Abstract
Mefloquine continues to be a key drug used for malaria chemoprophylaxis and treatment, despite reports of adverse events like depression and anxiety. It is unknown how mefloquine acts within the central nervous system to cause depression and anxiety or why some individuals are more vulnerable. We show that intraperitoneal injection of mefloquine in mice, when coupled to subthreshold social defeat stress, is sufficient to produce depression-like social avoidance behavior. Direct infusion of mefloquine into the nucleus accumbens (NAc), a key brain reward region, increased stress-induced social avoidance and anxiety behavior. In contrast, infusion into the ventral hippocampus had no effect. Whole cell recordings from NAc medium spiny neurons indicated that mefloquine application increases the frequency of spontaneous excitatory postsynaptic currents, a synaptic adaptation that we have previously shown to be associated with increased susceptibility to social defeat stress. Together, these data demonstrate a role for the NAc in mefloquine-induced depression and anxiety-like behaviors.
Keywords: mefloquine, social defeat stress, nucleus accumbens, anxiety, depression
INTRODUCTION
Neuropsychiatric adverse events such as depression, anxiety, psychosis, and suicidality have been reported in patients taking mefloquine for malaria treatment and prophylaxis (1–3). Such cases compelled the U.S. Food and Drug Administration to issue a warning for the drug, cautioning physicians to use mefloquine only with appropriate monitoring for adverse events like depression (4). Mefloquine continues to be widely prescribed and remains one of a handful of key drugs available for treating chloroquine-resistant malaria (5, 6). The U.S. military widely prescribed mefloquine for malaria prophylaxis during the 2007 war in Afghanistan, and there is evidence of severe neuropsychiatric reactions in soldiers with a prior history of depression (7, 8). Reviews of the clinical data conclude that there is also increased risk of depression side effects following long-term prophylactic use in tourists traveling to malaria endemic regions (9).
However, it is currently unknown why mefloquine causes depression or anxiety. A better understanding of the drug actions in the brain might aid in identifying susceptibility traits and developing new therapies for depression and stress disorders. Here we use the preclinical model of social defeat stress to investigate mefloquine-induced depression and anxiety-related behavioral changes. In addition, because our previous studies have identified increased frequency of excitatory postsynaptic currents (EPSCs) on NAc MSNs to be associated with susceptibility to chronic social defeat stress (10, 11), we performed whole cell electrophysiology recordings from NAc medium spiny neurons following bath application of mefloquine. Together, these findings highlight a novel, clinically relevant connection between the NAc and depression- and anxiety-like behaviors resulting from mefloquine exposure.
EXPERIMENTAL PROCEDURES
Animals
Seven to eight week-old C57BL/6J male mice (Jackson Laboratories, Bar Harbor, Maine) were used for all behavioral experiments. Mice were group housed before the start of all experiments and maintained on a 12h light/dark cycle with ad libitum access to food and water. 4 month-old retired male CD-1 breeders (Charles River Laboratories, Wilmington, Massachusetts) were singly housed and used as aggressors in the subthreshold social defeat stress paradigm. Behavioral assessments and tissue collection were performed during the animals’ light phase (0700–1900 hours). Mouse procedures were performed in accordance with the Institutional Animal Care and Use Committee guidelines of the Icahn School of Medicine at Mount Sinai.
Drug
Mefloquine hydrochloride, Lot #111M4707V, (Sigma-Aldrich, St. Louis, Missouri) was dissolved in dimethyl sulfoxide (DMSO) for a 38mg/mL stock solution. Intraperitoneal injections (drug: 20 mg/kg mefloquine or 5 mg/kg mefloquine in 0.9% saline, or vehicle: 5% DMSO in 0.9% saline) were administered 20 minutes prior to stress. Direct cannula microinfusions (150 µM mefloquine or 1% DMSO in artificial cerebrospinal fluid; see Electrophysiology methods) were performed using a minipump (Harvard Apparatus, Holliston, Massachusetts) and internal cannula (Plastics One, Roanoke, Virginia) at a rate of 0.1 mL/minute for 5 minutes followed by a 5 minute rest period with the cannula still in place. Drug dosing was based on the doses used for malaria treatment (20 mg/kg) and prophylaxis (5 mg/kg) in humans, as well as previous literature using mefloquine to block gap junctions in mice (12, 13). Since mefloquine has a long elimination half-life of approximately two to three weeks (14), the drug was given only once as a pretreatment prior to subthreshold social defeat stress. Subsequent behavioral tests were performed without additional drug administrations.
Cannula Surgery
Eight-week old C57BL/6J male mice were anesthetized with a mixture of ketamine (100mg/kg) and xylazine (10mg/kg) in 0.9% saline. Mice were positioned in a small animal stereotaxic instrument (David Kopf Instruments, Tujunga, California), and the skull surface was exposed. Bilateral guide cannulas from Plastics One were implanted so the tip of the cannulas reached the nucleus accumbens (bregma coordinates: anterior, 1.5mm; mediolateral, 0.8mm; dorsoventral, 3.6mm) or ventral hippocampus (bregma coordinates: posterior, 3.2mm; mediolateral, 3.0mm; dorsoventral, 4.2mm). Ventral hippocampal coordinates were based on these published studies (12, 15).
Behavioral Testing
Mice underwent a single pairing of mefloquine administration with stress, followed by behavioral testing.
Subthreshold Social Defeat Stress
Subthreshold social defeat stress is a well-validated model for studying vulnerability factors in mice (16–19). Control animals do not develop social interaction deficits after subthreshold defeat, but manipulations that promote susceptibility will result in social avoidance. We used a subthreshold social defeat stress to measure increased susceptibility to stress as previously described (20, 21). Mice were single-housed one day prior to beginning stress experiments. Mice were then exposed to a novel CD-1 aggressor for 5 minutes followed by 10 minutes rest in the home cage. Exposure to the CD-1 aggressor was repeated for a total of 3 physical interactions. All aggressors were screened for aggressive behavior prior to use according to published protocols (22).
Social Interaction
Twenty-four hours following subthreshold social defeat stress, mice underwent the social interaction test as previously described (21, 22). Mice were placed into a novel open field arena with a small animal cage at one end. Their movement was recorded for 2.5 minutes in the absence of a social target (target absent trial), followed by 2.5 minutes in the presence of a novel CD-1 mouse (target present trial). Duration spent in the interaction zone (in seconds) as well as distance traveled (in centimeters) was measured using Ethovision software (Noldus Information Technology, Leesburg, Virginia). Heat maps were generated using the “heat map” function on the Ethovision software to create a representative image of the animals’ movements during the target present trial. Heat map scale bar represents the normalized time spent at each XY coordinate during the trial. Social interaction ratio was calculated as duration in the interaction zone during the target present trial divided by duration in the interaction zone during the target absent trial. A ratio less than 1 indicates social avoidance.
Elevated Plus Maze
The elevated plus maze consisted of two, straight intersecting runways positioned 60 centimeters above the floor and divided into two open and two closed arms. Mice were placed into the center of the maze and allowed to explore for a period of 5 minutes. Duration spent and entrances in the open arms and the closed arms were recorded using Ethovision software under red-light conditions. Heat map scale bar represents the normalized duration at each XY coordinate during the trial.
Perfusion and Tissue Processing
Following behavioral studies, mice were anesthetized with 15% chloral hydrate. Transcardial perfusion was performed with cold phosphate-buffered saline (pH 7.4) followed by 4% paraformaldehyde in phosphate-buffered saline. Brains were dissected and post fixed overnight in 4% paraformaldehyde. Each brain was cut on a vibratome (Leica) into 50 µm coronal slices to validate cannula placement site.
Electrophysiology
Seven week-old C57BL/6J male mice were anesthetized using isoflurane and perfused for 1 minute with ice-cold artificial cerebrospinal fluid (aCSF) containing in mM: 128 NaCl, 10 D-Glucose, 1.25 NaH2PO4, 25 NaHCO3, 2 MgSO4.7H20, 3 KCl, 2 CaCl2.2H20 (pH 7.35, 295–305 mOsm, oxygenated with 95% O2 and 5% CO2). 250 µm acute brain slices containing the NAc were cut in sucrose-aCSF containing 254 mM sucrose instead of NaCl. Slices were incubated in the holding chamber for 1 hour at 32 degrees Celsius. 25 µM mefloquine, or vehicle, was added to the holding chamber and incubated for 1 hour at room temperature. The dose and protocol for mefloquine application was based on previously published slice electrophysiology studies (23, 24). Slices were then transferred to a recording chamber with a constant flow rate (2mL/min) of carbogenated aCSF at room temperature, containing either 25 µM mefloquine or vehicle. NAc shell MSNs were identified by their location and size using infrared differential interference contrast microscopy, and confirmed by the presence of inward rectification. Patch clamp recordings were made in whole-cell configuration using glass microelectrodes (3–5 MΩ) filled with an internal solution containing (mM): 115 potassium gluconate, 20 KCl, 1.5 MgCl2, 10 phosphocreatine, 10 HEPES, 2 magnesium ATP, and 0.5 GTP (pH 7.2, 285 mOsm). 50 µM picrotoxin was added to the bath to isolate spontaneous excitatory postsynaptic currents (sEPSCs). Recordings were made with a computer-controlled amplifier (MultiClamp 700B), digitized (Digidata 1440), and acquired with Axoscope 10.1 (Molecular Devices) at a sampling rate of 10 kHz. The frequency and amplitude of sEPSCs were analyzed using MiniAnalysis software (Synaptosoft). Analysis was performed on 18 cells from 3 mice (n= 3 animals, 9 cells per group).
Statistics
All data are expressed as the mean ± SEM. Mean differences between groups were determined using Student’s t-test, two-way analysis of variance (ANOVA) or repeated measures two-way ANOVA when appropriate, followed by Bonferroni post-tests if the main effect or interaction was significant at p<0.05. Statistical analyses were performed using Prism 5.0 (GraphPad Software).
RESULTS
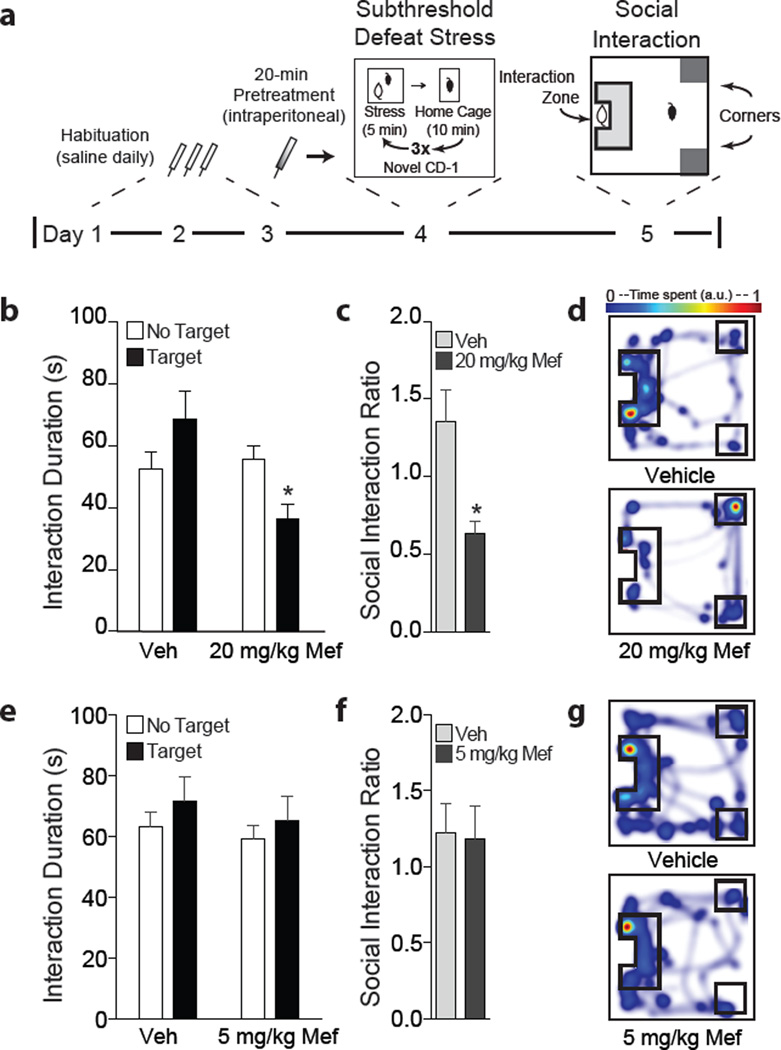
In order to examine pro-depressant effects of mefloquine in mice, we first delivered an intraperitoneal injection of either mefloquine or vehicle and exposed mice to subthreshold social defeat stress (Figure 1). We assayed doses that in humans reflect a malaria treatment dose of 20 mg/kg and a prophylactic dose of 5 mg/kg (25, 26). One day following social defeat, mice were tested for social interaction behavior (Figure 1a). Mice that received intraperitoneal injection of 20 mg/kg mefloquine, compared to vehicle, displayed social avoidance behavior after the subthreshold stress. We found a main effect of drug for time spent in the interaction zone with a novel animal present (two-way repeated measures ANOVA: F(1,16) = 15.09, p<0.01; Figure 1b). Post hoc analysis revealed that mice receiving mefloquine displayed social avoidance behavior as measured by a significant reduction in social interaction ratio (Student’s t-test, p<0.01; Figure 1c). When animals were administered the lower 5 mg/kg dose of mefloquine prior to stress, there was no effect on either interaction time (two-way repeated measures ANOVA, p<0.05; Figure 1e) or social interaction ratio (Student’s t-test, p<0.05; Figure 1f). In addition, we observed no difference in total distance traveled or mean velocity between groups during the “no target” trial, demonstrating that the drug does not affect basal locomotor activity (Supplemental Figure 1a–d).
Figure 1.
Single intraperitoneal injection of mefloquine promotes social avoidance behavior following subthreshold social defeat stress. (a) Experimental timeline. (b) Animals that received 20 mg/kg mefloquine prior to stress display decreased time in the interaction zone with a target animal present (*p<0.01, two-way repeated measures ANOVA, n=9 mice/group). (c) Mice that received 20 mg/kg mefloquine also showed a decrease in social interaction ratio (*p<0.01, Student’s t-test). (d) Representative heat maps from the target present trial. (e) Animals that received 5 mg/kg mefloquine prior to stress did not display reduced social interaction (p<0.05, two-way repeated measures ANOVA, n=9–10 mice/group). (f) 5 mg/kg mefloquine did not affect social interaction ratio (p<0.05, Student’s t-test). (g) Representative heat maps from the target present trial. Data are represented as group means. Error bars represent SEM.
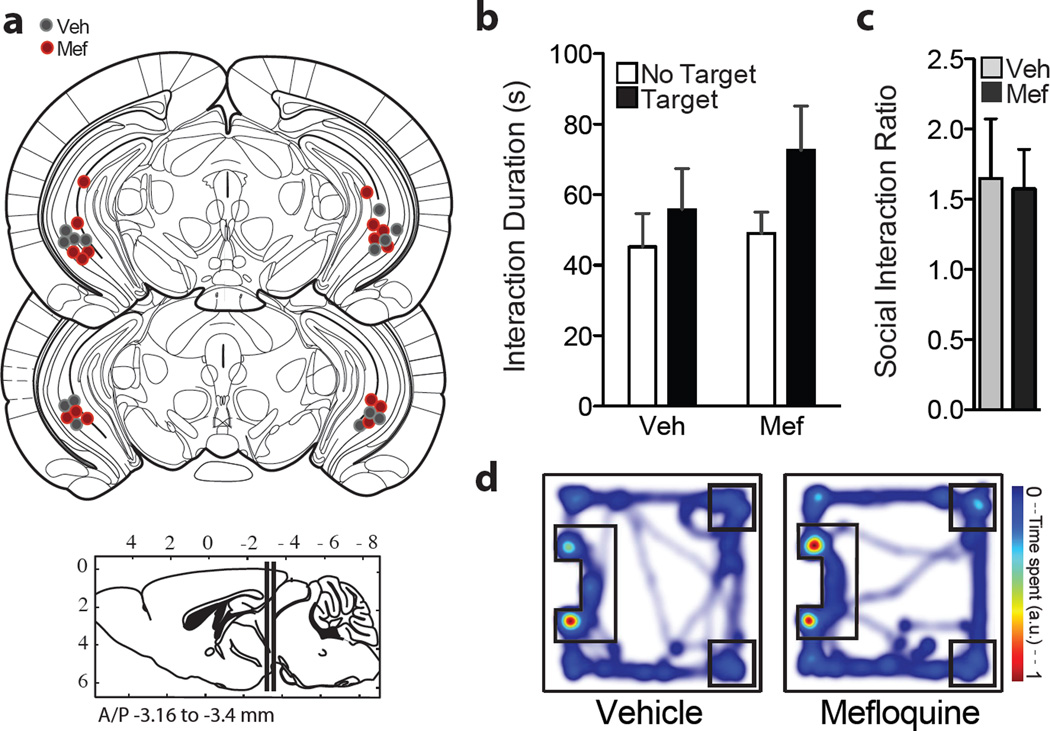
Since the nucleus accumbens (NAc) is a brain region that has been implicated in depression as well as social defeat stress (10, 18, 27, 28) we examined the effects of direct mefloquine infusion into the NAc on stress-induced social avoidance. Bilateral guide cannulas were implanted in the NAc and appropriate placement was assessed by post-hoc histology following all behavioral studies (Figure 2a). Direct infusion of mefloquine into the NAc, coupled to subthreshold social defeat stress, resulted in decreased time spent in the interaction zone with a social target present (two-way repeated measures ANOVA: F(1,11) = 13.12, p<0.01; Figure 2c). Bonferroni posttests revealed a significant effect of mefloquine. In addition, mefloquine-infused animals had a lower social interaction ratio compared to the vehicle group (Student’s t-test, p<0.01; Figure 2d).
Figure 2.
Direct infusion of mefloquine into nucleus accumbens promotes social avoidance behavior following subthreshold social defeat stress. (a) Schematic depicting bilateral cannula placements for all animals included in analysis (n=6–7 mice/group). Images are modified from Paxinos and Franklin, 2001. (b) Experimental timeline. (c) Mefloquine administration decreased interaction duration in the social interaction test one day following stress and drug infusion (*p<0.01, two-way repeated measures ANOVA, n=6–7 mice/group). (d) Animals that received mefloquine had a lower social interaction ratio (*p<0.01, Student’s t-test). (e) Representative heat maps from the target present trial. Data are represented as group means. Error bars represent SEM.
In a parallel study, direct infusion of mefloquine into the ventral hippocampus prior to subthreshold social defeat stress did not result in social interaction deficits when assayed in the social interaction test the following day (Figure 3). Both vehicle and drug-infused groups spent similar time in the interaction zone with a social target present (two-way repeated measures ANOVA: p>0.05; Figure 3b), and we observed no differences in social interaction ratios (Student’s t-test, p>0.05; Figure 3c). When exploring the arena without a target present, all animals that received cannula infusions of vehicle or drug in either NAc or ventral hippocampus showed equivalent locomotor activity, supporting the conclusion that these changes in social interaction behavior are not due to differences in basal locomotor activity (Supplemental Figure 1e–h). Together, these data support a selective role for the NAc in mediating the effects of mefloquine on social avoidance behavior.
Figure 3.
Direct infusion of mefloquine into ventral hippocampus has no effect on social avoidance behavior following subthreshold social defeat stress. (a) Schematic depicting bilateral cannula placements in the ventral hippocampus (n = 7–8 mice/group). Images are modified from Paxinos and Franklin, 2001. (b) There was no effect of mefloquine on time spent in the interaction zone during the social interaction test (p>0.05, two-way repeated measures ANOVA, n=8–9 mice/group). (c) Vehicle and mefloquine-infused mice had similar social interaction ratios (p>0.05, Student’s t-test). (d) Representative heat maps from the target present trial. Data are represented as group means. Error bars represent SEM.
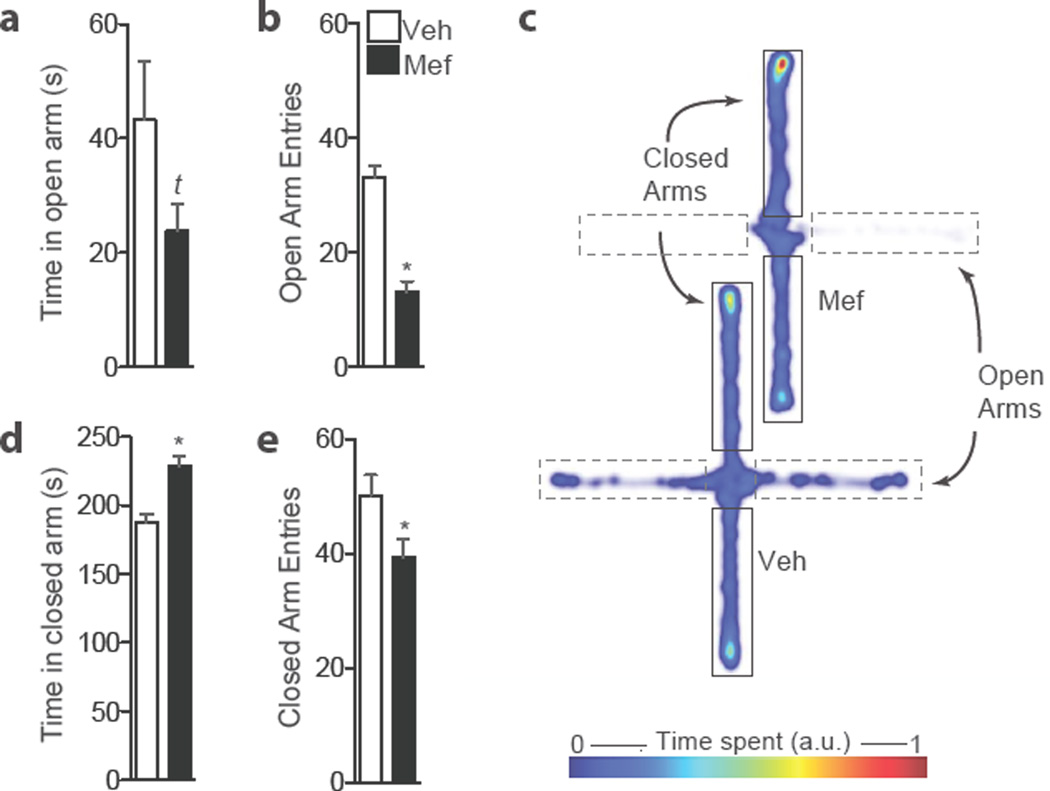
Direct infusion of mefloquine into the NAc also increased anxiety behavior as measured by the elevated plus maze test (Figure 4). Mice that had previously received mefloquine coupled to subthreshold social defeat stress trended towards less time in the open arms (Student’s t-test, p = 0.13; Figure 4a) and entered the open arms less frequently than vehicle-infused mice (Student’s t-test, p<0.01; Figure 4b). Mefloquine-infused mice also spent more time in the closed arms (Student’s t-test, p<0.01; Figure 4c). Overall, these data show that a single infusion of mefloquine into the NAc produces both depression- and anxiety-like behaviors.
Figure 4.
Direct infusion of mefloquine into nucleus accumbens coupled to stress is anxiogenic. (a) Stressed mice that received direct infusion of mefloquine into nucleus accumbens trend towards less time in the open arms (tp=0.13, Student’s t-test, n = 6–7 mice/group). (b) Drug-treated animals display decreased frequency of open arm entries (*p<0.01, Student’s t-test, n=6–7 mice/group). (c) Drug-treated animals spend greater time in the closed arms of the elevated plus maze (*p<0.01, Student’s t-test, n=6–7 mice/group) and (d) have a lower number of closed arm entries (*p<0.05, Student’s t-test, n=6–7mice/group). (e) Representative heat maps. Data are shown as means. Error bars represent SEM.
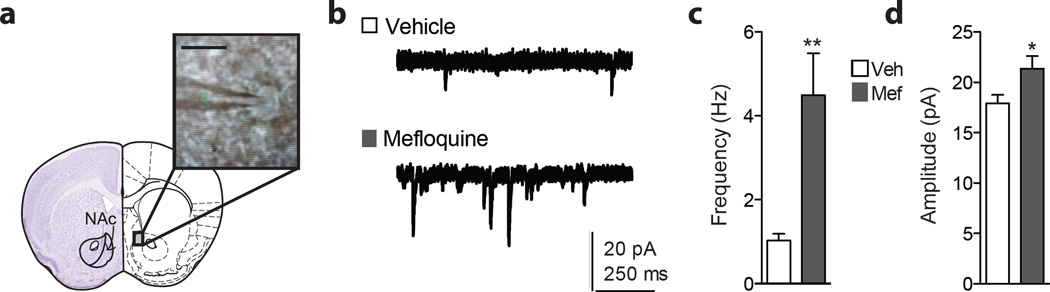
To assess whether mefloquine alters synaptic plasticity in the NAc, which we have shown previously to regulate stress-induced depression-like behavior (10, 11), we performed whole cell electrophysiological recordings from NAc MSNs following bath application of mefloquine (Figure 5). Mefloquine or DMSO as vehicle was added to the bath solution for 1 hour prior and perfused throughout recordings, using a protocol based on studies using mefloquine in slice electrophysiology (23, 29). We observed a significant increase in sEPSC frequency on NAc MSNs in the mefloquine-treated cells (Student’s t-test, p<0.01; Figure 5c). There was an additional increase in sEPSC amplitude compared to vehicle cells (Student’s t-test, p<0.05; Figure 5d), suggesting an increase in the strength and number of presynaptic excitatory events following mefloquine exposure.
Figure 5.
(a) Schematic depicting whole cell recordings from NAc shell medium spiny neurons. Scale bar: 20 µm. (b) Representative traces. Scale: 20 pA, 250 ms. (c) Cells from slices incubated in 25 µM mefloquine display increased sEPSC frequency compared to DMSO-vehicle (**p<0.01, Student’s t-test, n = 9 cells per group). (d) An additional increase in sEPSC amplitude was observed (*p>0.05, Student’s t-test, n = 9 cells per group). Data are represented as means. Error bars represent SEM. Atlas image modified from Paxinos and Franklin, 2001.
DISCUSSION
Mefloquine remains a widely prescribed treatment for malaria despite adverse psychiatric outcomes (6). It is also one of the antimalarial drugs approved for use by pregnant women and infants (30, 31). However, there is controversy regarding mefloquine’s safety and the prevalence of adverse effects (5, 32, 33). Using a preclinical model of psychiatric illness, we show that a single intraperitoneal injection of mefloquine is sufficient to produce social avoidance behavior induced by social defeat stress. This effect was only observed at the higher dose used for malaria treatment and not at a prophylactic dose. Since long-term prophylaxis is associated with psychiatric episodes, it is possible repeated exposure to the lower dose is needed to promote social avoidance. In addition, we show that the NAc is an important site of action for the pro-depressant and anxiogenic effects of mefloquine, since direct NAc infusion promoted these behaviors while ventral hippocampal infusion had no effect. Whole cell electrophysiological recordings suggest that mefloquine exposure induces functional alterations in synaptic transmission on NAc MSNs.
Changes in excitatory synaptic transmission in the NAc have been shown to underlie stress susceptibility and the manifestation of depression-like social avoidance and anxiety-like behaviors (10, 11, 16, 18). These previous studies showed that mice displaying social avoidance behavior exhibited an increase in the number of excitatory synapses in the NAc and pruning of these newly formed synapses was sufficient to reverse social avoidance. Here we observe that mefloquine increases sEPSC frequency and amplitude on NAc MSNs, pointing either to an increase in presynaptic glutamate release or the strength of excitatory synapses on NAc MSNs. In line with this hypothesis, previous electrophysiological studies have found that bath application of mefloquine increases spontaneously occurring activity in cortical cells (23). One likely mechanism of action may involve altered calcium homeostasis, since mefloquine has been shown to increase intracellular calcium (29, 34). Increased calcium could lead to an increase in sEPSC frequency by promoting synaptic vesicle release. Future studies will expand upon these correlative findings to determine whether altered excitatory synaptic transmission in the NAc as a result of mefloquine exposure promotes depression- and anxiety-like behavior.
Interestingly, recurring depression was observed years after taking mefloquine for malaria prophylaxis (3). Together with our data, this suggests that mefloquine might induce an alteration of neurocircuitry, potentially involving dysregulation of the NAc and mesolimbic reward circuits (35). Alternatively, patients with a history of major depressive disorder may be at higher risk of developing psychiatric side effects of mefloquine (8) because the drug exacerbates preexisting neurocircuitry deficits in reward processing. Further studies are needed to investigate the mechanism and time course underlying mefloquine-induced depression effects in the NAc.
Currently, mefloquine is still used for the treatment and prophylaxis of malaria (36). Thus, it is important that we gain a greater understanding of the drug’s mechanism at a preclinical level, which will better inform clinical practice and potentially identify new strategies to prevent or reduce psychiatric side effects. In addition, a greater understanding of the mechanisms and brain regions involved in mefloquine-induced depression and anxiety will provide insights into depression pathophysiology in general, leading to identification of novel targets for therapeutic development.
Supplementary Material
Supplementary Figure 1. There is no effect of mefloquine on baseline motor behavior as assessed during the target absent trial of the social interaction test. (a) Distance traveled and (b) mean velocity of animals that received intraperitoneal injection of either vehicle or 20 mg/kg mefloquine coupled to stress. (c) Distance traveled and (d) mean velocity of animals that received intraperitoneal injection of either vehicle or 5 mg/kg mefloquine coupled to stress. (e) Distance traveled and (f) mean velocity of animals that received intra- NAc infusion of either vehicle or mefloquine coupled to stress. (g) Distance traveled and (h) mean velocity of animals that received intra-hippocampal infusion of either vehicle or mefloquine coupled to stress. Data are represented as group means. Error bars represent SEM (p>0.05, Student’s t-test, n = 6–10 mice/group).
Highlights.
Mefloquine coupled to social defeat stress caused social avoidance behavior
Social avoidance and anxiety was seen after infusion into nucleus accumbens
Mefloquine infusion into ventral hippocampus did not affect social interaction
These findings provide a basis to study the neuropsychiatric effects of mefloquine
Acknowledgments
FUNDING
This research was supported by US National Institute of Mental Health and National Center for Complementary and Integrative Health Grants: RO1MH090264 and 1P50AT008661-01 (to S.J.R.), T32 MH087004 (to M.L.P. and M.H.), T32MH096678 (to M.L.P.) and 5F30MH100835 (to M.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Caillon E, Schmitt L, Moron P. Acute depressive symptoms after mefloquine treatment. The American journal of psychiatry. 1992;149(5):712. doi: 10.1176/ajp.149.5.712a. [DOI] [PubMed] [Google Scholar]
- 2.Croft AM, World MJ. Neuropsychiatric reactions with mefloquine chemoprophylaxis. Lancet. 1996;347(8997):326. doi: 10.1016/s0140-6736(96)90500-0. [DOI] [PubMed] [Google Scholar]
- 3.Whitworth AB, Aichhorn W. First-time diagnosis of severe depression: induced by mefloquine? Journal of clinical psychopharmacology. 2005;25(4):399–400. doi: 10.1097/01.jcp.0000169619.07325.ff. [DOI] [PubMed] [Google Scholar]
- 4.FDA requires warnings on anti-malaria drug Lariam. Consumer reports. 2004;69(1):45. [PubMed] [Google Scholar]
- 5.Chen LH, Wilson ME, Schlagenhauf P. Controversies and misconceptions in malaria chemoprophylaxis for travelers. Jama. 2007;297(20):2251–2263. doi: 10.1001/jama.297.20.2251. [DOI] [PubMed] [Google Scholar]
- 6.Jacquerioz FA, Croft AM. Drugs for preventing malaria in travellers. The Cochrane database of systematic reviews. 2009;4:CD006491. doi: 10.1002/14651858.CD006491.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Nevin RL. Mefloquine prescriptions in the presence of contraindications: prevalence among US military personnel deployed to Afghanistan, 2007. Pharmacoepidemiology and drug safety. 2010;19(2):206–210. doi: 10.1002/pds.1879. [DOI] [PubMed] [Google Scholar]
- 8.Peterson AL, Seegmiller RA, Schindler LS. Severe neuropsychiatric reaction in a deployed military member after prophylactic mefloquine. Case reports in psychiatry. 2011;2011:350417. doi: 10.1155/2011/350417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringqvist A, Bech P, Glenthoj B, Petersen E. Acute and long-term psychiatric side effects of mefloquine: A follow-up on Danish adverse event reports. Travel medicine and infectious disease. 2015;13(1):80–88. doi: 10.1016/j.tmaid.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(1):314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, et al. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nature neuroscience. 2015;18(7):962–964. doi: 10.1038/nn.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenfeld TJ, Kloth AD, Hsueh B, Runkle MB, Kane GA, Wang SS, et al. Gap junctions in the ventral hippocampal-medial prefrontal pathway are involved in anxiety regulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(47):15679–15688. doi: 10.1523/JNEUROSCI.3234-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bissiere S, Zelikowsky M, Ponnusamy R, Jacobs NS, Blair HT, Fanselow MS. Electrical synapses control hippocampal contributions to fear learning and memory. Science. 2011;331(6013):87–91. doi: 10.1126/science.1193785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karbwang J, White NJ. Clinical pharmacokinetics of mefloquine. Clin Pharmacokinet. 1990;19(4):264–279. doi: 10.2165/00003088-199019040-00002. [DOI] [PubMed] [Google Scholar]
- 15.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65(2):257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christoffel DJ, Golden SA, Heshmati M, Graham A, Birnbaum S, Neve RL, et al. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(12):2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, et al. beta-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. 2014;516(7529):51–55. doi: 10.1038/nature13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nature medicine. 2013;19(3):337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, et al. A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(25):9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 22.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nature protocols. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(33):12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison DW, Wilcox RS, Ellefsen KL, Askew CE, Hansen DM, Wilcox JD, et al. Mefloquine effects on ventral tegmental area dopamine and GABA neuron inhibition: a physiologic role for connexin-36 GAP junctions. Synapse. 2011;65(8):804–813. doi: 10.1002/syn.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlagenhauf P, Adamcova M, Regep L, Schaerer MT, Bansod S, Rhein HG. Use of mefloquine in children - a review of dosage, pharmacokinetics and tolerability data. Malaria journal. 2011;10:292. doi: 10.1186/1475-2875-10-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dow G, Bauman R, Caridha D, Cabezas M, Du F, Gomez-Lobo R, et al. Mefloquine induces dose-related neurological effects in a rat model. Antimicrobial agents and chemotherapy. 2006;50(3):1045–1053. doi: 10.1128/AAC.50.3.1045-1053.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biological psychiatry. 2010;67(2):110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(2):368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 29.Caridha D, Yourick D, Cabezas M, Wolf L, Hudson TH, Dow GS. Mefloquine-induced disruption of calcium homeostasis in mammalian cells is similar to that induced by ionomycin. Antimicrobial agents and chemotherapy. 2008;52(2):684–693. doi: 10.1128/AAC.00874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radeva-Petrova D, Kayentao K, ter Kuile FO, Sinclair D, Garner P. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. The Cochrane database of systematic reviews. 2014;10:CD000169. doi: 10.1002/14651858.CD000169.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gosling RD, Gesase S, Mosha JF, Carneiro I, Hashim R, Lemnge M, et al. Protective efficacy and safety of three antimalarial regimens for intermittent preventive treatment for malaria in infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374(9700):1521–1532. doi: 10.1016/S0140-6736(09)60997-1. [DOI] [PubMed] [Google Scholar]
- 32.Schneider C, Adamcova M, Jick SS, Schlagenhauf P, Miller MK, Rhein HG, et al. Antimalarial chemoprophylaxis and the risk of neuropsychiatric disorders. Travel medicine and infectious disease. 2013;11(2):71–80. doi: 10.1016/j.tmaid.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Toovey S. Mefloquine neurotoxicity: a literature review. Travel medicine and infectious disease. 2009;7(1):2–6. doi: 10.1016/j.tmaid.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Dow GS, Hudson TH, Vahey M, Koenig ML. The acute neurotoxicity of mefloquine may be mediated through a disruption of calcium homeostasis and ER function in vitro. Malaria journal. 2003;2:14. doi: 10.1186/1475-2875-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews Neuroscience. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlagenhauf P, Adamcova M, Regep L, Schaerer MT, Rhein HG. The position of mefloquine as a 21st century malaria chemoprophylaxis. Malaria journal. 2010;9:357. doi: 10.1186/1475-2875-9-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. There is no effect of mefloquine on baseline motor behavior as assessed during the target absent trial of the social interaction test. (a) Distance traveled and (b) mean velocity of animals that received intraperitoneal injection of either vehicle or 20 mg/kg mefloquine coupled to stress. (c) Distance traveled and (d) mean velocity of animals that received intraperitoneal injection of either vehicle or 5 mg/kg mefloquine coupled to stress. (e) Distance traveled and (f) mean velocity of animals that received intra- NAc infusion of either vehicle or mefloquine coupled to stress. (g) Distance traveled and (h) mean velocity of animals that received intra-hippocampal infusion of either vehicle or mefloquine coupled to stress. Data are represented as group means. Error bars represent SEM (p>0.05, Student’s t-test, n = 6–10 mice/group).