Abstract
Suboptimal maternal nutrition exerts lasting impacts on obesity risk in offspring, but the direction of the effect is determined by the timing of exposure. While maternal undernutrition in early pregnancy is associated with increased body mass index, in later pregnancy it can be protective. The importance of the timing of maternal undernutrition is also observed in rodents, however, many of the processes that occur in the last trimester of human gestation are delayed to the postnatal period. Neonatal leptin administration exerts lasting impacts on susceptibility to obesity in rodents. Although leptin can influence the formation of hypothalamic circuits involved in homeostatic control of feeding during the postnatal period, these effects are too late to account for its ability to reverse adverse metabolic programming due to early gestational exposure to maternal undernutrition. This review presents an alternative framework for understanding the effects of neonatal leptin through influences on developing thermoregulatory circuits.
Keywords: maternal programming, undernutrition, catch-up growth, obesity, leptin, brown adipose tissue, thermogenesis
Introduction
The rapid increase in the prevalence of childhood obesity and the concomitant rise in type 2 diabetes/obesity-related medical morbidities and costs, lend urgency to the need for new insights into the causes and potential preventive measures for this disease. Because the trajectory of this increase is very steep- as much as 60% between 1988 and 2000 in some populations - it is unlikely that genetic and conventional environmental factors are sufficient to explain these trends [1]. There is a growing appreciation that maternal nutritional and metabolic status during gestation can exert lasting effects on susceptibility to obesity in offspring. In their “thrifty phenotype hypothesis”, Hales and Barker propose that maternal influences on the developing hypothalamus, pancreatic islets, adipose tissue and liver result in metabolic adaptations in the progeny that promote survival under conditions of limited nutrient availability, but render them vulnerable to nutritional excess later in life [2].
In rodents, the nutritional environment during lactation (roughly equivalent to the third trimester of human gestation) has lasting impacts on body weight and susceptibility to diet-induced weight gain [3,4]. As the amount of milk consumed during lactation determines the level of voluntary food intake after weaning [5], efforts to elucidate the mechanism of maternal programming of obesity have often focused on hypothalamic feeding circuits [6]. This review will explore the possibility that susceptibility to obesity due to suboptimal nutrition during development is mediated via effects on thermoregulatory circuits.
1. Association between suboptimal fetal growth and susceptibility to obesity in humans
1.1. Gestational undernutrition followed by catch-up growth
Suboptimal nutrition during gestation, due to famine or other factors that reduce birth weight, has been associated with long-term impacts on body mass index (BMI). However, in some situations maternal undernutrition is linked to increased body weight in progeny, while in others it is associated with reduced body weight. The timing of developmental exposure to famine and the abundance of food in the postnatal environment appear to determine whether offspring are at increased or decreased risk of obesity. The 5-month period of extreme food shortage in the Dutch Winter Hunger of 1944-45 afforded the unique opportunity to parse the consequences of severe maternal undernutrition in early vs. late gestation on offspring outcomes. Whereas maternal exposure to famine in early gestation is associated with increased BMI, exposure late in gestation and early infancy appears to be protective with respect to obesity, but is linked to impaired glucose tolerance [7,8,9]. These observations support the idea that maternal programming of obesity and metabolic dysregulation are mediated via effects on distinct developmental processes. This review will focus on programming of obesity-related endpoints, while the Brüning review in this issue will focus on glucose tolerance.
Another important determinant of the impact of maternal undernutrition on offspring BMI is the abundance of food in the postnatal environment. Maternal programming of increased BMI is typically observed in situations when the period of famine is followed by relative nutritional abundance, as in the Dutch Winter Hunger. On the other hand, long-lasting famines involving exposure to undernutrition in both the gestational and postnatal periods, such as that caused by the Siege of Leningrad from 1941-5 [10], are not associated with increased BMI in offspring. The lasting impact of gestational undernutrition is not limited to famine conditions, but can also be observed in small for gestational age (SGA) babies that received suboptimal fetal nutrition but ample postnatal nutrition [2]. These observations are consistent with the idea that the undernourished fetus develops in anticipation of limited nutrient availability in later life [2]. While these adaptations promote survival under conditions of food scarcity, they are not well-suited to a nutrient-rich environment and thus increase susceptibility to diet-induced weight gain [2,11].
1.2. Rapid weight gain in infancy as a risk factor for obesity
One common feature of SGA babies and those exposed to the Dutch Winter Hunger early in gestation is that readily available food sources in the postnatal environment promote rapid “catch-up growth”. Fast growth trajectories in early infancy are associated with increased prevalence of obesity, even in babies with normal birth weights [12,13,14,15,16]. It has been proposed that preferential deposition of fat mass in neonates as compared to older infants underlies this observation [17,18]. In support of this idea, rapid weight gain during the first three months of life is associated with a higher percentage of body fat and more central adiposity in early adulthood than weight gain that is distributed evenly throughout the entire first year [19]. Thus, constrained postnatal growth in infants born into famine conditions could underlie observations that these individuals have reduced BMI in adulthood [7,10]. Distinguishing between directs effects of maternal undernutrition on the development of circuits regulating energy balance and indirect effects due to increased risk of rapid growth in infancy could lead to more effective strategies to prevent obesity in at-risk SGA infants.
2. Rodent models of maternal undernutrition
Experimental animal models have been used to gain mechanistic insights into the fetal origins of adult obesity. Obesity-related outcomes in sheep models of maternal nutrient restriction depend on the timing of exposure, similar to observations from studies of the Dutch Winter Hunger (as discussed in section 1.1 of this paper and reviewed in [20]). Suboptimal nutrition during the first two-thirds of sheep gestation leads to increased adiposity that persists to adulthood [21], while restriction in late gestation results in reduced adiposity [22]. While the formation of neural circuits that regulate food intake and energy expenditure is largely completed at parturition in precocial newborns, such as sheep and humans, these processes continue into the suckling period in altricial newborns, such as mice and rats [23]. Because distinct developmental events occur in the gestational and postnatal periods in the rodent, they can be used to distinguish which periods, and therefore processes, are developmentally sensitive to maternal influences. At the same time, it is important to keep in mind that situations in which the early and late gestational environments in humans are discordant are the exception, rather than the rule. This review will focus on rodent models of maternal programming, although corresponding periods in precocial species will be discussed in section 9.
2.1. Gestational undernutrition followed by catch-up growth programs sensitivity to diet-induced obesity
Rodent models of maternal undernutrition recapitulate observations that the timing of developmental exposure and postnatal dietary factors determine whether the offspring exhibit susceptibility or resistance to diet-induced weight gain. Maternal nutrient restriction throughout gestation and lactation has little effect on offspring fed a chow diet, but leads to increased adiposity when challenged with high fat diet (HFD) in adulthood [24,25,26]. A rapid increase in the postnatal growth trajectory in rodents, whether or not it was preceded by growth retardation, can lead to increased body weight and adiposity on chow and susceptibility to HFD-induced weight gain [4,24,27,28,29].
2.2. Postnatal undernutrition can be protective against DIO
Models involving maternal dietary restriction during lactation and suckling in a large litter have been used to study the impacts of undernutrition in the suckling period. While postnatal undernutrition is consistently associated with reduced body weight at weaning [3,30], these effects do not always persist into adulthood [27,31]. Protective effects of reduced nutrition during lactation are more pronounced in mice with genetically- or diet-induced obesity (DIO) [32,33].
In summary, rodent models recapitulate fundamental observations in precocial species that maternal undernutrition early in gestation programs increased susceptibility to obesity, while late exposure programs resistance to HFD-induced weight gain. Consistent with delayed maturation of circuits regulating energy balance in altricial newborns, impacts associated with exposure in late human gestation are shifted to the suckling period in rodents. In both precocial and altricial species, the long-term impact of maternal undernutrition on obesity is most clearly observed when progeny are challenged with a calorically dense diet in adulthood.
3. Developmental influences on hypothalamic feeding circuits in rodents
Pioneering studies by Widdowson and McCance demonstrated that manipulations of litter size could be used to assess the long-term impact of changes in caloric intake during the suckling period [3]. As maternal milk supply is finite, the primary determinant of food intake in species with large litter sizes is the number of suckling pups [37]. Postnatal undernutrition (UN) by lactation in a large litter leads to persistent reductions in body weight [3]. Observations that large litter size programms reduced food intake [5] fostered interest in elucidating developmental influences on brain circuits regulating food intake.
3.1. Ontogeny of circuits regulating food intake
Feeding behavior is controlled by sensorimotor, neuroendocrine and cognitive systems that process and integrate signals from the external environment in the context of internal neuronal and humoral signals of energy availability [38]. Circuits regulating distinct aspects of feeding behavior are formed in different periods of development. The most basic type of control over food intake, meal initiation and termination, develops earliest. Autonomic circuits linking the stomach and feeding circuits in the hypothalamus and brainstem are observed at birth and expand rapidly within the first week of lactation [39,40]. Gastric distension can suppress food intake as early as postnatal day 1 (P1), while postabsorptive nutritional signals from the gut operate after P9-11 [41,42](reviewed in [40]). Homeostatic regulation of food intake in response to information about the availability of short- and long-term energy stores emerges in the peri-weaning period [43,44,45,46]. Projections from neurons in the arcuate nucleus of the hypothalamus that can sense nutrient (i.e. glucose, fatty acids) and hormonal (i.e. leptin, ghrelin and insulin) signals of energy status are first detected in preautonomic components of the feeding circuitry at P15-16 [47]. However, leptin’s ability to suppress food intake does not emerge until after weaning at P28 [44]. Finally, cognitive processes that control motivated aspects of feeding behavior are not developed until post-ingestive consequences can be reinforced by the action of corticolimbic circuits, which mature in the post-weaning period [48,49].
Lactation is an important period for the establishment of post-weaning levels of food intake [5] and the formation of circuits regulating homeostatic influences on feeding behavior [47,50]. These observations raise the possibility that maternal programming of susceptibility to obesity is mediated, in part, via effects on developing interoceptive neurons in the mediobasal hypothalamus. Efforts to study maternal influences on developing feeding circuits have centered on two neuronal populations in the arcuate nucleus of the hypothalamus (ARH) that have been implicated in homeostatic regulation of energy balance, NAG neurons co-expressing neuropeptide Y (NPY), agouti-related peptide (AgRP) and gamma-aminobutyric acid (GABA) and another population that expresses pro-opiomelanocortin (POMC).
NAG neurons are activated by signals of negative energy balance (i.e. ghrelin) and are inhibited by signals of an energy replete state (i.e. leptin) [51,52,53]. Central injection of NPY or AgRP [54,55,56] or stimulation of NAG neuronal activity is sufficient to drive acute and robust feeding behavior [57,58]. As functionally distinct sets of POMC neurons sense signals such as leptin, insulin and serotonin [59,60], dissecting the roles of POMC-expressing neurons in regulating food intake is more complicated. While POMC neurons mediate the effects of serotoninergic compounds on food intake [61], there is little evidence to support the idea that leptin- or insulin-sensing POMC neurons directly control food intake [62]. The failure of pharmacological and optogenetic regulation of POMC neuronal activity to acutely impact food intake [57,58] supports the idea that effects of POMC neurons on feeding are likely secondary to impacts on energy expenditure and/or glucose homeostasis.
3.2. Maternal influences on the ontogeny of hypothalamic circuits regulating energy balance
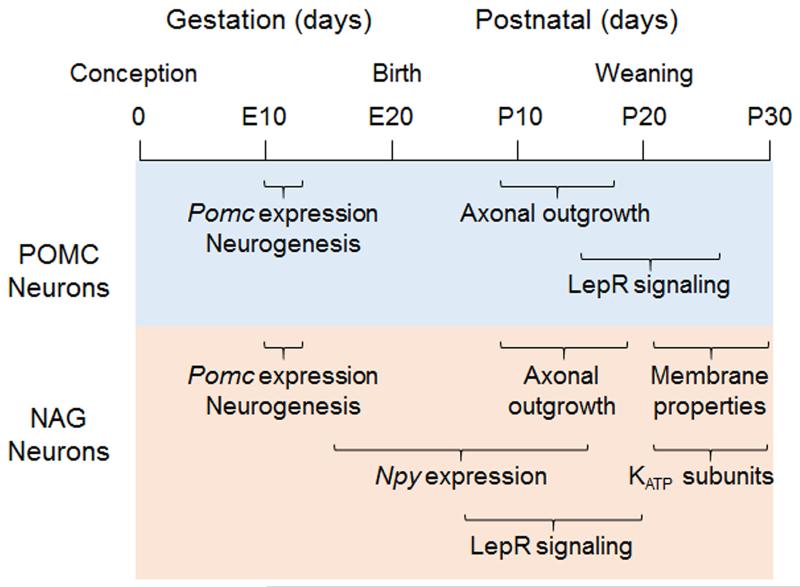
The development of NAG and POMC neurons spans gestation and lactation in the rodent (Figure 1), and maternal nutrient status is reported to impact several stages of neuronal differentiation and maturation. Most ARH neurons are born at embryonic day 11-12 (E11-12) and differentiate from a Pomc-expressing progenitor [63,64]. After E14, some neurons switch off Pomc expression and begin to express Npy; the number of NAG neurons reaches stable levels by P15 [64]. POMC vs. NAG cell fate decisions can be influenced by maternal signals during gestation [65], although these differences often do not persist to adulthood.
Figure 1. Ontogeny of leptin-sensing POMC and NAG neurons.
Both neuronal populations are born at the same time during gestation and extend axons to target nuclei during the second week of lactation. However, the timing of terminal differentiation is divergent. LepR expression and signaling is observed earlier in NAG neurons than POMC neurons. In addition, the onset of KATP channel subunit expression in the peri-weaning period coincides with a switch from leptin-mediated activation to inhibition of NAG neurons.
While many intra-hypothalamic projections are formed before P6 [50], those implicated in regulating food intake develop in the latter half of the suckling period. Projections from ARH neurons to the paraventricular nucleus of the hypothalamus (PVH) are first detected at P8-10 [47,50]. However, they do not innervate the origin of pre-autonomic projections that regulate feeding in the caudal PVH [66,67] until P15-16 [47]. Projections from NAG neurons to the lateral hypothalamic area (LHA), which can also stimulate feeding [68], are first detected at P12 [50]. As projections from ARH neurons that regulate food intake are formed during the suckling period, it raises the possibility that maternal influences on obesity are conveyed via effects on this process [6]. Exposure to undernutrition during lactation is associated with increases in the percent of NAG neurons in the ARH, the number of AgRP+ projections to the PVH and release of NPY/AgRP in the PVH at 3-4 weeks [30,33,69,70], This increase in NPY/AgRP tone could promote survival by stimulating food intake in undernourished animals. However, it cannot explain the protective effect of postnatal undernutrition against diet-induced obesity [32,33]. As the effects of postnatal undernutrition on the NPY/AgRP system are transient [26,71], it is likely that impacts of postnatal undernutrition on other determinants of energy balance underlie the long-lasting susceptibility to DIO.
4. Leptin in the neonatal period programs susceptibility to diet-induced obesity
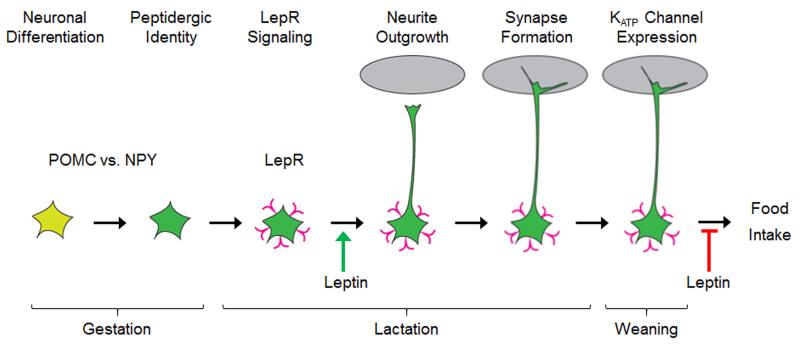
Exposure to leptin in the early postnatal period imparts lasting effects on susceptibility to obesity, although the direction of the outcome is dependent on the nutritional status of the dam during gestation. Daily leptin treatment in wild-type rodents raised in average-sized litters results in elevated Npy expression in the PVH and increased weight gain and adiposity on a HFD [25,72], similar to consequences of exposure to early restriction (as discussed in section 2.1). On the other hand, neonatal leptin treatment can reverse sensitivity to DIO programmed by exposure to UN during gestation [73]. To elucidate what is driving the differential effects of neonatal leptin administration, it is important to identify the developmental processes that are impacted. There is an endogenous surge in plasma leptin levels at the end of the first postnatal week in rodents, although there is some variability in the precise timing of the peak [25,43,74](Green arrow in Figure 2). Since leptin does not affect food intake at this time [45], understanding how leptin is regulated and what it is doing during this period can provide critical insights into the mechanism underlying its persistent effects on susceptibility to DIO.
Figure 2. Ontogeny of leptin-sensing NAG neurons.
Many NAG neurons are derived from a Pomc-expressing progenitor (yellow neuron); the decision to express Npy largely occurs during gestation and the early postnatal period (green neuron). LepR expression and signaling (pink “v” shape) is first observed at P5 and continues to increase throughout lactation. A leptin surge in the beginning of the second postnatal week (green arrow) activates NAG neurons and promotes neurite outgrowth. The expression of KATP channel subunits in the peri-weaning period coincides with leptin-mediated inhibition of NAG neurons (red stop arrow). The timing of NAG neuron maturation coincides with the onset of leptin’s effects on food intake.
4.1. Ontogeny and function of leptin signaling in homeostatic/hypothalamic feeding circuits
Leptin receptor (Lepr) expression and pSTAT3-mediated leptin signaling are first detected in the parenchyma of the ARH at P5 and markedly increase during lactation [74,75](Pink “v” shapes in Figure 2). The onset of leptin signaling in other hypothalamic nuclei is delayed relative to the ARH; P9 in the dorsomedial nucleus of the hypothalamus (DMH) and P13 in the ventromedial nucleus of the hypothalamus (VMH) [75].
Pioneering studies by Bouret and Simerly demonstrated that leptin administration from P4-P12 promotes axonal outgrowth from ARH neurons [76]. Follow-up studies reported that neonatal leptin preferentially impacts projections from NAG neurons to the autonomic compartment of PVH [67], which have been implicated in regulating food intake [66]. The preferential effect of neonatal leptin administration on NAG neurons is likely explained by the fact that the majority of leptin-sensing neurons in the neonatal ARH express Npy and not Pomc [77](Figure 1). The excitatory effect of leptin on neonatal NAG neurons [77] would be predicted to support axonal outgrowth. Finally, as NAG projections to the caudal PVH form between P10-15 [47](Figure 1), they would be maximally exposed to the growth-promoting effects of leptin treatment from P4-12.
During the transition to solid food at weaning, leptin’s effect on neuronal responses in NAG neurons switches from excitation to inhibition, which is the response elicited in mature NAG neurons [77,78,79](red arrow, Figure 2). The timing of the switch in NAG neuronal responsiveness to leptin coincides with the onset of leptin’s effects on food intake in the fourth postnatal week [44], raising the possibility that they are mechanistically linked.
4.2. Effect of neonatal leptin on ARH neurons is likely independent of processes that program susceptibility to obesity
While the data are compelling that leptin signals during the neonatal period promote outgrowth of NAG projections to pre-autonomic neurons in the PVH that regulate feeding [67,76], there are several reasons why leptin’s effects on feeding circuits cannot explain the lasting impact of neonatal leptin on obesity-related outcomes [25,73]. First, the effect of leptin treatment in wild-type mice raised in average-sized litters to increase susceptibility to HFD-induced weight gain does not involve changes in food intake [25,72]. Second, it is not clear how rescuing orexigenic NAG projections in leptin-deficient ob/ob mice could contribute to the long-lasting protective effects of leptin treatment in ob/ob neonates [67]. Finally, reduced ARH projections in ob/ob mice [76] do not impair the ability of leptin to normalize food intake in these animals [80,81].
To begin to elucidate how neonatal leptin programs susceptibility to obesity, it is critical to consider what is known about the source(s) and target(s) of leptin in the neonatal period. While milk-derived leptin is detected in pups and likely contributes to neonatal physiology [82], serum leptin levels during the postnatal surge do not correlate with milk levels [83]. In addition, litter size manipulations that alter fatty acid composition and insulin levels in milk do not impact leptin [83,84]. Finally, the absence of plasma leptin in ob/ob offspring of ob/+ dams provides strong evidence that it is pup-derived [85]. While white adipose tissue (WAT) is the major source of circulating leptin in the adult [86], there is very little WAT at the time of the neonatal leptin surge. Leptin is produced by the stomach, although this activity is less apparent in first half of the lactation period [87].
Studies from Zhang et al. provide compelling evidence that the main determinant of plasma leptin at P10 is leptin produced in brown adipose tissue (BAT) [88]. The earliest detectable impairments in LepR-deficient fa/fa rats are in BAT structure and function, consistent with the possibility that BAT is both a primary source and target for neonatal leptin action. BAT is the main site of non-shivering and adaptive thermogenesis in the rodent [89]. Thus, factors that establish baselines of BAT activity in the suckling period are well-positioned to exert a lasting impact on energy expenditure. To begin to evaluate whether effects of neonatal leptin on developing BAT and thermoregulatory circuits underlie its lasting effects on susceptibly to obesity, the timing of critical steps in the maturation of thermoregulatory circuits will be outlined below.
5. Ontogeny of BAT thermoregulatory circuits in rodents
5.1. Perinatal period – BAT recruitment
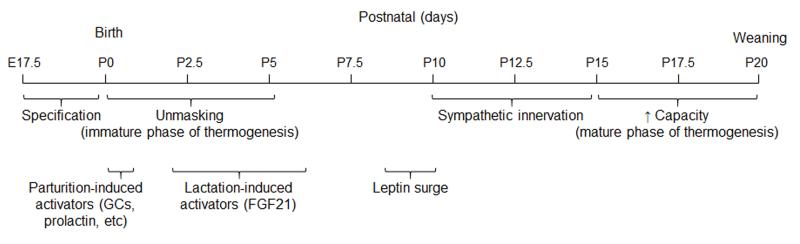
The brown adipose depot is formed during gestation [90], but the first few days after birth is a critical period for the recruitment of BAT in altricial newborns [89]. Uncoupling protein 1 (UCP1), the critical mitochondrial protein needed to produce heat in brown adipose tissue, is expressed in the final days of gestation [90](Figure 3), but neonates do not have the capacity to thermoregulate at birth [91]. There is a dramatic increase in Ucp1 expression in BAT within the first few hours after birth [91]. High levels of catecholamines [92], glucocorticoids [93] and prolactin [94] at parturition could all contribute to this process, either by direct effects on BAT or by activating autocrine factors in BAT (i.e. IGF2) [94](Figure 3). After birth, the transition to lactation exposes the neonates to fatty acids and ketone bodies [95], factors that promote the expression of Fibroblast growth factor 21 (Fgf21) by the liver [96]. FGF21 can be detected in plasma within 2 days of birth and is maximal at P6 [97](Figure 3). FGF21 treatment in neonates is sufficient to increase thermogenic genes and core body temperature [97]. The initial phase of BAT recruitment at parturition is likely independent of sympathetic inputs because there are very few autonomic projections to BAT that can be detected at birth [98]. Thus, the collective action of neuroendocrine, cold-induced and dietary factors within first 2 days of birth likely explain the onset of cold-induced thermogenesis at P2-5 [91,99], a behavior that is critical for survival.
Figure 3. Stages of BAT development.
The BAT depot is formed and produces thermogenic machinery by the end of gestation. Neuroendocrine factors produced at parturition and in response to the transition to a milk-based diet unmask BAT activity. This immature phase of BAT thermogenesis is critical to survival outside the womb and is largely unopposed. The surge of plasma leptin in the beginning of the second postnatal week immediately precedes the expansion of sympathetic projections onto BAT and a marked increase in thermogenic capacity and activity. The mature phase of thermogenesis is regulated, in large part, by sympathetic tone onto BAT.
5.2. Early lactation period – increased sympathetic input and capacity for cold-induced thermogenesis
Neonatal responses to cold are much weaker than those exhibited by adults. In the adult, input from the sympathetic nervous system (SNS) to BAT is the major driver of cold-induced thermogenesis [89]. Sympathetic nerves innervate BAT by P6, and the dramatic increase in the capacity for cold-induced thermogenesis between P10-15 [99] coincides with an expansion in the catecholaminergic innervation of the BAT parenchyma [98](Figure 3). Ucp1, a molecular indicator of BAT activity, reaches adult levels by P16 [100]. Together these observations are consistent with the idea that factors driving autonomic innervation of BAT between P5-15 (such as cold) are major determinants of thermogenic activity.
5.3. Peri-weaning period – critical period of development for baselines of activity in thermogenic circuits
Baselines of activity in thermoregulatory circuits appear to be established in the peri-weaning period. The transition from a ketogenic diet during lactation to a carbohydrate–based diet is likely responsible for reduced circulating levels of FGF21 in weanlings, as this decrease can be prevented if animals are weaned onto a HFD [97]. Maintenance of elevated levels of this BAT-activating factor could underlie observations that weaning onto a HFD results in increased thermogenic capacity and protection from DIO [101]. Exposure to cold in the peri-weaning period is also associated with lasting increases in thermogenic capacity [102,103]. While HFD-feeding at weaning is protective against diet-induced weight gain [101], cold-rearing leads to increased susceptibility [103]. Observations that early cold exposure leads to increased catecholaminergic innervation of BAT [103,104] raise the possibility that early elevations of sympathetic tone lead to the establishment of high thresholds for activity – a phenomenon also described as norepinephrine (NE) resistance [105,106].
6. Leptin influences BAT development and activity
6.1 Stages of BAT development
There at least four stages of BAT development (Figure 3). The gestational phase is characterized by the formation of the tissue depot and production of key components of the thermogenic machinery (i.e. UCP1). Hormones secreted at parturition (i.e. glucocorticoids, prolactin) and following the transition to a milk-based diet (i.e. FGF21) are likely responsible for “unmasking” BAT activity in the neonatal phase (P0-10). The mid-lactation phase (P10-17) is characterized by the establishment of sympathetic projections and SNS-dependent stimulation of BAT activity. Environmental factors in the peri-weaning period, including diet and temperature, influence baselines of activity in thermogenic circuits. Gain- and loss-of-function studies provide insight into leptin’s influence on these processes.
6.1.1. Gestational Phase
Total mitochondrial protein and UCP1 levels are not altered in leptin signaling-deficient fa/fa rats and ob/ob mice [107,108], arguing against a major role for leptin in the earliest phase of brown adipocyte proliferation and differentiation.
6.1.2. Neonatal Phase
Impairments in BAT mitochondrial function and reduced energy expenditure at thermoneutrality have been detected in LepR-deficient rats as early as P2 [109,110]. Because deficits in energy expenditure are not associated with a decrease in total mitochondrial protein or UCP1 [107,108], perinatal leptin signals may promote the “unmasking” of BAT activity. By P7-8, leptin-deficient models exhibit increased lipid deposition [111,112,113] and modest reductions in thermoregulatory thermogenesis [109,110,112]. Leptin administration to lean rats from P1 reduces adiposity by P7 [114,115], but does not increase energy expenditure [44]. These observations support the idea that leptin plays a role in “unmasking” BAT after birth and maintaining baseline thermogenic activity of BAT. In principle, leptin could mediate these effects via direct effects on BAT to promote lipolysis [116] and/or indirect stimulation of the earliest sympathetic projections to BAT [98].
6.1.3. Mid-Lactation Phase
The period from P10-17 is characterized by an expansion of sympathetic projections onto BAT and a marked increase in thermogenic capacity and activity. At P10, leptin mRNA expression in BAT and circulating protein levels in plasma are elevated in LepR-deficient rats [117]. Exposure to NE, but not cold, can restore lower levels of leptin expression in BAT of fa/fa rats at this age, consistent with the idea that leptin conveys a cold-induced negative feedback signal via sympathetic neurons [88]. Increased lipid accumulation in fa/fa and ob/ob mutants is accompanied by impaired BAT mitochondrial structure at this stage [99,118], although it is not clear whether the two are causally related.
Reduced sympathetic tone onto BAT is readily observed in leptin mutants at P15 [119]. Interestingly, this decrease is relatively specific to BAT, as rates of NE turnover in WAT, liver, and pancreas of ob/ob mice are similar to those in lean mice [119]. Thermogenic capacity of BAT in fa/fa rats can largely be restored by treatment with a β-agonist from P8-16 [120], consistent with the idea that this deficit is due to reduced sympathetic tone. The severity of the deficit in thermoregulatory thermogenesis in leptin mutants increases after P16-17, in parallel with the marked increase in sympathetic innervation of BAT [121,122,123]. P17 also represents the earliest age at which leptin injection is reported to acutely increase energy expenditure in lean or ob/ob mice [44]. Together, these observations support the idea that leptin plays a critical role in mediating sympathetic signals onto BAT that promote adaptive thermogenesis, which becomes more prominent in the transition to weaning.
6.1.4. Peri-weaning phase
Exposure to cold in the peri-weaning period leads to lasting increases in thermogenic capacity [102,103]. However, the influence of leptin on this process has not been explored. This is an important area for future research.
6.2. Sites mediating leptin’s effects on BAT thermogenic circuits
The gradual emergence of impairments in BAT structure and activity across lactation in genetic mutants lacking leptin signals is consistent with the idea that leptin influences several distinct phases of the development of BAT thermoregulatory circuits. As the initiation of LepR signaling in the brain and periphery spans the period of gestation and lactation, temporal patterns of expression can point toward sites where leptin might act to influence a particular process. Leptin likely promotes the “unmasking” of BAT activity that occurs shortly after birth. LepR is not yet detected in the mediobasal hypothalamus at this time [74,75]. The observation that neonatal BAT does not receive catecholaminergic input at birth [98], argues against a role for leptin signaling in other brain regions in the initial phase of BAT recruitment. It is possible that early effects of leptin are mediated by direct signaling within BAT [116] or indirect actions on other endocrine organs.
The earliest deficit in mutant models that can be attributed to a central action of leptin is the suppression of Leptin expression in BAT at P7, which is mediated by sympathetic inputs to BAT [88]. Brain regions that project to BAT and express LepR by P10 include the median preoptic area (mPOA) and the nucleus of the solitary tract (NTS), but not the DMH [75,124,125,126]. While LepR is expressed in a subset of NAG neurons at P10 [77], projections to the autonomic compartment of the caudal PVH are not formed until P15-16 [47]. Recent studies support the idea that leptin can act directly on LepR-expressing sympathetic axons to promotes axonal outgrowth [127]. While this capability was not assessed in projections onto BAT, it raises the possibility that leptin secreted from BAT during the surge (P8-10) contributes to the dramatic increase in sympathetic innervation of the BAT parenchyma observed after this period [98]. If true, it could explain why the deficit in SNS tone in ob/ob at P14 is restricted to BAT and is not observed in other organs that receive sympathetic input [119].
In theory, two modes of leptin action could contribute to deficits in thermoregulatory thermogenesis observed in leptin mutants after P15 [99]. First, it is possible that reduced sympathetic innervation due to the loss of LepR in sympathetic axons causes lasting decreases in sympathetic tone onto BAT. Exploring this possibility is an important area for future research. Second, impaired LepR-dependent transmission of cold-induced signals in the DMH and/or mPOA, and possibly BAT-projecting neurons in the retrochiasmatic area, Edinger-Westphal nucleus or the NTS, could also impede adaptive thermogenic responses [126].
6.3. Role of leptin in developing thermoregulatory circuits
Several lines of evidence support the hypothesis that neonatal leptin administration programs susceptibility or resistance to obesity via effects on developing BAT thermoregulatory circuits. Lean mice raised in average-sized litters that received daily leptin injections from P3-13 [72] or P5-15 [25] have no detectable change in obesity-related endpoints under chow-feeding conditions, but develop increased adiposity in response to a HFD challenge. As enhanced sensitivity DIO in these mice is not accompanied by hyperphagia, it supports the idea that deficits in adaptive thermogenesis are responsible. Conversely, neonatal leptin administration (P4-14) to ob/ob mice is associated with long-lasting improvements in BAT morphology and enhanced thermogenic responses to nutritional and cold challenges [67].
In mature animals, leptin action in the CNS can promote thermogenesis by modulating sympathetic tone onto BAT [128]. However, this activity is not observed until P15, when the BAT parenchyma is densely innervated by sympathetic projections [44,99]. The consequences of leptin treatment in neonates are opposite from those observed in mature animals. Neonatal leptin administration in rats (P1-10) and sheep is reported to reduce BAT UCP1 protein and thyroid hormones that promote thermogenesis [129,130]. An inverse relationship between Leptin and Ucp1 gene expression in BAT has also been noted in older (7 weeks) rats exposed to different temperatures [131]. These observations raise the possibility that BAT-derived leptin may act locally to suppress Ucp1 and BAT thermogenesis, although this idea has not been directly tested.
What can explain the switch in leptin’s effect on thermogenic endpoints in the last week of lactation? This transition is characterized by a gradual decrease in BAT-derived leptin, which had been acting to suppress thermogenesis, and a concomitant increase in LepR-mediated increases in sympathetic tone that promote BAT activity. As LepR signaling is weak in BAT-activating neurons of the DMH in neonates [75,124], the putative local inhibitory effect of leptin on Ucp1 would be predicted to function unopposed. Sympathetic projections that form in the first postnatal week may provide weak negative feedback to leptin expression in BAT by P7 [88]. LepR-expressing neurons in the mPOA or NTS could mediate this signal, although this has not been examined directly [75,124]. The onset of LepR signaling in the DMH [75] and the dramatic increase in sympathetic inputs to BAT after P10 [98] may act to suppress leptin expression in BAT [88] and thus promote Ucp1 expression and thermogenesis.
If BAT-derived leptin promotes innervation by sympathetic axons [127], it would accelerate the arrival of negative feedback signals to drive the sharp decline in plasma leptin that marks the end of the surge [43]. If true, this system would allow maximal activation of BAT in response to parturition/lactation-related signals, which is critical to promote survival. SNS-dependent down-regulation of BAT-derived leptin could then act to shut off the early phase of unopposed thermogenesis when the CNS-regulated phase is able to take over. Thus, the neonatal leptin surge could act to coordinate the transition between these phases of thermoregulatory thermogenesis. In theory, the high plasma levels of leptin in the neonatal surge could ensure that circuits driving food intake develop in time to meet the increased energetic needs driven by the action of circuits regulating energy expenditure.
7. The importance of peri-weaning growth rates in programming susceptibility to obesity
It has been difficult to formulate a model to explain the contribution of changes in leptin signaling to the programming of obesity susceptibility because of the variability in the outcomes of studies using different experimental paradigms. For example, neonatal leptin administration improves deficits in BAT due to leptin deficiency in ob/ob mice [67] or severe intrauterine growth retardation (IUGR) [73]. However, the same treatment leads to impaired BAT thermogenesis and increased susceptibility to DIO when given to normally-nourished wild type mice [25,72].
There are also conflicting reports of the impact of maternal dietary restriction on the timing and size of the leptin surge. Some groups observed a premature leptin surge [25], while other groups reported a delay [83]. These inconsistencies may stem from the fact that the plasma leptin surge is derived from the pup and not the dam [85], and thus variations in the timing, severity and nature of the maternal restriction could lead to different impacts on leptin production in pups. It is not clear what factors are driving leptin expression in neonatal BAT, but they may be independent of diet, as fasting does not impact leptin levels at this stage [43]. In light of these observations, influences of leptin on the formation of circuits regulating energy intake and expenditure are likely not the primary determinant of susceptibility vs. resistance to diet-induced weight gain in adulthood.
A recent paper provides compelling evidence to support the idea that the rate of growth in the peri-weaning period is predictive of responses to a HFD challenge [132]. In comparisons between experimental groups that were exposed to IUGR and/or neonatal leptin treatment, those that exhibited growth restriction in the peri-weaning period were protected from HFD-induced weight gain, while rapid growth in this period correlates with increased susceptibility [132]. Consistent with this theory, persistent improvements in BAT structure and capacity due to neonatal leptin treatment in ob/ob mice are also associated with restricted growth in the peri-weaning period [67]. The post-weaning period is characterized by a high rate of linear growth, a process that is energetically costly. Elucidation of the relationship between circuits regulating growth, adiposity and thermogenesis during development is an important area for future research.
8. Overview of the ontogeny of thermoregulatory circuits in rodents
A working model of the ontogeny of thermoregulatory circuits is presented below. While this formulation was informed by published information, many aspects of the model have not yet been examined directly. There are at least two phases of BAT thermogenesis, an immature phase that is critical for extrauterine survival and the transition to a fat-based diet, and a mature phase that is regulated by combined actions of sympathetic and circulating signals [89,133]. The immature phase is induced by hormones released at parturition (i.e. glucocorticoids, prolactin) and factors released in response to the ketogenic diet of lactation (i.e. FGF21) and does not appear to be impacted by maternal undernutrition [134]. It is possible that leptin works with these factors in the initial phase of BAT activity “unmasking” [109,110]. Some of these activating factors may drive expression of leptin in BAT, which may act locally to suppress Ucp1 expression [129,130] and serves as the major source of plasma leptin in the neonate [88]. BAT-derived leptin may act locally to increase innervation by sympathetic projections [127] and via the circulation to promote axonal outgrowth in homeostatic feeding circuits [67,76]. Increased LepR expression in cold-activated neurons in the DMH [75] and sympathetic inputs to BAT after P17 [98], could act to robustly suppress leptin expression in BAT [88] and stimulate thermogenesis [126], providing for smooth transition to CNS-driven thermoregulation. Interactions between circuits regulating growth, adiposity and energy expenditure during the peri-weaning period likely impart significant and lasting effects on susceptibility to obesity [132]. However, little is known about the molecular and neuronal factors involved.
9. Translation to humans
Although the timing of developmental processes is different in rodents and humans, early nutritional restriction followed rapid growth is associated with increased susceptibility to obesity, while exposure that is limited to later developmental stages is associated with protection. Therefore, it is possible that effects of early suboptimal nutrition to depress BAT activity in rodents could underlie increased risk of obesity observed in precocial species as well. In support of this idea, reduced BAT function in children is associated with increased obesity [135,136,137].
9.1. Ontogeny of BAT in precocial species
Precocial newborns, including sheep, non-human primates and humans, exhibit active BAT from birth. Brown fat is first detected histologically in human fetuses at 20 weeks of gestation and is fully formed by 35 weeks of gestation [138]. As in rodents [134], BAT histology is not impacted by IUGR [138]. Serum leptin concentrations in humans are high at birth and rapidly decline [139,140]. Fetal leptin levels are elevated in growth-restricted humans [141] and sheep [142]. It is not clear whether there is a rodent correlate of this phenomenon; however, there are several reasons to think it may be similar to the leptin surge observed at the end of the first postnatal week in rodents. As in rodents, the peak of leptin at birth in sheep is followed by a rapid suppression of Ucp1 expression such that the neonatal phase of declines after P7 [143]. Leptin treatment in neonatal sheep can drive a dramatic decrease in Ucp1 [130], reminiscent of effects in rodents [129].
While it is tempting to speculate that the leptin surge in human births is analogous to the rodent surge at P8-10, there are some notable differences. For example, the leptin surge during parturition of precocial species is not likely to drive outgrowth of sympathetic innervation of BAT or AgRP projections to the autonomic compartment of the PVH, as these projections are established before birth in precocial species [144,145]. Gestational timing for influences on sympathetic innervation of BAT is supported by observations that reductions in catecholaminergic pathways in BAT due to intrauterine growth restriction are evident at birth in sheep [145]. Together, these observations support the idea that leptin signals in precocial newborns contribute to the process whereby the immature phase of BAT thermogenesis is extinguished, but do not influence axonal outgrowth or persistent patterns of BAT activity. This interpretation is consistent with reports that leptin levels at birth (i.e. cord blood) reflect birth weight [146,147], but do not predict later adiposity [148].
Several lines of evidence support the idea that the peak of BAT activity in humans occurs in infancy. Although the BAT depot is formed during gestation, it is likely activated by factors associated with parturition, as evidenced by lower UCP1 levels in BAT of pre-term and still-born infants than in older infants [149]. The maximal number of brown adipocytes detected histologically in infants [150,151] coincides with peaks of UCP1 protein levels in BAT [149] and plasma levels of FGF21 [152]. It has also been reported that FGF21 is expressed in BAT and beige/BRITE adipocytes in human neonates and that FGF21 levels are correlated with UCP1 expression [153]. The peak of plasma FGF21 is observed at 6 months of age, with reduced levels detected by 12 months [152]. Finally, analyses of BAT histology [150,151] and thermal imaging of the supraclavicular region [154] across the lifespan are consistent with the idea that BAT activity declines with age.
9.2. Impacts of catch-up growth on BAT in humans
In rodent models and humans, rapid weight gain in the postnatal period is associated with increased deposition of fat and susceptibility to obesity, independent of birth weight [14,16,18,19,28,29,132,155]. The idea that increased fat deposition associated with catch-up growth [17,18] programs impaired BAT function is supported by the observation that malnourished toddlers that experience catch-up growth exhibit increased leptin and low FGF21 as compared to those who maintain a slow growth trajectory [156]. The early phase of BAT development is likely important, because failure to develop active BAT in children is correlated with increased BMI [135,136,137]. Thus, these observations are consistent with the hypothesis that increased fat deposition during rapid growth in infancy programs diminished BAT capacity and reduced ability to increase energy expenditure when confronted with a higher nutritional plane.
10. Summary
Increased risk of obesity programmed by early exposure to suboptimal maternal nutrition in humans is also observed in animal models with altricial (rats and mice) and precocial (sheep and non-human primates) newborns. Leptin administration to normally-nourished rodents in the neonatal period, roughly equivalent to the last trimester in humans, is sufficient to program increased susceptibility to diet-induced weight gain. The rodent neonate expresses high levels of leptin in BAT that acts locally to activate an immature phase of thermogenesis and may also promote innervation by sympathetic inputs. BAT-derived leptin is also released into the serum and acts to promote projections from NAG neurons to pre-autonomic neurons in the PVH that regulate food intake. Effects of neonatal leptin on developing circuits regulating energy intake and expenditure can be overridden by nutrient and external environment factors that influence growth rates in the peri-weaning period, which are predictive of susceptibility to diet-induced weight gain in adulthood.
The relationship between increased fat deposition during catch-up growth, decreased BAT activity and risk of obesity in human infants is strikingly similar to observations about the lasting impact of growth rates in the peri-weaning period on susceptibility to diet-induced weight gain in rodents. Since interventions in the peri-weaning period that enhance thermogenic capacity can be protective against obesity in rats, it is important to explore whether similar processes can be invoked in humans. In theory, observations that slow rates of infant growth associated with exposure to famine late in gestation/early infancy are protective against obesity could be mediated via impacts on BAT development during this critical period. If true, a better understanding of the relationship between growth in infancy, fat deposition, and thermoregulatory circuits in humans could lead to novel strategies to combat childhood obesity.
Highlights.
Timing of exposure to maternal undernutrition programs susceptibility to obesity.
Neonatal leptin programs susceptibility to diet-induced weight gain in rodents.
Leptin’s effects on hypothalamic neurons are too late to impact this process.
Neonatal leptin impacts brown adipose structure and function.
Influences on thermoregulatory circuits could program obesity susceptibility.
Acknowledgments
The writing of this article was supported by a grant from the NIH (DK089038).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and Trends in Overweight Among US Children and Adolescents, 1999-2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- [2].Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- [3].Widdowson EM, McCance RA. The Effect of Finite Periods of Undernutrition at Different Ages on the Composition and Subsequent Development of the Rat. Proc R Soc Lond B Biol Sci. 1963;158:329–342. doi: 10.1098/rspb.1963.0051. [DOI] [PubMed] [Google Scholar]
- [4].Stephens DN. Growth and the development of dietary obesity in adulthood of rats which have been undernourished during development. Br J Nutr. 1980;44:215–227. doi: 10.1079/bjn19800034. [DOI] [PubMed] [Google Scholar]
- [5].Oscai LB, McGarr JA. Evidence that the amount of food consumed in early life fixes appetite in the rat. Am J Physiol. 1978;235:R141–144. doi: 10.1152/ajpregu.1978.235.3.R141. [DOI] [PubMed] [Google Scholar]
- [6].Bouret SG. Nutritional programming of hypothalamic development: critical periods and windows of opportunity. International Journal of Obesity Supplements. 2012;2:S19–24. doi: 10.1038/ijosup.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- [8].Ravelli ACJ, van der Meulen JHP, Michels RPJ, Osmond C, Barker DJP, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. The Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- [9].Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- [10].Stanner SA, Yudkin JS. Fetal programming and the Leningrad Siege study. Twin Res. 2001;4:287–292. doi: 10.1375/1369052012498. [DOI] [PubMed] [Google Scholar]
- [11].Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- [12].Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr. 2003;77:1374–1378. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- [13].Sachdev HS, Fall CH, Osmond C, Lakshmy R, Dey Biswas SK, Leary SD, Reddy KS, Barker DJ, Bhargava SK. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- [14].Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- [15].Ekelund U, Ong KK, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab. 2007;92:98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- [16].Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, Rich-Edwards JW, Gillman MW. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165:993–998. doi: 10.1001/archpediatrics.2011.167. [DOI] [PubMed] [Google Scholar]
- [17].Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27:101–140. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- [18].Gillman MW. The first months of life: a critical period for development of obesity. Am J Clin Nutr. 2008;87:1587–1589. doi: 10.1093/ajcn/87.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301:2234–2242. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- [20].Ojha S, Robinson L, Symonds ME, Budge H. Suboptimal maternal nutrition affects offspring health in adult life. Early Hum Dev. 2013;89:909–913. doi: 10.1016/j.earlhumdev.2013.08.022. [DOI] [PubMed] [Google Scholar]
- [21].Gnanalingham MG, Mostyn A, Symonds ME, Stephenson T. Ontogeny and nutritional programming of adiposity in sheep: potential role of glucocorticoid action and uncoupling protein-2. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1407–1415. doi: 10.1152/ajpregu.00375.2005. [DOI] [PubMed] [Google Scholar]
- [22].Brennan KA, Gopalakrishnan GS, Kurlak L, Rhind SM, Kyle CE, Brooks AN, Rae MT, Olson DM, Stephenson T, Symonds ME. Impact of maternal undernutrition and fetal number on glucocorticoid, growth hormone and insulin-like growth factor receptor mRNA abundance in the ovine fetal kidney. Reproduction. 2005;129:151–159. doi: 10.1530/rep.1.00229. [DOI] [PubMed] [Google Scholar]
- [23].Symonds ME. Brown adipose tissue growth and development. Scientifica (Cairo) 2013;2013:305763. doi: 10.1155/2013/305763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- [25].Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [26].Ikenasio-Thorpe BA, Breier BH, Vickers MH, Fraser M. Prenatal influences on susceptibility to diet-induced obesity are mediated by altered neuroendocrine gene expression. J Endocrinol. 2007;193:31–37. doi: 10.1677/joe.1.07017. [DOI] [PubMed] [Google Scholar]
- [27].Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, Faucette RR, Reamer C, Przybyla R, Ruest S, Barry K, Otis JP, Patti ME. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49:1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- [28].Isganaitis E, Jimenez-Chillaron J, Woo M, Chow A, DeCoste J, Vokes M, Liu M, Kasif S, Zavacki AM, Leshan RL, Myers MG, Patti ME. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes. 2009;58:1192–1200. doi: 10.2337/db08-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cottrell EC, Martin-Gronert MS, Fernandez-Twinn DS, Berends LM, Ozanne SE. Leptin independent programming of adult body weight and adiposity in mice. Endocrinology. 2011 doi: 10.1210/en.2010-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dorner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999;11:541–546. doi: 10.1046/j.1365-2826.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- [31].Prior LJ, Velkoska E, Watts R, Cameron-Smith D, Morris MJ. Undernutrition during suckling in rats elevates plasma adiponectin and its receptor in skeletal muscle regardless of diet composition: a protective effect? Int J Obes (Lond) 2008;32:1585–1594. doi: 10.1038/ijo.2008.141. [DOI] [PubMed] [Google Scholar]
- [32].Johnson PR, Stern JS, Greenwood MR, Zucker LM, Hirsch J. Effect of early nutrition on adipose cellularity and pancreatic insulin release in the Zucker rat. J Nutr. 1973;103:738–743. doi: 10.1093/jn/103.5.738. [DOI] [PubMed] [Google Scholar]
- [33].Patterson CM, Bouret SG, Park S, Irani BG, Dunn-Meynell AA, Levin BE. Large litter rearing enhances leptin sensitivity and protects selectively bred diet-induced obese rats from becoming obese. Endocrinology. 2010;151:4270–4279. doi: 10.1210/en.2010-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet. 2011;2:27. doi: 10.3389/fgene.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li M, Sloboda DM, Vickers MH. Maternal obesity and developmental programming of metabolic disorders in offspring: evidence from animal models. Exp Diabetes Res. 2011;2011:592408. doi: 10.1155/2011/592408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ojha S, Saroha V, Symonds ME, Budge H. Excess nutrient supply in early life and its later metabolic consequences. Clin Exp Pharmacol Physiol. 2013;40:817–823. doi: 10.1111/1440-1681.12061. [DOI] [PubMed] [Google Scholar]
- [37].Fiorotto ML, Burrin DG, Perez M, Reeds PJ. Intake and use of milk nutrients by rat pups suckled in small, medium, or large litters. Am J Physiol. 1991;260:R1104–1113. doi: 10.1152/ajpregu.1991.260.6.R1104. [DOI] [PubMed] [Google Scholar]
- [38].Schwartz GJ, Zeltser LM. Functional organization of neuronal and humoral signals regulating feeding behavior. Annu Rev Nutr. 2013;33:1–21. doi: 10.1146/annurev-nutr-071812-161125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rinaman L, Roesch MR, Card JP. Retrograde transynaptic pseudorabies virus infection of central autonomic circuits in neonatal rats. Brain Res Dev Brain Res. 1999;114:207–216. doi: 10.1016/s0165-3806(99)00039-5. [DOI] [PubMed] [Google Scholar]
- [40].Rinaman L. Ontogeny of hypothalamic-hindbrain feeding control circuits. Dev Psychobiol. 2006;48:389–396. doi: 10.1002/dev.20146. [DOI] [PubMed] [Google Scholar]
- [41].Phifer CB, Browde JA, Jr., Hall WG. Ontogeny of glucose inhibition of independent ingestion in preweanling rats. Brain Res Bull. 1986;17:673–679. doi: 10.1016/0361-9230(86)90199-1. [DOI] [PubMed] [Google Scholar]
- [42].Swithers SE, Hall WG. A nutritive control of independent ingestion in rat pups emerges by nine days of age. Physiol Behav. 1989;46:873–879. doi: 10.1016/0031-9384(89)90051-6. [DOI] [PubMed] [Google Scholar]
- [43].Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mistry AM, Swick A, Romsos DR. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol. 1999;277:R742–747. doi: 10.1152/ajpregu.1999.277.3.R742. [DOI] [PubMed] [Google Scholar]
- [45].Ahima RS, Hileman SM. Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul Pept. 2000;92:1–7. doi: 10.1016/s0167-0115(00)00142-7. [DOI] [PubMed] [Google Scholar]
- [46].Steculorum SM, Bouret SG. Developmental effects of ghrelin. Peptides. 2011;32:2362–2366. doi: 10.1016/j.peptides.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79:47–63. doi: 10.1016/s0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- [48].Rozin P, Kalat JW. Specific hungers and poison avoidance as adaptive specializations of learning. Psychol Rev. 1971;78:459–486. doi: 10.1037/h0031878. [DOI] [PubMed] [Google Scholar]
- [49].Ogawa H, Hasegawa K, Ohgushi M, Murayama N. Changes in properties of neuronal responses in two cortical taste areas in rats of various ages. Neurosci Res. 1994;19:407–417. doi: 10.1016/0168-0102(94)90082-5. [DOI] [PubMed] [Google Scholar]
- [50].Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- [52].Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- [53].Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann N Y Acad Sci. 2003;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- [54].Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- [55].Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci U S A. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- [57].Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011 doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C Receptor Activates a Distinct Population of Arcuate Pro-opiomelanocortin Neurons via TRPC Channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK. 5-HT2CRs expressed by pro opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shimada M, Nakamura T. Time of neuron origin in mouse hypothalamic nuclei. Exp Neurol. 1973;41:163–173. doi: 10.1016/0014-4886(73)90187-8. [DOI] [PubMed] [Google Scholar]
- [64].Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Carmody JS, Wan P, Accili D, Zeltser LM, Leibel RL. Respective Contributions of Maternal Insulin Resistance and Diet to Metabolic and Hypothalamic Phenotypes of Progeny. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012 doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bouyer K, Simerly RB. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. J Neurosci. 2013;33:840–851. doi: 10.1523/JNEUROSCI.3215-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lopez M, Seoane LM, Tovar S, Garcia MC, Nogueiras R, Dieguez C, Senaris RM. A possible role of neuropeptide Y, agouti-related protein and leptin receptor isoforms in hypothalamic programming by perinatal feeding in the rat. Diabetologia. 2005;48:140–148. doi: 10.1007/s00125-004-1596-z. [DOI] [PubMed] [Google Scholar]
- [70].Cripps RL, Martin-Gronert MS, Archer ZA, Hales CN, Mercer JG, Ozanne SE. Programming of hypothalamic neuropeptide gene expression in rats by maternal dietary protein content during pregnancy and lactation. Clin Sci (Lond) 2009;117:85–93. doi: 10.1042/CS20080393. [DOI] [PubMed] [Google Scholar]
- [71].Remmers F, Verhagen LA, Adan RA, Delemarre-van de Waal HA. Hypothalamic neuropeptide expression of juvenile and middle-aged rats after early postnatal food restriction. Endocrinology. 2008;149:3617–3625. doi: 10.1210/en.2007-1388. [DOI] [PubMed] [Google Scholar]
- [72].Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- [73].Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- [74].Cottrell EC, Cripps RL, Duncan JS, Barrett P, Mercer JG, Herwig A, Ozanne SE. Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat. Am J Physiol Regul Integr Comp Physiol. 2009;296:R631–639. doi: 10.1152/ajpregu.90690.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Frontini A, Bertolotti P, Tonello C, Valerio A, Nisoli E, Cinti S, Giordano A. Leptin-dependent STAT3 phosphorylation in postnatal mouse hypothalamus. Brain Res. 2008;1215:105–115. doi: 10.1016/j.brainres.2008.03.078. [DOI] [PubMed] [Google Scholar]
- [76].Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- [77].Baquero AF, de Solis AJ, Lindsley SR, Kirigiti MA, Smith MS, Cowley MA, Zeltser LM, Grove KL. Developmental switch of leptin signaling in arcuate nucleus neurons. J Neurosci. 2014;34:9982–9994. doi: 10.1523/JNEUROSCI.0933-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- [79].Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- [80].Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- [81].Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- [82].Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, Casanueva FF. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab. 1997;82:4270–4273. doi: 10.1210/jcem.82.12.4590. [DOI] [PubMed] [Google Scholar]
- [83].Bautista CJ, Boeck L, Larrea F, Nathanielsz PW, Zambrano E. Effects of a maternal low protein isocaloric diet on milk leptin and progeny serum leptin concentration and appetitive behavior in the first 21 days of neonatal life in the rat. Pediatr Res. 2008;63:358–363. doi: 10.1203/01.pdr.0000304938.78998.21. [DOI] [PubMed] [Google Scholar]
- [84].Purcell RH, Sun B, Pass LL, Power ML, Moran TH, Tamashiro KL. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav. 2011;104:474–479. doi: 10.1016/j.physbeh.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cottrell EC, Mercer JG, Ozanne SE. Postnatal development of hypothalamic leptin receptors. Vitam Horm. 2010;82:201–217. doi: 10.1016/S0083-6729(10)82011-4. [DOI] [PubMed] [Google Scholar]
- [86].Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- [87].Oliver P, Pico C, De Matteis R, Cinti S, Palou A. Perinatal expression of leptin in rat stomach. Dev Dyn. 2002;223:148–154. doi: 10.1002/dvdy.1233. [DOI] [PubMed] [Google Scholar]
- [88].Zhang Y, Hufnagel C, Eiden S, Guo KY, Diaz PA, Leibel R, Schmidt I. Mechanisms for LEPR-mediated regulation of leptin expression in brown and white adipocytes in rat pups. Physiol Genomics. 2001;4:189–199. doi: 10.1152/physiolgenomics.2001.4.3.189. [DOI] [PubMed] [Google Scholar]
- [89].Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- [90].Giralt M, Martin I, Iglesias R, Vinas O, Villarroya F, Mampel T. Ontogeny and perinatal modulation of gene expression in rat brown adipose tissue. Unaltered iodothyronine 5′-deiodinase activity is necessary for the response to environmental temperature at birth. Eur J Biochem. 1990;193:297–302. doi: 10.1111/j.1432-1033.1990.tb19336.x. [DOI] [PubMed] [Google Scholar]
- [91].Vinter J, Hull D, Elphick MC. Onset of thermogenesis in response to cold in newborn mice. Biol Neonate. 1982;42:145–151. doi: 10.1159/000241588. [DOI] [PubMed] [Google Scholar]
- [92].Skala JP. Mechanisms of hormonal regulations in brown adipose tissue of developing rats. Can J Biochem Cell Biol. 1984;62:637–647. doi: 10.1139/o84-085. [DOI] [PubMed] [Google Scholar]
- [93].Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- [94].Viengchareun S, Servel N, Feve B, Freemark M, Lombes M, Binart N. Prolactin receptor signaling is essential for perinatal brown adipocyte function: a role for insulin-like growth factor-2. PLoS One. 2008;3:e1535. doi: 10.1371/journal.pone.0001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yubero P, Hondares E, Carmona MC, Rossell M, Gonzalez FJ, Iglesias R, Giralt M, Villarroya F. The developmental regulation of peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression in the liver is partially dissociated from the control of gluconeogenesis and lipid catabolism. Endocrinology. 2004;145:4268–4277. doi: 10.1210/en.2004-0099. [DOI] [PubMed] [Google Scholar]
- [96].Domouzoglou EM, Maratos-Flier E. Fibroblast growth factor 21 is a metabolic regulator that plays a role in the adaptation to ketosis. Am J Clin Nutr. 2011;93:901S–905. doi: 10.3945/ajcn.110.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Derry DM, Daniel H. Sympathetic nerve development in the brown adipose tissue of the rat. Can J Physiol Pharmacol. 1970;48:160–168. doi: 10.1139/y70-028. [DOI] [PubMed] [Google Scholar]
- [99].Hull D, Vinter J. The development of cold-induced thermogenesis and the structure of brown adipocyte mitochondria in genetically-obese (ob/ob) mice. Br J Nutr. 1984;52:33–39. doi: 10.1079/bjn19840068. [DOI] [PubMed] [Google Scholar]
- [100].Xiao XQ, Williams SM, Grayson BE, Glavas MM, Cowley MA, Smith MS, Grove KL. Excess weight gain during the early postnatal period is associated with permanent reprogramming of brown adipose tissue adaptive thermogenesis. Endocrinology. 2007;148:4150–4159. doi: 10.1210/en.2007-0373. [DOI] [PubMed] [Google Scholar]
- [101].Rothwell NJ, Stock MJ. Effects of early overnutrition and undernutrition in rats on the metabolic responses to overnutrition in later life. J Nutr. 1982;112:426–435. doi: 10.1093/jn/112.3.426. [DOI] [PubMed] [Google Scholar]
- [102].Doi K, Kuroshima A. Lasting effect of infantile cold experience on cold tolerance in adult rats. Jpn J Physiol. 1979;29:139–150. doi: 10.2170/jjphysiol.29.139. [DOI] [PubMed] [Google Scholar]
- [103].Morrison SF, Ramamurthy S, Young JB. Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue. J Neurosci. 2000;20:9264–9271. doi: 10.1523/JNEUROSCI.20-24-09264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ashwell M, Dunnett SB. Fluorescent histochemical demonstration of catecholamines in brown adipose tissue from obese (ob/ob) and lean mice acclimated at different temperatures. J Auton Nerv Syst. 1985;14:377–386. doi: 10.1016/0165-1838(85)90083-9. [DOI] [PubMed] [Google Scholar]
- [105].Levin BE, Sullivan AC. Dietary obesity and neonatal sympathectomy. II. Thermoregulation and brown adipose metabolism. Am J Physiol. 1984;247:R988–994. doi: 10.1152/ajpregu.1984.247.6.R988. [DOI] [PubMed] [Google Scholar]
- [106].Young JB, Weiss J, Boufath N. Effects of rearing temperature on sympathoadrenal activity in young adult rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1198–1209. doi: 10.1152/ajpregu.00525.2001. [DOI] [PubMed] [Google Scholar]
- [107].Bazin R, Eteve D, Lavau M. Evidence for decreased GDP binding to brown-adipose-tissue mitochondria of obese Zucker (fa/fa) rats in the very first days of life. Biochem J. 1984;221:241–245. doi: 10.1042/bj2210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ashwell M, Holt S, Jennings G, Stirling DM, Trayhurn P, York DA. Measurement by radioimmunoassay of the mitochondrial uncoupling protein from brown adipose tissue of obese (ob/ob) mice and Zucker (fa/fa) rats at different ages. FEBS Lett. 1985;179:233–237. doi: 10.1016/0014-5793(85)80525-1. [DOI] [PubMed] [Google Scholar]
- [109].Moore BJ, Horwitz BA, Stern JS. Brown fat thermogenesis and its role in the development of obesity. Brain Res Bull. 1985;14:577–583. doi: 10.1016/0361-9230(85)90107-8. [DOI] [PubMed] [Google Scholar]
- [110].Berce PJ, Moore BJ, Horwitz BA, Stern JS. Metabolism at thermoneutrality and in the cold is reduced in the neonatal preobese Zucker fatty (fa/fa) rat. J Nutr. 1986;116:2478–2485. doi: 10.1093/jn/116.12.2478. [DOI] [PubMed] [Google Scholar]
- [111].Le Marchand-Brustel Y, Jeanrenaud B. Pre- and postweaning studies on development of obesity in mdb/mdb mice. Am J Physiol. 1978;234:E568–574. doi: 10.1152/ajpendo.1978.234.6.E568. [DOI] [PubMed] [Google Scholar]
- [112].Planche E, Joliff M, de Gasquet P, Leliepvre X. Evidence of a defect in energy expenditure in 7-day-old Zucker rat (fa/fa) Am J Physiol. 1983;245:E107–113. doi: 10.1152/ajpendo.1983.245.2.E107. [DOI] [PubMed] [Google Scholar]
- [113].Meierfrankenfeld B, Abelenda M, Jauker H, Klingenspor M, Kershaw EE, Chua SC, Jr., Leibel RL, Schmidt I. Perinatal energy stores and excessive fat deposition in genetically obese (fa/fa) rats. Am J Physiol. 1996;270:E700–708. doi: 10.1152/ajpendo.1996.270.4.E700. [DOI] [PubMed] [Google Scholar]
- [114].Yuan CS, Attele AS, Zhang L, Lynch JP, Xie JT, Shi ZQ. Leptin reduces body weight gain in neonatal rats. Pediatr Res. 2000;48:380–383. doi: 10.1203/00006450-200009000-00021. [DOI] [PubMed] [Google Scholar]
- [115].Schmidt I, Fritz A, Scholch C, Schneider D, Simon E, Plagemann A. The effect of leptin treatment on the development of obesity in overfed suckling Wistar rats. Int J Obes Relat Metab Disord. 2001;25:1168–1174. doi: 10.1038/sj.ijo.0801669. [DOI] [PubMed] [Google Scholar]
- [116].Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW, Cusin I, Rohner-Jeanrenaud F, Burger AG, Zapf J, Meier CA. Direct effects of leptin on brown and white adipose tissue. J Clin Invest. 1997;100:2858–2864. doi: 10.1172/JCI119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhang Y, Olbort M, Schwarzer K, Nuesslein-Hildesheim B, Nicolson M, Murphy E, Kowalski TJ, Schmidt I, Leibel RL. The leptin receptor mediates apparent autocrine regulation of leptin gene expression. Biochem Biophys Res Commun. 1997;240:492–495. doi: 10.1006/bbrc.1997.7622. [DOI] [PubMed] [Google Scholar]
- [118].Goodbody AE, Trayhurn P. Studies on the activity of brown adipose tissue in suckling, pre-obese, ob/ob mice. Biochim Biophys Acta. 1982;680:119–126. doi: 10.1016/0005-2728(82)90002-0. [DOI] [PubMed] [Google Scholar]
- [119].Knehans AW, Romsos DR. Norepinephrine turnover in obese (ob/ob) mice: effects of age, fasting, and acute cold. Am J Physiol. 1983;244:E567–574. doi: 10.1152/ajpendo.1983.244.6.E567. [DOI] [PubMed] [Google Scholar]
- [120].Charon C, Dupuy F, Marie V, Bazin R. Effect of the beta-adrenoceptor agonist BRL-35135 on development of obesity in suckling Zucker (fa/fa) rats. Am J Physiol. 1995;268:E1039–1045. doi: 10.1152/ajpendo.1995.268.6.E1039. [DOI] [PubMed] [Google Scholar]
- [121].Schmidt I, Stahl J, Kaul R, Carlisle HJ. Cold-rearing normalizes capacity for norepinephrine-stimulated thermogenesis but not body temperature in 16-day-old fatty Zucker rats. Life Sci. 1986;38:129–136. doi: 10.1016/0024-3205(86)90004-4. [DOI] [PubMed] [Google Scholar]
- [122].Kaul R, Heldmaier G, Schmidt I. Defective thermoregulatory thermogenesis does not cause onset of obesity in Zucker rats. Am J Physiol. 1990;259:E11–18. doi: 10.1152/ajpendo.1990.259.1.E11. [DOI] [PubMed] [Google Scholar]
- [123].Kortner G, Petrova O, Vogt S, Schmidt I. Sympathetically and nonsympathetically mediated onset of excess fat deposition in Zucker rats. Am J Physiol. 1994;267:E947–953. doi: 10.1152/ajpendo.1994.267.6.E947. [DOI] [PubMed] [Google Scholar]
- [124].Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 2010;518:459–476. doi: 10.1002/cne.22219. [DOI] [PubMed] [Google Scholar]
- [125].Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- [126].Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Pellegrino MJ, McCully BH, Habecker BA. Leptin stimulates sympathetic axon outgrowth. Neurosci Lett. 2014;566:1–5. doi: 10.1016/j.neulet.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Rezai-Zadeh K, Munzberg H. Integration of sensory information via central thermoregulatory leptin targets. Physiol Behav. 2013;121:49–55. doi: 10.1016/j.physbeh.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Toste FP, Alves SB, Dutra SC, Bonomo IT, Lisboa PC, Moura EG, Passos MC. Temporal evaluation of the thyroid function of rats programmed by leptin treatment on the neonatal period. Horm Metab Res. 2006;38:827–831. doi: 10.1055/s-2006-956502. [DOI] [PubMed] [Google Scholar]
- [130].Mostyn A, Bispham J, Pearce S, Evens Y, Raver N, Keisler DH, Webb R, Stephenson T, Symonds ME. Differential effects of leptin on thermoregulation and uncoupling protein abundance in the neonatal lamb. FASEB J. 2002;16:1438–1440. doi: 10.1096/fj.02-0077fje. [DOI] [PubMed] [Google Scholar]
- [131].Cancello R, Zingaretti MC, Sarzani R, Ricquier D, Cinti S. Leptin and UCP1 genes are reciprocally regulated in brown adipose tissue. Endocrinology. 1998;139:4747–4750. doi: 10.1210/endo.139.11.6434. [DOI] [PubMed] [Google Scholar]
- [132].Ellis PJ, Morris TJ, Skinner BM, Sargent CA, Vickers MH, Gluckman PD, Gilmour S, Affara NA. Thrifty metabolic programming in rats is induced by both maternal undernutrition and postnatal leptin treatment, but masked in the presence of both: implications for models of developmental programming. BMC Genomics. 2014;15:49. doi: 10.1186/1471-2164-15-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- [134].Felipe A, Villarroya F, Mampel T. Effects of maternal hypocaloric diet feeding on neonatal rat brown adipose tissue. Biol Neonate. 1988;53:105–112. doi: 10.1159/000242769. [DOI] [PubMed] [Google Scholar]
- [135].Drubach LA, Palmer EL, 3rd, Connolly LP, Baker A, Zurakowski D, Cypess AM. Pediatric brown adipose tissue: detection, epidemiology, and differences from adults. J Pediatr. 2011;159:939–944. doi: 10.1016/j.jpeds.2011.06.028. [DOI] [PubMed] [Google Scholar]
- [136].Chalfant JS, Smith ML, Hu HH, Dorey FJ, Goodarzian F, Fu CH, Gilsanz V. Inverse association between brown adipose tissue activation and white adipose tissue accumulation in successfully treated pediatric malignancy. Am J Clin Nutr. 2012;95:1144–1149. doi: 10.3945/ajcn.111.030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Robinson L, Ojha S, Symonds ME, Budge H. Body mass index as a determinant of brown adipose tissue function in healthy children. J Pediatr. 2014;164:318–322. e311. doi: 10.1016/j.jpeds.2013.10.005. [DOI] [PubMed] [Google Scholar]
- [138].Moragas A, Toran N. Prenatal development of brown adipose tissue in man. A morphometric and biomathematical study. Biol Neonate. 1983;43:80–85. doi: 10.1159/000241641. [DOI] [PubMed] [Google Scholar]
- [139].Schubring C, Siebler T, Kratzsch J, Englaro P, Blum WF, Triep K, Kiess W. Leptin serum concentrations in healthy neonates within the first week of life: relation to insulin and growth hormone levels, skinfold thickness, body mass index and weight. Clin Endocrinol (Oxf) 1999;51:199–204. doi: 10.1046/j.1365-2265.1999.00761.x. [DOI] [PubMed] [Google Scholar]
- [140].Hytinantti T, Koistinen HA, Koivisto VA, Karonen SL, Rutanen EM, Andersson S. Increased leptin concentration in preterm infants of pre-eclamptic mothers. Arch Dis Child Fetal Neonatal Ed. 2000;83:F13–16. doi: 10.1136/fn.83.1.F13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Hytinantti TK, Juntunen M, Koistinen HA, Koivisto VA, Karonen SL, Andersson S. Postnatal changes in concentrations of free and bound leptin. Arch Dis Child Fetal Neonatal Ed. 2001;85:F123–126. doi: 10.1136/fn.85.2.F123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Buchbinder A, Lang U, Baker RS, Khoury JC, Mershon J, Clark KE. Leptin in the ovine fetus correlates with fetal and placental size. Am J Obstet Gynecol. 2001;185:786–791. doi: 10.1067/mob.2001.117313. [DOI] [PubMed] [Google Scholar]
- [143].Clarke L, Buss DS, Juniper DT, Lomax MA, Symonds ME. Adipose tissue development during early postnatal life in ewe-reared lambs. Exp Physiol. 1997;82:1015–1027. [PubMed] [Google Scholar]
- [144].Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience. 2006;143:975–986. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- [145].Oyama K, Padbury J, Martinez A, Chappell B, Stein H, Humme J. Effects of fetal growth retardation on the development of central and peripheral catecholaminergic pathways in the sheep. J Dev Physiol. 1992;18:217–222. [PubMed] [Google Scholar]
- [146].Schubring C, Kiess W, Englaro P, Rascher W, Dotsch J, Hanitsch S, Attanasio A, Blum WF. Levels of leptin in maternal serum, amniotic fluid, and arterial and venous cord blood: relation to neonatal and placental weight. J Clin Endocrinol Metab. 1997;82:1480–1483. doi: 10.1210/jcem.82.5.3935. [DOI] [PubMed] [Google Scholar]
- [147].Mantzoros CS, Varvarigou A, Kaklamani VG, Beratis NG, Flier JS. Effect of birth weight and maternal smoking on cord blood leptin concentrations of full-term and preterm newborns. J Clin Endocrinol Metab. 1997;82:2856–2861. doi: 10.1210/jcem.82.9.4248. [DOI] [PubMed] [Google Scholar]
- [148].Boeke CE, Mantzoros CS, Hughes MD, L.R.-S. S, Villamor E, Zera CA, Gillman MW. Differential associations of leptin with adiposity across early childhood. Obesity (Silver Spring) 2013;21:1430–1437. doi: 10.1002/oby.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond) 1986;71:291–297. doi: 10.1042/cs0710291. [DOI] [PubMed] [Google Scholar]
- [150].Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- [151].Tanuma Y, Tamamoto M, Ito T, Yokochi C. The occurrence of brown adipose tissue in perirenal fat in Japanese. Arch Histol Jpn. 1975;38:43–70. doi: 10.1679/aohc1950.38.43. [DOI] [PubMed] [Google Scholar]
- [152].Mericq V, De Luca F, Hernandez MI, Pena V, Rossel K, Garcia M, Avila A, Cavada G, Iniguez G. Serum fibroblast growth factor 21 levels are inversely associated with growth rates in infancy. Horm Res Paediatr. 2014;82:324–331. doi: 10.1159/000367922. [DOI] [PubMed] [Google Scholar]
- [153].Hondares E, Gallego-Escuredo JM, Flachs P, Frontini A, Cereijo R, Goday A, Perugini J, Kopecky P, Giralt M, Cinti S, Kopecky J, Villarroya F. Fibroblast growth factor-21 is expressed in neonatal and pheochromocytoma-induced adult human brown adipose tissue. Metabolism. 2014;63:312–317. doi: 10.1016/j.metabol.2013.11.014. [DOI] [PubMed] [Google Scholar]
- [154].Symonds ME, Henderson K, Elvidge L, Bosman C, Sharkey D, Perkins AC, Budge H. Thermal imaging to assess age-related changes of skin temperature within the supraclavicular region co-locating with brown adipose tissue in healthy children. J Pediatr. 2012;161:892–898. doi: 10.1016/j.jpeds.2012.04.056. [DOI] [PubMed] [Google Scholar]
- [155].Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- [156].Stein K, Vasquez-Garibay E, Kratzsch J, Romero-Velarde E, Jahreis G. Influence of nutritional recovery on the leptin axis in severely malnourished children. J Clin Endocrinol Metab. 2006;91:1021–1026. doi: 10.1210/jc.2005-1394. [DOI] [PubMed] [Google Scholar]