Abstract
We conducted in vitro studies and a clinical trial for patients with squamous cell carcinoma of the head and neck (SCCHN) to study the relationship between FcγRIIIa polymorphisms and antibody-dependent cellular cytotoxicity (ADCC). In vitro, FcγRIIIa genotype was correlated with ADCC and innate cytotoxicity using natural killer (NK) cells harvested from healthy donors. In the phase II study, patients with recurrent or metastatic SCCHN were treated with cetuximab (500 mg/m2 i.v. every 2 weeks) and lenalidomide (25 mg daily). FcγRIIIa genotype and ex vivo ADCC were correlated with clinical response, progression-free survival (PFS), and overall survival (OS). In vitro, healthy donors with a FcγRIIIa 158-V allele demonstrated more effective ADCC against two colon cancer cell lines HT29 and SW480, mean cytotoxicity: FF 16.1%, VF/VV 24.3% (P = 0.015) and FF 11.7%, VF/VV 21.0% (P = 0.008), respectively. We observed a linear relationship between ADCC response and innate cytotoxicity. In the phase II trial, 40 patients received cetuximab and lenalidomide with median PFS of 7.2 weeks and OS of 16.4 weeks. Thirty-six patients had FcγRIIIa genotype: VV (2), VF (20), and FF (14), and 25 patients had sufficient NK-cell yield to perform ex vivo ADCC. FcγRIIIa genotype was not associated with any clinical outcomes. Patients mounting ex vivo ADCC response had a higher likelihood of stable disease (P = 0.01) and showed a trend toward increased PFS: 14 weeks versus 6.8 weeks, respectively (P = 0.13). Enhanced ex vivo ADCC and innate immunity responses were more predictive of clinical response than FcγRIIIa and may offer a functional assay to select patients suitable for cetuximab therapy.
Introduction
Cetuximab has established benefit in squamous cell carcinoma of the head and neck (SCCHN) and colorectal cancer and, by virtue of being an IgG1 antibody, can activate antibody-dependent cellular cytotoxicity (ADCC). In ADCC, the constant (Fc) region of IgG1-based mAbs that are bound to cell surface targets can engage and crosslink low-affinity canonical Fc receptors (FcγRIIIa) expressed on natural killer (NK) cells, resulting in NK activation, degranulation, and lysis of the target cell (1, 2). There has been considerable effort to identify biomarkers that reflect the capacity to mount ADCC. Presently, the most studied biomarkers are single amino acid polymorphisms at the 158 position of FcγRIIIa. Substitution of phenylalanine [F] by valine [V] increases FcγRIIIa affinity for mAb Fc, which affects ADCC and tumor regression (3). As an example, it is generally accepted that non-Hodgkin lymphoma patients who are homozygous for the high-affinity FcγRIIIa-158-V polymorphism have better response to rituximab, an IgG1 mAb directed against CD20, than do patients who carry low-affinity FcγRIIIa-158-F polymorphisms.
However, the association between FcγRIIIa-158 polymorphisms and ADCC response to mAb is less straightforward in other cancer types, including SCCHN, colorectal cancer, and breast cancer (4–9). Interestingly, in a previously published pilot study, we demonstrated that NK cells carrying the high-affinity FcγRIIIa-158-V polymorphism were more effective in killing K562 leukemia cells, even in the absence of antibody (innate immunity), compared with NK cells homozygous for the low-affinity F polymorphism (10). These data suggest that FcγRIIIa-158 polymorphisms may correlate not only with the FcγRIIIa-binding affinity, but also with an NK phenotype that has a broader cytotoxicity profile.
In this study, we sought to more clearly define the link between FcγRIIIa-158-V and F polymorphisms and ADCC response, both in vitro and in vivo. In addition, we studied colorectal cancer cells to expand our study to include another solid tumor system, in which cetuximab is used therapeutically. Based on our previous data, we hypothesized that NK cells carrying the FcγRIIIa-158-V polymorphism would both have a higher level of innate cytotoxicity against colon cancer cell lines and induce more potent cetuximab-mediated ADCC in vitro. We now report that the FcγRIIIa-158-V polymorphism is not the sole determinant of the magnitude of cetuximab-mediated ADCC in vitro. We observe NK cells with a phenotype of broad cytotoxic capacity that simultaneously share high innate cytotoxicity and enhanced ADCC independent of FcγRIIIa-158 genotype.
We sought to validate these findings clinically without the potentially confounding effects of cytotoxic chemotherapy in SCCHN because cetuximab is rarely administered as a single agent in colorectal cancer. Therefore, we initiated a multi-institutional phase II clinical trial evaluating the efficacy of cetuximab in patients with recurrent or metastatic SCCHN in combination with lenalidomide. The rationale for choosing lenalidomide in combination with cetuximab is that there is strong evidence that lenalidomide enhances ADCC in combination with IgG1 antibodies, including cetuximab, and the mechanism of action is through ADCC (11–15). Our clinical trial data also suggest that FcγRIIIa-158 polymorphisms alone are inadequate to predict clinical response in cetuximab and lenalidomide-treated patients. We obtained NK cells from enrolled patients to perform ex vivo ADCC; when NK cells from patients were able to effectively initiate ADCC ex vivo, they were described as “ADCC inducible.” Moreover, our data suggest that the capacity of a patient’s NK cells to mount ADCC ex vivo best predicted improved clinical response, regardless of FcγRIIIa polymorphisms. In concert, these data support that innate NK-cell cytotoxicity and the capacity to mount ADCC are more important than FcγRIIIa polymorphisms in determining clinical response to cetuximab.
Materials and Methods
ADCC assays
Whole blood was obtained from enrolled SCCHN patients for the isolation of NK cells to perform ex vivo ADCC assays, which in this study is distinguished from ADCC assays involving healthy blood donors utilized for in vitro colorectal cancer studies. From each enrolled patient, 150 mL of whole blood was processed and centrifuged; the buffy coat layer was isolated to harvest peripheral blood mononuclear cells (PBMC) using the Ficoll–Hypague centrifugation method; NK cells were negatively selected using a MACS human NK cell isolation kit (Miltenyi Biotec). ADCC assays were performed using SCCHN cells (TU167) as targets, and purified NK cells as effectors from enrolled SCCHN patients. ADCC assays were also performed using a leukemia cell line (K562) for positive controls, two colon cancer cell lines (HT29, SW480) as target cells, and purified NK cells from healthy donors. Target cells were incubated with 150 μCi of chromium-51 (51Cr; Amersham) at 37°C for 1 hour, mixing thoroughly every 15 minutes, and washed twice with media. Cells were subsequently incubated with 10 μg/mL of cetuximab, 10 μg/mL of control human IgG1 isotype, or with media alone for another 30 minutes at 37°C, and washed twice with media to remove unbound antibodies. The concentration of cetuximab was established based upon our prior work and also on physiologic serum concentration ranges: 4–8 μg/mL to 16–23 μg/mL for peak and trough, respectively, according to packet insert. Effector and target cells were plated at 50:1, 25:1, and 12.5:1 in 96-well plates and incubated for 14 to 16 hours. Each assay was performed in triplicate. Cell lysis supernatant was collected and mixed with the Optiphase Supermix scintillation fluid (Perkin Elmer) and counted in a MicroBeta 1450 scintillation counter (Wallac). Activity against NK-sensitive K562 tumor cell line was used as a positive control for all ADCC experiments and provided the measure for innate cytotoxicity. Results were expressed as the percentage of specific lysis according to the following formula:
Determination of FcγRIIIa polymorphisms
Genomic DNA from PBMCs obtained from enrolled SCCHN patients was isolated using the Qiagen DNA extraction Kit (Qiagen) and stored at −20°C. The FcγRIIIa valine, V, or phenylalanine, F, at position 158 was determined by PCR. Briefly, the PCR reaction was optimized using 250 ng of DNA, 0.5 mmol/L dNTPs, 1 unit GoTaq polymerase (Promega), and corresponding 1× buffer containing 1.5 mmol/L MgSO4 to a final volume of 50 μL. For PCR amplification, samples were subjected to an initial denaturation step at 94°C for 5 minutes, followed by 35 cycles at 94°C for 40 seconds, 52°C for 1 minute, 72°C for 1 minute, and a final extension at 72°C for 5 minutes. Amplified PCR samples were cleaned using phenol extraction and ethanol precipitation before restriction digestion. Two digestions per sample were performed to distinguish the FcγRIIIa polymorphisms. First, 10 μL of the PCR product was incubated at 37°C overnight with 10 units of RsaI with the appropriate 1× buffer (Promega) to a final volume of 20 μL. For the second digestion, 10 μL of the RsaI-digested sample was subsequently incubated at 37°C overnight with 40 units of Eco130I with the appropriate 1× buffer (Fermentas) to a final volume of 30 μL. Both RsaI- and RsaI/Eco130I-digested products were separated on a 3% agarose gel with ethidium bromide and visualized under UV light.
Cell culture
SCCHN cell line TU167 was obtained from The University of Texas MD Anderson Cancer Center (Houston, TX). Cells were cultured in RPMI 1640 complete media containing 10% heat-inactivated FBS (Atlanta Biologics), 1% L-glutamine (Gibco), 1% penicillin–streptomycin (Gibco), and HEPES buffer (Mediatech, Inc.). The K562, HT29, and SW480 cell lines were purchased from the ATCC, which characterizes the cell lines by various methods. No other authentication assays were performed.
Clinical trial design
The phase II multi-institutional trial enrolled patients with recurrent/metastatic SCCHN recruited at three cancer centers: University of Chicago Comprehensive Cancer Center, University of Maryland Greenebaum Cancer Center, and Medical College of Wisconsin Cancer Center. The primary endpoint was objective response rate (RR). Secondary endpoints included estimating progression-free survival (PFS), overall survival (OS), as well as the plausible association of ex vivo ADCC response (NK cells harvested from enrolled patients) with PFS, OS, and FcγRIIIa polymorphisms.
Patient eligibility
Patients with recurrent or metastatic squamous cell or undifferentiated carcinoma of the head and neck not amenable to curative therapy were screened for eligibility. Patients were eligible if they were at least 18 years of age, able to understand and provide voluntary consent, had an Eastern Cooperative Oncology Group (ECOG) status of at least 2, serum chemistry reflecting normal kidney, liver, and hematologic function, and had not received therapy (including radiation, hormonal therapy, and surgery) for at least 4 weeks before enrollment. In addition, patients had to have measurable disease as defined by RECIST (16). Patients were excluded if they had a secondary malignancy within 3 years of enrollment, with the exception of treated basal cell or squamous cell carcinoma of the skin, or carcinoma in situ of the cervix or breast. This study was conducted in accordance with the Institutional Review Board approval from each participating institution.
Treatment administration
Patients received cetuximab (500 mg/m2) i.v. infusions via infusion pump or gravity drip every 2 weeks. Lenalidomide, 25 mg, orally or via feeding tube once daily was provided in accordance with the RevAssist program of Celgene Corporation. Per standard RevAssist requirements, all physicians who prescribed lenalidomide for research subjects enrolled into this trial were registered in and complied with all requirements of the RevAssist program.
Evaluation during study and response assessment
Clinical response was evaluated with CT every 2 cycles (8 weeks). RECIST was used to determine response. At treatment discontinuation, a safety assessment was done approximately 30 days after the last dose of protocol therapy.
p16 determination for oropharyngeal SCCHN patients
After deparaffinization and rehydration, tissue sections were treated with antigen retrieval buffer (S1699; DAKO) and heated in a steamer for 20 minutes. Anti-p16 antibody (Santa Cruz Biotechnology; Cat#sc-56330, mouse IgG, JC8, 1:100) was applied on tissue sections for 1-hour incubation at room temperature in a humidity chamber. Following TBS wash, the antigen–antibody binding was detected with Envision+system (DAKO; K4001) and DAB+ chromogen (DAKO; K3468). Tissue sections were briefly immersed in hematoxylin for counterstaining and were covered with cover glasses to determine p16 positivity.
Statistical analysis
Formal power calculations, and resulting adequate sample size, were obtained based on our preliminary in vitro FcγRIIIa polymorphism ADCC studies. However, there are no published data regarding differential clinical response for FcγRIIIa polymorphic genotype in SCCHN patients treated with cetuximab. Power of the statistical test and a corresponding sample size were constructed to detect an improvement in median OS of 8 weeks for patients with a favorable FcγRIIIa polymorphic genotype. Thus, the total required accrual was 40 to 45 patients.
OS was defined as time from diagnosis to date of death from any cause, censored at the date the patient was last known to be alive. PFS was defined as time from the date of the first treatment dose administered to the earlier of either disease progression or death from any cause.
OS and PFS functions were estimated by the Kaplan–Meier method. OS and PFS medians with the corresponding confidence intervals (CI) were used to summarize the time-to-event distributions. Patients were grouped for the analyses according to their FcγRIIIa polymorphism status. The log-rank test was used to test whether distinct categories of patients had different OS and/or PFS experience. The multivariable Cox regression model was utilized to estimate HRs. The following risk factors were assessed in the regression model, FcγRIIIa genotype, ADCC inducibility, human papilloma virus (HPV) status, prior treatment, and tumor site. However, modeling options were very limited due to rather small number of events in the dataset. The general liner model approach was applied to estimate plausible differences (between patients’ categories) in continuous variables, and exact tests for rxc contingency tables were appropriate to use for the categorical ones. All hypothesis tests were conducted at the 0.05 level of statistical significance, were two-sided, and exact where appropriate. Statistical analyses were performed using SAS statistical software (SAS version 9.1; SAS Institute) and S-plus (TIBCO, v.8.2).
An intention-to-treat analysis, based on the initial treatment assignment and not on the treatment eventually received, was done on the distribution of FcγRIIIa polymorphisms, ADCC responders, and relevant demographic factors.
Results
Phase II trial of cetuximab and lenalidomide in SCCHN
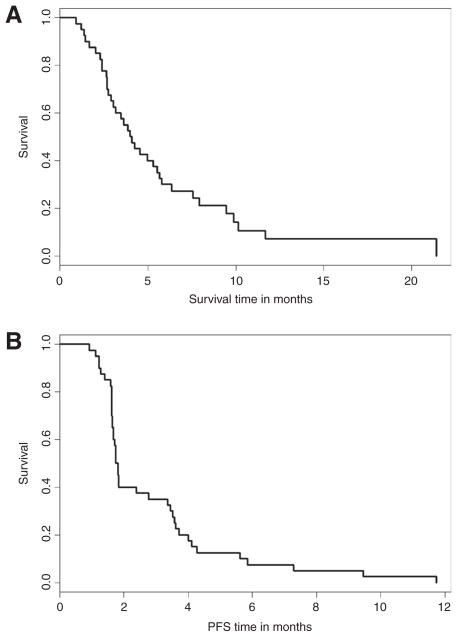
Based on these in vitro data, we decided to further evaluate the relationship between FcγRIIIa polymorphisms, ex vivo ADCC response, and clinical response in cetuximab-treated patients with recurrent or metastatic SCCHN in the context of the immune modulator, lenalidomide. Lenalidomide was selected for combination therapy with cetuximab because of evidence that it enhances ADCC in the presence of cetuximab. Forty-two patients were enrolled (Table 1). Two patients were removed from the study because their clinical status declined substantially during the interval between registration and initiation of therapy. For all patients enrolled, the median OS and PFS were 16.4 and 7.2 weeks, respectively (Fig. 1). In addition, no patients demonstrated complete or partial response to treatment, whereas 14 (39%) showed stable disease and 22 (61%) had progressive disease at first assessment.
Table 1.
Distribution of phase II trial clinical, molecular, and demographic characteristics (n = 42)
| Patient characteristics | Frequency (%) |
|---|---|
| Gender | |
| Female | 10 (23.8) |
| Male | 32 (76.2) |
| Race | |
| White | 30 (71.4) |
| African American or Black | 7 (16.7) |
| Asian | 1 (2.4) |
| American Indian or Alaska native | 1 (2.4) |
| Unknown | 3 (7.1) |
| Age range (years) | |
| 40–49 | 6 (14.3) |
| 50–59 | 17 (40.5) |
| 60–69 | 14 (33.3) |
| 70–79 | 2 (4.8) |
| >79 | 2 (4.8) |
| Unknown | 1 (2.4) |
| Age (years) | |
| N | 42 |
| Median | 59 |
| Range | 42–82 |
| Status | |
| Alive | 4 (9.5) |
| Dead | 36 (85.7) |
| Unknown | 2 (4.8) |
| Clinical measures | |
| Primary tumor site | |
| OP (oropharynx) | 16 (38.1) |
| Non-OP (all other sites) | 24 (57.1) |
| Not available | 2 (4.8) |
| Genotype | |
| FF | 14 (33.3) |
| VF | 19 (45.2) |
| VV | 3 (7.1) |
| Not available | 6 (14.3) |
| ADCC performed | |
| No | 17 (40.0) |
| Yes | 25 (60.0) |
| ADCC | |
| Noninducible | 17 (69.2) |
| Inducible | 8 (30.8) |
| Prior chemoradiation | |
| No | 3 (7.1) |
| Yes | 36 (85.7) |
| Unknown | 3 (7.1) |
| Prior surgery | |
| No | 14 (33.3) |
| Yes | 25 (59.5) |
| Unknown | 3 (7.1) |
| p16 staining | |
| Negative | 7 (16.7) |
| Positive | 6 (14.3) |
| Not tested | 29 (69.0) |
| ECOG status | |
| 0 | 13 (30.9) |
| 1 | 27 (64.3) |
| Unknown | 2 (4.8) |
| Disease location | |
| Local only | 5 (11.9) |
| Metastatic only | 21 (50.0) |
| Local and metastatic | 14 (33.3) |
| Unknown | 2 (4.8) |
Figure 1.
The Kaplan–Meier estimates of OS (A) and PFS (B) for all patients on the study.
FcγRIIIa genotype does not correlate with cetuximab-associated ADCC inducibility and clinical outcome
Determination of FcγRIIIa polymorphic genotype at position 158 was available for 36 patients: VV 2, VF 20, and FF 14. A total of 6 patients did not have genotyping performed (two of the shipped samples were not available within 24 hours, technical difficulties were encountered during the processing of two samples, and for 2 patients, adequate blood sample was not obtained during enrollment). Patients who were both homozygous and heterozygous for the high-affinity V allele at position 158 were grouped together and compared with those who were homozygous at the F allele because our previous data demonstrated an ADCC advantage if at least 1 V allele is present (10). When NK cells from trial patients were able to effectively initiate ADCC ex vivo, they were described as “ADCC inducible.” No association between FcγRIIIa genotype and OS (HR for FF 0.9; 95% CI, 0.4–1.9, P = 0.82), PFS (HR for FF 1.0; 95% CI, 0.5–2.1, P = 0.9), or RR (P = 0.1) was observed (Tables 2, 3, and 4). In addition, there was no association with ADCC inducibility and FcγIIIRa genotype. These data suggest that FcγRIIIa polymorphisms are not adequate to predict clinical outcome in cetuximab/ lenalidomide-treated SCCHN patients.
Table 2.
Results of the univariate Cox regression model for PFSa (n = 42)
| N | HR (95% CI) | P value | |
|---|---|---|---|
| Patient characteristics | |||
| Gender | |||
| Male | 31 | 1.0 | 0.68 |
| Female | 9 | 0.8 (0.4–1.9) | |
| Race | |||
| White | 30 | 1.0 | 0.73 |
| Black | 6 | 0.9 (0.4–2.1) | |
| Race | |||
| White | 30 | 1.0 | 0.44 |
| Other | 10 | 0.8 (0.4–1.6) | |
| Age | 40 | 1.0 (0.9–1.0) | 0.18 |
| Clinical measures | |||
| Primary tumor site | |||
| Non-OP | 24 | 1.0 | 0.53 |
| OP | 16 | 1.2 (0.6–2.3) | |
| Polymorphism | |||
| FF | 14 | 1.0 | 0.90 |
| FV or VV | 21 | 1.0 (0.5–2.1) | |
| Surgery (in addition to treatment) | |||
| No surgery | 14 | 1.0 | 0.81 |
| Surgery | 25 | 0.9 (0.5–1.8) | |
| P16 staining | |||
| Negative | 7 | 1.0 | 0.71 |
| Positive | 6 | 0.8 (0.3–2.6) | |
| ADCC response | |||
| No response | 18 | 1.0 | 0.13 |
| Response | 8 | 0.6 (0.2–1.3) | |
Abbreviation: OP, oropharynx.
PFS is defined as time from the start of treatment until progression or death. For cases without progression, follow-up was censored at date patient was last known to be progression free.
Table 3.
Results of the univariate Cox regression model for OSa (n = 42)
| n | HR (95% CI) | P value | |
|---|---|---|---|
| Patient characteristics | |||
| Gender | |||
| Male | 31 | 1.0 | 0.44 |
| Female | 9 | 0.7 (0.3–1.7) | |
| Race | |||
| White | 30 | 1.0 | 0.77 |
| Black | 6 | 0.9 (0.3–2.3) | |
| Race | |||
| White | 30 | 1.0 | 0.98 |
| Other | 10 | 1.0 (0.5–2.2) | |
| Age | 40 | 1.0 (0.9–1.0) | 0.28 |
| Clinical measures | |||
| Primary tumor site | |||
| Non-OP | 24 | 1.0 | 0.74 |
| OP | 16 | 0.9 (0.4–1.8) | |
| Polymorphism | |||
| FF | 14 | 1.0 | 0.82 |
| FV or VV | 21 | 0.9 (0.4–1.9) | |
| Surgery (in addition to treatment) | |||
| No surgery | 14 | 1.0 | 0.86 |
| Surgery | 25 | 0.9 (0.5–1.9) | |
| P16 staining | |||
| Negative | 7 | 1.0 | 0.09 |
| Positive | 6 | 0.3 (0.1–1.2) | |
| ADCC response | |||
| No Response | 17 | 1.0 | 0.85 |
| Response | 8 | 1.1 (0.4–2.9) | |
Abbreviation: OP, oropharynx.
OS is defined from the date of start of treatment till the date of death or censored at the date last known alive.
Table 4.
Contingency table for best response to treatment
| Factor | Best response status
|
Total | |
|---|---|---|---|
| SD | PD | ||
| Polymorphism | |||
| FF | 5 | 8 | 13 |
| FV or VV | 8 | 10 | 18 |
| Total | 13 | 18 | 31 |
| HPV | |||
| Negative | 2 | 4 | 6 |
| Positive | 3 | 3 | 6 |
| Total | 5 | 7 | 12 |
| ADCC induciblea | |||
| No | 3 | 11 | 14 |
| Yes | 6 | 2 | 8 (P = 0.01) |
| Total | 9 | 13 | 22 |
NOTE: No association was observed between best response (RR), FcγRIIIa genotype, and HPV status (both exact and two-sided P values are 1.0).
However, there was a better chance of having SD (stable disease response) versus disease progression (PD) for those patients who were ADCC inducible (P = 0.01, exact and two-sided).
Ex vivo ADCC predicts clinical outcome
Twenty-five patients had adequate harvest of NK cells to perform ex vivo ADCC against SCCHN cell lines (Table 5). It was our goal to determine if patients who were able to initiate effective ADCC ex vivo would also have improved clinical outcomes, such as clinical response, OS, and PFS. Seventeen patients did not have ex vivo ADCC performed, with the most common reason being inadequate NK-cell yield to perform ADCC from 13 patients; shipped samples from 2 patients were not available within 24 hours; and blood sample was not taken from 2 patients during enrollment. In order to determine if any bias existed for the patients for whom we were able to perform ex vivo ADCC versus those who were not, we estimated and compared the OS and PFS experience for the two groups of patients, and demonstrated that no difference existed in OS or PFS between groups (Supplementary Fig. S1).
Table 5.
Ex vivo ADCC assays from patients enrolled in phase II trial
| Genotype | Media (−) | IgG (−) | Cetuximab | K562 (+) |
|---|---|---|---|---|
| VF | 11% | 9% | 25% | 17% |
| VV | 0% | 0% | 15% | 1 |
| FF | 9% | 1% | 3% | 0 |
| FF | 6% | 5% | 8% | 4 |
| VF | 7% | 5% | 11% | 7 |
| VF | 2% | 4% | 10% | 8 |
| FF | 8% | 6% | 54% | 32 |
| VF | 2% | 1% | 12% | 14 |
| VF | 6% | 6% | 3% | 2 |
| VF | 0% | 4% | 34% | 30 |
| VF | 4% | 4% | 33% | 28 |
| FF | 1% | 0% | 16% | 3 |
| FF | 4% | 5% | 54% | 47 |
| FF | 8% | 9% | 38% | 30 |
| FF | 5% | 7% | 11% | 12 |
| FF | 8% | 2% | 3% | 6 |
| FF | 3% | 3% | 2% | 2 |
| VF | 3% | 1% | 46% | 49 |
| VF | 0% | 8% | 17% | 6 |
| VF | 4% | 3% | 0% | 2 |
| VF | 11% | 1% | 23% | 19 |
| VF | 0% | 0% | 19% | 21 |
| FF | 12% | 4% | 13% | 8 |
| VF | 9% | 5% | 31% | 51 |
| VF | 6% | 7% | 41% | 51 |
NOTE: Results from ex vivo chromium-51 ADCC assays performed for 25 patients from phase II trial when adequate sample was available. First column indicates FcγRIIIa genotype, subsequent columns represent percentage of specific cell lysis for negative controls (media and IgG), cetuximab, and positive controls (K562), respectively. Bold rows indicate ADCC-inducible ex vivo assays.
Among the 25 patients who had adequate NK-cell yield to perform ADCC, 8 showed enhanced cytotoxic activity (>30% cytotoxicity) and were termed “ADCC inducible.” Interestingly, ADCC inducibility was the most predictive marker for clinical effectiveness. Compared with the 17 patients who did not mount an ex vivo ADCC response, those who were ADCC inducible showed a trend toward increased PFS (3.5 vs. 1.7 months; HR = 0.6; 95% CI, 0.6–1.3, P = 0.13; Table 2). Furthermore, there was a significantly greater likelihood of having stable disease versus progressive disease for patients who were ADCC inducible compared with those who did not mount an ex vivo ADCC response (P = 0.01, exact and two-sided). No association was found with OS. These data suggest that the capacity to mount ADCC ex vivo may correlate with improved clinical outcome. This, together with the finding that FcγRIIIa genotype did not correlate with ADCC response, provides further rationale to identify biomarkers beyond the FcγRIIIa genotype that define innate NK-cell cytotoxicity and potential ADCC response.
HPV status and outcome
In this study, 16 of the 42 patients enrolled had primary tumors originating in the oropharynx. Because HPV is associated with oropharyngeal SCCHN and has been established to correlate with better survival, we determined the HPV status of the patients with oropharyngeal cancers by staining for the presence of p16. We were able to determine HPV status for 13 of the 16 patients with oropharyngeal SCCHN. Six patients were p16 positive, and these patients showed a trend toward increased OS (HR, 0.3; P = 0.09; Table 3). There was no association between HPV status and FcγRIIIa genotype or objective RR. Table 4 provides a contingency table for clinical RR by HPV status, ADCC inducibility, and FcγRIIIa genotype.
Toxicities
Of the 40 patients that received cetuximab/lenalidomide, all had at least one adverse event (AE), and of these, 39 (99%) patients had an AE related to cetuximab/lenalidomide (Table 6). Six grade 4 AEs were observed. One patient developed an oropharyngeal hemorrhage due to a pseudoaneurysm of the left internal maxillary artery, which was not attributed to treatment. Seventeen patients (43%) had serious AEs (SAE) that did not result in death, and 5 of these SAEs (13%) were deemed related to cetuximab/lenalidomide. The most frequent SAE was neutropenia (2/40, 5%). Other SAEs related to cetuximab/ lenalidomide included an infusion-related reaction (1/40, 3%), muscle weakness (1/40, 3%), and deep vein thrombosis (1/40, 3%). A total of 9 patients (23%) died during the study, and one of the deaths was associated with multiple deep vein thrombosis and pulmonary thromboembolism was considered possibly related to cetuximab/lenalidomide. Causes of death included pulmonary edema, aspiration, and progressive disease; 5 patients died of unspecified causes.
Table 6.
Summary of AEs
| Cetuximab ± lenalidomide (N = 40)
|
||
|---|---|---|
| All | Grades 3–4 | |
| Total | 40 (100%) | 26 (65%) |
| Fatigue | 33 (82%) | 2 (5%) |
| Rash | 32 (80%) | 0 (0%) |
| Anemia | 27 (67%) | 3 (8%) |
| Constipation | 18 (45%) | 0 (0%) |
| Anorexia | 15 (32%) | 1 (3%) |
| Nausea | 11 (28%) | 0 (0%) |
| Mucositis | 10 (25%) | 0 (0%) |
| Hypoalbuminemia | 10 (25%) | 0 (0%) |
| Leukopenia | 10 (25%) | 5 (13%) |
| Lymphopenia | 9 (23%) | 6 (15%) |
| Neutropenia | 8 (20%) | 5 (13%) |
| Thrombocytopenia | 8 (20%) | 4 (10%) |
| Vomiting | 7 (20%) | 0 (0%) |
| Hyponatremia | 8 (20%) | 3 (8%) |
| Diarrhea | 8 (20%) | 1 (3%) |
| Xerostomia | 6 (15%) | 0 (0%) |
| Hypomagnesemia | 3 (8%) | 0 (0%) |
| Infusion reaction | 2 (5%) | 1 (3%) |
| Thromboembolic events | 1 (3%) | 0 (0%) |
In vitro ADCC and innate cytotoxicity
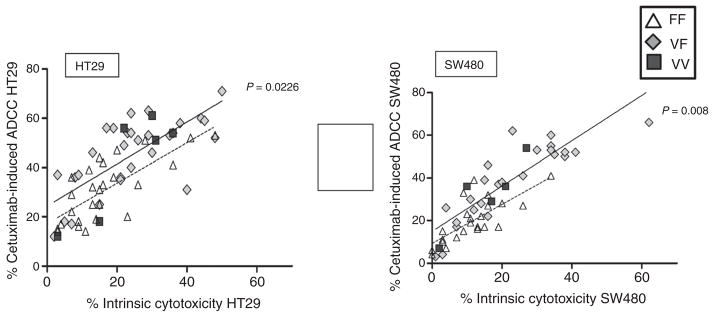
Our previously published work showed that NK cells carrying at least one FcγRIIIa-158-V allele are more effective at killing (positive control) NK-sensitive K562 leukemia cells than FF genotype NK cells. More specifically, even in the absence of tumor antigen–targeted mAbs, cytotoxic activity of VV and VF NK cells was 41% compared with 21% for FF (P = 0.04), suggesting that FcγRIIIa polymorphisms may be associated with enhanced NK-cell cytotoxicity through mechanisms unrelated to FcγRIIIa engagement (10). To test this concept further, we evaluated the relationship between FcγRIIIa polymorphisms and cytotoxicity in the absence of antibody (innate cytotoxicity) against K562 leukemia cells, as well as cetuximab-induced ADCC using a larger cohort of NK-cell healthy donors against two separate colon cancer cell lines, HT29 (N = 27 FF and 35 VV/VF) and SW480 (N = 23 FF and 30 VV/VF).
Consistent with our previous data, the FcγRIIIa-158-V polymorphism in vitro was associated with higher innate cytotoxicity against the highly sensitive K562 cells compared with the FcγRIIIa-158-F polymorphism in this larger cohort (FF 40%; VF and VV = 47%, P = 0.054). Similarly, we found that FcγRIIIa-158-V--expressing NK cells had a higher cytotoxic effect against the examined colon cancer cell lines compared with the FF genotype [HT29 mean cytotoxicity: FF 16.1%, VF/VV = 24.3% (P = 0.015) and SW480: FF 11.7%, VF/VV = 21.0% (P = 0.008)]. Thus, when we examined the relationship between FcγRIIIa genotype and cetuximab-induced ADCC, we observed that donor NK cells carrying the FcγRIIIa-158-V polymorphism were generally more effective at inducing ADCC against colon cancer cell lines than those carrying only the FcγRIIIa-158-F polymorphism.
Interestingly, we also found that innate cytotoxicity (against K562) positively correlated with the magnitude of cetuximab-induced ADCC (Fig. 2). Despite our validation that the FcγRIIIa-158-V polymorphism is associated with higher innate NK and cetuximab-induced ADCC against colorectal cancer cell lines in vitro, the observed cytotoxicity was highly variable between the individual donors, regardless of their genotype. Specifically, many donors who were homozygous for the FcγRIIIa-158-F polymorphism demonstrated both high innate cytotoxicity and high cetuximab-induced ADCC. Figure 2 shows the linear relationship between innate cytotoxicity (without antibody) and the magnitude of cetuximab-induced ADCC. Innate cytotoxicity rather than FcγRIIIa polymorphisms had the strongest relationship for predicting the magnitude of cetuximab-mediated ADCC response. Some NK cells expressing FcγRIIIa-158-FF demonstrating high innate cytotoxicity could also mount ADCC more effectively than VF or VV NK cells with low innate cytotoxicity. These in vitro data suggest that FcγRIIIa-158 polymorphisms alone are insufficient to predict the magnitude of ADCC response for colorectal cancer cell lines and provide rationale to identify biomarkers independent of FcγRIIIa polymorphisms that reflect the level of innate NK-cell cytotoxicity.
Figure 2.
ADCC versus innate cytotoxicity (K562) against colorectal cancer cell lines SW480 and HT29.
Discussion
Previous work has demonstrated that cetuximab can induce ADCC in vitro; however, it remained uncertain whether ADCC effects translated into clinically meaningful tumor regression in patients. Moreover, although FcγRIIIa-158-V and F polymorphisms have been associated with clinically relevant ADCC in lymphoma patients, their predictive value in solid organ malignancies remains less defined (7, 17, 18). Here, we demonstrate, in colon cancer cell lines, that FcγRIIIa polymorphisms alone are insufficient to predict ADCC response and that innate NK-cell cytotoxicity, a property for which there are no validated biomarkers, may be more important. In fact, NK cells expressing low-affinity FcγRIIIa-158-F receptors could mount robust ADCC if they had high innate cytotoxicity. Furthermore, in a phase II clinical trial using cetuximab and lenalidomide in SCCHN patients, our findings support that it is possible that the capacity of NK cells to induce ADCC ex vivo independently correlates with improved clinical outcomes irrespective of FcγRIIIa polymorphisms. Taken together, these data provide clinical evidence of the relevance of ADCC to the mechanism of action of cetuximab and suggest that functional measures of ADCC and NK-cell innate cytotoxicity, rather than FcγRIIIa polymorphisms, may provide a more reliable means of selecting patients who will benefit from this drug.
The observation that innate NK cytotoxicity is independently correlated with ADCC may partly explain why there are conflicting data regarding NK FcγRIIIa polymorphisms and prognosis in cancer patients treated with tumor antigen–targeted mAbs. For example, although the FcγRIIIa VV polymorphism is clearly associated with better prognosis in rituximab-treated patients with follicular lymphoma or with Waldenstrom macroglobulinemia, the same does not hold true in patients with lymphocytic leukemia (4, 5, 19–23). Similarly, two recent studies evaluating clinical responses in cetuximab-treated, KRAS-mutant colon cancer patients yielded conflicting results (6, 7). Ferris and colleagues (24) recently reported results from a phase III RTOG trial of SCCHN patients randomized to receive radiation and cisplatin or radiation, cisplatin, and cetuximab to evaluate the FcγRIIa-H131R and FcγRIIIa-V158F polymorphisms as predictive biomarkers for cetuximab response. They found that polymorphisms in FcγRIIa and FcγRIIIa did not correlate with OS or PFS and concluded that other immune- and non–immune-mediated mechanisms are likely responsible for clinical activity following treatment with cetuximab (24). We postulate that individual specific variation in innate NK-cell cytotoxicity and tumor susceptibility to ADCC may play a role. In other words, although the FcγRIIIa-V polymorphism accurately characterizes FcγRIIIa-binding affinity, it does not reliably predict a phenotype with enhanced ADCC responsiveness against different tumor types. The significance of this finding is that to reliably identify patients who could benefit from ADCC-inducing antibodies, it may be necessary to characterize innate NK-cell cytotoxicity or ex vivo ADCC as well as the FcγRIIIa genotype.
There may be a variety of factors related to why no association was found between FcγRIIIa polymorphisms and clinical outcomes in this study. First, the relatively small sample of patients in our trial may have not been sufficient to detect a difference, if one exists; however, the HRs obtained did not even suggest a trend toward a difference based on polymorphism. Second, the patients in this study had significant tumor burden and a high rate of previous chemotherapy exposures, both likely resulting in dysfunction of their NK responses compared with those of healthy donors and newly diagnosed patients. Of course, the overall milieu of inflammatory cells within the tumor, which is difficult to measure, may influence FcγRIIIa polymorphism response to cetuximab, as well as NK-cell behavior. For instance, in one study, CD163-postive M2 macrophages with FcγRIIIa V allele had a pronounced tumor-promoting response in the presence of cetuximab (18). Although it is possible that the addition of lenalidomide might conceivably affect the influence of FcγRIIIa genotype, other studies suggest that a negative interaction is unlikely (25). In this study, we present data that suggest that other factors may be more influential and predictive for tumoricidal activity than NK FcγRIIIa polymorphisms.
We also explored the potential interaction of HPV and outcome in our patients with recurrent or metastatic oropharyngeal SCCHN receiving cetuximab and lenalidomide. There is emerging evidence that even among patients with recurrent and metastatic oropharyngeal SCCHN, HPV status portends better OS (26, 27). In this trial, HPV status was the only clinical factor that demonstrated a trend for an association with OS (HR = 0.3; 95% CI, 0.1–1.2, P = 0.09). We did not observe a relationship between HPV status and ADCC inducibility.
Our current data show that the strongest predictor of preclinical and clinical efficacy in the presence of cetuximab is ADCC inducibility. In SCCHN patients, an ex vivo functional assay establishing ADCC inducibility was linked with a greater likelihood of stable clinical disease and improved PFS. In addition, our measure of innate immunity correlated more closely with ADCC effectiveness in vitro than FcγRIIIa genotype, suggesting that other factors may be more influential in predicting ADCC response. Therefore, discovering functional assays or markers that predict ADCC inducibility may identify patients who are good candidates for cetuximab-based treatments.
Supplementary Material
Acknowledgments
Grant Support
This work was supported in part by funding from the NCI, Early Therapeutics Development with Phase II Emphasis (NO1 CM62201; W. Stadler, Principal Investigator). In addition, a portion of this work was supported by funding from the Orokawa Foundation. This work was also supported by Celgene Corporation.
Footnotes
Note: Supplementary data for this article are available at Cancer Immunology Research Online (http://cancerimmunolres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: A. Jain, O. Goloubeva
Development of methodology: A. Jain, M. Nagilla
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A. Jain, L. Silpino, J. de Souza, T. Seiwert, V. Villaflor
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A. Jain, O. Goloubeva, S. Kronsberg, M. Nagilla, J. de Souza, T. Seiwert, V. Villaflor
Writing, review, and/or revision of the manuscript: A. Jain, O. Goloubeva, S. Kronsberg, J. de Souza, T. Seiwert
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S. Kronsberg, M. Nagilla, L. Silpino
References
- 1.Iannello A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005;24:487–99. doi: 10.1007/s10555-005-6192-2. [DOI] [PubMed] [Google Scholar]
- 2.Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist. 2007;12:1084–95. doi: 10.1634/theoncologist.12-9-1084. [DOI] [PubMed] [Google Scholar]
- 3.Lin W, Voskens CJ, Zhang X, Schindler DG, Wood A, Burch E, et al. Fc-dependent expression of CD137 on human NK cells: insights into “agonistic” effects of anti-CD137 monoclonal antibodies. Blood. 2008;112:699–707. doi: 10.1182/blood-2007-11-122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–23. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlotti E, Palumbo GA, Oldani E, Tibullo D, Salmoiraghi S, Rossi A, et al. FcgammaRIIIA and FcgammaRIIA polymorphisms do not predict clinical outcome of follicular non-Hodgkin’s lymphoma patients treated with sequential CHOP and rituximab. Haematologica. 2007;92:1127–30. doi: 10.3324/haematol.11288. [DOI] [PubMed] [Google Scholar]
- 6.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–9. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–8. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 8.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–67. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemenceau B, Gallot G, Vivien R, Gaschet J, Campone M, Vié H. Long-term preservation of antibody-dependent cellular cytotoxicity (ADCC) of natural killer cells amplified in vitro from the peripheral blood of breast cancer patients after chemotherapy. J Immunother. 2006;29:53–60. doi: 10.1097/01.cji.0000175686.13368.5c. [DOI] [PubMed] [Google Scholar]
- 10.Taylor RJ, Chan SL, Wood A, Voskens CJ, Wolf JS, Lin W, et al. FcgammaR-IIIa polymorphisms and cetuximab induced cytotoxicity in squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2009;58:997–1006. doi: 10.1007/s00262-008-0613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acebes-Huerta A, Huergo-Zapico L, Gonzalez-Rodriguez AP, Fernandez-Guizan A, Payer AR, López-Soto A, et al. Lenalidomide induces immunomodulation in chronic lymphocytic leukemia and enhances antitumor immune responses mediated by NK and CD4 T cells. Biomed Res Int. 2014;2014:265840. doi: 10.1155/2014/265840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treon SP, Soumerai JD, Branagan AR, Hunter ZR, Patterson CJ, Ioakimidis L, et al. Lenalidomide and rituximab in Waldenstrom’s macroglobulinemia. Clin Cancer Res. 2009;15:355–60. doi: 10.1158/1078-0432.CCR-08-0862. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–7. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 14.Wu L, Parton A, Lu L, Adams M, Schafer P, Bartlett JB. Lenalidomide enhances antibody-dependent cellular cytotoxicity of solid tumor cells in vitro: influence of host immune and tumor markers. Cancer Immunol Immunother. 2011;60:61–73. doi: 10.1007/s00262-010-0919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Qian Z, Cai Z, Sun L, Wang H, Bartlett JB, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84:553–9. doi: 10.1002/ajh.21468. [DOI] [PubMed] [Google Scholar]
- 16.Bogaerts J, Ford R, Sargent D, Schwartz LH, Rubinstein L, Lacombe D, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer. 2009;45:248–60. doi: 10.1016/j.ejca.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Weng WK, Czerwinski D, Timmerman J, Hsu FJ, Levy R. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22:4717–24. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Pander J, Heusinkveld M, van der Straaten T, Jordanova ES, Baak-Pablo R, Gelderblom H, et al. Activation of tumor-promoting type 2 macrophages by EGFR-targeting antibody cetuximab. Clin Cancer Res. 2011;17:5668–73. doi: 10.1158/1078-0432.CCR-11-0239. [DOI] [PubMed] [Google Scholar]
- 19.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–14. [PubMed] [Google Scholar]
- 20.Farag SS, Flinn IW, Modali R, Lehman TA, Young D, Byrd JC. Fc gamma RIIIa and Fc gamma RIIa polymorphisms do not predict response to rituximab in B-cell chronic lymphocytic leukemia. Blood. 2004;103:1472–4. doi: 10.1182/blood-2003-07-2548. [DOI] [PubMed] [Google Scholar]
- 21.Treon SP, Hansen M, Branagan AR, Verselis S, Emmanouilides C, Kimby E, et al. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom’s macroglobulinemia. J Clin Oncol. 2005;23:474–81. doi: 10.1200/JCO.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 22.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 23.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Ferris RL, Zhang Q, Rosenthal D, Gildener-Leapman N, Poveda Gibson S, Singh AK, et al. Correlation Of Fc gamma receptor (FcγR) IIa and IIIa polymorphisms with clinical outcome in patients treated with cetuximab-based chemoradiation in the RTOG 0522 trial [abstract]. 2014 Multidisciplinary Head and Neck Cancer Symposium; Scottsdale, AZ. 2014. [Google Scholar]
- 25.Tuscano JM, Dutia M, Chee K, Brunson A, Reed-Pease C, Abedi M, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol. 2014;165:375–81. doi: 10.1111/bjh.12755. [DOI] [PubMed] [Google Scholar]
- 26.Fakhry C, Zhang Q, Nguyen-Tân P, Rosenthal D, El-Naggar A, Garden A. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma management of recurrent head-and-neck squamous cell carcinoma. Int J Radiat Oncol. 2014;88:466. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehra R, Egloff AM, Li S, Yang D, Wang L, Zhu F, et al. Analysis of HPV and ERCC1 in recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) J Clin Oncol. 2013;31(suppl):abstr 6006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.