Abstract
Previous studies have indicated systemic deregulation of the proinflammatory or anti-inflammatory balance in individuals with first-episode psychosis (FEP) that persists 12 months later. To identify potential risk/protective factors and associations with symptom severity, we assessed possible changes in plasma levels of neurotrophins (brain-derived neurotrophic factor [BDNF] and nerve growth factor [NGF]) and their receptors in peripheral blood mononuclear cells (PBMCs). Expression of the 2 forms of BDNF receptors (active TrkB-FL and inactiveTrkB-T1) in PBMCs of FEP patients changed over time, TrkB-FL expression increasing by 1 year after diagnosis, while TrkB-T1 expression decreased. The TrkB-FL/TrkB-T1 ratio (hereafter FL/T1 ratio) increased during follow-up in the nonaffective psychosis group only, suggesting different underlying pathophysiological mechanisms in subgroups of FEP patients. Further, the expression of the main NGF receptor, TrkA, generally increased in patients at follow-up. After adjusting for potential confounders, baseline levels of inducible isoforms of nitric oxide synthase, cyclooxygenase, and nuclear transcription factor were significantly associated with the FL/T1 ratio, suggesting that more inflammation is associated with higher values of this ratio. Interestingly, the FL/T1 ratio might have a role as a predictor of functioning, a regression model of functioning at 1 year suggesting that the effect of the FL/T1 ratio at baseline on functioning at 1 year depended on whether patients were treated with antipsychotics. These findings may have translational relevance; specifically, it might be useful to assess the expression of TrkB receptor isoforms before initiating antipsychotic treatment in FEPs.
Key words: inflammation, BDNF, NGF, first-episode psychosis, antipsychotic, schizophrenia
Introduction
In recent years, there have been changes in the approach to schizophrenia, with a focus on the search for biological markers. Specifically, the National Institute of Mental Health has emphasized the need to explore the neurobiological basis of psychiatric disorders, calling for an effort to understand the underlying pathophysiological mechanisms.1 Although many biomarkers have been proposed, in the last 3 years particular attention has been paid to inflammatory processes.2,3
Research on the early stages of psychosis tries to capture the pathophysiological changes when signs and symptoms first appear, allowing us to study this complex illness before progression, avoiding potential confounding factors, such as prolonged treatments with antipsychotics and concomitant pathologies.
Regarding inflammation, several studies have found evidence of active inflammatory processes in schizophrenia, mainly at the cytokine level, but also changes in other inflammatory mediators and subsequent oxido-nitrosative stress-related damage,4,5 even at illness onset.6 Excessively long or intensive inflammation damages tissues triggering protective or repair systems.6–9 In this regard, several experimental,10 and clinical studies11,12 have indicated that neurotrophins may be involved in repair mechanisms.
Brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are neurotrophins that play critical roles in neurodevelopment and adult brain plasticity, survival and differentiation of neurons, neuronal function, and repair mechanisms.13 Both of these proteins act after the activation of their tyrosine kinase-type receptors, TrkB and TrkA respectively.13,14 In the case of TrkB, in addition to the active full-length form of the receptor (TrkB-FL), there is a truncated form (TrkB-T1); this lacks kinase activity and inhibits the function of TrkB-FL by competing in binding to BDNF.15 In the developing primate brain, TrkB-T is a negative effector of TrkB-FL16. An excess of the TrkB-T1 isoform can lead to neuronal death17 and elevated levels of truncated isoforms have been found in the prefrontal cortex of individuals with schizophrenia.18 On the other hand, TrkA mediates the multiple effects of NGF, including neuronal differentiation and the inhibition of programmed cell death.19
Preclinical and clinical studies have found changes in plasma levels of neurotrophins, as well as decreases in protein expression in certain brain areas of patients with schizophrenia.18,20–22 Moreover, there may be a relationship between inflammation and changes in the expression of neurotrophins in schizophrenia.18 Indeed, alterations in neurotrophin levels seem to increase with age in schizophrenia, independent of medication dosage.23 In addition, an imbalance between BDNF receptor isoforms has been related to neuronal death17 and schizophrenia.18 Considering the possible counterbalancing role of neurotrophins, it is plausible that early inflammation triggers an activation of neurotrophic pathways, which can also be affected by antipsychotic treatment.24 The aim of this study was to evaluate the expression of BDNF and NGF receptors TrkA and TrkB (FL and T1), and the relationship between proinflammatory and anti-inflammatory dysregulation in first-episode psychosis (FEP) patients during the first episode and 1 year later. Further, we explored potential correlations with clinical symptoms and their response to treatment.
Subjects and Methods
Subjects
A complete description of the protocol for our multicentre longitudinal study of FEP has been published previously.6 This Flamm-PEPs study included 117 patients during the first year after their FEP, and 106 healthy controls matched for gender, race, and age. The inclusion criteria were: (1) positive psychotic symptoms for less than 12 months, (2) 9–35 years at illness onset, (3) speaking Spanish fluently, and (4) giving written informed consent. Exclusion criteria were: (1) mental retardation (DSM-IV criteria), (2) history of head trauma with loss of consciousness, and (3) systemic disease with mental repercussions. Psychotic symptoms were assessed with the Positive and Negative Symptoms Scale (PANSS).25
Inclusion criteria for healthy controls were: (1) no past or current psychiatric disorder (DSM-IV criteria), (2) speaking Spanish fluently, and (3) giving written informed consent. Exclusion criteria for healthy controls were the same as for patients, and (4) history of psychotic episodes among first-degree relatives.
No participants had fever or any allergies, ongoing infections, or other serious physical conditions, and they had not received immunosuppressive drugs or vaccines for at least 6 months or anti-inflammatory drugs for at least 2 days before blood sampling. The study was approved by the Ethics Committees of the participating hospitals. In the case of minors, parents, or legal guardians gave written informed consent and participants gave their assent.
This follow-up substudy included the patients of the Flamm-PEPS study who provided blood samples and completed clinical assessments at baseline and 12 months later. Ninety-four patients and 80 healthy controls satisfied these conditions.
Sample Collection
Venous blood samples (10mL) were collected between 8 Am and 10 Am, after overnight fasting. Sample collection and preparation procedures have been described previously.6 See supplementary information for more details.
Biochemical Measurements in Plasma
Plasma levels of BDNF and NGF were determined using enzymatic assays (RayBiotech), according to the manufacturer’s instructions, and measured using a Synergy 2 system (BioTek).
Measurement of TrkA, TrkB-FL, and TrkB-T1 Receptor Expression in PBMCs
Protein levels of receptors were quantified by Western blot analysis. In brief, 12.5 µg of cytosolic extracts were loaded onto electrophoresis gels. Protein samples were separated and transferred onto nitrocellulose membrane (Amersham-Ibérica). After blocking, membranes were incubated with specific antibodies. Blots were imaged using an Odyssey® Fc System (Li-COR Biosciences) and quantified by densitometry (ImageJ®, NIH). We used β-actin as a loading control. All densitometry results are expressed as a percentage of the control.
Given the counterbalancing effect of TrkB-T1 and TrkB-FL, we chose the ratio of TrkB-FL to TrkB-T1 expression (hereafter FL/T1 ratio) as our index variable for describing BDNF receptor expression.
Measurement of Inflammatory and Anti-inflammatory Parameters
As we have described previously,6 inflammatory and anti-inflammatory parameters were assessed in plasma and peripheral blood mononuclear cells (PBMCs) of all patients and controls of the Flamm-PEPs cohort. In brief, we measured the activity of transcription factor NFκB and the protein expression of inflammatory and oxidative/nitrosative inducible isoforms of cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) enzymes. Furthermore, we measured the plasma level of an anti-inflammatory endogenous ligand for peroxisome proliferator-activated receptor gamma (PPARγ), namely, COX-derived 15-deoxy-delta 12, 14 prostaglandin-12 J2 (15d-PGJ2). See supplementary information for more details.
Assessment of General Functioning
To assess patient functioning, we used the Global Assessment of Functioning (GAF) scale, which gives a score between 0 and 100 taking into account different aspects of daily life including social relations and employment status. Based on a revision of Endicott’s Global Assessment Scale,26 the GAF scale became the basis of Axis-V diagnoses in DSM-III-R,27 DSM-IV,28 and DSM-IV-TR.29 A higher score indicates better functioning.
Statistical Analyses
Demographic variables are described with means (SD) and percentages. Differences between controls and patients were assessed with t tests for independent samples, and differences in patients between baseline and at 1 year of follow-up with t tests for paired samples.
To explore possible relationships between the expression of neurotrophin receptors and proinflammatory or anti-inflammatory parameters, we calculated the arithmetic sum of the levels of only 3 proinflammatory variables identified as elevated in the FEP patients at baseline (iNOS, COX2 and NFκB, expressed in arbitrary units).6 Moreover, due to the small sample size for which we had IκBα and PPARγ data, we analysed only the main variable 15d-PGJ2 as an indicator of anti-inflammatory activity. All the biochemical values of iNOS, COX2, NFκB and 15d-PGJ2 appear in supplementary table 1.
Multiple linear regression models were built to assess the association between inflammation status at baseline and the FL/T1 ratio both at baseline and 1 year, and the influence of the FL/T1 ratio on global functioning (GAF) and clinical status (PANSS) at 1 year. To obtain the adjusted model, a stepwise procedure was used including all the potential confounding variables considered. Only variables that were significant or produced significant changes in the coefficient of the independent variable were included in the regression model. Finally, the interaction terms were evaluated. For the final model, the fit of the residuals and their normality were analysed. The β coefficient, 95% CI and P value were calculated from t tests.
When the interaction term was significant, the effect size and 95% CI were calculated for each group. In addition, the effect size of the main group on the dependent variable was estimated for different values of the independent variable (3rd, 25th, 50th, 75th, and 97th percentiles). Data analyses were conducted with Stata 12.1, setting the level of significance at P < .05.
Results
Sample Characteristics
The 94 patients included in the analysis did not differ in sociodemographic characteristics from the 23 who dropped out. Participating patients had a mean body mass index (BMI) of 25.02 (3.97), their mean age was 23.78 (5.81) years and 65 (69.1%) of them were men. The mean baseline GAF score was 68.90 (13.14), and the mean baseline PANSS total score was 50.44 (16.89). At 1 year, the mean GAF score had increased to 72.03 (16.29), but this increase was not significant. However, the PANSS total score had decreased significantly to 38.53 (23.27) (t = 4.21, df = 67, P < .001). Regarding diagnosis, 76 patients (80.9%) had nonaffective psychosis (table 1). Seventy-five patients were taking atypical antipsychotics (risperidone, paliperidone, clozapine, aripiprazole, ziprasidone, and/or quetiapine) at a median chlorpromazine equivalent dose of 349.20 (277.64) mg/day.
Table 1.
Baseline Characteristics of the Sample
| Patients (n = 94) | Controls (n = 80) | Total Sample (n = 174) | Statistics | ||
|---|---|---|---|---|---|
| Gender, N (%) | Female | 29 (30.9%) | 27 (33.8%) | 56 (32.2%) | χ2 = 0.17,df = 1, P = .683 |
| Male | 65 (69.1%) | 53 (66.3%) | 118 (67.8%) | ||
| Age (y), mean (±SD) | 23.78±5.81 | 25.69±7.07 | 24.66±6.47 | t = −1.96, df = 172 P = 0.052 | |
| Socioeconomic level, N (%) | Low | 28 (29.8%) | 19 (23.8%) | 47 (27.0%) | χ2 = 1.66, df = 2, P = 0.436 |
| Medium | 38 (40.4%) | 40 (50%) | 78 (44.8%) | ||
| High | 28 (29.8%) | 21 (26.3%) | 49 (28.2%) | ||
| Body mass index, mean (±SD) | 25.02±3.97 | 23.08±3.07 | 24.12±3.70 | t = 3.38, df = 155, P = 0.001 | |
| Cannabis use, N (%) | Yes | 23 (24.5%) | 13 (17.3%) | 36 (20.6%) | χ2 = 1.27, df = 1, P = 0.260 |
| Age at onset (y), mean (±SD) | 24.45±5.80 | ||||
| Duration of untreated psychosis (d), mean (±SD) | 91.26±97.05 | ||||
| Diagnosis, N (%) | Affective psychosisa | 18 (19.1%) | |||
| Nonaffective psychosisb | 76 (80.9%) | ||||
| PANSS positive score, mean (±SD) | 10.21±4.99 | ||||
| PANSS negative score, mean (±SD) | 14.11±5.73 | ||||
| PANSS total score, mean (±SD) | 50.44±16.89 | ||||
| Montgomery-Asberg depression rating scale score, mean (±SD) | 6.31±6.37 | ||||
| Global Assessment of Functioning, mean (±SD) | 68.90±13.14 | ||||
Note: PANSS, Positive and Negative Symptoms Scale. The bold values in the table represent the values reaching statistical significance (P value < .05).
aAffective psychosis: bipolar disorder, psychotic depression, or schizoaffective disorder.
bNonaffective psychosis: schizophrenia, schizophreniform disorder, and psychotic disorder not otherwise specified.
Plasma Levels of Neurotrophic Factors and Receptor Expression in PBMCs
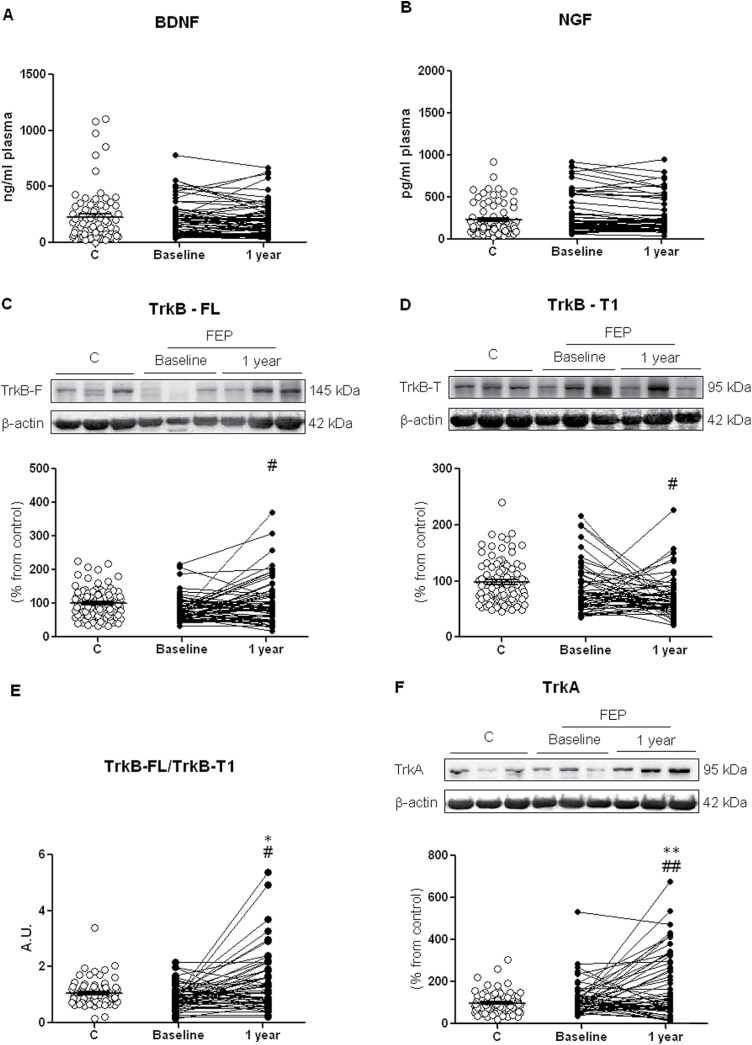
Although no significant differences were seen between patients and controls in plasma levels of either neurotrophin at baseline or follow-up (figure 1A and 1B), the expression of receptors in PBMCs of FEP patients changed with time, the expression of TrkB-FL increasing (t = −2.53, df = 59, P = .014; figure 1C), while at this stage of the illness, TrkB-T1 expression decreased over time (t = 1.22, df = 59, P =.028; figure 1D). Accordingly, the mean FL/T1 ratio in all FEP patients increased during follow-up (t = −3.14, df = 59, P = .030), the difference compared to the control group reaching significance (t = 2.49, df = 100.21, P = .014; figure 1E).
Fig. 1.
Plasma levels of BDNF (A) and NGF (B); neurotrophin receptor expression of full-length (FL) (C) and truncated (T1) (D) TrkB receptor; FL/T1 ratio (E); protein levels of receptor TrkA (F) in PBMCs from controls (C group), and first-episode psychosis (FEP) patients at baseline and the 1-year follow-up. The densitometric data of the respective band of interest are normalized by β-actin (lower band). *P < .05 vs Control; #P < .05, ##P < .01 vs Baseline. Paired sample t tests. For the control group, the mean ± SD are plotted.
To explore possible differences between diagnoses, we stratified the analysis and found that the FL/T1 ratio in PBMCs had only significantly increased in the nonaffective subgroup of FEP patients, from 0.92 (0.41) at baseline to 1.53 (1.33) 1 year later (t = −3.17, df = 49, P = .003)
Finally, there were no significant differences between patients and controls in PBMC expression of the TRkA receptor at baseline, but an increase was observed in patients at 1 year (t = −2.95, df = 53, P = .005), the difference with controls also reaching significance (t = 2.82, df = 98.84, P = .006; figure 1F).
Relation Between Inflammation and the FL/T1 Ratio
After adjusting for potential confounders (smoking, use of other substances and BMI), a significant positive association was found between the anti-inflammatory marker, 15d-PGJ2 (plasma levels) and the FL/T1 ratio at baseline (β = 0.0008; P = .015; 95% CI: 0.0002, 0.0014; supplementary table 2). However, the association with the ratio became nonsignificant by 1 year. Despite BMI not being significant, we decided to include it in the model of the association between this ratio and the inflammatory markers at baseline (iNOS, COX2, NFκB) as inflammation lost significance when BMI was not entered. After controlling for potential confounders, baseline iNOS + COX2 + NFκB was significantly associated with the FL/T1 ratio, suggesting that more inflammation is associated with a higher FL/T1 ratio (β = 0.0168; P = .037; 95% CI: 0.0001, 0.0033). Similarly, as occurred with anti-inflammatory parameters, there was no significant association between baseline inflammation and the FL/T1 ratio at 1 year (β = 0.0009; P = .591; 95% CI: −0.0024, 0.0041; supplementary table 2). Finally, we observed no significant interactions of baseline anti- and proinflammatory variables with smoking or BMI.
FL/T1 Ratio as a Predictor of Functioning
The final regression model of functioning (GAF) at 1 year included baseline FL/T1 ratio, BMI, smoking status, and antipsychotic use. This model indicated a significant positive interaction between the FL/T1 ratio and antipsychotic treatment (β = 41.46; P = .004; 95% CI: 14.327, 68.590), which suggests that the effect of the FL/T1 ratio at baseline on functioning at 1 year depended on whether patients were treated with antipsychotics. Specifically, in patients who did not take antipsychotics (N = 19), a 1-unit increase in the ratio was associated with a significant decrease in GAF score (by 30.59 points, 95% CI: −55.390, −5.787). In contrast, among patients on antipsychotics, for each 1-unit increase in the ratio GAF increased by 3.74 points, although this increase was not significant.
For patients with low values of the ratio at baseline (0.18), those who were taking antipsychotics had poorer functioning than those who were not (effect size: −30.26; 95% CI: −52.903, −30.259). However, taking antipsychotics seemed to have the opposite effect among individuals with high baseline ratios (1.96), patients who were on antipsychotics having better functioning at 1 year than those who were not (effect size: 43.54; 95% CI: 12.189, 74.887) (table 2). Thus, functioning at 1 year is related to baseline values of this ratio in a different way depending on whether or not the individual is taking antipsychotics. Additionally, neither the value of the ratio nor antipsychotic use independently have any effect on functioning, neither of them being significant when running the regression equation without the interaction term. Regarding the PANSS total score at the end of the follow-up, no association was found with the FL/T1 ratio at baseline.
Table 2.
Effect Sizes of Taking Antipsychotics on Global Assessment of Functioning (GAF) Scores at 1 Year (and 95% CI) for Selected Values of the Ratio of TrkB-FL to TrkB-T1 Expression (FL/T1 ratio)a
| FL/T1 Ratio | Taking Antipsychoticsb |
|---|---|
| 3rd Percentile (=0.18) | −30.26 (−52.903, −30.259) |
| 25th Percentile (=0.67) | −9.94 (−22.573, 2.685) |
| Median (=0.90) | −0.41 (−11.369, 10.552) |
| 75th Percentile (=1.11) | 8.30 (−4.142, 20.737) |
| 97th Percentile (=1.96) | 43.54 (12.189, 74.887) |
Note: aAdjusted for potential confounders
bEffect size is interpreted as the difference in average GAF scores between first-episode psychosis patients treated with antipsychotics and those who did not take antipsychotics.
Discussion
In this study, we found evidence of systemic changes in the expression of the receptors of neurotrophins BDNF and NGF in PBMCs in a cohort of FEP patients during the first year after diagnosis. Specifically, we observed an increase in the functional form of the BDNF receptor along with a decrease in the truncated isoform. Further, levels of the main NGF receptor appeared to be elevated by the end of the follow-up period.
Some aspects of our results may have translational importance because the multiple linear regression models indicated an association of systemic inflammatory status6 with the changes observed in neurotrophins and patients’ global functioning at follow-up. Firstly, a significant positive correlation was found between the anti-inflammatory marker 15d-PGJ2 and the FL/T1 ratio at baseline after adjusting for confounders. Secondly, the effect of the functioning of BDNF receptors (FL/T1 ratio) was studied in relation to antipsychotics and the clinical status of patients, indicating that the presence of a higher FL/T1 ratio in patients was associated with a better clinical response to antipsychotics. However, the study design precludes drawing conclusions about causality and there may be other neurobiological mechanisms involved. Moreover, there is evidence of a relationship between antipsychotic treatment and signalling pathways of neurotrophic factors.36,43,45 Although longer longitudinal studies should be conducted, this is, to our knowledge, the first time that the functioning of BDNF receptors in PBMCs of FEP patients is described as a possible biomarker of clinical response to antipsychotics.
Plasma levels of neurotrophins have been extensively used in the search for biomarkers of neuropsychiatric disorders.30,31 To simplify the analysis of BDNF levels, it is assumed that peripheral levels of neurotrophins and their receptors mirror their brain concentrations32 and there is evidence suggesting that brain and plasma contain comparable levels of such biomarkers,33 evidence supported by genetic findings.34
In this study, no differences were found in plasma levels of BDNF or NGF between patients and controls. In line with this, previous research found mixed results, depending on the type of biological samples used, the stage of the illness, gender, and whether pharmacological treatment was given.35 A recent meta-analysis36 concluded that peripheral serum and plasma BDNF levels were moderately lower than in controls. The few data available on NGF levels in schizophrenia also indicate that they are lower than normal.12,37 However, studies on neurotrophin levels in early onset psychosis patients are scarce, have mainly focused on BDNF-gene polymorphisms, and have produced inconsistent and even contradictory results.38,39 In the case of NGF, the data available indicate decreases in serum NGF levels.40–42
Interestingly, alterations in the expression of functional and nonfunctional neurotrophin receptors in PBMCs appeared in our FEP patients at 1 year. In particular, we observed increases in the levels of functional receptors (TrkB-FL and TrkA) for BDNF and NGF and a decrease in the level of a nonfunctional BDNF receptor (TrkB-T1). The FL/T1 ratio of the TrkB receptors is a useful indicator of the real functioning of BDNF.
Although no previous studies have assessed levels in PBMCs in schizophrenia, a few studies have shown increases in truncated TrkB isoforms,18 along with decreases in TrkB-FL levels in the prefrontal cortex22 in individuals with schizophrenia. Moreover, a nonsignificant decrease in TrkB immunoreactivity was found in the cerebellum in this population.21 Taken together with these previous findings, our results suggest that blocking of excessive TrkB-T1 or enhancement of TrkB-FL function might be a future therapeutic target to restore deficits in BDNF signalling in schizophrenia.
At the clinical level, scales assessing patient functioning provide a measure of how an illness affects patients’ daily life. Among individuals with high FL/T1 ratios, patients who took antipsychotics had significantly higher GAF scores after 1 year than those who did not (table 2). In contrast, taking antipsychotics was not associated with better GAF scores and may even have been detrimental in patients with low FL/T1 ratios. Thus, regarding patient functioning, a desirable response to antipsychotics was only observed in patients with high FL/T1 ratios. These are, in our opinion, very interesting translational findings that should be explored in future research. The implications are especially important in patients with low FL/T1 ratios, because the findings indicate that for adequate neurotrophin signalling, it is not only necessary to increase BDNF levels but also adjust the proportion of the functional and truncated forms of the receptor. In line with previous reports,43 our results suggest that increasing the functional form and decreasing the truncated form could be a future therapeutic goal to improve BDNF signalling in psychotic patients.
Another interesting finding is that the FL/T1 ratio in the nonaffective group increased during follow-up, to values even exceeding that of the control group, due to expression of the FL form increasing and that of the T1 isoform decreasing. Similarly, Mackin et al44 found related changes in BDNF expression in schizophrenia but not in bipolar patients, likely due to different underlying pathophysiological mechanisms. Previous research in this cohort6 identified robust phenotypic differences in the inflammatory pathways at the cellular machinery level in PMBCs. In particular, we found significantly higher levels of intracellular components of proinflammatory pathways, along with significantly lower levels of components of anti-inflammatory pathways. In this new study, levels of the anti-inflammatory prostaglandin, 15d-PGJ2, were positively correlated with the FL/T1 ratio; given its soluble nature, 15d-PGJ2 should be explored as a plasma biomarker in FEP.
Taken as a whole, the results of this study suggest that compensatory mechanisms are activated in psychosis at very early stages of the illness, possibly to repair neuronal damage, as has been described in other experimental settings.10 Indeed, such compensatory processes have been identified in other neurological and neuropsychiatric conditions.45–47 The compensatory mechanisms involved include increases not only in anti-inflammatory prostaglandins, but also in neurotrophin receptors. It is possible that in the initial stages of the illness the body tries to counteract the deleterious effects of inflammatory processes by increasing the ratio of functional to nonfunctional forms of the receptor, preserving BDNF signalling in cells. Previous studies in patients with schizophrenia have observed both high brain levels of the truncated isoforms of the TrkB receptor18 and low levels of the FL form.22 Reduced TrkB expression has also been found in the brain of patients with bipolar disorder.21 These findings suggest that dysregulation of TrkB-mediated neurotrophic signalling could play a role in the pathophysiology of both psychiatric illnesses. Although levels of the truncated form were not significantly higher at baseline, the FL/T1 ratio was lower and might have prognostic value. In our sample, having higher levels of the T1 isoform (lower ratio) is related to a poorer functional response in patients taking antipsychotics. It seems that patients with high FL/T1 ratios respond better to antipsychotic treatment. Therefore, if patients have a high FL/T1 ratio, it may be particularly important to prescribe antipsychotics and ensure good adherence, because not taking such drugs is associated more strongly with functional impairment.
Previous studies have focused on the relationship between antipsychotic treatment and BDNF levels in schizophrenia patients, and found contradictory results. BDNF levels have been observed to increase after treatment with olanzapine,48 lurasidone49, or clozapine50; while other studies found unchanged levels51,52; or even decreased levels.53 To our knowledge, this is the first study assessing the expression of the TrkB receptor isoforms and their relationship with clinical features in FEP patients. Given the emerging interest in neuroplasticity mechanisms in schizophrenia as well as in the imbalance in proinflammatory and anti-inflammatory mechanisms, understanding the role(s) of neurotrophins may lead to more effective diagnoses and treatments. The results presented here suggest that it would be interesting to assess the expression of TrkB receptor isoforms (in terms of the FL/T1 ratio) before initiating antipsychotic treatment. This could be a biomarker of the response to antipsychotic drugs, but clearly further research is needed to assess its specificity and sensitivity as a biomarker.
In line with Kapur et al,54 this study contributes to the characterization of biological subtypes of this psychiatric disorder related to specific phenotypes. Furthermore, the study has the strengths that it was conducted in a homogeneous sample of patients with FEP, with fewer confounding factors than chronic patients and that we have controlled for other possible confounding factors.
A potential source of bias is that the expression of BDNF could be modified in substance abusers,55,56 and for this reason, we adjusted the statistical model for substance use. A limitation of the study concerns evidence that BDNF serum levels are related to traumatic experiences57 and this factor has not been explored in this research.
Conclusion
Inflammatory processes, functioning of neurotrophin pathways, and functional status seem to be related and this is of major translational relevance.
In particular, the level of expression of TrkB receptor isoforms should be taken into account before initiating antipsychotic treatment in FEPs.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by the Spanish Ministry of Economy and Competiveness and the Carlos III Health Institute through CIBERSAM, and the Foundation Santander-UCM (GR 58/08), and partly supported by the Spanish Ministry of Economy and Competiveness directly, and by the European Regional Development Fund, FEDER (PI10/01746, PI11/01977, PI11/02708,PI12/02077), local grants from the Department of Education, Linguistic Policy and Culture of the Basque Country Government (2010111170, 2010112009), the Basque Foundation for Health Innovation and Research (BIOEF), ) and the University of the Basque Country (GIC10/80, GIC12/84).
Conflict of interest statement
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Supplementary Material
References
- 1. Reardon S. NIH rethinks psychiatry trials. Nature. 2014;507:288. [DOI] [PubMed] [Google Scholar]
- 2. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev. 2014;38:72–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Müller N. Immunology of schizophrenia. Neuroimmunomodulation. 2014;21:109–116. [DOI] [PubMed] [Google Scholar]
- 5. Suvisaari J, Mantere O. Inflammation theories in psychotic disorders: a critical review. Infect Disord Drug Targets. 2013;13:59–70. [DOI] [PubMed] [Google Scholar]
- 6. García-Bueno B, Bioque M, Mac-Dowell KS, et al. Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull. 2014;40:376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meyer U. Anti-inflammatory signaling in schizophrenia. Brain Behav Immun. 2011;25:1507–1518. [DOI] [PubMed] [Google Scholar]
- 9. Martínez-Gras I, Pérez-Nievas BG, García-Bueno B, et al. The anti-inflammatory prostaglandin 15d-PGJ2 and its nuclear receptor PPARgamma are decreased in schizophrenia. Schizophr Res. 2011;128:15–22. [DOI] [PubMed] [Google Scholar]
- 10. Gomes JR, Costa JT, Melo CV, et al. Excitotoxicity downregulates TrkB.FL signaling and upregulates the neuroprotective truncated TrkB receptors in cultured hippocampal and striatal neurons. J Neurosci. 2012;32:4610–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palomino A, Vallejo-Illarramendi A, González-Pinto A, et al. Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients. Schizophr Res. 2006;86:321–322. [DOI] [PubMed] [Google Scholar]
- 12. Martinotti G, Di Iorio G, Marini S, Ricci V, De Berardis D, Di Giannantonio M. Nerve growth factor and brain-derived neurotrophic factor concentrations in schizophrenia: a review. J Biol Regul Homeost Agents. 2012;26:347–356. [PubMed] [Google Scholar]
- 13. Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res. 2007;4:143–151. [DOI] [PubMed] [Google Scholar]
- 14. Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 2012;846:1–12. [DOI] [PubMed] [Google Scholar]
- 15. Carim-Todd L, Bath KG, Fulgenzi G, et al. Endogenous truncated TrkB.T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J Neurosci. 2009;29:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohira K, Shimizu K, Hayashi M. Change of expression of full-length and truncated TrkBs in the developing monkey central nervous system. Brain Res Dev Brain Res. 1999;112:21–29. [DOI] [PubMed] [Google Scholar]
- 17. Vidaurre OG, Gascón S, Deogracias R, et al. Imbalance of neurotrophin receptor isoforms TrkB-FL/TrkB-T1 induces neuronal death in excitotoxicity. Cell Death Dis. 2012;3:e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong J, Rothmond DA, Webster MJ, Weickert CS. Increases in two truncated TrkB isoforms in the prefrontal cortex of people with schizophrenia. Schizophr Bull. 2013;39:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu T, Calvo L, Anta B, et al. Regulation of trafficking of activated TrkA is critical for NGF-mediated functions. Traffic. 2011;12:521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mondelli V, Cattaneo A, Belvederi Murri M, et al. Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. J Clin Psychiatry. 2011;72:1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soontornniyomkij B, Everall IP, Chana G, Tsuang MT, Achim CL, Soontornniyomkij V. Tyrosine kinase B protein expression is reduced in the cerebellum of patients with bipolar disorder. J Affect Disord. 2011;133:646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weickert CS, Ligons DL, Romanczyk T, et al. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637–650. [DOI] [PubMed] [Google Scholar]
- 23. Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960–972. [DOI] [PubMed] [Google Scholar]
- 24. Pillai A. Brain-derived neurotropic factor/TrkB signaling in the pathogenesis and novel pharmacotherapy of schizophrenia. Neurosignals. 2008;16:183–193. [DOI] [PubMed] [Google Scholar]
- 25. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 26. Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. [DOI] [PubMed] [Google Scholar]
- 27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R), Text Revision. 3rd ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 28. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV, 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29. American Psychiatric Association. DSM-IV-TR Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 30. Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960–972. [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci. 2010;64:341–357. [DOI] [PubMed] [Google Scholar]
- 32. Klein AB, Williamson R, Santini MA, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14:347–353. [DOI] [PubMed] [Google Scholar]
- 33. Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, Bahn S. Comparison of peripheral and central schizophrenia biomarker profiles. PLoS One. 2012;7:e46368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rollins B, Martin MV, Morgan L, Vawter MP. Analysis of whole genome biomarker expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:919–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Favalli G, Li J, Belmonte-de-Abreu P, Wong AH, Daskalakis ZJ. The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res. 2012;46:1–11. [DOI] [PubMed] [Google Scholar]
- 36. Fernandes BS, Steiner J, Berk M, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications [published online ahead of print September 30, 2014]. Mol Psychiatry. doi:10.1038/mp.2014.117. [DOI] [PubMed] [Google Scholar]
- 37. Lee BH, Kim YK. Increased plasma brain-derived neurotropic factor, not nerve growth factor-Beta, in schizophrenia patients with better response to risperidone treatment. Neuropsychobiology. 2009;59:51–58. [DOI] [PubMed] [Google Scholar]
- 38. Yi Z, Zhang C, Wu Z, et al. Lack of effect of brain derived neurotrophic factor (BDNF) Val66Met polymorphism on early onset schizophrenia in Chinese Han population. Brain Res. 2011;1417:146–150. [DOI] [PubMed] [Google Scholar]
- 39. Vyas NS, Puri BK. Evidence for an association between brain-derived neurotrophic factor Val66Met gene polymorphism and general intellectual ability in early-onset schizophrenia. Isr J Psychiatry Relat Sci. 2012;49:137–142. [PubMed] [Google Scholar]
- 40. Parikh V, Evans DR, Khan MM, Mahadik SP. Nerve growth factor in never-medicated first-episode psychotic and medicated chronic schizophrenic patients: possible implications for treatment outcome. Schizophr Res. 2003;60:117–123. [DOI] [PubMed] [Google Scholar]
- 41. Xiong P, Zeng Y, Zhu Z, et al. Reduced NGF serum levels and abnormal P300 event-related potential in first episode schizophrenia. Schizophr Res. 2010;119:34–39. [DOI] [PubMed] [Google Scholar]
- 42. Xiong P, Zeng Y, Wan J, et al. The role of NGF and IL-2 serum level in assisting the diagnosis in first episode schizophrenia. Psychiatry Res. 2011;189:72–76. [DOI] [PubMed] [Google Scholar]
- 43. Pandya CD, Kutiyanawalla A, Pillai A. BDNF-TrkB signaling and neuroprotection in schizophrenia. Asian J Psychiatr. 2013;6:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mackin P, Gallagher P, Watson S, Young AH, Ferrier IN. Changes in brain-derived neurotrophic factor following treatment with mifepristone in bipolar disorder and schizophrenia. Aust N Z J Psychiatry. 2007;41:321–326. [DOI] [PubMed] [Google Scholar]
- 45. Kim SW, Cho KJ. Activity-dependent alterations in the sensitivity to BDNF-TrkB signaling may promote excessive dendritic arborization and spinogenesis in fragile X syndrome in order to compensate for compromised postsynaptic activity. Med Hypotheses. 2014;83:429–435. [DOI] [PubMed] [Google Scholar]
- 46. Faria MC, Gonçalves GS, Rocha NP, et al. Increased plasma levels of BDNF and inflammatory markers in Alzheimer’s disease. J Psychiatr Res. 2014;53:166–172. [DOI] [PubMed] [Google Scholar]
- 47. Charoenphandhu N, Nuntapornsak A, Wongdee K, Krishnamra N, Charoenphandhu J. Upregulated mRNA levels of SERT, NET, MAOB, and BDNF in various brain regions of ovariectomized rats exposed to chronic aversive stimuli. Mol Cell Biochem. 2013;375:49–58. [DOI] [PubMed] [Google Scholar]
- 48. González-Pinto A, Mosquera F, Palomino A, et al. Increase in brain-derived neurotrophic factor in first episode psychotic patients after treatment with atypical antipsychotics. Int Clin Psychopharmacol. 2010;25:241–245. [DOI] [PubMed] [Google Scholar]
- 49. Luoni A, Berry A, Calabrese F, et al. Delayed BDNF alterations in the prefrontal cortex of rats exposed to prenatal stress: preventive effect of lurasidone treatment during adolescence. Eur Neuropsychopharmacol. 2014;24:986–995. [DOI] [PubMed] [Google Scholar]
- 50. Pedrini M, Chendo I, Grande I, et al. Serum brain-derived neurotrophic factor and clozapine daily dose in patients with schizophrenia: a positive correlation. Neurosci Lett. 2011;491:207–210. [DOI] [PubMed] [Google Scholar]
- 51. Pirildar S, Gönül AS, Taneli F, Akdeniz F. Low serum levels of brain-derived neurotrophic factor in patients with schizophrenia do not elevate after antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:709–713. [DOI] [PubMed] [Google Scholar]
- 52. Yoshimura R, Ueda N, Hori H, Ikenouchi-Sugita A, Umene-Nakano W, Nakamura J. Different patterns of longitudinal changes in plasma levels of catecholamine metabolites and brain-derived neurotrophic factor after administration of atypical antipsychotics in first episode untreated schizophrenic patients. World J Biol Psychiatry. 2010;11:256–261. [DOI] [PubMed] [Google Scholar]
- 53. Tan YL, Zhou DF, Cao LY, Zou YZ, Zhang XY. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett. 2005;382:27–32. [DOI] [PubMed] [Google Scholar]
- 54. Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17:1174–1179. [DOI] [PubMed] [Google Scholar]
- 55. Ricci V, Martinotti G, Gelfo F, et al. Chronic ketamine use increases serum levels of brain-derived neurotrophic factor. Psychopharmacology (Berl). 2011;215:143–148. [DOI] [PubMed] [Google Scholar]
- 56. Angelucci F, Ricci V, Martinotti G, et al. Ecstasy (MDMA)-addicted subjects show increased serum levels of brain-derived neurotrophic factor, independently from a rise of drug-induced psychotic symptoms. Addict Biol. 2010;15:365–367. [DOI] [PubMed] [Google Scholar]
- 57. Angelucci F, Ricci V, Gelfo F, et al. BDNF serum levels in subjects developing or not post-traumatic stress disorder after trauma exposure. Brain Cogn. 2014;84:118–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.