Abstract
Although the precise mechanisms for control of consciousness are not fully understood, emerging data show that conscious information processing depends on the activation of certain networks in the brain and that the impairment of consciousness is related to abnormal activity in these systems. Epilepsy can lead to transient impairment of consciousness, providing a window into the mechanisms necessary for normal consciousness. Thus, despite differences in behavioral manifestations, cause, and electrophysiology, generalized tonic–clonic, absence, and partial seizures engage similar anatomical structures and pathways. We review prior concepts of impaired consciousness in epilepsy, focusing especially on temporal lobe complex partial seizures, which are a common and debilitating form of epileptic unconsciousness. We discuss a “network inhibition hypothesis” in which focal temporal lobe seizure activity disrupts normal cortical–subcortical interactions, leading to depressed neocortical function and impaired consciousness. This review of the major prior theories of impaired consciousness in epilepsy allows us to put more recent data into context and to reach a better understanding of the mechanisms important for normal consciousness.
Keywords: consciousness, epilepsy, neuronal activity
Introduction
We are all familiar with the notion of consciousness; however, the term consciousness has always been difficult to define and explain. Many question whether the philosophical state of the mind can ever be directly linked to neurological phenomena.1–3 Despite historic and continued uncertainty surrounding this mind–body connection, observation of behavior is the generally accepted method of consciousness assessment.4,5 Consciousness is often measured by assessing a person’s ability to maintain alertness, attention, and awareness of self and environment by continuously monitoring behavioral interactions with the surroundings. Accepting the assumption that there is a correlation between change in mental and neural states, we use epilepsy to study the neurobiological basis for the alteration of consciousness. While it is appropriate to contrast the different symptoms and characteristics of absence, tonic–clonic, and complex partial seizures, it is also important to recognize what is common to these different types of epileptic seizures. Despite their differences, the same anatomical networks have been suggested to be involved in impaired consciousness in these three types of seizures, through either abnormal increases or abnormal decreases in neuronal activity.6–8 Comparative study of these types of seizures may lead to better therapeutic interventions for all types of epilepsy and better understanding of the mechanisms for unconsciousness.
In this paper, we will first outline the most prevalent types of seizures associated with impaired consciousness, and we will then review the major recent ideas of consciousness in epilepsy, chronologically exploring the progression of the research. Our discussion will emphasize mechanisms of impaired consciousness in complex partial temporal lobe seizures, since impaired consciousness in generalized seizures has been discussed extensively elsewhere.7,9,10,85 Concepts from generalized epilepsy have relevance for understanding impaired consciousness in partial seizures. The historical “highest centres” philosophy of Hughlings Jackson and the “centrencephalic theory” of Penfield and Jasper created the framework for contemporary refinement of theories concerning the control of seizures and consciousness. Ideas about hemisphere lateralization in impaired consciousness and the roles of the midbrain and thalamus stemmed from these earlier findings. We review these ideas and the challenges that have arisen, emphasizing recent conceptions of consciousness based on the relationship between impairment and altered function in specific brain regions. We conclude with our proposal of a network inhibition or network disruption by seizure hypothesis as a plausible mechanism for impaired consciousness in temporal lobe complex partial seizures.
Epileptic Seizures Associated with Impaired Consciousness
Epilepsy and seizures are often categorized as either generalized or partial. Generalized tonic–clonic (GTC), or grand mal seizures, are widespread neuronal discharges that include abnormal activity throughout widespread areas of the brain. The tonic portion of these seizures refers to the sustained muscle contractions with 10–20 s of high-frequency electroencephalogram (EEG) activity. Clonic activity follows: limbs contract rhythmically as brain activity settles into poly-spike-and-wave EEG discharges. After a minute or two, clinical and EEG seizure activity usually stops, and the patient typically remains deeply lethargic and unresponsive for several minutes or longer into the postictal period. Tonic–clonic seizures can be primarily generalized, or secondarily generalized, with onset in one focus in the brain and then spread to other regions. Because of the large brain area involved in these generalized seizures, nearly all GTC seizures result in impaired consciousness.85
Occasionally, patients undergoing GTC seizures will retain consciousness ictally.11 For example, seizures beginning in central, extratemporal brain regions with limited effects outside the bilateral motor pathways were found to be spared many behavioral changes associated with loss of consciousness. The regions activated were close to the motor cortex, possibly accounting for the tonic–clonic behavioral components of the seizure. Yet consciousness, language, and memory processing were relatively unaffected.11
Another type of generalized seizure, although very different in manifestations, is the absence seizure. Behaviorally marked by a brief slip in awareness and few motor changes, typical absence seizures are electrographically characterized by large-amplitude spike–waves at approximately 3 Hz. Consciousness is often but not always impaired during an absence seizure.7,12,13,86
Partial seizures may secondarily generalize into GTC seizures as abnormal discharges spread from the focal origin, or they may remain localized to one region of the brain. Partial seizures are classified further as simple partial seizures, with retained consciousness, or complex partial seizures, defined by a loss of consciousness. Temporal lobe seizures, a common type of partial seizure, can likewise be either simple or complex. Simple partial seizures of the temporal lobe are often characterized by autonomic and/or psychic symptoms as well as epigastric sensations. Complex partial temporal lobe seizures often begin with an inhibition of motor activity with noticeable ictal behavioral automatisms and unresponsiveness.14 With complex partial seizures, postictal behavior often continues to show impairment of consciousness, complete with confusion and often amnesia of the event. While impaired consciousness in generalized seizures, with widespread bilateral involvement of cortical and subcortical structures, is not surprising, it has been more of a challenge to explain why focal temporal lobe seizures should commonly lead to impaired consciousness. We will first discuss historical origins of the concept of cortical–subcortical interactions in epileptic unconsciousness in general before returning in detail to more recent advances in explaining impaired consciousness in temporal lobe epilepsy.
John Hughlings Jackson’s “Highest Centers”
Many of the modern ideas of epilepsy anatomy and physiology originated from the work of John Hughlings Jackson (1835–1911), who proposed in his work On the Anatomical, Physiological, and Pathological Investigation of Epilepsies that an epileptic seizure be defined as a “sudden, excessive and rapid discharge of grey matter of some part of the brain; it is a local discharge.”15 His work came at a time when empirical science had begun to displace superstition as the dominant framework for interpreting the physical world; formerly, epilepsy and many other disorders were blamed on witchcraft.16 Jackson explored the origins and outcomes of the seizures he observed in his patients, noting that these seizures often led to an impairment of consciousness. A few of Jackson’s contemporaries agreed that consciousness was the essential feature of epilepsy,17 but many focused on motor convulsions as the primary clinical manifestation (for a review, see reference 16). Despite their disagreement on the fundamental nature of the condition, Jackson and contemporaries agreed that the different portions of the brain played unique roles in epileptic seizures. Due to the technological limitations at the time, neurologists could rely only on clinical observation and anatomical postmortem studies to assess the functions of specific brain structures.
From his many observations of epileptic seizures, Jackson proposed that consciousness was impaired when higher cortical function became disorganized and lacked integrative ability.18 He considered the substrata of consciousness to be the highest nervous center, representing the most complex region responsible for the coordination of various inputs and outputs throughout the body, and hypothesized that impaired consciousness was the result of discharge in this region. Jackson believed that these higher processes evolved from the lower, simpler processes and together formed a continuous network throughout the brain. The degree of consciousness impairment would depend on the seat of the discharging lesion, as well as on the extent and speed by which this discharge spreads. If the discharge began in a subordinate structure or series of structures, consciousness would be lost either once this discharge spread to a higher process or when a sufficiently large quantity of subordinate structures became involved.18,19 The importance Jackson placed on the study of consciousness in epilepsy paved the way for the development of an entire field of study, promoting greater understanding of an important yet previously overlooked component of epileptic disorders.
Penfield and Jasper’s Centrencephalic Theory
During the 1930s to the 1950s, Wilder Penfield and Herbert Jasper posited that a system existed within the brain stem that could be responsible for integrating the actions of the two hemispheres of the brain. They saw that epileptic patients who had had large portions of cerebral cortex or corpus callosum removed suffered little to no impairment of consciousness, while pressure applied to the brain stem resulted in immediate and reversible loss of consciousness.20 Based on this observation, Penfield and Jasper deduced that consciousness control in the brain involved more than just the cerebral cortex, which was known to control many cognitive processes.21 Instead, they argued that the “indisputable substratum” of consciousness was rooted in the diencephalon and upper brain stem. While they acknowledged that the cortex was involved in high-level functioning, Penfield suggested that networks of neurons contained in the diencephalon and the brain stem integrated the activities of the two hemispheres, thereby controlling states of consciousness.20,21 Jasper also posited that activation of only the amygdala and hippocampus was insufficient to induce automatisms with amnesia. Instead, the impairment of consciousness required the involvement of widespread structures, particularly through involvement of the aforementioned mesial diencephalon and brain stem.22 He suggested that seizure discharge must affect the temporal lobes bilaterally in order to effect loss of consciousness, which he termed epileptic automatism with amnesia.22
Jasper and Penfield termed this complex network of interactions between cortical and subcortical structures “centrencephalic,” drawing attention to the complex integration of several systems required to facilitate the spread of epileptic activity throughout the brain.20,21,23 Several contemporary studies found evidence of a diffuse thalamo–cortical projection system in the intralaminar regions of the thalamus.24 Stimulation of the intralaminar thalamus at 3 Hz reproduced bilateral spike–wave patterns similar to those seen in petit mal (absence) epilepsy.25,26 Furthermore, neurochemical studies suggested chemically specific ascending and diffuse modulatory systems projecting from the diencephalon and brain stem that interact with, but are anatomically separate from, the sensory and motor systems.21 Experimenting on cats, Moruzzi and Magoun in 1949 showed that stimulating the ascending reticular relays in the brain stem can activate or desynchronize EEG recordings. This causes low-voltage fast activity to replace high-voltage slow waves, particularly in the cortex, in a process that is mediated by diffuse projections.27 They postulated that this ascending activation system may be responsible for wakefulness and that the reduction of activity in this activation system may cause sleep or other impairment of awareness.27
These early studies by Penfield, Jasper, and contemporaries suggested that control of consciousness could occur outside the cerebral cortex. Subsequent to their work, many studies have substantiated the importance of subcortical structures in modulating consciousness.
Effects and Importance of Lateralization
The two hemispheres of the brain, although virtually symmetrical, have slightly differentiated functions. Early hemispherectomy studies as treatment for severe epilepsy showed that a single hemisphere and intact brain stem connections are sufficient to sustain normal consciousness.28 However, the specific features of consciousness retained posthemispherectomy may depend on the side of the brain removed. Although, interestingly, Jackson believed that most verbal losses occurred when the right hemisphere was impaired,18 work since then has confirmed that the left hemisphere is usually responsible for verbal consciousness while the right hemisphere is linked to basic awareness of corporeal and emotional self.29 A patient without a left hemisphere, for example, would likely suffer a loss in the auditory and verbal language skills that are often used as a method of measuring conscious response while still retaining emotional awareness of self.30 On the other hand, a patient without a right hemisphere would be able to vocalize responses, but would lack emotional attributions, when presented with stimuli.29
Others have gone so far as to suggest that the left hemisphere, typically dominant for language, is also dominant for consciousness.31–33 By using a right-side intracarotid amytal injection to cause left hemiplegia in a case study, Franczek and colleagues found right hemisphere EEG slowing without change in consciousness or language.34 When the same process was performed on the opposite side, the patient was unresponsive to verbal commands and to noxious somatic stimulation for 63 s, without global aphasia. Although open to interpretation, the authors inferred dominance for consciousness based on these results.34
Since the temporal lobe is known to be important for relating current and past experiences and the assessment of consciousness often relies on the recollection of past events, the temporal lobe has been considered an important structure in the regulation of consciousness. Some believe that the specific temporal lobe (left versus right) involved or the one most involved in the seizure is a predictor for the impairment of consciousness, while others believe that it is the number of temporal lobes (one versus two) affected that better predicts the outcome.
Lux and colleagues found that 39% of left temporal lobe seizures resulted in impaired consciousness, while only 12% of right temporal seizures did the same.35 All patients with bitemporal seizure activity showed ictal impairment, but when activity was confined to the right temporal lobe consciousness was more often spared.35 Because involvement of the left temporal lobe was more likely to lead to impaired consciousness, they concluded that unimpaired consciousness requires normal function of the left temporal lobe. The sparing of both temporal lobes would be sufficient to retain consciousness, because that would include by definition the sparing of the left temporal lobe; the involvement of both temporal lobes includes the involvement of the left temporal lobe, hence the resulting impairment of consciousness.35 Inoue and Mihara argued that unilateral temporal seizure activity was sufficient to impair consciousness and was more likely to do so if activity was located in the left temporal lobe than in the right.36 Others found that contralateral focal jerking and tonic head deviation were more common when temporal lobe seizures occurred in the left temporal lobe and that seizures in the left lobe were more likely to generalize.37 In addition, postictal aphasia occurred exclusively in left temporal seizures, whereas ictal speech quickly returned to normal after offset in right temporal seizures.37 Because they found that seizure activity involving the right temporal lobe had comparable rates of consciousness impairment to frontal seizures, while the seizures affecting the left temporal lobe had far greater rates of impaired consciousness,35,36 these investigators concluded that the right temporal lobe has only a small role in modulating consciousness, while the left temporal lobe is much more directly implicated in consciousness control.
Impairment of consciousness has also been linked to bilateral, rather than unilateral, mesial temporal lobe discharges, as these discharges may limit the patient’s postictal response to the environment and recollection of events.38 Herbert Jasper also posited that epileptic seizure discharge must reach both temporal lobes in order to cause epileptic automatism to occur with amnesia.22 Gloor and others suggested that ictal loss of consciousness without secondary generalization most often occurs when there is bilateral involvement of limbic and neocortical components of the temporal lobes. Others have confirmed that during temporal epileptic seizures, consciousness is found to be impaired in up to 81% of patients with bilateral seizure activity.36,38–41 Over 50% of seizures with impaired consciousness involve parts of the contralateral hemisphere, often the temporal lobe.39
Assessment Challenges
In the lateralization studies discussed above, those who found consciousness to be linked to the dominant hemisphere often relied on verbal determinants of arousal to measure conscious response. In some studies, when response to noxious stimulation was substituted for response to verbal stimulation, no difference was found between seizures originating in the left and right hemispheres.42 In most studies, no function that would have been expected to be impaired during right seizure activity was assessed, such as the memory for faces or figures, which has been localized to the right temporal lobe.35 The reliance on verbal measures of consciousness seems then to be an obvious limitation of many consciousness studies and something researchers should consider when interpreting their results.37 Because pain and noxious stimuli activate regions throughout the brain bilaterally, they may be better measures of reaction than verbal response.43
Pierre Gloor44 reviewed the difficulty of assessing consciousness in others and hypothesized that the only person who can truly determine a patient’s consciousness is the patient himself. Although a lack of response is often considered to be an indication of impaired consciousness, a patient could be unresponsive for other reasons, such as lack of motor control or aphasia. Another measure of consciousness is the recall of past events, specifically, those that occur during a seizure. Yet the inability to recall may be due to amnesia rather than a lack or impairment of consciousness at the time of the event. Frequent interactions with patients are the only reliable way to assess responsiveness over a period of time. Close observations of patient responses and actions are the best, although still imperfect, method to distinguish impaired and unimpaired consciousness.44 Since patient examination is inherently subjective, this can be problematic and in some cases may even lead to erroneously inferring impaired consciousness when psychological factors prevail.45
Some have suggested, on the contrary, that this problem can be alleviated by taking into account the subjective experience of the patient. Assuming that all psychological phenomena originate from biological changes within the brain, a change of subjective awareness reflects a change in brain function. This suggests that underlying biological phenomena can be deduced from patients’ descriptions of events before, during, and after their seizures. Consequently, instead of having someone else assess a patient’s mental state, some researchers have relied on the patient’s own report of the seizure as the most informative record of ictal events.46,47,87 Yet this strategy is useful only with patients who remember the details of the seizure and thus cannot be applied to a representative sample of all patients undergoing epileptic seizures, because many do not retain sufficient awareness or cannot remember postictally. While use of subjective patient accounts may certainly be useful in some situations, the emergence of improved neuroimaging technology offers better opportunities to visualize objective phenomena in the brain, with new approaches that were not available at the time of Pierre Gloor’s somewhat pessimistic review.
SPECT Analysis and the Midbrain–Thalamus Hypothesis
Single photon emission computed tomography (SPECT), an imaging technique that uses gamma rays to create a three-dimensional picture of brain blood flow, has been particularly important in evaluating brain function during seizures. Ictal SPECT has an advantage over other methods, such as positron emission tomography or functional magnetic resonance imaging (fMRI), because the SPECT radiotracer injection can be done during the seizure but imaging is done later, alleviating problems with movement artifact during seizures. Several SPECT studies have shown that impaired consciousness in temporal lobe seizures is correlated with secondary hyperperfusion in the thalamus and upper brain stem.48–50 Medial temporal and medial diencephalic connections may be required for the generation and propagation of limbic seizures. It seems plausible that the spread of epileptic discharge or activation to these regions is the mechanism by which consciousness is impaired or altered.48–50
SPECT imaging has also been useful in the study of temporal lobe seizures, because it allows for the analysis of cerebral blood flow (CBF) changes within and beyond the temporal lobe. Seizures with impaired consciousness showed CBF increases in the temporal lobe, with increases in bilateral midline subcortical structures, while CBF decreases occurred bilaterally in the frontal and parietal association cortex.48 This CBF decrease has been found to be correlated with CBF increases in midline structures, such as the mediodorsal thalamus. In patients with hippocampal sclerosis, complex partial seizures were related to larger decreases in CBF in the frontal association cortex than simple partial seizures. Thus, these widespread CBF changes, including midline increases and cortical decreases, were not present in temporal lobe seizures where consciousness was spared; in such cases, changes were much more likely to be confined to the temporal lobe.48 This suggests that perhaps the involvement of the midline structures interacting with the cortex is related to the impairment of consciousness.
Many specific thalamo–cortical connections exist from the various nuclei that compose the thalamus. Tae and colleagues51 noted ictal hyperperfusion and interictal hypoperfusion in the ipsilateral temporal lobe, insula, putamen, bilateral thalami, and in the bilateral precentral gyri. Hypoperfusion between seizures was hypothesized to be due to repetitive ictal discharges generating and propagating through this thalamo–hippocampal insular network.51 Hogan and colleagues also found that SPECT hyperperfusion occurs in the brain stem tegmentum and bilateral thalamus during medial temporal seizures, confirming the involvement of these regions.52
Rat models have also shown that the midline thalamus is involved in limbic seizure activity.53,54 Significant neuronal loss has been found in the medial dorsal and rhomboid nuclei and lidocaine infusions into the midline thalamus reduced the duration of discharges, suggesting that this region of the thalamus is important in limbic seizures.53 Recent neuroimaging in a rat model of limbic seizures showed increased activity in the thalamus along with decreased activity in the frontal association cortex, similar to humans, likely due to long-range network effects.54
Guye and colleagues analyzed thalamic activity during human temporal lobe seizures, correlating intracerebral EEG recordings with behavioral observations, and found marked synchronization of signals from temporal lobe structures and the thalamus, particularly in the last phase of the seizure.55 Specifically, they found the medial pulvinar to be involved in all seizures that affected the associative portions of the temporal lobe, suggesting that its connections with associative cortices may be the pathway for the spreading of the seizure and that this may be true for other thalamic nuclei as well. When consciousness was lost within the first 10 s of the seizure, significantly more synchrony occurred.55
The Network Inhibition Hypothesis
We have proposed that anatomical structures important for consciousness be referred to as the “consciousness system,” in analogy to the motor, sensory, and limbic systems, which similarly involve a network of cortical and subcortical structures.8,56 If we assess consciousness as the ability to maintain an alert state, attention, and awareness of self and environment, the system responsible for regulating consciousness must include the structures known to be responsible for these capacities, such as the frontal and parietal association cortex, cingulate gyrus, precuneus, and activating systems located in the basal forebrain, thalamus hypothalamus, midbrain, and upper pons.6,57,58 Additionally, since electrical stimulation of the limbic structures correlates with unconsciousness, it has been suggested that the limbic system is important in the maintenance of awareness of environment.44 If we consider controlled attention to be a prerequisite for consciousness, the consciousness system must also include the basal ganglia and cerebellum, as these structures have also been recognized to play a role in regulating attention.59–61
As previously discussed, SPECT studies of partial seizures have shown that when normal consciousness is retained, changes are more likely to be confined to the temporal lobe, without fronto–parietal or midline subcortical changes. Complex partial seizures, with impaired consciousness, are more likely to include changes outside the temporal lobe, including CBF increases in the upper brain stem and medial thalamus and decreases in the fronto–parietal association cortex and the anterior and posterior interhemispheric regions.48
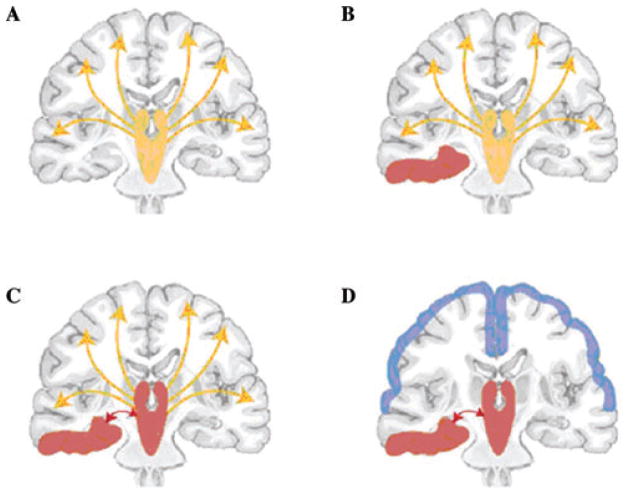
We have summarized our work and that of others by proposing a “network inhibition hypothesis,” or what should perhaps more appropriately be termed the “network inhibition or network disruption by seizures hypothesis” for impaired consciousness in temporal lobe seizures (Fig. 1).6,62 Normally, the upper brain stem–diencephalic activating systems interact with the cortex to maintain normal consciousness (Fig. 1A). Temporal lobe seizures (Fig. 1B) propagate through known pathways63,64 to reach the midline subcortical structures (Fig. 1C), disrupting the normal activating systems and thus leading to decreased activity or inhibition of the fronto–parietal association cortex (Fig. 1D). The resulting decreased activity in the fronto–parietal cortex is poorly understood but may be caused by increased inhibitory inputs and/or decreased excitatory inputs.
Figure 1.
Network inhibition hypothesis for loss of consciousness in complex partial seizures. (A) Under normal conditions, the upper brain stem–diencephalic activating systems interact with the cerebral cortex to maintain normal consciousness (yellow represents normal activity). (B) A focal seizure (red) involving the mesial temporal lobe unilaterally. (C) Propagation of seizure activity from the mesial temporal lobe to midline subcortical structures. (D) Disruption of the normal activating functions of the midline subcortical structures leads to depressed activity (blue) in bilateral regions of the fronto–parietal association cortex, leading to loss of consciousness. Reproduced with permission from Ref. 6. (In color in Annals online.)
Human intracranial EEG studies of temporal lobe epilepsy seizures have lent some additional support to the concept of temporal lobe seizures producing remote network effects in the neocortex. These studies have shown ictal high-frequency discharges in the temporal lobe, along with slow-frequency waves in the association cortex, which are accompanied by decreased CBF.48,50,65–70 Because these slow waves lack spikes or sharp components, differing drastically from the high-frequency ictal discharges in the temporal lobe, we believe them not to be a propagation of seizure activity outside the temporal lobe but rather a type of EEG pattern similar to that seen in sleep, coma, or encephalopathy. Quantitative analysis of intracranial EEG data has confirmed that the activity patterns in the temporal lobe and association cortex are distinct.71,72 Recent animal models of limbic seizures have also shown slow activity in the frontal cortex with decreased CBF and decreased frontal neuronal firing, resembling the effects of deep anesthesia or natural sleep.54 If these slow waves are suggestive of the brain being in a deep sleep-like state, the loss of consciousness may be a remote network effect on cortical structures secondary to the fast activity in the temporal lobe. We previously likened this mechanism to diaschisis following stroke, in which pathology in one focal region of the brain can lead to functional inhibition of remotely located brain structures.62,73 We hypothesize that the generation of slow activity through abnormal neocortical–subcortical interactions is the route by which fast temporal seizure activity impairs consciousness. Further work is needed in both humans and in animal models to more completely investigate this hypothesis and to determine the fundamental network and cellular and neurochemical mechanisms involved.
Summary and Conclusions
Although we have discussed the major theories of consciousness control in epileptic seizures separately, we do not intend to suggest that these ideas operate independently of each other. Instead, each theory of consciousness, including our own, is closely related to other work. For example, the centrencephalic theory influenced later studies showing midbrain and thalamic involvement during temporal lobe seizures and our subsequent network inhibition or network disruption by seizure hypothesis. Our proposed “consciousness system” is a coarse outline of structures, including the medial thalamus andbreak upper brain stem, interhemispheric regions (medial frontal cortex, cingulate, and precuneus), and lateral frontal and parietal association cortex. Diverse seizure types can cause impaired consciousness when abnormal activity occurs in the structures that are involved in moderating the controlled awareness of the environment. Despite having different behavioral manifestations, absence, GTC, and temporal lobe complex partial seizures all cause abnormal increases in the activity of the medial thalamus and upper brain stem and decreased activity in interhemispheric regions.7,8,10,48,74,85,86 The lateral frontal and parietal cortex show abnormal changes in both directions during absence seizures: increases during GTC seizures and decreases during complex partial temporal lobe seizures. Following most GTC and complex temporal lobe seizures, there is a noticeable postictal period with impaired consciousness, accompanied by decreased activity across the frontal and parietal association cortex. However, this postictal impairment is not present after most absence seizures.
It is important to better understand mechanisms of impaired consciousness in epilepsy so that we can eventually prevent its negative effects. Being epileptic correlates with having a shorter life expectancy, greater risk of injuries such as burns, falls, and vehicular accidents, and an overall reduced quality of life.75–77 Patients with seizures with impaired consciousness are often restricted from driving, and the risk of motor vehicle accidents is higher in seizures with impaired consciousness, such as complex partial and GTC seizures, than in seizures with retained consciousness.78,79 Fear of losing consciousness in public is also a concern to patients who suffer from complex and generalized seizures.80 This type of concern can be stigmatizing and can increase the suffering of patients already dealing with a debilitating illness.
Patients’ quality of life would greatly improve if seizures with impaired consciousness could be prevented. Review of these mechanisms of consciousness control has facilitated the development of methods designed to block several types of seizures, especially those that include loss of consciousness. For example, some investigators have developed deep brain stimulators to treat epilepsy and other disorders of consciousness.81–84 As these neuronal networks are studied, medicines can be developed that more accurately target the specific regions involved. Although ideally all seizures, whether or not they impair consciousness, might be prevented, even a smaller improvement, such as minimizing the impairment of consciousness, would make a noticeable impact on patient well-being.
In conclusion, reviewing and understanding the various perceptions of the root of impaired consciousness in epilepsy help us to see recent research in a historical context. Integration of these various concepts directs our efforts to uncover more precise evidence of the neuronal networks responsible for regulation of conscious behavior. It is hoped that further investigation will lead to the development of treatments and preventative techniques that will improve the quality of life for those living with this disorder. By comparing brain activity when consciousness is normal and when impaired, we can effectively model the systems implicated in control of consciousness.
Acknowledgments
This work was supported by NIH grants R01 NS055829, R01 NS049307, and P30 NS052519, the Donaghue Foundation, Mark Loughridge, and Michele Williams.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Dennett DC. Kinds of Minds: Toward an Understanding of Consciousness. Basic Books; New York, NY: 1996. [Google Scholar]
- 2.Chalmers DJ. The Conscious Mind: In Search of a Fundamental Theory. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- 3.Searle JR. Reductionism and the irreducibility of consciousness. In: Block N, Flanagan OJ, Guzeldere G, editors. The Nature of Consciousness. MIT Press; Cambridge, MA: 1997. [Google Scholar]
- 4.Gray J. Consciousness on the scientific agenda. Nature. 1992;358:277. doi: 10.1038/358277a0. [DOI] [PubMed] [Google Scholar]
- 5.Singer W. Consciousness from a neurobiological perspective. In: Gazzaniga M, Altman J, editors. Brain and Mind: Evolutionary Perspectives. HFSP; Strasbourg, Germany: 1998. pp. 72–88. [Google Scholar]
- 6.Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? Neuroscientist. 2003;9:301–310. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- 7.Blumenfeld H. Consciousness and epilepsy: Why are patients with absence seizures absent? Prog Brain Res. 2005;150:271–286. doi: 10.1016/S0079-6123(05)50020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenfeld H. Epilepsy and consciousness. In: Laureys S, Tononi G, editors. The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. Elsevier, Ltd; Amsterdam, Netherlands: 2009. pp. 247–260. [Google Scholar]
- 9.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46:21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 10.Blumenfeld H, Westerveld M, Ostroff RB, et al. Selective frontal, parietal and temporal networks in generalized seizures. Neuroimage. 2003;19:1556–1566. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- 11.Bell WL, Walczak TS, Shin C, et al. Painful generalised clonic and tonic-clonic seizures with retained consciousness. J Neurol Neurosurg Psychiatry. 1997;63:792–795. doi: 10.1136/jnnp.63.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vuilleumier P, Assal F, Blanke O, et al. Distinct behavioral and EEG topographic correlates of loss of consciousness in absences. Epilepsia. 2000;41:687–693. doi: 10.1111/j.1528-1157.2000.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 13.Gokygit A, Caliskan A. Diffuse spike-wave status of 9-year duration without behavioral change or intellectual decline. Epilepsia. 1995;36:210–213. doi: 10.1111/j.1528-1157.1995.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 14.International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 15.Jackson J. On the anatomical, physiological, and pathalogical investigations of epilepsies. West Riding Lunatic Asylum Med Rep. 1873;3:315–339. [Google Scholar]
- 16.Eadie MJ, Blandin PF. A Disease Once Sacred: A History of the Medical Understanding of Epilepsy. John Libbey and Company Ltd; Eastleigh, UK: 2001. [Google Scholar]
- 17.Todd RB. On the pathology and treatment of convulsive diseases. Delivered at the Royal College of Physicians, London. Epilepsia. 2005;46:995–1009. doi: 10.1111/j.1528-1167.2005.10205.x. [DOI] [PubMed] [Google Scholar]
- 18.Jackson JH, et al. Selected Writings of John Hughlings Jackson. Hodder and Stoughton; London, UK: 1931. [Google Scholar]
- 19.Yamauchi T. Impairment of consciousness during epileptic seizures with special reference to neuronal mechanisms. Epilepsia. 1998;39:16–20. doi: 10.1111/j.1528-1157.1998.tb05144.x. [DOI] [PubMed] [Google Scholar]
- 20.Penfield W. Centrencepalic intergrating system. Brain. 1958;81:231–234. doi: 10.1093/brain/81.2.231. [DOI] [PubMed] [Google Scholar]
- 21.Jasper HH. Current evaluation of the concepts of centrencephalic and corticoreticular seizures. Electroencephalogr Clin Neurophysiol. 1991;78:2–11. doi: 10.1016/0013-4694(91)90012-s. [DOI] [PubMed] [Google Scholar]
- 22.Jasper HH. Some physiological mechanisms involved in epileptic automatisms. Epilepsia. 1964;5:1–20. doi: 10.1111/j.1528-1157.1964.tb04341.x. [DOI] [PubMed] [Google Scholar]
- 23.Penfield W. Epileptic automatism and the centrencephalic integrating system. Res Publ Assoc Res Nerv Ment Dis. 1950;30:513–528. [PubMed] [Google Scholar]
- 24.Morrison RS, Dempsey EW. Mechanism of thalamocortical augmentation and repitition. Am J Physiol. 1943;138:297–308. [Google Scholar]
- 25.Jasper HH, Droogleever-Fortuyn J. Experimental studies on the functional anatomy of petit mal epilepsy. Res Publ Assoc Res Nerv Ment Dis. 1947;26:272–298. [Google Scholar]
- 26.Penfield WG. Highest level seizures. Assoc Nerv Ment Dis Proc. 1947;26:252–271. [Google Scholar]
- 27.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 28.Austin G, Grant F. Physiologic observations following total hemispherectomy in man. Surgery. 1955;38:239–258. [PubMed] [Google Scholar]
- 29.Devinsky O. Right cerebral hemisphere dominance for a sense of corporeal and emotional self. Epilepsy Behav. 2000;1:60–73. [Google Scholar]
- 30.Basso A, Spinnler H, Vallar G, et al. Left hemisphere damage and selective impairment of auditory verbal short-term memory. A case study. Neuropsychologia. 1982;20:263–274. doi: 10.1016/0028-3932(82)90101-4. [DOI] [PubMed] [Google Scholar]
- 31.Ebner A, Dinner DS, Noachtar S, et al. Automatisms with preserved responsiveness: A lateralizaing sign in psychomotor seizures. Neurology. 1995;45:61–64. doi: 10.1212/wnl.45.1.61. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz B. Hemispheric dominance and consciousness. Acta Neurol Scand. 1967;43:513–525. doi: 10.1111/j.1600-0404.1967.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 33.Albert ML, Silverberg R, Reches A, et al. Cerebral dominance for consciousness. Arch Neurol. 1976;33:453–454. doi: 10.1001/archneur.1976.00500060059013. [DOI] [PubMed] [Google Scholar]
- 34.Franczek S, Demakis GJ, Pennell EB, et al. Revisited: Cerebral dominance for consciousness. Electroencephalogr Clin Neurophysiol. 1997;102:24P–25P. [Google Scholar]
- 35.Lux S, Kurthen M, Helmstaedter C, et al. The localizing value of ictal consciousness and its constituent functions: A video-EEG study in patients with focal epilepsy. Brain. 2002;125:2691–2698. doi: 10.1093/brain/awf276. [DOI] [PubMed] [Google Scholar]
- 36.Inoue Y, Mihara T. Awareness and responsiveness during partial seizures. Epilepsia. 1998;39:7–10. doi: 10.1111/j.1528-1157.1998.tb05142.x. [DOI] [PubMed] [Google Scholar]
- 37.Fakhoury T, Abou-Khalil B, Peguero E. Differentiating clinical features of right and left temporal lobe seizures. Epilepsia. 1994;35:1038–1044. doi: 10.1111/j.1528-1157.1994.tb02552.x. [DOI] [PubMed] [Google Scholar]
- 38.Gloor P, Olivier A, Ives J. Loss of consciousness in temporal lobe epilepsy: Observations obtained with stereotaxic depth electrode recordings and stimulations. In: Canger R, Angeleri F, Penry JK, editors. Advances in Epileptology: The XIth Epilepsy International Symposium. Raven Press; New York, NY: 1980. pp. 349–353. [Google Scholar]
- 39.Munari C, Bancaud J, Bonis A, et al. Impairment of consciousness in temporal lobe seizures: A stereoelectroencephalographic study. In: Canger R, Angeleri F, Penry JK, editors. Advances in Epileptology: The XIth Epilepsy International Symposium. Raven Press; New York, NY: 1980. pp. 111–114. [Google Scholar]
- 40.Bancaud J, Brunet-Bourgin F, Chauvel P, et al. Anatomical origin of deja vu and vivid ‘memories’ in human temporal lobe epilepsy. Brain. 1994;117:71–90. doi: 10.1093/brain/117.1.71. [DOI] [PubMed] [Google Scholar]
- 41.Pedley TA. Classifications of seizures and epilepsy. In: Resor S, Kutt H, editors. The Medical Treatment of Epilepsy. Vol. 8. Informa Health Care; New York, NY: 1992. [Google Scholar]
- 42.Salazar AM, Grafman JH, Vance SC, et al. Consciousness and amnesia after penetrating head injury: Neurology and anatomy. Neurology. 1986;36:178–187. doi: 10.1212/wnl.36.2.178. [DOI] [PubMed] [Google Scholar]
- 43.Coghill RC, Sang CN, Maisoq JM, et al. Pain intensity processing within the human brain: A bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 44.Gloor P. Consciousness as a neurological concept in epileptology: A critical review. Epilepsia. 1986;27:S14–S26. doi: 10.1111/j.1528-1157.1986.tb05737.x. [DOI] [PubMed] [Google Scholar]
- 45.Kapur J, Pillai A, Henry TR. Psychogenic elaboration of simple partial seizures. Epilepsia. 1995;36:1126–1130. doi: 10.1111/j.1528-1157.1995.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 46.Johanson M, Revonsuo A, Chaplin J, et al. Level and contents of consciousness in connection with partial epileptic seizures. Epilepsy Behav. 2003;4:279–285. doi: 10.1016/s1525-5050(03)00106-9. [DOI] [PubMed] [Google Scholar]
- 47.Frith C, Perry R, Lumer E. The neural correlates of conscious experience: An experimental framework. Trends Cogn Sci. 1999;3:105–114. doi: 10.1016/s1364-6613(99)01281-4. [DOI] [PubMed] [Google Scholar]
- 48.Blumenfeld H, McNally KA, Vanderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- 49.Lee KH, Meador KJ, Park YD, et al. Pathophysiology of altered consciousness during seizures: Subtraction SPECT study. Neurology. 2002;59:841–846. doi: 10.1212/wnl.59.6.841. [DOI] [PubMed] [Google Scholar]
- 50.Mayanagi Y, Watanabe E, Kaneko Y. Mesial temporal lobe epilepsy: Clinical features and seizure mechanism. Epilepsia. 1996;37(Suppl 3):57–60. doi: 10.1111/j.1528-1157.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 51.Tae WS, Joo EY, Kim JH, et al. Cerebral perfusion changes in mesial temporal lobe epilepsy: SPM analysis of ictal and interictal SPECT. Neuroimage. 2005;24:101–110. doi: 10.1016/j.neuroimage.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Hogan RE, Kaiboriboon K, Bertrand ME, et al. Composite SISCOM perfusion patterns in right and left temporal seizures. Arch Neurol. 2006;63:1419–1426. doi: 10.1001/archneur.63.10.1419. [DOI] [PubMed] [Google Scholar]
- 53.Bertram EH, Mangan PS, Zhang D, et al. The midline thalamus: Alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42:967–978. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- 54.Englot DJ, Mishra AM, Mansuripur PK, et al. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28:9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guye M, Regis J, Tamura M, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129:1917–1928. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- 56.Blumenfeld H. Epilogue: A Simple Working Model of the Mind Neuroanatomy through Clinical Cases. Sinauer Assoc. Publ., Inc; Sunderland, MA: 2002. [Google Scholar]
- 57.Blumenfeld H. Neuroanatomy through Clinical Cases. Sinauer Associates Inc; Sunderland, MA: 2002. [Google Scholar]
- 58.Plum F, Posner JB. The Diagnosis of Stupor and Coma. Davis; Philadelphia, PA: 1982. [Google Scholar]
- 59.Ring HA, Serra-Mestres J. Neuropsychiatry of the basal ganglia. J Neurol Neurosurg Psychiatry. 2002;72:12–21. doi: 10.1136/jnnp.72.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bischoff-Grethe A, Ivry RB, Grafton ST. Cerebellar involvement in response reassignment rather than attention. J Neurosci. 2002;22:546–553. doi: 10.1523/JNEUROSCI.22-02-00546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dreher JC, Grafman J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci. 2002;16:1609–1619. doi: 10.1046/j.1460-9568.2002.02212.x. [DOI] [PubMed] [Google Scholar]
- 62.Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- 63.Zhang DX, Bertram EH. Midline thalamic region: Widespread excitatory input to the entorhinal cortex and amygdala. J Neurosci. 2002;22:3277–3284. doi: 10.1523/JNEUROSCI.22-08-03277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cassidy RM, Gale K. Mediodorsal thalamus plays a critical role in the development of limbic motor seizures. J Neurosci. 1998;18:9002–9009. doi: 10.1523/JNEUROSCI.18-21-09002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sperling MR, Chung H-J, Temple JP. Atlas of Electroencephalography. Elsevier Science; Amsterdam, Netherlands: 1999. [Google Scholar]
- 66.Van Paesschen W, Dupont P, Van Driel G, et al. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 2003;126:1103–1111. doi: 10.1093/brain/awg108. [DOI] [PubMed] [Google Scholar]
- 67.Markand ON, Andersen AR, Spencer SS. SPECT in epilepsy. J Neuroimag. 1995;5:S23–S34. doi: 10.1111/jon19955s1s23. [DOI] [PubMed] [Google Scholar]
- 68.Avery RA, Zubal IG, Stokking R, et al. Decreased cerebral blood flow during seizures with ictal SPECT injections. Epilepsy Res. 2000;40:53–61. doi: 10.1016/s0920-1211(00)00109-1. [DOI] [PubMed] [Google Scholar]
- 69.Lieb JP, Dasheiff RM, Engel J., Jr Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991;32:822–837. doi: 10.1111/j.1528-1157.1991.tb05539.x. [DOI] [PubMed] [Google Scholar]
- 70.Blumenfeld H, Rivera M, McNally KA, et al. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004;63:1015–1021. doi: 10.1212/01.wnl.0000141086.91077.cd. [DOI] [PubMed] [Google Scholar]
- 71.Golomb JD, Shamy M, Levin AR, et al. Quantitative analysis of intracranial EEG patterns: Evidence for neocortical slowing in temporal lobe seizures. Soc Neurosci Abs. 2005 http://web.sfn.org/
- 72.Golomb JD, Shamy M, Levin AR, et al. Neocortical slowing during temporal lobe seizures demonstrated by quantitative analysis of intracranial EEG. Epilepsia. 2005 AES abstracts. [Google Scholar]
- 73.Komaba Y, Osono E, Kitamura S, et al. Crossed cerebellocerebral diaschisis in patients with cerebellar stroke. Acta Neurol Scand. 2000;101:8–12. doi: 10.1034/j.1600-0404.2000.00002.x. [DOI] [PubMed] [Google Scholar]
- 74.Enev M, McNally KA, Varghese G, et al. Imaging onset and propagation of ECT-induced seizures. Epilepsia. 2007;48:238–244. doi: 10.1111/j.1528-1167.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 75.Vickrey BG, Berg AT, Sperling MR, et al. Relationships between seizure severity and health-related quality of life in refractory localization-related epilepsy. Epilepsia. 2000;41:760–764. doi: 10.1111/j.1528-1157.2000.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 76.Sperling MR. The consequences of uncontrolled epilepsy. CNS Spectrums. 2004;9:98–101. doi: 10.1017/s1092852900008464. [DOI] [PubMed] [Google Scholar]
- 77.Theodore WH, Spencer SS, Wiebe S, et al. Epilepsy in North America: A report prepared under the auspices of the Global Campaign against Epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia. 2006;47:1700–1722. doi: 10.1111/j.1528-1167.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 78.Gastaut H, Zifkin BG. The risk of automobile accidents with seizures occurring while driving: Relation to seizure type. Neurology. 1987;37:1613–1616. doi: 10.1212/wnl.37.10.1613. [DOI] [PubMed] [Google Scholar]
- 79.Berkovic SF. Epilepsy syndromes: Effects on cognition, performance and driving ability. Med Law. 2000;19:757–761. [PubMed] [Google Scholar]
- 80.Jacoby A, Snape D, Baker GA. Epilepsy and social identity: The stigma of a chronic neurological disorder. Lancet Neurol. 2005;4:171–178. doi: 10.1016/S1474-4422(05)01014-8. [DOI] [PubMed] [Google Scholar]
- 81.Yamamoto T, Katayama Y. Deep brain stimulation therapy for the vegetative state. Neuropsychol Rehabil. 2005;15:406–413. doi: 10.1080/09602010443000353. [DOI] [PubMed] [Google Scholar]
- 82.Murphy JV, Patil A. Stimulation of the nervous system for the management of seizures: Current and future developments. CNS Drugs. 2003;17:101–115. doi: 10.2165/00023210-200317020-00003. [DOI] [PubMed] [Google Scholar]
- 83.Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. Erratum, 452: 120, 2008. [DOI] [PubMed] [Google Scholar]
- 84.Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. 2004;3:111–118. doi: 10.1016/s1474-4422(03)00664-1. Erratum, 3: 332. [DOI] [PubMed] [Google Scholar]
- 85.Blumenfeld H, Varghese G, Purcaro MJ, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009 doi: 10.1093/brain/awp028. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berman R, Negishi M, Vestal M, et al. Simultaneous EEG, fMRI, and behavior in typical childhood absence seizures. 2009 doi: 10.1111/j.1528-1167.2010.02652.x. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cavanna AE, Mula M, Servo S, et al. Measuring the level and contents of consciousness during epileptic seizures: the Ictal Consciousness Inventory. Epilepsy Behav. 2008;13:184–188. doi: 10.1016/j.yebeh.2008.01.009. [DOI] [PubMed] [Google Scholar]