brain samples. Error bars represent standard error of the mean (SEM). b Significantly higher Aurora kinase A mRNA expression is seen in common ATRT cell lines by qRT-PCR
Inhibition of Aurora Kinase A suppresses ATRT cell growth
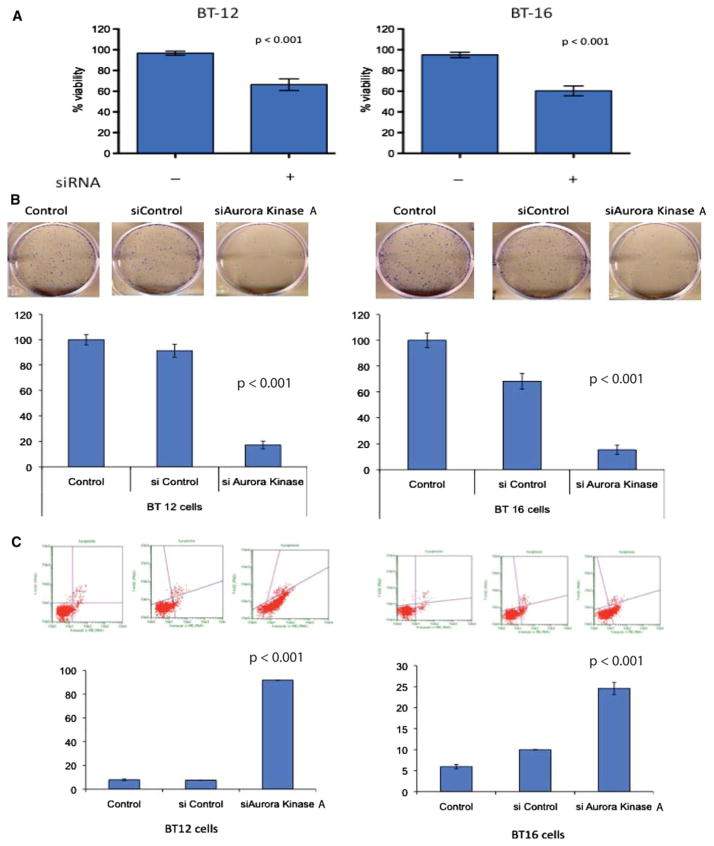
To examine whether Aurora Kinase A was functionally important for ATRT tumorigenesis, we initially decreased expression of Aurora Kinase A mRNA by siRNA in BT12 an BT16 cells. We confirmed loss of SMARCB1 in these cells (Supplementary Figure S1A). Using the MTS assay we found a significant decrease in cell proliferation of both ATRT cell lines treated with Aurora Kinase A siRNA compared to control siRNA (Fig. 2a). Knockdown of Aurora Kinase A mRNA expression was verified by qRT-PCR and by western blot analysis (Figure S1B). The cell proliferation assays were performed after 72 h of transfection. To evaluate a longer-term impact we then performed colony formation assays on cells transfected with control or Aurora Kinase A siRNA. Once again, inhibition of Aurora Kinase A mRNA by RNAi significantly decreased ATRT cell growth as measured by the ability to form colonies (Fig. 2b). Interestingly in ATRT cells there was a much more marked inhibition in the colony formation capability compared to measuring just cell proliferation (‘60% inhibition vs. 20% inhibition, respectively). These data may reflect the impact of Aurora Kinase A on tumor cell self-renewal compared to just mitosis.
Fig. 2.
Decrease in ATRT cell growth following Aurora Kinase A inhibition. a A significant decrease in relative cell number was seen by MTS assay 72 h following siRNA-mediated inhibition of Aurora Kinase A mRNA in BT12 and BT16 cells. Error bars represent SEM. b Long-term decrease in growth as seen through a colony formation assay following knockdown of Aurora Kinase A mRNA with siRNA. Representative pictures for each cell line are below the quantifying graphs. Error bars represent SEM. c Increased apoptosis following Aurora Kinase A inhibition. Representative flow cytometry plots for two cell lines stained with Guava Nexin reagent. Quantifying bar graphs below showing a significant increase in the percentage of apoptotic/dead cells following Aurora Kinase A mRNA knockdown with siRNA
Aurora Kinase A inhibition induces apoptosis in ATRT cells
Inhibition of Aurora Kinase A induces the spindle checkpoint with subsequent induction of cell death [22]. To evaluate whether decreased expression of Aurora Kinase A mRNA resulted in apoptosis in ATRT, we analyzed Annexin V expression on ATRT cells by flow cytometry. As shown in the representative plots in Fig. 2c, siAurora Kinase A, but not non-silencing control, potently induced apoptosis as detected by increased Annexin V expression in both BT12 and BT16 ATRT cell lines. The difference in apoptosis between non-silencing control and si Aurora Kinase A was statistically significant in multiple independent replicates (Fig. 2c). These data suggest that Aurora Kinase A could indeed be a promising therapeutic target for ATRT.
Small molecule inhibitor MLN 8237 is a potent inhibitor of ATRT cell growth
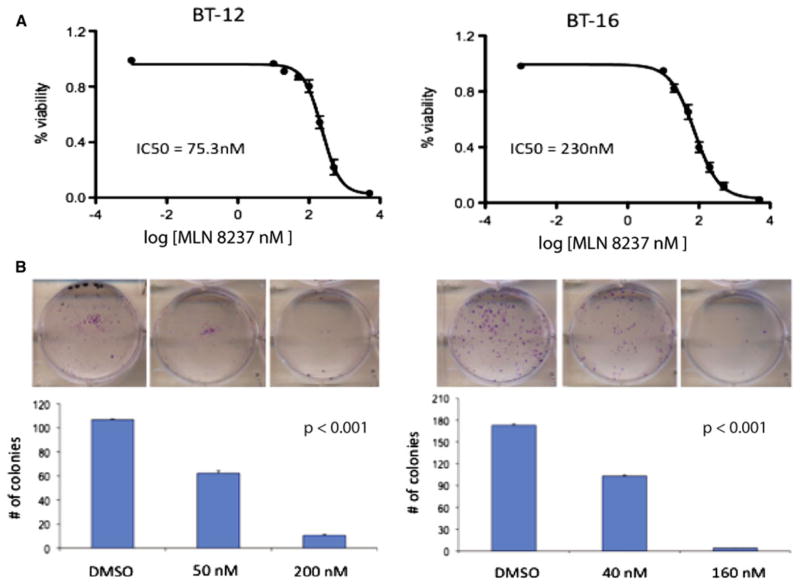
Recently, several inhibitors of Aurora Kinase A have been described. Among these is MLN 8237 (Millennium Pharmaceuticals, MA). We determined whether MLN 8237, like Aurora Kinase A siRNA, decreases proliferation of ATRT cells. BT12 and BT 16 cells were treated with varying concentrations of MLN 8237 and cell proliferation was evaluated by the MTS assay. MLN 8237 potently inhibited cell growth with an IC50 of 75.3 nM for BT12 and 230 nM for BT16 cells after 72 h of treatment (Fig. 3a). To further examine the effects of MLN 8237 on ATRT cells, we performed colony formation assays. Representative colonies are shown in Fig. 3b. We found that MLN 8237 strongly suppressed the ability of ATRT cells to form colonies (Fig. 3b).
Fig. 3.
Inhibition of Aurora Kinase A with MLN-8237 decreases cell growth and increases apoptosis. a Decrease in the relative cell number of two ATRT cell lines through a wide range of MLN-8237 concentrations. Error bars represent SEM. b Representative pictures from colony formation assay with quantifying bar graph showing a significant decrease in the ability of two ATRT cell lines in forming colonies following 48 h treatment with MLN-8237
MLN 8237 enhances radiation sensitivity of ATRT cells
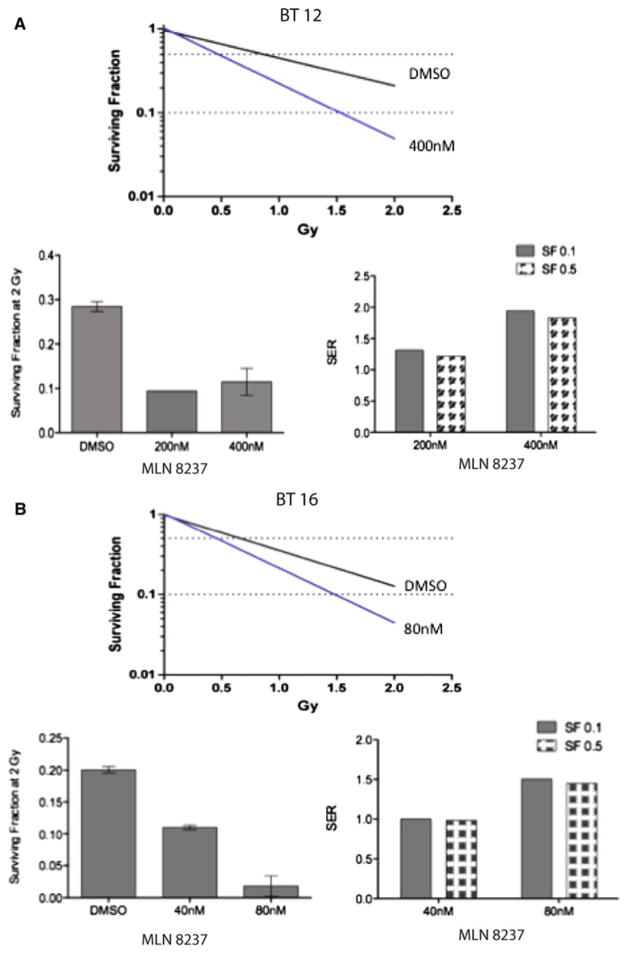
MLN 8237 is a promising agent for combination therapy in ATRT. To investigate whether MLN 8237 enhances cellular sensitivity to ionizing radiation, ATRT cells were exposed to MLN 8237 for 24 h before irradiation and the effects evaluated using the colony formation assay. We found that the survival fractions (SF) were reduced in BT12 and BT16 cells after they were exposed to MLN 8237. Survival fractions of MLN 8237-pretreated cells after two Gray (Gy) radiation were significantly lower than those of untreated cells (Fig. 4A-i, B-i, respectively). Nonlinear regressions were fit to the curves to assist in calculating the SER (Fig. 4A-ii, B-ii). The SERs were 1.9 for BT-12 at 10% cell survival (SF0.1) and 1.8 at 50% cell survival (SF0.5) with 400 nM MLN 8237 pre-treatment (Fig. 4A-iii). For BT16 cells pretreated with 80 nM MLN 8237, the SERs were 1.5 and 1.45 for SF0.1 and SF0.5, respectively (Fig. 4B-iii). The SER demonstrates the effect of the sensitizing agent relative to control vehicle in the presence of radiation. A SER of greater than one indicates a synergistic effect of the sensitization agent with radiation. Thus, the radiation survival curves obtained by the colony formation assay showed that MLN 8237 pretreatment sensitized human ATRT cells to ionizing radiation.
Fig. 4.
Pretreatment with MLN-8237 enhances radiation sensitivity in ATRT cell lines. Nonlinear regression lines for ATRT cells treated with MLN-8237 then irradiated (A-i = BT12; B-i = BT16). Bar graphs show the quantification of the surviving fraction following 2 Gray irradiation (left) and the sensitizer enhancement ratio (SER; right) for BT12 cells (A-ii and A-iii) and BT16 cells (B-ii and B-iii)
Inhibition of Aurora Kinase A alters pro-growth signaling in ATRT cells
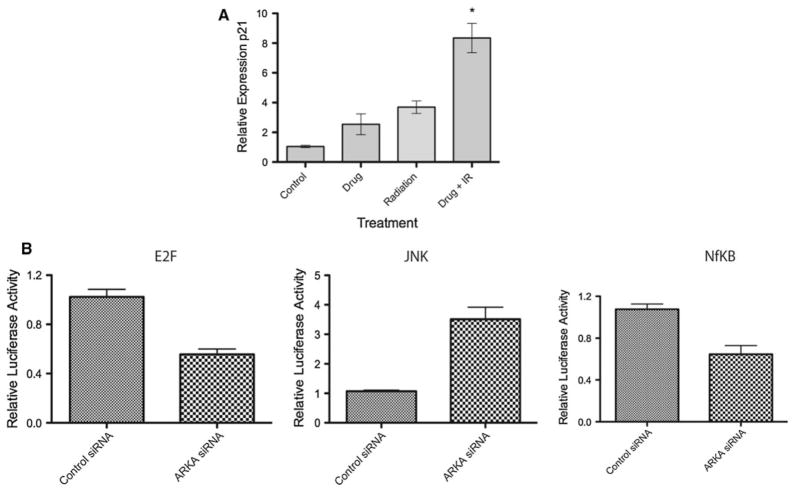
To evaluate the mechanisms by which Aurora Kinase A inhibition might impact ATRT cell growth, we evaluated the activity of cancer associated signaling pathways in BT12 cells. Treatment with radiation induced p21 gene expression as expected (Fig. 5a). Co-treatment with radiation and MLN 8237 potently increased the gene expression of p21 indicating that inhibition of Aurora Kinase A may potentiate radiation by modulating the DNA damage response pathway. Next, using luciferase reporter assays (Cignal Cancer Pathway Finder; SA Biosciences) we examined other cellular signaling mechanisms that may be impacted by Aurora Kinase A inhibition. This assay measure the activity of transcription factors associated with known cancer pathways. ATRT cells were seeded into 96 well plates containing cancer associated transcription factor activated promoter luciferase reporters along with surefect transfection reagent. 12 h later cells were treated with MLN 8237 (IC30) or DMSO control and cells incubated for a further 24 h. Luciferase activity was measured using the Dual Luciferase Assay system (Promega) on a Glomax multi luminometer (Promega). We found that inhibition of Aurora Kinase A resulted in significantly decreased E2F1 regulated promoter activity indicating an impairment of RB-Cyclin D1 responsive signaling (Fig. 5b). Similarly, NFkB modulated promoter activity was also down-regulated by Aurora Kinase A inhibition (Fig. 5b) while JNK activity was increased. These findings suggest a shift to a pro-apoptotic state in ATRT cells.
Fig. 5.
Signaling pathways impacted by inhibition of Aurora Kinase A. a Inhibition of Aurora Kinase A in BT12 cells resulted in significantly increased p21 gene expression in response to radiation. b Altered cellular signaling in response to Aurora Kinase A inhibition. Reporter activity was performed in quadruplicate using the Cignal pathway finder (SA Biosciences) luciferase constructs
Discussion
Atypical teratoid rhabdoid tumor (AT/RT) of the CNS is a highly malignant neoplasm of childhood with median survival of 6 to 11 months. Despite advances in surgery, radiation, and chemotherapy, little progress has been made in advancing therapy for these tumors. Current therapy consists of intensive chemotherapy in combination with RT where possible [5]. Initial data on this approach shows an improvement in progression free survival (PFS) at 2 years [5]. However there were significant toxicities reported including one therapy related death [5]. Improvements in outcomes with a decrease in toxicities will require additional therapies. In that regard we have found that inhibition of Histone deacetylases inhibit growth of ATRT cells and can potentiate radiation (Knipstein et al. accepted, NeuroOncology). Protein kinases provide a promising target for exploration. We found many kinases to be differentially expressed when compared to normal brain tissue. Among these, Aurora Kinase A was significantly elevated in expression in ATRT tumor samples. Aurora Kinase A is important in maintaining proper chromosome segregation and has been associated with many different cancers. We have previously shown that Aurora Kinase A is an adverse marker in medulloblastoma and glioblastoma [10, 11].
In this report, we demonstrate that Aurora Kinase A is a potential therapeutic target for ATRT treatment. We show that inhibition of Aurora Kinase A suppress ATRT cell growth and induces a cell cycle arrest. Interestingly Aurora Kinase A was recently found to be a direct target of SMARCB1 in kidney and muscle rhabdoid tumors further indicating the key role for Aurora Kinase in rhabdoid tumors in general [19]. These data also provide a strong biological rationale for targeting Aurora Kinase A in ATRT. Importantly, we found that Aurora Kinase A inhibition potently sensitized ATRT cells to radiation. RT plays an important role in the treatment of ATRT [2, 5, 23]. Radiation dose is typically limited by normal tissue toxicity, but the addition of radiosensitizers can potentiate the effectiveness of RT without significant increase in toxicity. The radiosensitization seen with the inhibition of Aurora Kinase A suggests that combined therapy with Aurora Kinase A inhibitors and RT should be explored in future clinical trials. Our data suggest a role for Aurora Kinase A inhibitors in combination with standard radiation in the treatment of ATRT and provide rationale for clinical trials of Aurora Kinase A inhibitors. By radiosensitizing cells, lower amounts of radiation would be required, thus lowering toxicity.
Recent studies have identified a host of Aurora Kinase inhibitors as reviewed by Taylor et al. [24]. Some inhibitors such as ZM447439 (AstraZeneca, Boston, MA) and VX-680 (Merck, Rahway, NJ) inhibit both Aurora Kinase A and B [24]. MLN-8237 (Millennium Pharmaceuticals, Cambridge, MA) has been shown to have significant selectivity for Aurora Kinase A. These and other newer compounds are promising potential agents for ATRT therapy. We show that MLN 8237 is a promising small molecule inhibitor for ATRT therapy. Previous work by us and others has shown that MLN8237 is a useful drug around which to build clinical trials [10, 25].
We identified several mechanisms by which Aurora Kinase A may control ATRT cell growth. We show that pro-proliferative and anti-apoptotic signaling mechanisms are down-regulated by inhibition of Aurora Kinase A and conversely pro-apoptotic signaling mechanisms are induced. Whether these effects are a direct result of Aurora Kinase A inhibition is currently under investigation by us.
In future clinical trails of Aurora Kinase A inhibitors the patient population in which these agents are tested has to be carefully chosen. One approach would be to treat only those patients in whom Aurora Kinase A has increased expression compared with normal tissue. Moreover our data suggest that pre-clinical studies and early trial design should include a combination therapy approach with radiation and Aurora Kinase A inhibition. In summary we have established a proof of principle that inhibition of Aurora Kinase A is a viable strategy for ATRT therapy.
Supplementary Material
Acknowledgments
The authors thank the Department of Pediatrics and The Children’s Hospital Denver for their support and Morgan Adams Foundation (to NF and RV); National Institutes of Health (KO8 NS59790 to RV) for funding.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-011-0795-y) contains supplementary material, which is available to authorized users.
Contributor Information
Sujatha Venkataraman, Department of Pediatrics, Children’s Hospital Colorado, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA.
Irina Alimova, Department of Pediatrics, Children’s Hospital Colorado, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA.
Tiffany Tello, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA.
Peter S. Harris, Department of Pediatrics, Children’s Hospital Colorado, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA
Jeffrey A. Knipstein, Department of Pediatrics, Children’s Hospital Colorado, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA
Andrew M. Donson, Department of Pediatrics, Children’s Hospital Colorado, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA
Nicholas K. Foreman, Department of Pediatrics, Children’s Hospital Colorado, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA
Arthur K. Liu, Department of Radiation Oncology, University of Colorado, Anschutz Medical Campus, Aurora, CO, USA
Rajeev Vibhakar, Email: rajeev.vibhakar@ucdenver.edu, Department of Pediatrics, Children’s Hospital Colorado, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA. Department of Pediatrics, University of Colorado Denver, 12800 19th Ave, Mail Stop 8302, Aurora, CO 80045, USA.
References
- 1.Bambakidis NC, Robinson S, Cohen M, Cohen AR. Atypical teratoid/rhabdoid tumors of the central nervous system: clinical, radiographic and pathologic features. Pediatr Neurosurg. 2002;37:64–70. doi: 10.1159/000065107. [DOI] [PubMed] [Google Scholar]
- 2.Hilden JM, Meerbaum S, Burger P, Finlay J, Janss A, Scheithauer BW, Walter AW, Rorke LB, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004;22:2877–2884. doi: 10.1200/JCO.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 3.Reddy AT. Atypical teratoid/rhabdoid tumors of the central nervous system. J Neurooncol. 2005;75:309–313. doi: 10.1007/s11060-005-6762-8. [DOI] [PubMed] [Google Scholar]
- 4.Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008;51:235–240. doi: 10.1002/pbc.21578. [DOI] [PubMed] [Google Scholar]
- 5.Chi SN, Zimmerman MA, Yao X, Cohen KJ, Burger P, Biegel JA, Rorke-Adams LB, Fisher MJ, Janss A, Mazewski C, Goldman S, Manley PE, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27:385–389. doi: 10.1200/JCO.2008.18.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- 7.Biegel JA, Tan L, Zhang F, Wainwright L, Russo P, Rorke LB. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res. 2002;8:3461–3467. [PubMed] [Google Scholar]
- 8.Fujisawa H, Misaki K, Takabatake Y, Hasegawa M, Yamashita J. Cyclin D1 is overexpressed in atypical teratoid/rhabdoid tumor with hSNF5/INI1 gene inactivation. J Neurooncol. 2005;73:117–124. doi: 10.1007/s11060-004-4276-4. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312:1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 10.Barton VN, Foreman NK, Donson AM, Birks DK, Handler MH, Vibhakar R. Aurora kinase A as a rational target for therapy in glioblastoma. J Neurosurg Pediatr. 2010;6:98–105. doi: 10.3171/2010.3.PEDS10120. [DOI] [PubMed] [Google Scholar]
- 11.El-Sheikh A, Fan R, Birks D, Donson A, Foreman NK, Vibhakar R. Inhibition of aurora kinase A enhances chemosensitivity of medulloblastoma cell lines. Pediatr Blood Cancer. 2010;55:35–41. doi: 10.1002/pbc.22465. [DOI] [PubMed] [Google Scholar]
- 12.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Wang JH, Chu XY, Geng HC, Chen LB. Expression of STK15 mRNA in hepatocellular carcinoma and its prognostic significance. Clin Biochem. 2009;42:641–647. doi: 10.1016/j.clinbiochem.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Baba Y, Nosho K, Shima K, Irahara N, Kure S, Toyoda S, Kirkner GJ, Goel A, Fuchs CS, Ogino S. Aurora-A expression is independently associated with chromosomal instability in colorectal cancer. Neoplasia. 2009;11:418–425. doi: 10.1593/neo.09154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogawa E, Takenaka K, Katakura H, Adachi M, Otake Y, Toda Y, Kotani H, Manabe T, Wada H, Tanaka F. Perimembrane aurora-A expression is a significant prognostic factor in correlation with proliferative activity in non-small-cell lung cancer (NSCLC) Ann Surg Oncol. 2008;15:547–554. doi: 10.1245/s10434-007-9653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milam MR, Gu J, Yang H, Celestino J, Wu W, Horwitz IB, Lacour RA, Westin SN, Gershenson DM, Wu X, Lu KH. STK15 F31I polymorphism is associated with increased uterine cancer risk: a pilot study. Gynecol Oncol. 2007;107:71–74. doi: 10.1016/j.ygyno.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Yang SB, Zhou XB, Zhu HX, Quan LP, Bai JF, He J, Gao YN, Cheng SJ, Xu NZ. Amplification and overexpression of aurora-A in esophageal squamous cell carcinoma. Oncol Rep. 2007;17:1083–1088. [PubMed] [Google Scholar]
- 18.Kitzen JJ, de Jonge MJ, Verweij J. Aurora kinase inhibitors. Crit Rev Oncol Hematol. 2009;73:99–110. doi: 10.1016/j.critrevonc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Cimica V, Ramachandra N, Zagzag D, Kalpana GV. Aurora A is a repressed effector target of the chromatin remodeling protein INI1/hSNF5 required for rhabdoid tumor cell survival. Cancer Res. 2011;71:3225–3235. doi: 10.1158/0008-5472.CAN-10-2167. [DOI] [PubMed] [Google Scholar]
- 20.Barton VN, Donson AM, Kleinschmidt-DeMasters BK, Birks DK, Handler MH, Foreman NK. Unique molecular characteristics of pediatric myxopapillary ependymoma. Brain Pathol. 2010;20:560–570. doi: 10.1111/j.1750-3639.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birks DK, Kleinschmidt-Demasters BK, Donson AM, Barton VN, McNatt SA, Foreman NK, Handler MH. Claudin 6 is a positive marker for atypical teratoid/rhabdoid tumors. Brain Pathol. 2010;20:140–150. doi: 10.1111/j.1750-3639.2008.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Erikson RL. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc Natl Acad Sci USA. 2002;99:8672–8676. doi: 10.1073/pnas.132269599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Squire SE, Chan MD, Marcus KJ. Atypical teratoid/rhabdoid tumor: the controversy behind radiation therapy. J Neurooncol. 2007;81:97–111. doi: 10.1007/s11060-006-9196-z. [DOI] [PubMed] [Google Scholar]
- 24.Taylor S, Peters JM. Polo and aurora kinases: lessons derived from chemical biology. Curr Opin Cell Biol. 2008;20:77–84. doi: 10.1016/j.ceb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Maris JM, Morton CL, Gorlick R, Kolb EA, Lock R, Carol H, Keir ST, Reynolds CP, Kang MH, Wu J, Smith MA, Houghton PJ. Initial testing of the aurora kinase A inhibitor MLN8237 by the pediatric preclinical testing program (PPTP) Pediatr Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.