Abstract
Human leukocyte antigen (HLA) class I molecules are ligands for antigen receptors of cytotoxic T cells (CTL) and inhibitory receptors of natural killer (NK) cells. The high degree of HLA class I polymorphism allows for the selection of distinct and diverse sets of antigenic peptide ligands for presentation to CTL. The extensive polymorphisms of the HLA class I genes also result in large variations in their intracellular folding and assembly characteristics. Recent findings indicate that North American HLA-B variants differ significantly in the stabilities of their peptide-deficient forms and in the requirements for the endoplasmic reticulum (ER)-resident factor tapasin for proper assembly. In HIV-infected individuals, the presence of tapasin-independent HLA-B allotypes links to more rapid progression to death. Further studies are important to better understand how the intrinsic structural characteristics of HLA class I folding intermediates affect immune responses mediated by CTL and NK cells.
MHC class I molecules as ligands for cytotoxic T cell (CTL) and natural killer (NK) cell receptors
Major histocompatibility complex (MHC) class I molecules are ligands for antigen receptors of CD8+CTL(Bjorkman, 1997; Rossjohn et al., 2014) and for inhibitory receptors of natural killer (NK) cells(Parham and Moffett, 2013). In a normal healthy cell, MHC class I heavy chains and light chains (β2-microglobulin; β2m) form heterodimers that bind short peptides derived from self-proteins. In cells infected with an intracellular pathogen or in cancer cells, a subset of the cellular peptides bound to MHC class I molecules are replaced by pathogen-derived or tumor-specific peptide sequences, which can trigger the activation of T cell receptors (TCR) of specific CTL. Cell surface MHC class I molecules also engage selected inhibitory receptors of NK cells, thereby maintaining NK cells in a quiescent state(Li and Mariuzza, 2014). Viruses and cancers that interfere with the assembly and cell surface expression of MHC class I molecules reduce the engagement of inhibitory receptors, thereby driving NK cellstowards activation. Thus, MHC class I molecules are key regulators of the activation of two major subsets of immune cells.
Three sets of genes encode human and mouse MHC class I heavy chains; these are respectively human HLA-A, HLA-B and HLA-C and murine H2-K, H2-L and H2-D. Human MHC class I genes, encoded on chromosome 6, are highly polymorphic. The HLA-B locus is the most polymorphic. Based on current estimates, there are over 3000 allelic variants of HLA-B(Robinson et al., 2015). The heavy chains of MHC class I molecules comprise three domains; α1 and α2, which contain a peptide-binding groove formed by anti-parallel β–strands that are topped with two helices, and α3, an immunoglobulin(Ig)-like membrane proximal domain (Figure 1A). β2m also forms an Ig-like domain(Bjorkman, 1997). The majority of polymorphic residues are located within the peptide binding grooves of MHC class I molecules(for example, those depicted in Figure 1B) and determine the specificities for peptide binding. Each MHC class I variant binds to a large number of peptides with common sequence motifs(Rammensee et al., 1999), called anchor residues,that form specific interactions with MHC class I residues contained within the peptide binding grooves. The peptide binding groove compositions of individual MHC class I allotypes allow for the selection of peptides with distinct sequence motifs. Expression of MHC class I alleles is codominant, and because of the high degree of polymorphism of the HLA-A, HLA-B and HLA-C genes, most humans are heterozygous at each locus. Thus, human cells typically express six different MHC class I variants to enable presentation of a diverse set of peptide antigens to CTL. Antigen receptors of CTL, TCRs, have extracellular domains that resemble antibody Fab regions in their overall structure(Bjorkman, 1997; Rossjohn et al., 2014). Like antibodies, TCRs contain variable domains that are generated during T cell development by the recombination of multiple gene segments. Hypervariable loops of the TCR variable domains interact with the α1 and α2 domains of the MHC class I heavy chain as well as with bound peptide (Figure 1A). TCRs are exquisitely specific for particular MHC class I-peptide combinations(Bjorkman, 1997; Rossjohn et al., 2014).
Figure 1. Structures of MHC class I molecules and their distinct intracellular assembly modes.
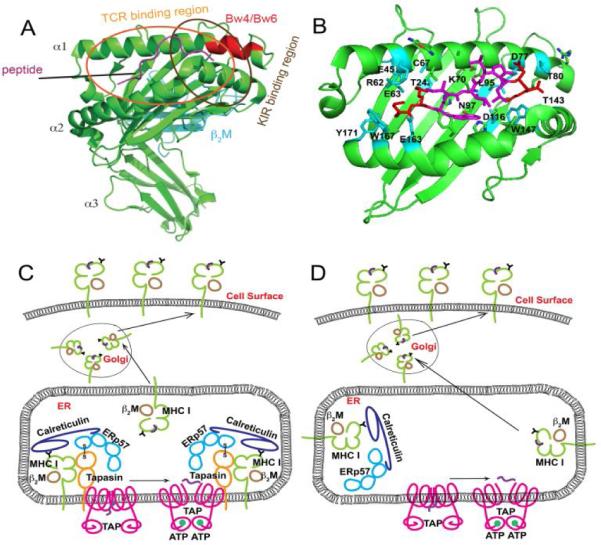
A) Structure of HLA-B*2705 (PDB 4G9D), with heavy chains indicated in green and β2m in cyan. The Bw4/Bw6 epitope region is shown in red. Bound peptide (KRWIILGLNK) is indicated in magenta. Approximate TCR and KIR footprints are specified by ovals. B) Peptide binding groove (α1 and α2 domains) of HLA-B*2705 with bound KRWIILGLNK. Several polymorphic HLA-B*2705 residues important for peptide binding specificity are indicated as sticks. Polymorphic residues interacting with peptide N- and C-termini are shown in cyan. HLA-B*2705 has cysteine, lysine and asparagine respectively at positions 67, 70 and 97. These residuesare shown to be important in control of AIDS progression. Bound peptide is shown in magenta and red, with terminal residues colored red and the central residues colored magenta. C) Conventional tapasin-dependent assembly of HLA-B molecules such as HLA-B*4402 and HLA-B*5701. D) Tapasin-independent assembly of HLA-B molecules such as HLA-B*3501 and HLA-B*4405.
Antigen recognition by NK cells is driven by the net balance of signals emanating from the engagement of activating and inhibitory NK cell receptors. The inhibitory NK receptors that engage MHC class I molecules are structurally distinct in mice and humans. Murine Ly49 receptors belong to the lectin superfamily, whereas human killer-cell immunoglobulin-like receptors (KIR) are members of the immunoglobulin superfamily(Li and Mariuzza, 2014). The human KIR gene family is encoded on chromosome 19 within the leukocyte receptor complex (LRC). There are four phylogenetically distinct lineages of KIR, with lineage II and III KIRs bearing specificity for polymorphic MHC class I molecules(Parham and Moffett, 2013). Among lineage II KIRs, KIR3DL1 recognizes HLA-B molecules and a few HLA-A molecules containing a “Bw4” epitope, which is a specific sequence motif contained within residues 77-83 of the heavy chain (Figure 1A). KIR3DL2 recognizes HLA-A11 and HLA-A3. Lineage III KIRs, KIR2DL1 and KIR2DL2/3,are largely specific for HLA-C variants(Parham and Moffett, 2013). KIR2DL1 recognizes HLA-C molecules with lysine at position 80 (the C2 epitope), whereas KIR2DL2/L3 recognize HLA-C variants with valine at position 76 and asparagine at position 80 (the C1 epitope), as well as certain C2 and two Bw6 allotypes(Li and Mariuzza, 2014; Parham and Moffett, 2013). MHC class recognition by KIR can typically be mediated by a large number of peptides present in complex with cell surface MHC class I, although C-terminal peptide residues can also interfere with binding, via steric blockage(Li and Mariuzza, 2014).
MHC class I assembly in the ER
The assembly of MHC class I molecules occurs within the ER of cells, and involves the assembly factors TAP (transporter associated with antigen presentation) (Mayerhofer and Tampe, 2015) and tapasin(Blum et al., 2013), that are also encoded within the MHC gene complex, that includes the MHC class I heavy chain genes. In the endogenous pathway of antigen presentation, peptides that derive from cytosolic proteolysis are transported into the ER by TAP. TAP is comprised of two subunits, TAP1 and TAP2, each comprising multiple membrane-spanning segments and a cytoplasmic ATP binding domain(Mayerhofer and Tampe, 2015). In the resting state, the cytosol-facing surface of the transmembrane regions of TAP1/TAP2 contains a peptide-binding site with broad specificity for short peptide sequences. The binding of both peptides and ATP induces conformational reorganizations within the transmembrane domains of TAP (Geng et al., 2013) that expose the peptide binding site to the ER lumen, simultaneously reducing the TAP binding affinity for peptide, which allow peptide release into the ER lumen (Figure 1C). ATP hydrolysis resets TAP to the resting state.
Following the assembly of MHC class I heavy chains and β2m, the heterodimers, which are typically unstable in the absence of peptides, are recruited into a multiprotein complex called the peptide loading complex (PLC). The PLC includes TAP, tapasin, the thiol-oxidoreductase ERp57 and the lectin chaperone calreticulin(Blum et al., 2013) (Figure 1C). Tapasin is an ER-resident type I transmembrane domain protein.TAP and tapasin form a complex via their transmembrane regions. The ER lumenal domain of tapasin binds to MHC class I molecules. Tapasin also forms a disulfide-linked heterodimer with ERp57. Calreticulin contain binding sites for the conserved MHC class I glycanas well as for ERp57 within the tapasin-ERp57 complex, and thus helps stabilize interactions within the PLC(Blum et al., 2013; Raghavan et al., 2013) (Figure 1C). Interactions of MHC class I with the PLC facilitate MHC class I-peptide assembly via multiple mechanisms. These include an increased local concentration of peptides (via localization in the vicinity of TAP), stabilization of peptide-deficient heterodimers and optimization of the peptide repertoire in favor of high affinity peptide sequences(Blum et al., 2013). Cells expressing a soluble forms of tapasin are able to form sub-complexes containing MHC class I, tapasin, calreticulin and ERp57, although the complex is only partly functional forthe induction of MHC class I assembly. Similarly, a tapasin construct that is unable to recruit ERp57 and calreticulin into the PLC is only partially functional at inducing MHC class I assembly(Blum et al., 2013; Raghavan et al., 2013). Thus, tapasin is central to the formation and the function of the PLC. Optimal function of the PLC requires both the transmembrane and ER lumenal interactions mediated by tapasin.
TAP preferentially transports 8-16 mer peptide sequences(Mayerhofer and Tampe, 2015), but the majority of peptides bound to MHC class I molecules are 8-10 mer sequences(Rammensee et al., 1999). The activity of an ER aminopeptidase (ERAAP) is important for trimming peptides to amore optimal size for MHC class I binding(Nagarajan and Shastri, 2013; van Endert, 2011).
MHC Polymorphisms and Assembly Variations
Human TAP and tapasin sequences have no known functional polymorphisms. Rat TAP2 and chicken TAP1 and TAP2 sequences have functional polymorphisms that are known to affect peptide transport specificity. Chicken MHC haplotypes have TAP1, TAP2 and tapasin allele variants that are optimally configured for the transport and binding of peptide sequences relevant to the encoded MHC class I (Kaufman, 2015). Further studies of such functional polymorphisms are expected to provide insights into peptide transport and MHC class I assembly mechanisms.
Given the thousands of human MHC class I variants that exist in the human population, it is an important question whether all variants assemble via similar mechanisms described above(Figure 1C). Early studies had indicated differences in the requirement for tapasin for cell surface expression of selected HLA-B variants, defining allotypes that are tapasin-dependent or independent in their assembly characteristics(Thammavongsa et al., 2006; Williams et al., 2002; Zernich et al., 2004). Based on these findings, we recently conducted a systematic analysis of the tapasin dependence of HLA-B allotypes that occur at high frequencies in North America. Retroviral constructs encoding individual HLA-B molecules were used to infect a tapasin-deficient cell line, and cell surface HLA-B expression was measured under conditions of tapasin-deficiency or following reconstitution of the cells with human tapasin, using a second retroviral construct. These studies defined a spectrum of tapasin dependencies among HLA-B variants(Rizvi et al., 2014). Several allotypes such as HLA-B*5701 were strongly tapasin-dependent for their assembly and cell surface expression. Other allotypes such as HLA-B*3501 were expressed at high levels even in the absence of tapasin, and introduction of tapasin had a comparatively small effect on inducing the allotype cell-surfaceexpression. Additionally, significant differences in the stabilities of the peptide-deficient forms were noted following the refolding of soluble forms of several HLA-B molecules after their expression in E. coli. Whereas some peptide-deficient HLA-B molecules migrated primarily as heterodimers, other HLA-B molecules were aggregated, with a significant fraction migrating in the column void volume. In general, tapasin-independent allotypes were less aggregated compared to their tapasin-dependent counterparts, suggesting that tapasin-dependence of assembly relates at least in part to the stabilities of peptide-deficient MHC class I molecules(Rizvi et al., 2014). Molecular dynamics simulations have also suggested conformational differences between peptide-free versions of tapasin-dependent and tapasin-independent MHC class I molecules, particularly in the region near the F-pocket (Abualrous et al., 2015; van Hateren et al., 2015).
In summary, HLA-B molecules can be grouped into those with low, high or intermediate intrinsic stabilities. In turn, stabilityinfluences the extent of dependence on tapasinfor folding and cell surface expression(Rizvi et al., 2014). Several HLA-B molecules are able to successfully fold viaa cellular pathway that is tapasin-independent, which may involve transient associations with a calreticulin-ERp57 complex (Figure 1D). It is noteworthy that tapasin-dependent assembly is generally also associated with a slower rate of intracellular maturation of HLA-B allotypes and stronger steady state bindingto TAP (Thammavongsa et al., 2006; Williams et al., 2002; Zernich et al., 2004).
HLA-B Polymorphisms and AIDS outcomes
The extreme polymorphism of the MHC class I molecules reflects evolutionary adaptations of T and NK cell responses to pathogens, in addition to NK cell functions during reproduction(Parham and Moffett, 2013). Since different combinations of HLA-A, B and C variants are present within individuals in a population, distinct sets of pathogen-derived peptides are presented during infections, resulting in the induction of CTL responses with distinct specificities. Thus, it might be expected that HLA genotypes of individuals are key determinants of susceptibility of individuals to particular pathogens. Indeed, in HIV infections, a number of studies have shown strong genetic associations between the presence of particular HLA class I genotypes and progression to different AIDS outcomes. Compared with HLA-A and HLA-C molecules, HLA-B molecules have stronger influences on AIDS outcomes (Kiepiela et al., 2004). Individuals expressing HLA alleles such as HLA-B*57 and HLA-B*27 showed slow progression to AIDS, while those expressing HLA alleles such as HLA-B*35 progressed rapidly to AIDS(Carrington and Walker, 2012). A genome wide association analysis among HIV controllers and progressors identified single nucleotide polymorphisms within the MHC as major genetic determinants of HIV-1 control. Three amino acids of the HLA-B (positions 67, 70, and 97) (Figure 1B) showed significant associations with host control of HIV infection(Pereyra et al., 2010). All these residues are located in the peptide-binding groove and are important for determining specificity for peptide binding and conformations of peptide-bound HLA class I molecules. Thus, peptide selectivity differences between HLA-B variants are undoubtedly key factors that account for some of the influences of HLA-B genotypes upon rapid or slow progression to AIDS outcomes.
If HLA-B molecules display intrinsic folding and assembly differences as noted in the previous section, a relevant question is whether such differences, in addition to peptide selectivity differences, can affect the abilities of individual allotypes to mediate immune responses. To address this question, individuals from different AIDS cohorts were assigned tapasin-independence scores based on their known HLA-B genotypes. Statistical models were used to examine the effects of tapasin-independent HLA-B expression as a continuous variable upon progression to different AIDS outcomes. These analyses suggested that in HIV infections, tapasin-independent HLA-B assembly confers more rapid progression to death(Rizvi et al., 2014).
HLA-B molecules are grouped into the HLA-Bw4 or HLA-Bw6 serotypes, based on the sequences of residues 77-83 of the heavy chains (Figure 1A). All HLA-B molecules fall into the HLA-Bw4 or the HLA-Bw6 serotype. It is of interest that, tapasin-dependence as well as reduced stability of the peptide-deficient form is more prevalent within the HLA-Bw4 group compared to the HLA-Bw6 group (Rizvi et al., 2014). HLA-Bw4 but not HLA-Bw6 molecules are ligands for KIR3DL1 receptors of NK cells(Parham and Moffett, 2013). HLA class I is known to be down-modulated in HIV-infected cells(Collins and Collins, 2014) and NK cells from subjects expressing high levels of KIR3DL1 and HLA-B*57 were more effective at suppressing HIV-1 replication, compared to NK cells from HLA-Bw6 homozygous individuals(Song et al., 2014). The engagement of MHC class I by KIR receptors during NK cell development is thought to be important for licensing the activity of NK cells, and thus NK cells from HLA-Bw6 homozygous individuals may be less effectively licensed(Kim et al., 2008). Since tapasin-independent assembly is more prevalent within the HLA-Bw6 group, the linkage of tapasin-independent assembly to more rapid progression to death following HIV infections could thus reflect an NK surveillance disadvantage. On the other hand, previous studies have suggested that tapasin-independent assembly confers advantages of increased MHC class I cell surface expression under conditions where viral immune evasion strategies target ER assembly of MHC class I, such as in herpes simplex virus and human cytomegalovirus infection(Park et al., 2004; Zernich et al., 2004). Further studies are required to better understand why evolution has maintained HLA-B molecules with the distinct stability characteristics, and the specific immune advantages conferred by each type of assembly mode.
In summary, North American HLA-B molecules have varying intrinsic stabilities and can begrouped into those with more stringent (Figure 1C) or less stringent (Figure 1D) assembly requirements involving the PLC(Rizvi et al., 2014). Indeed assembly is so efficient that interactions with PLC components in the steady state are essentially undetectable for some allotypes (Thammavongsa et al., 2006; Williams et al., 2002; Zernich et al., 2004). Each mode of assembly may confer selective immune advantages for CTL or NK recognition. Further studies are needed to better understand how the distinct stability and assembly patterns affect immune functions in HIV infections as well as in other infections.
Highlights.
Peptide-deficient forms of HLA-B molecules have varying stabilities.
HLA-B molecules can assemble via tapasin-dependent or tapasin-independent routes.
Assembly modes may confer selective immune advantages for CTL or NK recognition.
Acknowledgements
This work was funded by a NIH grant AI044115 (to MR). This work was supported in part by the University of Michigan Medical School Fast Forward Protein Folding Diseases Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abualrous ET, Fritzsche S, Hein Z, Al-Balushi MS, Reinink P, Boyle LH, Wellbrock U, Antoniou AN, Springer S. F pocket flexibility influences the tapasin dependence of two differentially disease-associated MHC Class I proteins. Eur J. Immunol. 2015;45:1248–1257. doi: 10.1002/eji.201445307. [DOI] [PubMed] [Google Scholar]
- Bjorkman PJ. MHC restriction in three dimensions: a view of T cell receptor/ligand interactions. Cell. 1997;89:167–170. doi: 10.1016/s0092-8674(00)80195-6. [DOI] [PubMed] [Google Scholar]
- Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington M, Walker BD. Immunogenetics of spontaneous control of HIV. Annu Rev Med. 2012;63:131–145. doi: 10.1146/annurev-med-062909-130018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DR, Collins KL. HIV-1 accessory proteins adapt cellular adaptors to facilitate immune evasion. PLoS Pathog. 2014;10:e1003851. doi: 10.1371/journal.ppat.1003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Sivaramakrishnan S, Raghavan M. Analyses of conformational states of the transporter associated with antigen processing (TAP) protein in a native cellular membrane environment. J Biol Chem. 2013;288:37039–37047. doi: 10.1074/jbc.M113.504696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. What chickens would tell you about the evolution of antigen processing and presentation. Curr Opin Immunol. 2015;34C:35–42. doi: 10.1016/j.coi.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, Rathnavalu P, Moore C, Pfafferott KJ, Hilton L, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432:769–775. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- Kim S, Sunwoo JB, Yang L, Choi T, Song YJ, French AR, Vlahiotis A, Piccirillo JF, Cella M, Colonna M, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mariuzza RA. Structural basis for recognition of cellular and viral ligands by NK cell receptors. Front Immunol. 2014;5:123. doi: 10.3389/fimmu.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer PU, Tampe R. Antigen Translocation Machineries in Adaptive Immunity and Viral Immune Evasion. J Mol Biol. 2015;427:1102–1118. doi: 10.1016/j.jmb.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Nagarajan NA, Shastri N. Immune surveillance for ERAAP dysfunction. Mol Immunol. 2013;55:120–122. doi: 10.1016/j.molimm.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B, Kim Y, Shin J, Lee S, Cho K, Fruh K, Lee S, Ahn K. Human cytomegalovirus inhibits tapasin-dependent peptide loading and optimization of the MHC class I peptide cargo for immune evasion. Immunity. 2004;20:71–85. doi: 10.1016/s1074-7613(03)00355-8. [DOI] [PubMed] [Google Scholar]
- Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan M, Wijeyesakere SJ, Peters LR, Del Cid N. Calreticulin in the immune system: ins and outs. Trends Immunol. 2013;34:13–21. doi: 10.1016/j.it.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- Rizvi SM, Salam N, Geng J, Qi Y, Bream JH, Duggal P, Hussain SK, Martinson J, Wolinsky SM, Carrington M, et al. Distinct Assembly Profiles of HLA-B Molecules. J Immunol. 2014;192:4967–4976. doi: 10.4049/jimmunol.1301670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SG. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015;43:D423–431. doi: 10.1093/nar/gku1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T Cell Antigen Receptor Recognition of Antigen-Presenting Molecules. Annu Rev Immunol. 2014 doi: 10.1146/annurev-immunol-032414-112334. [DOI] [PubMed] [Google Scholar]
- Song R, Lisovsky I, Lebouche B, Routy JP, Bruneau J, Bernard NF. HIV protective KIR3DL1/S1-HLA-B genotypes influence NK cell-mediated inhibition of HIV replication in autologous CD4 targets. PLoS Pathog. 2014;10:e1003867. doi: 10.1371/journal.ppat.1003867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammavongsa V, Raghuraman G, Filzen TM, Collins KL, Raghavan M. HLA-B44 polymorphisms at position 116 of the heavy chain influence TAP complex binding via an effect on peptide occupancy. J Immunol. 2006;177:3150–3161. doi: 10.4049/jimmunol.177.5.3150. [DOI] [PubMed] [Google Scholar]
- van Endert P. Post-proteasomal and proteasome-independent generation of MHC class I ligands. Cell Mol Life Sci. 2011;68:1553–1567. doi: 10.1007/s00018-011-0662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hateren A, Bailey A, Werner JM, Elliott T. Plasticity of empty major histocompatibility complex class I molecules determines peptide-selector function. Mol Immunol. 2015 doi: 10.1016/j.molimm.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity. 2002;16:509–520. doi: 10.1016/s1074-7613(02)00304-7. [DOI] [PubMed] [Google Scholar]
- Zernich D, Purcell AW, Macdonald WA, Kjer-Nielsen L, Ely LK, Laham N, Crockford T, Mifsud NA, Bharadwaj M, Chang L, et al. Natural HLA class I polymorphism controls the pathway of antigen presentation and susceptibility to viral evasion. J Exp Med. 2004;200:13–24. doi: 10.1084/jem.20031680. [DOI] [PMC free article] [PubMed] [Google Scholar]


