Abstract
Patients with schizophrenia are known to have increased prevalence of abnormalities in midline brain structures, such as a failure of the septum pellucidum to fuse (cavum septum pellucidum) and the absence of the adhesio interthalamica. This is the first study to investigate the prevalence of these abnormalities across a large multidiagnostic sample. Presence of cavum septum pellucidum and absence of the adhesio interthalamica was assessed in 639 patients with chronic schizophrenia, delusional disorder, schizoaffective disorder, bipolar disorder, major depressive disorder, or a first episode of psychosis, mania or unipolar depression. This was compared with 223 healthy controls using logistic-regression-derived odds ratios (OR). Patients with psychotic or mood disorders showed an increased prevalence of both abnormalities (OR of cavum septum pellucidum = 2.1, OR of absence of the adhesio interthalamica = 2.6, OR of both cavum septum pellucidum and absence of the adhesio interthalamica = 3.8, all P < .001). This increased prevalence was separately observed in nearly all disorders as well as after controlling for potential confounding factors. This study supports a general increased prevalence of midline brain abnormalities across mood and psychotic disorders. This nonspecificity may suggest that these disorders share a common neurodevelopmental etiology.
Key words: structural abnormalities, midline brain, cavum septum pellucidum, absent adhesio interthalamica, psychotic disorders, mood disorders
Introduction
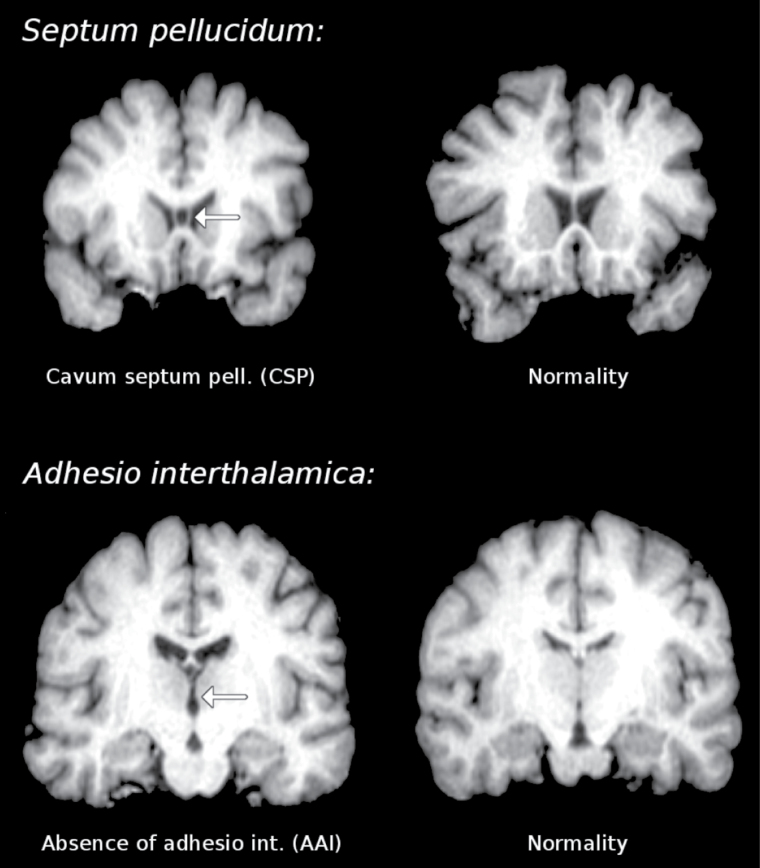
A large body of literature has emphasized the role of aberrant neurodevelopmental processes in the pathophysiology of psychosis.1 Only few of these studies have specifically examined the prevalence of abnormalities in midline brain structures such as the septum pellucidum and the adhesio interthalamica. The septum pellucidum is a component of the limbic system that forms the medial walls of the lateral ventricles and consists of 2 layers or laminae of both gray and white matter. During fetal development there is a space between the 2 laminae which is normally fused in an anterior to posterior fashion by the age of 3–6 months.2 When these laminae fail to fuse, they form a cavity known as cavum septum pellucidum (CSP3) or ‘fifth ventricle’ (figure 1 top). The incidence of CSP among healthy individuals varies considerably and some authors argue that a very small CSP (1–1.4mm) is common with an incidence of 60%–80% and is therefore considered as part of the normal anatomy of the brain.4 The adhesio interthalamica, or massa intermedia, is a flattened gray matter band, thought to connect both thalami across the third ventricle, which generally fuses by the 13th week of gestation5 (figure 1 bottom). Postmortem studies have shown that the adhesio interthalamica is absent in 15%–25% of humans,6 suggesting the possibility of developmental problems during early gestation.
Fig. 1.
Real examples of cavum septum pellucidum (CSP) and absence of adhesio interthalamica (AAI) in coronal magnetic resonance slices.
Consistent with the neurodevelopmental paradigm of psychosis, the presence of CSP and absent adhesio interthalamica (AAI) in schizophrenia has been related to abnormalities in the corpus callosum and limbic structures, such as the thalamus and the hippocampus, during fetal development, a time when risk factors for schizophrenia reportedly have their effect on the disorder.7 However, prevalence of CSP in psychosis is still controversial with some reports suggesting a higher8,9 and others providing a similar prevalence rate when compared with the normal population.10,11 A meta-analysis of CSP in schizophrenia spectrum disorders reported a higher prevalence of CSP of any size in 6 out of 15 included studies with 5 out of 15 showing greater occurrence of large CSP.12 Broader consensus prevails in the literature regarding the prevalence of AAI in psychosis. A meta-analysis found a higher prevalence of AAI in patients with schizophrenia spectrum disorders in relation to healthy controls in 9 out of 11 included studies.13 Further studies have examined longitudinally these midline brain abnormalities in patients with a first episode of psychosis progressing to chronic schizophrenia, detecting that the length of the AAI, but not the CSP, can change during the course of the illness.14,15 So far, no studies have separately assessed the midline abnormalities in other schizophrenia spectrum disorders such as schizoaffective and delusional disorders.
Less attention has been paid to the study of mood disorders from a neurodevelopmental perspective.16 The literature available on midline brain abnormalities in these populations is scarce and the results are inconsistent with some studies reporting increased prevalence of CSP of any size in bipolar disorder and major depression17,18 while others found no evidence of CSP abnormalities.19,20 To our knowledge, only 2 studies to date have examined adhesio interthalamica abnormalities in mood disorders, detecting shorter adhesio interthalamica in bipolar patients and currently depressed major depression patients18,20 but not in remitted major depression patients.20
To clarify and widen the prior findings and to get a more complex understanding of the nature of these midline brain abnormalities in severe mental disorders, we investigated the prevalence of CSP and AAI in a large cross sectional multidiagnostic sample. Specifically, we included 639 patients with schizophrenia, delusional disorder, schizoaffective disorder, bipolar disorder, major depressive disorder, or a first episode of psychosis, mania, or unipolar depression, along with a sample of 223 healthy controls.
Methods
Subjects
Data for this study have been previously analyzed in a series of neuroimaging studies evaluating structural and functional differences between patients with psychotic or mood disorders and healthy controls (table 1).21–25 In these studies all adult patients had been scanned using the same resonance imaging (MRI) scanner and met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (or in case of schizoaffective disorder additionally also Research Diagnostic Criteria) for their corresponding disorders. Patients had been recruited in hospitals and outpatient facilities in Barcelona and surrounding areas. Adult healthy controls had been recruited from nonmedical staff working in the hospital, their relatives and acquaintances, plus independent sources in the community. Controls had been questioned and excluded if they reported a history of mental illness and/or treatment with psychotropic medication. After complete description of the study, written informed consent had been obtained from all participants and the studies had been approved by the local research ethics committee.
Table 1.
Demographical, Clinical, and Neuropsychological Description of the Sample
| Age (y) | Sex (women) | University Studiesa | Premorbid IQb (TAP) | Current IQc (WAIS-III) | Executive Functiond (BADS) | Memorye (WMS) | Age of Onsetf (y) | Duration of the Diseasef (y) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean | SD | n | % | N | % | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | |
| Healthy controls (n = 223) | 36.0 | 11.3 | 124 | 55.6 | 74 | 43.3 | 23. 7 | 4.5 | 108.5 | 14.7 | 100.8 | 12.6 | 42.1 | 8.0 | — | — | — | — |
| All patients (n = 639) | 39.4 | 11.9 | 259 | 40.5 | 88 | 18.2 | 21.5 | 5.1 | 91.2 | 16.9 | 78.3 | 22.2 | 29.1 | 8.5 | 25.6 | 9.3 | 13.4 | 11.6 |
| First episode of psychosis (n = 85) | 26.9 | 8.2 | 27 | 31.8 | 11 | 14.5 | 20.8 | 4.4 | 89.1 | 15.7 | 83.9 | 19.7 | 27.6 | 7.4 | 26.7 | 8.6 | 0.5 | 1.3 |
| First episode of mania (n = 38) | 30.6 | 9.6 | 16 | 42.1 | 7 | 33.3 | 22.8 | 5.3 | 98.4 | 18.8 | 88.4 | 19.1 | 33.6 | 9.8 | 30.5 | 10.0 | 0.2 | 1.0 |
| First episode of depression (n = 14) | 44.2 | 13.6 | 5 | 35.7 | 3 | 25.0 | 18.6 | 4.4 | 90.2 | 18.1 | 79.1 | 22.0 | 31.1 | 9.8 | 44.0 | 12.9 | 0.7 | 1.0 |
| Schizophrenia (n = 219) | 41.1 | 10.9 | 60 | 27.4 | 16 | 11.2 | 20.0 | 5.1 | 87.6 | 16.8 | 71.8 | 23.7 | 27.0 | 8.3 | 22.5 | 6.9 | 17.8 | 11.0 |
| Delusional disorder (n = 24) | 47.2 | 11.0 | 15 | 62.5 | 3 | 18.8 | 20.5 | 6.0 | 101.1 | 17.9 | 86.2 | 26.6 | 33.6 | 8.8 | 32.8 | 11.9 | 17.5 | 13.2 |
| Schizoaffective disorder (n = 47) | 41.9 | 9.6 | 20 | 42.6 | 2 | 5.4 | 21.4 | 5.3 | 88.4 | 15.4 | 73.2 | 22.6 | 29.6 | 7.4 | 22.9 | 7.4 | 18.7 | 10.9 |
| Bipolar disorder (n = 189) | 41.5 | 10.3 | 99 | 52.4 | 45 | 28.7 | 23.5 | 4.4 | 94.9 | 16.6 | 82.2 | 19.5 | 30.4 | 8.3 | 26.0 | 9.0 | 15.3 | 10.1 |
| Major depressive disorder (n = 23) | 51.3 | 10.9 | 17 | 73.9 | 1 | 4.8 | 21.7 | 4.1 | 92.7 | 11.7 | 76.9 | 17.7 | 34.5 | 7.9 | 33.6 | 12.5 | 17.3 | 10.8 |
Note: n, number of cases; BADS, Behavioural Assessment of the Dysexecutive Syndrome; TAP, “Test de Acentuacion de Palabras” (Word Accentuation Test); WMS, Wechsler Memory Scale.
aInformation missing for 208 (24.1%) individuals.
bInformation missing for 113 (13.1%) individuals.
cInformation missing for 141 (16.4%) individuals.
dInformation missing for 238 (27.6%) individuals.
eInformation missing for 289 (33.5%) individuals.
fInformation missing for 111 (17.4%) patients.
The following information had been retrieved from both patients and controls: age, sex, education level (whether the participant had completed university studies or not), premorbid IQ, current IQ, and memory and executive function scores. Premorbid IQ had been estimated using the ‘Test de Acentuación de Palabras’ (Word Accentuation Test)26 which requires pronunciation of Spanish words whose accents have been removed. Current IQ was measured using 4 subtests of the Wechsler Adult Intelligence Scale III (vocabulary, similarities, block design, and matrix reasoning). Memory had been assessed using the Spanish version of the third edition of the Wechsler Memory Scale (WMS-III),27 and executive function had been tested using the Behavioural Assessment of the Dysexecutive Syndrome (BADS), which has also been adapted for the use in Spanish populations.28
The following information had been additionally retrieved for patients: age of onset, duration of the disease, and whether the patient had been scanned during the first episode or during the chronic phase of the disorder. No information could be retrieved regarding cumulative lifetime medication.
MRI Data Acquisition and Assessment
All individuals had been scanned in the same 1.5 Tesla GE Signa scanner (General Electric Medical Systems) located at the Sant Joan de Déu Hospital in Barcelona. A high-resolution structural T1 MRI sequence with the following parameters had been used: number of axial slices = 180; slice thickness = 1mm, slice gap = 0mm, matrix size = 512×512; voxel resolution 0.5×0.5×1mm3; echo time (TE) = 4ms, repetition time (TR) = 2000ms, flip angle = 15°.
Structural images had the nonbrain matter removed with the ‘brain extraction tool’ (BET), were affine-registered to a standard 1×1 × 1mm3 MNI template, had their intensity standardized to a 0–255 scale, and were sliced for presentation in a zoomable and scrollable computer screen. Scans from 49 (5.5%) of the initial 898 individuals included in the study had to be discarded because motion artifacts prevented a correct evaluation of the midline abnormalities, but valid subsequent longitudinal scans could be retrieved for 13 of these individuals.
The anatomical slices were presented and assessed in coronal plane. In the instances when the image was unclear it was also assessed in other planes and in native space and further discussed with other researchers if required, blind to the group condition. When assessing CSP, all degrees of abnormality were labeled as abnormal (even the mildest ones), but those abnormal CSPs with an anteroposterior length > 5mm were additionally considered ‘large’. Presence of AAI and CSP was established when it could be identified on at least 1 slice. The same researcher (R.L.-R.) assessed all the images, blind to any information of the individuals. Prior to this assessment, 1 subsample of 50 cases had been analyzed by 2 of the researchers (R.L.-R. and J.R.) and discrepancies discussed to reach consensus; this process was repeated with 2 further random subsamples of 100 individuals until complete inter-rater reliability was achieved.
Statistical Analysis
Being a case-control study, associations between prevalence of midline abnormalities and disease were assessed using odds ratios (OR) rather than relative risks or hazard ratios. Note however that odds ratios may exaggerate the relative risk. Thus, for example, an OR = 3 may mean that patients have more chances to show midline abnormalities (or the other way round), but probably not as much as 3 times more. OR for the prevalence of CSP or AAI were estimated by means of logistic regressions, and they were re-evaluated including potential confounding factors as covariates in case of statistical significance. These (age, sex, education, premorbid IQ, IQ, BADS, and WMS) had been previously assessed in the sample of healthy controls. Two clinical factors (age of onset and duration of disease) were also assessed.
Given that this study investigated 2 brain abnormalities in 8 disorders, only P values ≤ .003 (ie, 0.05/16) were considered statistically significant, with the exception of the study of confounding factors where no Bonferroni correction for multiple comparisons was applied in order to maximize the detection of potential confounding factors.
Results
Psychotic and mood disorders were moderate-to-strongly associated with the prevalence of both CSP (of any size) and AAI: OR of CSP = 2.1, OR of AAI = 2.6, and OR of both CSP and AAI = 3.8 (all P < .001). Investigation of potential confounding factors in healthy controls showed a strong relationship between age and AAI (OR = 3.1; table 2) and a moderate relationship between premorbid or current IQ and CSP (OR = 0.6). Given the strong correlation between premorbid and current IQ (P < .001), only the former was considered. Inclusion of these potential confounding factors in the comparisons between patients and controls did not substantially alter the results. To note, there was no association between CSP and AAI (only controls: χ2 = 0.02, p = .90; only patients: χ2 = 0.30, P = .58; all together: χ2 = 1.4, P = .23).
Table 2.
Prevalence of Midline Brain Abnormalities Across Mental Disorders and Healthy Controls
| CSP (of any size) | AAI | |||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence | OR (95% CI) | P | Prevalence | OR (95% CI) | P | |||
| Potential confounding factors in healthy controls, n = 223 | n | % | N | % | ||||
| Agea (>40 y vs ≤40 y) | 23/73 vs 48/150 | 32 (vs 32) | 1.0 (0.5–1.8) | n.s. | 28/73 vs 25/150 | 38 (vs 17) | 3.1 (1.6–5.9) | <.001b |
| Sex (women vs men) | 34/124 vs 37/99 | 27 (vs 37) | 0.6 (0.4–1.1) | n.s. | 25/124 vs 28/99 | 20 (vs 28) | 0.6 (0.3–1.2) | n.s. |
| University studies vs no university studies | 21/74 vs 39/97 | 28 (vs 40) | 0.6 (0.3–1.1) | n.s. | 16/74 vs 25/97 | 22 (vs 26) | 0.8 (0.4–1.6) | n.s. |
| Premorbid IQa (TAP > 22) | 33/127 vs 21/55 | 26 (vs 38) | 0.6 (0.3–1.1) | .030 | 29/127 vs 14/55 | 23 (vs 25) | 0.9 (0.4–1.8) | n.s. |
| Current IQa, (>100) | 29/119 vs 18/49 | 24 (vs 37) | 0.6 (0.3–1.1) | .042 | 29/119 vs 10/49 | 24 (vs 20) | 1.3 (0.6–2.9) | n.s. |
| (covarying by TAP) | 0.7 (0.3–1.7) | n.s. | ||||||
| BADSa (>100) | 22/92 vs 21/61 | 24 (vs 34) | 0.6 (0.3–1.2) | n.s. | 18/92 vs 17/61 | 20 (vs 28) | 0.6 (0.3–1.4) | n.s. |
| WMSa (>40) | 17/77 vs 21/63 | 22 (vs 33) | 0.6 (0.3–1.2) | n.s. | 15/77 vs 18/63 | 19 (vs 29) | 0.6 (0.3–1.3) | n.s. |
| Patients vs controls and clinical factors (n = 862) | ||||||||
| All patients, n = 639 | 314/639 vs 71/223 | 49 (vs 32) | 2.1 (1.5–2.9) | <.001b | 285/639 vs 53/223 | 45 (vs 24) | 2.6 (1.8–3.7) | <.001b |
| (covarying by TAP | age) | 51 (vs 32) | 2.2 (1.5–3.2) | <.001b | 42 (vs 24) | 2.3 (1.6–3.3) | <.001b | ||
| First episode of psychosis, n = 85 | 48/85 | 56 (vs 32) | 2.8 (1.7–4.7) | <.001b | 36/85 | 42 (vs 24) | 2.4 (1.4–4.0) | .002b |
| (covarying by TAP | age) | 54 (vs 32) | 2.6 (1.4–4.6) | .001b | 57 (vs 24) | 4.2 (2.4–7.4) | <.001b | ||
| First episode of mania, n = 38 | 12/38 | 32 (vs 32) | 1.0 (0.5–2.0) | n.s. | 16/38 | 42 (vs 24) | 2.3 (1.1–4.7) | .020 |
| (covarying by age) | 51 (vs 24) | 3.4 (1.6 − 7.1) | .001b | |||||
| First episode of depression, n = 14 | 8/14 | 57 (vs 32) | 2.9 (1.0–9.0) | n.s. | 9/14 | 64 (vs 24) | 5.8 (1.9–19.5) | .002b |
| (covarying by age) | 58 (vs 24) | 4.3 (1.4–15.4) | .016 | |||||
| Schizophrenia, n = 219 | 103/219 | 47 (vs 32) | 1.9 (1.3–2.8) | .001b | 95/219 | 43 (vs 24) | 2.5 (1.6–3.7) | <.001b |
| (covarying by TAP | age) | 48 (vs 32) | 2.0 (1.3–3.1) | .002b | 38 (vs 24) | 2.0 (1.3–3.1) | .002b | ||
| Delusional disorder, n = 24 | 16/24 | 67 (vs 32) | 4.3 (1.8–11.0) | .001b | 14/24 | 58 (vs 24) | 4.5 (1.9–11.0) | .001b |
| (covarying by TAP | age) | 68 (vs 32) | 4.5 (1.8–12.6) | .002b | 49 (vs 24) | 3.1 (1.2–8.1) | .018 | ||
| Schizoaffective disorder, n = 47 | 19/47 | 40 (vs 32) | 1.5 (0.8–2.8) | n.s. | 19/47 | 40 (vs 24) | 2.2 (1.1–4.2) | .021 |
| (covarying by age) | 34 (vs 24) | 1.7 (0.8–3.3) | n.s. | |||||
| Bipolar disorder, n = 189 | 98/189 | 52 (vs 32) | 2.3 (1.5–3.5) | <.001b | 82/189 | 43 (vs 24) | 2.5 (1.6–3.8) | <.001b |
| (covarying by TAP | age) | 55 (vs 32) | 2.6 (1.7–4.1) | <.001b | 38 (vs 24) | 2.0 (1.3–3.0) | .003b | ||
| Major depressive disorder, n = 23 | 10/23 | 43 (vs 32) | 1.6 (0.7–3.9) | n.s. | 14/23 | 61 (vs 24) | 5.0 (2.1–12.6) | <.001b |
| (covarying by age) | 43 (vs 24) | 2.4 (1.0–6.4) | n.s. | |||||
| Age of onseta (>25 y; covarying by diagnosis) | 102/207 vs 159/321 | 49 (vs 50) | 1.0 (0.7–1.4) | n.s. | 99/207 vs 122/321 | 48 (vs 38) | 1.4 (1.0–2.0) | .020 |
| (covarying by both diagnosis and age) | 0.8 (0.5–1.2) | n.s. | ||||||
| Duration of the diseasea (>15 y; covarying by diagnosis) | 105/215 vs 156/313 | 49 (vs 50) | 1.1 (0.7–1.6) | n.s. | 111/215 vs 110/313 | 52 (vs 35) | 2.5 (1.7–3.8) | <.001b |
| (covarying by both diagnosis and age) | 1.6 (1.0–2.5) | n.s. | ||||||
Note: AAI, absent adhesio interthalamica; CSP, Cavum Septum Pellucidum; n.s., not significant.
aFor descriptive purposes, prevalence of midline brain abnormalities have been calculated after binarizing the age, TAP, IQ, BADS, WMS, age of onset, and duration of the disease, but P values have been calculated using the original continuous variables to avoid a reduction in statistical power.
bStatistical significance after correction for multiple comparisons.
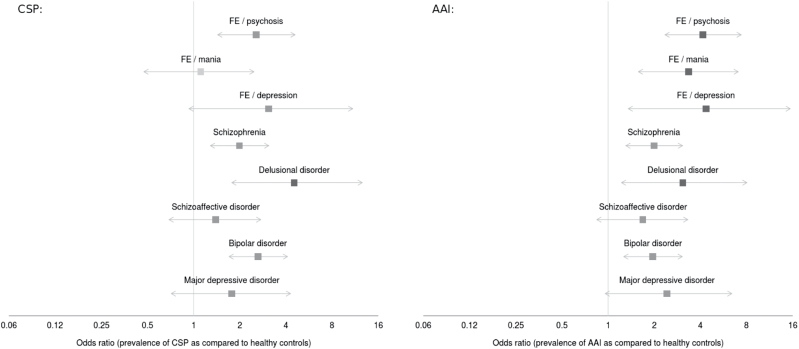
As shown in figure 2 and table 2, the higher prevalence of CSP in patients than in controls could be separately observed for all disorders (except the first episode of mania), with similar results obtained after controlling for premorbid IQ. Specifically, the difference between patients and controls was strong and statistically significant in delusional disorder (OR = 4.3), moderate and statistically significant in first episode of psychosis, schizophrenia and bipolar disorder (OR = 1.9–2.8), and moderate but not reaching statistical significance in the remaining, smaller-sized samples (OR = 1.5–2.0). This higher prevalence could be separately observed in bipolar patients with current Young Mania Rating Scale29 (YMRS) ≥20 (n = 41; OR = 3.9 after controlling by premorbid IQ, P < .001), patients with current Hamilton Rating Scale for Depression30 (HRSD) ≥20 (n = 45, OR = 3.9, P < .001), and patients with both YMRS < 20 and HRSD < 20 (n = 79, OR = 2.3, P = .004); these differences between the so-defined manic/depressed and euthymic patients did not reach statistical significance (P = .026).
Fig. 2.
Odds ratios of the prevalence of cavum septum pellucidum of any size and absent adhesio interthalamica in psychotic and mood disorders. Left: cavum septum pellucidum (CSP; of any size) odds ratios corrected for premorbid IQ (TAP score, see Text). Right: absent adhesio interthalamica (AAI) odds ratios corrected for age. FE: first episode.
Since AAI OR and estimated prevalence substantially changed after controlling for age, only findings using age as covariate are summarized here (see table 2 for complete results). Again, higher prevalence in patients than in controls could be separately observed for all disorders (OR = 2.0–4.2), though statistical significance was only reached for first episode of psychosis or mania and for schizophrenia (and almost reached for bipolar disorder). Again, the higher prevalence could be separately observed in bipolar patients with current Y-MRS ≥ 20 (OR = 2.3; after controlling for age, P = .024), patients with current HRSD ≥ 20 (OR = 2.0, P = .050), and patients with both YMRS < 20 and HRSD < 20 (OR = 1.8, P = .040).
The prevalence of AAI was the same in patients with a chronic disorder than in patients with a first episode (45% vs 45%, χ2 = 0, P = 1). This was striking given that patients with a chronic disorder were substantially older than patients with a first episode (42.1 vs 29.7 years, t = 12, P < .001) and that we had found a strong association between age and prevalence of AAI both in controls and in patients (>40 years OR = 3.1 and 2.6, both P < .001). To further assess these apparently contradictory results we fitted a multiple logistic regression of the presence of AAI as a function of having a disorder, of having the first episode of the disorder, and of age, with the odds ratios of the 3 variables being >1 and statistically significant (P ≤ .002).
No statistically significant differences in the prevalence of midline abnormalities were observed between first episode of psychosis and first episode of mania, between schizophrenia and bipolar disorder, or between psychotic and mood disorders. There were neither statistically significant differences between those patients with a first episode of psychosis who subsequently developed schizophrenia and those who did not.
We further examined the effects of age of onset and duration of the disease for each specific disorder (table 3). There were no statistically significant effects in the prevalence of CSP of any size and age of onset or duration of disease in any of the diagnostic categories. Similarly, we found no significant associations between AAI and age of onset in any specific disorder although there was a moderate but not reaching statistical significance effect between older age of onset and AAI in first episode psychosis (OR = 2.1) and first episode mania (OR = 2.6). On the other hand, there was a moderate and significant effect between the prevalence of AAI and a longer duration of the disease in schizophrenia and bipolar disorder. The lack of statistical power due to small sample sizes prevented these analyses in the first episode depression and delusional disorder groups. Finally, age of onset and duration of illness had no relationship with any midline abnormality after controlling for age.
Table 3.
Effects of the Age of Onset and Duration of the Disease for Each Specific Specific Disorders
| Cavum Septum Pellucidum (of any size) | Absent Adhesio Interthalamica | |||||
|---|---|---|---|---|---|---|
| Prevalence (%) | OR (95% CI) | P | Prevalence | OR (95% CI) | P | |
| Age of onset (>25 y) | ||||||
| All patients (covarying by diagnosis, n = 528) | 49 vs 50 | 1.0 (0.7 − 1.4) | n.s. | 48 vs 38 | 1.4 (1.0 − 2.0) | .020 |
| First episode of psychosis (n = 75) | 66 vs 53 | 1.7 (0.7 − 4.4) | n.s. | 50 vs 33 | 2.1 (0.8 − 5.4) | n.s. |
| First episode of mania (n = 25) | 38 vs 22 | 2.1 (0.4 − 17.3) | n.s. | 56 vs 33 | 2.6 (0.5 − 15.9) | n.s. |
| Schizophrenia (n = 186) | 43 vs 50 | 0.7 (0.4 − 1.4) | n.s. | 38 vs 40 | 0.9 (0.5 − 1.9) | n.s. |
| Schizoaffective disorder (n = 36) | 43 vs 36 | 1.3 (0.3 − 5.2) | n.s. | 29 vs 45 | 0.5 (0.1 − 1.9) | n.s. |
| Bipolar disorder (n = 170) | 48 vs 54 | 0.8 (0.4 − 1.5) | n.s. | 49 vs 36 | 1.7 (0.9 − 3.2) | n.s. |
| Major depressive disorder (n = 21) | 43 vs 29 | 1.9 (0.3 − 16.4) | n.s. | 57 vs 57 | 1.0 (0.1 − 6.4) | n.s. |
| Duration of the disease (>15 y) | ||||||
| All patients (covarying by diagnosis, n = 528) | 49 vs 50 | 1.1 (0.7 − 1.6) | n.s. | 52 vs 35 | 2.5 (1.7 − 3.8) | <.001 |
| Schizophrenia (n = 186) | 50 vs 46 | 1.2 (0.6 − 2.1) | n.s. | 48 vs 28 | 2.4 (1.3 − 4.5) | .008 |
| Schizoaffective disorder (n = 36) | 45 vs 31 | 1.8 (0.5 − 7.5) | n.s. | 50 vs 25 | 3.0 (0.8 − 13.8) | n.s. |
| Bipolar disorder (n = 170) | 50 vs 52 | 0.9 (0.5 − 1.7) | n.s. | 54 vs 32 | 2.5 (1.4 − 4.8) | .002 |
| Major depressive disorder (n = 21) | 27 vs 50 | 0.4 (0.1 − 2.2) | n.s. | 73 vs 40 | 4.0 (0.7 − 28.6) | n.s. |
Note: For descriptive purposes, prevalence of midline brain abnormalities have been calculated after binarizing the age of onset/duration of illness, but P values have been calculated using the original continuous variables to avoid a reduction in statistical power. First episode of depression and delusional disorder are not shown because age of onset/duration of illness was available only in 11 and 4 patients.
The low prevalence of large CSP (80 patients and 14 controls) prevented a robust assessment of group differences, but frequencies seemed also higher in the group of patients (12.5% in patients vs 6.3% in controls, OR = 2.1, P = .012).
Discussion
To our knowledge, this is the first study examining the prevalence of CSP and AAI in a large cross sectional multidiagnostic sample of patients with mood and psychotic disorders using homogeneous MRI methods of acquisition and assessment. In addition, reported results also include first evidence of higher prevalence of these midline brain abnormalities in patients with some specific disorders such as schizoaffective or delusional disorder.
Our findings show that a diagnosis of psychotic or mood disorder is associated with a higher prevalence of CSP and AAI, even after controlling for potential confounding factors such as age or premorbid IQ. Splitting psychotic and mood disorders into discrete DSM-IV diagnoses resulted in moderate to strong associations with CSP in all conditions except in patients with a first manic episode. There were also moderate to strong associations between presence of AAI and any of the studied disorders.
CSP results in psychotic disorders are in agreement with previous studies finding increased prevalence in first episode psychosis31 and schizophrenia32 although several other studies had found no significant differences.4,10,19 In their recent meta-analysis, Trzesniak and colleagues12 attributed these conflicting results to a significant variability in methods, to small sample sizes and to publication bias. They concluded that only a large CSP is associated with psychosis while a small CSP could be considered a normal neuroanatomical variation. Our results, however, show a significantly increased prevalence of CSP of any size in schizophrenia spectrum disorders. In agreement with this meta-analysis,13 our results also suggest a greater prevalence of AAI in schizophrenia spectrum disorders.
The increased prevalence of CSP in bipolar disorder in our study is also supported by previous studies based on large samples and adequate MRI methods.17,18 This is in contrast with the observed relationship between prevalence of CSP (of any size) and major depression, which conflicts with previous studies.19,33 These studies, however, had used small sample sizes and broad diagnostic categories (eg, mixing schizoaffective, bipolar and unipolar affective disorders). Finally, as far as we know, only 1 study has previously examined the prevalence of AAI in bipolar disorder,18 and another one in major depression,20 finding in both cases a shorter adhesio interthalamica but reporting no significant differences in prevalence. It can be argued that in unipolar depression the rather late occurrence of unipolar depression together with its association with dementia may suggest that this disorder, in contrast to the other ones, is not or less neurodevelopmental in origin. As we tested effects between increased prevalence of midline brain abnormalities and later onset of disease or disease severity without significant effects, we add evidence favoring an underlying neurodevelopmental theory also in unipolar depression.
The present study is also the first to report increased prevalence of midline brain abnormalities in pure samples of schizoaffective disorder and delusional disorder patients. Schizoaffective disorder was associated with a moderately increased prevalence of CSP and AAI in relation to healthy controls, and delusional disorder ranked among those disorders with highest prevalence in CSP and AAI. In comparison to other psychoses, both schizoaffective and delusional disorders have been scarcely investigated using brain imaging techniques. However, recent VBM and functional MRI studies have reported similar changes in brain volume and activity in schizophrenia and schizoaffective disorder,23,34–36 supporting the idea that schizoaffective disorder might resemble more schizophrenia than bipolar disorder. The few structural imaging studies that have investigated delusional disorder37 have also shown evidence of convergent brain abnormality in the medial frontal/anterior cingulate cortex and bilateral insula, which are also regions abnormal in schizophrenia.38–41
The findings of the present study are relevant to the discussion on the neurodevelopmental versus neurodegenerative course of psychiatric disorders. The neurodevelopmental models attribute the pathology to alterations in the prenatal-to-early adolescent development while the neurodegenerative models identify progressive neurodegeneration as the core attribute. Abnormal neurodevelopment is present in many children and adolescents who eventually develop psychosis.42 Our findings underline this widely accepted concept and provide strong evidence that this may be also true in a wider array of diagnoses including mood, schizoaffective, and delusional disorders. Early insults to the brain have been argued to produce dynamic alterations rather than static ones in the brain ontogeny.16 Such dynamic processes may explain our remarkable finding of first episode patients presenting with a higher prevalence of AAI than patients in chronic phases when controlling for age effects (ie, for a given age, first episode patients have higher prevalence of AAI than chronic patients). As a potential explanation for such a result, one may speculate on an initial separation of both thalami triggered by the first episode onset that would subsequently stabilize or even revert (to some extent). However, unless replicated, this may very well be considered a spurious result, especially because the effects of age should be taken with caution as they were not found in the meta-analysis by Trzesniak and colleagues.13
In order to resolve whether CSP and AAI abnormalities remain stable over time or are influenced by neurodegeneration or neuroplasticity, future studies should use a longitudinal follow-up of cases ‘at risk’ on the basis of the presence of these midline brain abnormalities, starting in critical ages of the neurodevelopment such as in childhood and adolescence. To complete the diagnostic spectrum, further studies might also include other populations with abnormal neurodevelopmental trajectories such as autism spectrum disorders and attention deficit hyperactivity disorder, and examine the relationship between these midbrain abnormalities and other relevant neighbouring regions such as the thalamic nuclei, the hippocampus and the corpus callosum. Such approaches will need to take into account that a progressive disease inherent component may also play a role in the neuropathology of mental disorders, at least in other brain regions such as the cortex in schizophrenia but also in other diseases.
Strengths of the study are the inclusion of a large cross sectional multidiagnostic sample (639 patients with several mood and psychotic disorders and 223 healthy controls), and the rigorous approach and definitions adopted for assessing the abnormalities, now considered the golden standard.19 Several limitations must be also highlighted. First, even though moderate to strong relationships could be separately observed for nearly all conditions, some of them did not reach statistical significance due to their relatively smaller sample size. Future studies with larger samples of patients with these disorders may confirm our findings. Second, we decided to code brain abnormalities as binary variables (i.e. presence vs. absence), whereas other studies have adopted continuous approaches such as measuring the length of the adhesio interthalamica. Interestingly, these studies observed that patients with psychosis spectrum disorders have shorter adhesio interthalamica than controls.43–45 Third, it must be noted that the prevalence of AAI in our sample is at the very high end of that reported in previous studies.46 This estimated higher prevalence may reflect a true higher prevalence in our sample, but it could also be related to differences in imaging procedures or to our criterion to establish AAI even when it could be identified on only 1 slice. Forth, as stated earlier the relationship between AAI and age found in this paper was not found in the meta-analysis by Trzesniak and colleagues13 Further studies may confirm or reject this specific relationship, but in any case patients showed increased prevalence of AAI independently of the inclusion of age as a covariate. Fifth and as stated before, we could not assess the effects of the lifetime intake of medication as this information was not available. This has to be taken into account because some authors have suggested that antipsychotic medication eg, influences volumetric brain structure.47 Indeed, antipsychotic medications have been related to morphological alterations in hippocampus,48 which could influence measures of CSP,49 and a negative correlation between daily medication dosage and the length of the adhesio interthalamica in chronic schizophrenia patients has been actually reported.46 Furthermore, the significant effect between a higher prevalence of AAI in patients with schizophrenia and bipolar disorder with longer disease duration might be also meditated by the longer duration of the pharmacological treatment.
To sum up, this study supports a general increased prevalence of structural midline brain abnormalities across mood and psychotic disorders. This finding may be relevant as this lack of specificity may suggest that these disorders share a common neurodevelopmental etiology.
Funding
Catalonian Government (2014-SGR-1573 to the Research Unit of FIDMAG) and several grants from the Plan Nacional de I+D+i and co-funded by the Instituto de Salud Carlos III-Subdirección General de Evaluación y Fomento de la Investigación, Plan Nacional 2008 -2011 and 2013 -2016; European Regional Development Fund (FEDER): Stabilization Contract grant (CES12/024 to B.L.A.); Miguel Servet research contracts (CPII13/00018 to R.S., CP10/00596 to E.P.-C., CP14/00041 to J.R.) and Research Projects (PI11/01766 and PI14/00292 to J.R., PI07/1278 and PI10/02622 to B.L.A., PI10/01058 and PI14/01148 to E.P.-C., PI05/1874, PI10/01071, PI14/01151 to R.S.). These funding organizations played no role in the study design, in the collection, analysis and interpretation of the data, in the writing of the manuscript, and in the decision to submit the article for publication.
Acknowledgments
We acknowledge the generous support by the Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Instituto Carlos III, Madrid, Spain and FEDER Fund. We also would like to sincerely acknowledge Dr Tsutomu Takahashi for his helpful comments on an early version of this manuscript. Dr B.L. Amann has served as speaker for Janssen, Lundbeck and Otsuka. Dr Vieta has received grants and served as consultant, advisor or CME speaker for the following entities: AstraZeneca, Bristol-Myers Squibb, Elan, Eli Lilly, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Sunovion, Takeda, Teva, the Spanish Ministry of Science and Innovation (CIBERSAM), the Seventh European Framework Programme (ENBREC), and the Stanley Medical Research Institute. The rest of the authors have declared that there are no conflicts of interest in the last 3 years.
References
- 1. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [DOI] [PubMed] [Google Scholar]
- 2. Shaw CM, Alvord EC., Jr Cava septi pellucidi et vergae: their normal and pathogical states. Brain. 1969;92:213–223. [DOI] [PubMed] [Google Scholar]
- 3. Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. J Comp Neurol. 1968;132:45–72. [DOI] [PubMed] [Google Scholar]
- 4. Nopoulos P, Swayze V, Flaum M, Ehrhardt JC, Yuh WT, Andreasen NC. Cavum septi pellucidi in normals and patients with schizophrenia as detected by magnetic resonance imaging. Biol Psychiatry. 1997;41:1102–1108. [DOI] [PubMed] [Google Scholar]
- 5. Rosales RK, Lemay MJ, Yakovley PI. The development and involution of massa intermedia with regard to age and sex. J Neuropathol Exp Neurol. 1968;27:166. [PubMed] [Google Scholar]
- 6. Samra KA, Cooper IS. Radiology of the massa intermedia. Radiology. 1968;91:1124–1128. [DOI] [PubMed] [Google Scholar]
- 7. Wright P, Takei N, Rifkin L, Murray RM. Maternal influenza, obstetric complications, and schizophrenia. Am J Psychiatry. 1995;152:1714–1720. [DOI] [PubMed] [Google Scholar]
- 8. Rajarethinam R, Miedler J, DeQuardo J, et al. Prevalence of cavum septum pellucidum in schizophrenia studied with MRI. Schizophr Res. 2001;48:201–205. [DOI] [PubMed] [Google Scholar]
- 9. Shioiri T, Oshitani Y, Kato T, et al. Prevalence of cavum septum pellucidum detected by MRI in patients with bipolar disorder, major depression and schizophrenia. Psychol Med. 1996;26:431–434. [DOI] [PubMed] [Google Scholar]
- 10. de Souza Crippa JA, Zuardi AW, Busatto GF, et al. Cavum septum pellucidum and adhesio interthalamica in schizophrenia: an MRI study. Eur Psychiatry. 2006;21:291–299. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi T, Suzuki M, Hagino H, et al. Prevalence of large cavum septi pellucidi and its relation to the medial temporal lobe structures in schizophrenia spectrum. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1235–1241. [DOI] [PubMed] [Google Scholar]
- 12. Trzesniak C, Oliveira IR, Kempton MJ, et al. Are cavum septum pellucidum abnormalities more common in schizophrenia spectrum disorders? A systematic review and meta-analysis. Schizophr Res. 2011;125:1–12. [DOI] [PubMed] [Google Scholar]
- 13. Trzesniak C, Kempton MJ, Busatto GF, et al. Adhesio interthalamica alterations in schizophrenia spectrum disorders: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:877–886. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi T, Nakamura K, Ikeda E, et al. Longitudinal MRI study of the midline brain regions in first-episode schizophrenia. Psychiatry Res. 2013;212:150–153. [DOI] [PubMed] [Google Scholar]
- 15. Trzesniak C, Schaufelberger MS, Duran FL, et al. Longitudinal follow-up of cavum septum pellucidum and adhesio interthalamica alterations in first-episode psychosis: a population-based MRI study. Psychol Med. 2012;42:2523–2534. [DOI] [PubMed] [Google Scholar]
- 16. Arango C, Fraguas D, Parellada M. Differential neurodevelopmental trajectories in patients with early-onset bipolar and schizophrenia disorders. Schizophr Bull. 2014;40(suppl 2):S138–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim MJ, Lyoo IK, Dager SR, et al. The occurrence of cavum septi pellucidi enlargement is increased in bipolar disorder patients. Bipolar Disord. 2007;9:274–280. [DOI] [PubMed] [Google Scholar]
- 18. Takahashi T, Malhi GS, Wood SJ, et al. Midline brain abnormalities in established bipolar affective disorder. J Affect Disord. 2010;122:301–305. [DOI] [PubMed] [Google Scholar]
- 19. Kwon JS, Shenton ME, Hirayasu Y, et al. MRI study of cavum septi pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. Am J Psychiatry. 1998;155:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takahashi T, Yücel M, Lorenzetti V, et al. Midline brain structures in patients with current and remitted major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1058–1063. [DOI] [PubMed] [Google Scholar]
- 21. Pomarol-Clotet E, Canales-Rodríguez EJ, Salvador R, et al. Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Mol Psychiatry. 2010;15:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodríguez-Cano E, Sarró S, Monté GC, et al. Evidence for structural and functional abnormality in the subgenual anterior cingulate cortex in major depressive disorder. Psychol Med. 2014;44:3263–3273. [DOI] [PubMed] [Google Scholar]
- 23. Amann BL, Canales-Rodriguez EJ, Madre M, et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder [published online ahead of print May 13, 2015]. Acta Psychiatr Scand. doi:10.1111/acps.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radua J, Canales-Rodriguez EJ, Pomarol-Clotet E, Salvador R. Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage. 2004;86:81–90. [DOI] [PubMed] [Google Scholar]
- 25. Canales-Rodriguez EJ, Pomarol-Clotet E, Radua J, et al.Structural abnormalities in bipolar euthymia: a multicontrast molecular diffusion imaging study. Biol Psychiatry. 2014;76:239–248. [DOI] [PubMed] [Google Scholar]
- 26. Gomar JJ, Ortiz-Gil J, McKenna PJ, et al. Validation of the Word Accentuation Test (TAP) as a means of estimating premorbid IQ in Spanish speakers. Schizophr Res. 2011;128:175–176. [DOI] [PubMed] [Google Scholar]
- 27. Pereña J, Seisdedos N., Corral S., Arribas D., Santamaría P., Sueiro M. Spanish Adaptation of the Wechsler Memory Scale. Madrid, Spain: TEA Ediciones; 2004. [Google Scholar]
- 28. Vargas ML, Sanz JC, Marín JJ. Behavioral assessment of the dysexecutive syndrome battery (BADS) in schizophrenia: a pilot study in the Spanish population. Cogn Behav Neurol. 2009;22:95–100. [DOI] [PubMed] [Google Scholar]
- 29. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 30. Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41:21–24. [PubMed] [Google Scholar]
- 31. Kasai K, McCarley RW, Salisbury DF, et al. Cavum septi pellucidi in first-episode schizophrenia and first-episode affective psychosis: an MRI study. Schizophr Res. 2004;71:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeLisi LE, Hoff AL, Kushner M, Degreef G. Increased prevalence of cavum septum pellucidum in schizophrenia. Psychiatry Res. 1993;50:193–199. [DOI] [PubMed] [Google Scholar]
- 33. Brisch R, Bernstein HG, Krell D, et al. Volumetric analysis of septal region in schizophrenia and affective disorder. Eur Arch Psychiatry Clin Neurosci. 2007;257:140–148. [DOI] [PubMed] [Google Scholar]
- 34. Madre M, Pomarol-Clotet E, McKenna P, et al. Brain functional abnormality in schizo-affective disorder: an fMRI study. Psychol Med. 2013;43:143–153. [DOI] [PubMed] [Google Scholar]
- 35. Madre M, Radua J, Landin-Romero R, et al. Trait or state? A longitudinal neuropsychological evaluation and fMRI study in schizoaffective disorder. Schizophr Res. 2014;159:458–464. [DOI] [PubMed] [Google Scholar]
- 36. Yuhui D, Jingyu L, Jing S, Hao H, Pearlson GD, Calhoun VD. Exploring Difference and Overlap Between Schizophrenia, Schizoaffective and Bipolar Disorders Using Resting-State Brain Functional Networks. 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL; 26–30 August 2014; 2014:1517–1520. http://embc.embs.org/2014/. [DOI] [PubMed] [Google Scholar]
- 37. Vicens V, Radua J, Salvador R, et al. Structural and functional brain changes in delusional disorder Br J Psychiatry. In press. [DOI] [PubMed] [Google Scholar]
- 38. Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. [DOI] [PubMed] [Google Scholar]
- 39.Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36:2325–2333. [DOI] [PubMed] [Google Scholar]
- 41.Palaniyappan L, Balain V, Radua J, Liddle PF. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr Res. 2012;137:169–173. [DOI] [PubMed] [Google Scholar]
- 42. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shimizu M, Fujiwara H, Hirao K, et al. Structural abnormalities of the adhesio interthalamica and mediodorsal nuclei of the thalamus in schizophrenia. Schizophr Res. 2008;101:331–338. [DOI] [PubMed] [Google Scholar]
- 44. Takahashi T, Suzuki M, Nakamura K, et al. Association between absence of the adhesio interthalamica and amygdala volume in schizophrenia. Psychiatry Res. 2008;162:101–111. [DOI] [PubMed] [Google Scholar]
- 45. Takahashi T, Suzuki M, Zhou SY, et al. Prevalence and length of the adhesio interthalamica in schizophrenia spectrum disorders. Psychiatry Res. 2008;164:90–94. [DOI] [PubMed] [Google Scholar]
- 46. Takahashi T, Yücel M, Yung AR, et al. Adhesio interthalamica in individuals at high-risk for developing psychosis and patients with psychotic disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1708–1714. [DOI] [PubMed] [Google Scholar]
- 47. Chakos MH, Schobel SA, Gu H, et al. Duration of illness and treatment effects on hippocampal volume in male patients with schizophrenia. Br J Psychiatry. 2005;186:26–31. [DOI] [PubMed] [Google Scholar]
- 48. McClure RK, Phillips I, Jazayerli R, Barnett A, Coppola R, Weinberger DR. Regional change in brain morphometry in schizophrenia associated with antipsychotic treatment. Psychiatry Res. 2006;148:121–132. [DOI] [PubMed] [Google Scholar]
- 49. Dickey CC, McCarley RW, Xu ML, et al. MRI abnormalities of the hippocampus and cavum septi pellucidi in females with schizotypal personality disorder. Schizophr Res. 2007;89:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]