Abstract
The enormous variability in electrical properties of neurons is largely affected by a multitude of potassium channel subunits. Kv2.1 is a widely expressed voltage-dependent potassium channel and an important regulator of neuronal excitability. The Kv2.1 auxiliary subunit AMIGO constitutes an integral part of the Kv2.1 channel complex in brain and regulates the activity of the channel. AMIGO and Kv2.1 localize to the distinct somatodendritic clusters at the neuronal plasma membrane. Here we have created and characterized a mouse line lacking the AMIGO gene. Absence of AMIGO clearly reduced the amount of the Kv2.1 channel protein in mouse brain and altered the electrophysiological properties of neurons. These changes were accompanied by behavioral and pharmacological abnormalities reminiscent of those identified in schizophrenia. Concomitantly, we have detected an association of a rare, population-specific polymorphism of KV2.1 (KCNB1) with human schizophrenia in a genetic isolate enriched with schizophrenia. Our study demonstrates the involvement of AMIGO-Kv2.1 channel complex in schizophrenia-related behavioral domains in mice and identifies KV2.1 (KCNB1) as a strong susceptibility gene for schizophrenia spectrum disorders in humans.
Key words: KCNB1, susceptibility gene, mouse behavior, AMIGO, Kv2.1
Introduction
Characteristics of schizophrenia include “positive” symptoms such as delusions and hallucinations, “negative” symptoms such as social withdrawal, blunted affect and diminished motivation, and cognitive problems such as impaired working memory. Currently the disease mechanism is poorly understood and the pharmacological interventions typically relieve only positive symptoms.1 A critical challenge in schizophrenia research is to create animal models to illuminate the underlying pathophysiology and to develop new treatments.2 Modeling psychiatric disorders in animals is obviously challenging.3 However, several characteristics of schizophrenia have correlates in mice such as abnormal social behavior, impaired working memory, and defective prepulse inhibition (PPI).4–6 Positive symptoms are traditionally modeled in mice by studying dopamine-related behavior such as locomotor activity.5,7 Schizophrenia is highly heritable and genetic factors contribute to about 80% of the liability to the illness.8 However, the genetic etiology is complex and still largely unknown.
Potassium channels are key determinants of neuronal excitability. The significance of voltage-dependent potassium channels is demonstrated by several diseases, such as arrhythmia, periodic paralysis, episodic ataxia, sensorineural deafness and epilepsy, which are caused by mutations in genes coding for potassium channels or their auxiliary subunits.9 Kv2.1 is a widely expressed voltage-dependent potassium channel.10–12 In mammalian brain, Kv2.1 is present in most neurons, including both pyramidal cells and interneurons.11,13 Kv2.1 is specifically localized to unique clusters at neuronal perisomatic membrane, including cell soma, proximal dendrites, and axon initial segment.11,14 Kv2.1 channels constitute an essential component of the somatodendritic delayed rectifier current (IK) in several neuronal types15–18 and regulate excitability especially during periods of high frequency firing.16,19 Kv2.1 channels are subjected to dynamic activity-dependent modulation and are thought to function as a homeostatic regulator of neuronal excitability.20–22
The Kv2.1 auxiliary subunit AMIGO is a transmembrane protein containing a leucine-rich repeat domain and an Ig-domain on the extracellular side and a short cytoplasmic tail.23 AMIGO constitutes an integral part of the Kv2.1 channel complex in brain and modifies the channel function.24 In this study, we have generated mice lacking the AMIGO gene. To further understand the physiological role of AMIGO in mouse brain, we have studied the molecular, electrophysiological, morphological, and behavioral properties of these knockout (KO) mice. Absence of AMIGO clearly reduced the amount of the Kv2.1 channel protein in mouse brain and altered the electrophysiological properties of neurons. These changes were accompanied by behavioral and pharmacological abnormalities related to schizophrenia.
Our study demonstrated the involvement of AMIGO-Kv2.1 channel complex in schizophrenia-related behavioral domains in mice and identified AMIGO1 and KV2.1 (KCNB1) as candidate genes for human schizophrenia and related psychiatric disorders. Out of them, KV2.1 (KCNB1) has recently emerged as a new candidate gene for schizophrenia, as common variants located at chromosome 20q13.13 in the vicinity of KCNB1 and PTGIS genes were found to associate modestly with schizophrenia (OR = 1.07) in a large international meta-analysis,25 although the common variants from that chromosomal region did not show association in the Finnish families included in that study (P > .5). Only about one-third of heritability of schizophrenia has been estimated to be captured by the common variations included in genotyping platforms used in the large-scale genome-wide analyses, and relatively rare, high-impact variations have been suggested to contribute to the missing heritability of schizophrenia.25,26 The variants are typically distributed in varying frequencies among different populations. The unique population history, distinctive pattern of rare functional variants27 and presence of regional isolates with manifold risk for schizophrenia28 makes Finns an ideal population to target these rare disease-causing variants for schizophrenia, as was recently demonstrated for population from Northern Finland.29
Interestingly, 2 individuals homozygous for a KV2.1 (KCNB1) variation substituting the penultimate amino acid serine 857 with asparagine have been identified in an earlier study on the low voltage alpha EEG trait, and one of the Asn857/Asn857 homozygotes was reported to have schizophrenia and the other had paranoia.30 However, the association of the corresponding single nucleotide polymorphism (SNP) rs34280195 with schizophrenia has not been studied before. We hypothesized that the allele Asn857 of variation rs34280195 would comprise a rare, high-impact genetic risk factor for schizophrenia. According to the hypothesis, the minor allele of rs34280195, associated significantly with schizophrenia and schizophrenia spectrum disorders in the Finnish schizophrenia families.
Methods
A full description of the materials and methods is available in the supplementary material.
Animals
Animal care and handling were performed according to guidelines of the EU Commission recommendation and approved by the National Animal Experiment Board of Finland. The AMIGO KO mice were backcrossed to the C57BL/6J strain at least 8 generations before being used for experiments. Behavioral phenotyping was started at the age of 8 weeks.
Antibodies
The chicken polyclonal antibody to AMIGO was produced against the cytoplasmic tail of mouse AMIGO (aa 394–492) and was affinity purified as described previously.24 Kv2.1 K89/34 and Kv1.2 K14/16 were obtained from UC Davis/NIH NeuroMab Facility (Western blotting). Kv2.1 (APC-012) was obtained from Alomone Labs (immunohistochemistry).
Immunohistochemistry
Floating sections (40 µm) of perfusion fixed mouse brains were stained with rabbit antibody to Kv2.1 (Alomone Labs) followed by Alexa Fluor 488 conjugated donkey anti-rabbit antibody (2 µg/ml, Invitrogen).
Protein Analysis
Mouse brain samples were analyzed with Western blotting. Equal amount of protein was loaded in each lane. Even loading was confirmed with Ponceau staining and blotting with control antibodies (Kv1.2, PSD95).
Histology
Paraffin sections (10 µm) of perfusion fixed mouse brains were stained with 1% Luxol fast blue and 0.5% cresyl violet acetate.
Electrophysiology
Outward potassium currents were recorded from acute hippocampal slices prepared from 7- to 9-week old AMIGO male KO and wild-type (WT) mice using the whole-cell patch-clamp technique. Hippocampal slices (350–400 µM) were cut with a vibratome. The slices were superfused with artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 15 d-glucose, and 2 CaCl2; 5% CO2–95% O2, at a rate of 1–2mL/min (32°C). Whole-cell recordings were obtained from CA1 pyramidal neurons with pipettes with tip resistance of 3–4 MΩ and currents were recorded with MultiClamp 700A amplifier (Molecular Devices, Inc) and were sampled at 12.5kHz. The pipette solution contained (in mM) 130 potassium gluconate, 10 NaCl, 2 Na2-ATP, 0.3 Na-GTP, 10 Hepes and 0.6 EGTA, pH 7.4. The cells were held at −70 mV and step depolarized for 400ms with 20 mV increments until +110 mV. The interpulse interval was 5 s. pCLAMP 9.2 software (Molecular Devices, Inc) was used for acquisition and analysis of currents. The current amplitude was determined as the mean amplitude between 310–360ms. Statistical comparisons were performed using the 2-tailed, unpaired Student’s t test.
Behavioral Tests
The following behavioral tests were carried out: anxiety level and locomotor activity (open field, elevated plus maze, light–dark box), motor coordination (Rota-Rod, beam walking test), nociception (hot plate), sensorimotor gating (PPI), learning and memory (Morris water maze, IntelliCage), behavioral despair (forced swim test), spontaneous alternation (Y-maze), and social behavior (tube test, resident intruder test).
The behavioral tests were carried out as described previously31,32 with the exception of beam walking test, and adaptation to drinking session and patrolling in IntelliCage. The detailed description of individual behavioral tests and protocols applied in IntelliCage is included in the supplementary material.
One cohort of mice (11 WT + 13 KO) was used for original behavioral phenotyping with tests arranged in the following order with 2–3 days interval: open field, elevated plus maze, light/dark test, Y-maze, hot plate, Rota-Rod, beam walking test, PPI, Morris water maze, and forced swim test. Additional mice were used for the open field and PPI validation and pharmacology (number of animals presented in figure 2). Separate mice were used for resident-intruder test and tube test (12 WT + 13 KO). Females from social tests and additional females were used in IntelliCage (12 WT + 15 KO).
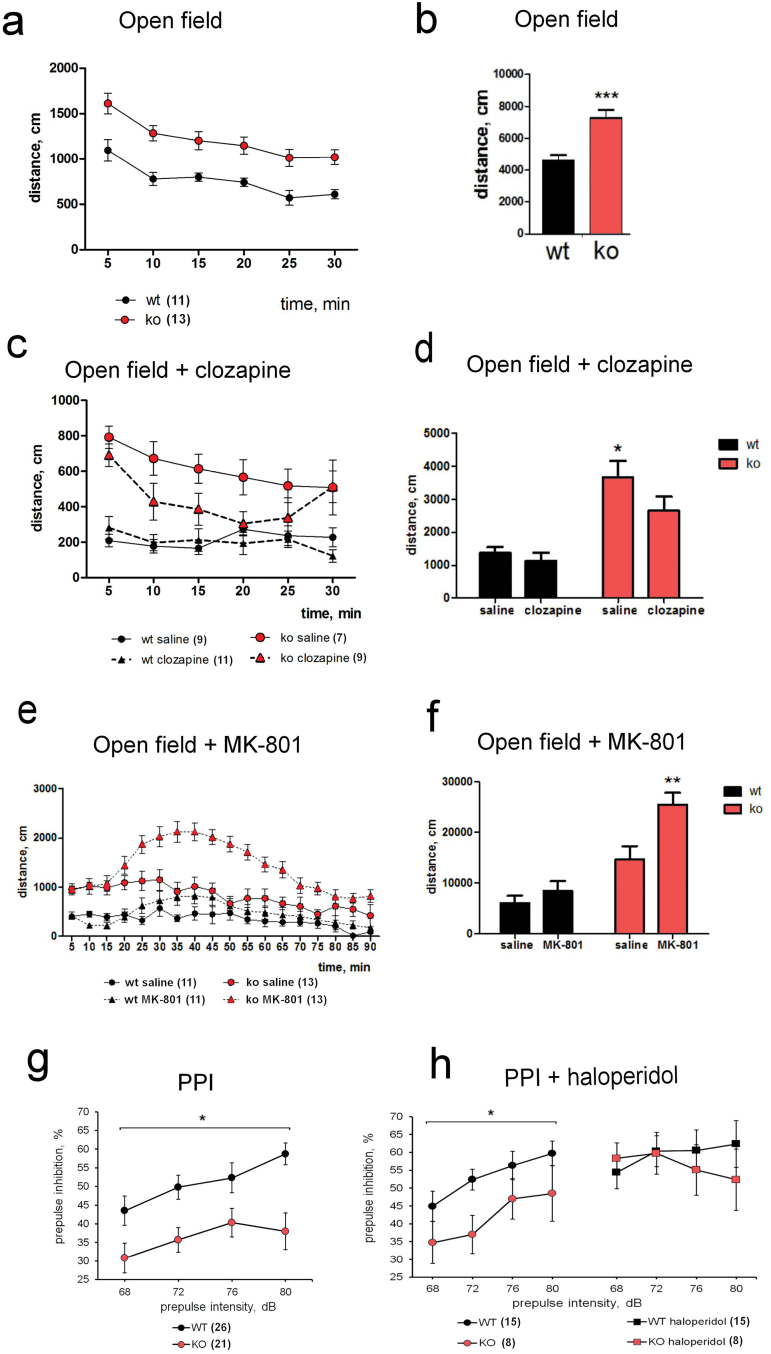
Fig. 2.
AMIGO-deficient mice display increased locomotor activity, sensitivity to psychotomimetic MK-801 and defective prepulse inhibition (PPI). (a, b) AMIGO knockout (KO) mice were hyperactive in the open field. (b)Total distance traveled during 30min was significantly higher in KO mice (158%; P = .0004). (c, d) In the open field, 1mg/kg of the antipsychotic drug clozapine reduced the locomotor activity of the KO mice, which could not be observed in the wild-type (WT) mice. (d) The total distance travelled was reduced 28% in the KO mice by the application of clozapine (P = .04, post hoc). (e, f) AMIGO KO mice were more sensitive to the locomotor activating effect of psychotomimetic MK-801 (0.2mg/kg) in the open field (f) In the WT animals, a low dose of MK-801 (0.2mg/kg) slightly increased the total distance traveled, but the effect was not significantly different from saline. In the KO animals, the same dose significantly increased the distance traveled (P = .001, post hoc). (g) The AMIGO KO mice had reduced PPI compared to the WT littermates (see table 1 for P values) (h) Antipsychotic drug haloperidol (1mg/kg) improved the impaired PPI. The number of animals used in each experiment is indicated in parentheses. *P < .05, **P < .01, ***P < .001.
Drug Treatments
All drugs were dissolved in saline (0.9% NaCl) and injected intraperitoneally in the volume of 10ml/kg. MK-801 (0.2mg/kg) was administered immediately before starting the 90min observation period in the open field. Another group of animals was used to test the effect of clozapine. Clozapine (1mg/kg) was injected 30min before the open field or PPI tests. Haloperidol (1mg/kg) injections were done 30min before the PPI test. Baseline PPI test was followed by testing with haloperidol.
Statistics
Factorial analysis of variance (ANOVA) with genotype, sex and treatment (if needed) as independent variables was used for analysis of the behavioral data. The normality of data distribution was tested with the Kolmogorov–Smirnov test. Repeated measures ANOVA was applied in analysis of locomotor activity in the open field and PPI. To assess drug effects, the repeated and factorial ANOVA with following Newman–Keuls post hoc test were used. Most parameters obtained from IntelliCages for female mice were analyzed by t test. Data with nonnormal distributions were analyzed with Mann–Whitney test.
Determination of Monoamines and Their Metabolites
The concentrations of monoamines were analyzed as described earlier.33
Sample
The Schizophrenia family sample is a systematically collected sample of Finnish schizophrenia families. Families were identified through a search of nationwide health care and population registries. All individuals born in Finland between 1940 and 1976 were screened for hospitalization during the period from 1969 to 1998 (Hospital Discharge Register), for use of free antipsychotic medication (Medication Reimbursement Register), or disability pension (Pension Register) due to schizophrenia, schizoaffective disorder, or schizophreniform disorder. Free outpatient antipsychotic medication means that the person is entitled to 100% reimbursement of the medication price from the Social Insurance Institution; in practice, the medication is free for the patient. Pedigrees were constructed by linking the personal identification numbers of the affected individuals to their parents and siblings, derived from the Population Register Centre. Two samples of subjects with schizophrenia and their relatives were contacted. The first sample consisted of families with at least 2 siblings with schizophrenia and their first-degree relatives from the whole geographical area of Finland. The second sample comprised patients and their relatives from families with at least 1 member with schizophrenia from an isolated region in the north-eastern part of the country with an exceptionally high lifetime risk of schizophrenia.34,35
The sample consists of 3335 individuals (776 with schizophrenia and 1209 with schizophrenia spectrum diagnosis) from 918 families, of which 1/3 come from the isolate (IS) and 2/3 from the All Finland (AF) subsamples (N ind = 1009, N fam = 264 from internal isolate).34–36 The collected data includes DNA, accurate data on genealogy, family structures, and best-estimate lifetime diagnoses assessed according to the DSM-IV criteria.
Genotyping and Data Analysis
The samples were genotyped with homogeneous mass extension using the MassARRAY System (Sequenom). Genotyping was performed by following the manufacturer’s guidelines in 384-well plates using a total reaction volume of 5 µl including 20ng of genomic DNA. For quality control, duplicate DNA samples, control DNA, and water controls were included in each plate.
We genotyped 3335 individuals. The success rates for the SNPs were 98.7% for rs34280195, 98.3% for rs112735799, and 98.8% for rs34467662. Hardy–Weinberg equilibrium (HWE) was tested using 1142 unrelated individuals. All of the 3 SNPs were in HWE (P values .51, .62, and .58 for rs34280195, rs34467662, and rs112735799, respectively). Minor allele frequencies for the SNPs in the whole sample were 0.047 for rs34280195, 0.030 for rs34467662, and 0.030 for rs112735799.
The association between the SNPs and schizophrenia or schizophrenia spectrum diagnosis was analyzed with Pseudomarker 2.0.37–39 Pseudomarker is a powerful analysis software that is capable of analyzing large pedigrees and jointly modeling linkage and linkage disequilibrium. To examine the association of the 3 SNPs to schizophrenia and schizophrenia spectrum disorders we used recessive model and studied association in the presence of linkage.
We first analyzed the associations in the complete data set, and, in case of a significant or borderline significant finding we further analyzed the association within the 2 subpopulations of IS and AF families. In the analysis of the IS sample, we used 614 unrelated unaffected controls from the AF families in addition to the 697 genotyped unaffected IS controls. To control the P values for multiple testing we used Bonferroni-correction and multiplied the P values by 6 (3 SNPs, 2 diagnosis groups). All the P values we present in the tables and in the text are uncorrected P values, unless otherwise notified.
Results
AMIGO-Deficient Mice Display Reduced Amount of KV2.1 Protein and Altered Electrophysiological Properties of Neurons
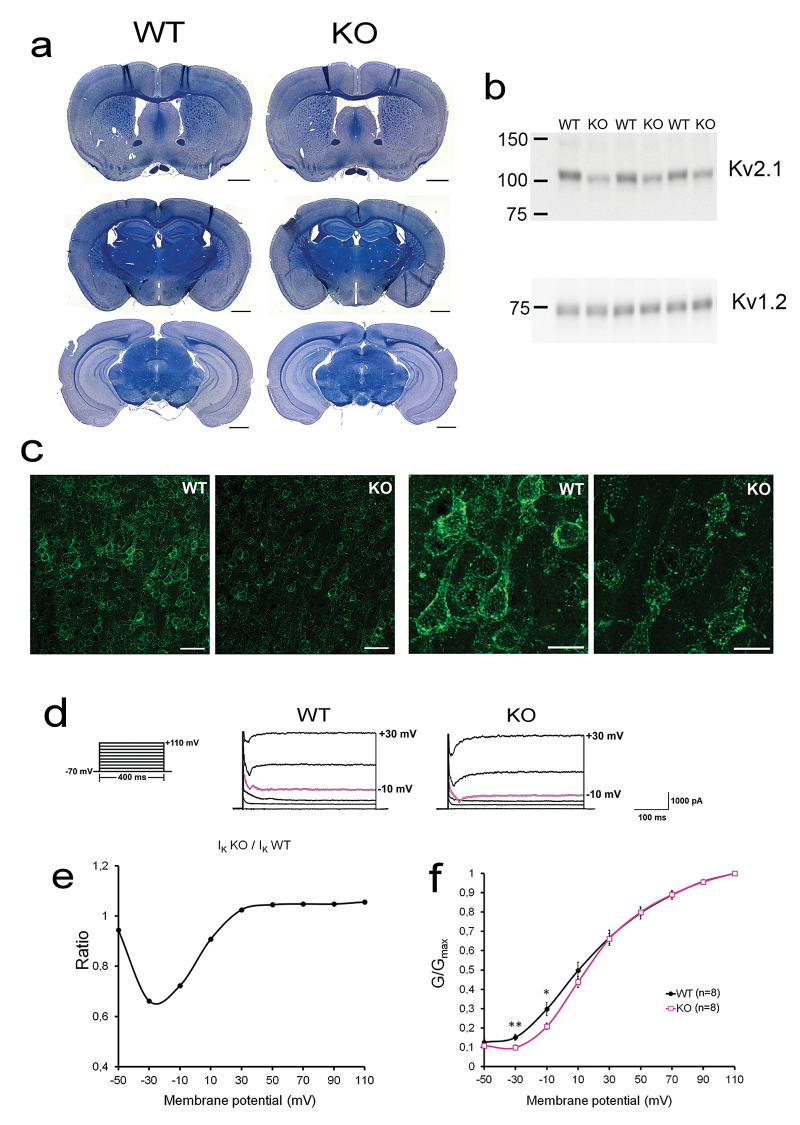
We generated mice lacking the AMIGO gene (supplementary figure 1). There were no apparent morphological differences between the AMIGO KO and WT brains (figure 1a). The HPLC analysis of monoamine neurotransmitters and their metabolites from different brain areas revealed that the serotonin concentration was increased in prefrontal and temporal cortex of the KO mice (supplementary table 1). Notably, the amount of the Kv2.1 protein in AMIGO KO brains was reduced to about half of the amount in the WT brains (to 55%, P < .05, figure 1b). The intensity of Kv2.1 immunostaining was clearly lower in the AMIGO KO brain (figure 1c). However, the characteristic somatodendritic and clustered localization of Kv2.1 was not altered.
Fig. 1.
Decreased expression of Kv2.1 and altered IK current in AMIGO knockout (KO) mouse brain. (a) Histological sections from the wild-type (WT) (left panels) and AMIGO KO (right panels) mouse brain. There were no gross morphological changes in the AMIGO KO mouse brain. (b) Upper panel: Immunoblotting of membrane protein samples from the AMIGO KO and WT mouse brain with Kv2.1 antibody. The amount of Kv2.1 protein in AMIGO KO brains was reduced to 55% (P = .018) of the level detected in the WT brains. Lower panel: control immunoblot with Kv1.2 antibody. (c) Immunohistochemistry of Kv2.1 in cerebral cortex of the WT and the AMIGO KO mouse brain. Right side panels represent higher magnification. (d) Representative current traces from CA1 pyramidal neurons in acute hippocampal slices from AMIGO WT and KO mice at depolarization steps from −70 to +30 mV. The pulse protocol is shown on the left. (e) IK currents from CA1 pyramidal neurons from WT (IKWT) and AMIGO KO (IKKO) mice. Ratio of average current densities (IKKO to IKWT) at different membrane potentials. At membrane potential ranging from −30 mV to −10 mV, the current density in the KO mice was significantly lower (27%–34%) as compared to the WT mice, whereas at membrane potentials over 10 mV the current was close to WT mice values. (f) Normalized conductance-voltage relationship for AMIGO KO (□) or WT mice (●). *P < .05, **P < .02. Error bars represent SEM. Scale bars 1mm (a), 500 µm (c—left side panels) and 200 µm (c—right side panels).
Kv2.1 channels constitute an essential component of the delayed rectifier current (IK) in hippocampal neurons.16,18 We have previously shown that iRNA inhibition of AMIGO expression alters the voltage-dependent activation of the IK current in cultured hippocampal neurons.24 We recorded neuronal IK currents from pyramidal neurons in acute hippocampal slices from WT and AMIGO KO mice (figures 1d–f). In agreement with previous results, we found 27%–33% decreased current density in the KO mice at the threshold of activation (from −30 mV to −10 mV, P < .05). The activation of neuronal IK thus required stronger depolarization in the AMIGO KO mice than in the WT controls.
AMIGO-Deficient Mice Display Several Schizophrenia-Related Features
AMIGO KO mice were subjected to an extensive behavioral test battery (see table 1). The mice performed normally in a number of behavioral tasks. However, the AMIGO KO mice displayed many schizophrenia-related behavioral abnormalities.
Table 1.
Behavioral Characterization of AMIGO-Deficient Mice
| Test and Parameter | KO vs WT | WT | KO | F Value | P Value |
|---|---|---|---|---|---|
| Elevated plus maze | |||||
| Distance, cm | ↗ | 1154.6±77.7 | 1577.9±133.6 | F(1,22) = 6.81 | .016 |
| Open arm latency, s | 142.5±36 | 69.2±20.33 | ns | ||
| Open entries, % | 21.5±4.5 | 31±3.2 | ns | ||
| Open arm time, s | ↗ | 31.5±7.8 | 57.8±7.9 | F(1,22) = 5.78 | .025 |
| Rearings, s | 20.5±2.7 | 23.3±2.5 | ns | ||
| Open field | |||||
| Distance, cm | ↗ | 4602.3±339.2 | 7279.4±520.3 | F(1,22) = 17.12 | .0004 |
| Distance in center, % | ↗ | 22.6±1.7 | 17.2±1.2 | F(1,22) = 7.61 | .0115 |
| Latency to center, s | 16.5±4.5 | 14.5±4.7 | ns | ||
| Time in center,s | 272.1±30.7 | 223.2±21 | ns | ||
| Center entries, n | ↗ | 410.7±13.4 | 569.6±34.1 | F(1,22) = 16.40 | .0005 |
| Rearing, s | 225.5±26.9 | 273.8±22.4 | ns | ||
| Light–dark | |||||
| Latency to dark, s | 30.8±16.4 | 11±1.8 | ns | ||
| Light–dark transitions, n | 11.4±2 | 15±1.8 | ns | ||
| Distance in light, cm | 977.4±97.1 | 1001.2±119.6 | ns | ||
| Time in light, s | ↘ | 182.2±23.9 | 114.8±11.3 | F(1,22) = 7.21 | .013 |
| Rearing, s | 15.6±3.7 | 13±2.6 | ns | ||
| Y-maze | |||||
| Spontaneous alternation, % | 67.5±4 | 59.5±3.5 | ns | ||
| Hot plate | |||||
| Latency to reaction, s | 15.29±1 | 19.58±3.7 | ns | ||
| Rota-Rod | |||||
| Latency to fall, first trial, s | 83.8±21.6 | 86.5±17.9 | ns | ||
| Latency to fall, last trial, s | 205.9±27.3 | 239.7±25.4 | ns | ||
| Beam walking test | |||||
| Time to fall 1st trial, s | 94.1±10 | 98.5±11.8 | ns | ||
| Time to fall 2nd trial, s | 97.2±10.2 | 92.4±12.5 | ns | ||
| Crossed marks 1st trial, n | 36.3±7.6 | 26.7±7.9 | ns | ||
| Crossed marks 2nd trial, n | 36.8±6.1 | 21±5.2 | ns | ||
| Startle response | ↘ | 1611.9±183.3 | 691.6±106.9 | F(1,45) = 16.61 | .0002 |
| Prepulse inhibition | |||||
| PPI 68 dB | ↘ | 43.7±3.6 | 30.8±3.8 | F(1,45) = 6.07 | .0176 |
| PPI 72 dB | ↘ | 49.6±3.2 | 35.8±3.2 | F(1,45) = 9.23 | .0039 |
| PPI 76 dB | ↘ | 52.4±3.8 | 40.3±3.8 | F(1,45) = 4.98 | .0307 |
| PPI 80 dB | ↘ | 58.7±3.1 | 38.2±5 | F(1,45) = 13.14 | .0007 |
| Forced swim test | |||||
| Immobility time, s | 73.5±15.4 | 97.5±10.5 | ns | ||
| Tube test | |||||
| Wins, % | ↘ | 73.1±8.3 | 24.9±5.1 | F(1,23) = 23.55 | .0001 |
| Resident-intruder test | |||||
| Time in social activity, % | 42.3±4.3 | 34.1±3.9 | ns | ||
| Time in nonsocial activity, % | 12.0±2.8 | 10.2±1.4 | ns | ||
| IntelliCage | |||||
| Corner visits, n | 663.2±65.8 | 733.3±71 | ns | ||
| Corner visits with drinking, % | 0.309±0.019 | 0.276±0.038 | ns | ||
| Drinking, n | 751.3±58.5 | 854.4±55.7 | ns | ||
| Corner visits for 30min, n | 7.3±4.6 | 5.7±2 | ns | ||
| Corner visits till first darkness. n | 29.7±10.6 | 35.4±14.2 | ns | ||
| Latency to first visit, s | 2328.5±1205.5 | 17461.7±11169.6 | ns | ||
| Latency to all 4 corner visits, s | 7526.7±4077.6 | 25020.2±16.852.8 | ns | ||
| Latency to drinking, s | 55432.9±21857.2 | 34910.4±15741.3 | ns | ||
| Spontaneous alteration, % | 13.3±4.1 | 13.8±7.6 | ns | ||
| Place learning | 38.8±0.8 | 40.4±1.7 | ns | ||
| Patrolling | ↘ | 41.1±0.9 | 35.9±1.1 | F(1,11) = 13.38 | .0038 |
Note: ↗ increased in the KO mice; ↘ decreased in the KO mice; KO, knockout; ns, statistically not significant; PPI, prepulse inhibition; WT, wild-type. Nociception (Hot plate) and motor coordination (Rota-Rod, Beam walking test) of the AMIGO KO mice were normal. Several tests (Elevated plus maze, Open field, Prepulse inhibition, Tube test, Patrolling in IntelliCage) detected abnormalities in schizophrenia-related behavioral domains. From the 2 tests modeling anxiety-related behavior, the light/dark test suggested that the AMIGO KO mice are more anxious. However, the elevated plus maze suggested that the AMIGO KO mice are less anxious. The discrepancy between these 2 tests may be due to the hyperactivity of the AMIGO KO mice and dependency of the used tests on general locomotor activity. Values are presented as mean ± SEM.
Increased locomotor activity of the KO mice was seen in 2 different tests. In open field, the KO mice traveled significantly more than the WT littermates (158%, P < .001, table 1, figures 2a and 2b). The difference was also evident in elevated plus maze (138%, P < .05, table 1). The hyperactivity of the AMIGO KO mice was reduced by the application of the antipsychotic drug clozapine (Open field, 28%, P < .05, figures 2c and 2d).
We then tested the effects of the psychotomimetic drug MK-801 in the AMIGO KO and WT mice (figures 2e and 2f). MK-801 is a noncompetitive NMDA receptor antagonist producing psychosis in humans. Noncompetitive NMDA antagonists are used to model several aspects of schizophrenia in mice and have been shown to exacerbate symptoms in schizophrenic patients.40 The AMIGO KO mice were more sensitive to the locomotor activating effect of MK-801 than the WT mice.
PPI is a widely used endophenotype of schizophrenia.41 Several studies have demonstrated the impaired PPI in schizophrenic patients.42 The AMIGO KO mice had reduced PPI compared to the WT littermates (figure 2g, see table 1 for P values). Antipsychotic drugs improve the impaired PPI in schizophrenic patients and in animal models of schizophrenia. Following the application of the antipsychotic drug clozapine (1mg/kg), the PPI of the AMIGO KO mice was not anymore statistically different from the WT mice (P = .24), but the improvement was not statistically significant. We therefore next tested the antipsychotic drug haloperidol. We found that haloperidol (1mg/kg) improved the reduced PPI in the AMIGO KO animals (P < .05, figure 2h).
We then studied the social behavior of the AMIGO KO mice. In resident-intruder test there was no significant difference between the genotypes in time that the animals spent in social or nonsocial activity (table 1). However, this test can be affected by increased activity of the AMIGO KO mice. Social dominance was measured using the tube test. The test demonstrated that the AMIGO KO mice were significantly more submissive than the WT mice (P < .001). The WT mice won in 73% of trials whereas the KO mice won only in 25% (table 1).
We also detected a specific cognitive deficit in the AMIGO KO mice. The KO mice did not differ from the WT littermates in Morris water maze (data not shown). For further analysis, the mice were placed in IntelliCage platform which enables automated monitoring of spontaneous and learning behavior in a home cage-like environment. There was no difference between the KO and WT mice in many of the IntelliCage behavioral parameters including group learning and relearning in corner preference task. However, the AMIGO KO mice had significantly impaired performance in patrolling task (P < .01, table 1). The patrolling task is comparable with 8-arm radial maze: both of them test behavioral flexibility and working memory.43,44
rs34280195 is Significantly Associated With Schizophrenia
It is quite intriguing to find this many schizophrenia-related abnormalities, relevant to all 3 major symptom clusters, in a mouse line lacking a single gene. Even the mouse models created by deleting established schizophrenia-associated genes do not generally recapitulate the breadth of the clinical profile of schizophrenia.45 Our results clearly identified AMIGO1 and KV2.1 (KCNB1) as candidate genes for human schizophrenia and related psychiatric disorders. In previous literature, a possible role in psychiatric disease has been suggested for the KV2.1 (KCNB1) variation substituting the penultimate amino acid serine 857 with asparagine.30 However, the association of the corresponding SNP rs34280195 with schizophrenia has not been studied before.
We hypothesized that the allele Asn857 of variation rs34280195 would comprise a rare, high-impact genetic risk factor for schizophrenia and genotyped rs34280195 as well as 2 other nonsynonymous SNPs (rs34467662 and rs112735799) of KV2.1 (KCNB1) in a Finnish schizophrenia family sample comprising 3335 individuals (1209 with schizophrenia spectrum disorder) from families with multiple cases of schizophrenia (table 2). According to the hypothesis, the minor allele of rs34280195, corresponding to Asn857 associated significantly with schizophrenia and schizophrenia spectrum disorders (P = .0019 and P = .0087, respectively; Bonferroni-corrected P values .01 and .05) in the Finnish schizophrenia families. Two other variations did not associate with schizophrenia or schizophrenia spectrum disorders. The association of rs34280195 was strongest in families from an internal isolate with high prevalence of schizophrenia and a very limited number of founder chromosomes27 (P = 1.2×10−5 and P = 2.2×10−5, for schizophrenia and schizophrenia spectrum disorders, respectively; P = .035 for schizophrenia spectrum disorder in the families outside the isolate). Furthermore, rs34280195 was found to be enriched in the IS families (f = 0.078 in the IS families; f = 0.034 in the families outside the isolate; f = 0.012 in Finns from 1000 Genomes database [N = 93] and f = 0.005 in all reported populations [N = 1089]). Thus, the functional polymorphism rs34280195 associated highly significantly with schizophrenia and schizophrenia spectrum disorders in families from an internal isolate of Finland, and the disease-associated allele was enriched particularly in these families.
Table 2.
The Association of KCNB1 SNPs With Schizophrenia and Schizophrenia Spectrum Disorders in the Finnish Schizophrenia Family Sample
| Schizophrenia | Schizophrenia Spectrum | |||||
|---|---|---|---|---|---|---|
| N cases a | N unaffected a | P b | N cases a | N unaffected a | P b | |
| rs34280195 | 768 | 2099 | .0019c | 1193 | 2099 | .0087d |
| rs34467662 | 769 | 2100 | .42 | 1195 | 2100 | .53 |
| rs112735799 | 764 | 2090 | .24 | 1188 | 2090 | .04e |
Note: AF, families from the whole geographical area of Finland; IS, families from the internal isolate; SNP, single nucleotide polymorphism. Additional unrelated unaffected controls (N = 614) from the AF families were used in the analysis of the IS sample.
aWith genotypes.
b P(LD|Linkage).
c P(IS families) = .000012, P(AF families) = .15.
d P(IS families) = .000022, P(AF families) = .035.
e P(IS families) = .78, P(AF families) = .06.
Discussion
The phenotypic characteristics found in the AMIGO KO mice demonstrate the involvement of AMIGO-Kv2.1 channel complex in schizophrenia-related behavioral disturbances in mice. In agreement with our finding, a recent study has demonstrated that the Kv2.1-deficient mice are strikingly hyperactive.46 Kv2.1-deficient mice also exhibit defects in spatial learning. However, the behavioral phenotype of the Kv2.1-deficient mice has not been previously compared to schizophrenia.
We have detected an association of a rare coding variant of KV2.1 (KCNB1) with schizophrenia and schizophrenia spectrum disorders. Including our study and recent findings on other channels, there is now emerging evidence that voltage-dependent potassium channels and their interaction partners may contribute to the pathophysiology of schizophrenia and related psychiatric disorders. Kv11.1 (KCNH2) is linked to an increased risk of schizophrenia and shown to affect cortical physiology and cognition.47 Caspr2 (CNTNAP2), an adhesion protein interacting with Kv1 potassium channels, has been associated with schizophrenia and epilepsy.48 Kv7.2 (KCNQ2) has been associated with bipolar disorder.49
Some studies have observed that potassium channel activators have antipsychotic effects.50–52 It is also suggested that the current antipsychotic drugs could mediate part of their therapeutic actions by affecting potassium channels because genetic variation in Kv11.1 (KCNH2) modulates antipsychotic treatment response in patients with schizophrenia.53 Additionally, it has been shown that the amount of Kv3.1 channels is reduced in patients with untreated schizophrenia and normalized with antipsychotic drugs.54 Our findings emphasize the role of potassium channels as attractive targets for antipsychotic treatment development, and identify 2 novel target candidates; AMIGO and Kv2.1. Based on our studies in mice, agents modifying the function of AMIGO-Kv2.1 channel complex might have broader effects on schizophrenia symptoms than traditional antipsychotics.
Kv2.1 regulates excitability during periods of high-frequency firing and is suggested to function as a component of homeostatic plasticity.16,22 Altered Kv2.1 channel complex is thus expected to lead to a situation where neurons are more prone to high-frequency firing without proper homeostatic control. Since Kv2.1 channels are widely expressed in different brain regions, and present in pyramidal neurons as well as inhibitory interneurons, altered Kv2.1 activity could have complex effects on overall brain function. Neural oscillations are linked to several important brain functions disturbed in schizophrenia such as attention, memory and sensory processing.55,56 Since potassium channels play a central role in neuronal synchronization, the schizophrenia-like behavior resulting from AMIGO/Kv2.1 disruption could at least partially be accounted for by disturbed synchrony and cortical oscillations associated with schizophrenia.56
The phosphorylation, localization and activity of Kv2.1 are shown to be coupled.20,21 In this respect, it is relevant that the Ser857Asn variation replaces a phosphorylation target serine with asparagine. Disrupted Kv2.1 phosphorylation could affect the localization and activity of the channel and thus alter the neuronal excitability.
Hypoxia-ischemia-related fetal and neonatal complications are associated with increased risk of schizophrenia.57,58 Predisposing genetic factors might interact with hypoxia in increasing the risk of schizophrenia. Interestingly, the phosphorylation, localization and activity of Kv2.1 are shown to be strongly regulated by hypoxia/ischemia.20,59,60 Kv2.1 is suggested to function as a mechanism to suppress pathological hyperexcitability of central neurons during ischemic conditions.60 We hypothesize that disrupted Kv2.1 phosphorylation could affect the channel responsiveness to hypoxia and thus act as a predisposing mechanism to hypoxia-related complications and increase the risk of schizophrenia.
We have established the role of KV2.1 (KCNB1) as a schizophrenia susceptibility gene. Our convergent findings in humans and mice suggest a role for AMIGO-Kv2.1 potassium channel complex in pathophysiology of schizophrenia. Furthermore, these findings suggest AMIGO and Kv2.1 as potential drug targets for schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Sigrid Jusélius Foundation; the Academy of Finland (259581); the Finnish Cultural Foundation; and the Alfred Kordelin Foundation.
Supplementary Material
Acknowledgments
We thank Seija Lågas, Erja Huttu, and Eeva-Liisa Saarikalle for excellent technical assistance. The authors declare no conflict of interest.
References
- 1. Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17:1206–1227. [DOI] [PubMed] [Google Scholar]
- 2. Kas MJ, Kahn RS, Collier DA, et al. Translational Neuroscience of Schizophrenia: seeking a meeting of minds between mouse and man. Sci Transl Med. 2011;3:102mr3. [DOI] [PubMed] [Google Scholar]
- 3. Gass P, Wotjak C. Rodent models of psychiatric disorders–practical considerations. Cell Tissue Res. 2013;354:1–7. [DOI] [PubMed] [Google Scholar]
- 4. Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiatry. 2006;59:1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Tuathaigh CM, Moran PM, Waddington JL. Genetic models of schizophrenia and related psychotic disorders: progress and pitfalls across the methodological "minefield". Cell Tissue Res. 2013;354:247–257. [DOI] [PubMed] [Google Scholar]
- 7. Pratt J, Winchester C, Dawson N, Morris B. Advancing schizophrenia drug discovery: optimizing rodent models to bridge the translational gap. Nat Rev Drug Discov. 2012;11:560–579. [DOI] [PubMed] [Google Scholar]
- 8. Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. [DOI] [PubMed] [Google Scholar]
- 9. Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bocksteins E, Raes AL, Van de Vijver G, Bruyns T, Van Bogaert PP, Snyders DJ. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am J Physiol Cell Physiol. 2009;296:C1271–C1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan L, Figueroa DJ, Austin CP, et al. Expression of voltage-gated potassium channels in human and rhesus pancreatic islets. Diabetes. 2004;53:597–607. [DOI] [PubMed] [Google Scholar]
- 13. Du J, Tao-Cheng JH, Zerfas P, McBain CJ. The K+ channel, Kv2.1, is apposed to astrocytic processes and is associated with inhibitory postsynaptic membranes in hippocampal and cortical principal neurons and inhibitory interneurons. Neuroscience. 1998;84:37–48. [DOI] [PubMed] [Google Scholar]
- 14. Sarmiere P, Weigle C, Tamkun M. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neuroscience. 2008;9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baranauskas G, Tkatch T, Surmeier DJ. Delayed rectifier currents in rat globus pallidus neurons are attributable to Kv2.1 and Kv3.1/3.2 K(+) channels. J Neurosci. 1999;19:6394–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J Physiol. 2000;522:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guan D, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons. J Physiol. 2007;581:941–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murakoshi H, Trimmer JS. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci. 1999;19:1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan D, Armstrong WE, Foehring RC. Kv2 channels regulate firing rate in pyramidal neurons from rat sensorimotor cortex. J Physiol. 2013;591:4807–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misonou H, Mohapatra DP, Park EW, et al. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. [DOI] [PubMed] [Google Scholar]
- 21. Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005;26:743–752. [DOI] [PubMed] [Google Scholar]
- 22. Surmeier DJ, Foehring R. A mechanism for homeostatic plasticity. Nat Neurosci. 2004;7:691–692. [DOI] [PubMed] [Google Scholar]
- 23. Kuja-Panula J, Kiiltomäki M, Yamashiro T, Rouhiainen A, Rauvala H. AMIGO, a transmembrane protein implicated in axon tract development, defines a novel protein family with leucine-rich repeats. J Cell Biol. 2003;160:963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peltola MA, Kuja-Panula J, Lauri SE, Taira T, Rauvala H. AMIGO is an auxiliary subunit of the Kv2.1 potassium channel. EMBO Rep. 2011;12:1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ripke S, O’Dushlaine C, Chambert K, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim ET, Würtz P, Havulinna AS, et al. Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet. 2014;10:e1004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perälä J, Saarni SI, Ostamo A, et al. Geographic variation and sociodemographic characteristics of psychotic disorders in Finland. Schizophr Res. 2008;106:337–347. [DOI] [PubMed] [Google Scholar]
- 29. Stoll G, Pietilainen OPH, Linder B, et al. Deletion of TOP3[beta], a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat Neurosci. 2013;16:1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazzanti CM, Bergen A, Enoch MA, Michelini S, Goldman D. Identification of a Ser857-Asn857 substitution in DRK1 (KCNB1), population frequencies and lack of association to the low voltage alpha EEG trait. Hum Genet. 1996;98:134–137. [DOI] [PubMed] [Google Scholar]
- 31. Kulesskaya N, Rauvala H, Voikar V. Evaluation of social and physical enrichment in modulation of behavioural phenotype in C57BL/6J female mice. PLoS One. 2011;6:e24755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kulesskaya N, Võikar V, Peltola M, et al. CD73 is a major regulator of adenosinergic signalling in mouse brain. PLoS ONE. 2013;8:e66896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Airavaara M, Mijatovic J, Vihavainen T, Piepponen TP, Saarma M, Ahtee L. In heterozygous GDNF knockout mice the response of striatal dopaminergic system to acute morphine is altered. Synapse. 2006;59:321–329. [DOI] [PubMed] [Google Scholar]
- 34. Arajärvi R, Suvisaari J, Suokas J, et al. Prevalence and diagnosis of schizophrenia based on register, case record and interview data in an isolated Finnish birth cohort born 1940–1969. Soc Psychiat Epidemiol. 2005;40:808–816. [DOI] [PubMed] [Google Scholar]
- 35. Hovatta I, Varilo T, Suvisaari J, et al. A genomewide screen for schizophrenia genes in an isolated Finnish subpopulation, suggesting multiple susceptibility loci. Am J Hum Genet. 1999;65:1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paunio T, Ekelund J, Varilo T, et al. Genome-wide scan in a nationwide study sample of schizophrenia families in Finland reveals susceptibility loci on chromosomes 2q and 5q. Hum Mol Genet. 2001;10:3037–3048. [DOI] [PubMed] [Google Scholar]
- 37. Gertz EM, Hiekkalinna T, Digabel SL, Audet C, Terwilliger JD, Schäffer AA. PSEUDOMARKER 2.0: efficient computation of likelihoods using NOMAD. BMC Bioinformatics. 2014;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Göring HH, Terwilliger JD. Linkage analysis in the presence of errors IV: joint pseudomarker analysis of linkage and/or linkage disequilibrium on a mixture of pedigrees and singletons when the mode of inheritance cannot be accurately specified. Am J Hum Genet. 2000;66:1310–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hiekkalinna T, Schäffer AA, Lambert B, Norrgrann P, Göring HH, Terwilliger JD. PSEUDOMARKER: a powerful program for joint linkage and/or linkage disequilibrium analysis on mixtures of singletons and related individuals. Hum Hered. 2011;71:256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. [DOI] [PubMed] [Google Scholar]
- 41. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 42. Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, "just the facts": what we know in 2008 Part 3: neurobiology. Schizophr Res. 2008;106:89–107. [DOI] [PubMed] [Google Scholar]
- 43. Too LK, Ball HJ, McGregor IS, Hunt NH. A novel automated test battery reveals enduring behavioural alterations and cognitive impairments in survivors of murine pneumococcal meningitis. Brain Behav Immun. 2014;35:107–124. [DOI] [PubMed] [Google Scholar]
- 44. Weyer SW, Klevanski M, Delekate A, et al. APP and APLP2 are essential at PNS and CNS synapses for transmission, spatial learning and LTP. EMBO J. 2011;30:2266–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carpenter WT, Koenig JI. The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacology. 2008;33:2061–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Speca DJ, Ogata G, Mandikian D, et al. Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability. Genes Brain Behav. 2014;13:394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huffaker SJ, Chen J, Nicodemus KK, et al. A primate-specific, brain isoform of KCNH2 affects cortical physiology, cognition, neuronal repolarization and risk of schizophrenia. Nat Med. 2009;15:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Friedman JI, Vrijenhoek T, Markx S, et al. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2008;13:261–266. [DOI] [PubMed] [Google Scholar]
- 49. Borsotto M, Cavarec L, Bouillot M, et al. PP2A-Bgamma subunit and KCNQ2 K+ channels in bipolar disorder. Pharmacogenomics J. 2007;7:123–132. [DOI] [PubMed] [Google Scholar]
- 50. Akhondzadeh S, Mojtahedzadeh V, Mirsepassi GR, Moin M, Amini-Nooshabadi H, Kamalipour A. Diazoxide in the treatment of schizophrenia: novel application of potassium channel openers in the treatment of schizophrenia. J Clin Pharm Ther. 2002;27:453–459. [DOI] [PubMed] [Google Scholar]
- 51. Sotty F, Damgaard T, Montezinho LP, et al. Antipsychotic-like effect of retigabine [n-(2-amino-4-(fluorobenzylamino)-phenyl)carbamic acid ester], a KCNQ potassium channel opener, via modulation of mesolimbic dopaminergic neurotransmission. J Pharmacol Exp Ther. 2009;328:951–962. [DOI] [PubMed] [Google Scholar]
- 52. Vukadinovic Z, Rosenzweig I. Abnormalities in thalamic neurophysiology in schizophrenia: could psychosis be a result of potassium channel dysfunction? Neurosci Biobehav Rev. 2012;36:960–968. [DOI] [PubMed] [Google Scholar]
- 53. Apud JA, Zhang F, Decot H, Bigos KL, Weinberger DR. Genetic variation in KCNH2 associated with expression in the brain of a unique hERG isoform modulates treatment response in patients with schizophrenia. Am J Psychiatry. 2012;169:725–734. [DOI] [PubMed] [Google Scholar]
- 54. Yanagi M, Joho RH, Southcott SA, Shukla AA, Ghose S, Tamminga CA. Kv3.1-containing K(+) channels are reduced in untreated schizophrenia and normalized with antipsychotic drugs. Mol Psychiatry. 2014;19:573–579. [DOI] [PubMed] [Google Scholar]
- 55. Lakatos P, Schroeder CE, Leitman DI, Javitt DC. Predictive suppression of cortical excitability and its deficit in schizophrenia. J Neurosci. 2013;33:11692–11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. [DOI] [PubMed] [Google Scholar]
- 57. Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157:196–202. [DOI] [PubMed] [Google Scholar]
- 58. Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000;26:351–366. [DOI] [PubMed] [Google Scholar]
- 59. Misonou H, Thompson SM, Cai X. Dynamic regulation of the Kv2.1 voltage-gated potassium channel during brain ischemia through neuroglial interaction. J Neurosci. 2008;28:8529–8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Misonou H, Mohapatra DP, Menegola M, Trimmer JS. Calcium- and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J Neurosci. 2005;25:11184–11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.