Abstract
In recent years, use of psychoactive synthetic stimulants has grown rapidly. 5-(2-Aminopropyl)indole (5-IT) is a synthetic drug associated with a number of fatalities, that appears to be one of the newest 3,4-methylenedioxymethamphetamine (MDMA) replacements. Here, the monoamine-releasing properties of 5-IT, its structural isomer 6-(2-aminopropyl)indole (6-IT), and MDMA were compared using in vitro release assays at transporters for dopamine (DAT), norepinephrine (NET), and serotonin (SERT) in rat brain synaptosomes. In vivo pharmacology was assessed by locomotor activity and a functional observational battery (FOB) in mice. 5-IT and 6-IT were potent substrates at DAT, NET, and SERT. In contrast with the non-selective releasing properties of MDMA, 5-IT displayed greater potency for release at DAT over SERT, while 6-IT displayed greater potency for release at SERT over DAT. 5-IT produced locomotor stimulation and typical stimulant effects in the FOB similar to those produced by MDMA. Conversely, 6-IT increased behaviors associated with 5-HT toxicity. 5-IT likely has high abuse potential, which may be somewhat diminished by its slow onset of in vivo effects, whereas 6-IT may have low abuse liability, but enhanced risk for adverse effects. Results indicate that subtle differences in the chemical structure of transporter ligands can have profound effects on biological activity. The potent monoamine-releasing actions of 5-IT, coupled with its known inhibition of MAO A, could underlie its dangerous effects when administered alone, and in combination with other monoaminergic drugs or medications. Consequently, 5-IT and related compounds may pose substantial risk for abuse and serious adverse effects in human users.
Keywords: 5-(2-Aminopropyl)indole (5-IT); 6-(2-Aminopropyl)indole (6-IT); 3,4-Methylenedioxymethamphetamine (MDMA); Locomotor activity; Monoamine releaser; Synthetic stimulants
1.0 Introduction
In recent years, the non-medical use of products containing psychoactive synthetic stimulants has grown rapidly (Baumann et al., 2013a, 2014; EMCDDA, 2014; UNODC, 2014). A range of stimulant classes has appeared in the recreational drug marketplace, including synthetic cathinones, piperazines, and phenethylamines (EMCDDA, 2013b; Hill and Thomas, 2011; Rosenbaum et al., 2012; Shanks et al., 2012). Many of the newer synthetic stimulants have been marketed as substitutes for 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”) including piperazines such as m-chlorophenylpiperazine and benzylpiperazine (BZP), and various synthetic cathinones (Bossong et al., 2010; Brunt et al., 2011, 2012; EMCDDA, 2013b; German et al., 2014). High doses or chronic use of synthetic stimulants can produce adverse effects including agitation, delusions, violent behaviors, cardiovascular stimulation, hyperthermia, organ damage, and even death (EMCDDA, 2014; Miotto et al., 2013; Rosenbaum et al., 2012). More than 200 new psychoactive substances have been identified in Europe and the United States, with new compounds discovered weekly (Iversen et al., 2014). In 2014 alone, 31 new synthetic stimulants were reported in Europe and added to the growing list monitored by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (EMCDDA, 2015).
Synthetic stimulants produce effects that are similar to those of prototypical stimulants like amphetamine and cocaine. BZP and synthetic cathinones from the methcathinone structural class induce the transporter-mediated release of dopamine (DA), norepinephrine (NE), and serotonin (5-HT), similar to the actions of methamphetamine and MDMA. By comparison, synthetic cathinones from the pyrovalerone structural class block transporter-mediated uptake of DA, NE, and 5-HT, similar to the actions of cocaine (Baumann et al., 2004, 2012, 2013b; Eshleman et al., 2013; Marusich et al., 2014; Simmler et al., 2013). Behavioral studies in rodents have shown that BZP and synthetic cathinones increase locomotor activity and are self-administered, indicating a propensity for abuse and dependence (Aarde et al., 2013a, 2013b; 2015; Creehan et al., 2015; Fantegrossi et al., 2005, 2008, 2013; López-Arnau et al., 2013; Marusich et al., 2012, 2014; Watterson et al., 2012, 2014; Yarosh et al., 2007).
5-(2-Aminopropyl)indole (5-API or 5-IT) is a new synthetic compound that emerged in 2012 (EMCDDA, 2013a) and gained attention due to its intoxicating and life-threatening adverse effects (Coppola and Mondola, 2013; EMCDDA, 2014). Specifically, a cluster of fifteen fatalities associated with the misuse of 5-IT was reported in Sweden in 2012 (Seetohul and Pounder, 2013). Forensic and pathological investigation of these deaths revealed that a majority involved the presence of 5-IT in combination with other drugs of abuse or prescribed medications, whereas only two cases involved 5-IT alone. Reports of non-fatal intoxications with 5-IT are consistent with a sympathomimetic toxidrome similar to the effects of prototypic stimulant drugs (Bäckberg et al., 2014; EMCDDA, 2014; Seetohul and Pounder 2013). 5-IT, originally synthesized as a stimulant in the 1960s (Hofmann and Troxler 1963; Troxler et al. 1968), now joins the list of psychoactive substances being diverted and sold for recreational purposes, and may to be one of the newest MDMA replacements (EMCDDA, 2013a, 2014; Seetohul and Pounder, 2013). 5-IT is sold as “research chemicals,” “benzo fury,” and “ecstasy” indicating that it is used for both stimulant and MDMA-like entactogenic effects (Bäckberg et al., 2014; EMCDDA, 2013a, 2014; Seetohul and Pounder, 2013).
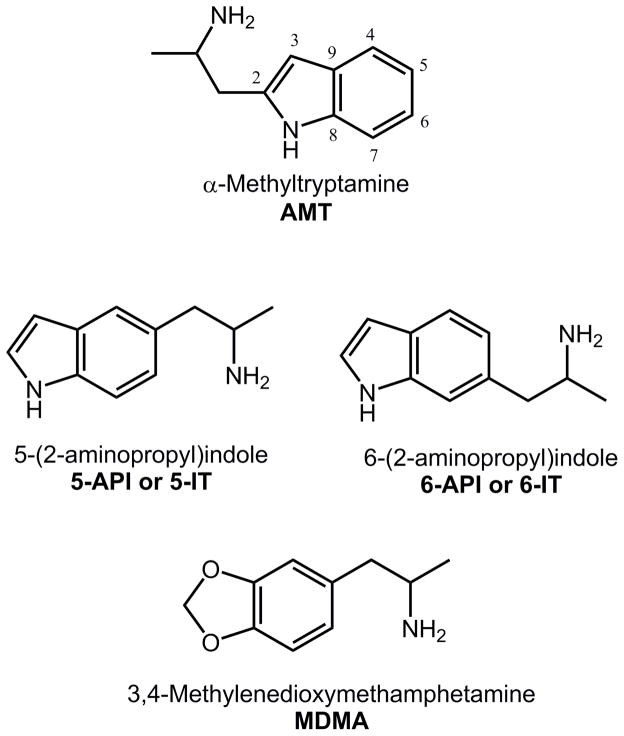
Only a few scientific articles (Banks et al., 2014; Herraiz and Brandt, 2014), reports from the EMCDDA (EMCDDA, 2014), and anecdotal reports from online drug user fora (http://www.bluelight.org/vb/threads/616728-The-Big-amp-Dandy-5-IT-5-API-Thread) are available with regard to the pharmacology of 5-IT or its structural isomer 6-(2-aminopropyl)indole (6-API or 6-IT). 5-IT and 6-IT are synthetic indoles, and positional isomers of α-methyltryptamine (AMT), with structural similarity to MDMA (see Figure 1). Importantly, 5-IT and 6-IT contain the substructure of α-methylphenethylamine (i.e., amphetamine), suggesting the compounds may have stimulant or hallucinogenic properties (EMCDDA, 2014; Glennon, 2014; Rothman and Baumann, 2003). Older research shows that 6-IT is more potent than 5-IT with respect to antagonizing the pro-convulsant effects of reserpine, and inhibiting the activity of monoamine oxidase (Cerletti et al., 1968). More recent studies confirm that 5-IT acts as a selective, reversible and competitive inhibitor of monoamine oxidase A (MAO A) (Herraiz and Brandt, 2014), and 6-IT was shown to evoke release of DA, NE and 5-HT (Banks et al., 2014). Here, we compared the monoamine-releasing properties of 5-IT, 6-IT, and MDMA using in vitro release assays for DA transporters (DAT), NE transporters (NET) and 5-HT transporters (SERT) in rat brain synaptosomes (Baumann et al., 2012, 2013b). The in vivo pharmacology of these same compounds was assessed by measuring locomotor activity and effects in a functional observational battery (FOB) in mice (Marusich et al., 2012, 2014).
Figure 1.
Chemical structures of AMT, 5-IT, 6-IT, and MDMA.
2.0 Methods and Materials
2.1 Subjects
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing 300–400 g (total n=28) were housed three per cage. Adult male ICR mice (Harlan, Frederick, MD, USA) weighing 30–55 g (total n= 104) were housed individually. Animals were housed in polycarbonate cages with hardwood bedding. All animals were drug and test naïve, and were housed in temperature-controlled conditions (20–24°C) with a 12 h standard light-dark cycle. Animals had ad libitum access to food and water in their home cages at all times. Rat experiments were approved by the Institutional Animal Care and Use Committee at NIDA IRP, while mouse experiments were approved by the Institutional Animal Care and Use Committee at Mispro Biotech. All research was conducted as humanely as possible, and followed the principles of laboratory animal care (National Research Council, 2011). The authors consulted the ARRIVE guidelines for reporting experiments involving animals, and all efforts were made to minimize animal suffering, reduce the number of animals used, and utilize alternatives to in vivo techniques, if available.
2.2 Drugs
Racemic 5-(2-Aminopropyl)indole (5-IT) and 6-(2-Aminopropyl)indole (6-IT) were synthesized and characterized following established procedures (Elliott et al. 2013; Scott et al. 2014) and were > 97% pure. Racemic 3,4-methylenedioxy-N-methylamphetamine HCl (MDMA) was purchased from Cayman Chemical (Ann Arbor, MI, USA). For in vitro assays, 10 mM stock solutions of 5-IT, 6-IT, and MDMA were prepared in DMSO and frozen. Drug dilutions were prepared in assay buffer using aliquots of stock solutions. For in vivo studies, 5-IT and 6-IT were suspended in a vehicle of 7.8 % tween 80 N.F. (VWR, Radnor, PA) and 92.2% sterile saline USP (Butler Schein, Dublin, OH). MDMA was dissolved in sterile saline. Doses are expressed as mg/kg of the salt, and were administered at a volume of 10 ml/kg in mice. Sterile saline (100%) and 7.8 % tween 80 + 92.2% sterile saline were used as comparisons for all drugs for in vivo studies.
2.3 In Vitro Transporter Release Assays
Rats were euthanized by CO2 narcosis, and brains were processed to yield synaptosomes as previously described (Baumann et al., 2013b; Rothman et al., 2003). Synaptosomes were prepared from rat striatum for the DAT assays, whereas synaptosomes were prepared from whole brain minus striatum and cerebellum for the NET and SERT assays. For release assay procedures, 9 nM [3H]1-methyl-4-phenylpyridinium ([3H]MPP+) was used as the radiolabeled substrate for DAT and NET, while 5 nM [3H]serotonin was used as a substrate for SERT. All buffers used in the release assay methods contained 1 μM reserpine to block vesicular uptake of substrates. The selectivity of release assays was optimized for a single transporter by including unlabeled blockers to prevent the uptake of [3H]MPP+ or [3H]serotonin by competing transporters. Synaptosomes were preloaded with radiolabeled substrate in Krebs-phosphate buffer for 1 h (steady state). Release assays were initiated by adding 850 μl of preloaded synaptosomes to 150 μl of test drug; incubations were allowed to proceed for 30 min for DAT and NET assays, or 5 min for SERT assays. Release was terminated by vacuum filtration and retained radioactivity was quantified by liquid scintillation counting.
2.4 Apparatus for Behavioral Testing
Mouse locomotor activity was assessed in clear Plexiglass open field activity chambers measuring 47×25.5×22 cm. San Diego Instruments Photobeam Activity System software (model 2325-0223, San Diego, CA, USA) was used to calculate beam breaks. Each chamber contained two 4×8 beam infrared arrays that monitored horizontal movement. The FOB was conducted during handling, and in a clear Plexiglas open field measuring 47×25.5×22 cm.
2.5 Locomotor Activity and FOB
Mice were randomly assigned to receive a single dose of a particular drug, saline vehicle, or tween 80 vehicle (n=8 per group), and the same cohort of mice was used for all behavioral assessments. Locomotor activity was quantified by an automated system which provides a general measure of movement in the horizontal plane across time. Locomotor activity tests were conducted during week 1. The FOB consisted of observations by a trained technician and was conducted during week 2. In total, each mouse was given 2 administrations of drug or vehicle, with a minimum 6-day wash out between each administration. All compounds were administered intraperitoneally (i.p.), and doses were chosen based on in vitro research from the present study.
For initial locomotor assessments (week 1), mice received their assigned drug dose and immediately thereafter were placed individually into locomotor activity chambers for a 90-min test, conducted by a technician who was blind to treatment. Doses examined were saline vehicle, tween vehicle, 3.0–30.0 mg/kg MDMA, and 1.0–10.0 mg/kg 5-IT and 6-IT.
An FOB (week 2), modified from a procedure commonly used by the Environmental Protection Agency (U.S. Environmental Protection Agency, 1998a, 1998b), was used to classify observable effects of the drugs, as determined 20 min post-injection. This assay allowed for assessment of a wide range of drug effects, and provided an overall behavioral profile for each compound, with an emphasis on detection of potential safety concerns. All measures were scored using an ordinal scale, with 1 = normal/no drug effect, 2 = minor-moderate drug effect, and 3 = major drug effect. Methods were similar to those used in our previous studies (Marusich et al., 2012; 2014). Doses examined were 3.0–30.0 mg/kg MDMA, 3.0–30.0 mg/kg 5-IT, 1.0–5.6 mg/kg 6-IT, saline vehicle, and tween vehicle (n=8 for all doses except tween vehicle n=16 and 5.6 mg/kg 6-IT n=7). FOBs were scored by a trained technician who was blind to treatment. Dependent measures included ataxia, bizarre behavior (e.g. jumping, rearing while facing away from wall), circular ambulations, convulsions, ejaculation, exploration (e.g. excessive sniffing or reorienting of the head), flattened body posture, forepaw treading, grooming, hind limb splay, hyperactivity, hypoactivity, muscle relaxation, piloerection, retropulsion, salivation, self-injury, stereotyped biting, stereotyped head circling, stereotyped head weaving, stereotyped licking, stimulation (e.g. increased heart rate, tense body), Straub tail, and tremor.
2.6 Data Analysis
For in vitro release assays, statistical analyses were carried out using GraphPad Prism (v. 5.0; GraphPad Scientific, San Diego, CA, USA). EC50 values for stimulation of release were calculated based on non-linear regression analysis. The EC50 value for release was used as a measure of substrate potency because previous findings have shown that transporter-mediated release of the [3H]transmitter is directly coupled to the movement of substrate drug molecules through the channel of the transporter (Reith et al., 2015; Sitte and Freissmuth, 2015). For in vivo assays, statistical analyses were conducted using NCSS (2004; Number Cruncher Statistical Systems, Kaysville, Utah, USA). To facilitate comparison, data from the tween 80 vehicle group was included in graphs and analyses for both 5-IT and 6-IT, and data from the saline vehicle group was included in graphs and analyses for MDMA. Locomotor activity was expressed as total beam breaks per 10-min bin. Mixed model analysis of variance (ANOVA) was used to analyze dose effect and time course locomotor data, with time as the within-subject factor and dose as the between-subject factor. All tests were considered significant at p<0.05.
FOB data were analyzed with Kruskal–Wallis one-way ANOVA by ranks for each dependent measure, corrected for ties. No dose of any drug produced convulsions, ejaculation, muscle relaxation, self-injury, or stereotyped biting; therefore, these measures were not analyzed. Measures were grouped into four domains, and the alpha level was controlled within each domain: CNS activity (α=0.0125; exploration, grooming, hyperactivity, hypoactivity), CNS excitability (α=0.0056; bizarre behavior, circular ambulations, retropulsion, stereotyped head circling, stereotyped head weaving, stereotyped licking, stimulation, Straub tail, tremor), autonomic effects (α=0.025; piloerection, salivation), and muscle tone/equilibrium (α=0.0125; ataxia, flattened body posture, forepaw treading, hindlimb splay) (Bowen et al., 1996). When ANOVAs revealed significant main effects or interactions, Tukey’s post hoc test was used to determine differences between group means.
3.0 Results
3.1 In Vitro Transporter Assays
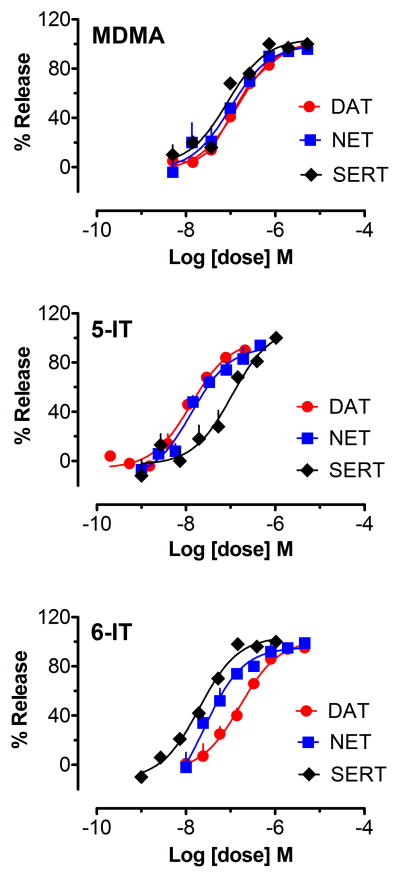
Values for the potency (i.e., EC50) of test drugs to stimulate release at DAT, NET and SERT are summarized in Table 1, with data for amphetamine provided as a comparison, while dose-response curves for stimulation of release activity are depicted in Figure 2. Amphetamine was previously shown to be a potent and selective releaser of [3H]MPP+ at DAT and NET (Baumann et al., 2013b), whereas MDMA was a non-selective releaser at DAT, NET and SERT. 5-IT was more potent at releasing [3H]MPP+ via DAT when compared to its effects at SERT (Fig. 2), with a DAT/SERT ratio indicating 8-fold selectivity for DAT-mediated release. By contrast, 6-IT was more potent at releasing via SERT when compared to its effects at DAT (Fig. 2), with a DAT/SERT ratio indicating a 10-fold selectivity for SERT-mediated release. It is noteworthy that 5-IT was more potent than MDMA as a DAT substrate, whereas 6-IT was more potent than MDMA as a SERT substrate. Finally, all of the test drugs were capable of full efficacy releasing activity.
Table 1.
Effects of amphetamine, MDMA, 5-IT and 6-IT on the release of [3H]MPP+ from DAT and NET, and [3H]5-HT from SERT in rat brain synaptosomes. Data are expressed as nM concentrations (mean±SD) for n=3 experiments performed in triplicate.
| Test Drug | [3H]MPP+ release, EC50 at DAT nM | [3H]MPP+ release, EC50 at NET nM | [3H]5-HT release, EC50 at SERT nM | DAT/SERT ratioa |
|---|---|---|---|---|
| Amphetamineb | 5.8±0.4 | 6.6±0.7 | 698±71 | 120 |
| MDMA | 143.1±10.2 | 98.3±15.0 | 85.0±13.3 | 0.6 |
| 5-IT | 12.9±1.5 | 13.3±1.8 | 104.8±18.2 | 8 |
| 6-IT | 164.0±15.2 | 25.6±4.2 | 19.9±1.6 | 0.1 |
DAT/SERT ratio was determined by [DAT EC50]−1/[SERT EC50]−1 such that higher numerical value equals greater DAT selectivity
Amphetamine data from Baumann et al. (2013b)
Figure 2.
Effects of test drugs on release of [3H]MPP+ via DAT and NET, and [3H]5-HT via SERT. Data are mean±SD expressed as a % of maximal release response for n=3 experiments performed in triplicate.
3.2 Locomotor Dose Response and Time Course
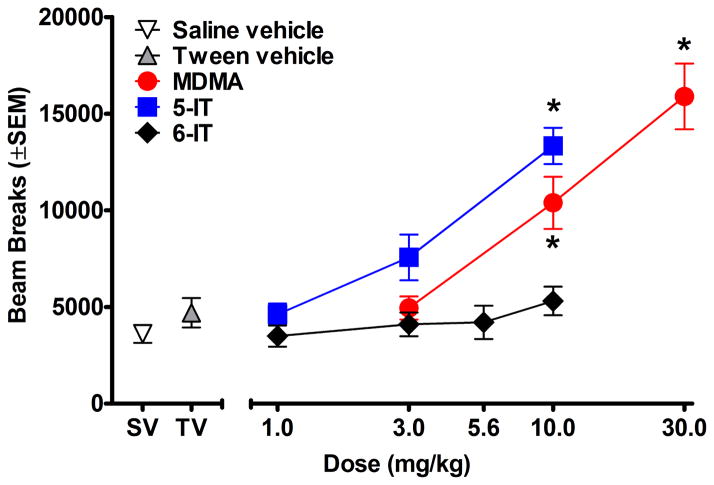
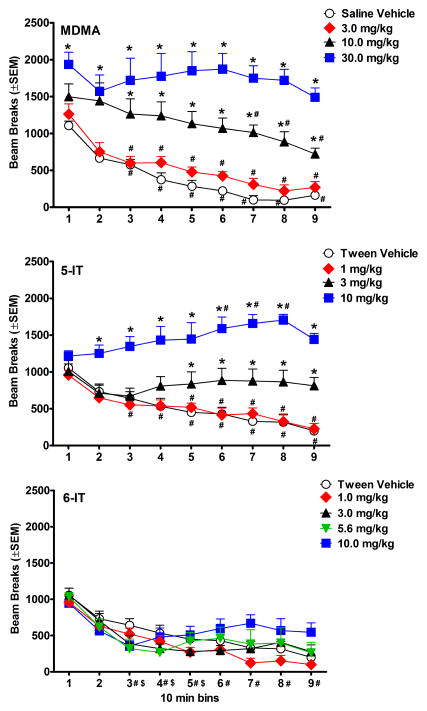
Figures 3 and 4 show the effects of test compounds on locomotor activity. As depicted in Figure 3, MDMA and 5-IT produced dose dependent increases in beam breaks [MDMA:F(3, 32)=23.95, p<0.001; 5-IT:F(3, 32)=21.04, p<0.001]. All compounds showed an effect of bin [MDMA:F(8, 224)=31.14, p<0.001; 5-IT:F(8, 224)=9.10, p<0.001; 6-IT:F(8, 280)=42.58, p<0.001] and an interaction between dose and bin [MDMA:F(8, 224)=3.54, p<0.001; 5-IT:F(8, 224)=8.36, p<0.001; 6-IT:F(8, 280)=2.24, p<0.001] (Fig. 4). For MDMA and 5-IT, the dose by time interaction was dose dependent. Higher doses increased beam breaks for a greater number of bins compared to lower doses. Similarly, higher doses of MDMA showed less attenuation across the session. Unlike many of the doses studied here, 10.0 mg/kg 5-IT produced a slow increase in beam breaks during much of the session with a significant increase in beam breaks during 60–80 min post-injection compared to the first 10 min bin. Only 1.0 mg/kg 5-IT showed attenuation in beam breaks over time. 6-IT did not produce a significant difference in beam breaks compared to tween vehicle; however, all doses produced some attenuation in beam breaks over time (Fig. 4).
Figure 3.
Effects of test drugs on cumulative locomotor activity during 90 min sessions, plotted as a function of dose. * represents doses that produced significant increases in beam breaks compared to vehicle. SV stands for saline vehicle and TV stands for tween vehicle.
Figure 4.
Time course of locomotor activity, plotted as a function of 10 min bins. Values represent mean±SEM expressed as number of beam breaks for each dose (n=8 per dose). * indicates significant increases in beam breaks compared to vehicle at the same time point. # represents significant attenuation of the effect of the dose compared to its first 10 min bin. The top panel shows data for MDMA, middle panel shows data for 5-IT, and lower panel shows data for 6-IT. For 6-IT, # on the x axis indicates significant attenuation compared to the first 10 min bin for 1.0–5.6 mg/kg, and $ indicates significant attenuation compared to the first 10 min bin for 10.0 mg/kg.
Doses of 5.6–10.0 mg/kg 6-IT led to adverse effects indicative of 5-HT toxicity including salivation, tremor, hind limb splay, Straub tail, and flattened body posture that were noted after the locomotor session or the following day. One mouse administered 5.6 mg/kg and two mice administered 10.0 mg/kg 6-IT were euthanized due to the severity of these effects. Their locomotor data were included in graphs and analyses due to the similarity to data from other subjects given the same doses.
3.3 Functional Observational Battery
Results of significant drug effects for the FOB are displayed in Table 2 along with data for methamphetamine from a previous study for comparison (Marusich et al., 2012). Within the CNS activity domain, all compounds produced significant increases in exploration compared to vehicle [MDMA: H(3)=25.76, p<0.0125; 5-IT:H(3)=11.83, p<0.0125; 6-IT:H(3)=12.01, p<0.0125]. MDMA also increased hyperactivity [H(3)=23.41, p<0.0125].
Table 2.
Effects of MDMA, 5-IT, 6-IT, and methamphetamine (METH) in the FOB. Doses (mg/kg) that produced a significant difference from vehicle are shown in columns with the corresponding average percent of vehicle shown in parenthesis. The corrected alpha level for each domain is noted in parenthesis in the first column. For comparison purposes, doses at which beam breaks were significantly increased during the second 10-min bin of the locomotor activity sessions are also shown. Only behaviors that were significantly affected by at least one drug are included.
| Dependent variable | MDMA | 5-IT | 6-IT | METHa |
|---|---|---|---|---|
| Beam Breaks (10–20 min) | 10.0 (217) 30.0 (236) |
10.0 (171) | 1.0 (419) 3.0 (469) 5.6 (284) |
|
| CNS Activity (α=0.0125) | ||||
| Exploration | 10.0 (238) 30.0 (300) |
30.0 (157) | 5.6 (179) | |
| Hyperactivity | 10.0 (200) 30.0 (240) |
1.0 (180) 3.0 (220) 10.0 (230) |
||
| CNS Excitability (α=0.0056) | ||||
| Circular Ambulations | 10.0 (173) 30.0 (200) |
1.0 (250) 3.0 (225) 10.0 (250) |
||
| Stereotyped Head Circling | 10.0 (178) | 1.0 (238) 3.0 (250) 10.0 (300) |
||
| Stereotyped Head Weaving | 10.0 (250) 30.0 (275) |
10.0 (142) 30.0 (150) |
1.0 (175) 3.0 (175) 10.0 (263) |
|
| Stimulation | 10.0 (175) 30.0 (200) |
1.0 (238) 3.0 (288) 10.0 (300) |
||
| Autonomic Effects (α=0.025) | ||||
| Piloerection | 3.0 (150) 30.0 (220) |
3.0 (160) | 5.6 (174) | * |
| Salivation | 30.0 (225) | 10.0 (238) | ||
| Muscle tone/Equilibrium (α=0.0125) | ||||
| Flattened Body Posture | 10.0 (156) 30.0 (178) |
|||
| Forepaw Treading | 30.0 (213) | 10.0 (180) | * |
Methamphetamine data from Marusich et al. (2012).
Forepaw treading and piloerection were not measured in the previous study.
For the CNS excitability domain, MDMA increased circular ambulations [H(3)=18.02, p<0.0056]. MDMA [H(3)=24.93, p<0.0056] and 5-IT [H(3)=13.71, p<0.0056] elevated stereotyped head weaving. 5-IT also increased stereotyped head circling [H(3)=13.62, p<0.0056]. Finally, MDMA elevated stimulation [H(3)=22.93, p<0.0056].
In the autonomic effects domain, MDMA [H(3)=19.20, p<0.025], 5-IT [H(3)=13.93, p<0.025], 6-IT [H(3)=16.98, p<0.025] increased piloerection compared to vehicle. Furthermore, MDMA increase salivation [H(3)=21.26, p<0.025]. For muscle tone/equilibrium effects, MDMA elevated flattened body posture [H(3)=15.16, p<0.0125], and MDMA [H(3)=16.65, p<0.0125] and 5-IT [H(3)=17.59, p<0.0125] increased forepaw treading.
4.0 Discussion
The present study demonstrates that 5-IT and 6-IT are potent substrates at DAT, NET, and SERT, thereby causing non-exocytotic transmitter release by reversing the normal direction of transporter flux (Baumann et al., 2013a; Glennon, 2014). Despite their close structural similarities, 5-IT displays preference for releasing activity at DAT over SERT, while 6-IT displays preference for SERT over DAT. Both drugs have similar potency at NET. Our data for 6-IT are consistent with the findings of Banks et al. (2014) who showed the drug (i.e., PAL-571) is a monoamine releasing agent with a preference for SERT over DAT. Blough et al. (2014) demonstrated that AMT, a structural isomer of 5-IT and 6-IT (see Figure 1), is a non-selective transporter substrate and a potent agonist at 5-HT2 receptor subtypes. The effects of 5-IT and 6-IT at various monoamine receptor subtypes are not known, and therefore warrant further study.
The differential releasing potencies of 5-IT and 6-IT at DAT and SERT contrast with the similar releasing potencies of MDMA at all three transporters. Importantly, 5-IT is more potent than MDMA at DAT whereas 6-IT is more potent than MDMA at SERT. Our results indicate that 5-IT and 6-IT are mechanistically similar to MDMA, BZP, and the synthetic cathinones mephedrone and methylone (Baumann et al., 2004, 2012). Conversely, our test compounds are mechanistically distinct from synthetic cathinones in the pyrovalerone structural class including 3,4-methylenedioxypyrovalerone (MDPV), and the more recent pyrrolidinophenones (e.g., α-PVP), which exert their effects by blocking the reuptake of catecholamine transporters (Baumann et al., 2013b; Marusich et al., 2014).
The potency and selectivity of 5-IT and 6-IT for monoamine transporters were predictive of their in vivo effects. For example, the high DAT potency for 5-IT correlated with increases in locomotor activity, similar to what has been found for a variety of new psychoactive stimulants (Baumann et al., 2004, 2012, 2013b; Marusich et al., 2012, 2014; Yarosh et al., 2007). 5-IT also produced typical stimulant effects in the FOB similar to those produced by MDMA and methamphetamine (Marusich et al., 2012). In contrast, the high SERT potency for 6-IT correlated with behaviors associated with 5-HT toxicity observed following the locomotor session (i.e., after 90 min), but not increased locomotor activity during the session (Green et al., 2003; Ma et al., 2008). Salivation, tremor, hind limb splay, Straub tail, and flattened body posture were noted after the locomotor session or the following day. Surprisingly, 6-IT only increased one 5-HT-associated sign in the FOB at 20 min post-injection, piloerection. The time points at which these serotonergic behaviors occurred indicate that the 20 minute pretreatment time for FOB evaluation was too early for capturing the peak effects of 6-IT, which appears to have a slow onset and persistent effects in vivo. It is tempting to speculate that combined effects of transporter-mediated release and MAO inhibition may contribute to the sustained effects of 6-IT. Further studies should determine the precise underpinnings of the adverse effects of 6-IT in rodent models.
It is well established that monoamine releasers with high selectivity for DAT/NET versus SERT function as strong positive reinforcers in animal models, and typically have high abuse liability in humans (Banks et al., 2014; Negus et al., 2007; Negus and Miller, 2014; Wee et al., 2005). Furthermore, compounds that produce locomotor increases are likely to be reinforcing (Wise and Bozarth, 1987). Therefore, given the preference for DAT-mediated release and motor stimulatory effects of 5-IT, it is likely that this drug has high abuse potential. On the other hand, the slow onset of in vivo effects for 5-IT may serve to diminish its abuse potential to some extent. Previous research in humans and monkeys has shown that a rapid rate of stimulant drug delivery is associated with greater reinforcing effects (Abreu et al., 2001; Nelson et al., 2006; Woolverton and Wang, 2004). The onset of stimulant effects is reportedly the most pleasurable aspect of the drug-taking experience, and a short duration of action allows for more opportunities to self-administer, and consequently re-experience the onset of effects (Fischman, 1989). Peak locomotor effects of 5-IT did not occur until approximately 80 min post-injection, which is considerably longer than that for MDMA, methamphetamine, BZP, and synthetic cathinones with releasing properties (Marusich et al., 2012, 2014; Yarosh et al., 2007). Thus, 5-IT may serve as an interesting pharmacological tool for investigating the role of pharmacokinetics in animal models of drug reinforcement due to its unique time course.
In contrast with the neurochemical profile of 5-IT, the preference for SERT-mediated release exhibited by 6-IT may dampen dopaminergic effects of the drug. For mixed 5-HT/DA releasers, previous research has found that 5-HT plays a key role in reducing typical DA-mediated behaviors such as locomotor stimulation and self-administration (Baumann et al., 2011; Rothman et al., 2007; Rothman and Baumann, 2006; Wee et al., 2005). This is consistent with the ability of 6-IT to decrease intracranial self-stimulation in rats, and its failure to substitute for cocaine in drug discrimination in monkeys (Banks et al., 2014). Evidence suggests that activation of 5-HT2C receptors are responsible for the inhibitory actions of 5-HT on DA-mediated behaviors (Filip et al., 2010; Howell and Cunningham, 2015), and it is feasible that 6-IT may have direct actions at specific 5-HT receptor subtypes in addition to its 5-HT releasing activity. Overall, results of the present study combined with those of previous studies indicate that 6-IT may have low abuse liability, but enhanced risk for adverse effects.
A noteworthy finding of the present study is that small structural changes in a drug molecule can greatly impact transporter activity. Previous studies on synthetic stimulants have found similar results. Repositioning of the 3,4-methylenedioxy group in MDMA to create 2,3-methylenedioxymethamphetamine (2,3-MDMA) substantially decreases 5-HT uptake while NET uptake remains relatively constant (Montgomery et al., 2007). Similarly, a shift in the position of the flouro group in 3-fluoromethcathinone to create 4-fluoromethcathinone greatly diminishes DAT inhibition (Simmler et al., 2013, 2014). These combined results highlight the importance of including isomer identification in toxicological analyses. As the market for new psychoactive substances continues to evolve, it is likely that structural isomers of currently available compounds will appear in the street drug marketplace as a means to evade regulatory control. Including new potential isomers in toxicology screens will allow more rapid identification of novel compounds. One caveat of the results of the present study is that the in vitro release assays were conducted in rat brain synaptosomes, whereas the in vivo assays were conducted with mice. Mice were chosen for in vivo testing because the quantity of the test compounds was not sufficient for testing in rats. The differences in brain circuitry between mice and rats could complicate the interpretation of these data.
A number of drug tablets confiscated by European authorities have been found to contain 5-IT, and these tablets resemble those being sold as “ecstasy” in the street drug marketplace. Users who take such tablets may be exposed to MDMA, various ring-substituted cathinones, and/or 5-IT, among other compounds (EMCDDA, 2014). While it is difficult to postulate the health effects of these compounds when administered alone or in combination, methamphetamine and cocaine taken in combination with MDMA increase the risk of acute 5-HT toxidrome (i.e., 5-HT syndrome), a condition in which high levels of 5-HT accumulate in the brain causing symptoms ranging from mild shivering and diarrhea to severe muscle rigidity, fever, seizures, and death (Boyer and Shannon, 2005; Silins et al., 2007). Importantly, the ability of 5-IT to simultaneously release monoamines and inhibit MAO A will exacerbate the serotonergic effects of co-administered drugs, especially when administered at high doses. Therefore, 5-IT taken in combination with MDMA or other serotonergic compounds may lead to significant risk of toxicity.
5.0 Conclusion
5-IT and 6-IT are potent monoamine releasers which produce a variety of adverse effects in an FOB, and 5-IT is also a locomotor stimulant. 5-IT and 6-IT are structurally very similar, yet their transporter releasing activities at DAT and SERT are the opposite (i.e., 5-IT is DAT-preferring while 6-IT is SERT-preferring), indicating that subtle differences in chemical structure can have profound effects on biological activity. Thus, the prospect of inferring pharmacology from drug structure is a complicated endeavor. The potent monoamine-releasing actions of 5-IT, coupled with its known inhibition of MAO A (Herraiz and Brandt, 2014), could underlie its dangerous effects when administered alone, and in combination with other monoaminergic drugs or medications. Consequently, 5-IT and related compounds may pose substantial risk for abuse and serious adverse effects in human users.
Recreational use of synthetic stimulants has grown rapidly in recent years.
Structurally related novel indoles were studied in pharmacological assays.
5-IT and 6-IT were potent substrates at DAT, NET, and SERT.
5-IT produced typical stimulant effects, while 6-IT produced serotonin toxicity.
These compounds pose substantial risk for abuse or adverse effects in human users.
Acknowledgments
The authors thank Tim Lefever and Tony Landavazo for technical assistance. Research was generously supported by the Intramural Research Program at NIDA, RTI International internal research and development funds, and NIH/NIDA Grant DA12970. These sources of funding did not play any role in study design, data collection, analysis, and interpretation, in writing the report, or in the decision to submit the article for publication.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013a;18:786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013b;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology. 2015 doi: 10.1007/s00213-00015-03944-00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology. 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Bäckberg M, Beck O, Hultén P, Rosengren-Holmberg J, Helander A. Intoxications of the new psychoactive substance 5-(2-aminopropyl)indole (5-IT): a case series from the Swedish STRIDA project. Clin Toxicol. 2014;52:6618–6624. doi: 10.3109/15563650.2014.920088. [DOI] [PubMed] [Google Scholar]
- Banks ML, Bauer CT, Blough BE, Rothman RB, Partilla JS, Baumann MH, Negus SS. Abuse-related effects of dual dopamine/serotonin releasers with varying potency to release norepinephrine in male rats and rhesus monkeys. Exp Clin Psychopharmacol. 2014;22:274–284. doi: 10.1037/a0036595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB. Effects of “Legal X” piperazine analogs on dopamine and serotonin release in rat brain. Ann N Y Acad Sci. 2004;1025:189–197. doi: 10.1196/annals.1316.024. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. Eur J Pharmacol. 2013a;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Srihari RT, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. 2013b;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Solis E, Jr, Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL. Baths salts, spice, and related designer drugs: the science behind the headlines. J Neurosci. 2014;34:15150–15158. doi: 10.1523/JNEUROSCI.3223-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB. Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology. 2014;231:4135–4144. doi: 10.1007/s00213-014-3557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, Brunt TM, Van Dijk JP, Rigter SM, Hoek J, Goldschmidt HM, Niesink RJ. mCPP: an undesired addition to the ecstasy market. J Psychopharmacol. 2010;24:1395–1401. doi: 10.1177/0269881109102541. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Evans EB, Tokarz ME, Balster RL. Functional observational battery comparing effects of ethanol, 1,1,1-trichloroethane, ether, and flurothyl. Neurotoxicol Teratol. 1996;18:577–585. doi: 10.1016/0892-0362(96)00064-5. [DOI] [PubMed] [Google Scholar]
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J Psychopharmacol. 2011;25:1543–1547. doi: 10.1177/0269881110378370. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Koeter MW, Niesink RJ, van den Brink W. Linking the pharmacological content of ecstasy tablets to the subjective experiences of drug users. Psychopharmacology. 2012;220:751–762. doi: 10.1007/s00213-011-2529-4. [DOI] [PubMed] [Google Scholar]
- Cerletti A, Taeschler M, Weidmann H. Pharmacologic studies on the structure-activity relationship of hydroxyindole alkylamines. Adv Pharmacol. 1968;6:233–246. doi: 10.1016/s1054-3589(08)60322-1. [DOI] [PubMed] [Google Scholar]
- Coppola M, Mondola R. A new stimulant of abuse: 5-(2-aminopropyl) indole. Am J Psychiatry. 2013;170(2):226. doi: 10.1176/appi.ajp.2012.12091168. [DOI] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015;92:90–97. doi: 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SP, Brandt SD, Freeman S, Archer RP. AMT (3-(2-aminopropyl)indole) and 5-IT (5-(2-aminopropyl)indole): an analytical challenge and implications for forensic analysis. Drug Test Anal. 2013;5:196–202. doi: 10.1002/dta.1420. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) EMCDDA-Europol Joint Report on a new psychoactive substance: 5-(2-aminopropyl)indole. Lisbon: 2013a. [Accessed 22 June 2015]. Available at: http://www.emcdda.europa.eu/publications/joint-reports/5-IT. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) EU drug markets report: a strategic analysis. Lisbon: 2013b. [Accessed 22 June 2015]. Available at: http://www.emcdda.europa.eu/publications/joint-publications/drug-markets. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Report on the risk assessment of 5-(2-aminopropyl)indole in the framework of the Council Decision on new psychoactive substances. Lisbon: 2014. [Accessed 15 June 2015]. 5-(2-Aminopropyl)indole (5-IT) Available at: http://www.emcdda.europa.eu/attachements.cfm/att_222688_EN_TDAK13002ENN-1_.pdf. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2015) European Monitoring Centre for Drugs and Drug Addiction (2015) An update from the EU Early Warning System. Lisbon: Mar, 2015. [Accessed 15 June 2015]. New psychoactive substances in Europe. Available at: http://www.emcdda.europa.eu/attachements.cfm/att_235958_EN_TD0415135ENN.pdf. [Google Scholar]
- Fantegrossi WE, Winger G, Woods JH, Woolverton WL, Coop A. Reinforcing and discriminative stimulus effects of 1-benzylpiperazine and trifluoromethylphenylpiperazine in rhesus monkeys. Drug Alcohol Depend. 2005;77:161–168. doi: 10.1016/j.drugalcdep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, 2nd, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151:533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Alenina N, Bader M, Przegaliński E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict Biol. 2010;15:227–249. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Fischman MW. Relationship between self-reported drug effects and their reinforcing effects: studies with stimulant drugs. NIDA Res Monogr. 1989;92:211–230. [PubMed] [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2014;97:2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA. Bath salts, mephedrone, and methylenedioxypyrovalerone as emerging illicit drugs that will need targeted therapeutic intervention. In: Dwoskin LP, editor. Abuse Advances in Pharmacology: Emerging Targets & Therapeutics in the Treatment of Psychostimulant. Academic Press; San Diego: 2014. pp. 581–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Herraiz T, Brandt SD. 5-(2-Aminopropyl)indole (5-IT): a psychoactive substance used for recreational purposes is an inhibitor of human monoamine oxidase (MAO) Drug Test Anal. 2014;6:607–613. doi: 10.1002/dta.1530. [DOI] [PubMed] [Google Scholar]
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol. 2011;49(8):705–719. doi: 10.3109/15563650.2011.615318. [DOI] [PubMed] [Google Scholar]
- Hofmann A, Troxler F. FR1344579 Nouveaux dérivés de l’indole et leur préparation (patent) 1963
- Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67:176–197. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L, White M, Treble R. Designer psychostimulants: Pharmacology and differences. Neuropharmacology. 2014;87:59–65. doi: 10.1016/j.neuropharm.2014.01.015. [DOI] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Carbó M, Pubill D, Escubedo E, Camarasa J. An integrated pharmacokinetic and pharmacodynamic study of a new drug of abuse, methylone, a synthetic cathinone sold as “bath salts”. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:64–72. doi: 10.1016/j.pnpbp.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Ma Z, Zhang G, Jenney C, Krishnamoorthy S, Tao R. Characterization of serotonin-toxicity syndrome (toxidrome) elicited by 5-hydroxy-l-tryptophan in clorgyline-pretreated rats. Eur J Pharmacol. 2008;588:198–206. doi: 10.1016/j.ejphar.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto K, Striebel J, Cho AK, Wang C. Clinical and pharmacological aspects of bath salt use: a review of the literature and case reports. Drug Alcohol Depend. 2013;132:1–12. doi: 10.1016/j.drugalcdep.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Montgomery T, Buon C, Eibauer S, Guiry PJ, Keenan AK, McBean GJ. Comparative potencies of 3, 4-methylenedioxymethamphetamine (MDMA) analogues as inhibitors of [3H] noradrenaline and [3H] 5-HT transport in mammalian cell lines. Br J Pharmacol. 2007;152:1121–1130. doi: 10.1038/sj.bjp.0707473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academies Press; Washington (DC): 2011. [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE, et al. Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug Alcohol Depend. 2006;82:19–24. doi: 10.1016/j.drugalcdep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, et al. Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter. Drug Alcohol Depend. 2015;147:1–19. doi: 10.1016/j.drugalcdep.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow… and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between Dopamine and Serotonin Release Modulates Behavioral Effects of Amphetamine-Type Drugs. Ann NY Acad Sci. 2006;1074(1):245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS J. 2007;9(1):E1–E10. doi: 10.1208/aapsj0901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Scott KR, Power JD, McDermott SD, O’Brien JE, Talbot BN, Barry MG, Kavanagh PV. Identification of (2-aminopropyl)indole positional isomers in forensic samples. Drug Test Anal. 2014;6:598–606. doi: 10.1002/dta.1508. [DOI] [PubMed] [Google Scholar]
- Seetohul LN, Pounder DJ. Four fatalities involving 5-IT. J Anal Toxicol. 2013;37:447–451. doi: 10.1093/jat/bkt053. [DOI] [PubMed] [Google Scholar]
- Shanks KG, Dahn T, Behonick G, Terrell A. Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. J Anal Toxicol. 2012;36:360–371. doi: 10.1093/jat/bks047. [DOI] [PubMed] [Google Scholar]
- Silins E, Copeland J, Dillon P. Qualitative review of serotonin syndrome, ecstasy (MDMA) and the use of other serotonergic substances: hierarchy of risk. Aust N Z J Psychiatry. 2007;41:649–655. doi: 10.1080/00048670701449237. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology. 2014;79:152–160. doi: 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol Sci. 2015;36:41–50. doi: 10.1016/j.tips.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler F, Harnisch A, Bormann G, Seemann F, Szabo L. Synthesen von Indolen mit (2-Aminoäthyl)-, (2-Aminopropyl)-oder Alkanolamin-Seitenketten am Sechsring. 5. Mitt. über synthetische Indol-Verbindungen. Helv Chim Acta. 1968;51:1616–1628. [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) 2014 Global Synthetic Drugs Assessment: Amphetamine-type stimulants and new psychoactive substances. Vienna: 2014. [Accessed 22 June 2015]. Available at: https://www.unodc.org/documents/scientific/2014_Global_Synthetic_Drugs_Assessment_web.pdf. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Risk assessment forum. Washington, DC: U.S. EPA; 1998a. Guidelines for neurotoxicity risk assessment. 630/R-95/001F. [Google Scholar]
- U.S. Environmental Protection Agency (U.S. EPA) Health effects guidelines: neurotoxicity screening battery. Washington, DC: U.S. EPA; 1998b. EPA 712-C-98-238. OPPTS 870.6200. [Google Scholar]
- Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts”. J Addict Res Ther Suppl. 2012;9 doi: 10.4172/2155-6105.S9-002. pii: 002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Yarosh HL, Katz EB, Coop A, Fantegrossi WE. MDMA-like behavioral effects of N-substituted piperazines in the mouse. Pharmacol Biochem Behav. 2007;88:18–27. doi: 10.1016/j.pbb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]