Abstract
Background. Immune markers have been associated with schizophrenia, but few studies have examined multiple markers in both recent onset and chronic schizophrenia patients. Methods. The sample of 588 individuals included 79 with recent onset psychosis, 249 with chronic schizophrenia, and 260 controls. A combined inflammation score was calculated by principal components factor analysis of the levels of C-reactive protein, Pentraxin 3, and IgG antibodies to gliadin, casein, and Saccharomyces cerevisiae measured in blood samples. Inflammation scores among groups were compared by multivariate analyses. Results. The chronic schizophrenia group showed significant elevations in the combined inflammation score compared with controls. The recent onset group surprisingly showed a reduction in the combined inflammation score. Consistent with these findings, the chronic schizophrenia group had significantly increased odds of a combined inflammation score greater than the 75th and the 90th percentile of that of the controls. The recent onset group had significantly increased odds of a combined inflammation score less than the 10th and the 25th percentile level of the controls. Conclusions. The recent onset of psychosis may be associated with inherent deficits in innate immunity. Individuals later in the course of disease may have increased levels of innate immunity. The reasons for these changes are not known with certainty but may be related to compensatory increases as the disease progresses. Longitudinal studies are needed to determine the course of immune abnormalities in schizophrenia and their role in the clinical manifestations of the disorder.
Key words: psychosis, schizophrenia, inflammation, immunity
Introduction
Schizophrenia is a disease of unknown etiology. Immunological abnormalities have been identified from blood samples and may contribute to the pathophysiology of the disorder.1–6 Evidence for a role of immunologic factors in schizophrenia also comes from epidemiological studies that show an association between several perinatal infectious exposures and the development of schizophrenia in the offspring.7 Epidemiologic studies also show an increased schizophrenia risk in persons who have a history of severe infection or autoimmune disease.8,9 In addition, the areas of the genome that show the strongest association with schizophrenia risk are those involved in the major histocompatibility complex (MHC) and other immune regulatory regions.10 Aspects of both the innate and the adapative immune system may be implicated in schizophrenia.3
A number of immune markers have been associated with schizophrenia. In a previous study, we found that individuals with established schizophrenia have levels of C-reactive protein (CRP), a pentraxin protein which is a nonspecific marker of inflammation, that are significantly increased compared with controls.11 The individuals with schizophrenia also had increased odds of having elevated CRP relative to both the 75th and the 90th percentile level of the controls; these analyses were adjusted for demographic factors and also body mass index (BMI) and cigarette smoking. Elevated CRP in schizophrenia has been found by other researchers and substantiated by a recent meta-analysis.1,12 Pentraxin 3 is a related acute phase pentraxin protein that has been much less studied than CRP. Like CRP, Pentraxin 3 is an important component of the innate immunity system and provides early defense against infections.13
Several markers of intestinal inflammation have also been implicated in schizophrenia. Gliadin is a glycoprotein derived from the ingestion of gluten from dietary wheat and related grains. An autoimmune response to gliadin and other related antigens is found in some gastrointestinal disorders such as celiac disease. In previous studies, we have found elevated levels of IgG antibodies to gliadin, but not other markers of celiac disease, in individuals with chronic schizophrenia and recent onset psychosis.14 An autoimmune response to casein, the family of proteins in cow’s milk, is also associated with gastrointestinal inflammation. In a previous study, we found elevated IgG antibodies to casein proteins, particularly to whole casein and the alpha(s) subunit, in individuals with established schizophrenia and recent onset psychosis.15 Unlike antibodies to casein and gliadin, antibodies to the yeast Saccharomyces cerevisiae (ASCA) are not dependent on dietary intake as Saccharomyces cerevisiae is a normal inhabitant of the GI tract. We have found elevated levels of ASCA in some schizophrenia patients adding further evidence for the possible role of intestinal inflammation in schizophrenia.16
In this study we examined the role that inflammation plays in schizophrenia by comparing several markers of inflammation in schizophrenia patients at different points in the disease course, those with recent onset of psychosis and those with chronic schizophrenia, to individuals without a history of psychiatric disorder. We compared the levels of each of 5 inflammatory markers in these 3 participant groups, as well as a composite inflammation score that was calculated from the levels of the 5 markers.
Methods
The study population consisted of 588 individuals: 79 with a recent onset of psychosis, 249 with chronic schizophrenia, not of recent onset, and 260 controls without a history of psychiatric disorder. The details of the recruitment and evaluation of individuals in these groups have been previously described.14
The participants having a recent onset of psychosis met the following criteria: (1) onset of psychotic symptoms for the first time within the past 24 months defined as the presence of a positive psychotic symptom of at least moderate severity that lasted through the day for several days or occurred several times a week and could not have been limited to a few brief moments; (2) age between 18 and 45 inclusive; and (3) absence of substance-induced psychosis or of psychotic symptoms which occurred only in the context of intoxication or withdrawal. All of the recent onset patients were receiving anti-psychotic medication at the time of study participation.
The individuals with chronic schizophrenia met the following criteria: (1) age between 18–65 inclusive; (2) diagnosis of schizophrenia or schizoaffective disorder meeting criteria in the Diagnostic and Statistical Manual of Mental Disorder Fourth Edition (DSM-IV); (3) onset of psychotic symptoms more than 24 months earlier; and (4) currently receiving antipsychotic medication treatment.
The nonpsychiatric control sample met these criteria: (1) age between 18–65 inclusive and (2) absence of a current or past psychiatric disorder as confirmed by screening with the Structured Clinical Interview for DSM-IV Axis I Disorders Non-Patient edition (SCID-I/NP).17
All participants met the following additional criteria: (1) absence of current substance dependence over the past 1 month and of any history of intravenous substance abuse; (2) absence of mental retardation; (3) absence of apparent acute infection; and (4) absence of a serious medical disorder that would affect cognitive functioning.
The participants with recent onset psychosis were recruited from inpatient and day hospital programs at a large psychiatric health system in Baltimore, Maryland. The participants with chronic schizophrenia were recruited from these same programs and at affiliated outpatient treatment sites in the region. The control participants were recruited using posted announcements at local health care facilities and universities in the same geographic area as the sites from where the individuals with recent onset psychosis and with schizophrenia were drawn.
The studies were approved by the Institutional Review Boards of the Sheppard Pratt Foundation and the Johns Hopkins Medical Institutions following established guidelines. All participants provided written informed consent after the study procedures were explained.
A blood sample was obtained from all participants and analyzed as described below. Pentraxin 3 and high-sensitivity CRP levels were measured by means of commercially available enzyme immunoassay kit obtained from IBL America. IgG antibodies to gliadin, casein, and Saccharomyces cerevisiae (ASCA) were measured by means of solid phase immunoassays as previously described.16 Samples from different groups were run on the same plate to minimize experimental variation.
Participants were asked about their educational level, other demographic variables, current cigarette smoking status as well as the presence of co-occurring medical conditions. Height and weight were used to calculate BMI. All participants were individually administered a brief cognitive battery, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, Form A).18 Participants in the psychiatric groups were also interviewed and rated on the Positive and Negative Syndrome Scale (PANSS)19 to assess current psychiatric symptoms; medication data were recorded from their clinical charts.
Statistical Analyses
Five markers were measured from the blood samples and included in the calculation of the composite score. The markers were selected based on their significant individual association with schizophrenia in previous studies as noted in the introduction.
In order to obtain a single composite measure of all 5 markers, principal components analysis (PCA) was performed on the entire sample. PCA uses measured data to generate “components” which function as summary scores of the observed variables. PCA is a commonly used technique for data reduction (ie, generating a small number of summary scores from a larger number of measurements) and is preferable to factor analysis for this purpose.20 A 1-component solution was specified in order to obtain a single composite score; because the solution contained only 1 component, rotation was not possible, since rotation determines how the components are allowed to correlate with one another. The factor loadings were used to calculate a composite score of inflammation for each participant, which is a weighted sum of each marker, with the loadings used as weights.
ANOVA were used to determine the associations between inflammation composite score and each individual marker with diagnostic group. Post hoc means comparisons tests examined pair wise group differences; P values were adjusted for multiple comparisons using Tukey’s honest significant difference (HSD). Linear regression was used to assess the degree of association between diagnostic group and the composite score and individual markers after adjusting for the covariates of age, gender, race, maternal education and also current cigarette smoking status, and BMI as these variables may be associated with immunity and potential confounders in the analysis. The 90 missing BMI values were imputed as previously described.21 Logistic regression models were used to determine the association between the inflammation score and diagnostic group after adjusting for the same covariates.
Logistic regression was also used to determine the odds ratios associated with increased and decreased levels of individual markers and the composite marker in the individuals with recent onset psychosis and those with chronic schizophrenia. For these analyses, increased levels of antibodies were defined as levels greater than or equal to the 75th and 90th percentiles of the controls; decreased levels were defined as lesser than or equal the 10th and the 25th percentile levels of the controls. The covariates age, gender, race, maternal education, smoking status, and BMI were also included in these analyses.
Results
Sample Characteristics
The study sample consisted of 588 participants, 79 with a recent onset of psychosis, 249 with chronic schizophrenia, and 260 controls. The demographic and clinical characteristics of the study populations are presented in table 1.
Table 1.
Characteristics of Study Groups
| Recent Onset of Psychosis (N = 79) | Chronic Schizophrenia (N = 249) | Nonpsychiatric Controls (N = 260) | |
|---|---|---|---|
| Mean (SD) or Number (%) | |||
| Age, years | 24.0 (6.2) | 41.4 (11.8) | 32.1 (11.4) |
| Male | 52 (66%) | 148 (59%) | 102 (39%) |
| Caucasian race | 40 (51%) | 127 (51%) | 136 (52%) |
| Years maternal education | 13.9 (2.9) | 12.4 (2.7) | 13.7 (2.7) |
| Duration of illness | 7.3 mo (7.2) | 20.6 y (10.7) | — |
| BMIa | 26.1 (7.5) | 30.8 (7.1) | 27.5 (6.8) |
| Cigarette smoker | 32 (41%) | 159 (64%) | 40 (15%) |
Note: BMI, body mass index.
a N = 225 chronic schizophrenia, 194 controls.
Within the recent onset group, participants had the following diagnoses: schizophreniform disorder (n = 15, 19%); schizophrenia (n = 18, 23%); schizoaffective disorder (n = 12, 15%); bipolar I disorder, most recent episode manic (n = 9, 11%); bipolar I disorder, most recent episode depressed (n = 3, 4%); bipolar disorder single manic episode (n = 4, 5%); major depression with psychotic features (n = 11, 14%); delusional disorder (n = 1, 1%); brief reactive psychosis (n = 2, 3%); and psychotic disorder not otherwise specified (n = 4, 5%).
Within the chronic schizophrenia group, the patients had the following diagnoses: paranoid subtype (n = 42, 17%); undifferentiated subtype (n = 64, 26%); schizoaffective disorder (n = 135, 54%); and other schizophrenia subtype (n = 8, 3%).
All persons in the psychiatric groups were receiving psychotropic medication at the time of the study assessment. A total of 72 (91%) of the recent onset and 198 (80%) of the schizophrenia group were receiving a second generation antipsychotic medication. The following medications were the most commonly received: risperidone, n = 29 (37%) of the recent onset and n = 62 (25%) of the chronic schizophrenia group; olanzapine, n = 14 (18%) of the recent onset and n = 44 (18%) of the schizophrenia group; ziprasidone, n = 4 (5%) of the recent onset and n = 14 (6%) of the schizophrenia group; clozapine, n = 2 (3%) of the recent onset and n = 48 (19%) of the schizophrenia group; valproate, n = 3 (4%) of the recent onset and n = 49 (20%) of the schizophrenia group.
Principal Components Analysis Results
First the distributions of the 5 immunological markers were examined. All analytes were right-skewed, so all markers were log-transformed to normalize their distributions. Once transformed, the measures showed reasonably bell-shaped distributions without outliers or missing data. Next, the correlation matrix for the 5 transformed markers was examined. The correlations were generally weak to moderate, ranging from r = 0.023 (antibodies to gliadin and CRP) to r = 0.27 (antibodies to gliadin and casein). Generally, CRP was the most weakly correlated with the other measures, while the correlation between the level of casein antibodies and other measures was the strongest.
In order to obtain a single composite inflammation score, a one-component solution was specified. The factor loadings were as follows: ASCA, 0.4641; pentraxin 3, 0.4465; antibodies to gliadin, 0.6348; antibodies to casein, 0.7017; and CRP, 0.3493. Next, the factor loadings were used to calculate a composite score of inflammation markers for each participant. This score is a weighted sum of each marker (log-transformed) multiplied by its factor loading. The composite score was normally distributed with a mean value of −0.57 and SD of 1.14. The composite score is positively correlated with each of the individual markers, so a higher composite score reflects higher levels of the individual markers. The inflammation composite score was not significantly associated with any participant demographic variables: age, race, gender, or maternal education. The inflammation composite score was also not significantly associated with body mass index, cigarette smoking status, RBANS cognitive score, PANSS symptom score, or the receipt of individual medications or classes of medications including risperidone, olanzapine, clozapine, valproate, atypical anti-psychotics in the chronic schizophrenia or the recent onset group.
Inflammatory Markers
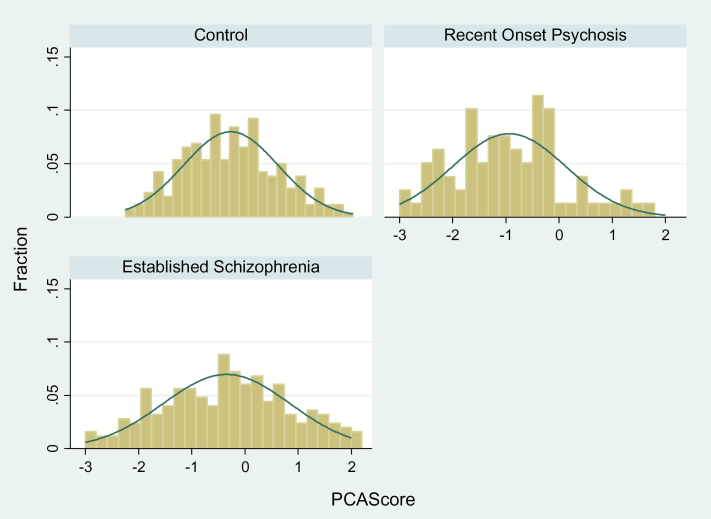
The distributions of the unadjusted composite inflammation scores for each of the diagnostic groups are shown in figure 1. The inflammation composite score was significantly associated with diagnostic group (F [2,587] = 10.86, P < .0001). Post hoc means comparisons tests (Tukey’s HSD) showed the schizophrenia group (mean = −0.34±0.07) to be significantly different from both the recent onset group (mean = −0.94±0.13, P = .0001), as well as from the control group (mean = −0.68±0.07, P = .0017). In both of these instances, the schizophrenia group had higher levels of the composite inflammation score. The recent onset group did not differ significantly from the controls (P = .18), probably due in part to the small sample size of this group and a lack of statistical power to detect a difference.
Fig. 1.
Distribution of composite inflammation score in study populations. Control group n = 260; recent onset psychosis n = 79; and chronic schizophrenia n = 249. The x axis represents the composite score from the principal components analysis (PCA). The y axis represents the proportion of the population under study.
Each log-transformed marker and the composite score was compared between the recent onset group and the control group and the chronic schizophrenia group and the control group using linear regression adjusting for age, gender, race, maternal education, smoking status, and BMI. As shown in figure 2, the recent onset group differed from the control group for composite inflammation score (coefficient −.298, P = .049). This value indicates that the level of the composite score was lower in the recent onset group than the control group. The chronic schizophrenia group differed from the control group for antibodies to gliadin (coefficient = .333, P = .007); CRP (coefficient = .469, P = <.001); ASCA (coefficient = .382, P = .005); pentraxin 3 (coefficient = .118, P = .023); and the composite inflammation score (coefficient = .453, P = <.001). The level of these markers was higher in the chronic schizophrenia group than in the control group
Fig. 2.
Regression coefficients of individual markers and composite inflammation score for recent onset and chronic schizophrenia groups.
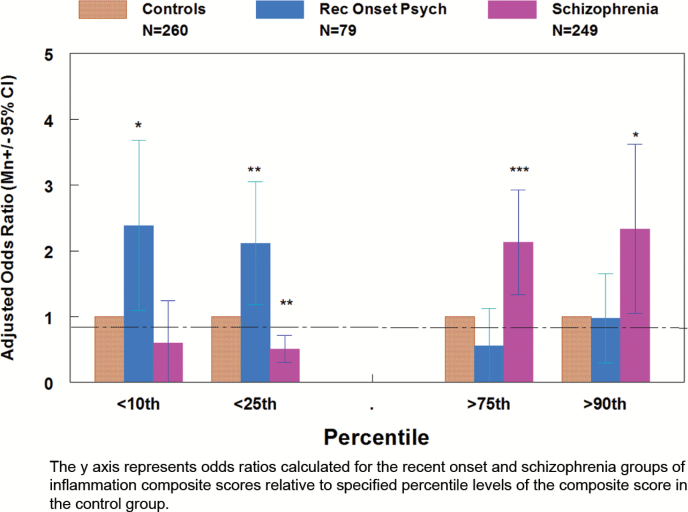
Logistic regression was also used to determine the odds ratios associated with having a relatively high combined inflammation score (defined as greater than or equal to the 75th and 90th percentile of the level in the control group) and a low inflammation score (defined as lesser than or equal to the 10th and the 25th percentile level of the control group) adjusting for age, gender, race, maternal education, smoking status, and BMI. As shown in figure 3, the chronic schizophrenia group had a significantly decreased odds of having a composite inflammation score lesser than or equal to the 25th percentile of the level of the control group (OR = 0.509, 95% CI .303, .852, P = .010) and increased odds of having a composite inflammation score greater than or equal to 75th percentile level and the 90th percentile level of the control group (OR = 2.13, 95% CI 1.38, 3.40, P = .001; OR = 2.33, 95% CI 1.05, 5.20, P = .038). The recent onset group had increased odds of a composite inflammation score lesser than or equal to the 10th percentile and the 25th percentile of the level of the control group (OR = 2.38, 95% CI 1.09, 5.22, P = .030; OR = 2.18, 95% 1.18, 3.79, P = .012).
Fig. 3.
Odds ratios of high and low composite inflammation scores in recent onset and chronic shizophrenia groups.
In terms of the individual markers, the chronic schizophrenia group had increased odds of a pentraxin 3 level greater than or equal to the 75th percentile and the 90th percentile of that of the control group (OR = 2.19, 95% CI 1.33, 3.61, P = .002; OR = 2.05, 95% CI 1.10, 4.14, P = .047) and also of having antibodies to gliadin >= the 75th percentile of that of the control group (OR = 1.76, 95% CI 1.09, 2.84, P = .020. The recent onset group had increased odds of having antibodies to casein lesser than or equal to the 25th percentile of the control group (OR = 1.96, 95% CI 1.11, 3.51, P = .020). The chronic schizophrenia group had decreased odds of a CRP level lesser than or equal to the 25th percentile of the level of the control group (OR = .482, 95% CI .261, .890, P = .020) and increased odds of having a CRP level greater than or equal to the 75th percentile and the 90th percentile of the control group (OR = 2.09, 95% CI 1.27, 3.39, P = .003; OR = 2.12, 95% CI 1.15, 3.92, P = .016). The recent onset group had increased odds of a CRP level lesser than or equal to 25th percentile of the control group (OR = 2.46, 95% CI 1.36, 4.42, P = .003) and decreased odds of a CRP level greater than or equal to 75th percentile level of the controls (OR = .363, 95% CI .154, .855, P = .020). The other comparisons were not significant (P > .05).
Discussion
Our study documents that immune abnormalities are present in individuals with recent onset psychosis and those with chronic schizophrenia compared to nonpsychiatric controls. We employed a factor score to document these abnormalities. This factor score consists largely of measures of innate immunity, which is the component of the immune system largely responsible for the initial response to pathogens and other danger signals.22 Our findings confirm previous genetic and functional studies indicating altered innate immunity in many individuals with schizophrenia23,24 although the immune system is highly complex and the adaptive immune system may also be implicated in schizophrenia.3 Our findings are also consistent with studies indicating that altered innate immunity can alter behavior and cognitive performance in animal model systems.25
It is also of note that there may be other reasons for differences in antibody levels in the population in addition to those related to innate immunity. For example, the dose of antigen, time interval since exposure, genetic factors, and nutritional status may all affect antibody levels.26,27
We also found that, while both individuals with resent onset psychosis and chronic schizophrenia had immune abnormalities as measured by the factor score, the direction of the factor score differed in that individuals with recent onset psychosis had lower scores and individuals with chronic schizophrenia had elevated scores as compared with controls. While our study was cross-sectional in nature, our findings suggest that innate immunity may be depressed early in the course of a psychotic illness and elevated later in the schizophrenia disease process. It is possible that these patients manifest an inherent deficit in innate immunity, a finding which is consistent with genetic studies indicating that individuals with schizophrenia have polymorphisms in genes encoding Toll-like receptors, major constituents of the innate immune response.28 This deficit in innate immunity may coexist with other inherited or acquired immune alterations.29
Innate immunity comprises the first line of resistance against pathogens and plays a key role in activation and orientation of adaptive immunity and in the maintenance of tissue integrity and repair.13 Such a deficit in innate immunity might mean that there is more replication of an infectious agent within the central nervous system before the generation of an effective immune response. For example, exposure to several individual infectious agents such as Toxoplasma gondii in the perinatal period has been associated with an increased risk for schizophrenia.30–32 While exposure to an infectious agent by itself is unlikely to be causative, the immune response to the exposure may exacerbate symptoms. In addition, a decreased level of immunity may lead to an increased pathogen burden due to a decreased ability to clear microbial organisms from the CNS. For example, Toll-like receptors and other components of the innate immune system have been found to be critical components of the individual response to infection with Toxoplasma gondii in humans and experimental animals.33–35 Innate immunity has also been shown to be critical for the replication of other microbial agents within the central nervous system, including viruses24 and bacteria.36 Innate immunity may also be essential for the control of autoimmune processes through the suppression of potentially damaging self-antigens.37
It is of note that there were stronger associations for certain inflammatory markers (CRP, ASCA, antibodies to gliadin) than others (pentraxin, antibodies to casein), the explanation for which is uncertain. Pentraxin3 may be less susceptible to inflammatory signals compared to CRP; in addition, the level of antibodies to casein could be related to genetic differences or differences in exposure.38
The elevation of the inflammation composite score in individuals with chronic schizophrenia confirms and expands on previous studies performed by us and by others which have found elevations of individual markers in this population.1,11,12,14 The reason for the increase in markers of innate immunity during the course of disease are not known with certainty but may be related to a homeostatic increase in immune activation in response to persistent immune suppression or in persistent neuroinflammation resulting from increased exposure to infectious agents.39 It is also possible that environmental exposures related to hospitalization, medications, or other life-style factors result in increased immune activation. It is uncertain if immune dysfunction is part of a causative pathway or the result of protracted illness and consequent life circumstances. A role of inflammation in the causative pathway is supported by perinatal studies which show an association between some immune and infectious markers and the later development of schizophrenia in the offspring,7,40,41 as well as by epidemiological studies showing an increased rate of autoimmune disorders in persons prior to the onset of schizophrenia.8 Support for a causal role also comes from the results of genome-wide association studies which show that the areas of the genome with the strongest association with schizophrenia risk are those involved in the MHC and other immune regulatory regions.10 However, the answer to this important question cannot be determined from a cross sectional study.
Regardless of its role in the etiology of schizophrenia, the elevated inflammation has implications for the physical health of persons with schizophrenia and their risk of cardiac disease and cardiovascular mortality.42,43 Careful monitoring and treatment of conditions associated with cardiac disease is essential in order to reduce the premature mortality found in this population.
Our study was limited by the relatively small sample size of the recent onset group. Also, the study was cross-sectional and we cannot determine the course of immune markers from the time of psychosis onset to later phases of schizophrenia. It is also of note that not all of the participants in the recent onset group had a diagnosis of schizophrenia, so the groups are not comparable in this regard. In addition, we did not control for all of the factors that may contribute to elevations in inflammatory markers. However, we did adjust for demographic variables and also for smoking status and for BMI. The differences between the recent onset and chronic schizophrenia groups in immune markers are not likely to be due to the effects of medication or treatment setting as both groups were receiving antipsychotic medication treatment and were recruited from many of the same treatment settings. However, we cannot rule out that the duration of medication exposure, which differed between the recent onset and the schizophrenia groups, influenced the level of the markers. Strengths of the study include our measurement of multiple immune markers, the laboratory method in which samples from different groups were run together, and the relatively large number of individuals with chronic schizophrenia and nonpsychiatric controls.
To follow up on the findings from this study, further investigations are needed to follow individuals longitudinally from the time of psychosis onset, or preferably earlier, through later phases of schizophrenia to determine the course of immune abnormalities. Additional studies should also be performed to determine if low CRP, possibly reflecting greater susceptibility to infections, combined with elevated antibody levels to toxoplasmosis confers greater risk for schizophrenia.
Funding
Stanley Medical Research Institute (# 07R-1690).
Acknowledgments
R.Y. is a member of the Stanley Medical Research Institute Board of Directors and Scientific Advisory Board. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1. Suvisaari J, Loo BM, Saarni SE, et al. Inflammation in psychotic disorders: a population-based study. Psychiatry Res. 2011;189:305–311. [DOI] [PubMed] [Google Scholar]
- 2. Singh B, Chaudhuri TK. Role of C-reactive protein in schizophrenia: an overview. Psychiatry Res. 2014;216:277–285. [DOI] [PubMed] [Google Scholar]
- 3. Müller N. Immunology of schizophrenia. Neuroimmunomodulation. 2014;21:109–116. [DOI] [PubMed] [Google Scholar]
- 4. Müller N, Myint AM, Schwarz MJ. Inflammation in schizophrenia. Adv Protein Chem Struct Biol. 2012;88:49–68. [DOI] [PubMed] [Google Scholar]
- 5. Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73:951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kinney DK, Hintz K, Shearer EM, et al. A unifying hypothesis of schizophrenia: abnormal immune system development may help explain roles of prenatal hazards, post-pubertal onset, stress, genes, climate, infections, and brain dysfunction. Med Hypotheses. 2010;74:555–563. [DOI] [PubMed] [Google Scholar]
- 7. Miller BJ, Culpepper N, Rapaport MH, Buckley P. Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:92–100. [DOI] [PubMed] [Google Scholar]
- 8. Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–1310. [DOI] [PubMed] [Google Scholar]
- 9. Benros ME, Eaton WW, Mortensen PB. The epidemiologic evidence linking autoimmune diseases and psychosis. Biol Psychiatry. 2014;75:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corvin A, Morris DW. Genome-wide association studies: findings at the major histocompatibility complex locus in psychosis. Biol Psychiatry. 2014;75:276–283. [DOI] [PubMed] [Google Scholar]
- 11. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is elevated in schizophrenia. Schizophr Res. 2013;143:198–202. [DOI] [PubMed] [Google Scholar]
- 12. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7:223–230. [DOI] [PubMed] [Google Scholar]
- 13. Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–183. [DOI] [PubMed] [Google Scholar]
- 14. Dickerson F, Stallings C, Origoni A, et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68:100–104. [DOI] [PubMed] [Google Scholar]
- 15. Severance EG, Dickerson FB, Halling M, et al. Subunit and whole molecule specificity of the anti-bovine casein immune response in recent onset psychosis and schizophrenia. Schizophr Res. 2010;118:240–247. [DOI] [PubMed] [Google Scholar]
- 16. Severance EG, Alaedini A, Yang S, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012;138:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. First M, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical interview for DSM-IV Disorders, Non-Patient Edition. New York, NY: Biometrics Research; 1998. [Google Scholar]
- 18. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 20. Fidell BTL. Using Multivariate Statistics (4th Edition). Boston, MA: Allyn and Bacon; 2001. [Google Scholar]
- 21. Rubin D. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489 [Google Scholar]
- 22. Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci. 2013;53:52–62. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Zhou K, Zhang Z, et al. Label-free quantitative proteomic analysis reveals dysfunction of complement pathway in peripheral blood of schizophrenia patients: evidence for the immune hypothesis of schizophrenia. Mol Biosyst. 2012;8:2664–2671. [DOI] [PubMed] [Google Scholar]
- 24. Carty M, Reinert L, Paludan SR, Bowie AG. Innate antiviral signalling in the central nervous system. Trends Immunol. 2014;35:79–87. [DOI] [PubMed] [Google Scholar]
- 25. Lin CW, Chen CY, Cheng SJ, Hu HT, Hsueh YP. Sarm1 deficiency impairs synaptic function and leads to behavioral deficits, which can be ameliorated by an mGluR allosteric modulator. Front Cell Neurosci. 2014;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pothlichet J, Quintana-Murci L. The genetics of innate immunity sensors and human disease. Int Rev Immunol. 2013;32:157–208. [DOI] [PubMed] [Google Scholar]
- 27. Huang E, Wells CA. The ground state of innate immune responsiveness is determined at the interface of genetic, epigenetic, and environmental influences. J Immunol. 2014;193:13–19. [DOI] [PubMed] [Google Scholar]
- 28. Kang WS, Park JK, Lee SM, Kim SK, Park HJ, Kim JW. Association between genetic polymorphisms of Toll-like receptor 2 (TLR2) and schizophrenia in the Korean population. Gene. 2013;526:182–186. [DOI] [PubMed] [Google Scholar]
- 29. Smyth AM, Lawrie SM. The neuroimmunology of schizophrenia. Clin Psychopharmacol Neurosci. 2013;11:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–1037. [DOI] [PubMed] [Google Scholar]
- 31. Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. [DOI] [PubMed] [Google Scholar]
- 32. Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, et al. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry. 2007;61:688–693. [DOI] [PubMed] [Google Scholar]
- 33. Atmaca HT, Kul O, Karakuş E, Terzi OS, Canpolat S, Anteplioğlu T. Astrocytes, microglia/macrophages, and neurons expressing Toll-like receptor 11 contribute to innate immunity against encephalitic Toxoplasma gondii infection. Neuroscience. 2014;269:184–191. [DOI] [PubMed] [Google Scholar]
- 34. Beiting DP, Peixoto L, Akopyants NS, et al. Differential induction of TLR3-dependent innate immune signaling by closely related parasite species. PLoS One. 2014;9:e88398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat Rev Immunol. 2014;14:109–121. [DOI] [PubMed] [Google Scholar]
- 36. Cooley ID, Chauhan VS, Donneyz MA, Marriott I. Astrocytes produce IL-19 in response to bacterial challenge and are sensitive to the immunosuppressive effects of this IL-10 family member. Glia. 2014;62:818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Poggi A, Zocchi MR. NK cell autoreactivity and autoimmune diseases. Front Immunol. 2014;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rubicz R, Yolken R, Alaedini A, et al. Genome-wide genetic and transcriptomic investigation of variation in antibody response to dietary antigens. Genet Epidemiol. 2014;38:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shastri A, Bonifati DM, Kishore U. Innate immunity and neuroinflammation. Mediators Inflamm. 2013;2013:342931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown AS, Hooton J, Schaefer CA, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–895. [DOI] [PubMed] [Google Scholar]
- 41. Gardner RM, Dalman C, Wicks S, Lee BK, Karlsson H. Neonatal levels of acute phase proteins and later risk of non-affective psychosis. Transl Psychiatry. 2013;3:e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dickerson F, Stallings C, Origoni A, Schroeder J, Khushalani S, Yolken R. Mortality in schizophrenia: clinical and serological predictors. Schizophr Bull. 2014;40:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. [DOI] [PubMed] [Google Scholar]