Abstract
Context
Healthcare worker exposure to antineoplastic drugs continues to be reported despite safe handling guidelines published by several groups. Sensitive sampling and analytical methods are needed so that occupational safety and health professionals may accurately assess environmental and biological exposure to these drugs in the workplace.
Objective
To develop liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) analytical methods for measuring five antineoplastic drugs in samples from the work environment, and to apply these methods in validating sampling methodology. A single method for quantifying several widely used agents would decrease the number of samples required for method development, lower cost, and time of analysis. Methods for measuring these drugs in workers’ urine would also be useful in monitoring personal exposure levels.
Results
LC-MS/MS methods were developed for individual analysis of five antineoplastic drugs in wipe and air sample media projected for use in field sampling: cyclophosphamide, ifosfamide, paclitaxel, doxorubicin, and 5-fluorouracil. Cyclophosphamide, ifosfamide, and paclitaxel were also measured simultaneously in some stages of the work. Extraction methods for air and wipe samples were developed and tested using the aforementioned analytical methods. Good recoveries from the candidate air and wipe sample media for most of the compounds, and variable recoveries for test wipe samples depending on the surface under study, were observed. Alternate LC-MS/MS methods were also developed to detect cyclophosphamide and paclitaxel in urine samples.
Conclusions
The sampling and analytical methods were suitable for determining worker exposure to antineoplastics via surface and breathing zone contamination in projected surveys of healthcare settings.
Keywords: Antineoplastic drugs, analytical methods, biomonitoring, healthcare workers, exposure assessment
Introduction
Since their introduction in the 1940s, antineoplastic drugs (AD) have been widely used to treat cancers. They have also been shown to exhibit a number of acute and chronic toxic effects including carcinogenicity, mutagenicity, and teratogenicity. Based upon animal and human studies, the International Agency for Research on Cancer (IARC) has classified many of these drugs as known or probable human carcinogens.1 In addition, some of these compounds have been associated with adverse reproductive outcomes, such as fetal loss, congenital malformations, and infertility.2 In the late 1970s it was realized that healthcare workers are potentially exposed to AD while preparing and administering these drugs.3
The Occupational Safety and Health Administration (OSHA) issued guidelines for safe handling of hazardous drugs in 1986 and revised them in 1995 and 1999.4–6 The National Institute for Occupational Safety and Health (NIOSH) published a NIOSH Alert in 2004 on handling hazardous drugs, which include many AD.2 However, a number of recent studies show that handling AD still involves potential healthcare worker exposure risk. A surface contamination study conducted in US and Canadian cancer centers showed that even when OSHA guidelines were followed, pharmacies, administrative offices, and other areas of the hospital were contaminated with measurable levels of AD.7 Additionally, several European studies and two American studies have documented not only surface and airborne contamination, but have shown that healthcare workers who handle these drugs excrete measurable quantities in their urine. Intake here is probably primarily via dermal adsorption.8–15
Potential worker exposure to AD is only expected to increase in the future, as the number of patients under treatment is likely to rise as the population ages. The World Health Organization (WHO) estimates that 11 million new cancer cases are diagnosed each year, and the number is expected to rise to 16 million cases by 2030.16 Higher doses of AD and the use of combinations of several drugs have also increased, while new, more potent drugs are being developed. The noncancer applications of AD have increased substantially,17 as has their use in veterinary oncology.18 One potential overlooked source of worker exposure in the pharmacy may be vial contamination. It has been reported that outer surfaces of vials containing AD as shipped from manufacturers may be contaminated with powdered and lyophilized drug.19
An extensive survey to determine AD contamination in healthcare work environments was recently conducted by the lead authors, involving evaluation of the drugs on contaminated surfaces, air in personnel breathing zones (aerosols and vapors), and in personnel urine (as the parent compounds). Reliable quantification of AD of interest (determined by inquiring which drugs are used in greatest quantity at the participating institutions) was necessary. Numerous analytical methods for AD assessment have been published: one report which involves monitoring assorted drugs in surface wipe samples, for instance, utilizes gas chromatography-mass spectrometry, liquid chromatography-ultraviolet detection, and immunoassay to quantify four different compounds.20 Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is useful for the quantification of polar and ionic organic compounds, including many important AD, and provides high sensitivity (detection limits of low ng/mL or better in solution) and specificity even in complex sample matrices. Sample workup often requires nothing more complex than brief extraction of the sampling media using a suitable solvent with no further media exchange or pre-concentration required. LC-MS/MS methods and applications for a wide variety of AD have been reported.15,21–24 Depending upon mass spectrometer capabilities and chromatographic parameters, simultaneous determination of multiple AD in a sample can readily be performed, with reports demonstrating as many as eight analytes quantified in a single run.25
This publication describes development of LC-MS/MS methods for five AD, cyclophosphamide (CP), ifosfamide (IF), doxorubicin (DX), 5-fluorouracil (FU), and paclitaxel (PA), all of which required quantification in samples collected during the aforementioned survey. Although a single method for simultaneous analysis of all the AD in air and wipe samples was initially desired for economy and efficiency, this proved problematic due to wide variation in the chromatographic retention behavior of the drugs. Methods for analysis of one AD at a time were then developed for convenience to expedite field sample analysis, although a method for simultaneous analysis of three of the AD was subsequently developed and used for some applications as described. After LC-MS/MS methods had been developed, they were used to determine the AD recoveries for (1) extraction from wipe media, (2) wipe samples of healthcare environment surrogate material surfaces using the aforementioned media, and (3) extraction from combined sorbent/filter media used for air sampling, using appropriate test samples. LC-MS/MS methods for individual analysis of CP and PA in healthcare personnel urine specimens were also developed separately.
Experimental
Analytical methods for CP, IF, DX, and PA in work environment samples
All quantitative analyses for AD were performed via LC-MS/MS using fragment ions produced via collision-induced fragmentation. For in-house work (NIOSH laboratory), Optima grade methanol and acetonitrile (Fisher Scientific, Pittsburgh, PA) and Nanopure® water from a Type D4700 purification system (Barnstead/Thermolyne, Dubuque, IA) were used for LC solvents and sample preparation. AD for preparing stock solutions were supplied by Sigma-Aldrich (St. Louis, MO for FU), Bristol-Myers-Squibb (New York, NY, clinical grade CP and IF), and Bedford Laboratories (Bedford, OH, clinical grade DX and PA). Clinical grades of AD typically also contained assorted additives (e.g., solubilizers and antioxidants). Hexamethylphosphoramide (HMPA, 99%) was obtained from Sigma-Aldrich.
Stocks of AD were initially prepared in 100% methanol at 100 µg/mL, except PA for which the stock was prepared in 100% acetonitrile. All further AD standards were serially diluted using a solution of methanol (25%), acetonitrile (10%), and pH 6.0 buffer (10 mM NH4Ac in Nanopure® water) (65%), matching the solvent employed in workup of wipe and air samples (see below). All in-house dilutions and liquid volume transfers were performed using automatic pipettors (EDP-2, Rainin Inc, MA) with disposable polypropylene tips from the same vendor, to eliminate cross-contamination. All in-house LC-MS/MS analyses used polypropylene autosampler vials (Thermo Fisher) and were reported as the mean result of triplicate injections. Calibration plots were generated and method development samples quantified using the ratio of peak heights for the analyte and internal standard.
Methods were developed in-house to individually quantify CP, IF, DX, and PA in samples from healthcare work sites. A method developed to simultaneously measure CP, IF, and PA in a single run was also used for certain applications as specified below. In-house analyses were performed with a LCQ Duo ion-trap mass spectrometric detector, interfaced with a Surveyor Pump plus with built-in solvent degasser and a Micro AS autosampler (Thermo Fisher Scientific, Waltham, MA). Table 1 summarizes the relevant parameters of each method. HMPA at 10 ng/mL was employed as an internal standard since ion-trap systems cannot simultaneously fragment multiple precursor ions of differing mass which negated the use of isotopically labeled analytes which would co-elute with the respective AD. HMPA ionizes well in reverse-phase LC elution solvents, yields relatively intense fragment ions for high sensitivity, and can be separated chromatographically from the target AD as required by measurement using the ion-trap system. It was thus suitable as an internal standard for our needs, following the example of De Jonge et al.26 for analysis of AD and metabolites presumably when labeled standards were unavailable.
Table 1.
Summary of LC-MS/MS analytical method conditions
| Analyte | Sample media |
MS detector |
Analyte precursor > product ions |
Internal standard precursor > product ions |
Summary of HPLC conditions |
|---|---|---|---|---|---|
| CP | Wipe | LCQa | Positive ion 261 > 140 | HMPAd 180 > 135 + 167 summed | NIOSH default LC parameterse; 55/45% (v/v) methanol/10 mM NH4Acd pH 6.0 buffer (isocratic) |
| IF | Wipe | LCQ | Positive ion 261 > 182 | HMPA 180 > 135 + 167 summed | NIOSH default LC parameters; 52.5/47.5% (v/v) methanol/10 mM NH4Ac pH 6.0 buffer (isocratic) |
| DX | Wipe | LCQ | Positive ion 544 > 397 | HMPA 180 > 135 + 167 summed | NIOSH default LC parameters; 62/38% (v/v) methanol/10 mM NH4Fd pH 4.2 buffer (isocratic) |
| PA | Wipe | LCQ | Positive ion 854 > 551 + 569 summed | HMPA 180 > 135 + 167 summed | NIOSH default LC parameters; 75/25 % (v/v) methanol/10 mM NH4Ac pH 6.0 buffer (isocratic) |
| CP, IF, and PA together | Wipe (Storage stability study) and Air | LCQ | Positive ion 261 > 140 (CP), 261 > 182 (IF), 854 > 551 + 569 summed (PA) | HMPA 180 > 135 + 167 summed | NIOSH default LC parameters; 55% A/45% B 0–5 min, step to 75% A/25% B at 5 min, step to 55% A/45% B at 9 min, hold to 15 min. A = 100% MeOH, B = 10 mM NH4F pH 4.2 buffer |
| FU | Wipe and Air | Quattrob | Negative ion 129 > 42 | FU-15N2 131 > 43 | Waters YMC ODS-AQ (250 × 2.0 mm2, 5 µm), 300 µL/min, 83% (v/v) 2.0 mM NH4Ac/17% MeOH, 30°C, 15 µL injection volume |
| CP | Urine | APIc | Positive ion 261 > 140 | CP-d4 265 > 140 | Phenomenex Luna C18 (150 × 3.0 mm, 3 µm), either single column or two connected in tandem. Mobile phase A = 95/5% (v/v) aqueous 2 mM NH4F (pH 3.3)/acetonitrile, mobile phase B = 100% acetonitrile. Single column conditions: Flow rate 1.0 mL/min. 0–1 min, 0–30% B; 1–5 min, 30–70% B then return to 100% A at 5.5 min, 50 µL injection volume. Tandem column conditions: Flow rate 0.5 mL/min. 0–2 min, 0 to 30% B; 2–10 min, 30–70% B then return to 100% A, 20 µL injection volume |
| PA | Urine | API | Positive ion 854.2 > 105 | PA-d5 859.2 > 105 | Agilent Eclipse C18 (50 × 4.6 mm, 1.8 µm), Flow rate 1.0 mL/min., mobile phase A = 95/5% (v/v) 2 mM NH4F (pH 3.3)/acetonitrile, mobile phase B = 100% acetonitrile: 0–1.0 min, 20–80% B; 1.0–1.5 min, 80% B isocratic hold, reequilibration at 20% B from 1.5– 2.0 min, 50 µL injection volume |
Notes: All analyses via electrospray ionization.
Thermo Electron LCQ Duo ion trap mass spectrometer coupled with Surveyor LC pump and Micro AS autosampler at the NIOSH laboratory.
Micromass Quattro-LC triple quadrupole detector coupled with Waters 2795 Alliance Separations Model at Bureau Veritas North America Inc.
Applied Biosystems API 3200 Q-Trap mass spectrometer coupled with Agilent 1100/1200 HPLC system at Duke Comprehensive Cancer Center.
HMPA = Hexamethylphosphoramide, NH4Ac = ammonium acetate, NH4F = ammonium formate.
Default LC parameters for analyses at NIOSH laboratory: Phenomenex Luna ODS-2 column (150 × 2.0 mm, 5 µm) column at 30°C, 230 µL/min, 10 µL injection volume.
Chromatography was developed for each AD which allowed adequate separation of the analyte and HMPA. The linear response of a candidate method was tested by running seven or more calibration standards up to 1.0 µg/mL, with a majority at concentrations 100 ng/mL and below. The linear regression plot of the data was examined, and a correlation coefficient (r2) of 0.990 or better was sought for at least two successive trials (in practice, 0.998 or better was often obtained). All AD gave linear response at least up to 750 ng/mL in repeated trials. Higher levels digressed from linearity in some trials; thus 750 ng/mL was considered to be the reliable upper limit of linear dynamic ranges. Detection limits for the LC-MS/MS methods were then determined as detailed below. Test AD samples (typically six, prepared identically to calibration standards but at different levels within the linear range) were subsequently analyzed, and recoveries within ±10% of the nominal concentrations for a majority of these samples was taken to indicate acceptable performance for use in subsequent sampling method validation.
Analytical method for FU for work environment samples
The key fragment ion of FU at m/z 42 could not be monitored by the in-house instrument which had a lower limit of m/z 50. Thus LC-MS/MS analysis of FU was performed by Bureau Veritas North America (BVNA, Novi, MI) using a Quattro-LC triple-quadruple mass spectrometric detector (Micromass/Waters) interfaced with a Waters 2795 Alliance Separation Module (Waters, Milford, MA). This unit was capable of reading the FU fragment ion, and analysis was performed via electrospray in negative ion mode. The highly polar FU is not retained well on standard reverse-phase LC columns, so BVNA developed a reliable LC method using a YMC-ODS-AQ (Waters) column which addressed this issue (Table 1). Triple-quadruple units circumvent the aforementioned limitation of ion trap systems regarding labeled standards, thus 5-Fluorouracil-15N2 (Isotec Inc., Miamisburg, OH) was employed as the internal standard. Calibration standards prepared at BVNA and aliquots of test samples and quality controls shipped from our site for FU analysis were fortified with 50 ng/mL 5-Fluorouracil-15N2. FU for calibration standards was provided from our in-house supply and was serially diluted by BVNA using the same solvent mixture employed for sample extraction (see below).
Biological monitoring methods for CP and PA
LC-MS/MS methods for CP and PA in urine were developed by the Duke Comprehensive Cancer Center, Clinical Research PK/PD Laboratory, Durham, NC, using an Applied Biosystems API 3200 Q-Trap mass spectrometer coupled with Agilent 1100/1200 HPLC system. Analysis was via electrospray in positive ion mode, and operating the instrument in triple-quadruple mode permitted the use of isotopically labeled internal standards. For these methods CP was purchased from Sigma/Aldrich, and cyclophosphamide-d4 was kindly provided by Dr Susan Ludeman, Duke University Medical Center, Durham, NC. Other chemicals and sources included: PA and paclitaxel-d5 from Toronto Research Chemicals Inc (Toronto, ON, Canada); formic acid and ammonium hydroxide (both LC-MS grade) from Fluka (Milwaukee, WI); LC-MS grade water from J.T. Baker (Phillipsburg, NJ); acetonitrile and methyl tert-butyl ether (MTBE) from Sigma/Aldrich; HPLC grade ethyl acetate from Fisher; and phosphate-buffered saline (PBS, pH 7.4) from Gibco (Carlsbad, California). Analytical conditions for the CP and PA methods are described in Table 1.
For CP, a 4.95mL aliquot of a urine specimen was combined with 50 µL of 10 ng/mL cyclophosphamide-d4 (internal standard) and 1.00 mL of 0.5 M phosphate-buffered saline (PBS, pH 7.4), vortex-mixed, and transferred into a pre-conditioned (6 mL methanol followed by 6 mL deionized water) solid-phase extraction cartridge (Waters Sep-Pak, 500 mg C18, 6 cc). The sample was applied to the cartridge and eluted by gentle vacuum, followed by rinsing with 6mL of deionized water. The cartridge was left to dry under vacuum for 5 min and was then eluted using 5mL of ethyl acetate, which was evaporated to dryness on a Speed-Vac rotary evaporator at 40°C. The sample was reconstituted in 100 µL of mobile phase A (Table 1), vortex-mixed, and centrifuged at 3000 g for 5 min at 4°C. It was then transferred into a polypropylene autosampler vial, centrifuged again at 3000 g for 5 min at 4°C, and placed in the refrigerated autosampler tray at 4°C. A set of calibration samples was prepared by adding 50 µL of concentrated CP stock solutions to 4.95 mL aliquots of CP-free urine to obtain CP levels ranging from 0.00 to 0.20 ng/mL prior to sample workup. These calibration samples were processed and analyzed along with, and by exactly the same procedure as, the samples from study subjects. A linear curve was fit onto the resulting data, and the lowest calibration standard for which experimental and nominal values agreed within 80–120% was taken as the lower limit of quantification (LLOQ). During subsequent analysis of urine samples from the field study, LLOQ of 0.050 ng/mL was obtained. Samples that appeared to exhibit peak signals at the appropriate retention time for CP were further examined by being reanalyzed with two HPLC columns in series (Table 1) to improve detection by further separating the analyte peak from any co-eluting concomitants.
For PA, a 50 µL volume of 100 ng/mL paclitaxel-d5 (internal standard) and 5 mL of MTBE were added to a 4.95 mL aliquot of urine sample. After rotary agitation for 10 min at room temperature and subsequent centrifugation at 3000 g at room temperature for 5 min, most of the upper ether layer was transferred into a 10 × 60 mm2 lime-glass test tube. The solvent was evaporated to dryness by means of a stream of nitrogen at room temperature. The dry residue was reconstituted by adding 40 µL of acetonitrile with vortex-mixing, adding 160 µL of mobile phase A (Table 1) with vortex-mixing, and finally centrifugation at 3000 g for 5 min at 4°C. The sample was transferred into a polypropylene autosampler vial, centrifuged again at 3000 g for 5 min at 4°C, and placed in the refrigerated autosampler tray at 4°C. Calibration samples, ranging from 0.00 to 0.40 ng/mL of PA in urine prior to sample workup, were prepared as described for CP. Analysis and LLOQ determination also followed as described above. In subsequent analysis of field urine samples, LLOQ of 0.050 ng/mL was obtained.
Wipe media and surface sampling recovery
Preparation
The method selected for performing and processing wipe samples was adapted from Larson et al.27 In this method, two filter paper circles (Whatman 42, 55 mm diameter, Fisher) are used to wipe a templated area of 100 cm2, with 250 µL of an extraction solvent (65% 10 mM ammonium acetate buffered to pH 6.0/25% methanol/10% acetonitrile) scattered over the area prior to each wiping. For the present work, the solvent was identical to that used in the reference except that potassium-based buffer was replaced with ammonium; this was due to the generally detrimental effects of potassium salts in LC-MS relative to more volatile buffering compounds. The extraction solvent had previously been found by Larson et al.28 to give adequate wipe sample recoveries for most of the AD of interest in the current study.
To test extraction efficiency from wipe media during method development or to prepare quality control samples for surface wiping studies (below), two filter paper wipes were each spiked with 250 µL of the appropriate AD stock solution prepared in extraction solvent at concentrations selected to provide pre-determined target levels assuming quantitative extraction. The wipes were placed together into a 125 mL polypropylene screw-cap jar (Fisher) and extracted immediately or stored at −8°C until processing.
To evaluate recoveries for the full surface wiping procedure, three materials were selected to represent surfaces likely to experience AD contamination in the workplaces surveyed: stainless steel as a surrogate for biological safety cabinets (Type 304 #3 satin finish, McMaster-Carr Supply, Cleveland, OH), Formica™ for countertops, and vinyl for resilient floor tiles (Imperial Texture, Armstrong World Industries Canada, Montreal, Quebec). Tiles were cut to 10 × 10 cm2 from sheets of the stock materials, washed with methanol, and dried in air prior to use. The tiles were spiked with 250 µL of AD stock solutions (prepared in methanol to hasten evaporation) that would yield selected target concentrations, assuming that pickup from the surface during wiping and subsequent extraction of the wipes were both quantitative. After 2 h, 250 µL of the extraction solvent was scattered over the tile, which was wiped dry with a circle of filter paper. This procedure was repeated, and both wipes were placed together in a polypropylene jar.
Processing
The sample jars were thawed if necessary, and 9.5 mL of extraction solvent was added, for a total of 10 mL including liquid present in the wipes. The jar was closed and swirled to remove any air trapped beneath or between the wipes, and suspended in a sonication bath for 30 min, at a depth which ensured that liquid in the jar was entirely immersed below water level. After sonication the jars were tilted and rolled to pick up droplets of condensation from the inner walls, and the supernatant was filtered directly into 15 mL centrifuge tubes through 0.22 µm Millex PVDF syringe filters (Millipore Corp, Bedford, MA) using 10 mL polypropylene disposable syringes (Becton-Dickinson & Co., Franklin Lakes, NJ), to remove particulates likely to interfere with autosampler operation. Typically only 8–9mL of supernatant was recovered, with the remainder held up in the wipes, but because sonication ensured homogeneity of the extract, this would not affect analyte recovery measured as concentration.
For in-house analyses, an aliquots (1.8 mL) of each filtered extract was transferred to a fresh centrifuge tube and fortified with 10 ng/mL internal standard (200 µL of 100 ng/mL HMPA prepared in extraction solvent). This addition slightly diluted the analyte concentration, which was taken into account in calculating recoveries. The sample was mixed by vortexing for 30 s and analyzed immediately or refrigerated at 4°C. For samples deliberately prepared to yield concentrations exceeding the linear dynamic range of the analytical method (to test method performance and analyst proficiency in the event that field samples yielded such levels), the necessary volume of original extract was transferred to a fresh centrifuge tube, 200 µL of the HMPA solution was added, and blank extraction solvent was added to yield a total volume of 2.0 mL, thus keeping internal standard concentration at the correct level in rediluted samples. For FU analyses, 2 mL aliquot of the original filtered extract were shipped to BVNA.
Recovery studies for wipe samples
Three replicate spiked wipe samples were prepared for each of six or seven target concentration levels, including at least one level intended to exceed the demonstrated linear dynamic range of the analytical methods, thus requiring the extract to be rediluted for accurate quantification. Extraction and analysis was conducted as described above. Spiked wipe samples were processed immediately or stored at −8°C and thawed before workup.
Spiked tile samples were prepared using a different surrogate material on each of three successive days. For each material, five spiked tiles were prepared for each of five target concentration levels (for CP six levels were used). In practice, these tile spike levels ranged from 0.40 to 600 ng/cm2 expressed as mass/area. The highest concentrations were selected to exceed analytical method linear ranges after processing, thus requiring redilution. Three tiles spiked with pure methanol were also prepared for each set as blanks. Tile wiping and extraction proceeded as described above. Spiked wipes at known concentration levels were also prepared and extracted along with spiked tile samples for use as quality controls (below).
Storage stability study for wipe samples
Following method development and validation, a set of field wipe samples were analyzed for several drugs including CP, IF, and PA.29 Wipes spiked with these compounds were prepared as process quality controls (PQC) to be extracted and analyzed along with field samples. Extra spiked wipes were simultaneously prepared at several concentrations covering the linear range of the analytical methods, set aside, and kept frozen (at −8°C) for several months. These were prepared over a period of several weeks as each drug in the field samples was analyzed in succession. After 7–8 months (depending on the preparation date of each group), all these samples were thawed together, processed as described, and analyzed as a measure of how much recovery loss could be expected due to prolonged storage. Samples were quantified using the analytical method for simultaneous analysis of all three AD (Table 1).
Air sampling media recovery
Preparation
OSHA versatile sampler (OVS) sorbent tubes provided by SKC (Eighty Four, PA) containing 200 mg of Anasorb 108 sorbent material were used for air sampling.30 The tubes were spiked with 250 µL of AD stock solutions (in 100% methanol to promote evaporation) at concentrations designed to provide total masses of analyte on the tubes corresponding to desired target concentrations assuming quantitative extraction, and were allowed to dry at least 2 h before processing.
Processing
The sorbent tubes were disassembled, and the filter pad which collects particulate analyte and the sorbent which collects vapor phase analyte were transferred together into a 125 mL polypropylene jar. For air samples, the initial extraction solvent was modified to 70% methanol/29.5% acetonitrile/0.25% formic acid/0.25% acetic acid. About 10mL of this solvent was used to rinse the inside of the sorbent tube into the jar. Pre-concentration was deemed necessary to improve the likelihood of detecting any AD captured during air sampling in the projected field survey, and the modified extraction solvent eliminated aqueous content, allowing efficient evaporation of the extract via subsequent blowdown.
The jars containing sorbent tube contents and solvent were closed and placed on a rotary shaker at 100 RPM for 30 min at room temperature. The supernatants were then filtered into centrifuge tubes as described above for wipe sample processing. Approximately 9 mL was typically recovered, which was reduced to dryness in a water bath at 30°C under ultrapure nitrogen, and the residues were reconstituted in 2.5 mL of 65% pH 6.0 buffer (10 mM ammonium acetate)/25% methanol/10% acetonitrile, pre-concentrating the original extract fourfold. After reconstitution, significant amounts of white flocculent precipitate were noted in almost all samples, which were subsequently refiltered through 0.22 µm PVDF syringe filters. For in-house analyses, 900 µL of the filtered reconstituted extract was added to a fresh centrifuge tube and combined with 100 µL of 100 ng/mL HMPA internal standards prepared in the reconstitution solvent. After vortex mixing, 500 µL was transferred to a polypropylene autosampler vial for analysis, and CP, IF, and PA were quantified using the method for simultaneous analysis of all three AD (Table 1). For FU analyses, 1 mL aliquots of reconstituted sample extract were sent to BVNA
Recovery studies from air sampling media
For recovery studies, test samples were prepared by spiking sorbent tubes as described above to produce levels of 12.5, 25, 50, 100, and 200 ng of all the AD of interest. Five sorbent tubes were spiked for each target level, and three tubes were spiked only with blank methanol.
Determination of detection limits
For in-house LC-MS/MS methods, six or more low-level analyte standards including an appropriate blank were analyzed, a linear regression plot was generated, and the instrumental detection limit (LOD) is reported as [(YB + 3SB)/m] where YB is the intercept and SB its standard deviation and m is the plot slope. Lower limit of quantification (LLOQ) is then estimated in the same way using 10SB, which corresponds to 3.3 LOD. The LOD was determined on at least two successive days for each analyte, and the average result is reported (Table 2). For LC-MS/MS analyses of FU at BVNA, instrumental LOD was determined in the same way using four standards ranging up to 5 ng/mL, with LLOQ following as above. This value was determined each time a set of method development or field sample analyses was performed, giving slightly different values in each session; thus an average instrumental LOD is reported.
Table 2.
Detection limits for antineoplastic drugs
| Analyte | Instrumental LODa (ng/mL) |
Wipes,b mass per area (ng/cm2) |
Air,c mass on sample tube (ng) |
Air,d per m3 (ng/m3) |
|---|---|---|---|---|
| Cyclophosphamide | 1.0 | 0.10 | 2.5 | 5.2 |
| Ifosfamide | 1.0 | 0.10 | 2.5 | 5.2 |
| Paclitaxel | 0.70 | 0.07 | 1.8 | 3.7 |
| Doxorubicin | 2.0 | 0.20 | 5.0 | 10.4 |
| 5-Fluorouracil | 0.60 | 0.06 | 1.5 | 3.1 |
Instrumental values for CP, IF, PA, DX are means of two determinations at NIOSH laboratory using 10 µL injections on LC. Instrumental value for FU is the mean of four determinations at BVNA laboratory using 15 µL injections on LC. Table omits values for urine sample analysis.
Determined as described in text.
Based on 100 cm2 sample area with extraction into 10 mL solvent, no further pre-concentration.
Based on extraction into 10 mL solvent and subsequent four-fold preconcentration.
0.480 m3 was chosen as standard volume for all air samples in projected field study and is thus used for calculation of these LOD.
Surface wipe samples were extracted into 10 mL total volume and extracts analyzed without further pre-concentration. All wipe samples were obtained from an area of 100 cm2, hence:
A concentration value X ng/mL corresponds to 1/10 of the value as mass/area. Thus instrumental LOD of 1.0 ng/mL for a compound equals mass/area LOD of 0.10 ng/cm2, and so on.
For air samples, the original sorbent tube extract was pre-concentrated by a factor of four via solvent blowdown and reconstitution, hence the LOD expressed as mass is:
where X ng/mL is the instrumental LOD. Detection limit as mass/(air sample volume) depends on the total volume sampled. For the projected field study, this was standardized at 480 L, thus LOD = (2.5 × ng)/(0.480 m3). See Table 2 for a summary of the instrumental LOD determined as described above and corresponding sampling media-based LOD. Values calculated for the latter assume for simplicity that sampling efficiency for and extraction of AD from media is essentially quantitative; this was not always the case as demonstrated below.
Quality controls
Two sets of quality control samples were used for analysis of wipe and air samples. Laboratory control samples (LCS) were prepared identically to calibration standards but with different concentration levels. PQC were prepared as wipes or sorbent tubes spiked with AD to yield known levels assuming quantitative extraction recovery. This approach allowed recovery issues due to instrumental performance and due to workup to be tracked separately, and was intended to compensate for the inability to use labeled internal standards for in-house analyses. Both LCS and PQC were prepared at multiple concentrations which covered the linear range of the respective analytical methods. Typically six levels of each type were prepared; the recovery tables (3–5) show exact numbers in each case. For FU, BVNA additionally tested method performance by selecting a single calibration standard that was rerun periodically during sample analyses (continuing calibration verification, CCVBVNA). Quality control sample mean recoveries of 75–125% were considered to indicate acceptable analytical performance.
Table 3.
Results for wipe extraction recovery studies
| Analyte/trial | Type | Number of concentrations tested |
Recovery range (%) |
Mean recovery and RSD (%) |
|---|---|---|---|---|
| Cyclophosphamide | ||||
| Trial 1 | Spiked wipes | 7 | 91.9–112.6 | 100.3 (8.1) |
| Trial 2 | LCS | 5 | 83.1–108.1 | 95.3 (10.2) |
| Spiked wipes | 7 | 86.7–102.7 | 96.1 (6.5) | |
| Trial 3 | LCS | 5 | 86.4–106.1 | 97.0 (7.0) |
| Spiked wipes | 7 | 98.0–106.6 | 107.4 (9.6) | |
| Ifosfamide | ||||
| Trial 1 | LCS | 6 | 96.9–113.4 | 103.7 (2.9) |
| Spiked wipes | 7 | 97.3–117.6 | 105.5 (7.8) | |
| Trial 2 | LCS | 7 | 96.9–104.1 | 101.3 (2.9) |
| Spiked wipes | 7 | 83.2–118.5 | 100.4 (12.1) | |
| Doxorubicin | ||||
| Trial 1 | LCS | 5 | 89.1–103.1 | 95.6 (5.2) |
| Spiked wipes | 7 | 55.1–89.1 | 74.2 (13.6) | |
| Trial 2 | LCS | 5 | 88.2–100.2 | 94.2 (5.8) |
| Spiked wipes | 6 | 66.1–74.9 | 72.4 (6.0) | |
| Trial 3 | LCS | 5 | 84.2–96.9 | 90.6 (5.9) |
| Spiked wipes | 6 | 66.2–83.2 | 74.3 (8.3) | |
| Paclitaxel | ||||
| Trial 1 | LCS | 6 | 107.6–122.4 | 115.4 (6.7) |
| Spiked wipes | 6 | 100.2–133.0 | 115.6 (6.3) | |
| Trial 2 | LCS | 6 | 104.6–123.5 | 121.7 (8.8) |
| Spiked wipes | 6 | 98.1–134.9 | 118.8 (9.4) | |
| 5-Fluorouracil | ||||
| CCVBVNA | 1 | 95.6–105.2 | 100.2 (3.0) | |
| LCS | 6 | 91.8–98.3 | 94.8 (2.7) | |
| Spiked wipes | 6 | 91.7–100.5 | 98.5 (3.9) |
LCS = Laboratory control samples. Each LCS concentration level was run once with the indicated set of wipe sample extracts and the range of individual recoveries and their mean and RSD are reported. CCVBVNA = Continuing calibration verification standard: 10 ng/mL FU run periodically during the sample queue. Three spiked wipe samples were prepared for each target concentration. Average recovery for each concentration was determined and the recovery range of these values is reported, along with the mean and RSD of these averages.
Note: No LCS accompanied the first trial of spiked wipe recovery for cyclophosphamide.
Table 5.
Results for air sampling sorbent tube extraction
| Analyte | Type | Number of concentrations tested | Recovery range (%) | Mean recovery & (RSD)% |
|---|---|---|---|---|
| Cyclophosphamide | LCS | 6 | 102.6–121.8 | 114.8 (7.0) |
| Spiked tubes | 5 | 99.4–106.5 | 103.9 (2.8) | |
| Ifosfamide | LCS | 6 | 77.9–119.6 | 108.0 (14.1) |
| Spiked tubes | 5 | 93.5–109.6 | 99.9 (6.3) | |
| Paclitaxel | LCS | 6 | 98.9–128.6 | 119.5 (8.9) |
| Spiked tubes | 5 | 61.9–86.5 | 74.7 (13.3) | |
| 5-Fluorouracil | LCS | 6 | 97.4–109.2 | 104.4 (3.3) |
| CCVBVNA | 1 | 98.4–108 | 103.0 (3.9) | |
| Spiked tubes | 5 | 88.1–98.4 | 92.9 (4.0) |
LCS = Laboratory Control Samples. LCS at each concentration level were run once and the range of individual recoveries and their mean and RSD are reported. CCVBVNA = Continuing calibration verification standard: 50 ng/mL FU run periodically during the sample queue. Five spiked sorbent tubes were prepared for each concentration. Average recovery for each concentration was determined and the recovery range of these values is reported, along with the mean and RSD of these averages.
Results
Sensitivity
LOD for each AD of interest were calculated as described above for air and wipe sampling based on the instrumental LOD and the applicable workup steps for each media and are summarized in Table 2. As intended, LC-MS/MS provided adequate sensitivity for the detection of the drugs in healthcare work environments with relatively simple workup of sample media.
Wipe sample processing: Possible Effects of Workup Steps
Possible irretrievable losses of each AD to the plastic ware used in the extraction process or to the filter paper wipe media were investigated prior to other stages of method development. Aliquots of several known concentrations prepared in the extraction solvent were stored in the screw-cap polypropylene jars for 30 min, in the polypropylene centrifuge tubes overnight, pressed through the syringe filters, etc., after which concentrations were determined and compared against the original levels. In most cases no demonstrable losses were observed, confirming that workup steps alone would not affect quantitative analysis of the AD within the concentration range tested. The exception was DX: when two filter paper wipes were added to 10 mL aliquots of test solutions (at levels covering the analytical method range) for 30 min, a consistent drop in concentration resulted. This was observed with and without sonication and was not strongly dependant on the original concentration. For higher levels of the brightly colored drug, retention in the wipes was evident as a pinkish tint. It was shown by further experiment that approximately 14% of the DX concentration was lost for each filter paper wipe added. For our standard extraction procedure using two wipes processed together, this would imply a loss of nearly 30% aside from any further losses in recovery from surfaces. The other tests performed with DX indicated that plastic ware had no effect on recovery and that the wipes were the sole contributors to loss.
Wipe extraction recoveries
Two or three trials were conducted on successive days for wipe recovery tests for each AD (one trial for FU since it was necessary to ship the extracts offsite for analysis). LCS were prepared and analyzed along with each batch of spiked wipe samples. As indicated in Table 3, wipe extraction recoveries were good except for the significant recovery losses for DX (which were expected due to the preliminary tests described above) and a moderate high bias for PA. The latter was reflected in the LCS as well, suggesting a systematic bias in the analysis rather than issues in the spiking or processing of the wipes, and was evident in two successive recovery trials for PA.
Tile wipe recoveries
For each AD, wipe samples from steel, Formica™, and vinyl were prepared and analyzed on successive days. One set of LCS and one set of PQC for spiked wipes were prepared and analyzed along with all three sets of samples. For FU, wipe samples for all three surfaces were analyzed in a single batch at BVNA. Good recoveries were found for all AD tested on the three surfaces, except markedly low values generally for DX and for all AD from the vinyl (Table 4). Larson et al.27 previously demonstrated that certain surfaces such as vinyl could strongly affect analyte recoveries. Recoveries for DX spiked wipes which were prepared and run as PQC along with the DX spiked tile samples were as expected (Table 3), given the irreversible loss of the compound into the filter paper wipes as previously demonstrated. Recoveries of DX from spiked tiles indicated further serious losses of the analyte to tile materials (incomplete pickup). The high RSD reflect the wide ranges of DX recoveries relative to mean values at all concentration levels.
Table 4.
Results for tile wipe recovery studies
| Analyte | Type | Number of concentrations tested |
Recovery range (%) |
Mean recovery and RSD (%) |
|---|---|---|---|---|
| Cyclophosphamide | ||||
| Stainless Steel | LCS | 6 | 88.1–101.7 | 94.9 (6.1) |
| PQC | 6 | 75.8–100.8 | 91.4 (10.4) | |
| Spiked tiles | 6 | 88.4–100.6 | 92.5 (4.6) | |
| Formica™ | LCS | 6 | 90.8–110.5 | 100.4 (7.2) |
| PQC | 6 | 83.2–109.3 | 95.0 (10.5) | |
| Spiked tiles | 6 | 78.1–120.4 | 93.8 (15.5) | |
| Vinyl | LCS | 6 | 90.2–113.3 | 100.2 (8.0) |
| PQC | 6 | 81.2–102.7 | 90.2 (7.8) | |
| Spiked tiles | 6 | 40.9–61.8 | 50.3 (18.3) | |
| Ifosfamide | ||||
| Stainless Steel | LCS | 7 | 91.2–104.5 | 95.5 (4.6) |
| PQC | 6 | 96.4–109.8 | 102.5 (5.8) | |
| Spiked tiles | 5 | 97.6–103.1 | 99.7 (2.1) | |
| Formica™ | LCS | 7 | 90.0–99.8 | 95.5 (4.2) |
| PQC | 6 | 93.4–116.0 | 102.5 (7.3) | |
| Spiked tiles | 5 | 92.5–102.9 | 97.6 (4.2) | |
| Vinyl | LCS | 7 | 91.2–105.4 | 99.4 (4.7) |
| PQC | 6 | 100.0–119.9 | 112.9 (7.1) | |
| Spiked tiles | 5 | 28.3–38.3 | 31.0 (13.5) | |
| Doxorubicin | ||||
| Stainless Steel | LCS | 6 | 93.9–99.4 | 97.6 (2.1) |
| PQC | 6 | 67.4–81.4 | 73.3 (8.4) | |
| Spiked tiles | 5 | 16.3–44.6 | 26.3 (42.7) | |
| Formica™ | LCS | 6 | 95.1–103.4 | 100.4 (7.2) |
| PQC | 6 | 65.8–86.0 | 73.8 (10.3) | |
| Spiked tiles | 5 | 30.7–43.8 | 35.1 (15.0) | |
| Vinyl | LCS | 6 | 95.8–101.2 | 98.9 (2.5) |
| PQC | 6 | 67.9–82.0 | 72.7 (7.8) | |
| Spiked tiles | 5 | 26.0–40.3 | 31.3 (17.7) | |
| Paclitaxel | ||||
| Stainless Steel | LCS | 6 | 87.8–117.2 | 106.6 (10.6) |
| PQC | 6 | 105.3–124.1 | 113.4 (7.3) | |
| Spiked tiles | 5 | 77.4–106.0 | 97.7 (12.0) | |
| Formica™ | LCS | 6 | 102.2–126.9 | 118.7 (7.2) |
| PQC | 6 | 120.0–132.4 | 124.2 (6.0) | |
| Spiked tiles | 5 | 92.0–115.8 | 109.1 (9.1) | |
| Vinyl | LCS | 6 | 80.3–98.3 | 90.2 (8.5) |
| PQC | 6 | 89.8–103.0 | 94.5 (6.0) | |
| Spiked tiles | 5 | 10.7–22.1 | 15.6 (28.2) | |
| 5-Fluorouracila | ||||
| CCVBVNA | 1 | 92.5–101 | 96.9 (2.2) | |
| PQC | 5 | 103.5–110.2 | 106.7 (2.9) | |
| Stainless Steel | Spiked tiles | 5 | 92.4–103.9 | 98.5 (5.0) |
| Formica™ | Spiked tiles | 5 | 78.2–94.6 | 85.6 (7.9) |
| Vinyl | Spiked tiles | 5 | 23.5–49.4 | 31.2 (33.9)b |
LCS = Laboratory control samples. PQC = Process quality controls (spiked wipes). LCS and PQC at each prepared concentration level were run once with the indicated set of spiked tile sample extracts, and the range of recoveries and their mean and RSD are reported. CCVBVNA = Continuing calibration verification standard: 50 ng/mL FU run periodically during the sample queue. Five spiked tile samples were prepared for each concentration. Average recovery for each concentration was determined and the recovery range of these values is reported, along with the mean and RSD of these averages.
No LCS were provided to the contract laboratory for the analysis of the FU samples.
Except for the highest concentration level (49.4%), mean recoveries for FU on vinyl did not exceed 30%. Total mean is 26.6%, and RSD is 10.4% if that highest recovery is omitted.
Storage stability study for spiked wipe samples
No degradation of recovery was observed for the three drugs tested in this study as demonstrated by the approximately quantitative average recoveries, 103.7% (CP, 9 concentration levels, RSD 4.3%), 111.4% (IF, 6 levels, RSD 6.8%), and 108.4% (PA, 3 levels of three samples each, RSD 5.0%). Results for accompanying LCS quality control samples demonstrated similar average recoveries in each case.
Aerosol sorbent tube recovery studies
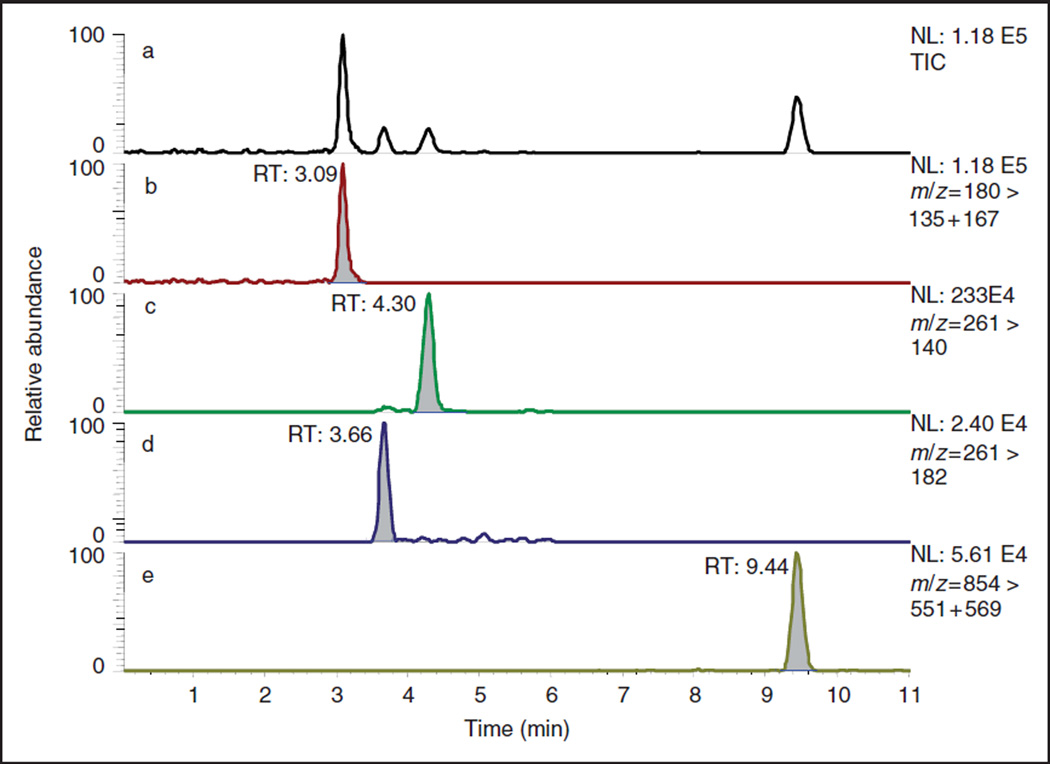
Figure 1 shows an example (one of the calibration plot standards) from in-house analysis of CP, IF, and PA using the LC-MS/MS method which allowed simultaneous quantification of all three AD. For the air sampling media, CP and IF recoveries were essentially quantitative, but significant losses were observed for PA, which also showed the widest variation in recovery across the concentration range (Table 5).
Figure 1.
Analysis of a multiple antineoplastic drug standard (20 ng/mL of CP, IF, and PA) with 10 ng/mL HMPA internal standard using LC-MS/MS method for simultaneous analysis of the three analytes. (a) total ion chromatogram, (b) selected signal for HMPA (m/z 135 + 167), (c) signal for IF (m/z 182), (d) signal for CP (m/z 140), (e) signal for PA (m/z 551 + 569). Raw data has been smoothed using 7-point boxcar algorithm supplied with Xcalibur software. NL = normalized ion current, RT = retention times.
For FU, the intensities of internal standard (5-fluorouracil-15N2) ion signals for calibration plot standards and LCS quality controls were compared with those of the recovery test samples (spiked sorbent tubes). The results suggested that the workup procedure for the air sampling media produced an unidentified co-eluting interferant that suppressed FU ion intensity, a problem not observed for wipe sample extraction. Nevertheless, observed FU recoveries for the test samples were not severely affected, due to the use of labeled, co-eluting internal standard for quantification via response ratio, and were well within acceptable limits at all levels tested.
Conclusions
Method development performed for CP, IF, PA, and FU indicated that the method used to extract the AD from the selected wipe sampling media was essentially quantitative. DX, however, showed a significant loss due to unrecoverable retention in the filter paper. A separate test suggested that this may also reflect readsorption of DX from solution into the paper. Subsequent tests of wiping efficiency, from three materials used as surrogates for work environment surfaces, demonstrated that recoveries for all AD tested varied from quantitative to poor depending on the material. DX recovery from all surrogate materials was severely affected in addition to the loss already observed for extraction from the wipes, suggesting that quantitative analysis of DX in field wipe samples by this sampling protocol would be unreliable. This led to the decision not to investigate DX in the storage stability study for wipe media. No losses were observed for long-term frozen storage of the wipe media spiked with CP, IF, or PA. For air sampling media, extraction recoveries for the four AD tested were nearly quantitative except for PA, which demonstrated a moderate loss. It is hypothesized that this loss may have been due to partial adsorption of the somewhat hydrophobic PA on the precipitate which appeared after reconstitution of the initial air sample extract and which was subsequently filtered out. Analysis of FU in air sampling media extracts did indicate moderate suppression of analyte ion signal due to an unidentified concomitant, but using labeled internal standard and response ratio appears to compensate for any effect on quantification in the quality controls, suggesting that field samples can be quantified accurately for the drug.
Attempts were made to address the poor extraction recoveries observed for DX from wipe media using different extraction solvent mixtures or buffer pH and other variants on the method, but no improvements were observed (data not shown). The stabilities of all the AD examined here during long-term storage on the air sampling media have not yet been tested, nor those of FU or DX on wipe sample media; these should be documented in future research. Finally, no studies have been performed regarding the efficacy of the air sampling media for quantitatively capturing AD present in environmental air, or to ensure that AD captured on the sampling media would not subsequently be lost via degradation or evaporative loss under continuing air flow before sampling is concluded. This was beyond the scope of the present study.
The LC-MS/MS method developed for FU analysis has good sensitivity and does not require elimination or exchange of the polar extraction solvent used in sampling. Except for DX, the sampling and extraction methods presented for surface wipes showed good recoveries for steel and Formica™ if not for vinyl, and thus have potential use in field investigations of AD contamination in work environments. Sensitive LC-MS/MS methods for monitoring CP and PA in urine have also been demonstrated. A full report of AD sampling results from the field survey which prompted development of these methods has been published.29
Although a number of studies have reported methods for sampling and analysis of AD from workplace surfaces, only a small number have documented recoveries from these surfaces. Schmaus et al.31 evaluated the recovery of CP, IF, and FU from glass surfaces as a prelude to investigating contamination in hospital pharamacies and found generally good recoveries with some concentration dependence. Hedmer et al.32 evaluated recovery of CP from three different types of wiping materials and three working surfaces (stainless steel, laminate, and plastic flooring), performing the wipe while the applied drug was still in liquid form (as water solution) or after the liquid had been allowed to evaporate (from methanol solution). Recoveries for the former were slightly higher on average than those obtained after evaporation for the three types of wiping materials tested. Similar surface materials were examined in the current study using several drugs applied from methanol and dried, and recovery rates from each material approximately followed the pattern reported by Hedmer (excepting DX, which exhibited sharply reduced recoveries from all tested materials as described above).
Acknowledgments
Our gratitude to our colleagues Jennifer Roberts, Christine Toennis, and John Clark for their assistance in preparing method development samples for shipment to BVNA for FU analysis. Our thanks to Jerome Kratzer for machining surrogate surface materials.
Footnotes
Disclaimers: Mention of company names and/or products does not constitute endorsement by the National Institute for Occupational Safety and Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Contributor Information
Jack R Pretty, Division of Applied Research and Technology, National Institute for Occupational Safety and Health, U.S. Centers for Disease Control and Prevention, Cincinnati, OH, USA.
Thomas H Connor, Division of Applied Research and Technology, National Institute for Occupational Safety and Health, U.S. Centers for Disease Control and Prevention, Cincinnati, OH, USA.
Ivan Spasojevic, Duke Comprehensive Cancer Center, Clinical Research PK/PD Laboratory, Durham, NC, USA.
Kristine S Kurtz, Bureau Veritas North America, Inc., Novi, MI, USA.
Jeffrey L McLaurin, Division of Applied Research and Technology, National Institute for Occupational Safety and Health, U.S. Centers for Disease Control and Prevention, Cincinnati, OH, USA.
Clayton B’Hymer, Division of Applied Research and Technology, National Institute for Occupational Safety and Health, U.S. Centers for Disease Control and Prevention, Cincinnati, OH, USA.
D Gayle Debord, Division of Applied Research and Technology, National Institute for Occupational Safety and Health, U.S. Centers for Disease Control and Prevention, Cincinnati, OH, USA.
References
- 1.Connor TH, McDiarmid MA. Preventing occupational exposures to antineoplastic drugs in health care settings. CA Cancer J Clin. 2006;56:354–365. doi: 10.3322/canjclin.56.6.354. [DOI] [PubMed] [Google Scholar]
- 2.NIOSH Alert. Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings. 2004 Sep; DHHS (NIOSH) Pub No. 2004-165. [Google Scholar]
- 3.Falck K, Gröhn P, Sorsa M, Vainio H, Heinonen E, Holsti LR. Mutagenicity in urine of nurses handling cytostatic drugs. Lancet. 1979;1:1250–1251. doi: 10.1016/s0140-6736(79)91939-1. [DOI] [PubMed] [Google Scholar]
- 4.OSHA. Guidelines for cytotoxic (antineoplastic) drugs. 1986 Jan; Publication No. 8-1.1. [Google Scholar]
- 5.OSHA Directorate of Technical Support. Controlling occupational exposure to hazardous drugs. Instruction TED 1.15. 1995 Sep
- 6.OSHA Directorate of Technical Support. Controlling occupational exposure to hazardous drugs. Instruction TED 1.15. 1999 Jan
- 7.Connor TH, Anderson RW, Sessink PJM, Broadfield L, Power LA. Surface contamination with antineoplastic agents in six cancer treatment centers in the United Sates and Canada. Am J Health Syst Pharm. 1999;56:1427–1432. doi: 10.1093/ajhp/56.14.1427. [DOI] [PubMed] [Google Scholar]
- 8.Sottani C, Rinaldi P, Leoni E, et al. Simultaneous determination of cyclophosphamide, ifosfamide, doxorubicin, epirubicin and daunorubicin in human urine using high-performance liquid chromatography/electrospray ionization tandem mass spectrometry: bioanalytical method validation. Rapid Commun Mass Spectrom. 2008;22:2645–2659. doi: 10.1002/rcm.3657. [DOI] [PubMed] [Google Scholar]
- 9.Mason HJ, Blair S, Sams C, et al. Exposure to antineoplastic drugs in two UK hospital pharmacy units. Ann Occup Hyg. 2005;49:603–610. doi: 10.1093/annhyg/mei023. [DOI] [PubMed] [Google Scholar]
- 10.Sottani C, Tranfo G, Bettinelli M, Farranda P, Spagnoli M, Minoia C. Trace determination of anthracyclines in urine: a new high-performance liquid chromatography/tandem mass spectrometry method for assessing exposure of hospital personnel. Rapid Commun Mass Spectrom. 2004;18:2426–2436. doi: 10.1002/rcm.1642. [DOI] [PubMed] [Google Scholar]
- 11.Favier B, Gilles L, Gesage M, Latour JF. Analysis of cyclophosphamide in the urine of antineoplastic drug handlers. Bull Cancer. 2003;90:905–909. [PubMed] [Google Scholar]
- 12.Pethran A, Schierl R, Hauff K, Grimm C-H, Boos K-S, Nowak D. Uptake of antineoplastic agents in pharmacy and hospital personnel. Part I: monitoring of urinary concentrations. Int Arch Occup Environ Health. 2003;76:5–10. doi: 10.1007/s00420-002-0383-8. [DOI] [PubMed] [Google Scholar]
- 13.Wick C, Slawson MH, Jorgenson JA, Tyler LS. Using a closed-system protective device to reduce personnel exposure to antineoplastic agents. Am J Health Syst Pharm. 2003;60:2314–2320. doi: 10.1093/ajhp/60.22.2314. [DOI] [PubMed] [Google Scholar]
- 14.Nyman H, Jorgenson J, Slawson MH. Workplace contamination with antineoplastic agents in a new cancer hospital using a closed-system drug transfer device. Hosp Pharm. 2007;42:219–225. [Google Scholar]
- 15.Turci R, Sottani C, Ronchi A, Minoia C. Biological monitoring of hospital personnel occupationally exposed to antineoplastic agents. Toxicol Lett. 2002;134:57–64. doi: 10.1016/s0378-4274(02)00163-7. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Are the number of cancer cases increasing or decreasing? 2008; Cancer: WHO cancer control programme. [accessed February 2010];Online Referencing. http://www.who.int/features/qa/15/en/index.html.
- 17.Abel EA. Immunosuppressant and cytotoxic drugs: unapproved uses or indications. Clin Dermatol. 2000;18:95–101. doi: 10.1016/s0738-081x(99)00098-x. [DOI] [PubMed] [Google Scholar]
- 18.Lucroy MD. Chemotherapy safety in veterinary practice: hazardous drug preparation. Comp Cont Educ Pract Vet. 2001;24:140–146. [Google Scholar]
- 19.Connor TH, Sessink PJM, Harrison BR, et al. Surface contamination of chemotherapy drug vials and evaluation of new vial-cleaning techniques: results of three studies. Am J Health Syst Pharm. 2005;62:475–484. doi: 10.1093/ajhp/62.5.475. [DOI] [PubMed] [Google Scholar]
- 20.Zeedijk M, Greidjanus B, Steenstra FB, Uges DRA. Monitoring exposure of cytostatics on the hospital ward: measuring surface contamination of four different cytostatic drugs from one wipe sample. Eur J Hosp Pharm Sci. 2005;11:18–22. [Google Scholar]
- 21.Minoia C, Turci R, Sottani C, et al. Application of high performance liquid chromatography/tandem mass spectrometry in the environmental and biological monitoring of health care personnel occupationally exposed to cyclophosphamide and ifosfamide. Rapid Commun Mass Spectrom. 1998;12:1485–1493. doi: 10.1002/(SICI)1097-0231(19981030)12:20<1485::AID-RCM333>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 22.Turci R, Sottani C, Spagnoli G, Minoia C. Biological and environmental monitoring of hospital personnel exposed to antineoplastic agents: a review of analytical methods. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789:169–209. doi: 10.1016/s1570-0232(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 23.Sabatini L, Barbieri A, Tosi M, Violante FS. A new high-performance liquid chromatographic/electrospray ionization tandem mass spectrometric method for the simultaneous determination of cyclophosphamide, methotrexate and 5-fluorouracil as markers of surface contamination for occupational exposure monitoring. J Mass Spectrom. 2005;40:669–674. doi: 10.1002/jms.840. [DOI] [PubMed] [Google Scholar]
- 24.Sottani C, Turci R, Schierl R, et al. Simultaneous determination of gemcitabine, taxol, cyclophosphamide and ifosfamide in wipe samples by high-performance liquid chromatography/tandem mass spectrometry: protocol of validation and uncertainty of measurement. Rapid Commun Mass Spectrom. 2007;21:1289–1296. doi: 10.1002/rcm.2960. [DOI] [PubMed] [Google Scholar]
- 25.Fransman W, Huizer D, Tuerk J, Kromhaut H. Inhalation and dermal exposure to eight antineoplastic drugs in an industrial laundry facility. Int Arch Occup Environ Health. 2007;80:396–403. doi: 10.1007/s00420-006-0148-x. [DOI] [PubMed] [Google Scholar]
- 26.De Jonge ME, Van Dam SM, Hillebrand MJX, Rosing H, Hultema ADR, Rodenhuls S, et al. Simultaneous quantification of cyclophosphamide, 4-hydroxycyclophosphamide, N, N’, N”-triethylenethiophosphoramide and N, N’, N”-triethylenephosphoramide in human plasma by high-perfomance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2004;39:262–271. doi: 10.1002/jms.570. [DOI] [PubMed] [Google Scholar]
- 27.Larson RR, Khazaeli MB, Dillon HK. Monitoring method for surface contamination caused by selected antineoplastic agents. Am J Health Syst Pharm. 2002;59:270–277. doi: 10.1093/ajhp/59.3.270. [DOI] [PubMed] [Google Scholar]
- 28.Larson RR, Khazaeli MB, Dillon HK. Development of an HPLC method for simultaneous analysis of five antineoplastic agents. Appl Occup Environ Hyg. 2003;18:109–119. doi: 10.1080/10473220301432. [DOI] [PubMed] [Google Scholar]
- 29.Connor TH, DeBord DG, Pretty JR, Rogers B, Escalante CP, Lees PSJ, et al. Evaluation of antineoplastic drug exposure of healthcare workers at three university-based U.S. cancer centers. J Occup Exp Monitoring. 2010;52:1019–1027. doi: 10.1097/JOM.0b013e3181f72b63. [DOI] [PubMed] [Google Scholar]
- 30.Larson RR, Khazaeli MB, Dillon HK. A new monitoring method using solid sorbent media for evaluation of airborne cyclophosphamide and other antineoplastic agents. Appl Occup Environ Hyg. 2003b;18:120–131. doi: 10.1080/10473220301435. [DOI] [PubMed] [Google Scholar]
- 31.Schmaus G, Schierl R, Funck S. Monitoring surface contamination by antineoplastic drugs using gas chromatography-mass spectrometry and voltammetry. Am J Health Syst Pharm. 2002;59:956. doi: 10.1093/ajhp/59.10.956. [DOI] [PubMed] [Google Scholar]
- 32.Hedmer M, Jonsson BAG, Nygren O. Development and validation of methods for environmental monitoring of cyclophosphamide in workplaces. J Environ Monit. 2004;6:979–984. doi: 10.1039/b409277e. [DOI] [PubMed] [Google Scholar]