Abstract
Objective: Recent studies question whether the risk for psychotic disorder associated with prenatal exposure to infection are due to infections per se, or to shared susceptibility of both infections and psychiatric disorders. Moreover, the potential link between prenatal infection and serious infections during childhood, another alleged risk factor for psychotic disorder, remains unknown. The aim of this study was to investigate the role of maternal infections during pregnancy in context of parental psychiatric disorders and subsequent childhood infections. Method: All children born in Sweden 1978–1997 were linked to the National Patient Register. Hazard ratios of nonaffective psychosis were estimated in relation to maternal infection during pregnancy and odds ratios of childhood infection were calculated in relation to maternal infection during pregnancy. Relative excess risk due to interaction (RERI) estimated biological synergism between parental psychiatric disorder and maternal infection during pregnancy, and between maternal infection during pregnancy and childhood infection. Results: Maternal infection during pregnancy was not statistically significantly associated with offspring psychosis (adjusted hazard ratio: 1.06, 95% CI 0.88–1.27). However, maternal infection during pregnancy and maternal psychiatric disorders acted synergistically in offspring psychosis development (RERI 1.33, 95% CI 0.27–2.38). Maternal infection during pregnancy increased the risk of offspring childhood infections (OR 1.50, 95% CI 1.45–1.54). These 2 factors also interacted in psychosis development (RERI 0.63, 95% CI 0.12–1.14). Conclusions: Among mothers with a history of psychiatric disease, infection during pregnancy increases the risk of psychosis in offspring. Maternal infections during pregnancy appear to contribute to the risk of childhood infections, which together render the child more vulnerable to psychosis development.
Key words: schizophrenia, psychosis, prenatal, fetal, interaction
Introduction
Numerous studies, with different designs, have examined the association between various types of maternal infections during pregnancy and offspring psychotic disorder. Some studies report an association while others do not, reviewed by Khandaker et al.1
Clarke et al,2 found no significant association between maternal pyelonephritis during pregnancy and schizophrenia or psychotic disorder in the offspring. However, they did report a synergistic effect between a family history of psychosis and maternal pyelonephritis on the risk of schizophrenia, suggesting that those who have a familial liability of psychotic disorder are more sensitive to exposure to certain infections during fetal life than those without.
Nielsen et al3 recently reported that any maternal hospitalization for infection during pregnancy was modestly associated with later development of schizophrenia in offspring. They also found equally strong associations between maternal and paternal infection before, during, or after pregnancy and schizophrenia development in the child and suggest a genetic propensity for infection as an explanation to their finding, rather than the maternal infection during pregnancy per se.
In addition to data from genetic studies implying a role for the immune system in the etiology of psychosis,4 recent studies indicate that defects in innate immunity among individuals with nonaffective psychosis are present already at birth.5,6 These findings, along with the observation that hospitalization for infection during both childhood and adult life are associated with psychotic disorder,7–10 not only supports the hypothesis of deficient immune responses in schizophrenia11 but also hints at a “multiple-hit” scenario,12 where exposure to maternal infection during fetal life may increase both susceptibility and neurodevelopmental vulnerability to infections later in life.
Current knowledge, however, leaves many questions unanswered: Are maternal infections during pregnancy associated with psychotic disorders in the offspring when important confounders have been taken into account? Is type of infection (bacteria or virus) or its timing (with regard to trimester) important? Are previously reported synergistic effects between parental history of psychosis and maternal infection observed in both mothers and fathers? Are maternal infections before or during pregnancy associated with infections during childhood (perhaps because of genetic vulnerability or fetal programming) and are such infections interacting in the causation of psychosis? In an attempt to address these outstanding questions, we here present findings from a longitudinal study using a large population-based cohort of nearly 2 million individuals and their parents. We investigate the role of maternal infections during pregnancy in the context of parental psychiatric disorder, familial susceptibility to infections, and subsequent childhood infections. We consider multiple confounding factors including factors related to the likelihoods of someone contracting infections and being admitted to a hospital, such as; socio economic position, urban birth, parental immigration as well as hospitalization for other causes, all of which are also associated with psychosis.
Methods
Registers
This study is based on Psychiatry Sweden, a linkage of several health and population registers created for studies of the etiology of psychiatric disorders). The National Patient Register (NPR) was used for identification of infection and nonaffective psychoses. The NPR includes virtually all inpatient care in Sweden since 1973, and psychiatric outpatient visits since 2001.13 Data on perinatal variables was retrieved from the Medical Birth Register (MBR), initiated in 1973 and including data from the prenatal, delivery, and neonatal periods from almost all deliveries in Sweden.14 We obtained data on single households from The Population and Housing Census, administered every 5 years and including, by law, all individuals registered and living in Sweden with information on demographic data.15 Data on socioeconomic status in terms of disposable income adjusted for family size and highest education level among parents were retrieved from the Longitudinal Integration Database for Health Insurance and Labor market studies, updated annually since 1990 and integrating information from the labor market, educational and social sectors.15 We used the Total Population Register, initiated 1968,15 to determine year of emigration if any. Date of death was retrieved from the National Cause of Death Register.
Study Population
All children born in Sweden between 1978 and 1997 who were alive on their 13th birthday were identified in the MBR. Individuals without a registered biological mother and father, and those not living in Sweden at age 13 were excluded.
Assessment of Exposure
All diagnoses of infection were identified according to ICD-8, -9, and -10 codes (supplementary table S1). We omitted all codes including “sequel” and “post-”, and extracted the primary diagnoses except when the pregnant women had been given a primary diagnosis of obstetric conditions (ICD-8 630–679, ICD-9 630–677, and ICD-10 Chapter O). In those cases, the first diagnosis of infection of the 7 secondary diagnoses was extracted. Pregnancy was defined as first day of last menstrual period (LMP) before pregnancy up until the day of delivery. “Trimesters” were defined as: zeroth: LMP-35days (before uteroplacental circulation is established), first: 36–97 days, second: 98–188 days, and third: 189 days-delivery.
Psychotic Disorder
Caseness was defined as receipt of in- or outpatient care with diagnoses of nonaffective psychosis, ICD-10 F20-29 and ICD-9 295, 297, and 298 except 298A and B.
Covariates
Based on the literature, the following covariates were considered as potential confounding factors: winter birth (ie, child born December–May16); urban birth (child born in municipality with ≥200 000 inhabitants in 1980)17; small for gestational age (SGA)18; mother or father ≥ 35 years at time of birth19; either parent born outside Sweden20; single-parent household21; disposable family income on individual level (in quintiles according to birth year using the highest quintile as reference)21; highest education among parents (<9 y, 9 y, 10–12 y, >12 y [reference], and missing [“missing” was given a value because of a rather large amount of missing information on this variable in the registers])21; and parental diagnoses of psychotic (ICD-10 F20-29) or other psychiatric disorders (ICD-10 F00-99) until December 31, 2011.22 Family tendency of seeking hospital care or becoming hospitalized was parameterized as hospitalization of either parent with any diagnosis other than infection or a psychiatric disorder up to 5 years before or during pregnancy.10
Statistical Methods
We calculated hazard ratios (HR) and 95% CI for nonaffective psychosis using Cox regression. Children were followed from 13 years of age until diagnosis of nonaffective psychosis, death, emigration, or December 31, 2011, whichever came first. In the basic model, all HRs were adjusted for sex of child and birth year as a centered, quadratic term (in order to allow for potential nonlinear relationships between the diagnosis of psychosis and time). Adjusted models included the basic adjustment as well as the covariates described in the preceding section. In order to separate the potential effects of infections during pregnancy from a high familial liability to serious infections, the analyses examining the risk of psychosis in relation to maternal infections were repeated after excluding mothers with a hospital diagnosis of infection during the 5 years prior to the index pregnancy (referred to as the restricted analyses in figures 1A, 1B, and 2A). Conversely, in order to examine the potential influence of familial liability to infection separate from possible biological mechanisms that could occur with infection during pregnancy, mothers with infection during pregnancy were excluded in restricted analyses examining the risk of psychosis in relation to parental infections occurring prior to pregnancy (referred to as the restricted analyses in figures 1C, 1D, and 2B).
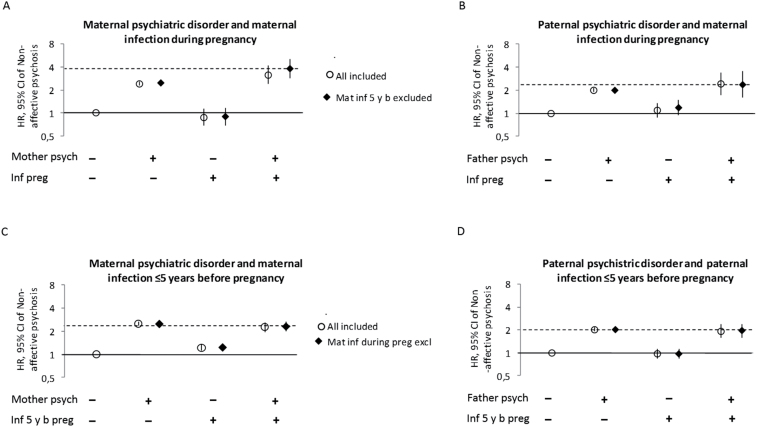
Fig. 1.
Risk of nonaffective psychoses (hazard ratio and 95% CI) among individuals with and without maternal infection during pregnancy, parental infection sometime in the 5 y before pregnancy, and parental psychiatric disorder. Fully adjusted for birth year, sex, urban birth, winter birth, parental age ≥ 35 y, small for gestational age, parent born outside Sweden, low socioeconomic status, any parent any inpatient care before or during pregnancy except for treatment of infection or psychiatric care. Circles represent the general population and diamonds the restricted model.
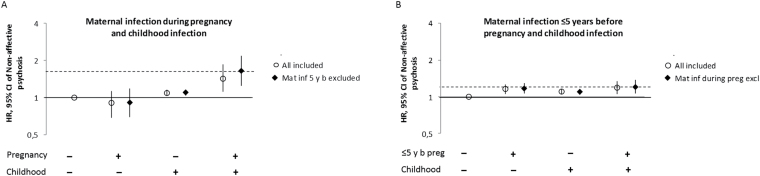
Fig. 2.
Risk of nonaffective psychoses (hazard ratio and 95% CI) following exposure to maternal infection during pregnancy (A) or before pregnancy (B), and childhood infection. Fully adjusted for birth year, sex, urban birth, winter birth, parental age ≥ 35 y, small for gestational age, parental history of psychiatric disorder, parent born outside Sweden, low socioeconomic status, any parent any inpatient care before or during pregnancy except for treatment of infection or psychiatric care, and for the child during childhood (0–13 y). Circles represent the general population and diamonds the restricted model.
The association between maternal infection during pregnancy and childhood infection among offspring was calculated as an OR and 95% CI using logistic regression, adjusted as detailed above. We then stratified the population on the basis of a diagnosis of nonaffective psychosis and repeated the analyses. To test for interaction on the additive scale, implicating causal or biological interaction, the relative excess risk due to interaction (RERI = RR11 − RR10 − RR01 + 1) was calculated for the risks of offspring nonaffective psychosis associated with parental infection prior to or during pregnancy, and/or parental psychiatric disorders.23 Both the point estimate and the 95% CI of RERIs were assessed using a method accounting for the asymmetric distribution of confidence limits for hazard ratios.24 Likewise, a possible interaction between maternal infections prior to or during pregnancy and childhood infections in the development of nonaffective psychosis was calculated using RERI. RERI > 0 implies presence of positive additive interaction. Statistical analyses were made using IBM SPSS statistics 22.0 (IBM).
Approval
Ethical approval has been sought and granted from the Regional Ethics Committee of Stockholm, EPN. Dnr 2010/1185-31/5.
Results
A total of 1 971 623 children were followed up in the registers. Altogether, 8330 (0.4%) of the children were subsequently diagnosed with nonaffective psychosis between ages 13 and 33. As expected, children who later developed nonaffective psychosis tended to be male, have older parents and parental histories of immigration, psychiatric disorders and hospital admissions prior to the birth of the index child. These children were also more often born in urban environments, born small for gestational age and brought up in a family with low socio-economic status (table 1). Associations between the covariates and the exposures and the outcome can be found in supplementary table S2.
Table 1.
Characteristics of the Study Population
| All Children Born in Sweden 1978–1997a, N = 1 971 623 | ||||||
|---|---|---|---|---|---|---|
| No Diagnosis of Nonaffective Psychosisb (n =1 963 293, 99.6%) | Diagnosis of Nonaffective Psychosisb (n = 8330, 0.4%) | |||||
| n | % | n | % | |||
| Male | 1 007 074 | 51.3 | 4872 | 58.5 | ||
| Urban birthc | 303 258 | 15.4 | 1601 | 19.2 | ||
| Born December–May | 1 015 884 | 51.7 | 4306 | 51.7 | ||
| Small for gestational age | With information | 53 217 | 2.7 | 339 | 4.1 | |
| Missing | 49 120 | 2.5 | 191 | 2.3 | ||
| Parent ≥35 y at time of birth | Mother | 221 721 | 11.3 | 1065 | 12.8 | |
| Father | 525 930 | 26.8 | 2449 | 29.4 | ||
| Parent born outside Sweden | Motheri | 236 555 | 12.0 | 1443 | 17.3 | |
| Father | 250 346 | 12.8 | 1579 | 19.0 | ||
| Parent psychotic disorderd | Mother | 16 020 | 0.8 | 420 | 5.0 | |
| Father | 13 372 | 0.7 | 288 | 3.5 | ||
| Parent psychiatric disordere | Mother | 160 795 | 8.2 | 1736 | 20.8 | |
| Father | 171 465 | 8.7 | 1636 | 19.6 | ||
| Socioeconomic status | Any parent highest education | <9 y | 37 501 | 1.9 | 331 | 4.0 |
| 9 y | 141 629 | 7.2 | 828 | 9.9 | ||
| 10–12 y | 959 081 | 48.9 | 3881 | 46.6 | ||
| >12 y | 823 026 | 41.9 | 3272 | 39.3 | ||
| missing | 2056 | 0.1 | 18 | 0.2 | ||
| Single-parent household | 175 564 | 8.9 | 1397 | 16.8 | ||
| Disposable incomef | First quintile (lowest) | 392 659 | 20.0 | 1926 | 23.1 | |
| Second quintile | 392 659 | 20.0 | 1726 | 20.7 | ||
| Third quintile | 392 658 | 20.0 | 1644 | 19.7 | ||
| Fourth quintile | 392 658 | 20.0 | 1582 | 19.0 | ||
| Fifth quintile (highest) | 392 659 | 20.0 | 1452 | 17.4 | ||
| Maternal hospital admission with infectiong during pregnancy | 23 667 | 1.2 | 117 | 1.4 | ||
| Hospital admission with infectiong 5 y prior to pregnancy | Mother | 130 532 | 6.6 | 706 | 8.5 | |
| Father | 64 894 | 3.3 | 283 | 3.4 | ||
| Any inpatient careh up to 5 y before pregnancy | Mother | 388 796 | 19.8 | 1830 | 22.0 | |
| Father | 267 522 | 13.6 | 1238 | 14.9 | ||
| Any inpatient careh during pregnancy | Mother | 118 972 | 6.1 | 691 | 8.3 | |
| Father | 50 055 | 2.5 | 270 | 3.2 | ||
| Admission with infectiong during childhood 0–13 y | 460 416 | 23.5 | 2335 | 28.0 | ||
Note: aWith information on biological parents and living in Sweden at 13 y of age. Individuals with missing information on variables were excluded, 1046 in total).
bICD-10 F20-29.
c>200 000 habitants 1980.
dICD-10 F20-29.
eICD-10 F00-99.
fPer family member.
gSee supplementary table S1.
hExcept with diagnoses ICD-10 F00-99, or with infection.g
i67% Europe (40% from Finland), 20% Asia (60% from the Middle East), 6% Africa (22% from Etiopia), 5% South America (65% from Chile), 1% North America (86% from the United States).
In the total population, there was a small but significantly increased risk of developing nonaffective psychosis among children whose mothers were hospitalized for infection during pregnancy, HR 1.26, 95% CI: 1.05, 1.52 (table 2). However, this association disappeared in the adjusted model. Adjustments for parental psychiatric disorder, previous parental hospital admission, and low socioeconomic status among parents attenuated the association the most. Risk for nonaffective psychosis did not vary by type of infection (bacterial or viral; table 2). Infection during the third trimester tended to increase risk of nonaffective psychosis in the offspring, (HR 1.23, 95% CI: 0.96, 1.58), whereas infections during other trimesters did not (table 2). Frequences of the most common diagnoses of infection among the pregnant women can be found in supplementary table S3. Maternal, but not paternal, admission for infection up to 5 years prior to pregnancy was weakly associated with offspring psychosis (HR 1.14, 95% CI: 1.05, 1.23) in the fully adjusted model (table 2).
Table 2.
Associations Between Nonaffective Psychosis and Parental Hospital Admission With Infection Among Individuals Born in Sweden 1978–1997, Hazard Ratios (HR) and 95% CI
| Mother Hospital Admitted With Infection During Pregnancy | N, Nonaffective Psychosis/ No Diagnosis of Nonaffective Psychosis | Basic Modela | Model 1b | Model 2c | Model 3d | Model 4e |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Any infection | 117/ 23 667 | 1.26 (1.05, 1.52) | 1.23 (1.02, 1.48) | 1.13 (0.94, 1.35) | 1.07 (0.89, 1.28) | 1.06 (0.88, 1.27) |
| Bacterial infection | 66/ 13 018 | 1.31 (1.02, 1.66) | 1.27 (1.00, 1.62) | 1.15 (0.90, 1.47) | 1.09 (0.85, 1.38) | 1.08 (0.84, 1.37) |
| Viral infection | 18/ 4041 | 1.04 (0.66, 1.66) | 1.00 (0.63, 1.58) | 0.91 (0.58, 1.45) | 0.86 (0.54, 1.36) | 0.85 (0.54, 1.36) |
| Other infection | 32/ 6694 | 1.26 (0.89, 1.78) | 1.23 (0.87, 1.74) | 1.15 (0.81, 1.63) | 1.11 (0.78, 1.57) | 1.10 (0.78, 1.56) |
| Zeroth trimester | 7/ 1424 | 1.10 (0.53, 2.32) | 1.03 (0.49, 2.16) | 0.92 (0.44, 1.93) | 0.86 (0.41, 1.81) | 0.85 (0.40, 1.79) |
| First trimester | 12/ 3369 | 0.91 (0.52, 1.60) | 0.88 (0.50, 1.55) | 0.79 (0.45, 1.39) | 0.74 (0.42, 1.30) | 0.73 (0.41, 1.28) |
| Second trimester | 38/ 8354 | 1.15 (0.84, 1.58) | 1.11 (0.81, 1.53) | 1.02 (0.74, 1.40) | 0.96 (0.70, 1.32) | 0.95 (0.69, 1.31) |
| Third trimester | 61/ 11 164 | 1.42 (1.11, 1.83) | 1.40 (1.09, 1.81) | 1.29 (1.00, 1.66) | 1.24 (0.96, 1.59) | 1.23 (0.96, 1.58) |
| Mother hospitalized with infection sometime in the 5 y before pregnancy | 706/ 130 532 | 1.33 (1.23, 1.44) | 1.29 (1.19, 1.39) | 1.19 (1.10, 1.28) | 1.13 (1.05, 1.22) | 1.14 (1.05, 1.23) |
| Father hospitalized with infection sometime in the 5 y before pregnancy | 283/ 64 894 | 1.09 (0.97, 1.23) | 1.06 (0.94, 1.19) | 1.00 (0.89, 1.13) | 0.97 (0.86, 1.09) | 0.97 (0.86, 1.09) |
Note: SES, socioeconomic status.
aAdjusted for year of birth and sex.
bAdditionally adjusted for mother or father with psychotic disorder (ICD-10 F20-29).
cAdditionally adjusted for mother or father with psychiatric disorder (ICD-10 F00-99).
dAdditionally adjusted for SES, and any parent any inpatient care before or during pregnancy except for treatment of infection or psychiatric care.
eAdditionally adjusted for urban birth, winter birth, parental age ≥ 35 y, small for gestational age, and parent born outside Sweden.
In contrast, among individuals whose parents were diagnosed with a psychiatric disorder, exposure to maternal infection during pregnancy was significantly associated with psychosis development in the fully adjusted model, HR 1.30, 95% CI: 1.02, 1.66. Among individuals with parents without a psychiatric disorder there was no association, HR 0.84, 95% CI: 0.63, 1.11.
Further exploration of the role of psychiatric disorder in the family indicated an interaction between maternal psychiatric disorder and maternal infection during pregnancy on the child’s risk of developing psychosis (RERI 0.79, 95% CI: −0.02, 1.60) (figure 1A), particularly among mothers who were not hospitalized for infections before pregnancy (RERI 1.33, 95% CI: 0.27, 2.39), and therefore not predisposed to inpatient care for infections. Interestingly, psychiatric disorders among fathers did not interact with maternal infection during pregnancy on the risk for psychoses in the offspring (RERI 0.14 95% CI: −0.59, 0.87, figure 1B). Moreover, maternal, but not paternal, infection during 5 years prior to pregnancy was weakly but significantly associated with psychosis development in offspring, irrespective of maternal psychiatric disorder (figures 1C and 1D). There was no indication of synergism between maternal psychiatric disorder and maternal infection prior to pregnancy (RERI −0.50, 95% CI: −0.82, −0.17) or between paternal psychiatric disorder and paternal infection prior to pregnancy (RERI −0.08, 95% CI: −0.49, 0.32) on psychosis risk in the offspring.
We next investigated whether maternal infections during pregnancy per se or maternal predisposition for infections (ie, infections prior to pregnancy) were associated with increased risk of childhood infections. The odds of being hospitalized with infection during childhood increased following exposure to maternal infection during prenatal life (OR 1.50, 95% CI: 1.45, 1.54), particularly among children who would later develop nonaffective psychosis (OR 2.12, 95% CI: 1.46, 3.07; table 3). Maternal infection prior to pregnancy increased the risk of childhood infection, both in the general population (OR 1.37, 95% CI: 1.35, 1.38) and, to a similar extent, among individuals who later developed nonaffective psychosis (OR 1.29, 95% CI: 1.10, 1.53; table 3). Finally, we analyzed if the combination of maternal and childhood exposures to infections further increased the risk for psychoses in the child. We observed an interaction between maternal infection during pregnancy and childhood infection in terms of the risk of nonaffective psychosis (RERI 0.45, 95% CI: 0.03, 0.87) (figure 2A). The relationship was stronger when women who had been hospitalized for infections before pregnancy were excluded from the analysis (RERI 0.63, 95% CI: 0.12, 1.14). There was no evidence of interaction between maternal infection during 5 years prior to pregnancy and later childhood infection in terms of psychosis risk (RERI −0.07, 95% CI: −0.25, 0.11) (figure 2B).
Table 3.
Associations Between Maternal Infection During Pregnancy or Maternal Infection Sometime in the 5 y Before Pregnancy and Childhood Infection Among the Total Population and Among Individuals With Nonaffective Psychosis, OR and 95% CI
| Maternal Infection During Pregnancy | N, Childhood Infection/ No Childhood Infection | Basic Modela | Adjusted Modelb | Maternal Infection ≤5 y Before Pregnancy | N, Childhood Infection/ No Childhood Infection | Basic Modela | Adjusted Modelb | |
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||
| Total population | Unexposed | 454 825/ 1 493 014 | 1 (reference) | 1 (reference) | Unexposed | 422 024/ 1 418 361 | 1 (reference) | 1 (reference) |
| Exposed | 7926/ 15 858 | 1.66 (1.61, 1.70) | 1.49 (1.45, 1.53) | Exposed | 40 727/ 90 511 | 1.51 (1.49, 1.53) | 1.37 (1.35, 1.38) | |
| Individuals with nonaffective psychosis | Unexposed | 2277/ 5936 | 1 (reference) | 1 (reference) | Unexposed | 2081/ 5543 | 1 (reference) | 1 (reference) |
| Exposed | 58/ 59 | 2.58 (1.79, 3.72) | 2.12 (1.46, 3.07) | Exposed | 254/ 452 | 1.49 (1.27, 1.76) | 1.29 (1.10, 1.53) |
Note: aAdjusted for birth year and sex.
bAdditionally adjusted for urban birth, winter birth, small for gestational age, parental age ≥ 35 y, parental history of psychiatric disorder, parent born outside Sweden, low SES, any parent any inpatient care before or during pregnancy except for treatment of infection or psychiatric care, and for the child during childhood (0–13 y).
Discussion
In this, the largest population-based cohort study to date, we found no evidence that hospitalization for infection during pregnancy was associated with nonaffective psychosis in the offspring overall. Consideration of the type of pathogen (bacteria, virus, or other) and timing of infection did not change this null association. Previous studies have found an association between schizophrenia or nonaffective psychoses in the offspring and specific groups of maternal infections and it is possible that specific maternal infections, not captured by our current approach, are more hazardous to the fetus than others. For example, acute infection with influenza during pregnancy25 and chronic infection (where initial exposure can occur outside of pregnancy) with Toxoplasma gondii 26–28 have previously been associated with the development of psychoses in the offspring. Nielsen et al, who previously also investigated any maternal infection, did find a modest association with schizophrenia. However, they did not adjust for some of the confounders included here, which may explain the somewhat discrepant results between their study and ours. In particular, adjustment for parental psychiatric disorders (not only psychotic disorders), low socioeconomic status, and parental hospital care-seeking patterns attenuated the associations between maternal infection during pregnancy and nonaffective psychoses in the offspring in our study. Solely adjusting for parental psychotic disorders does not appear to fully account for confounding by familial mental and social disadvantages.
We found evidence for a modest familial susceptibility to be hospitalized for infection among individuals with nonaffective psychosis; admission with maternal infection during the 5 years prior to pregnancy was weakly associated with offspring psychosis, but the fact that paternal admission with infection before pregnancy was not associated speaks against a general parental liability for infections among patients with nonaffective psychoses, as reported for patients with schizophrenia by Nielsen et al.3 Their study, however, included parental infections “after birth” of the index child, where familial transmission of infections can occur.29,30
A genetic predisposition to psychosis in conjunction with 1 or more environmental insults have been proposed in the etiology of psychosis.31 We found evidence for synergism between maternal psychiatric disorder (a proxy marker of genetic predisposition in the mother as well as the child) and infection during pregnancy on psychosis risk in the offspring, which is in agreement with previous observations by Clarke et al2 and Nielsen et al.3 Importantly, exposure to maternal infection did not appear to interact with paternal psychiatric disorder (a proxy for genetic predisposition in the father as well as the child). Neither did synergism occur between parental psychiatric disorder and parental infections prior to pregnancy, which would indeed be unlikely to influence a future pregnancy unless the infectious agent resulted in a persistent infection.32 Taken together, these results indicate an effect of acute infections during pregnancy specific to vulnerable mothers on the risk for the offspring to later develop nonaffective psychosis.
Many of the genetic loci that so far have been associated with schizophrenia are located within the major histocompatibility (MHC) region on chromosome 6, a region dense with genes linked to both immunity and brain development.4 Interestingly, genetic risk for schizophrenia, both within and outside the MHC-region, appears to overlap with risk for other psychiatric diagnoses,33 which suggests that genetic variation in many regions, including the MHC region, may explain some of the interaction between maternal psychiatric disease and infection. Indeed both common and rare genetic variation in innate and adaptive immune response genes have been reported to contribute to both susceptibility and outcome of infectious disease with a major, but not exclusive, role for genes in the MHC region, reviewed in ref.34 However, a number of environmental factors among mothers with psychiatric disease, such as medication, life-style or body mass index, can also potentially explain the observed interaction, only partially accounted for by our adjustments.
While there are no studies on acute maternal infections, some chronic maternal infections have been reported to have persistent effects on the child’s innate immune system for years in the absence of transmission of the pathogen.32 The biological mechanisms involved in the maternal-fetal interplay relating to the response to infection, and the specific role that the maternal environment might play, remain to be identified. One hypothesis posits that excessive maternal immune activation is the common effector mechanism linking exposure to a number of different agents to the pathogenesis of psychosis in the offspring. While strongly supported by experimental studies,35,36 there is so far little clinical support for this hypothesis.37–39
Regardless of the mechanisms acting during gestation, there are indications of deficiencies relating to innate immunity both among neonates who will develop nonaffective psychosis5 and among adults who have developed psychosis6 suggesting that these individuals are more vulnerable to infections.
Brain development proceeds through early adulthood40 and is therefore potentially continuously vulnerable to disruptive influences which may be more likely to occur in individuals with immune deficits. We and others have previously reported that there is indeed a weak association between hospitalization with infection during childhood or later in life and the later development of psychosis.9,10,41 In our previous study,10 this association was not confounded by parental psychiatric disorders or care seeking habits. The factors determining risk for such childhood infections were however not identified. Here we observed that individuals whose mothers were exposed to infections before and, particularly, during pregnancy were themselves more likely to be hospitalized for infections during childhood. Importantly, exposure to maternal infection during, but not before, pregnancy, and to a subsequent childhood infection acted synergistically on future psychosis risk. These observations indicate that maternal infection during pregnancy can modify, not only the future risk of the offspring to be hospitalized for infection, but also the long-term outcomes of such infections. Contrarily, Betts et al42, previously reported on the influence of maternal vaginal infection on offspring psychotic experience being mediated through childhood illness liability rather than synergism between the 2. However, they used self-reported information on possible exposure to infection in terms of experienced symptoms of vaginal infection during pregnancy and medical attention or symptoms of infection during the child’s first 6 months such as skin rashes, runny nose, and diarrhoea, which may explain our discrepant findings.
It is becoming increasingly apparent that a sensitization by multiple stressors eventually accumulate to the development of psychosis.43 If our observations replicate in independent data sets, they suggest that future studies of risk factors for nonaffective psychoses need to take on the challenging task of having to disentangle the complex interactions between gene variants and environmental exposures during pregnancy and during childhood. Moreover, we found many risk factors for psychosis to be associated with early infection. If their effect is mediated by infection or if there is a possible synergism between these risk factors and early infection in psychosis risk needs attention in future studies.
Limitations
The validity of infectious disease diagnoses in the NPR is high.44 However, our exposure variable included hospital-treated infections only, and hence likely only severe infections. Presumably, the majority of infections do not require hospital care, resulting in misclassification and perhaps underestimation of the association. Our results are not likely generalizable to the wide range of infections a woman could experience during pregnancy, particularly those that are more minor, uncomplicated, and easily resolved.
The proportion of individuals with a diagnosis of nonaffective psychosis (0.4%) in this data set is probably underestimated, mainly because of 2 factors. Firstly, the youngest individuals were only 14 years at the end of follow-up and had not had time to develop and receive a diagnosis. Using Cox regression in the analyses partly accounts for this, because the risks are estimated based on number of individuals per population at risk per day. Second, registration of diagnoses from outpatient care started in 2001 and reached complete coverage in 2005 in Sweden. Since about 25% of individuals with nonaffective psychosis only receive outpatient care,45 some individuals with nonaffective psychosis may not have been included as cases here.
Despite the large data set rather few mothers were hospital admitted for infection during pregnancy. However, the confidence intervals in the analyses are rather narrow, suggesting satisfying power.
Conclusions
In this largest population-based study to date, we report that infections during pregnancy among mothers with psychiatric disorders were associated with an increased risk for nonaffective psychosis in offspring. In contrast, no such association was seen in absence of a maternal psychiatric history.
Exposure to maternal infections before and during pregnancy was associated with subsequent childhood infections. Maternal infections during, but not before, pregnancy interacted with childhood infections in terms of the risk of later psychosis in the child. These findings imply that gestation may indeed be a critical period of exposure and support the “second hit” theory. The risk associated with maternal infections is probably not entirely attributable to familial liability to infections, as previously proposed. From a public health perspective, the implications of an interaction between maternal and childhood infections on future psychosis risk warrants further studies.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
The Swedish Research Council (2006-3002); the regional agreement on medical training and clinical research (20090401).
Supplementary Material
Acknowledgments
We acknowledge the expert statistics advice provided by Susanne Wicks. The authors report no competing interests, and no financial relationships with commercial interests.
References
- 1. Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025–1030. [DOI] [PubMed] [Google Scholar]
- 3. Nielsen PR, Laursen TM, Mortensen PB. Association between parental hospital-treated infection and the risk of schizophrenia in adolescence and early adulthood. Schizophr Bull. 2013;39:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GWAS Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner RM, Dalman C, Wicks S, Lee BK, Karlsson H. Neonatal levels of acute phase proteins and later risk of non-affective psychosis. Transl Psychiatry. 2013;3:e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Müller N, Wagner JK, Krause D, et al. Impaired monocyte activation in schizophrenia. Psychiatry Res. 2012;198:341–346. [DOI] [PubMed] [Google Scholar]
- 7. Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res. 2012;139:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang W, Chikritzhs T. Early childhood infections and risk of schizophrenia. Psychiatry Res. 2012;200:214–217. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen PR, Benros ME, Mortensen PB. Hospital contacts with infection and risk of schizophrenia: a population-based cohort study with linkage of Danish National Registers. Schizophr Bull. 2014;40:1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blomstrom A, Karlsson H, Svensson A, et al. Hospital admission with infection during childhood and risk for psychotic illness—a population-based cohort study. Schizophr Bull. 2014;40:1518–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kinney DK, Hintz K, Shearer EM, et al. A unifying hypothesis of schizophrenia: abnormal immune system development may help explain roles of prenatal hazards, post-pubertal onset, stress, genes, climate, infections, and brain dysfunction. Med Hypotheses. 2010;74:555–563. [DOI] [PubMed] [Google Scholar]
- 12. Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull. 2001;27:457–476. [DOI] [PubMed] [Google Scholar]
- 13. Kvalitet och innehåll i patientregistret 2008. www.socialstyrelsen.se. Accessed October 26, 2014.
- 14. The National Board of Health and Welfare. The Medical Birth Register 2011. http://www.socialstyrelsen.se/register/halsodataregister/medicinskafodelseregistret. Accessed November 3, 2014.
- 15. SCB-data för forskning 2011. In: SCB r, ed. Innehållsbeskrivning Av Olika Register. Örebro, Sweden: SCB, registerenhet; 2011. [Google Scholar]
- 16. Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29:587–593. [DOI] [PubMed] [Google Scholar]
- 17. Harrison G, Fouskakis D, Rasmussen F, Tynelius P, Sipos A, Gunnell D. Association between psychotic disorder and urban place of birth is not mediated by obstetric complications or childhood socio-economic position: a cohort study. Psychol Med. 2003;33:723–731. [DOI] [PubMed] [Google Scholar]
- 18. Dalman C, Allebeck P, Cullberg J, Grunewald C, Köster M. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56:234–240. [DOI] [PubMed] [Google Scholar]
- 19. Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB. Parental age and risk of schizophrenia: a case-control study. Arch Gen Psychiatry. 2003;60:673–678. [DOI] [PubMed] [Google Scholar]
- 20. Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24. [DOI] [PubMed] [Google Scholar]
- 21. Wicks S, Hjern A, Dalman C. Social risk or genetic liability for psychosis? A study of children born in Sweden and reared by adoptive parents. Am J Psychiatry. 2010;167:1240–1246. [DOI] [PubMed] [Google Scholar]
- 22. Dean K, Stevens H, Mortensen PB, Murray RM, Walsh E, Pedersen CB. Full spectrum of psychiatric outcomes among offspring with parental history of mental disorder. Arch Gen Psychiatry. 2010;67:822–829. [DOI] [PubMed] [Google Scholar]
- 23. Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. [DOI] [PubMed] [Google Scholar]
- 24. Zou GY. On the estimation of additive interaction by use of the four-by-two table and beyond. Am J Epidemiol. 2008;168:212–224. [DOI] [PubMed] [Google Scholar]
- 25. Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. [DOI] [PubMed] [Google Scholar]
- 26. Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. [DOI] [PubMed] [Google Scholar]
- 27. Xiao J, Buka SL, Cannon TD, et al. Serological pattern consistent with infection with type I Toxoplasma gondii in mothers and risk of psychosis among adult offspring. Microbes Infect. 2009;11:1011–1018. [DOI] [PubMed] [Google Scholar]
- 28. Blomström A, Karlsson H, Wicks S, Yang S, Yolken RH, Dalman C. Maternal antibodies to infectious agents and risk for non-affective psychoses in the offspring–a matched case-control study. Schizophr Res. 2012;140:25–30. [DOI] [PubMed] [Google Scholar]
- 29. Badger GF, Dingle JH, Feller AE, Hodges RG, Jordan WS, Jr, Rammelkamp CH., Jr A study of illness in a group of Cleveland families. IV. The spread of respiratory infections within the home. Am J Hyg. 1953;58:174–178. [DOI] [PubMed] [Google Scholar]
- 30. Gamba MA, Martinelli M, Schaad HJ, et al. Familial transmission of a serious disease–producing group A streptococcus clone: case reports and review. Clin Infect Dis. 1997;24:1118–1121. [DOI] [PubMed] [Google Scholar]
- 31. Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. [DOI] [PubMed] [Google Scholar]
- 32. Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12:330–340. [DOI] [PubMed] [Google Scholar]
- 33. Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chapman SJ, Hill AV. Human genetic susceptibility to infectious disease. Nat Rev Genet. 2012;13:175–188. [DOI] [PubMed] [Google Scholar]
- 35. Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. [DOI] [PubMed] [Google Scholar]
- 36. Bauman MD, Iosif AM, Smith SE, Bregere C, Amaral DG, Patterson PH. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry. 2014;75:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun. 2001;15:411–420. [DOI] [PubMed] [Google Scholar]
- 38. Brown AS, Hooton J, Schaefer CA, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–895. [DOI] [PubMed] [Google Scholar]
- 39. Canetta S, Sourander A, Surcel HM, et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014;171:960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–266. [DOI] [PubMed] [Google Scholar]
- 41. Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–1310. [DOI] [PubMed] [Google Scholar]
- 42. Betts KS, Williams GM, Najman JM, Scott J, Alati R. Maternal prenatal infection, early susceptibility to illness and adult psychotic experiences: a birth cohort study. Schizophr Res. 2014;156:161–167. [DOI] [PubMed] [Google Scholar]
- 43. Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jörgensen L, Ahlbom A, Allebeck P, Dalman C. The Stockholm non-affective psychoses study (snaps): the importance of including out-patient data in incidence studies. Acta Psychiatr Scand. 2010;121:389–392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.