Abstract
Introduction:
Hippocampal abnormalities have been widely studied in schizophrenia spectrum populations including those at ultrahigh risk (UHR) for psychosis. There have been inconsistent findings concerning hippocampal morphology prior to and during the transition to psychosis, and little is known about how specific subregions are related to the symptom progression.
Methods:
A total of 80 participants (38 UHR and 42 healthy controls) underwent a 3T MRI scan, as well as structured clinical interviews. Shape analysis of hippocampi was conducted with FSL/FIRST vertex analysis to yield a localized measure of shape differences between groups. A subgroup of the sample (24 UHR and 24 controls) also returned for a 12-month clinical follow-up assessment.
Results:
The UHR group exhibited smaller hippocampal volumes bilaterally, and shape analysis revealed significant inversion in the left ventral posterior hippocampus in the UHR group. Greater inversion in this subregion was related to elevated symptomatology at baseline and increased positive symptoms, negative symptoms, and impaired tolerance to normal stress 12 months later. These results did not hold when left hippocampal volume was used as a predictor instead.
Discussion:
This represents the first study to use vertex analysis in a UHR sample and results suggest that abnormalities in hippocampal shape appear to reflect underlying pathogenic processes driving the progression of illness. These findings suggest that examining shape and volume may provide an important new perspective for our conception of brain alterations in the UHR period.
Key words: Hippocampus, MRI, shape analysis, vertex analysis, ultra-high risk, psychosis
Introduction
Abnormalities in the hippocampal formation are one of the most widely cited brain findings implicated in the pathophysiology of psychosis.1–4 The structure plays a central role in leading neurodevelopmental models,4 being widely implicated as an interaction point between early vulnerability and later environmental stressors.5,6 There are a number of important reasons for investigating the hippocampus in the ultrahigh risk (UHR) period immediately preceding the onset of psychosis. Research during this period can provide a unique prospective view into etiological processes driving the onset of psychotic disorders because as many as 36% of individuals meeting criteria for a UHR syndrome will go on to develop a psychotic disorder such as schizophrenia within a 2-year period.7 Further, the UHR period offers an important opportunity to understand abnormalities in brain structure because the third variable confounds inherent in studies of formally psychotic patients (eg, long-term neuroleptic medication, drug, and alcohol effects on brain volume) are not as prevalent.8
Neural diathesis-stress conceptions of psychosis highlighting hippocampal vulnerability9–12 suggest that during the prodromal period, vulnerabilities in the hippocampus interact with normative and pathological adolescent neural and endocrine developmental factors and environmental stressors, ultimately driving the onset of psychosis. Cross-sectional studies with twins concordant and discordant for schizophrenia suggest that environmental stressors early in life play a role in reduced hippocampal volume.13–15 However, the body of empirical work in psychosis has yet to reach a consensus about the exact nature of impairment prior to the onset of the disorder.16,17 Specifically, while cross-sectional studies have reported evidence to suggest that a reduction in hippocampal volume occurs only after a first episode,18,19 other investigations have observed that smaller volume in the hippocampus is present prior to the onset of psychosis.18 Prospective studies focusing on the UHR period suggest that there is limited agreement about differences in the hippocampal structure between those who do and do not develop a psychotic disorder20–23 and schizophrenia specifically.24,25 Several studies have suggested that despite smaller hippocampal volumes bilaterally in UHR individuals, there were no differences between those who did and did not develop psychosis23,26 and schizophrenia.24 One prospective study suggests that greater left hippocampal volume is related to the transition to psychosis,19 while others have found that reduced medial temporal lobe grey matter volumes is related to eventual transition to psychosis20,22 and schizophrenia.25
It is important to consider that the UHR period often occurs during adolescence, a period characterized by rapid and significant neural reorganization.27 Measures of volume alone may miss subtle but important changes in hippocampal shape that occur during this dynamic stage. For example, when using a volumetric approach alone, subtle changes in 1 area of a structure may be missed entirely or increases in 1 area may effectively cancel out decreases in another area. The potential to examine the shape of the hippocampus during the UHR period is particularly important as the different subregions are responsible for a range of specific functions, may have heterogeneous developmental trajectories, and may be uniquely vulnerable to insult.28–30 Recent advances in neuroimaging modalities for investigating brain region of interests (ROIs) have become increasingly sophisticated31 and segmentation software packages now offer state-of-the-art shape analysis capability.32 Investigations of patients with schizophrenia and their unaffected family members suggest that an inward transformation of the hippocampus head bilaterally may be associated with vulnerability to schizophrenia.33–35 However, to date, this promising method has not been used in a UHR sample.
The current study recruited a total of 80 UHR and matched control participants—utilizing structural neuroimaging, innovative shape analysis software, and structured clinical interviews—to examine hippocampal shape abnormalities associated with UHR symptom domains. We hypothesized that hippocampal shape abnormalities would be related to more severe UHR positive and negative symptoms, as well as, impaired tolerance to normal stress (ITNS) based on previous work linking symptoms of psychosis and impaired stress reactivity with hippocampal abnormalities during the UHR period.4 Further, a subset of participants was followed for a 12-month period. We hypothesized that baseline hippocampal shape abnormalities would be associated with worsened positive, negative, and ITNS symptoms within the UHR group.
Methods
Participants
A total of 80 adolescent and young adult healthy control, and UHR participants between 16 and 21 years of age were recruited to the University of Colorado Boulder’s Adolescent Development and Preventive Treatment (ADAPT) research program (see table 1). Exclusion criteria consisted of head injury, the presence of a neurological disorder, lifetime substance dependence, and the presence of any contraindication to the magnetic resonance imaging environment. The presence or lifetime history of an Axis I psychotic disorder and use of any antipsychotic medication were exclusion criteria for UHR participants. The presence of a psychotic disorder in a first degree relative was an exclusion criterion for controls. The protocol and informed consent procedures were approved by the University of Colorado Boulder Institutional Review Board in accordance with the Belmont Report.36 Participants older than 18 years gave written consent to participate. A parent gave written consent for participants younger than 18 years of age, who gave written assents. Participants were compensated for their time with cash or gift cards.
Table 1.
Demographic Characteristics and Results of Hippocampal Volume Analysis
| UHR | Control | Statistic | P | |
|---|---|---|---|---|
| Age | ||||
| Mean (SD) | 18.92 (1.42) | 18.74 (1.93) | t(75.07) = .49 | NS |
| Gender | ||||
| Male | 22 | 21 | ||
| Female | 16 | 21 | ||
| Total | 38 | 42 | χ2(1, N = 80) = .50 | NS |
| Education (y) | ||||
| Mean (SD) | 12.75 (1.53) | 12.55 (2.23) | t(78) = .44 | NS |
| Ethnicity | ||||
| Hispanic | 8 | 15 | ||
| Non-Hispanic | 30 | 27 | χ2(1, N = 80) = 2.09 | NS |
| Parent education | ||||
| Mean (SD) | 15.99 (2.24) | 14.85 (3.74) | t(78) = 1.46 | NS |
| Race | ||||
| First Nations | 1 | 0 | ||
| East Asian | 2 | 4 | ||
| Southeast Asian | 0 | 2 | ||
| Black | 1 | 1 | ||
| Central/South American | 8 | 15 | ||
| West/Central Asia and Middle East | 1 | 2 | ||
| White | 25 | 18 | χ2(6, N = 80) = 7.09 | NS |
| Recruitment method | ||||
| Internet advertisements | 28 | 32 | ||
| Community fliers and bus/radio ads | 4 | 10 | ||
| Community professional referral | 6 | 0 | χ2(2, N = 80) = 8.66 | ≤.01 |
| Right hippocampus volume %TICV | ||||
| Mean (SD) | .0026 (.0002) | .0027 (.0002) | F(1, 79) = 8.48 | ≤.01 |
| Left hippocampus volume %TICV | ||||
| Mean (SD) | .0026 (.0002) | .0027 (.0002) | F(1, 79) = 4.67 | ≤.05 |
| Left ventral posterior hippocampus scalar value | ||||
| Mean (SD) | −.21 (.60) | .19 (.52) | F(1, 79) = 10.02 | ≤.01 |
| Range | −1.59 to .87 | −1.31 to .99 | — | — |
Note: UHR, ultrahigh risk; NS, not significant.
The ADAPT study is ongoing, and to date, 12 months have passed for 63 individuals who have completed a baseline assessment. Each of these individuals was invited back, and among the 63, a total of 50 UHR and healthy control participants between 17 and 22 years of age (M = 19.90 years, SD = 1.45 years) agreed to return to complete clinical interviews. Of the 50 who returned, 2 of the UHR participants had begun treatment with antipsychotic medication between the baseline and follow-up visits, and were excluded from follow-up analyses. The total follow-up sample included 48 participants (n = 24 UHR, 24 control). Those who were lost to attrition reported loss of interest in continuing with the study (n = 0 UHR and 2 controls), or could not be contacted (n = 4 UHR and 7 controls). A subset of the current participants in this study were also a part of a previous study designed to examine the relationship between medial temporal structure volume, and physical activity.37 This study did not evaluate shape, or examine multiple time points.
Clinical Interviews
At baseline, the Structured Interview for Prodromal Syndromes (SIPS)38 was administered to both UHR and control subjects to diagnose a UHR syndrome (the SIPS was used to rule out UHR symptoms in healthy controls). A total sum score for the positive and negative symptom domain was used as an indicator of the respective dimensions of symptomatology and 1 item from the general symptom scale, ITNS, was rated from 0 (absent) to 6 (extreme). The Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID)39 was administered to rule out a psychotic disorder diagnosis and to examine history of mood, and anxiety disorders.
At follow-up, the SIPS was administered to track UHR symptom changes over 12 months and the SCID was administered to assess for possible transition to psychosis. See supplemental material for more information regarding UHR criteria, and training of clinical interviewers.
Structural Imaging
Structural MRI scans were acquired on a Siemens 3-Tesla Magnetom TIM Trio MRI scanner (Siemens AG) with a 12-channel head coil. Please see supplemental material for the structural imaging parameters and processing. Target structures consisting of the left and right hippocampus and total intracranial volume (TICV; ie, the sum of whole-brain gray matter + white matter + cerebrospinal fluid) were segmented using the FreeSurfer 5.3.0 suite of automated tools.32 Each structure was divided by the participant’s TICV to control for whole-brain volume.
Shape (ie, vertex) analyses were carried out using the FMRIB Software Library (FSL) 5.0.7 FIRST tool.40 This tool allows for a model-based segmentation and registration of anatomical images, where volumetric labels are parameterized as surface meshes. Models for each subcortical structure are based on a training set of manually traced images. Vertex locations from each participant are projected onto the surface of the average shape transformed to Montreal Neurological Institute space. Scalar projection values were processed with univariate permutation methods using FSL’s randomise tool, corrected at the cluster-level using Threshold-Free Cluster Enhancement41 to a Family-Wise Error rate of P ≤ .05. Between-group comparisons were performed for both the left and right hippocampus with 5000 permutations. A mask of the hippocampal region showing a significant group difference was created. A mean vertex-level scalar projection value of this hippocampal region was extracted for each participant using the fslstats command line utility for summary statistics. The resulting values, where negative/positive values suggest greater inversion/eversion from the average hippocampal shape, were then examined in Statistical Package for the Social Sciences 22 to explore differences between groups, relationship to symptoms at baseline and follow-up, and symptom differences after 12 months.
Additional Data Analysis
Independent t tests and chi-square tests were employed to examine differences between groups in continuous and categorical demographic variables, respectively. ANOVA with age as a covariate of no interest was used to examine group differences for hippocampal volume and shape. Healthy controls reported limited positive, negative, or ITNS symptoms and analyses concerning the relationship between hippocampal shape and symptom severity focused on the UHR group alone. Relationships between hippocampal shape transformation and positive, negative, and ITNS symptoms at baseline were examined using one-tailed Pearson correlations with age as a covariate. A series of 3 hierarchical regression analyses were conducted within the UHR group alone. Positive, negative, and ITNS symptoms at the follow-up assessment were used as the dependent variables and the respective symptom variable for the baseline assessment was entered in the first block. In the second block, age was included as a covariate. In the third block, left hippocampal shape transformation value was entered as the predictor variable. With each analysis, the magnitude of R2 change (ΔR2) was tested for significance. This analytic approach tests the hypotheses that while controlling for the variance explained by symptoms and age at baseline, hippocampal shape abnormalities will be associated with respective symptoms 12 months later. To supplement this approach, symptom differences were also calculated by subtracting the baseline rating from the follow-up rating in order to examine relationship of hippocampal shape transformation to symptom changes. Positive differences indicate worsened symptoms at follow-up. Based on the results of this supplemental approach, the UHR group who returned for follow-up was divided between groups showing deteriorating symptoms, and those who improved or stayed the same. Analyses involved 1-tailed independent t tests comparing hippocampal shape inversion in UHR individuals with deteriorating positive, negative, and ITNS symptoms to those with improved or no changes in symptoms.
Results
Demographics
There were no significant differences between groups on demographic characteristics, except for recruitment method (see table 1). UHR and control participants who returned for follow-up did not differ from participants who did not return on baseline characteristics. UHR participants were rated significantly higher than controls on SIPS symptom domains positive, negative, and ITNS at baseline and at follow-up (see table 2 and figure 1). Within the UHR group, 7 participants at baseline, and 5 participants at follow-up had a first-degree relative with a psychotic disorder. Additionally, 7 UHR participants had a diagnosis of SPD at baseline, of which 5 participants returned for follow-up. Axis I disorders in the UHR group included a history of mood (66% of UHR group), and anxiety (42% of UHR group). Of the initial baseline sample, a total of 3 UHR participants (8% of UHR group) met criteria for a psychotic disorder at the follow-up assessment.
Table 2.
Symptom Progression for UHR and Healthy Control Participants
| UHR | Control | Statistic | P | |
|---|---|---|---|---|
| Baseline | ||||
| Positive | 11.26 (4.01) | .57 (1.40) | t(41.99) = 14.8 | ≤.001 |
| Negative | 9.34 (6.56) | .42 (.83) | t(35.99) = 7.91 | ≤.001 |
| Impaired tolerance to normal stress | 1.36 (1.22) | .10 (.37) | t(42.55) = 6.01 | ≤.001 |
| Follow-up | ||||
| Positive | 9.46 (6.47) | .17 (.48) | t(23.25) = 7.01 | ≤.001 |
| Negative | 6.25 (6.33) | .63 (1.61) | t(25.96) = 4.22 | ≤.001 |
| Impaired tolerance to normal stress | 1.29 (1.55) | .04 (.20) | t(23.80) = 3.93 | ≤.001 |
| Range of difference in UHR symptoms | ||||
| Positive | −10 to 10 | — | — | — |
| Negative | −16 to 7 | — | — | — |
| Impaired tolerance to normal stress | −2 to 4 | — | — | — |
Note: The SIPS was used to follow positive, negative, and impaired tolerance to normal stress (ITNS) symptoms over 12 mo; Mean (SD) for symptom totals within each group are presented; The range of differences between baseline and follow-up symptoms in the UHR group is included.
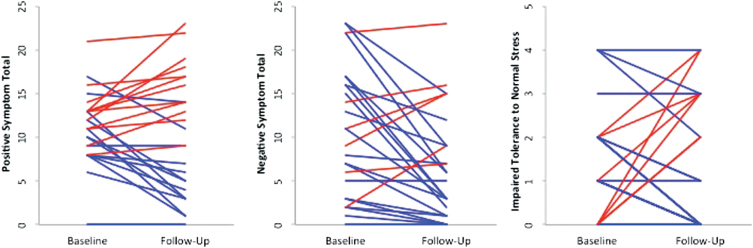
Fig. 1.
The figure shows the progression of positive, negative, and ITNS symptoms separately from the baseline to follow-up time-points in the UHR participants. A positive slope (red lines) indicates deteriorated symptoms, while a negative slope (blue lines) indicates improvement or no change in symptoms from baseline to follow-up assessments.
Hippocampal Volume and Shape
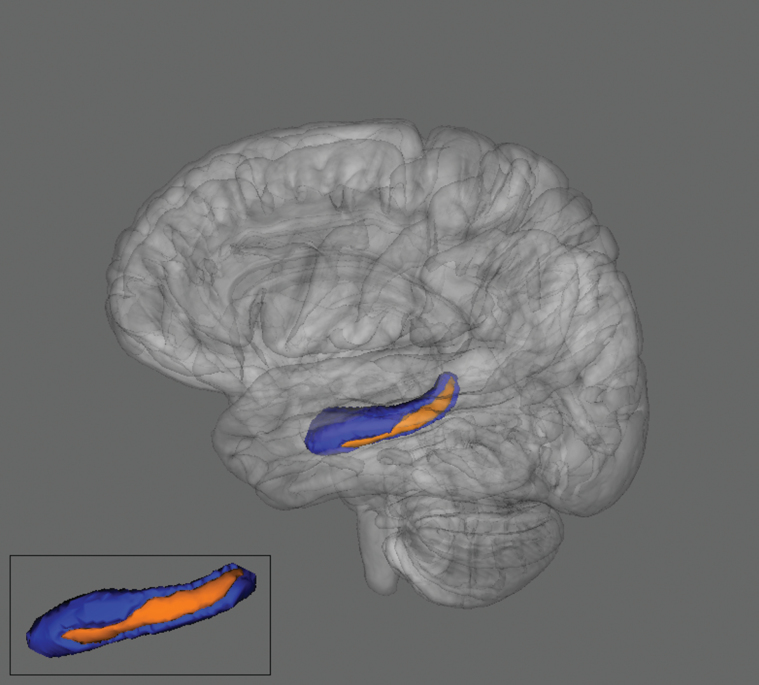
The UHR group showed significantly smaller hippocampal volume in the right and left hemispheres when compared with healthy controls (see table 1). Hippocampal shape results using FSL/FIRST vertex analysis revealed a significant inversion from the standard structure in the left ventral posterior hippocampus (LVPH) for UHR group compared with control participants. An F test using the scalar projection value of LVPH inversion with age as a covariate of no interest revealed that the UHR group showed a significant inward shape transformation when compared with the control group. Figure 2 illustrates the LVPH region (in orange) of shape transformation. See table 1 for results of volume and shape analysis.
Fig. 2.
Two different views of the left hippocampus. There was a significant shape inversion in the left posterior ventral hippocampus (in orange; Family-Wise Error corrected P ≤ .05) in the UHR group compared with healthy controls.
Left Ventral Posterior Hippocampus and Clinical Presentation
Because the shape differences were specific to LVPH, subsequent correlational and regression analyses used the scalar projection values for this region. Within the UHR group alone, greater inward transformation was significantly related to more severe positive symptoms at baseline r(35) = −.34, P ≤ .05. The relationship with baseline negative symptoms, r(35) = −.19, P = .13 and with ITNS r(35) = −.18, P = .15 was not significant.
Longitudinal Progression of Symptoms
Hierarchical linear regression was used to examine if the LVPH shape transformation at baseline was associated with symptoms at follow-up within the UHR group (see table 3). Greater inward LVPH transformation accounted for 10% of the variance for 12-month positive symptoms (β = −.33, P ≤ .05), 13% of the variance of 12-month negative symptoms (β = −.37, P ≤ .05), and 20% of the variance for 12-month ITNS (β = −.46, P ≤ .05). Additionally, there was a significant relationship between greater hippocampal shape inversion and worsened positive symptom difference at follow-up r(21) = −.42, P ≤ .05. The relationship between greater hippocampal shape inversion and elevated negative symptom difference at follow-up was not significant r(21) = −.23, P = .15, and there was a trend-linking-increased hippocampal shape inversion to worsened ITNS r(21) = −.34, P = .06. Additionally, there was significantly greater hippocampal shape inversion in the UHR individuals with deteriorating positive t(22) = 1.94, P ≤ .05 and negative t(22) = 1.73, P ≤ .05 symptoms and a trend level difference in UHR individuals who showed deteriorating ITNS t(22) = 1.45, P = .08 at follow-up compared with UHR individuals who improved or stayed the same.
Table 3.
Hierarchical Linear Regression Results
| Predicting 12-Month Variable | Block I: Baseline Symptoms | Block II: Age | Block III: Left Hippocampus Shape Transformation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R 2 | df | F | β | P | ΔR 2 | df | F | β | P | ΔR 2 | df | F | β | P | |
| Positive symptoms | .49 | 1, 22 | 21.00 | .70 | .0001 | .01 | 1, 21 | .59 | .14 | .45 | .10 | 1, 20 | 5.05 | −.33 | .04 |
| Negative symptoms | .32 | 1, 22 | 10.22 | .56 | .004 | .002 | 1, 21 | .07 | .05 | .79 | .13 | 1, 20 | 4.7 | −.37 | .04 |
| Impaired tolerance to normal stress | .12 | 1, 22 | 2.90 | .34 | .10 | .04 | 1, 21 | .95 | .20 | .34 | .20 | 1, 20 | 5.98 | −.46 | .02 |
| Block I: Baseline Symptoms | Block II: Age | Block III: Left Hippocampus Volume | |||||||||||||
| Predicting 12-month variable | R 2 | df | F | β | P | ΔR 2 | df | F | β | P | ΔR 2 | Df | F | β | P |
| Positive symptoms | .49 | 1, 22 | 21.00 | .70 | .0001 | .01 | 1, 21 | .59 | .14 | .45 | .02 | 1, 20 | .88 | −.15 | .36 |
| Negative symptoms | .32 | 1, 22 | 10.22 | .56 | .004 | .002 | 1, 21 | .07 | .05 | .79 | .03 | 1, 20 | .88 | −.17 | .36 |
| Impaired tolerance to normal stress | .12 | 1, 22 | 2.90 | .34 | .10 | .04 | 1, 21 | .95 | .20 | .34 | .05 | 1, 20 | 1.25 | −.22 | .28 |
Note: Linear regression was used to evaluate if left ventral posterior hippocampal shape inversion at baseline predicted worsening positive, negative, and ITNS symptoms at follow-up in the UHR group alone; Left hippocampal volume was used as a predictor variable to determine if shape transformation gave a unique perspective.
To determine if shape analysis provides a unique perspective on the progression of symptoms, the regression analyses and correlations between symptom difference scores were conducted within the UHR group with the left hippocampal volume as the predictor instead of the scalar projection value for LVPH. The results did not approach significance for left volume measurements associated with positive, negative, or ITNS symptoms at follow-up (P ≤ .5).
Discussion
The current study used a state-of-the-art vertex analysis approach to supplement a traditional volume based approach to examining hippocampal morphology in youth at risk for psychosis. We observed smaller hippocampi bilaterally in the UHR group when compared with matched healthy controls. When examining group differences in shape, we observed a significant inversion in the LVPH. Importantly, it was this difference in shape on the left hemisphere that was uniquely tied to symptom progression during the UHR period. Taken together, results suggest that there are significant abnormalities in structure and shape during the UHR period, and that subtle differences in morphology that may have been previously overlooked by less specific methods, may play an important role in the progression of UHR symptoms of psychosis.
Consistent with several related investigations,19,37,42 the UHR participants in the current study had smaller bilateral hippocampus volume measurements compared with controls. As noted, smaller hippocampal volumes have been found in a number of studies in schizophrenia patients, while in UHR groups, there remains some controversy as to when and what type of structural changes occur.18–21,23,26,43 One possibility is that the inconsistencies are due to varying study design, or sample characteristics. For example, cross-sectional studies examining differences between UHR and first episode psychosis (FEP) patients have found varied results, with 1 study finding that UHR individuals had smaller hippocampal volume compared with schizophrenia patients,17 while other prospective studies have noted that grey matter volume in the hippocampus was larger or not different in UHR who transitioned to psychosis than FEP.18,19 Other cross-sectional studies provide conflicting results when comparing UHR to healthy controls, finding either smaller18 or no difference between volume measurements.22
However, the noted prospectively designed studies of UHR individuals who did and did not transition to psychosis report just as varied results as cross-sectional studies. Recruitment methods of at-risk participants varies across prospective studies, with some studies recruiting biological relatives of schizophrenia patients (considered to be at high genetic risk for psychosis), with a subsection of those participants reporting attenuated psychosis symptoms and later developing psychosis.20,25 Other prospective studies rely on recruiting individuals meeting criteria for an UHR syndrome.21–24,26,44 Factors such as the use of neuroleptic medication in subsections of the sample may have also influenced results.17,18,26 While there have been some larger investigations21,24,26 a majority of the applicable investigations have had comparably sized samples to the current study. In cross-sectional studies, the range of sample sizes included 30–60 UHR individuals, while prospective studies ranged from 20 to 135 participants. The present prospective study was designed to address these confounds in previous studies by recruiting participants meeting UHR criteria, excluding participants taking antipsychotic medication, and including a moderate sample size (38 UHR baseline, 24 UHR follow-up).
Another possibility is that the lack of agreement may be due to the variety of methodological differences in image acquisition and processing. Manual tracing has been a predominant ROI method of investigating the hippocampus in prospective studies.21,23,25 In comparison, only 2 UHR cross-sectional studies, finding bilateral volume reduction in hippocampi, take advantage of segmentation programs.17,37 The findings in some prospective manual tracing studies of the hippocampus vary; some studies find that volumes did not differ between UHR (regardless of later transition) and healthy controls,21,26 or were larger for those who transitioned.18,19 However, a recent prospective study utilizing high resolution images and manual tracing found that UHR individuals had smaller hippocampal volumes when compared with controls, but volumetric measurements did not differ between those who did and did not develop psychosis.23 Only 1 cross-sectional study has used voxel-based morphometry (VBM) to examine grey matter, showing that at less-stringent threshold volume differences in the hippocampus were found between UHR and controls, but not between UHR and schizophrenia patients.44 While VBM methods have allowed localized analysis of grey matter changes in the temporal lobe more broadly in UHR samples related to transition,20,22,24 previous studies may not have benefited from high-resolution images, which can affect registration, and may hamper smoothing of grey and white matter.45 In contrast, the current methods use high resolution images registered in standard space and do not require smoothing, such that the hippocampal formation is subject to a more targeted analysis.40
Shape analysis of the hippocampi has been used effectively to illustrate abnormalities in brain structures including the putamen, caudate, and hippocampus related to disorders such as dementia, obsessive-compulsive disorder, and schizophrenia.5,46–49 The current results indicate that UHR participants show significant alteration in the structure of the LVPH. This is consistent with other investigations that found bilateral volume reduction in schizophrenia and UHR participants, and more localized changes to the anterior hippocampus,1,3,48–50 or greater volume asymmetry between left and right hippocampus in the clinical groups compared with healthy controls.5,23,51 Relevant research in schizophrenia patients and UHR individuals suggests that several factors may be attributed to greater inversion in hippocampal shape and size including altered neuron organization, synaptic pruning during the prodromal period, perfusion, and cerebral blood volume.2,25,52–55 Future work utilizing shape analysis across multiple time points will be integral for determining whether the anterior portion of the hippocampus is affected later in the progression of illness and if shape inversion remains a stable biomarker of psychosis.
A diathesis-stress model of psychosis suggests that early vulnerability due to genetic and prenatal factors present from birth interacts with environmental stressors, as well as normative and abnormal neural maturational factors during adolescence.12 This interaction takes place during a UHR period that is characterized by decreased social and role function and emergence of attenuated psychotic symptoms.11 With regards to the present findings, it is noteworthy that the hippocampus is related to greater severity of symptoms at the baseline and follow-up assessment. This finding supports the diathesis-stress model and is particularly important given the substantial pathogenic role the hippocampus plays in the etiology of psychosis including a sensitivity to early prenatal insult,21,42 and a role in glutamatergic and dopaminergic processes thought to underlie psychotic symptoms.56,57 The finding of hippocampal shape abnormality related to ITNS at follow-up is also significant because of the related hippocampal-mediated endocrine changes that occur during adolescence,11 which has a critical role in modulating the hypothalamic-pituitary-adrenal axis response to stress.4 The finding of LVPH abnormality is also interesting as this specific subregion is responsible for cognitive functions such as memory retrieval and spatial learning; domains also affected in psychosis.29 Future work including a targeted cognitive battery will be important for determining the effects that emerging shape abnormalities play during the UHR period.
The current study benefits from a number of strengths, most notably the use of a prospective design, neuroleptic-free sample, and an innovative regional shape analysis. As noted, while the current study is comparable in size to past work on hippocampal morphology in UHR samples,17,37 larger samples will be important for detecting subtle but clinically relevant group differences in structural morphology during this important period. The findings of this study suggest that hippocampal shape abnormalities are tied to worsening progression of UHR symptoms, yet it is currently not possible to definitely determine whether this shape abnormality can predict formal conversion to psychosis. Importantly, the current study is ongoing, and with more participants returning for their follow-up interviews, future work with a greater number of participants will focus on this important outcome variable. In a related point, the results suggests that there is greater hippocampal shape inversion in UHR individuals whose symptoms deteriorate compared with those who improve or stay the same; however, these results should be considered preliminary until larger follow-up samples can be examined. We assessed participants at 12 months, but current research suggests that while a proportion of UHR individuals show elevated symptoms in 12 months,58 a 2- to 3-year window is optimal for detecting disease progression in the UHR period; therefore, these results should be viewed as preliminary until future work is able to assess shape abnormalities over several time points. The current study included participants with and without a positive family history of psychosis as well as SPD traits; larger studies will be crucial for exploring differences related to these risk factors. Finally, it is important to consider that the UHR period is a time of rapid neural reorganization, and imaging data from several time points are necessary to determine how normative and pathological development in hippocampal volume and shape ultimately play a role in the progression of illness.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
National Institutes of Health (R01MH094650, R21/R33MH103231 to V.A.M.), (F32DA034412 to J.M.O), (F32MH102989 to J.A.B.), and (F31MH100821 to A.P.B.). The authors have no potential conflicts of interest to report.
References
- 1. Csernansky JG, Wang L, Jones D, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. [DOI] [PubMed] [Google Scholar]
- 2. Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl). 2004;174:151–162. [DOI] [PubMed] [Google Scholar]
- 3. Narr KL, Thompson PM, Szeszko P, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. [DOI] [PubMed] [Google Scholar]
- 4. Phillips LJ, McGorry PD, Garner B, et al. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. 2006;40:725–741. [DOI] [PubMed] [Google Scholar]
- 5. Ebner F, Tepest R, Dani I, et al. The hippocampus in families with schizophrenia in relation to obstetric complications. Schizophr Res. 2008;104:71–78. [DOI] [PubMed] [Google Scholar]
- 6. Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry. 2007;61:1179–1186. [DOI] [PubMed] [Google Scholar]
- 7. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
- 8. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. [DOI] [PubMed] [Google Scholar]
- 10. Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. [DOI] [PubMed] [Google Scholar]
- 12. Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–685. [DOI] [PubMed] [Google Scholar]
- 13. van Erp TG, Saleh PA, Huttunen M, et al. Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004;61:346–353. [DOI] [PubMed] [Google Scholar]
- 14. Van Erp TG, Saleh PA, Rosso IM, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–1520. [DOI] [PubMed] [Google Scholar]
- 15. McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000;157:203–212. [DOI] [PubMed] [Google Scholar]
- 16. Wood SJ, Pantelis C, Velakoulis D, Yücel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull. 2008;34:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Witthaus H, Kaufmann C, Bohner G, et al. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Res. 2009;173:163–169. [DOI] [PubMed] [Google Scholar]
- 18. Buehlmann E, Berger GE, Aston J, et al. Hippocampus abnormalities in at risk mental states for psychosis? A cross-sectional high resolution region of interest magnetic resonance imaging study. J Psychiatr Res. 2010;44:447–453. [DOI] [PubMed] [Google Scholar]
- 19. Phillips LJ, Velakoulis D, Pantelis C, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr Res. 2002;58:145–158. [DOI] [PubMed] [Google Scholar]
- 20. Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. [DOI] [PubMed] [Google Scholar]
- 21. Wood SJ, Yücel M, Velakoulis D, et al. Hippocampal and anterior cingulate morphology in subjects at ultra-high-risk for psychosis: the role of family history of psychotic illness. Schizophr Res. 2005;75:295–301. [DOI] [PubMed] [Google Scholar]
- 22. Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. [DOI] [PubMed] [Google Scholar]
- 23. Wood SJ, Kennedy D, Phillips LJ, et al. Hippocampal pathology in individuals at ultra-high risk for psychosis: a multi-modal magnetic resonance study. Neuroimage. 2010;52:62–68. [DOI] [PubMed] [Google Scholar]
- 24. Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. [DOI] [PubMed] [Google Scholar]
- 25. Lawrie SM, Whalley HC, Abukmeil SS, et al. Temporal lobe volume changes in people at high risk of schizophrenia with psychotic symptoms. Br J Psychiatry. 2002;181:138–143. [DOI] [PubMed] [Google Scholar]
- 26. Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. [DOI] [PubMed] [Google Scholar]
- 27. Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. [DOI] [PubMed] [Google Scholar]
- 29. Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci U S A. 1999;96:4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gogtay N, Nugent TF, 3rd, Herman DH, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. [DOI] [PubMed] [Google Scholar]
- 31. Morey RA, Petty CM, Xu Y, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 33. Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54:1234–1240. [DOI] [PubMed] [Google Scholar]
- 34. Styner M, Lieberman JA, Pantazis D, Gerig G. Boundary and medial shape analysis of the hippocampus in schizophrenia. Med Image Anal. 2004;8:197–203. [DOI] [PubMed] [Google Scholar]
- 35. Ho BC, Magnotta V. Hippocampal volume deficits and shape deformities in young biological relatives of schizophrenia probands. Neuroimage. 2010;49:3385–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Biomedical NCftPoHSoB, Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Bethesda, MD: ERIC Clearinghouse; 1978. [Google Scholar]
- 37. Mittal VA, Gupta T, Orr JM, et al. Physical activity level and medial temporal health in youth at ultra high-risk for psychosis. J Abnorm Psychol. 2013;122:1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller TJ, McGlashan TH, Woods SW, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. [DOI] [PubMed] [Google Scholar]
- 39. First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, January 1995 FINAL: SCID-I/P Version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 40. Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 42. Stefanis N, Frangou S, Yakeley J, et al. Hippocampal volume reduction in schizophrenia: effects of genetic risk and pregnancy and birth complications. Biol Psychiatry. 1999;46:697–702. [DOI] [PubMed] [Google Scholar]
- 43. Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Borgwardt SJ, Riecher-Rössler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. [DOI] [PubMed] [Google Scholar]
- 45. Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1462. [DOI] [PubMed] [Google Scholar]
- 46. Achterberg HC, van der Lijn F, den Heijer T, et al. Hippocampal shape is predictive for the development of dementia in a normal, elderly population. Hum Brain Mapp. 2014;35:2359–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zarei M, Mataix-Cols D, Heyman I, et al. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. 2011;70:1083–1090. [DOI] [PubMed] [Google Scholar]
- 48. Lee JM, Kim SH, Jang DP, et al. Deformable model with surface registration for hippocampal shape deformity analysis in schizophrenia. Neuroimage. 2004;22:831–840. [DOI] [PubMed] [Google Scholar]
- 49. Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54:1234–1240. [DOI] [PubMed] [Google Scholar]
- 50. Shenton ME, Gerig G, McCarley RW, Székely G, Kikinis R. Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry Res. 2002;115:15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Csernansky JG, Joshi S, Wang L, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci U S A. 1998;95:11406–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schobel SA, Lewandowski NM, Corcoran CM, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Théberge J. Perfusion magnetic resonance imaging in psychiatry. Top Magn Reson Imaging. 2008;19:111–130. [DOI] [PubMed] [Google Scholar]
- 54. Talati P, Rane S, Kose S, et al. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clin. 2014;5:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Talati P, Rane S, Skinner J, Gore J, Heckers S. Increased hippocampal blood volume and normal blood flow in schizophrenia. Psychiatry Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stone JM, Howes OD, Egerton A, et al. Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biol Psychiatry. 2010;68:599–602. [DOI] [PubMed] [Google Scholar]
- 57. Stone JM, Day F, Tsagaraki H, et al. ; OASIS. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–539. [DOI] [PubMed] [Google Scholar]
- 58. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.