Abstract
While cognitive ability is inversely associated with risk for schizophrenia (SZ), the association is less clear with other nonaffective psychoses (ONAP) and bipolar illness (BPI). Using national Swedish hospital registry data, we examined the prospective relationship between school achievement (SA) and development of SZ, ONAP, and BPI in 1800643 adolescents born 1972–1990. We used Cox proportional hazard and co-relative control models to predict onset of SZ, ONAP, and BPI from standardized SA scores at age 16. The hazard ratio (HRs; and 95% CIs) for first onset of SZ as a function of SA was 0.66 (0.64–0.68) for both sexes. For ONAP, the HRs equaled 0.66 (0.64–0.68) for males and 0.72 (0.70–0.75) for females. For BPI, parallel HRs were 0.81 (0.78–0.84) and 0.71 (0.70–0.73). The association between SA and risk was stronger in the lower vs the higher ranges of SA. In most analyses, moderate increases in risk were observed at the highest levels of SA, with the strongest evidence for females and risk of ONAP. Co-relative control analyses indicated that common genetic or familial-environmental effects only marginally confounded these associations. Consistent with prior studies, these results have 3 major implications for neurodevelopmental models: (1) adolescent cognitive deficits that increase risk are not the result of prodromal changes,( 2) individual specific environmental exposures are largely responsible for the association between low SA and psychosis risk, and (3) neurodevelopmental disturbances (as indicated by low SA) are not unique to SZ but also occur in ONAP and to a lesser degree BPI.
Key words: Cognitive ability, school performance, schizophrenia, bipolar illness, other nonaffective psychoses, prospective risk, co-relative control analysis
A series of meta-analyses have convincingly shown that cognitive ability is inversely related to risk for future schizophrenia (SZ) onsets1–4 although a question remains whether this relationship holds at the highest levels of cognitive performance.3,5,6
The association between cognitive functioning and risk for bipolar illness (BPI) is less consistent,7–10 with some evidence that higher cognitive functioning is associated with elevated risk.10,11 The relationship between cognitive performance and risk for other nonaffective psychosis (ONAP) has been less studied but extant research suggests that it resembles the relationship seen for SZ.9,12
In our prior study of cognitive ability indexed by IQ, IQ obtained at military conscription at age 18–20 had a strong and monotonic inverse relationship with risk for onset of SZ in Swedish males.6 Co-relative analyses suggested that the IQ-SZ relationship was not due to genetic or environmental familial risk factors that predisposed both to low IQ and SZ. While some prior reports, including a Finnish longitudinal study, found enrichment for cases of SZ among those with the highest level of cognitive functioning (indicating an inverted J-shaped relationship between performance and SZ risk),3,5 we found no evidence that high IQ is associated with increased risk for SZ. However, our study was unable to investigate this relationship at the highest end of the IQ distribution because the highest performing group in our study had a mean IQ of 129, slightly less than 2 standard deviations above the mean.
In the present study, we had access to a measure of aggregate school achievement (SA) at age 16 in a nearly complete sample of native Swedish children of both sexes born between 1972 and 1990 (n = 1800643). While substantially correlated, SA is not identical to IQ and has differential predictors including work habits, personality, and sociability.13 Our SA data includes a broader range of scores at the upper range of achievement than was available with the IQ data. In this sample, we attempted to extend and replicate our prior findings. We seek to address 3 major questions:
1. What is the relationship between SA and subsequent risk for SZ? Is there an inverse relationship between SA and SZ across the entire range, or is there an increased risk in those with very high achievement? Do these relationships differ in males vs females?
2. Is the association between SA, and ONAP and BPI similar to that seen for SZ?
3. Using co-relative analyses, can we clarify the degree to which the associations between SA and risk for SZ, ONAP, and BPI result from shared familial risk factors?
Methods
Sample
We used data from multiple Swedish nationwide registries linked by the unique individual Swedish 10-digit personal ID number assigned at birth or immigration to all Swedish residents. This ID number was replaced by a random number to preserve confidentiality. Our database was created by merging the following sources: the Multi-Generation Register, providing information on family relations; Swedish Hospital Discharge Register, containing all hospitalizations for Swedish inhabitants from 1964–2012; the Swedish Mortality Register, containing causes of death; and the National School Register, which contained information on educational achievement and performance. The database began with all individuals who were born in Sweden between 1972 and 1990, and had not died or migrated prior to the age of 16 (N = 1861934). 1800643 of these were registered in the National School Register and included in the analyses.
Measures
The National School Registry contained educational achievement (a grade point average) for all students at the end of grade 9 (usually at age 16) from 1988 to 2012. Students had an incentive to perform well in this school year because those with a high grades were more likely to gain admission to the desirable upper secondary schools.14 For each year and by gender we standardized the grade score into a Z score with mean 0 and SD 1. We call this variable school achievement (SA). From 1988 to 1997 the score was expressed on a scale between 1 (lowest) and 5 (overall mean was 3.2), and students were assessed by a peer referencing system.15 Grades awarded reflected the position of the student within Sweden and a set of correction factors were applied to ensure that the grades were equivalent between schools. Using this system, the grades had minimal grade inflation over time and were normally distributed. From 1998 and onwards the score was expressed on scale between 10 (lowest) and 320 (overall mean was 207) utilizing a criterion referenced system, in which students were assessed for their achievement of certain competencies.15 Scores were not standardized across schools or constructed to produce a normal distribution. Unlike the earlier system, this approach was vulnerable to grade inflation.
Schizophrenia (SZ) was defined in the Swedish Hospital Discharge Register by the following ICD codes: ICD9: 295B, 295C, 295D, 295G, and 295F and ICD10: F20.0, F20.1, F20.2, F20.3, F20.5, F20.8, and F20.9. Other Nonaffective psychosis (ONAP) was defined according to the following ICD codes: ICD9: all 295 categories excluding those meeting criteria for SZ, 297, 298C, 298E, 298W, 298X and ICD10: F2 excluding cases meeting criteria for SZ. Bipolar illness (BPI) was defined by the following ICD codes: ICD9: 296A, 296B, 296E, 296F, 296G, 296H, and 296W and ICD10: F31 and F30. We applied a lifetime hierarchical diagnostic approach so that individuals diagnosed at ONAP could never have received a diagnosis of SZ and individuals categorized as BPI could never have received a diagnosis of SZ or ONAP. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University.
Analysis
We investigated the relationship between SA and each diagnostic outcome separately. We excluded cases with onset prior to their age at which SA was assessed (SZ n = 192; ONAP n = 359; BP n = 237). We used Cox proportional hazards models to investigate the risk for future episodes of SZ, ONAP or BPI as a function of their SA in adolescence. Robust standard errors were used to account for the clustering of individuals within families, as the dataset included several types of relative pairs (eg, siblings). Follow-up time, in number of years, was measured from year of SA until year of first registration for SZ/ONAP/BP, death, or emigration, whichever came first. Observations were right censored at the end of follow-up (December 31, 2012). We investigated the proportionality assumption by including an interaction term between SA and the natural logarithm of time. If this term was significant, the effect of SA on risk of SZ/ONAP/BP was varying by time. To test for the equivalence of the association with psychiatric outcomes between the 2 measures of SA, we added a dummy variable indexing whether SA was measured in 1988–1997 or 1998 and onwards. We then examined the interaction between that dummy variable and SA scores in predicting risk for SA, ONAP, and BP.
We next added a quadratic function to capture the inverted J-shaped distribution seen in figure 1. We also fit a model with a spline term with 2 knots, one at the mean SA (0) and the other at +2 SDs. If the spline at +2 SD was significant and positive, this would be consistent with the hypothesized inverted J-shape relationship between SA and risk of the outcomes.
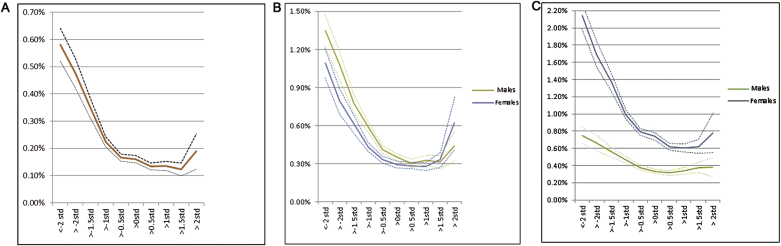
Fig. 1.
(a) Prospective risk for schizophrenia (± 95% CI) as a function of school achievement in both males and females. (b). Prospective risk for other nonaffective psychoses (±95% CI) as a function of school achievement separately in males and females. (c). Prospective risk for other bipolar illness (±95% CI) as a function of school achievement separately in males and females.
We then assessed the degree to which these regression results arose from confounding familial risk factors using a co-relative design. Using the Swedish Multi-Generation and Twin Registers, we identified all monozygotic (MZ) twin, full-sibling, half-sibling and first-cousin pairs. Using stratified Cox proportional hazards models, we refit all analyses within strata of specific relative pairs: MZ twins (n = 5312), full siblings (n = 1700176), half siblings (n = 333172) and first cousins (n = 5594814) discordant for their SA. The co-relative design allows us to contrast the rates of disorder in relatives with differing levels of SA. Within each relative pair strata, the Hazard ratio (HR) is adjusted for the familial cluster, and therefore, accounts for an array of unmeasured genetic and environmental factors shared by the pair. MZ twins share 100% of their genes while full siblings, half siblings, and cousins share, respectively, on average 50%, 25%, and 12.5% of their genes identical by descent. By modelling the genetic resemblance, all 4 types of relationship can be included in 1 model and we can obtain an improved estimate of the MZ twins where the data is sparse (5, 13, and 23 discordant pairs for SZ, ONAP, and BP, respectively). We assume that the parameter in the Cox regression is a linear function of genetic resemblance. To test whether this model well explained the observed data, we compared its fit with a model where the parameter for each relative type was independent (separate analyses for the 4 co-relative relationships) using Akaike’s Information Criterion16 where a lower number indicated an improved bit. If the linear model fitted well, we thereby obtain an improved estimate of the association among MZ twins, where the data was sparse. Statistical analyses were performed using SAS 9.3.17
Results
Descriptive Results
With follow-ups ranging from 1 to 25 years (mean: 14.4, SD: 5.8), the cumulative incidence of the 3 outcomes was 0.20% for SZ, 0.43% for ONAP, and 0.62% for BPI. SZ and ONAP were more common in men and BPI in women (table 1). The mean age at first diagnosis for all disorders was between 25 and 27 years, with little difference between sexes. Standardized across sexes, individuals who went on to develop SZ and ONAP had SA scores that were, respectively, 0.4–0.5 and 0.3–0.4 SDs lower than the mean. For BPI, the effect was weaker (0.2–0.3 SDs).
Table 1.
Descriptive Statistics for Population Born in Sweden from 1972 to 1990 and Registered for School Achievement
| N | SZ | ONAP | BPI | |
|---|---|---|---|---|
| Males | 922278 | 2,404 (0.26%) | 4,358 (0.47%) | 3,687 (0.40%) |
| Females | 878365 | 1,169 (0.13%) | 3,392 (0.39%) | 7,579 (0.86%) |
| Mean age at first reg (SD; Males) | 26.6 (5.0) | 25.6 (5.6) | 27.7 (5.7) | |
| Mean age at first reg (SD; Females) | 26.3 (5.4) | 25.3 (6.1) | 27.2 (5.7) | |
| Mean School Achivement (SD; Males) | −0.40 (1.1) | −0.41 (1.2) | −0.20 (1.1) | |
| Mean School Achivement (SD; Females) | −0.46 (1.2) | −0.34 (1.2) | −0.34 (1.1) |
Note: SZ, Schizophrenia; ONAP, Other Nonaffective Psychoses; BPI, Bipolar Illness.
Cox Proportional Hazard Analyses
Schizophrenia.
For a simple linear Cox model, controlling for sex, the HR (±95% CI) for SZ per SD increase in SA was 0.66 (0.64–0.68). The interaction between SA and the dummy variable indexing whether SA was assessed in 1988–1997 or after 1998 in the prediction of SZ was not significant (P = .13). We then examined the association between SA and SZ risk in both sexes combined (the relationship was similar for males and females), and a curvilinear relationship was apparent, with a strong linear inverse relationship between SA and SZ risk for SA scores between 2 and 0.5 SD below the mean, a more modest but still inverse relationship for SA scores between 0.5 SD below the mean and 1.5 SD above the mean (figure 1a). Furthermore, there was suggestive evidence that SA ≥ 2 SD above the mean was associated with increased risk of SZ, although the CIs around these estimates were very broad. Given these observations, we fitted a quadratic component to our Cox prediction model which was also significant (table 2). Next, we tested the proportionality assumption that the HR was constant over time. This failed, indicating that SA predicted the HR for SZ more robustly in the earlier than the later years of follow-up. This effect was modest as with a linear model, correcting for nonproportionality, the HR was estimated to be 0.55, 0.58, 0.60, and 0.61 at 0, 5, 10, and 15 years of follow-up. Finally, we formally tested the significance of the upswing of SZ risk at high levels of SA by a spline test. Setting splines at zero and +2 SDs of SA, the slope of the regression line above + 2 SDs of SA was substantial but not significant: 3.23 (0.93–14.91), P = 0.06.
Table 2.
Hazard Ratios and 95% CIs for Association Between SA (Coded as a Continuous Z score) and Risk for SZ
| Buffer Period (y) | Full Sample | Interactions With Sex, P Value | |
|---|---|---|---|
| Z score (SA) | 0 | 0.66 (0.64; 0.68) | .49 |
| Male vs Female | 0 | 1.95 (1.82; 2.10) | |
| Z score (SA) | 0 | 0.55 (0.49; 0.62) | |
| Male vs Female | 0 | 1.95 (1.82; 2.10) | |
| Log(time)a Z score | 1.09 (1.03; 1.14) | ||
| Z score | 5a | 0.67 (0.64; 0.69) | |
| Male vs Female | 5 | 2.05 (1.90; 2.21) | |
| Z score | 10a | 0.67 (0.64; 0.70) | |
| Male vs Female | 10 | 2.00 (1.81; 2.20) | |
| Z score (SA) | 0 | 0.73 (0.70; 0.76) | |
| Z score^2 | 0 | 1.09 (1.07; 1.11) | |
| Male vs Female | 0 | 1.97 (1.84; 2.12) | |
| Z score (SA) | 0 | 0.65 (0.58; 0.72) | |
| Z score^2 | 0 | 1.09 (1.07; 1.11) | |
| Log(time)a Z score | 0 | 1.06 (1.01; 1.10) | |
| Male vs Female | 0 | 1.97 (1.84; 2.12) |
Note: SA, School Achievement.
a423 (12%) cases of schizophrenia censored.
Other Nonaffective Psychosis.
For ONAP, the linear Cox model differed significantly in the sexes (table 3). For males, the effect size was identical to that seen in SZ. For females, it was weaker. The associations for the 2 sexes were plotted separately (figure 1b). The curve for ONAP in males closely resembles that seen for SZ with a modest upswing at SA ≥2 SDs. Compared with males, the association in females was weaker in those with low SA with a considerably stronger increased risk for those at high SA.). The interaction between SA and a dummy variable indexing whether SA was assessed in 1988–1997 or after 1998 was not significant in predicting ONAP in males (P = .75) but was in predicting ONAP in females (P < .001).
Table 3.
Hazard Ratios and 95% CIs for Association between SA (Coded as a Continuous Z Score) and Risk for ONAP
| Buffer Period (y) | Males | Females | Interactions With Sex, P Value | |
|---|---|---|---|---|
| Z score (SA) | 0 | 0.66 (0.64; 0.68) | 0.72 (0.70; 0.75) | .0003 |
| Z score (SA) | 0 | 0.64 (0.59; 0.69) | ||
| Log(time)a Z score | 0 | P = .90 | 1.07 (1.03; 1.11) | |
| Z score (SA) | 5a | 0.66 (0.64; 0.68) | 0.73 (0.71; 0.77) | |
| Z score (SA) | 10a | 0.66 (0.64; 0.68) | 0.73 (0.71; 0.77) | |
| Z score (SA) | 0 | 0.73 (0.71; 0.76) | 0.80 (0.77; 0.83) | |
| Z score^2 | 0 | 1.10 (1.08; 1.12) | 1.10 (1.08; 1.12) | |
| Z score | 0 | 0.73 (0.68; 0.79) | ||
| Z score^2 | 0 | 1.09 (1.07; 1.11) | ||
| Log(time)a Z score | 0 | P = .36 | 1.05 (1.01; 1.08) |
Note: a474 (17%) of male and 727 (22%) of female cases of ONAP censored.
Given the curves seen in figure 1b, we fitted a quadratic effect that was also significant. The test for the proportionality assumption was not significant in males but was in females. As with SZ, this effect in females was modest. With a linear model, correcting for nonproportionality, the HR was estimated to be 0.64, 0.66, 0.68, and 0.68 at 0, 5, 10, and 15 years of follow-up.
We tested the significance of the upswing of ONAP risk at very high levels of SA in the same way as for SZ. For males, the slope was substantial but not significant: 2.82 (0.92–8.61), P = .07. For females, the slope was stronger and significant: 11.19 (1.59–78.9), P = .02.
Bipolar Illness.
The interaction between SA and a dummy variable indexing whether SA was assessed in 1988–1997 or after 1998 was not significant in predicting BPI in either males (P = .54) or females (P = .91). The relationship between SA and risk of BPI was weaker than for SZ or ONAP and was appreciably stronger in females than in males (table 4). The shapes of the curves differed across the sexes (figure 1c). The SA-BPI association in females resembled the inverted J-shaped pattern seen for SZ (ie, a steep inverse relationship at SA scores below the mean, a flattening across the mean, and a slight uptick at SA scores ≥2 SD above the mean). In males, the curve was much flatter. Quadratic effects of SA on risk for BP were significant in both sexes but neither demonstrated any evidence for failure of the proportionality assumption. We tested the significance of the upswing of risk for BPI at very high levels of SA. For males, the slope was close to unity (0.86 [0.26–1.29], P = .81) with similar results for females (1.29 [0.22–8.61], P = .78).
Table 4.
Hazard Ratios and 95% CIs for Association between SA (Coded as a Continuous Z Score) and Risk for BPI
| Buffer Period (y) | Males | Females | Interactions With Sex, P Value | |
|---|---|---|---|---|
| Z score (SA) | 0 | 0.81 (0.78; 0.84) | 0.71 (0.70; 0.73) | <.0001 |
| Z score (SA) | 0 | |||
| Log(time)a Z score | 0 | P = .34 | P = .51 | |
| Z score (SA) | 5a | 0.81 (0.78; 0.84) | 0.72 (0.70; 0.74) | |
| Z score (SA) | 10a | 0.78 (0.75; 0.81) | 0.71 (0.68; 0.73) | |
| Z score (SA) | 0 | 0.85 (0.83; 0.88) | 0.77 (0.75; 0.79) | |
| Z score^2 | 0 | 1.08 (1.06; 1.10) | 1.06 (1.05; 1.08) | |
| Z score (SA) | ||||
| Z score^2 | 0 | |||
| Log(time)a Z score | 0 | P = .16 | P = .90 |
Note: a358 (10%) of male and 848 (11%) of female cases of BPI censored.
Impact of Possible Prodromal Syndromes
Could the observed SA-disorder associations arise as a result of prodromal symptoms (ie, reverse causation)? To address this question, we reran our linear Cox models building in a buffer of 5 and 10 years between SA assessment and the first illness onset counted in the model. As seen in tables 2–4, differences between our initial results and those obtained after adding these 2 buffer periods were minimal.
Co-relative Analyses
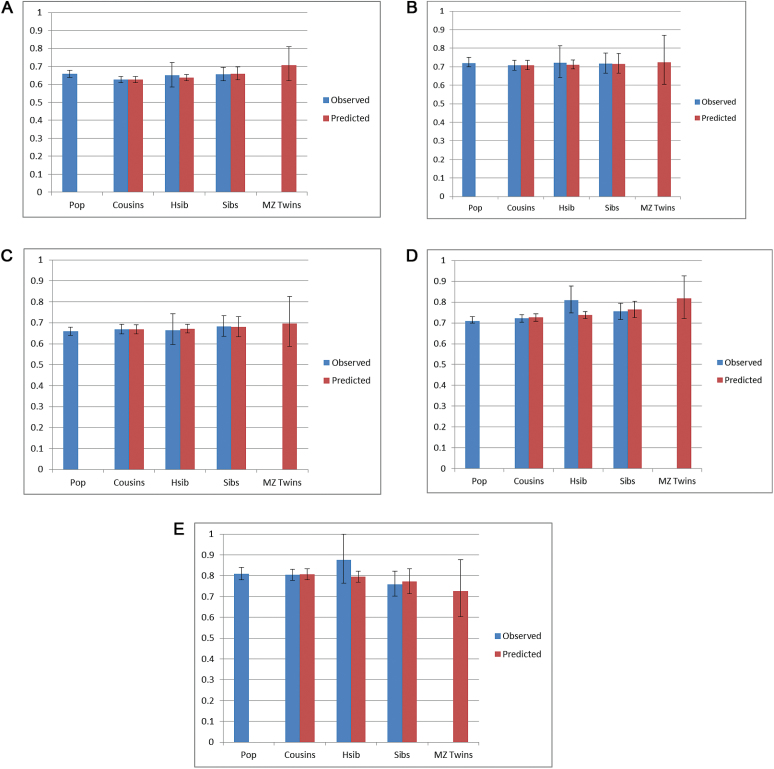
We next performed co-relative analyses that, for simplicity, utilized only the linear component of the association. For analyses where the proportionality assumption failed, the linear coefficient still well captured the average effect of SA over the follow-up period. If familial factors existed which predisposed both to low SA and to risk for SZ, we would predict an attenuation of the SA-SZ association in relatives of increasing relatedness. As seen in the blue bars in figure 2a, the magnitude of the observed association between SA and SZ was very similar in the general population and in pairs of cousins, half-siblings and full-siblings discordant for SA. Next, we fitted a model that estimated from the observed data the SA-SZ association from cousins, half siblings, full siblings and MZ twins discordant for SA based on their degree of genetic relationship (red bars in figure 2a). The model fitted the data better than the full model, which contained separate estimates for each relative class (AIC of 14678.7 vs 14681.2) showing that our model well described the observed associations. The results produced evidence for modest familial confounding in that a weak trend was seen for the HRs to become closer to unity among more closely than more distantly related relatives. However, even in MZ twin pairs discordant for SA, the model predicted a HR for the SA-SZ association only slightly larger than that seen in the general population.
Fig. 2.
(a) Co-relative control analyses (±95% CI) for the association between school achievement and schizophrenia based on a linear HR model for males and females combined. The observed results for the general population and cousins, hsib (half siblings) and full siblings (sibs) discordant for SA are presented in blue. The associations predicted from a model fitted to the observed data in cousins, half siblings, full siblings, and monozygotic (MZ) twins are presented in red. (b). Co-relative control analyses (±95% CI) in females for the association between school achievement and other nonaffective psychoses based on a linear HR model for males and females combined. The observed results for the general population and cousins, hsib (half siblings) and full siblings (sibs) discordant for SA are presented in blue. The associations predicted from a model fitted to the observed data in cousins, half siblings, full siblings, and monozygotic (MZ) twins are presented in red. (c). Co-relative control analyses (±95% CI) in males for the association between school achievement and other nonaffective psychoses based on a linear HR model for males and females combined. The observed results for the general population and cousins, hsib (half siblings) and full siblings (sibs) discordant for SA are presented in blue. The associations predicted from a model fitted to the observed data in cousins, half siblings, full siblings, and monozygotic (MZ) twins are presented in red. (d). Co-relative control analyses (±95% CI) in females for the association between school achievement and bipolar illness based on a linear HR model for males and females combined. The observed results for the general population and cousins, hsib (half siblings) and full siblings (sibs) discordant for SA are presented in blue. The associations predicted from a model fitted to the observed data in cousins, half siblings, full siblings, and monozygotic (MZ) twins are presented in red. (e). Co-relative control analyses (±95% CI) in males for the association between school achievement and bipolar illness based on a linear HR model for males and females combined. The observed results for the general population and cousins, hsib (half-siblings) and full siblings (sibs) discordant for SA are presented in blue. The associations predicted from a model fitted to the observed data in cousins, half-siblings, full siblings, and monozygotic (MZ) twins are presented in red.
For ONAP, neither the observed associations in females (figure 2b) nor in males (figure 2c) show appreciable evidence for familial confounding. In both sexes, our model fitted the data well (AIC of 6982.8 vs 6986.3 for females and 8855.3 vs 8858.5 in males). In females, the magnitude of the association was completely stable across the 4 classes of relatives. In males, a slight increase in the HR was predicted in closer relatives, especially in MZ pairs.
For BPI, the observed associations in females (figure 2d) suggested modest familial confounding as the HRs were modestly greater in full- and especially half-siblings compared with the general population and cousins. For BPI in males, no such consistent trend was seen (figure 2e). In both sexes, our model fit the data well (AIC of 14913.2 vs 14914.5 for females and 7568.5 vs 7569.2 in males). In females, a slight increase in the HR was seen, especially in MZ pairs. In males, no evidence of confounding was seen.
Discussion
We addressed 3 questions aimed at clarifying the nature of the association between SA assessed at age 16 and risk of developing SZ, ONAP, and BPI.
First, we sought to elucidate the nature of the association between SA and risk for SZ. Consistent with a large prior literature,1–4 we found a strong inverse association that was of similar magnitude in males and females, and considerably stronger in those with below than above average SA. Congruent with prior results from a Finnish cohort5 but not from our prior study with IQ assessed at age 18,6 we found a modestly increased risk for SZ in those with very high SA that was marginally significant. Cox modeling indicated that low SA was more strongly related to risk for SZ earlier than later in our follow-up period.
We next examined whether the relationship between SA, and risk for ONAP and BPI differed from that found for SZ. The association with premorbid cognitive functioning has been infrequently studied for ONAP.9,12 Consistent with most prior studies,9,18,19 we found that the association of ONAP with SA in males was identical to that seen for SZ. Interestingly, in females, it was modestly weaker. We found that within the spectrum of nonaffective psychoses, the association between low SA and risk was not restricted to the narrow SZ phenotype. Results for ONAP in females differed from all our other findings in showing statistically significant evidence for an increased risk associated with very high SA (≥2 SD). The magnitude of this increased risk, however, is known imprecisely, being based on only 35 cases of ONAP out of 5648 females with very high SA.
Prior studies of the association between premorbid cognitive functioning and risk for BPI are inconsistent,7–10 with some studies suggesting that higher functioning might be associated with increased risk.10,11 We found that the SA-BPI association differed appreciably by sex. In females, low SA predicted risk for BPI only modestly less robustly than seen in SZ. In males, by contrast, the association was considerably weaker. We found no appreciable evidence for increased risk for BPI for those with high SA. Our results are consistent with prior studies suggesting that cognitive functioning is less impaired in BPI than in SZ20,21 and congruent with a prior study of a smaller Swedish population cohort which found low IQ predicted increased risks of similar magnitude for SZ and ONAP, with a much weaker association with BPI.7
Prior studies suggest that in SZ, males have greater neurocognitive impairments than females.22,23 We found no such effect for premorbid SA and SZ but did see that for ONAP. Sex differences in cognitive performance in BPI has been rarely studied with most24,25 but not all studies26 finding, consistent with our results, that females demonstrate greater impairment.
A plausible explanation for the association of low SA with SZ, ONAP and BPI is that the poor academic performance was the result of prodromal symptoms. We evaluated this hypothesis by examining the associations after adding 5- and 10-year buffer periods between SA assessment and onset of illness. Consistent with recent meta-analysis from ultrahigh-risk and first-episode studies,27 we found no evidence that the observed relationship between SA and disease risk was mediated by prodromal processes.
To address our third question—clarifying the causes of the observed SA-disorder associations—we applied co-relative analyses. Consistent with our prior study of Swedish males, we found little evidence for familial confounding in the IQ-SZ association.6 Our current analyses using age 16 SA broadly replicates and extends these findings to SA in adolescence, a different measure of cognitive performance, and to both sexes. Our results suggest that only a small proportion of the association between lower cognitive performance and risk for SZ results from familial-environmental or genetic factors that predispose to both conditions. Rather, it indicates that this association arises from environmental experiences not shared with close relatives. Two causal hypotheses are most plausible. Either some environmental insults increase risk for both low SA and SZ, or there is a causal chain from the environmental adversities to low SA and then to SZ. Under the first model, low SA acts as an index of environmentally induced neurodevelopmental dysfunction. By contrast, the second model predicts that a comprehensive intervention in adolescence that improved SA could result in a lowered risk for subsequent SZ.28
We extended our prior work by applying the co-relative design, for the first time to our knowledge, to the associations between premorbid cognitive performance and risk for ONAP and BPI. The results were quite similar to that found for SZ. With the possible exception of BPI in females, the results indicated that little to none of the associations were the result of familial confounding.
This report provides suggestive evidence for an inverted J-shaped relationship between SA and risk of SZ which we did not see when we examined IQ6; there are 3 particularly plausible explanations for this difference in findings. First, SA has some predictors including personality, social skills and work drive that differentiate it from purer measures of cognitive performance like IQ.13 Twin studies have found that the genetic and environmental factors impacting on cognitive abilities and SA are partially distinct.29–31 For example, Krapohl and colleagues32 found that in addition to IQ, genetic influences on SA included measures of self-efficacy, positive personality features and self and low levels of parent-reported behavior problems. In the males in our sample, our measure of SA correlated with IQ scores obtained from the conscript registry at ages 18–20 only +0.63 suggesting a substantial proportion of nonshared variance.6 Second, our measure of SA covers a wide range of cognitive abilities, and thus we may have more power to pick up effects at the upper tail of the distribution. Third, cognitive performance at ages 16 might reflect different development processes for those at future risk for serious psychiatric disorders than measures of cognitive performance in early adulthood.
Among women, we found statistically significant evidence of an increased risk of ONAP associated with very high levels of SA. These results do provide limited empirical evidence in favor of the much debated association between “genius” and psychosis5,33–35 as does a recent report that polygenic risk scores for SZ predict membership in artistic and creative professions.36 However, our findings were based on a very small sample of high achieving subjects and were not stable across the 2 methods of SA assessment (see below). Furthermore, we found no convincing evidence for such an association with BPI, which is often more closely associated with genius and creativity in the literature than is SZ or ONAP.37
Gender differences in the association between SA and risk for ONAP and BPI were unexpected and, intriguingly, in opposite directions. Prevalence rates of ONAP were nearly equal across genders but, contrary to most prior literature, BPI was over twice as common in our sample in women as men.38 Further investigations will be needed to determine if these 2 observations are causally interrelated.
We are not the first to examine the association between SA and risk for psychotic disorders in Swedish data. MacCabe and colleagues12 studied SZ and ONAP in a smaller cohort with a shorter follow-up period. As expected, where our reports overlapped, our results were broadly confirmatory.
Limitations
These results should be interpreted in the context of 4 methodological limitations. First, our sample was restricted to Sweden and may not extrapolate to populations outside Western countries and cultures. Second, we relied on hospital diagnoses for SZ, ONAP, and BPI. We are aware of 2 studies which found, using record reviews39 and diagnostic interviews,40 that 96% and 94% of Swedish cases with hospital diagnoses of SZ, respectively, met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for SZ.41 One study examined by record review cases with 2 diagnoses of BPI and found positive predictive values for meeting DSM-IV criteria of 81%–92%.42 If we applied a narrow definition of SZ requiring ≥2 lifetime diagnoses and no BPI diagnosis, the HR for SA in a linear model strengthened slightly (0.65, 0.62–0.67). Given a narrow definition of BPI requiring ≥2 lifetime diagnoses and no SZ diagnosis, the HR for SA in a linear model was modestly weaker for males (0.85, 0.81–0.88) and slightly weaker for females (0.72, 0.71–0.74).
Furthermore, for SZ, ONAP, and BPI, age at first hospitalization will not always be a highly accurate index of age at first onset.
Third, because SA was evaluated in 2 different ways in Sweden in 1988–1997 vs 1998–2012, we examined whether the predictive relationships between SA and our psychiatric outcomes differed for individuals assessed in the 2 time periods. This analysis indicated that our findings did not differ across these 2 time periods for all outcomes except ONAP among females. That is, the association between higher SA and risk of ONAP was weaker in the early time period (HR = 0.77, 0.73–0.81) than in the later period (HR = 0.65, 0.63–0.68). Further analyses suggest that this difference likely arises from a modest increased risk for ONAP in high achieving females in 1988–1997 that was not seen in those assessed after 1998. Why this difference might occur only in women and only for ONAP (and not SZ or BPI) is not clear nor how it might relate to the differences in the 2 SA measures.
Fourth, our co-relative designs only examined the linear component of the SA-disorder association. It would have been very interesting to see how the observed quadratic effect—especially the small upswing in risk we sometimes observed at high SA—would behave in a co-relative design. We attempted this but the sample sizes were too small to produce meaningful results.
Conclusions
In a Swedish national sample, low SA measured at age 16 is a strong risk factor for subsequent onset of SZ, ONAP, and BPI. The relationship between SA and risk of these disorders is nonlinear and is considerably stronger at the lower than upper ends of achievement. These associations cannot be plausibly explained by the effects of a disease prodrome. The impact of SA on risk of SZ is similar for men and women, but this relationship is significantly stronger in males for ONAP and females for BPI. Modest increases in disease risk are seen at very high levels of SA for most disorders, but reach statistical significance only for ONAP in females and it is not consistently seen across the 2 types of SA assessment. Co-relative analyses indicate that genetic and shared environmental factors that predispose to both low SA and disease risk explain little of the observed association. These results have 2 major consequences for the neurodevelopmental model for SZ. First, they suggest that individual specific environmental factors rather than genetic or family environment are largely responsible for the association between poor cognitive performance and risk. Second, consistent with prior studies, they suggest that these neurodevelopmental processes are not unique to narrowly defined SZ but are also relevant to ONAP and to a lesser degree BPI.
Funding
Swedish Research Council (to J.S.); Swedish Research Council for Health, Working Life and Welfare (to J.S.); Swedish Research Council for Health, Working Life and Welfare (to K.S.); ALF funding from Region Skåne (to J.S. and K.S.). A career development award from the National Institute of Mental Health (K01- MH093642 to B.M.).
Acknowledgments
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Aylward E, Walker E, Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull. 1984;10:430–459. [DOI] [PubMed] [Google Scholar]
- 2. Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–587. [DOI] [PubMed] [Google Scholar]
- 3. Khandaker GM, Barnett JH, White IR, Jones PB. A quantitative meta-analysis of population-based studies of premorbid intelligence and schizophrenia. Schizophr Res. 2011;132:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med. 2012;42:743–755. [DOI] [PubMed] [Google Scholar]
- 5. Isohanni I, Järvelin MR, Jones P, Jokelainen J, Isohanni M. Can excellent school performance be a precursor of schizophrenia? A 28-year follow-up in the Northern Finland 1966 birth cohort. Acta Psychiatr Scand. 1999;100:17–26. [DOI] [PubMed] [Google Scholar]
- 6. Kendler KS, Ohlsson H, Sundquist J, Sundquist K. IQ and schizophrenia in a Swedish national sample: their causal relationship and the interaction of IQ with genetic risk. Am J Psychiatry. 2015;172:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zammit S, Allebeck P, David AS, et al. A longitudinal study of premorbid IQ Score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry. 2004;61:354–360. [DOI] [PubMed] [Google Scholar]
- 8. Reichenberg A, Caspi A, Harrington H, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacCabe JH, Wicks S, Löfving S, et al. Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry. 2013;70:261–270. [DOI] [PubMed] [Google Scholar]
- 10. Tiihonen J, Haukka J, Henriksson M, et al. Premorbid intellectual functioning in bipolar disorder and schizophrenia: results from a cohort study of male conscripts. Am J Psychiatry. 2005;162:1904–1910. [DOI] [PubMed] [Google Scholar]
- 11. MacCabe JH, Lambe MP, Cnattingius S, et al. Excellent school performance at age 16 and risk of adult bipolar disorder: national cohort study. Br J Psychiatry. 2010;196:109–115. [DOI] [PubMed] [Google Scholar]
- 12. MacCabe JH, Lambe MP, Cnattingius S, et al. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychol Med. 2008;38:1133–1140. [DOI] [PubMed] [Google Scholar]
- 13. Perry SR. Big Five Personality Traits and Work Drive as Predictors of Adolescent Academic Performance [dissertation]. Knoxville, TN: Graduate School at Trace, Tennessee Research and Creative Exchange, University of Tennessee; 2003. [Google Scholar]
- 14. Bjorklund A, Lindahl M, Sund K. Family background and school performance during a turbulent era of school reforms. Swedish Econ Pol Rev. 2003;10:111–136. [Google Scholar]
- 15. Wikipedia Contributors. Academic Grading in Sweden St. Petersburg, FL: Wikipedia Foundation; https://en.wikipedia.org/wiki/Academic_grading_in_Sweden Accessed May 27, 2015. [Google Scholar]
- 16. Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- 17. SAS Institute I. SAS/STAT User’s Guide, Version 9.3. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 18. Gunnell D, Harrison G, Rasmussen F, Fouskakis D, Tynelius P. Associations between premorbid intellectual performance, early-life exposures and early-onset schizophrenia. Cohort study. Br J Psychiatry. 2002;181:298–305. [DOI] [PubMed] [Google Scholar]
- 19. Fagerlund B, Pagsberg AK, Hemmingsen RP. Cognitive deficits and levels of IQ in adolescent onset schizophrenia and other psychotic disorders. Schizophr Res. 2006;85:30–39. [DOI] [PubMed] [Google Scholar]
- 20. Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80:137–149. [DOI] [PubMed] [Google Scholar]
- 21. Schretlen DJ, Cascella NG, Meyer SM, et al. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry. 2007;62:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. [DOI] [PubMed] [Google Scholar]
- 23. Goldstein JM, Seidman LJ, Goodman JM, et al. Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry. 1998;155:1358–1364. [DOI] [PubMed] [Google Scholar]
- 24. Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38:771–785. [DOI] [PubMed] [Google Scholar]
- 25. Bücker J, Popuri S, Muralidharan K, et al. Sex differences in cognitive functioning in patients with bipolar disorder who recently recovered from a first episode of mania: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM). J Affect Disord. 2014;155:162–168. [DOI] [PubMed] [Google Scholar]
- 26. Barrett SL, Kelly C, Bell R, King DJ. Gender influences the detection of spatial working memory deficits in bipolar disorder. Bipolar Disord. 2008;10:647–654. [DOI] [PubMed] [Google Scholar]
- 27. Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull. 2014;40:744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 29. Tambs K, Sundet JM, Magnus P, Berg K. Genetic and environmental contributions to the covariance between occupational status, educational attainment, and IQ: a study of twins. Behav Genet. 1989;19:209–222. [DOI] [PubMed] [Google Scholar]
- 30. Bartels M, Rietveld MJ, Van Baal GC, Boomsma DI. Heritability of educational achievement in 12-year-olds and the overlap with cognitive ability. Twin Res. 2002;5:544–553. [DOI] [PubMed] [Google Scholar]
- 31. Thompson LA, Detterman DK, Plomin R. Associations between cognitive abilities and scholastic achievement: genetic overlap but environmental differences. Psychol Sci. 1991;2:158–165. [Google Scholar]
- 32. Krapohl E, Rimfeld K, Shakeshaft NG, et al. The high heritability of educational achievement reflects many genetically influenced traits, not just intelligence. Proc Natl Acad Sci U S A. 2014;111:15273–15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waddell C. Creativity and mental illness: is there a link? Can J Psychiatry. 1998;43:166–172. [DOI] [PubMed] [Google Scholar]
- 34. Post F. Creativity and psychopathology. A study of 291 world-famous men. Br J Psychiatry. 1994;165:22–34. [DOI] [PubMed] [Google Scholar]
- 35. Nasar S. A Beautiful Mind. London: Faber and Faber; 1998. [Google Scholar]
- 36. Power RA, Steinberg S, Bjornsdottir G, et al. Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat Neurosci. 2015;18:953–955. [DOI] [PubMed] [Google Scholar]
- 37. Jamison KR. Touched with Fire: Manic-Depressive Illness and the Artistic Temperament. New York, NY: Simon & Schuster; 1996. [Google Scholar]
- 38. Tohen M, Angst J. Epidemiology of Bipolar Disorder. In: Tsuang MT, Tohen M, eds. Textbook in Psychiatric Epidemiology. 2nd ed. New York, NY: John Wiley & Sons, Inc; 2002:427–444. [Google Scholar]
- 39. Lichtenstein P, Björk C, Hultman CM, Scolnick E, Sklar P, Sullivan PF. Recurrence risks for schizophrenia in a Swedish national cohort. Psychol Med. 2006;36:1417–1425. [DOI] [PubMed] [Google Scholar]
- 40. Ekholm B, Ekholm A, Adolfsson R, et al. Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59:457–464. [DOI] [PubMed] [Google Scholar]
- 41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 42. Sellgren C, Landén M, Lichtenstein P, Hultman CM, Långström N. Validity of bipolar disorder hospital discharge diagnoses: file review and multiple register linkage in Sweden. Acta Psychiatr Scand. 2011;124:447–453. [DOI] [PubMed] [Google Scholar]