Abstract
Background
Amyotrophic lateral sclerosis (ALS), a debilitating neurodegenerative disorder of the motor neurons, leads to the disorganization of the neurofilament (NF) cytoskeleton, and – ultimately - the deterioration of the neuromuscular junction (NMJ). Some familial cases of ALS are caused by mutated FUS, TDP-43, or SOD1; it is thought that the mutated proteins inflict pathology either by gain or loss of function. The proper function of the NMJ requires sAPP, a soluble proteolytic fragment of the amyloid-β precursor protein (APP) - a transmembrane protein implicated in the pathology of Alzheimer’s disease (AD). Whether sAPP, FUS, TDP-43, and SOD1 are mechanistically linked in a common pathway deregulated in both AD and ALS is not known.
Summary
We show that sAPP, TDP-43, FUS, and SOD1 are transported to neurite terminals by a mechanism that involves endoplasmic reticulum (ER)-like tubules, and requires peripherin NFs. The transport of these proteins, and the translocation of the ER protein, Reticulon 4 (Rtn4) into neurites was studied in CAD cells, a brainstem-derived neuronal cell line highly relevant to AD and ALS. We show that a significant fraction of sAPP is generated in the soma, and accumulates in a juxtanuclear ER subdomain. In neurites, sAPP localizes to Rtn4-positive ER-like tubules that extend from the soma into the growth cone, and colocalizes with peripherin NFs. Knocking down peripherin disrupts the NF network, and diminishes the accumulation of sAPP, TDP-43, FUS, SOD1, and Rtn4 at terminals.
Key Messages
We propose that the impediment of a common, ER-mediated mechanism of transport of sAPP, TDP-43, FUS, and SOD1, caused by a disrupted NF network, could be part of the mechanisms leading to AD and ALS.
Keywords: Alzheimer’s disease, Amyotrophic Lateral Sclerosis, Amyloid-β Precursor Protein, FUS, TDP-43, SOD1, Motor neurons, Neurofilaments, Endoplasmic reticulum, Converging mechanisms of neurodegenerative diseases
Background and Objective
An emerging concept in the field of neurodegenerative diseases research is that many of these diseases display various degrees of overlapping pathology. Moreover, proteins linked to pathogenesis in one disease could also be implicated in other, apparently unrelated diseases. This suggests that the molecular mechanisms operating in distinct neurodegenerative diseases could share common components, from single proteins to entire signaling cascades or cellular functions. A case in point is that of AD and ALS, two unrelated neurodegenerative diseases that primarily target either the central nervous system (CNS; in AD) or the peripheral nervous system (PNS; in ALS). Both diseases are largely sporadic, meaning that they have no precisely identified genetic cause, although susceptibility genes exist. However, the familial types – which are inherited - point to genes that, when mutated, lead unavoidably to disease. One such gene codes for APP; certain mutations in APP gene cause early onset AD. The situation is similar in ALS, where a number of genes cause the disease, when mutated; among these are SOD1, FUS, TARDBP (encoding TDP-43), PRPH (encoding peripherin, a NF protein). Interestingly, while only a small fraction of the familial cases show mutations in genes encoding NF proteins, virtually all cases of ALS are afflicted by disorganized NF cytoskeleton [1].
A recent study proposed that APP, an AD-linked gene product, actively contributes to the neuronal pathology of certain forms of ALS [2]. Also, APP was proposed to be essential for the proper function of the NMJ, the primary target of ALS pathology (reviewed in [3]). Conversely, the abnormal expression and aggregation of the ALS-linked gene product, TDP-43, a DNA/RNA binding protein, was reported in some AD cases [4, 5], while SOD1 clearly regulates APP metabolism [6, 7], raising the possibility of converging mechanisms leading to disease in AD and ALS.
Here, we provide evidence that proteins linked to AD and ALS could use a common, yet unusual, form of axonal transport that relies on endoplasmic reticulum (ER)-like tubules as vehicle for transport, and is regulated by NFs. We propose that this transport mechanism, carrying sAPP (or other N-termial fragments - NTFs - of APP), as well as FUS, TDP-43, and SOD1 to the synaptic terminal, could become disrupted, and lead to disease. These results extend our previous studies pointing to overlapping mechanisms of disease in AD and ALS [8].
Methods
The neuronal cell line, CAD [9], extensively employed by us in AD-relevant work [10–12], was used throughout these studies. CAD neuronal cells are derived from the brainstem, a region that is afflicted with neuronal pathology both in AD and ALS. Similar to the motor neurons, they express all major NF proteins, including peripherin, as well as proteins relevant to ALS: FUS, TDP-43, SOD1. CAD neuronal cells also express APP (the 695 amino acids, neuronal isoform), which is cleaved by secretases to generate the characteristic, AD-relevant fragments: sAPPβ, C-terminal fragment-β (CTFβ), amyloid-β peptide (Aβ), and APP intracellular domain (AICD) [13–15]. Immunoblotting, immunocytochemistry, and transfection with dual-tagged APP (FLAG-APP-Myc) [16], or peripherin siRNA (Santa Cruz Biotechnology) plus GFP (Lonza; to visualize transfected cells), were done as described [17, 18]. The following antibodies were used: anti-APP N-terminal region (22C11; Millipore), anti-APP C-terminal region (Y188; Epitomics), anti-SOD1 (Santa Cruz), anti-FUS/TLS, and anti-TDP-43 (Proteintech). As previously shown, in CAD neuronal cells, the antibody 22C11 largely detects sAPP, rather than full-length APP [8, 13, 16]. To retest specificity of labeling, antibody 22C11 was pre-adsorbed on a membrane that contained the APP region of an overloaded transfer of rat brain lysate (similar to the blot in Fig. 2D in [13]) to remove the anti-APP immunoreactive species from the IgG fraction, or pre-adsorbed on a membrane that contained transferred BSA. The antibody pre-adsorbed on APP shows no labeling in immunocytochemistry of CAD cells (see fig. 1d). The anti-peripherin and one of the anti-APP C-terminal region (C9) antibodies were kindly provided by Dr. Robert Goldman (Northwestern University), and Dr. Denis Selkoe (Harvard Medical School), respectively. An additional anti-peripherin antibody (clone 8G2) was from Sigma. Anti-Rtn4 antibodies were either from LSBio, or kindly provided by Dr. Riqiang Yan (Cleveland Clinic Foundation). In some experiments, CAD cells were incubated with 10 μg/ml Brefeldin A (BFA; Sigma) for either 22 h or 1.5 h; in the latter case, a 1.5 h washout period followed, prior to fixing the cells. For immunocytochemistry experiments, digital images were acquired with an Olympus IX81 microscope equipped with Semrock filters, cooled CCD camera (Hamamatsu Photonics), and Image-Pro Plus software (Media Cybernetics). Box plots were generated with BoxPlotR, a web-tool of the Tyers (http://tyers.iric.ca/) and Rappsilber (http://rappsilberlab.org/) labs [19].
Figure 1.
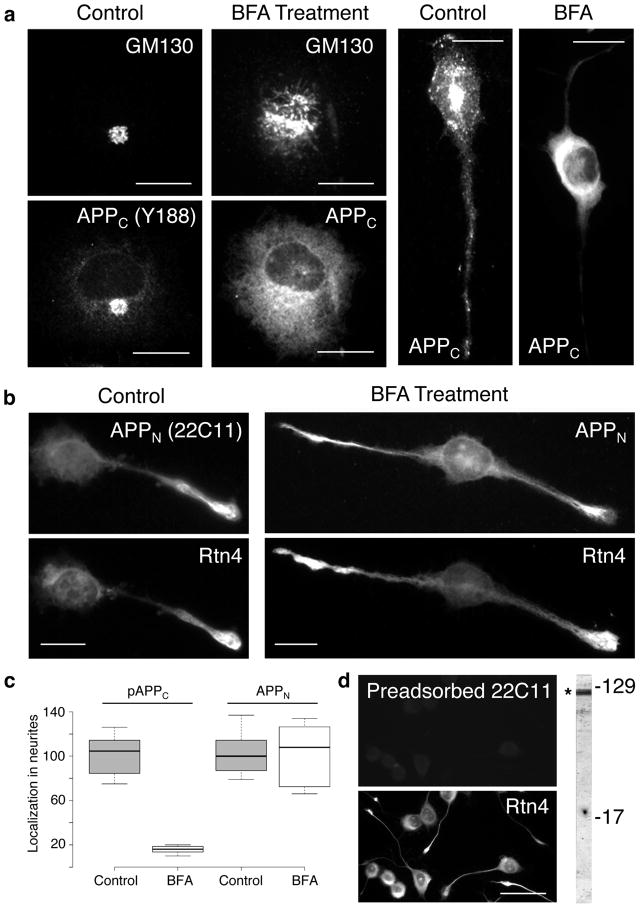
Transport into neurites of sAPP is not affected by Brefeldin A (BFA) treatment. CAD cells were subjected to BFA treatment for 22 hrs, and probed for the distribution of GM130 (a Golgi marker), APP C-terminal (APPC, detected with antibody Y188; a) and N-terminal (APPN, detected with antibody 22C11; b) epitopes, and Rtn4 (b). Controls without BFA treatment are also shown. The box plot in (c) shows that BFA treatment blocks translocation of phosphorylated APPC (pAPPC), but not of APPN into neurites. Translocation of nonphosphorylated APPC is also blocked by BFA treatment. (d) Control, dual labeling experiment, showing specificity of the 22C11 antibody for APP species. Antibody 22C11 was used after preadsorption on APP, a procedure that removed the anti-APP immunoreactive species from the IgG fraction (see Methods). Preadsorption on BSA revealed the typical immunolabeling with antibody 22C11, similar to that seen in (b) (not shown). The blot at right shows that antibody 22C11 detects polypeptides in the region of APP/sAPP (*). In a previous study, we showed that sAPPβ migrates within this region of the blot, and represents a substantial fraction of the immunolabeled band [16]. Molecular weight markers are given in kilodaltons. Bars = 20 μm (a and b); 50 μm (d).
Results
Using brainstem-derived CAD neuronal cells and primary neurons in culture, we have shown that a significant fraction of APP is cleaved in the neuronal soma, and that the generated sAPP segregates from the CTF [13, 16] (as revealed by immunocyto-chemistry with antibodies to epitopes in the N- and C-terminal region of APP, referred to as APPN and APPC, respectively; see supplementary fig. 1a), and accumulates in a cell compartment colocalized with the perinuclear peripherin NFs [8]. The identity of this compartment, and the mechanism of sAPP transport to the neurite terminals, is unknown. We now show that, in the soma, sAPP is enriched in an ER subdomain within the juxtanuclear region, where it largely colocalizes with Rtn4, a transmembrane protein specific for the tubular ER [20] (supplementary fig. 1b). We previously reported that sAPP – endogenous, or derived from exogenously expressed dual-tagged APP - localizes to filamentary structures that extend from the soma through the processes, and into the growth cone [16]. Surprisingly, Rtn4-positive, filament-like structures distribute in a similar pattern along the neurites (supplementary fig. 1c), suggesting that the transported sAPP could actually be localized within protruding ER tubules. If sAPP was transported into neurites within the ER, this form of transport should not be affected by treatment with BFA, a drug that inhibits ER to Golgi transport, and thus blocks the transport along the secretory route of conventional, trans-Golgi network (TGN)-derived vesicles [21], but does not affect the extension of the ER. As shown in fig. 1a and c, BFA induces the typical redistribution to the ER of Golgi markers (e.g., GM130), and of cargo proteins such as the CTFs – phosphorylated or not - that normally proceed along the secretory route, and blocks their transport into neurites. Unlike the CTFs, Rtn4- and sAPP-containing structures are unperturbed by BFA treatment, enter the neurites, and reach the terminals, as they do in control samples in the absence BFA (fig. 1b and c). These results are summarized in table 1, and indicate that sAPP is transported into neurites by a non-conventional mechanism that does not implicate TGN-derived secretory/transport vesicles, but which appears to involve extension of ER-like tubules.
Table 1. Similar effect of experimental manipulations that disrupt transport along the secretory route, or the neurofilament network, on the translocation of sAPP and ALS-relevant proteins, in CAD cell neurites.
Summary of outcome of experiments that test the translocation of AD- and ALS-relevant proteins into neurites, upon treatment of CAD cells with: BFA, BFA followed by a washout period, or peripherin-specific siRNA. Results were evaluated by comparison with non-treated CAD cells (endogenous condition, bottom row), where all tested proteins showed localization at neurite terminals (+). BFA treatment eliminates the localization of C-terminal APP epitopes (indicative of CTF), Thr668-phosphorylated CTF (pCTF), and JIP-1 (a kinesin-1 cargo, transported in association with TGN-derived vesicles). Penetration into neurites, and localization at terminals, of sAPP, FUS, TDP-43, and SOD1 is unaffected in BFA-treated cultures, but significantly diminished (downward-pointing arrows) upon transfection with peripherin-specific siRNA.
| Experimental manipulation | Localization at neurite terminals | ||||||
|---|---|---|---|---|---|---|---|
| sAPP | CTF | pCTF | JIP-1 | FUS | TDP-43 | SOD1 | |
| BFA | + | − | − | − | + | + | + |
| BFA plus washout | + | + | + | + | + | + | + |
| siRNA peripherin |
|
+ | + | + |
|
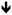
|
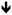
|
| Endogenous condition | + | + | + | + | + | + | + |
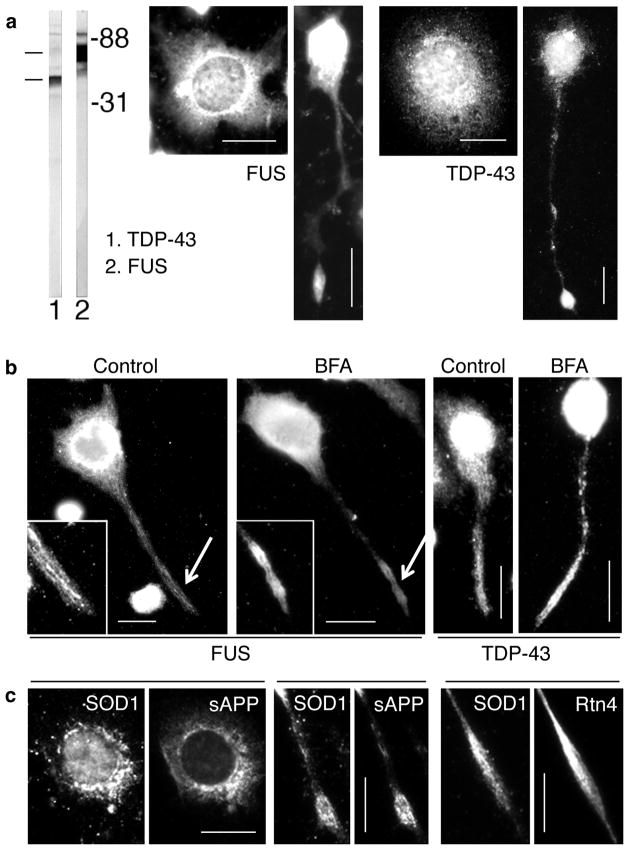
We also show that the ALS-relevant, DNA/RNA binding proteins FUS and TDP-43, while primarily being localized to the nucleus, also display a cytoplasmic distribution pattern reminiscent of that of sAPP and Rtn4 (fig. 2a). This suggests that these proteins could normally be delivered into neurites by associating with the ER. If so, BFA treatment, which blocks only the conventional vesicular transport along the secretory route, should not interfere with the extension of ER tubules in neurites, and with the accumulation of FUS and TDP-43 at terminals. As shown in fig. 2b and table 1, the penetration of FUS and TDP-43 into neurites is not blocked in BFA-treated CAD cells. The clear localization of both proteins to filamentous structures (fig. 2b), rather than the diffuse distribution typically seen for protein complexes free in the cytoplasm, indicates that FUS and TDP-43 are likely carried into the terminals by associating with protruding ER tubules.
Figure 2.
The distribution patterns of FUS, TDP-43, and SOD1 are similar to those of sAPP, and the tubular ER (Rtn4), both in the soma and the neurites, in CAD cells (see also supplementary fig. 1). (a) Localization to a broad region in the soma, and accumulation at neurite terminals, of FUS and TDP-43. In cells with flat appearance, the distribution is typical for the ER. The immunoblot shows FUS and TDP-43 in CAD cell extract. Molecular weight markers are given in kilodaltons. (b) BFA treatment does not affect the localization of FUS and TDP-43 to elongated structures at neurite terminals. The insets show higher magnifications of the terminals (arrows), at increased contrast and brightness. (c) Colocalization, to the same intracellular structure, of SOD1 with sAPP and Rtn4, in the soma and at neurite terminals. We note that conventional immunofluorescence microscopy - as used here - does not resolve the labeling of membrane-associated proteins, such as SOD1, from that of luminal or transmembrane proteins, such as sAPP and Rtn4. Bars = 20 μm.
About 20% of the familial cases of ALS are caused by mutations in SOD1 [22]. A recent study with the SOD1G93A mouse model of ALS indicated that APP contributes to the neuronal pathology [2], but the mechanism remains unresolved. Here, we find that SOD1 largely colocalizes with sAPP in the soma and neurites (fig. 2c); thus, like FUS and TDP-43, SOD1 appears to be transported into neurites attached to Rtn4-positive ER-like extensions. Indeed, SOD1 significantly colocalizes with Rtn4 in the neurites of CAD cells (fig. 2c). As with FUS and TDP-43, the translocation of SOD1 into neurites was not blocked by BFA treatment (table 1). Together, these results are consistent with a transport mechanism where SOD1 associates with the ER protrusions. Thus, within the neurites, SOD1, FUS, and TDP-43 behave like peripheral, ER membrane proteins, and reach the terminals - not by diffusion - but by binding to membrane-bounded carriers.
The formation of the ER networks is a microtubule-dependent process, which relies on microtubule motors [23]. The transport of sAPP also requires the anterograde motor, kinesin-1 [18]. This explains the partial colocalization of sAPP with microtubules – especially the acetylated microtubules - within the neurites, which we previously reported [13, 16]. However, the extensive colocalization of sAPP with NFs along neurites and at terminals, as we found here, is unexpected. This colocalization is particularly high with peripherin (supplementary fig. 2a), a NF subunit [24] present in PNS and brainstem neurons, and in retinal ganglion cells. Peripherin is required for the normal assembly of the NF network in these neurons [25]. We have previously shown that downregulation of peripherin expression eliminates the filamentary distribution of sAPP in the soma [8]. Here, we report that knocking down peripherin with siRNA in CAD cells - a procedure that decreases peripherin expression by ~60%, and disrupts peripherin-containing neurofilaments within neurites (supplementary fig. 2b and c) - also diminishes the translocation of sAPP into neurites, and its accumulation at terminals (supplementary fig. 2b). Likewise, the localization of FUS, TDP-3, and SOD1 at neurite terminals is decreased by ~ 50% in CAD cells transfected with peripherin siRNA (data not shown). This is a significant result, since NF proteins have low turnover, and are difficult to completely eliminate with RNAi. We conclude that NFs are required for the efficient translocation of sAPP, FUS, TDP-43, and SOD1 into the neurites.
Conclusions
This study identifies a common mechanism of transport into neurites of proteins linked to the pathology of AD (i.e., sAPP) and ALS (i.e., FUS, TDP-43, and SOD1). This novel form of transport likely relies on Rtn4-containing, ER-derived tubules penetrating into neurites, at long distance [26]. Interestingly, the Reticulon protein family, to which Rtn4 belongs, has been previously linked to both AD [27] and ALS [28]. The study also identifies the NFs – which are disorganized in all cases of ALS - as potential regulators of this mechanism of transport.
Previous studies have shown that a fraction of sAPP, mostly derived by secretase cleavage from the longer isoforms of APP (also known as protease nexin-II), is rapidly secreted into the extracellular space, via TGN-derived vesicles [29]. The reported, extremely fast, time course of secretion of protease nexin-II [29] suggests that this event occurs in the cell body. The TGN-independent transport pathway described by us carries sAPP – likely sAPPβ [16] - at long distance, to neurite terminals, and certainly occurs in addition to the traditional form of transport. Future studies are required to determine the extent of this novel mechanism of transport, and to precisely identify the sAPP species carried into neurites within ER tubules.
A deficient axonal transport is suspected to be part of the pathogenesis of several neurodegenerative diseases, although this remains to be unequivocally demonstrated [30]. Yet, it is unlikely that gross abnormalities of transport occurring throughout life underlie the pathology of old age diseases, since no signs of disease are seen until later in life. Rather, more subtle deficiencies that only become manifest over time, or during persistent stress (as occurs at old age), could ultimately lead to disease [31]. More specifically, the disease process likely affects transport of only some specific cargo. Here we tentatively identify a novel form of transport, carrying a defined - yet limited - set of cargo proteins: those that segregate into – or associate with – ER-like tubules extending into neurites, down to the terminal. How the intraluminal sAPP becomes concentrated in the tubular ER-like extensions that protrude into the neurites remains to be determined. Also, how soluble proteins present in the cytoplasm, such as FUS, TDP-43, and SOD1 are tethered to the translocating ER-derived tubules remains to be elucidated. An obvious possibility is that they directly bind to integral, ER membrane proteins present in the protruding tubules. RNA binding proteins have been previously shown to “hook” to the ER for transport of vegetal pole-localized RNA in Xenopus oocytes [32, 33]. We note that mitochondria could in principle also carry cytoplasmic proteins into neurites. Our findings do not exclude the possibility that the disease-related proteins investigated here do not also associate to some extent with mitochondria, in addition to the ER, as an alternative mechanism of transport into the neurites.
An interesting finding, to be expanded in future studies, is that NFs likely regulate the type of microtubule-based transport described here. This is a new role of the NFs, which have been consistently viewed as a cytoskeletal network with passive function. Importantly, the NFs appear to regulate the transocation into neurites not only of proteins linked to ALS (a disease where NFs are disorganized), but also of proteins relevant to AD (a disease with no obvious disruption of the NF cytoskeleton). This points to the necessity to investigate a possible “malfunction” of the NF network in AD, maybe in a more subtle way than in ALS, yet still causing disease. Indeed, the NFs appear to regulate both the proteolytic cleavage of APP [8], and the intracellular transport of sAPP (this report). We hope that this article will open new and important directions of study in AD and ALS.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health award AG039668 (Z.L.M.), National Science Foundation EAGER award IOS-1347090 (V.M., Z.L.M.), and New Jersey Health Foundation awards (Z.L.M., V.M.).
References
- 1.Xiao S, McLean J, Robertson J. Neuronal intermediate filaments and als: A new look at an old question. Biochim Biophys Acta. 2006;1762:1001–1012. doi: 10.1016/j.bbadis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Bryson JB, Hobbs C, Parsons MJ, Bosch KD, Pandraud A, Walsh FS, Doherty P, Greensmith L. Amyloid precursor protein (app) contributes to pathology in the sod1(g93a) mouse model of amyotrophic lateral sclerosis. Hum Mol Genet. 2012;21:3871–3882. doi: 10.1093/hmg/dds215. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell JH, Klevanski M, Saar M, Muller UC. Roles of the amyloid precursor protein family in the peripheral nervous system. Mech Dev. 2013;130:433–446. doi: 10.1016/j.mod.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. Tdp-43 immunoreactivity in hippocampal sclerosis and alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE. Temporal lobar predominance of tdp-43 neuronal cytoplasmic inclusions in alzheimer disease. Acta Neuropathol (Berl) 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami K, Murata N, Noda Y, Tahara S, Kaneko T, Kinoshita N, Hatsuta H, Murayama S, Barnham KJ, Irie K, Shirasawa T, Shimizu T. Sod1 (copper/zinc superoxide dismutase) deficiency drives amyloid beta protein oligomerization and memory loss in mouse model of alzheimer disease. The Journal of biological chemistry. 2011;286:44557–44568. doi: 10.1074/jbc.M111.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spisak K, Klimkowicz-Mrowiec A, Pera J, Dziedzic T, Aleksandra G, Slowik A. Rs2070424 of the sod1 gene is associated with risk of alzheimer’s disease. Neurol Neurochir Pol. 2014;48:342–345. doi: 10.1016/j.pjnns.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Muresan V, Villegas C, Ladescu Muresan Z. Functional interaction between amyloid-beta precursor protein and peripherin neurofilaments: A shared pathway leading to alzheimer’s disease and amyotrophic lateral sclerosis? Neurodegener Dis. 2014;13:122–125. doi: 10.1159/000354238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi Y, Wang JK, McMillian M, Chikaraishi DM. Characterization of a cns cell line, cad, in which morphological differentiation is initiated by serum deprivation. J Neurosci. 1997;17:1217–1225. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muresan Z, Muresan V. The amyloid-beta precursor protein is phosphorylated via distinct pathways during differentiation, mitosis, stress, and degeneration. Mol Biol Cell. 2007;18:3835–3844. doi: 10.1091/mbc.E06-07-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muresan Z, Muresan V. Seeding neuritic plaques from the distance: A possible role for brainstem neurons in the development of alzheimer’s disease pathology. Neurodegenerative Dis. 2008;5:250–253. doi: 10.1159/000113716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muresan Z, Muresan V. Cad cells are a useful model for studies of app cell biology and alzheimer’s disease pathology, including accumulation of aβ within neurites. Swan alzheimer knowledge base. Alzheimer research forum. 2009 Available at: Http://mind-swanweb1.Mgh.Harvard.Edu/swan/browser/showentity.Action?Objectid=urn%3alsid%3aswan.Org%3aresearchstatement%3aca7169f1-ff7d-456c-b3f3-c52bec1e8074.
- 13.Muresan V, Varvel NH, Lamb BT, Muresan Z. The cleavage products of amyloid-beta precursor protein are sorted to distinct carrier vesicles that are independently transported within neurites. J Neurosci. 2009;29:3565–3578. doi: 10.1523/JNEUROSCI.2558-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muresan Z, Muresan V. A phosphorylated, carboxy-terminal fragment of {beta}-amyloid precursor protein localizes to the splicing factor compartment. Hum Mol Genet. 2004;13:475–488. doi: 10.1093/hmg/ddh054. [DOI] [PubMed] [Google Scholar]
- 15.Muresan Z, Muresan V. Neuritic deposits of amyloid-β peptide in a subpopulation of central nervous system-derived neuronal cells. Mol Cell Biol. 2006;26:4982–4997. doi: 10.1128/MCB.00371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villegas C, Muresan V, Ladescu Muresan Z. Dual-tagged amyloid-beta precursor protein reveals distinct transport pathways of its n- and c-terminal fragments. Hum Mol Genet. 2014;23:1631–1643. doi: 10.1093/hmg/ddt555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muresan Z, Muresan V. C-jun nh2-terminal kinase-interacting protein-3 facilitates phosphorylation and controls localization of amyloid-beta precursor protein. J Neurosci. 2005;25:3741–3751. doi: 10.1523/JNEUROSCI.0152-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muresan Z, Muresan V. Coordinated transport of phosphorylated amyloid-beta precursor protein and c-jun nh2-terminal kinase-interacting protein-1. J Cell Biol. 2005;171:615–625. doi: 10.1083/jcb.200502043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer M, Wildenhain J, Rappsilber J, Tyers M. Boxplotr: A web tool for generation of box plots. Nat Methods. 2014;11:121–122. doi: 10.1038/nmeth.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri HP, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the er in the presence of brefeldin a suggests an er recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 22.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in cu/zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 23.Wozniak MJ, Bola B, Brownhill K, Yang YC, Levakova V, Allan VJ. Role of kinesin-1 and cytoplasmic dynein in endoplasmic reticulum movement in vero cells. J Cell Sci. 2009;122:1979–1989. doi: 10.1242/jcs.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escurat M, Djabali K, Gumpel M, Gros F, Portier MM. Differential expression of two neuronal intermediate-filament proteins, peripherin and the low-molecular-mass neurofilament protein (nf-l), during the development of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:764–784. doi: 10.1523/JNEUROSCI.10-03-00764.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan A, Sasaki T, Kumar A, Peterhoff CM, Rao MV, Liem RK, Julien JP, Nixon RA. Peripherin is a subunit of peripheral nerve neurofilaments: Implications for differential vulnerability of cns and peripheral nervous system axons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8501–8508. doi: 10.1523/JNEUROSCI.1081-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muresan V, Ladescu Muresan Z. Amyloid-beta precursor protein: Multiple fragments, numerous transport routes and mechanisms. Exp Cell Res. 2015;334:45–53. doi: 10.1016/j.yexcr.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He W, Lu Y, Qahwash I, Hu XY, Chang A, Yan R. Reticulon family members modulate bace1 activity and amyloid-beta peptide generation. Nat Med. 2004;10:959–965. doi: 10.1038/nm1088. [DOI] [PubMed] [Google Scholar]
- 28.Yang YS, Harel NY, Strittmatter SM. Reticulon-4a (nogo-a) redistributes protein disulfide isomerase to protect mice from sod1-dependent amyotrophic lateral sclerosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13850–13859. doi: 10.1523/JNEUROSCI.2312-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambamurti K, Shioi J, Anderson JP, Pappolla MA, Robakis NK. Evidence for intracellular cleavage of the alzheimer’s amyloid precursor in pc12 cells. J Neurosci Res. 1992;33:319–329. doi: 10.1002/jnr.490330216. [DOI] [PubMed] [Google Scholar]
- 30.Muresan V, Muresan Z. Is abnormal axonal transport a cause, a contributing factor or a consequence of the neuronal pathology in alzheimer’s disease? Future Neurology. 2009;4:761–773. doi: 10.2217/fnl.09.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muresan V, Muresan Z. A persistent stress response to impeded axonal transport leads to accumulation of amyloid-beta in the endoplasmic reticulum, and is a probable cause of sporadic alzheimer’s disease. Neurodegenerative Dis. 2012;10:60–63. doi: 10.1159/000332815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deshler JO, Highett MI, Abramson T, Schnapp BJ. A highly conserved rna-binding protein for cytoplasmic mrna localization in vertebrates. Curr Biol. 1998;8:489–496. doi: 10.1016/s0960-9822(98)70200-3. [DOI] [PubMed] [Google Scholar]
- 33.Deshler JO, Highett MI, Schnapp BJ. Localization of xenopus vg1 mrna by vera protein and the endoplasmic reticulum [see comments] Science. 1997;276:1128–1131. doi: 10.1126/science.276.5315.1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.