Abstract
Background. A dysfunctional differentiation between self-relevant and irrelevant information may affect the perception of environmental stimuli as abnormally salient. The aberrant salience hypothesis assumes that positive symptoms arise from an attribution of salience to irrelevant stimuli accompanied by the feeling of self-relevance. Self-referential processing relies on the activation of cortical midline structures which was demonstrated to be impaired in psychosis. We investigated the neural correlates of self-referential processing, aberrant salience attribution, and the relationship between these 2 measures across the psychosis continuum. Methods. Twenty-nine schizophrenia patients, 24 healthy individuals with subclinical delusional ideation, and 50 healthy individuals participated in this study. Aberrant salience was assessed behaviorally in terms of reaction times to task irrelevant cues. Participants performed a self-reference task during fMRI in which they had to apply neutral trait words to them or to a public figure. The correlation between self-referential processing and aberrant salience attribution was tested. Results. Schizophrenia patients displayed increased aberrant salience attribution compared with healthy controls and individuals with subclinical delusional ideation, while the latter exhibited intermediate aberrant salience scores. In the self-reference task, schizophrenia patients showed reduced activation in the ventromedial prefrontal cortex (vmPFC), but individuals with subclinical delusional ideation did not differ from healthy controls. In schizophrenia patients, vmPFC activation correlated negatively with implicit aberrant salience attribution. Conclusions. Higher aberrant salience attribution in schizophrenia patients is related to reduced vmPFC activation during self-referential judgments suggesting that aberrant relevance coding is reflected in decreased neural self-referential processing as well as in aberrant salience attribution.
Key words: psychosis, salience, vmPFC, self-referential processing, fMRI, psychosis continuum
Introduction
The aberrant salience hypothesis of psychosis1–3 suggests that delusional perception, acoustic hallucinations, and passivity symptoms result from dopamine mediated attribution of salience to certain perceptions and thoughts.4,5 However, attribution of salience to otherwise irrelevant stimuli does not explain why environmental perceptions are imbued with specific meaning centered around the psychotic person or why his/her thoughts appear to be transmitted from the outside or spoken aloud in the environment. Indeed, this failure to self-ascribe thoughts,6 as well as aberrant self-relevance attribution to environmental stimuli6,7 both point to a dysfunction of the self, which can also be observed in transcultural studies of schizophrenia.8
The concept of schizophrenia includes the notion of a disturbed basic sense of self as a core mechanism specific to schizophrenia.9–11 Difficulties in differentiating self-relevant from self-irrelevant information may render the perception of neutral environmental stimuli abnormally salient.2,3 Recently, a direct link between self-disturbances and the experience of aberrant salience has been proposed theoretically.12,13 Self-relevance can be tested with self-referential paradigms that require judgments about oneself and others.14 Meta-analyses show that the cortical midline structures (CMS) are involved in self-referential processing.15–17 The CMS comprise ventromedial and dorsomedial parts of the prefrontal cortex (vmPFC, dmPFC) and the anterior and posterior cingulate cortex.17,18 In self-referential studies, schizophrenia patients show a wide range of altered CMS activation19–25 but no change in self-referential judgments compared with healthy controls in nonpersonalized tasks.19–22,24,25 The most consistently reported hypoactivation in schizophrenia patients during self-referential tasks was found in the medial prefrontal cortex (mPFC).19,21–23,25 The vmPFC has thereby been identified as the core region of self-relevance processing,26–28 whereas the other regions are also engaged in social processing like thinking about other people.15 The vmPFC is claimed to assign personal value to self-related representations and therefore serves as the core region of self-relevance coding during self-reflection.27 More generally, the vmPFC is also involved in the computation of subjective value,29–32 which suggests a functional overlap in this region between self-relevance processing and the assignment of salience. Specifically investigating schizophrenia patients with delusions of reference, 1 study found that patients referred more nonpersonalized information to themselves and displayed a blunted differentiation between self-referred and non-self-referred information in the mPFC.23 This suggests a link between altered neural self-reference processing and aberrant assignment of salience.
According to the aberrant salience hypothesis psychotic symptoms arise from an attribution of salience to irrelevant environmental stimuli imbuing them with meaningfulness and relevance.1–3 A variety of phenomena observed in psychosis such as mismatch negativity33 and latent inhibition34 have been interpreted in this framework.12 In the context of associative learning, heightened attention to nonpredictive or irrelevant cues is taken to reflect aberrant salience. Previous results from reinforcement learning studies show elevated striatal activation to neutral cues in schizophrenia patients.35–39 However, these studies differed considerably in their operationalization of aberrant salience and used reinforcement learning paradigms with highly-reinforced compared with nonreinforced cues. The Salience Attribution Test (SAT) has been developed to specifically assess aberrant salience attribution to task-irrelevant or nonpredictive stimuli on the behavioral level. The SAT measures explicit salience attribution through subjective judgments about reward contingencies but also implicit salience attribution through changes in reaction times (eg, acceleration).40 The measurement of implicit mental processes via reaction times enables access to more automatic levels of processing (cf. Implicit Association Test).41 The SAT has been shown to be valid42 and appropriate for schizophrenia patients, whereby deluded schizophrenia patients exhibit increased explicit aberrant salience compared with patients without delusions.40
Taken together, there is mostly indirect support for aberrant salience attribution5,42–44 and evidence for diminished neural processing of self-relevant stimuli in psychosis.23,25 As both phenomena can be assumed to relate to each other as well as to symptoms of delusion, we intended to directly test these associations. By measuring salience attribution implicitly through reaction times in the SAT and analyzing self-relevance processing at brain level, we aimed at linking implicit cognitive processing to neural processing. A profound understanding of this neurocognitive implementation may substantially advance our knowledge of the formation of delusion even at subclinical thresholds. To further elucidate these processes at the subclinical level, our study includes healthy individuals with subclinical delusional ideation in addition to schizophrenia patients and healthy control participants. We thereby follow the notion of a personality continuum of psychotic experiences to investigate whether individuals with subclinical delusional ideation and schizophrenia patients share underlying neurocognitive mechanisms.45–47 An association between aberrant salience and self-referential processing would then support recent theoretical work12,13 proposing a close link between those 2 phenomena of psychotic experience.
First, we expected increased aberrant salience in schizophrenia patients compared with healthy controls. Second, we hypothesized that schizophrenia patients would show decreased activation in the mPFC during self-referential processing. Following the dimensional approach to psychosis, we expected individuals with subclinical delusional ideation to take an intermediate position compared with healthy controls and schizophrenia patients. Third, heightened levels of aberrant salience attribution were assumed to correlate with lower self-referential activation in the mPFC.
Methods and Material
Participants
In the present study, a total of 50 healthy individuals, 31 schizophrenia patients, and 24 individuals with subclinical delusional ideation were included. Schizophrenia patients fulfilled Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition and ICD-10 criteria for schizophrenia and had no other psychiatric axis I disorder.48 Psychopathological symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS).49 Patients were recruited at the Department of Psychiatry and Psychotherapy (Campus Charité Mitte) of the Charité-Universitätsmedizin Berlin.
Healthy controls were recruited via online advertisement and mailing lists. To recruit an extreme group of individuals with subclinical delusional ideation, 1059 individuals completed the 21-item version of the Peters Delusion Inventory (PDI)47 online (mean total PDI score: 6.75 ± standard deviation: 3.57). Individuals scoring in the fourth quartile with a total PDI score above nine were contacted and those not meeting exclusion criteria were included in the study as individuals with subclinical delusional ideation. Healthy controls and individuals with subclinical delusional ideation had no Axis I disorder and did not report any past event of neurological or psychiatric illness, or past or current substance abuse (assessed by the Structured Clinical Interview for DSM Disorders [SCID-I]).48
All participants completed the Schizotypal Personality Questionnaire (SPQ)50 to obtain general schizotypy. Handedness was assessed with the Edinburgh Handedness Inventory.51 We obtained a neuropsychological test battery including the Trail Making Tests A and B for attention and cognitive flexibility, a vocabulary test for verbal intelligence (Wortschatztest)52 and a digit-span test for working memory. In line with the literature53 schizophrenia patients showed reduced mean scores in all of the neuropsychological measures compared with both of the other groups (table 1 and table S1). Additional analyses of behavioral and MRI data using the neuropsychology as covariates can be found in the supplementary material. Exclusion criteria for all participants were standard contraindications for MRI scanning (eg, metal implants, cardiac pacemakers, or claustrophobia). All participants gave written informed consent to the study and received monetary compensation for study participation. The study was approved by the local Medical Ethics Committee.
Table 1.
Sociodemographic Characteristics of the Imaging Sample (Mean ± SD [Minimum/Maximum]).
| SZ (n = 22) | Individuals With Subclinical Delusional Ideation (PDI; n = 24) | HC (n = 42) | F (df) | P Value | |
|---|---|---|---|---|---|
| Age (y) | 33.50±7.31 (24/52) | 23.54±5.35 (18/40) | 28.21±6.99 (19/54) | 12.78 (2,85) | <.001a |
| Gender | 28 males, 4 females | 16 males, 8 females | 27 males, 15 females | — | .334 |
| Edinburgh Handedness Scale | 64±63.09 (-100/100) | 76.42±40.74 (-79/100) | 76.57±25.72(0/100) | 0.56 (2,63) | .575 |
| Verbal IQ | 98.82±10.39 (75/115) | 105.63±6.65 (85/115) | 104.31±6.56 (85/115) | 4.17 (2,76) | .02b |
| Educational years | 16.03±4.37(10/27) | 15.86±3.32 (12/23) | 16.88±3.04 (13/24) | 0.61 (2,64) | .55 |
| Trail Making Test A | 36.05±13.01 (19/62) | 25.46±7.04 (14/40) | 24.5±8.07 (10/40) | 11.18 (2,78) | <.001c |
| Trail Making Test B | 84.73±57.87 (27/304) | 49.25±12.08 (32/72) | 51.08±17.19 (13/93) | 8.97 (2,79) | <.001d |
| Digit Span | 6.5±2.11 (4/11) | 8.42±2.23 (5/12) | 7.78±2.31 (4/14) | 4.39 (2,80) | .016e |
| PDI total | 8.0±4.39 (1/19) | 12.33±1.99 (10/17) | 2.12±2.02 (0/6) | 105.09 (2,82) | <.001f |
| SPQ total | 30.82±15.2 (6/59) | 23.7±14.67 (5/73) | 9.08±8.722 (0/41) | 16.78 (2,63) | <.001g |
| PANSS total | 74.41±24.22 (33/118) | ||||
| PANSS positive | 19.91±7.79 (7/39) | ||||
| PANSS negative | 18.14±7.31(8/31) | ||||
| PANSS general | 36.36±12.14 (16/56) | ||||
| Age of onset (y) | 25.44±5.51 (18/37) | ||||
| Duration of illness (y) | 6.38±6.1 (0/19) | ||||
| Chlorpromazine equivalent | 384.81±218.16 (0/700) | ||||
| Duration of treatment (mo) | 35.26±38.90 (0/120) |
Note: HC, healthy controls; SZ, schizophrenia patients; SPQ, schizotypal personality questionnaire; PANSS, Positive and Negative Syndrome Scale; PDI, Peters Delusion Inventory.
Post hoc t tests of significant effects reported in the following order: HC compared with PDI, HC compared with SZ and PDI compared with SZ.
a t(64) = 2.83, P = .08; t(62) = 2.83, P = .003; t(44) = 5.3, P < .001.
b t(58) = 0.76, P = .45; t(53) = 2.25, P = .028; t(41) = 2.49; P = .017.
c t(58) = 0.74, P = .34; t(29.15) = 3.68, P = .001; t(29.85) = 3.33, P = .002.
d t(58) = 0.45, P = .65; t(23.29) = 2.66, P = .014; t(22.68) = 2.82, P = .01.
e t(59) = 1.06, P = .29; t(57) = 2.13, P = .038; t (44) = 2.99, P = .05.
f t(62) = 19.7, P < .001; t(24.5) = 5.82, P < .001; t(27.09) = 4.16, P < .001.
g t(34.9) = 4.17; P < .001, t(22.95) = 5.35, P < .001; t(38) = 1.5, P = .143
Most participants underwent both fMRI and SAT experimental sessions of the study (34 healthy controls, 24 individuals with subclinical delusional ideation, 20 schizophrenia patients). Subsamples did not differ in demographics (for sociodemographic characteristics and comparisons of these subsamples please see table S1).
Behavioral Paradigm SAT
The SAT is a reinforcement learning paradigm designed to measure aberrant and adaptive salience attribution implicitly via reaction time differences and explicitly via estimations on a visual analogue scale.40 Here, participants were instructed to increase their wins by rapid responses to a target stimulus that was preceded by conditioned stimuli. The conditioned stimuli varied along 2 dimensions: color and type. During the main experiment, 1 dimension was relevant with 1 reinforced (87.5% reward, eg, blue) and 1 nonreinforced (12.5% reward, eg, red) intradimensional manifestation whereas the other dimension was irrelevant with 2 equally reinforced manifestations (each with 50% reward eg, animals and objects). Twice during the task, participants were instructed to estimate on a visual analogue scale from 0% to 100% how often they thought that each of the 4 stimulus types (red animals, blue animals, red household objects, and blue household objects) had been reinforced. This measured explicit salience attribution, whereas reaction time differences in milliseconds reflected implicit salience attribution. Adaptive salience was measured by the difference between the reinforced and the nonreinforced stimuli of the relevant cue dimension, eg, blue > red. Aberrant salience attribution was measured via the absolute differences between the 2 equally reinforced conditioned stimuli of the irrelevant dimension, eg, |animals-objects|. The relevant and irrelevant dimensions were balanced over groups and their respective intradimensional manifestations were randomized across participants. The whole task consisted of 2 practice runs and 2 main experimental blocks with 64 trials each. For a more detailed description of the paradigm, see the supplementary material.
FMRI Paradigm: Self-Reference Task
To identify self-referential processing during fMRI, participants had to judge whether a neutral personality trait word was applicable to themselves (self), to Angela Merkel (other), or whether the word contained exactly 2 syllables (syllables; figure S2).14 These 3 conditions were presented in 18 alternating blocks. Each block was preceded by a cue indicating the task condition for 3s. During each block, 5 trait words were presented for 3 s each and participants responded to each word by a button press indicating either “yes” or “no”. Between blocks, a fixation cross was presented for 3 s (for depiction of the task see figure S2). Total task duration was approximately 6 min. All presented German trait words were rated as neutral in previous studies.54,55 The paradigm was implemented using the software Presentation (Version 0.70, www.neurobs.com). Analysis and results of behavioral data from the self-reference paradigm are reported in the supplementary material.
Data Analysis
Behavioral Paradigm: SAT
All data were analyzed using SPSS 19 (IBM Corp.). The implicit and explicit measures of aberrant salience (difference between the 2 stimulus manifestations of the task irrelevant dimension) were square root transformed to reduce skew and averaged across blocks. For implicit and explicit aberrant salience, 1-way analyses of variance including age as covariate were performed with group (healthy controls, individuals with subclinical delusional ideation, schizophrenia patients) as between-subject factor. Significance level for all analyses was P < .05 2-tailed. Post hoc analyses of significant effects were performed using t tests (Bonferroni-corrected for multiple comparisons). Correlational analyses with PANSS symptoms scores were performed using Spearman’s rho coefficients in the patient group. Here, we chose this coefficient due to its robustness with regard to extreme values measured by the PANSS. We calculated within-group correlations between implicit and explicit aberrant salience using Pearson’s correlation coefficient. For analyses concerning the adaptive salience measures, see the supplementary material.
MRI Acquisition
Anatomical and functional imaging was acquired using a 3 Tesla Siemens Trio System MRI-Scanner with a 12-channel head coil. Movement was minimized by vacuum pads on each side of the head. Functional images were acquired using T2*-weighted gradient echo planar imaging. Thirty-seven slices per volume were collected in descending order parallel to the AC-PC line (TR: 2250ms, TE: 25ms, flip angle: 80°, voxel size: 3mm3).
FMRI Data Analysis
Functional imaging data were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, Institute of Neurology; http://www.fil.ion.ucl.ac.uk/spm/). After correction for delay in slice-time acquisition, functional images were unwarped using the acquired field maps to correct for inhomogeneity of the magnetic field and its interaction with head movement. The individual anatomical T1 image was coregistered to the individual mean echo planar imaging (EPI) and normalized to the functional MNI template using the unified segmentation approach as implemented in SPM8.56 Spatial normalization parameters were applied to all EPI images and finally, all images were smoothed with a Gaussian kernel of 8mm full width at half maximum. Functional images were analyzed using the general linear model approach as implemented in SPM8. On the single subject level, the 3 conditions self, other, and syllables were modeled. Additionally, the 3 preceding cues were modeled and the 6 movement parameters were included as additional regressors of no interest. The individual contrast images of all 3 conditions were subjected to a second-level random effects model using a flexible factorial ANOVA design with group as the between-subjects factor (healthy controls, individuals with subclinical delusional ideation, and schizophrenia patients) and condition as the within-subjects factor (self, other, and syllables) including subjects as a random factor and age as a covariate. To probe neural correlates of self-referential processing, the t-contrast self > other was assessed combining all 3 groups at P < .05 family-wise error (FWE) whole brain corrected. Group differences were tested with an F-contrast for the interaction between group and the contrast self > other and are reported at P < .05 FWE corrected for the voxels showing a significant task effect (self > other at P < .05 FWE whole brain corrected). For whole brain correction see the supplementary material.
To probe a correlation between measures of aberrant salience attribution and brain activation during self-referential processing, mean parameter estimates were extracted from the peak of the contrast self>other (vmPFC at −9/44/−2) using a 10-mm sphere. Because groups differed in neuropsychological measures and age, we controlled for these differences and calculated within-group partial correlations between the individual implicit aberrant salience scores and vmPFC parameter estimates. In the following, we calculated Fisher’s z tests to test for differences of those correlation coefficients between groups. In an explorative approach, we also tested for a correlation of aberrant salience with other regions showing a significant activation during self > other (insula, middle cingulate cortex, and cerebellum, see table 2) by also using 10-mm spheres for extracting parameter estimates.
Table 2.
Brain Activation for the Contrast Self > Other Across All Participants Reported at P < .05 FWE Corrected for the Whole Brain (Cluster Size > 20)
| Anatomical Region | Cluster Size | MNI-Coordinates | R/L | t(1,170) | P FWE Whole Brain-Corrected | |||
|---|---|---|---|---|---|---|---|---|
| X | y | z | ||||||
| self > other | vmPFC | 4276 | −9 | 44 | −2 | L | 14.85 | <.001 |
| 9 | 41 | −5 | R | 14.78 | <.001 | |||
| 6 | 38 | 13 | R | 10.73 | <.001 | |||
| Middle cingulate cortex | 176 | 0 | −16 | 37 | 7.83 | <.001 | ||
| Insula | 83 | 42 | 11 | −5 | R | 6.23 | <.001 | |
| Cerebellum | 624 | −9 | −61 | 16 | L | 6.71 | <.001 | |
| Cerebellum | 154 | −30 | −64 | −26 | L | 5.76 | <.001 | |
Note: FWE, family-wise error; MNI, Montreal Neurological Institute; vmPFC, ventromedial prefrontal cortex.
Results
Behavioral Paradigm: SAT
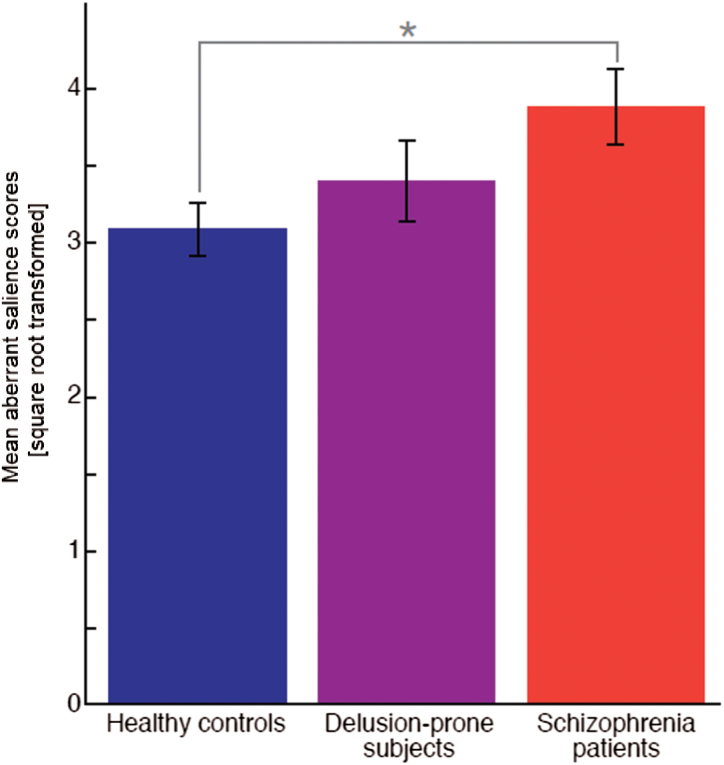
Groups differed in their implicit aberrant salience scores (F[2,99] = 3.521, P = .033). Schizophrenia patients displayed increased implicit aberrant salience scores compared with healthy controls (t[77] = 2.93, P = .012). The individuals with subclinical delusional ideation scored intermediately between patients and healthy controls and thus did not differ significantly from schizophrenia patients (t[47.7] = 2.01, P > .6) or healthy controls (t[72] = 0.801, P > .9; figure 1). Groups did not differ in explicit aberrant salience scores (F[2,99] = 0.809, P > .18). Aberrant salience attribution in patients was not correlated with psychopathology scores from the PANSS (P > .5). Implicit and explicit aberrant salience did not correlate within any of the groups (P > .1). For results concerning adaptive salience attribution, please see the supplementary material.
Fig. 1.
Mean square root transformed aberrant salience scores (± SE) for 50 healthy controls (3.08±0.17), 24 delusion-prone subjects (3.40±0.27), and 29 schizophrenia patients (3.89±0.25).
FMRI—Results: The Self-Reference Task
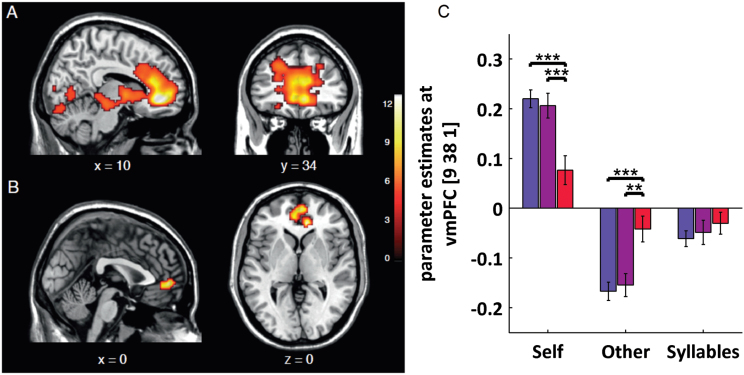
Across all participants, the contrast self > other yielded significant activation in the CMS containing the anterior cingulate cortex, vmPFC, insula, and cerebellum. The global maximum was located in the vmPFC (cluster size = 4276; peak at −9/44/−2; t[1,170] = 14.85, P FWE-corrected for the whole brain < .05; table 2, figure 2A).
Fig. 2.
(A) All participants taken together (n = 88) for the contrast self > other reported at P < .05 FWE corrected for the whole brain. (B) Group by self > other interaction in the right vmPFC (9/38/1, F[2,170] = 13.73, P FWE for self > other = .008) and left vmPFC (−3/50/−2, F[2,170] = 11.71, P FWE for self > other = .036; displayed at .001 uncorrected k > 50). (C) Parameter estimates in right vmPFC (at 9/38/1) for self, other, and syllables condition. For self, schizophrenia patients (red) showed decreased activation compared with healthy controls (blue; t[62] = 4.701, P < .001) and to individuals with subclinical delusional ideation (purple; t[44] = 3.65, P > .001). For other, schizophrenia patients displayed increased activation compared with healthy controls (t[62] = 3.95, P < .001) and compared with individuals with subclinical delusional ideation (t[44] = 3.26, P = .002). There were no differences between healthy controls and individuals with subclinical delusional ideation for self or other (P > .6) and no group differences for syllables (P > .2). Error bars show the standard error of the mean. Order of bars for each condition in panel C: healthy controls, individuals with subclinical delusional ideation, schizophrenia patients. (Note: For colour interpretation, please see figure online.)
Testing for group differences, we found a significant group by self>other interaction in the vmPFC bilaterally (9/38/1, F[2,170] = 13.73, P FWE for self > other = .008; 0/53/−1, F[2,170] = 12.2, P = .025; −3/50−2, F[2,170] = 11.71, P FWE for self > other = .036). Post hoc t tests revealed that this finding was due to reduced activation in schizophrenia patients compared with healthy controls (9/38/1, t[1,170] = 5.14, P FWE for self > other = .001; −6/47/−2, t[1,170] = 4.61, P FWE for self > other = .008) and individuals with subclinical delusional ideation (3/56/1, t[1,170] = 4.54, p FWE for self > other = .011; 9/41/−2, t[1,170] = 4.39, P FWE for self > other = .019). There were no differences between individuals with subclinical delusional ideation and healthy controls (t[1,170] = 3.19; P FWE for self > other > .5; figure 2B and 2C). For self, schizophrenia patients showed decreased vmPFC activation compared with healthy controls (t[62] = 4.701, P < .001) and to individuals with subclinical delusional ideation (t[44] = 3.65, P > .001). For other, schizophrenia patients displayed less deactivation compared with healthy controls (t[62] = 3.95, P < .001) and compared with individuals with subclinical delusional ideation (t[44] = 3.26, P = .002).
FMRI—The Relationship Between Self-Reference and Aberrant Salience Processing
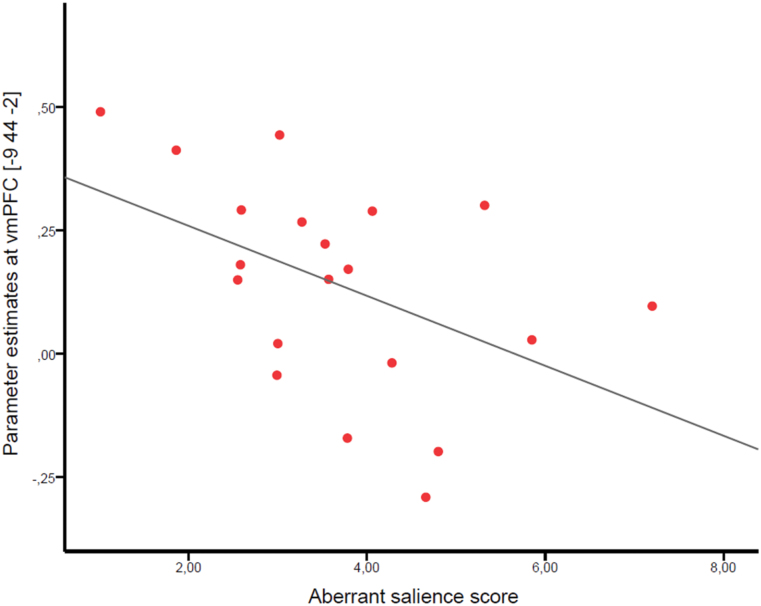
Within-group, we tested for a relationship between self-referential processing in the vmPFC (10-mm sphere around the peak at −9/44/−2) and aberrant salience attribution while controlling for differences in age and neuropsychology. There was a statistically significant correlation only in the schizophrenia patients (r[10] = −0.602, P = .038; figure 3). No significant correlations in individuals with subclinical delusional ideation (r[17] = −0.038, P > .8) or healthy controls (r[25] = 0.247, P > .2) were observed. No significant correlations were found between aberrant salience and other regions showing a significant task effect (supplementary material).
Fig. 3.
The correlation between reduced activation in the vmPFC for the contrast self> other (10-mm sphere around peak of the contrast at −9/44/−2) and the aberrant salience score in schizophrenia patients (n = 20, r[1,18] = −0.470, P = .037, R 2 = 0.221).
The difference in correlation coefficients between healthy controls and schizophrenia patients was statistically significant (z = −2.946, P = .02). There was also a significant difference in correlation coefficients between individuals with subclinical delusional ideation and schizophrenia patients (z = −1.908, P = .028). The correlation coefficients did not statistically differ between healthy controls and individuals with subclinical delusional ideation (z = −1.026, P > .1).
Discussion
In the present study, we investigated aberrant salience attribution and neural correlates of self-referential processing in schizophrenia patients, individuals with subclinical delusional ideation, and healthy controls. We report 3 key findings: first, schizophrenia patients showed increased aberrant salience attribution compared with healthy controls, with individuals with subclinical delusional ideation taking an intermediate position; second, patients displayed decreased activation during self-referential processing in the vmPFC compared with both healthy controls and individuals with subclinical delusional ideation; third, the activation in this region correlated negatively with aberrant salience in schizophrenia patients. These findings support the relevance of altered self-referential processing6,7 and aberrant salience attribution in schizophrenia.2,3
Heightened Aberrant Salience in Psychosis
To our knowledge, this is the first study to show heightened implicit aberrant salience in schizophrenia patients compared with healthy controls using a reaction time measurement taken from the SAT, an operationalization of aberrant salience attribution to irrelevant stimuli.40 Patients speeded up their reaction times following 1 out of 2 equally task-irrelevant stimulus manifestations. This result supports the aberrant salience hypothesis of schizophrenia.2,3 So far, the previous study investigating the SAT in schizophrenia patients found higher explicit aberrant salience in patients with delusions but did not report overall between-group differences either in implicit or in explicit aberrant salience measures.40 This might be due to differences in sample characteristics as indicated by higher positive symptoms in our sample: our patient sample displayed higher severity ratings for positive symptoms (19.9 compared with 14.8, respectively, using a formula specifically developed to compare Scale for the Assessment of Positive Symptoms and PANSS).57 Further, we found that individuals with subclinical delusional ideation displayed intermediate aberrant salience scores between schizophrenia patients and healthy controls. Albeit not statistically significant, this dimensional pattern is in accordance with previous studies investigating decision-making tasks (such as jumping to conclusions) across the psychosis continuum.58,59
It is important to note that we did not observe a significant correlation between aberrant salience measures and positive symptoms. This might be due to our cross-sectional design, where such correlations can be blurred due to multiple factors that develop at different trajectories in the course of the disorder, such as the appraisal of aberrant salience experience influenced by factors like reasoning bias, metacognition and social inclusion.60 Thus, the proposed association between aberrant salience and delusions may be more obvious at the beginning of the disorder, in a state that has been described as “delusional mood” and that is later followed by cognitive explanation models forming the actual delusions.2,3 Nevertheless, our finding of heightened aberrant salience in a rather stable and chronic schizophrenia sample suggests that aberrant salience is a continuing core feature of the disorder.
With respect to delusion-like ratings, schizophrenia patients displayed lower PDI scores compared with individuals with subclinical delusional ideation. Although the PDI was designed for healthy samples,61 this pattern suggests that a mere high delusion-like experience as in our high-functioning, high PDI sample, does not imply clinical impairment. In line with that, psychotic experiences occur in 7% of the general population46 but only very few of them seek clinical help and will meet complete diagnostic criteria for schizophrenia later in life,62,63 which is one important difference from high-risk individuals. Interestingly, we found decreased implicit adaptive salience attribution in schizophrenia patients as well as in individuals with subclinical delusional ideation. This may reflect a motivational deficit in reinforcement learning as Roiser and colleagues reported similar results,64 which is also in line with previous studies using other learning tasks.38,65
Decreased Activation in the vmPFC During Self-Referential Processing in Schizophrenia Patients
We found that schizophrenia patients showed reduced activation compared with healthy controls and individuals with subclinical delusional ideation in the vmPFC during self-referential processing. The finding of hypoactivation in anterior regions of the cortical midline structures in schizophrenia patients is consistent with previous studies on self-reference.19,21–23 Those findings varied with regard to task designs—especially the used control condition and the reported contrasts.19–25,66 We chose to investigate the contrast self > other due to its specificity to self-referential processing, because “thinking about myself” activates a similar neural pattern as “thinking about another person”.16 Moreover, we found that individuals with subclinical delusional ideation did not differ significantly from healthy controls in brain activation during self-referential processing. Healthy siblings of schizophrenia patients displayed no regional activation alterations for the contrast self > control compared with controls without genetic risk but heightened functional connectivity within the default mode network;67 the latter finding of hyperconnectivity in the default network was also observed in schizophrenia patients.68 Two previous studies found heightened PFC activation associated with psychosis proneness69 and positive schizotypy70 for the contrast self > control. These inconsistencies might be due to addressing social cognition instead of the self-referential processing that we decided to investigate.
In a study investigating self-referential processing in healthy individuals, vmPFC activation increased with higher subjective importance attached to the self-referred trait word.71 Thus, the vmPFC is conceptualized to assign personal value to self-related representations and therefore serves as the core region of self-relevance coding during self-reflection.27,72
Aberrant Salience Attribution Is Related to Reduced vmPFC Activation
We tested the hypothesis that self-referential processing and aberrant salience are related, more specifically that reduced activation in the self-reference network is correlated with heightened aberrant salience attribution. We found that in schizophrenia patients reduced vmPFC was associated with higher levels of aberrant salience attribution.
The vmPFC has been conceptualized as the core region of self-relevance coding27,72,73 and salience attribution during self-referential processing.23,26 In line with our finding, Menon and colleagues23 reported that schizophrenia patients felt that more neutral sentences were written specifically about them when compared with controls and showed reduced differences between self-referred and non-self-referred sentences in the mPFC. This resonates well with our finding of a negative correlation between aberrant salience attribution and blunted self-referential processing in the vmPFC in patients.
A disturbed sense of self-relevance with a blurred distinction between self-referencing and other referencing may affect aberrant salience attribution by assigning personal values to neutral events. Due to our correlational design, the causal direction of the observed effect cannot be inferred. However, in our opinion, it is conceivable that both phenomena are related to 1 core underlying process.12,13 In line with this, a recent finding suggests that the vmPFC codes reward values relevant for the current decision independent of whether the decision is carried out for oneself or another person.32 Transferring this to self-referential processing, vmPFC activation in healthy individuals elicited by judgments about oneself compared with another person could be driven by higher relevance attribution during self-reference processing. In schizophrenia patients, the blunted vmPFC activation might therefore indicate a disturbed attribution of relevance present in self-referential judgments as well as in learning environmental contingencies during the SAT.
The observed association between the 2 measures was only found in the schizophrenia patient group and was not explained by cognitive measures. Although aberrant salience measures were numerically increased in the individuals with subclinical delusional ideation, we did not observe an association with self-referential activation in the vmPFC. This suggests a specific role of altered relevance coding to schizophrenia patients.
Limitations
Several limitations of our article need to be addressed. First, most schizophrenia patients in our sample were medicated, potentially confounding group differences. Although this increases the ecological validity of our patient sample, studies in unmedicated patients are warranted. Nevertheless, because antipsychotic medication should rather act in reducing aberrant salience via its effect on positive symptoms, our finding of elevated aberrant salience in patients is unlikely to be due to medication effects. Controlling for Chlorpromazine-equivalent dosage did not change our results (supplementary material). Second, unlike Menon and colleagues,23 we did not find behavioral group differences in self-referential judgments. However, our behavioral results are in line with other studies using similar nonpersonalized task designs.19–22,24,25,66 Third, while the association between aberrant salience and vmPFC activation during self-referential processing seems to be specific for schizophrenia patients, comparable associations might be present in different brain regions in healthy individuals. Fourth, our measure of aberrant salience was derived from a probabilistic learning task, however no correlation between contingency ratings and our measure of implicit aberrant salience was observed in schizophrenia patients (P > .3); controlling for neuropsychological measures also did not alter our findings. Taken together, this suggests that our measure of aberrant salience goes beyond deficits in detecting contingencies. Finally, we have to caution that aberrant salience and the accompanying heightened sense of self to irrelevant stimuli are complex constructs with idiosyncratic aspects and future studies should therefore use multiple paradigms to cover their various facets.43
Conclusion
Our findings provide experimental evidence for the hypothesis of aberrant salience attribution1–3 and dysfunction of the self1,6–11 in schizophrenia. We demonstrate reduced neural self-referential processing in the vmPFC, the core region of self-relevance coding, which was associated with increased aberrant salience attribution in schizophrenia patients. The correlation between reduced vmPFC activation and aberrant salience in schizophrenia patients indicates disturbed attribution of relevance during self-reflection, as well as during detecting environmental contingencies.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
German Research Foundation (DFG SCHL1969/1-1, DFG SCHL 1969/2-2 to F.S., DFG GRK1123-2 to R.B.); Max Planck Society (to L.D. F.S.); Elsa Neumann Scholarship of the city of Berlin (to T.K.); German Federal Ministry of Education and Research (01GQ0411, 01QG87164, NGFN Plus 01 GS 08152, 01 GS 08159 to A.H.); Gerhard C. Starck Foundation and Konrad Adenauer Foundation (to S.D.); M.G. reports 2 travel grants from GlaxoSmithKline Foundation, outside the submitted work.
Supplementary Material
Acknowledgments
We thank Saineb Alaa-Eddine, Yu Fukuda, Jakob Kaminski, Judith K. Daniels, and Jan-Peter Lamke for help during data acquisition and Stefan Koch for task implementation. Furthermore, we thank Leila Shayegan for proof reading. All authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1. Gray JA. Integrating schizophrenia. Schizophr Bull. 1998;24:249–266. [DOI] [PubMed] [Google Scholar]
- 2. Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia--psychopathological and behavioral correlates. Eur Psychiatry. 2002;17:9–16. [DOI] [PubMed] [Google Scholar]
- 3. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 4. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gray DM. Failing to self-ascribe thought and motion: towards a three-factor account of passivity symptoms in schizophrenia. Schizophr Res. 2014;152:28–32. [DOI] [PubMed] [Google Scholar]
- 7. Mishara AL, Lysaker PH, Schwartz MA. Self-disturbances in schizophrenia: history, phenomenology, and relevant findings from research on metacognition. Schizophr Bull. 2014;40:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinz A, Bermpohl F, Frank M. Construction and interpretation of self-related function and dysfunction in Intercultural Psychiatry. Eur Psychiatry. 2012;27(suppl 2):S32–S43. [DOI] [PubMed] [Google Scholar]
- 9. Kraepelin E. Klinische Psychiatrie, Teil 2. Vol 8 Leipzig, Germany: Verlag von Johann Ambrosius Barth; 1913. [Google Scholar]
- 10. Bleuler E. Dementia praecox oder Gruppe der Schizophrenien. Leipzig, Germany: Franz Deuticke; 1911. [Google Scholar]
- 11. Berze J. Die primäre Insuffizienz der psychischen Aktivität: Ihr Wesen, ihre Erscheinungen und ihre Bedeutung als Grundstörung der Dementia praecox und der Hypophrenien überhaupt. Leipzig, Germany: Franz Deuticke; 1914. [Google Scholar]
- 12. Nelson B, Whitford TJ, Lavoie S, Sass LA. What are the neurocognitive correlates of basic self-disturbance in schizophrenia? Integrating phenomenology and neurocognition. Part 2 (Aberrant salience). Schizophr Res. 2014;152:20–27. [DOI] [PubMed] [Google Scholar]
- 13. Nelson B, Whitford TJ, Lavoie S, Sass LA. What are the neurocognitive correlates of basic self-disturbance in schizophrenia? Integrating phenomenology and neurocognition. Part 1 (Source monitoring deficits). Schizophr Res. 2014;152:12–19. [DOI] [PubMed] [Google Scholar]
- 14. Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. [DOI] [PubMed] [Google Scholar]
- 15. van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34:935–946. [DOI] [PubMed] [Google Scholar]
- 16. Murray RJ, Schaer M, Debbane M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev. 2012;36:1043–1059. [DOI] [PubMed] [Google Scholar]
- 17. Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. [DOI] [PubMed] [Google Scholar]
- 18. van Buuren M, Gladwin TE, Zandbelt BB, Kahn RS, Vink M. Reduced functional coupling in the default-mode network during self-referential processing. Hum Brain Mapp. 2010;31:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holt DJ, Cassidy BS, Andrews-Hanna JR, et al. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011;69:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shad MU, Keshavan MS, Steinberg JL, et al. Neurobiology of self-awareness in schizophrenia: an fMRI study. Schizophr Res. 2012;138:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blackwood NJ, Bentall RP, Ffytche DH, Simmons A, Murray RM, Howard RJ. Persecutory delusions and the determination of self-relevance: an fMRI investigation. Psychol Med. 2004;34:591–596. [DOI] [PubMed] [Google Scholar]
- 22. Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC Psychiatry. 2012;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Menon M, Schmitz TW, Anderson AK, et al. Exploring the neural correlates of delusions of reference. Biol Psychiatry. 2011;70:1127–1133. [DOI] [PubMed] [Google Scholar]
- 24. van der Meer L, de Vos AE, Stiekema AP, et al. Insight in schizophrenia: involvement of self-reflection networks? Schizophr Bull. 2013;39:1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pauly KD, Kircher TT, Schneider F, Habel U. Me, myself and I: temporal dysfunctions during self-evaluation in patients with schizophrenia. Soc Cogn Affect Neurosci. 2014;9:1779–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev. 2007;31:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front Hum Neurosci. 2013;7:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang L, Metzak PD, Woodward TS. Aberrant connectivity during self-other source monitoring in schizophrenia. Schizophr Res. 2011;125 136–142. [DOI] [PubMed] [Google Scholar]
- 29. Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 2014;9:1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barron HC, Dolan RJ, Behrens TE. Online evaluation of novel choices by simultaneous representation of multiple memories. Nat Neurosci. 2013;16:1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicolle A, Klein-Flugge MC, Hunt LT, Vlaev I, Dolan RJ, Behrens TE. An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron. 2012;75:1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaebler AJ, Mathiak K, Koten JW, Jr, et al. Auditory mismatch impairments are characterized by core neural dysfunctions in schizophrenia. Brain. 2015;138:1410–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gray N, Hemsley D, Gray J. Abolition of latent inhibition in acute, but not chronic, schizophrenics. Neurol Psychol Brain Res. 1992;1:83–89. [Google Scholar]
- 35. Romaniuk L, Honey GD, King JR, et al. Midbrain activation during Pavlovian conditioning and delusional symptoms in schizophrenia. Arch Gen Psychiatry. 2010;67:1246–1254. [DOI] [PubMed] [Google Scholar]
- 36. Diaconescu AO, Jensen J, Wang H, et al. Aberrant effective connectivity in schizophrenia patients during appetitive conditioning. Front Hum Neurosci. 2011;4:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jensen J, Willeit M, Zipursky RB, et al. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–479. [DOI] [PubMed] [Google Scholar]
- 38. Schlagenhauf F, Huys QJ, Deserno L, et al. Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. Neuroimage. 2014;89:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schlagenhauf F, Sterzer P, Schmack K, et al. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009;65:1032–1039. [DOI] [PubMed] [Google Scholar]
- 40. Roiser JP, Stephan KE, den Ouden HE, Barnes TR, Friston KJ, Joyce EM. Do patients with schizophrenia exhibit aberrant salience? Psychol Med. 2009;39:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74:1464–1480. [DOI] [PubMed] [Google Scholar]
- 42. Schmidt K, Roiser JP. Assessing the construct validity of aberrant salience. Front Behav Neurosci. 2009;3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Winton-Brown TT, Fusar-Poli P, Ungless MA, Howes OD. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014;37:85–94. [DOI] [PubMed] [Google Scholar]
- 44. Jensen J, Walter H. Incentive motivational salience and the human brain. Restor Neurol Neurosci. 2014;32:141–147. [DOI] [PubMed] [Google Scholar]
- 45. Johns LC, van Os J. The continuity of psychotic experiences in the general population. Clin Psychol Rev. 2001;21:1125–1141. [DOI] [PubMed] [Google Scholar]
- 46. van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. [DOI] [PubMed] [Google Scholar]
- 47. Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI). Schizophr Bull. 2004;30:1005–1022. [DOI] [PubMed] [Google Scholar]
- 48. First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. Washington, DC: American Psychiatric Publishing; 2012. [Google Scholar]
- 49. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 50. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 51. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 52. Schmidt K-H, Metzler P. Wortschatztest (WST). Weinheim, Germany: Beltz; 1992. [Google Scholar]
- 53. Bora E. Developmental lag and course of cognitive deficits from the premorbid to postonset period in schizophrenia. Am J Psychiatry. 2014;171:369. [DOI] [PubMed] [Google Scholar]
- 54. Gruhn D, Smith J. Characteristics for 200 words rated by young and older adults: age-dependent evaluations of German adjectives (AGE). Behav Res Methods. 2008;40:1088–1097. [DOI] [PubMed] [Google Scholar]
- 55. Vo ML, Conrad M, Kuchinke L, Urton K, Hofmann MJ, Jacobs AM. The Berlin Affective Word List Reloaded (BAWL-R). Behav Res Methods. 2009;41:534–538. [DOI] [PubMed] [Google Scholar]
- 56. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 57. van Erp TG, Preda A, Nguyen D, et al. Converting positive and negative symptom scores between PANSS and SAPS/SANS. Schizophr Res. 2014;152:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Balzan R, Delfabbro P, Galletly C, Woodward T. Confirmation biases across the psychosis continuum: the contribution of hypersalient evidence-hypothesis matches. Br J Clin Psychol. 2013;52:53–69. [DOI] [PubMed] [Google Scholar]
- 59. Balzan RP, Delfabbro PH, Galletly CA, Woodward TS. Illusory correlations and control across the psychosis continuum: the contribution of hypersalient evidence-hypothesis matches. J Nerv Ment Dis. 2013;201:319–327. [DOI] [PubMed] [Google Scholar]
- 60. Kuipers E, Garety P, Fowler D, Freeman D, Dunn G, Bebbington P. Cognitive, emotional, and social processes in psychosis: refining cognitive behavioral therapy for persistent positive symptoms. Schizophr Bull. 2006;32(suppl 1):S24–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peters ER, Joseph SA, Garety PA. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. Delusions Inventory). Schizophr Bull. 1999;25:553–576. [DOI] [PubMed] [Google Scholar]
- 62. Bak M, Delespaul P, Hanssen M, de Graaf R, Vollebergh W, van Os J. How false are “false” positive psychotic symptoms? Schizophr Res. 2003;62:187–189. [DOI] [PubMed] [Google Scholar]
- 63. Schultze-Lutter F, Michel C, Ruhrmann S, Schimmelmann BG. Prevalence and clinical significance of DSM-5-attenuated psychosis syndrome in adolescents and young adults in the general population: the Bern Epidemiological At-Risk (BEAR) study. Schizophr Bull. 2014;40:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull. 2013;39:1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Murray GK, Corlett PR, Clark L, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Murphy ER, Brent BK, Benton M, et al. Differential processing of metacognitive evaluation and the neural circuitry of the self and others in schizophrenia: a pilot study. Schizophr Res. 2010;116:252–258. [DOI] [PubMed] [Google Scholar]
- 67. van Buuren M, Vink M, Kahn RS. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr Res. 2012;142:237–243. [DOI] [PubMed] [Google Scholar]
- 68. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Modinos G, Renken R, Ormel J, Aleman A. Self-reflection and the psychosis-prone brain: an fMRI study. Neuropsychology. 2011;25:295–305. [DOI] [PubMed] [Google Scholar]
- 70. Debbané M, Vrtička P, Lazouret M, Badoud D, Sander D, Eliez S. Self-reflection and positive schizotypy in the adolescent brain. Schizophr Res. 2014;152:65–72. [DOI] [PubMed] [Google Scholar]
- 71. D’Argembeau A, Jedidi H, Balteau E, Bahri M, Phillips C, Salmon E. Valuing one’s self: medial prefrontal involvement in epistemic and emotive investments in self-views. Cereb Cortex. 2012;22:659–667. [DOI] [PubMed] [Google Scholar]
- 72. Abraham A. The world according to me: personal relevance and the medial prefrontal cortex. Front Hum Neurosci. 2013;7:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.