Abstract
Background
Anesthetic contact residues in γ-aminobutyric acid type A (GABAA) receptors have been identified using photolabels, including two propofol derivatives. O-propofol-diazirine labels H267 in β3 and α1β3 receptors, while m-azi-propofol labels other residues in intersubunit clefts of α1β3. Neither label has been studied in αβγ receptors, the most common isoform in mammalian brain. In αβγ receptors, other anesthetic derivatives photolabel m-azi-propofol labeled residues, but not βH267. Our structural homology model of α1β3γ2L receptors suggests that β3H267 may abut some of these sites.
Methods
Substituted cysteine modification-protection was used to test β3H267C interactions with four potent anesthetics: propofol, etomidate, alphaxalone, and R-5-allyl-1-methyl-5-(m-trifluoromethyl-diazirinylphenyl) barbituric acid (mTFD-MPAB). We expressed α1β3γ2L or α1β3H267Cγ2L GABAA receptors in Xenopus oocytes. We used voltage clamp electrophysiology to assess receptor sensitivity to GABA and anesthetics, and to compare para-chloromercuribenzenesulfonate (pCMBS) modification rates with GABA versus GABA plus anesthetics.
Results
Enhancement of GABA EC5 responses by equi-hypnotic concentrations of all four anesthetics was similar in α1β3γ2L and α1β3H267Cγ2L receptors (n ≥ 3). Direct activation of α1β3H267Cγ2L receptors, but not α1β3γ2L, by mTFD-MPAB and propofol was significantly greater than the other anesthetics. Modification of β3H267C by pCMBS (n ≥ 4) was rapid and accelerated by GABA. Only mTFD-MPAB slowed β3H267C modification (~2-fold; p = 0.011).
Conclusions
β3H267 in α1β3γ2L GABAA receptors contacts mTFD-MPAB, but not propofol. Our results suggest that β3H267 is near the periphery of one or both transmembrane inter-subunit (α+/β− and γ+/β−) pockets where both mTFD-MPAB and propofol bind.
Introduction
Propofol, etomidate, barbiturates, and alphaxalone enhance gamma-aminobutyric acid type A (GABAA) receptor gating, contributing to sedation, hypnosis, and immobilization 1–3. GABAA receptors are pentameric ligand-gated ion channels (pLGICs). The most common subtypes in mammalian brain contain two α, two β, and one γ subunit arranged as in Figure 1 4,5. Each subunit has an N-terminal extracellular domain and a four-helix (M1 to M4) transmembrane domain (TMD). Subunit interfacial surfaces are designated “plus; +” (M3 side) or “minus; −” (M1 side) 4. Current structural homology models of αβγ receptors, based on crystallized homomeric pLGICs from bacteria, nematodes, and humans β3, are similar 6–11.
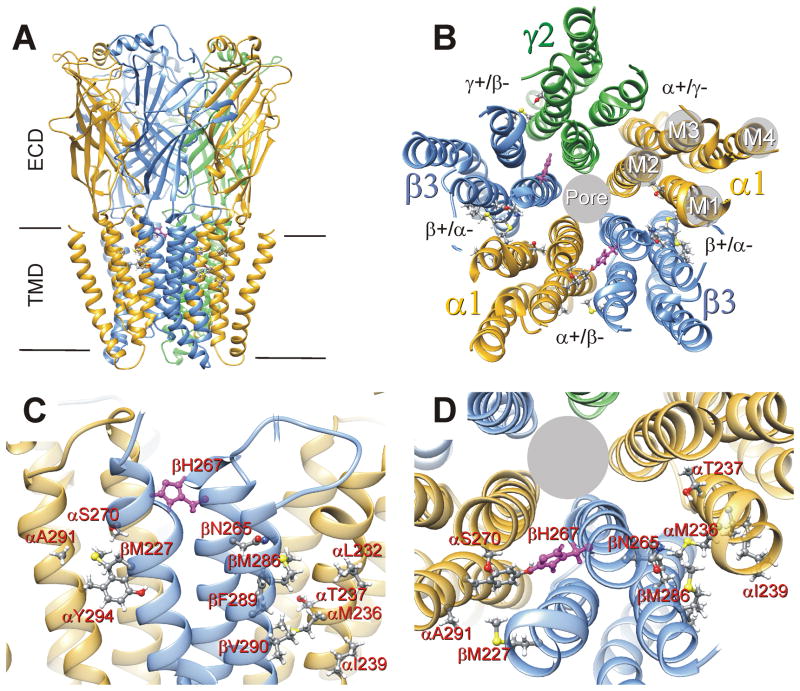
Figure 1. Anesthetic Binding Sites in a Structural Model of α1β3γ2L GABAA Receptors.
A: The panel depicts a structural homology model of α1β3γ2L GABAA receptors22, viewed from the side. Subunits are color-coded: α1 = gold, β3 = blue, and γ2 = green. The peptide chain backbones are depicted as ribbons and loops. The extracellular (ECD) and transmembrane (TMD) domains are labeled. Intracellular domains have been truncated to match those of the GluCl template. B: The transmembrane domain viewed from the extracellular space, depicting the established subunit arrangement, the four-helix bundles of each subunit, and the transmembrane pockets formed at subunit interfaces. Amino acid residues thought to interact with anesthetics based on either photolabeling or cysteine modification and protection (Table 1) are identified as ball-and-stick structures. The two β3H267 residues (highlighted in magenta) are located in the α+/β− and γ+/β− interfaces. C: A close-up view from a perspective similar to that in Panel A, identifying putative anesthetic contact residues in the α+/β− interface (on the left) and one of the β+/α− interfaces (on the right). D: A close-up view of the same two transmembrane interfacial pockets from the extracellular space. A subset of the putative anesthetic contact residues, including β3H267, is labeled.
Anesthetic binding residues in GABAA receptors (Fig 1C, D) have been identified using both photolabel derivatives (Fig 2, Table 1) and substituted cysteine modification-protection (Table 1). Two propofol derivatives, m-azi-propofol (azi-Pm) and o-propofol diazirine (o-PD), photolabel distinct residues 12,13. In α1β3 receptors, azi-Pm labels residues in β3-M3 (β3M286), α1-M1 (α1M236), and β3-M1 (β3M227) 12. These residues are also labeled in αβγ receptors by either azi-etomidate or the potent barbiturate R-5-allyl-1-methyl-5-(m-trifluoromethyl-diazirinylphenyl) barbituric acid (mTFD-MPAB) (Fig 1C, D and Table 1) 14,15. Propofol inhibits photolabeling by azi-Pm, azi-etomidate, or mTFD-MPAB 12,15,16. O-PD also inhibits azi-etomidate and mTFD-MPAB incorporation 12. However, in β3 homomers and α1β3 receptors o-PD uniquely labels β3H267 (M2–17′), which is not labeled by other anesthetics 13 (Table 1). To date, neither azi-Pm nor o-PD has been studied in αβγ GABAA receptors.
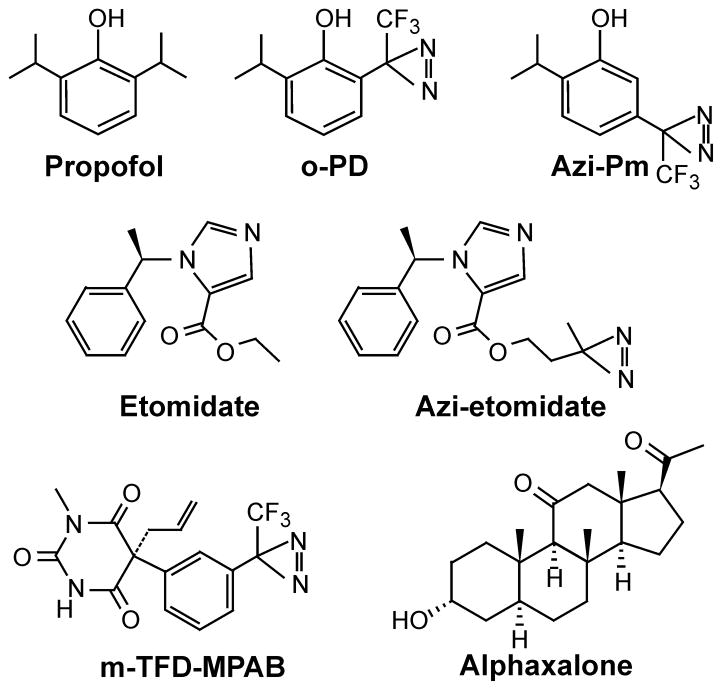
Figure 2. Potent General Anesthetics and Anesthetic Photolabels.
The chemical structures of three potent anesthetics (etomidate, propofol, and alphaxalone) and four diazirine photolabels (o-PD = o-propofol diazirine; azi-Pm = m-azi-propofol; azi-etomidate, and mTFD-MPAB = R-5-allyl-1-methyl-5-[m-trifluoromethyl-diazirinylphenyl] barbituric acid) are shown.
Table 1.
Anesthetic Contact Residues in GABAA receptors
| Residue | Receptor Type | Interfacial Sites | Photolabels | Substituted Cysteine Modification-Protection |
|---|---|---|---|---|
| α1L232 | α1β3γ2L | β+/α− | — | ETO20 |
| α1M236 | αβγ, α1β3 | β+/α− | Azi-ETO14 | ETO, PRO20,22 |
| α1β3 | “ | TDBzl-ETO32 | ND | |
| α1β3 | “ | Azi-Pm12 | ND | |
| α1T237 | α1β3γ2L | β+/α− | — | ETO20 |
| α1I239 | α1β3 | β+/α− | Azi-Pm12 | — 20, a |
| α1S291 | α1β3γ2L | α+/β−, α+/γ− b | mTFD-MPAB15 | ND |
| α1Y294 | α1β3γ2L | α+/β−, α+/γ− b | mTFD-MPAB15 | ND |
| β3M227 | α1β3 | α+/β−, β+/β− | Azi-Pm12 | ND |
| α1β3γ2L | α+/β−, γ+/β− | mTFD-MPAB15 | ||
| β3N265 | α1β3γ2L | β+/α− | — | ETO, PRO22, c |
| β3H267 | β3, α1β3 | β+/β−, α+/β− | o-PD13 | d |
| β3M286 | αβγ, α1β3 | β+/α−, β+/β− | Azi-ETO14 | ETO, PRO19,21 |
| α1β3 | “ | TDBzl-ETO32 | ND | |
| α1β3 | “ | Azi-Pm12 | ND | |
| α1β3 | β+/β− e | mTFD-MPAB12 | ND | |
| β3F289 | α1β3 | β+/β− e | mTFD-MPAB12 | ND |
| β3V290 | α1β3 | β+/α−, β+/β− | TDBzl-ETO32 | ND |
| γ2S301 | α1β3γ2L | γ+/β− | mTFD-MPAB15 | ND |
Azi-ETO = azi-etomidate; TDBzl-ETO = o-trifluromethyldiazirinylphenyl-etomidate; Azi-Pm = mazi-propofol; o-PD = o-propofol diazirine; mTFD-MPAB = R-5-allyl-1-methyl-5-(m-trifluoromethyl-diazirinylphenyl) barbituric acid; — Indicates negative modification or protection result; ND indicates no published data.
p-Chloromercuribenzenesulfate application to α1I239C did not alter function.
To date, there is no evidence of anesthetic contact with γ-M1 helix residues, so anesthetic binding in the α+/γ− interface remains speculative.
A binding role for βN265 was indirectly demonstrated using α1M236C protection.
Current study.
mTFD-MPAB did not photolabel βM286 or β3F289 in α1β3γ2L, but did in α1β3. Thus, incorporation into these residues was likely at the β+/β− interface.
Conflicting structural interpretations of propofol photolabeling results, and particularly the role of βH267, emerge from homology model analyses. In silico docking calculations for propofol in the β3 crystal structure suggest that H267 contributes to binding sites separate from those where azi-Pm binds 17. In contrast, our α1β3γ2L homology model (Fig. 1) locates β3H267 near and possibly within α+/β− and γ+/β− pockets containing residues labeled by both azi-Pm and mTFD-MPAB.
Substituted cysteine modification-protection is sensitive to steric interactions between anesthetics and putative contact residues. Sulfhydryl-specific reagents covalently modify accessible cysteine-substituted residues, usually producing functional changes 18. Bound anesthetic may hinder chemical modification of cysteines located near or within anesthetic sites. For example, both etomidate and propofol block modification of αM236C and βM286C in α1β2/3γ2 receptors 19–21 (Table 1). This approach also has identified several non-photolabeled anesthetic contact residues in β+/α− interfaces (Fig 1, Table 1) 20,22,23, but has not been reported for anesthetic interactions with other transmembrane interfacial pockets.
In the current study, we tested the hypothesis that in α1β3γ2L receptors β3H267 is near propofol and mTFD-MPAB sites in α+/β− and γ+/β− interfaces, but not those for etomidate or alphaxalone in β+/α− interfaces 24,25. Using voltage-clamp electrophysiology we pharmacologically characterized α1β3H267Cγ2L receptors and compared rates of β3H267C modification by para-chloromercuribenzesulfonate (pCMBS) in the absence vs. presence of anesthetics. The β3H267C mutation selectively sensitized α1β3γ2L to direct activation by propofol and mTFD-MPAB. Modification of β3H267C by pCMBS was rapid, enhanced by GABA, and slowed by mTFD-MPAB, but not other anesthetics. We infer that β3H267 is located in or near mTFD-MPAB binding sites in α1β3γ2L receptors.
Materials and Methods
Animal use
Female Xenopus laevis were used with approval from the Massachusetts General Hospital Institutional Animal Care & Use Committee. Frogs were housed in a veterinary-supervised environment in accordance with local and federal guidelines. Frogs were anesthetized by immersion in 0.2% tricaine (Sigma-Aldrich, St. Louis, MO) prior to mini-laparotomy to harvest oocytes.
Chemicals
R(+)-Etomidate was obtained from Bedford Laboratories (Bedford, OH). The clinical preparation in 35% propylene glycol was diluted directly into buffer. Propylene glycol at the resulting concentrations has no effect on GABAA receptor function 26. Propofol was purchased from Sigma Aldrich (St. Louis, MO) and alphaxalone was purchased from MP Biomedical (Solon, OH). Both propofol and alphaxalone were prepared as stock solutions in dimethylsufoxide. After dilution into electrophysiology buffer, dimethylsufoxideconcentrations were below 0.1%, and produced no effects on either wild-type or mutant GABAA receptors. R-mTFD-MPAB was a gift from Dr. Karol Bruzik, Ph.D. (Dept. of Medicinal Chemistry, Univ. Illinois Chicago, IL) and prepared as a 100 mM stock in methanol. After dilution for electrophysiology studies, methanol was below 0.01%, which produced no significant modulation of either wild-type or mutant GABAA receptors. Picrotoxin (PTX) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved (2 mM) in electrophysiology buffer. p-Chloromercuribenzenesulfonic acid sodium salt (pCMBS) was purchased from Toronto Research Chemicals (North York, Ontario, Canada). All other chemicals were purchased from Sigma-Aldrich.
Molecular Biology
Complementary DNAs for human GABAA receptor α1, β3, and γ2L subunits were cloned into pCDNA3.1 vectors (Invitrogen, Carlsbad, CA). A mutation encoding β3H267C was created with oligonucleotide-directed mutagenesis, using a QuikChange kit (Agilent Technologies, Santa Clara, CA). Several clones from the mutagenesis reaction were subjected to DNA sequencing through the entire β3 coding region to confirm the presence of the intended mutation and absence of stray mutations. A single mutant clone was selected for further use.
Oocyte Electrophysiology
Messenger RNA synthesis and Xenopus oocyte expression were performed as we have described 27. Electrophysiology experiments were conducted at room temperature (21–23 °C). Oocytes were voltage-clamped at −50 millivolts and signals were low-pass filtered at 1 kiloHertz (Model OC-725B, Warner Instruments, Hamden, CT). Electrophysiological signals were digitized at 200 Hertz (iWorx RA834, iWorx Systems Inc, Dover, NH) and recorded digitally on a personal computer running Labscribe v3 software (iWorx Systems Inc.). Oocyte superfusion in a custom-build flow chamber was software-controlled through the iWorx RA834 interface to solenoid switches (ALA-VM8, ALA Scientific Associates, Farmingdale, NY) and a sub-microliter dead-volume manifold. Five-fold data reduction and further low-pass (10 Hertz) digital filtering (using Clampfit 9.0, Molecular Devices, Sunnyvale, CA) were used in preparing traces for display in figures.
Electrophysiology solutions, including those containing GABA and/or anesthetics were based on ND96 (in mM: 96 NaCl, 2 KCl, 0.8 MgCl2, 1.8 CaCl2, 5 HEPES, pH 7.5). Peak current responses to GABA concentrations ranging from 0.1 μM to 3 mM, alone or co-applied with anesthetics, were assessed in Xenopus oocytes (n ≥ 3 from at least two frogs) using two microelectrode voltage clamp electrophysiology28. GABA applications varied in duration, depending on the time to reach steady-state peak current. Normalizing GABA responses at maximal GABA (1 mM), were recorded every 2nd or 3rd sweep. Picrotoxin-sensitive leak was measured using 2 mM PTX, followed by >5 minute washout and a maximal GABA response test. Propofol (5 μM) or alphaxalone (2 μM) were used as gating enhancers together with maximal GABA to assess GABA efficacy29. Direct activation and GABA enhancement were assessed in both wild-type and α1β3H267Cγ2L receptors using equi-potent anesthetic concentrations (2 × EC50 for loss of righting reflexes in Xenopus tadpoles = 2.5 μM alphaxalone, 5 μM propofol, 3.2 μM etomidate, and 8 μM mTFD-MPAB). The EC5 GABA concentration was identified for individual oocytes by testing GABA concentrations ranging from 2 to 4 μM. After establishing stable EC5 and 1 mM responses, oocyte currents were recorded during exposure to first anesthetic alone for 30s, followed by anesthetic combined with EC5 GABA for another 15 to 30s.
Electrophysiological Data Analysis
Analyses for agonist concentration-responses, and propofol-induced left shift followed our approach described elsewhere 27,29. Peak GABA-stimulated currents were normalized to maximal GABA responses, and GABA concentration-response data for individual oocytes in the absence and presence of propofol were fitted with logistic functions using non-linear least squares (Graphpad Prism v.5):
| Eq. 1 |
where EC50 is the half-maximal activating concentration and nH is Hill slope.
EC50 shift ratio was calculated from the difference in log(GABA EC50) values [Δlog(EC50)] measured in the presence of 5 μM propofol versus control.
Cysteine Modification with pCMBS
Voltage-clamped oocytes expressing GABAA receptors were repetitively activated with alternating EC5 and 1 mM GABA pulses every five minutes until at least three sequential sets of responses were constant (± 5%). Oocytes were then exposed to pCMBS (alone, with GABA, or with GABA + anesthetic) for 5 to 12s followed by 5 min ND96 wash. In oocytes expressing wild-type α1β3γ2L receptors, exposure to pCMBS (1 mM x 60 s, followed by a 5 minute wash in ND96 buffer) produced no significant changes in currents stimulated with low (EC5 = 4 μM) or 1 mM GABA. We tested a range of pCMBS concentrations on oocytes expressing α1β3H267Cγ2L receptors. Exposure to 1 μM pCMBS for 10 s resulted in an approximately 5-fold increase in response to low GABA (EC5 = 3 μM) relative to saturating GABA (1 mM). In most oocytes, the change in response ratio (I3μM/Imax) was associated with increased response to 3 μM GABA and a modest reduction in response to 1 mM GABA. Repeated 10s exposures to 1 μM pCMBS did not produce further change in response ratio, suggesting that β3H267C modification was complete after a single exposure. For experiments comparing the apparent initial covalent modification rates in α1β3H267Cγ2L receptors, we used a much lower pCMBS concentration of 10 nM. Two or three 5 to 12 s applications of 10 nM pCMBS (each followed by 5 min ND96 wash) typically resulted in less than a doubling of I3μM/Imax, i.e. less than 20% of the change associated with complete modification. After repeated exposures to 10 nM pCMBS, each oocyte was also exposed to 1 μM pCMBS for 10s to assess IEC5/Imax following full modification.
To test for anesthetic protection (inhibition of β3H267C modification), apparent modification rates with pCMBS plus 1 mM GABA were compared to rates with pCMBS plus 1 mM GABA and anesthetic. The GABA-bound receptor was chosen as the index condition, because GABA binding enhances the affinity of receptors for anesthetics, thereby increasing anesthetic site occupancy 21. The anesthetic concentrations used in protection studies were 10 μM alphaxalone, 10 and 30 μM etomidate, 5, 10, and 30 μM propofol, and 8 and 16 μM mTFD-MPAB. These anesthetic concentrations enhance activation of both wild-type and mutant GABAA receptors at least 10-fold (see results), and estimates of etomidate26 and propofol30 affinities for GABA-bound receptors suggest that over 90% of anesthetic sites are occupied under these conditions. For modification rate analysis, I3μM/Imax response ratios were normalized to the pre -modification control, and plotted against cumulative pCMBS exposure in units of nM × sec. Normalized response ratios were fitted by linear least squares to determine the apparent initial modification rate (slope, in M−1s−1). We fitted modification rates for both individual oocytes and for combined response ratio data from groups of oocytes for each condition. These resulted in slightly different mean and standard error values, due to differential data weighting, without affecting our overall conclusions.
Molecular Structural Modeling
We used a structural model for the α1β3γ2 GABAA receptor based on GluCl bound to ivermectin (PDB 3RHW)10, which we have described in a prior publication 22. The optimized structure was visualized and analyzed using University of California San Francisco Chimera v1.10. Optimized molecular structure models for the anesthetic drugs were built and analyzed using Avogadro v1.1.1 31.
Statistical Analysis
Oocytes were obtained from at least two frogs and randomly selected for each experiment. Blinding was not used during experiments or analysis. Group sizes (n ≥ 3 for functional characterization; n ≥ 4 for modification rate comparisons) were based on prior experience with these techniques. Additional control modification experiments (with GABA plus pCMBS) were performed with each set of protection studies. Results are reported as mean ± standard error unless otherwise noted. Statistical analyses were performed using Prism 5.02 (Graphpad Software, Inc., La Jolla, CA). Statistical comparisons of anesthetic direct activation and GABA enhancement in both wild-type and α1β3H267Cγ2L receptors was based on two-way ANOVA and pairwise Bonferroni post-tests. Apparent pCMBS modification rates measured under multiple conditions (i.e. sets of individual oocyte results) were compared using Kruskal-Wallis with Dunn’s Multiple Comparison test. Other pairwise comparisons were performed using Student’s t-tests or Mann-Whitney. Statistical significance was inferred at p < 0.05.
Results
Xenopus oocytes injected with messenger RNA mixtures encoding α1, wt β3 or β3H267C, and γ2L GABAA receptor subunits were studied using two-electrode voltage-clamp. In wild-type control experiments, α1β3γ2L receptors produced GABA-dependent currents with EC50 averaging 31 μM (data not shown; n = 3; 95% CI = 18 to 49 μM), consistent with previous reports 26. Propofol (5 μM) produced a 12-fol GABA EC50 shift in wild-type receptors (data not shown; n = 3, 95% CI = 6.3 to 23-fold).
Voltage-clamped oocytes expressing α1β3H267Cγ2L receptors produced inward currents in response to GABA, in a concentration-dependent and reversible manner (Fig. 3A). The fitted GABA EC50 value for α1β3H267Cγ2L receptors was 25 μM (n = 3; 95% CI = 19 to 32 μM), similar to wild-type. Co-application of GABA with propofol (5 μM) enhanced currents elicited by GABA concentrations below 100 μM (Fig 3B), producing a 15-fold (95% CI = 7.7 to 30-fold) leftward shift in the averaged concentration-response curve (Fig 3C) to 1.6 μM (n = 3; 95% CI = 0.83 to 3.2 μM). Again, this result does not significantly differ from wild-type, indicating that mutant receptors retain near-normal sensitivity to propofol. In oocytes expressing α1β3H267Cγ2L receptors with maximal peak currents over 5 μA, picrotoxin (2 mM) applied in the absence of GABA did not alter basal leak currents (Fig. 3D; n = 3), indicating that spontaneous receptor activation is below the detection threshold (about 5 nA or 0.1% of maximal peak). Currents elicited with 1 mM GABA were not enhanced by propofol, indicating that high GABA concentrations activated nearly 100% of α1β3H267γ2L receptors (Fig 3D). We have previously estimated that spontaneous activation of wild-type receptors has a probability below 0.01% and that maximal GABA efficacy in wild-type receptors is approximately 85% 26.
Figure 3. Functional Characterization of α1β3H267Cγ2L GABAA Receptors.
A: Traces are currents measured from a single voltage-clamped oocyte expressing α1β3H267Cγ2L GABAA receptors. Bars over the traces identify GABA concentration (μM) and period of exposure. B: Traces are recorded from the same oocyte as panel A, activated with various GABA concentrations combined with 5 μM propofol (PRO). C: Combined GABA concentration-responses from 3 oocytes in the absence and presence of propofol. Normalized data was fitted with Eq. 1 (methods). Fitted GABA EC50 values are 25 μM with GABA alone, and 1.6 μM in the presence of 5 μM propofol. D: Picrotoxin (PTX) application to a voltage-clamped oocyte expressing α1β3H267Cγ2L receptors reveals an absence of spontaneous gating activity. Combining propofol (10 μM) with maximal (1 mM) GABA does not enhance peak current, indicating that GABA alone activates nearly 100% of receptors.
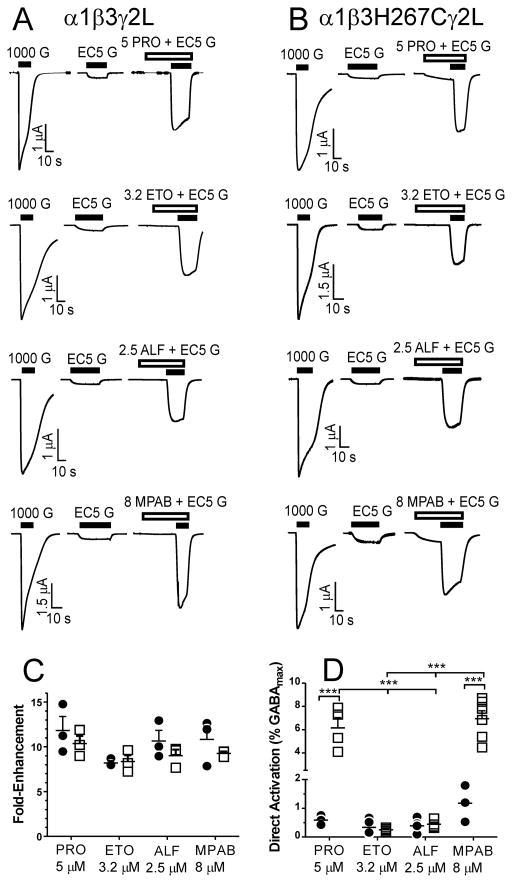
We extended our study of anesthetic interactions at β3H267 to three other potent anesthetics that modulate GABAA receptors: etomidate, alphaxalone, and mTFD-MPAB. Using voltage-clamp electrophysiology, we compared the effects of equipotent drug concentrations (2 × the EC50 for loss-of-righting-reflexes in tadpoles) in both wild-type α1β3γ2L (Fig. 4A) and α1β3H267Cγ2L (Fig. 4B) GABAA receptors. In current recordings where oocytes were first exposed to anesthetic for 30 s followed by anesthetic + EC5 GABA, we found that 5 μM propofol, 3.2 μM etomidate, 2.5 μM alphaxalone, and 8 μM mTFD-MPAB produce indistinguishable (~ 10-fold) enhancing effects on EC5 GABA responses in both α1β3γ2L and α1β3H267Cγ2L receptors (Fig 4C). These studies also revealed that both propofol and mTFD-MPAB directly activated α1β3H267Cγ2L receptors significantly more than the other anesthetics, and also far more than these drugs activated wild-type receptors (Fig 4D).
Figure 4. Anesthetic Direct Activation and Enhancement of GABA EC5 in α1β3γ2L and α1β3H267Cγ2L GABAA Receptors.
A: Each set of traces is from a single oocyte expressing α1β3γ2L receptors, tested with a different anesthetic drug (PRO = propofol; ETO = etomidate; ALF = alphaxalone; MPAB = R-5-allyl-1-methyl-5-[m-trifluoromethyl-diazirinylphenyl] barbituric acid). The first trace depicts response to 1 mM GABA, the second to EC5 GABA (ranging from 3 to 6 μM), and the third shows current elicited during exposure to anesthetic (at 2 × EC50 for loss of righting reflexes in tadpoles, indicated in μM) then anesthetic plus EC5 GABA. Anesthetic concentrations are indicated in μM. B: The traces are from oocytes expressing α1β3H267Cγ2L receptors, studied as described for panel A. C: A scatter plot showing all EC5 enhancement results with α1β3γ2L (solid circles) and α1β3H267Cγ2L (open squares), using equipotent concentrations of four anesthetics. Each drug produced similar EC5 enhancement in both receptors, and the amount of enhancement was similar among the four drugs (p >0.05 with two-way ANOVA). D: A scatter plot showing all direct activation results with α1β3γ2L (solid circles) and α1β3H267Cγ2L (open squares). Direct activation was similar for all drugs in α1β3γ2L, but both propofol and mTFD-MPAB activated α1β3H267Cγ2L receptors much more than the other drugs and more than wild-type receptors (p< 0.001 for both drug and receptor types, using two-way ANOVA and Bonferroni post-tests). *** p < 0.001.
After applying pCMBS (1 μM for 10 s) to voltage-clamped oocytes expressing α1β3H267Cγ2L receptors, followed by 5 min wash in electrophysiology buffer, we observed a 5-fold increase in the response to 3 μM GABA (approximate EC5) relative to the 1 mM GABA response (an example is shown in Fig 5A). Repeated exposure to 1 μM pCMBS (with post-exposure wash) did not further increase the normalized response ratio (I3μM/I1mM), indicating that a single 10 s exposure fully and irreversibly modified all receptors. In contrast, when oocytes expressing α1β3γ2L receptors were exposed to 1 mM pCMBS for up to 60 s, no changes were observed in spontaneous leak or current responses to low and high GABA (n = 3; not shown). Therefore, the effect of pCMBS on α1β3H267Cγ2L function was due to covalent bond formation at β3H267C.
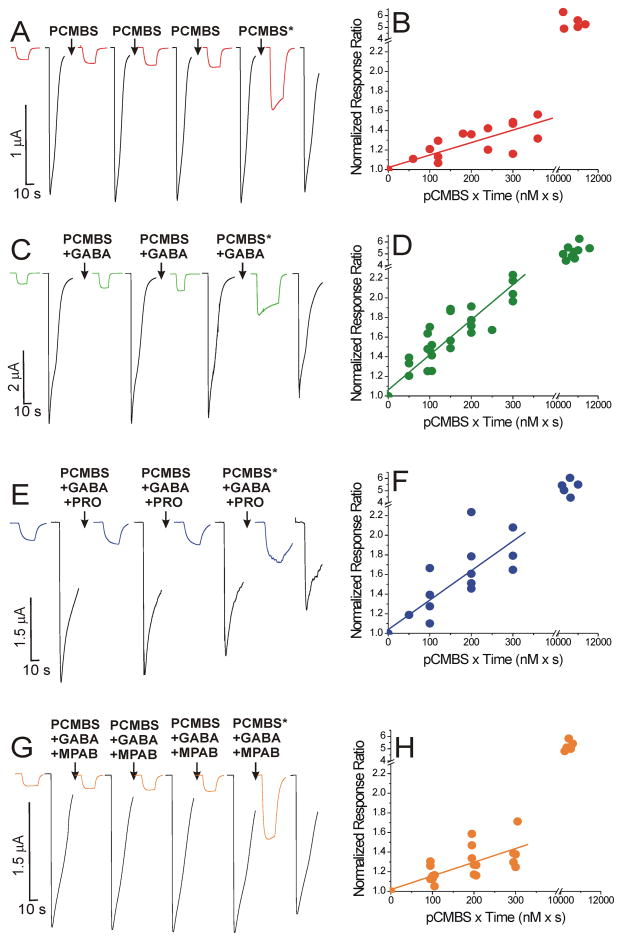
Figure 5. Modification of α1β3H267Cγ2L GABAA Receptors with pCMBS.
The panels on the left show examples of voltage-clamp current traces during modification under four different conditions. Colored traces are responses to 3 μM GABA, and black traces are responses to 1 mM GABA. Arrows indicate modification exposures, which were followed by 5 min wash. The starred arrows indicate exposure to 1 μM p-chloromercuribenzensulfonate (pCMBS) for 10 s. The panels on the right show the corresponding initial linear rate analyses for combined normalized response I3μM/I1mM ratios from all oocytes used for each condition. Points represent the ratio of I3μM:I1mM, normalized to the pre-modification control, and plotted against cumulative pCMBS exposure. Points in the upper right portion of the panel represent response ratios after modification with 1 μM pCMBS. A: Modification in the absence of GABA. Traces are recorded from one voltage-clamped oocyte expressing α1β3H267Cγ2L GABAA receptors before and after sequential 10 s exposures to 10 nM pCMBS. B: Initial modification rate analysis for combined data from all oocytes modified with pCMBS alone (n = 5). The line through the first four points has a fitted slope of 1.3 ± 0.19 × 106 M−1s−1. Maximal normalized response ratio = 5.4 ± 0.25 (n =5; mean ± sem). C: Modification in the presence of GABA. Current responses from a single oocyte during sequential 10 s exposures to 10 nM pCMBS plus 1 mM GABA. D: Initial modification rate analysis for all oocytes modified with pCMBS plus GABA (n = 9). The fitted linear slope is 3.6 ± 0.25 × 106 M−1s−1. Maximal normalized response ratio = 5.2 ± 0.24 (n =8; mean ± sem). E: Modification in the presence of GABA and propofol. Current responses from one oocyte before and after sequential 10 s exposures to 10 nM pCMBS plus 1 mM GABA plus 10 μM propofol (PRO). F: Initial modification rate analysis for all oocytes modified with pCMBS plus GABA and propofol (n = 5). The fitted linear slope is 3.0 ± 0.47 × 106 M−1s−1. Maximal normalized response ratio = 5.3 ± 0.27 (n =5; mean ± sem). G: Modification in the presence of GABA and mTFD-MPAB. Current responses from one oocyte before and after sequential 10 s exposures to 10 nM pCMBS plus 1 mM GABA plus 8 μM mTFD-MPAB (MPAB = R-5-allyl-1-methyl-5-[m-trifluoromethyl-diazirinylphenyl] barbituric acid). H: Initial modification rate analysis for all oocytes modified with pCMBS plus GABA and MPAB (n = 7). The fitted linear slope is 1.4 ± 0.22 × 106 M−1s−1. Maximal normalized response ratio = 5.2 ± 0.18 (n =5; mean ± sem).
In oocytes expressing α1β3H267Cγ2L receptors, initial pCMBS modification rates were assessed using repeated 5 to 12 s exposures to 10 nM pCMBS. At this concentration, the I3μM/I1mM response ratio increased by about 40% after a cumulative 30 s of exposure (Fig 5B). Linear fits to the normalized response ratios plotted against cumulative pCMBS exposure for all oocytes (Fig 5B; n = 5) indicated an apparent slope of (mean ± se) 1.3 ± 0.19 × 106 M−1 s−1. The average of individual oocyte modification rates (mean ± sem) was similar (1.3 ± 0.24 × 106 M−1 s−1). When pCMBS was co-applied with 1 mM GABA (e.g. Fig. 5C), the apparent rate of modification (all oocytes; n = 9) increased to 3.6 ± 0.25 × 106 M−1 s−1 (Fig 5D). The average of individual oocyte modification rates with GABA was 3.9 ± 0.58 × 106 M−1 s−1, three-fold higher (p = 0.0078; Mann-Whitney test) than the rate without GABA. The maximal change in normalized response ratio remained approximately 5-fold after co-application of 1 μM pCMBS with GABA (Fig 5D). Co-application of pCMBS with 1 mM GABA plus 10 μM propofol (e.g. Fig. 5E) resulted in an apparent rate of modification (all oocytes; n = 5) of 3.0 ± 0.47 × 106 M−1 s−1 (Fig 5F). The individual oocyte modification rates with GABA + propofol (3.5 ± 0.69 × 106 M−1 s−1) and the overall effect of modification were similar to those in the presence of GABA alone. Additional protection experiments using 30 μM propofol (not shown; n = 5) also indicated no reduction in the modification rate.
We also tested whether etomidate, alphaxalone, or mTFD-MPAB alter the rate of pCMBS modification in GABA-activated α1β3H267Cγ2L receptors, applying the same approach used for propofol. Etomidate (10 and 30 μM) and alphaxalone (10 μM) produced no changes, whereas mTFD-MPAB (8 μM; Fig 5G and 5H) reduced the average modification rate approximately 2-fold (Fig. 6; p = 0.011; Mann-Whitney test). Attempts to study protection using higher (16 μM) mTFD-MPAB concentrations were complicated by very slow drug washout producing residual direct activation and desensitization of α1β3H267Cγ2L receptors, resulting in widely varying apparent modification rates in repeated experiments.
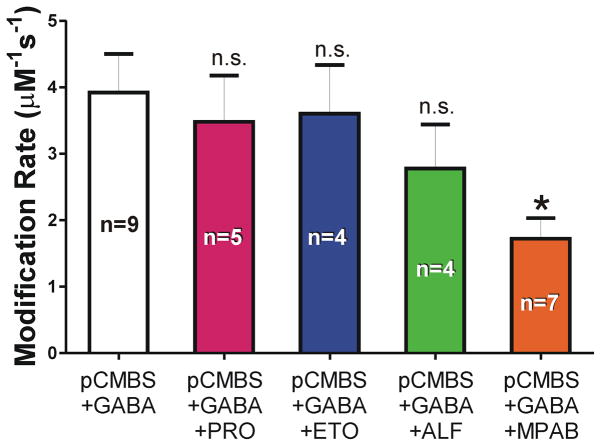
Figure 6. Anesthetic Effects on β3H267C Sulfhydryl Modification Rates.
Each column represents group mean ± sem calculated from individual oocyte modification rate results. Modification conditions are labeled: 10 nM pCMBS; 1 mM GABA; PRO = 10 μM propofol; ETO = 10 μM etomidate; ALF = 10 μM alphaxalone; MPAB = 8 μM mTFD-MPAB. Results with anesthetics were compared to pCMBS plus GABA (Kruskal Wallace with Dunn’s multiple comparisons), indicating that only MPAB significantly slowed modification. * p = 0.011.
Discussion
In a α1β3γ2L background, we investigated β3H267C effects on anesthetic sensitivity, and tested whether bound anesthetics protect this cysteine from modification by pCMBS. Although βH267 was photolabeled by o-PD in β3 and α1β3 receptors, we found that in α1β3γ2L, propofol did not protect β3H267C from chemical modification. In similar studies with etomidate, alphaxalone, and mTFD-MPAB, only mTFD-MPAB reduced the rate of β3H267C modification. This suggests that β3H267 is near at least one of the two mTFD-MPAB “β−” sites in α1β3γ2L, as predicted by our structural homology model (Fig 1D) 12,15. Our negative β3H267C protection results with etomidate and alphaxalone are also consistent with prior evidence that these anesthetics bind in β+/α− interfaces 14,24,25,32.
Earlier studies showed that βH267 mutations influence GABAA receptor modulation by both Zn2+ and protons 33–35. We also found that the β3H267C mutation selectively sensitized receptors to activation by both mTFD-MPAB and propofol, linking β3H267 to channel gating and the nearby α+/β− and γ+/β− sites where these anesthetics bind. The absence of β3H267C effects on receptor agonism by GABA, etomidate, and alphaxalone rules out global allosteric effects of the mutation. Indeed, the anesthetic specificity of both pharmacological effects (Fig 4) and biochemical protection (Fig 6) indicate local interactions of β3H267 with the “β−” anesthetic sites.
Consistent with our observations, a prior study of α1β1H267Cγ2 also reported enhanced channel gating after pCMBS modification 36. The pCMBS modification rate at β3H267C (~4 × 106 M−1s−1 with GABA) was about 10-fold faster than other TMD cysteine substitutions we have examined 20–22. The rapid modification of β3H267C indicates a relatively high degree of probe and water exposure for a TMD sidechain,18 but remains far slower than pCMBS reactions with free cysteine in bulk water at pH 7.5 (estimated near 108 M−1s−1) 37. GABA increased the rate of modification, indicating GABA-dependent structural rearrangements near β3H267. The dynamic structural changes in the GABAA receptor TMD that accompany channel activation and desensitization remain uncertain, although comparisons of crystallized GluCl structures9,10 and biophysical studies of bacterial pLGICs 38 in different states suggest that the extracellular ends of M2 and M3 helices tilt away from the pore, possibly expanding inter-subunit pockets and their water content.
The interpretation of our new results must consider limitations of photolabeling, cysteine modification-protection, and structural models of heteromeric GABAA receptors. Photolabeling is an unbiased method for identifying ligand contact loci. Photolabels must be structurally and pharmacologically similar to the “parent” drug of interest. Also required are sufficient target protein quantity and purity, efficient and stable photo-adduct formation, and a sensitive method for identifying incorporation sites. Limitations include the potential for photolabeling sites other than those where the parent drug acts, and for selective photochemical reactions with amino acids that may not exist in drug binding sites. The β3H267 residue was identified as the sole contact in β3 and α1β3 receptors photolabeled with o-PD using mass spectroscopic proteonomic analysis13. Subsequently, Jayakar et al reported that o-PD displaced azi-etomidate and mTFD-MPAB labeling in α1β3 receptors12, implying that o-PD interacts with residues other than β3H267 in heteromeric receptors (that contain a β3-β3 interface). Thus, o-PD photolabeling may have missed other contact residues due to technical limitations. Photolabeling results for azi-Pm and o-PD may also reflect different orientations of photo-reactive groups at ring positions 2 and 6 (presumably near βH267) relative to positions 3 and 5 when bound in the same site with steric constraints. Indeed, modifications at these propofol ring positions also produce distinct effects on drug potency/efficacy 39. Similarly, etomidate’s photolabel derivatives14,32 have identified only a portion of its currently known contact residues. Others were identified in αβγ receptors using cysteine modification-protection.
The substituted cysteine modification-protection strategy uses an unmodified ligand and sulfhydryl-selective chemistry to test interactions at putative contact residues. Important considerations for this method include: 1) that ligand binding is retained in the cysteine-substituted mutant receptor, 2) that ligand occupies a large fraction of its sites during protection experiments, and 3) that a similar mixture of receptor states is present during modification in both the absence and presence of ligand. In our current experiments, evidence indicates that all these conditions were met. Modulation of α1β3H267Cγ2L receptors by propofol and the other anesthetics was similar to that in wild-type GABAA receptors (Fig 4C), indicating minimal changes in affinity/binding. By using high GABA, we established conditions where nearly all α1β3H267Cγ2L receptors were either in open or desensitized states that have high anesthetic affinity relative to resting/closed receptors. Propofol modestly slows GABAA receptor desensitization without altering its extent 40, implying that both open and desensitized receptors bind propofol with similar affinities. Thus, similar receptor state mixtures were present during modification with or without anesthetics. Our prior estimate of the propofol dissociation constant for GABA-bound α1β2γ2L receptors (KP × d ≈ 2 μM)30, suggests that 10 μM propofol occupies ~83% of sites and 30 μM PRO occupies ~93% of sites. Photolabeling inhibition also indicates that propofol binds to both etomidate and mTFD-MPAB sites with similar affinities 15.
We studied propofol interactions with β3H267 in α1β3γ2L, and our results do not address β3 and α1β3 receptors that were photolabeled with o-PD13. Propofol contact might occur only within β/β interfaces that are absent in αβγ receptors. Even if propofol contacts β3H267 in wild-type receptors, the histidine-to-cysteine mutation reduces sidechain size and may also alter orientation, reducing contact in the mutant. Given that in β3 homomers H267 is positioned between the inter-subunit cleft and the ion channel6, it is conceivable that propofol binds near β3H267C but does not effectively protect the sulfhydryl group from pCMBS in the receptor pore. However, our “positive control” finding that mTFD-MPAB protects β3H267C indicates that this is unlikely and that the technique worked as intended. Moreover, a recent study of β3H267W effects in β3 and α1β3 also found no evidence for propofol interactions with this residue 41.
Photolabeling has established that in α1β3 receptors, propofol, azi-Pm, o-PD and mTFD-MPAB compete for binding sites in α+/β− and β+/β− interfaces12,15. Considering these data together with our current results suggests that β3H267 is located near the periphery of at least one of the mTFD-MPAB sites in α1β3γ2L and further from sub-regions of the β− pockets that interact with both mTFD-MPAB and propofol. In our structural homology model, contiguous cavities extend from β3H267 to residues photolabeled by mTFD-MPAB and azi-Pm (Fig. 7A, B), including α1S270, another residue thought to interact with anesthetics 42,43. The model-derived distances from the β3H267 imidazole to α1S270, α1A291, and α1Y294 range from 7.0 to 12.7 angstroms while from β3H267 to γ2S301 is 12.8 Å. The largest projection length of R-mTFD-MPAB is 10.9 Å, while that of propofol is 7.6 Å. Thus, mTFD-MPAB is large enough to bind near α1A291 or γ2S301 and impede pCMBS access to β3H267C, while propofol is smaller and may fail to obstruct this interaction. To fully reconcile photolabeling with our protection results we also posit that azi-Pm and o-PD both occupy β− sites overlapping those for propofol and mTFD-MPAB, yet photolabel different residues because of constrained binding orientations.
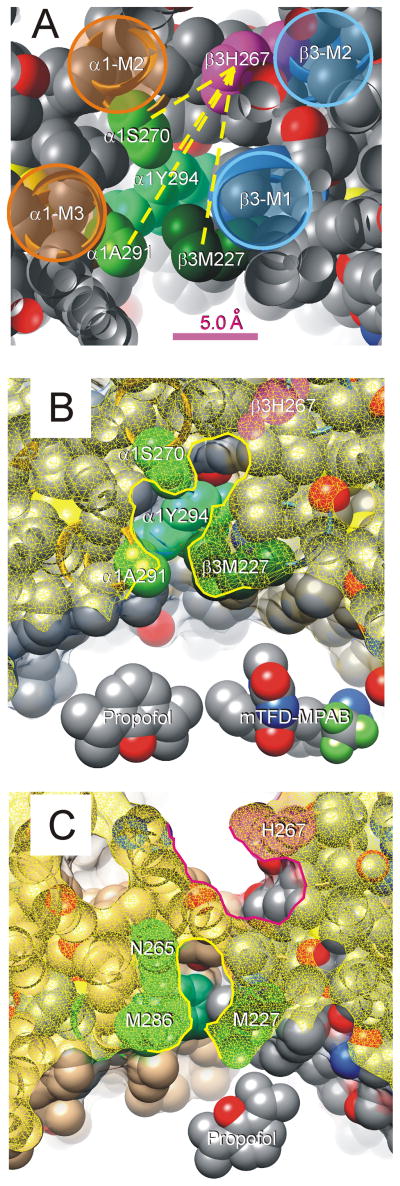
Figure 7. β3H267 and Other α+/β-Anesthetic Contact Residues Line a Contiguous Pocket.
A: A portion of our α1β3γ2L structural homology model is shown with peptide backbone as ribbons and side-chains depicted as spherical shells (hydrogens are hidden). The view is from the extracellular space, off-axis, through a planar cut (atoms cut by this plane appear hollow). The peptide backbones of transmembrane helices are highlighted and labeled. The side-chain of β3H267 is colored magenta, and other side-chains known to contribute to anesthetic binding are shaded in green and labeled. Other side-chain atoms are color coded (gray = carbon, red = oxygen; blue = nitrogen; yellow = sulfur). Some side-chains (β3L223, β3Q224, α1R274, α1M286, and α1D287) were hidden in order to un-roof the cavity that contacts residues of interest. Yellow dotted lines connecting β3H267 to other side-chains represent measured distances in the model, which range from 7.0 Å (to α1S270) to 12.8 Å (to α1A291). B: A view of our homology model similar to that in panel A is shown. The protein surface has been added and is depicted as a translucent film. The “cut plane” is about 1 helical turn (4 Å) more intracellular than that in panel A, and the cut surface shown as yellow mesh. The highlighted border of the cut surface outlines the proposed anesthetic-binding pocket that is lined by β3H267 (magenta) and the other residues that contribute to anesthetic binding (green). Models of propofol and mTFD-MPAB are included for size comparison. C: A “cut” view of the crystallized β3 homomeric receptor structure6. The cut surface is again shown as a yellow mesh. Note that H267 (magenta) forms part of a pocket (highlighted in red) adjacent to the ion channel. Sidechains of P228 and T266 separate the pocket containing H267 from another (highlighted in yellow) that includes other anesthetic photolabeled residues (green) and part of the lipid-protein interface.
Some alternative GABAA receptor structural models do not contain a contiguous pocket linking β3H267 with the residues labeled by mTFD-MPAB and azi-Pm. Franks17 conducted docking calculations for propofol in the β3 crystal structure that shows two separated pockets (Fig 7C) and found these consistent with o-PD photolabeling of β3 homomers 6. Jayakar et al12 also describe an α1β3 model based on Gloeobacter violaceus ligand gated ion channel where β3H267 forms part of a pocket adjacent to the ion channel and separated by intruding side-chains from inter-subunit residues photolabeled by azi-Pm. The accuracy of structural models vis-a-vis the various functional states of α1β3γ2L and other GABAA receptors remains speculative. Small helix rotations or side-chain rearrangements in the models in figures 7B and 7C could alter the shape and contiguity of the depicted pockets. Our current protection results favors a structure for α1β3γ2L receptors with “β−” anesthetic binding pockets contiguously linking the o-PD, azi-Pm, and mTFD-MPAB photolabeled residues.
Analysis of other β3H267 mutations in α1β3γ2L may provide further insights into its roles in anesthetic modulation. However, functional analysis alone may not distinguish between mutant-associated changes in anesthetic binding vs. transduction 22,29. This is because anesthetics are highly efficacious agonists of GABAA receptors, binding almost exclusively to activated and desensitized states. In contrast, cysteine modification-protection has identified likely anesthetic contact even at residues where cysteine substitution did not significantly alter sensitivity to anesthetic 20. This further highlights the importance of complementary methods to probe both functional and steric interactions between drug and receptor.
In summary, in cysteine modification-protection studies of α1β3H267Cγ2L GABAA receptors and four potent general anesthetics (propofol, etomidate, alphaxalone, and mTFD-MPAB), only mTFD-MPAB slowed β3H267C modification, indicating steric proximity. The β3H267C mutation also selectively enhanced direct agonism by both propofol and mTFD-MPAB. These results are consistent with a structural model locating β3H267 near the “β−” inter-subunit clefts where photolabeling indicates that both mTFD-MPAB and propofol (but not etomidate or alphaxalone) bind.
Acknowledgments
Research support: This work was supported by grant GM089745 from the National Institutes of General Medical Sciences (Bethesda, MD, USA).
Professor Karol Bruzik, Ph.D. and Pavel Svechenkov, Ph.D. (both at University of Illinois at Chicago, Chicago, IL) synthesized and provided mTFD-MPAB used in our studies. We thank Professor Keith Miller, D.Phil. (Massachusetts General Hospital, Boston, MA) and Professor Jonathan Cohen, Ph.D. (Harvard Medical School, Boston, MA) for comments and suggestions on the study and manuscript.
The authors thank Prof. Karol Bruzik, Ph.D. and Pavel Savechenkov, Ph.D. (both in the Dept. of Medicinal Chemistry and Pharmacognosy, University of Illinois at Chicag, Chicago, IL, USA) for providing access to mTFD-MPAB. We thank Professor Keith Miller, D.Phil. (Massachusetts General Hospital, Boston, MA) and Professor Jonathan Cohen, Ph.D. (Harvard Medical School, Boston, MA) for comments and suggestions on the study and manuscript. GABA receptor molecular graphics and distance analyses were performed with the University of California San Francisco Chimera package (v1.10). Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103311).
Footnotes
Prior Presentations: None.
Conflicts of Interest: The authors have no conflicts of interest related to this work.
Contributor Information
Alex T. Stern, Dept. of Anesthesia Critical Care & Pain Medicine, Massachusetts General Hospital, Boston, MA
Stuart A. Forman, Dept. of Anesthesia Critical Care & Pain Medicine, Massachusetts General Hospital, Boston, MA.
References
- 1.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 2.Zeller A, Arras M, Jurd R, Rudolph U. Identification of a molecular target mediating the general anesthetic actions of pentobarbital. Mol Pharmacol. 2007;71:852–9. doi: 10.1124/mol.106.030049. [DOI] [PubMed] [Google Scholar]
- 3.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen RW, Sieghart W. GABA(A) receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–8. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–5. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 6.Miller PS, Aricescu AR. Crystal structure of a human GABAA receptor. Nature. 2014;512:270–5. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–4. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 8.Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–8. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 9.Althoff T, Hibbs RE, Banerjee S, Gouaux E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature. 2014;512:333–7. doi: 10.1038/nature13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertaccini EJ, Yoluk O, Lindahl ER, Trudell JR. Assessment of homology templates and an anesthetic binding site within the gamma-aminobutyric acid receptor. Anesthesiology. 2014;119:1087–95. doi: 10.1097/ALN.0b013e31829e47e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayakar SS, Zhou X, Chiara DC, Dostalova Z, Savechenkov PY, Bruzik KS, Dailey WP, Miller KW, Eckenhoff RG, Cohen JB. Multiple Propofol Binding Sites in a gamma-Aminobutyric Acid Type A Receptor (GABAAR) Identified Using a Photoreactive Propofol Analog. J Biol Chem. 2014;289:456–68. doi: 10.1074/jbc.M114.581728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yip GM, Chen ZW, Edge CJ, Smith EH, Dickinson R, Hohenester E, Townsend RR, Fuchs K, Sieghart W, Evers AS, Franks NP. A propofol binding site on mammalian GABA receptors identified by photolabeling. Nat Chem Biol. 2013;9:715–20. doi: 10.1038/nchembio.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of intersubunit general anesthetic binding sites in the transmembrane domain of the human alpha1beta3gamma2 GABAA receptor. J Biol Chem. 2013;288:19343–57. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li GD, Chiara DC, Cohen JB, Olsen RW. Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem. 2010;285:8615–20. doi: 10.1074/jbc.M109.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franks NP. Structural Comparisons of Ligand-gated Ion Channels in Open, Closed, and Desensitized States Identify a Novel Propofol-binding Site on Mammalian gamma-Aminobutyric Acid Type A Receptors. Anesthesiology. 2015;122:787–94. doi: 10.1097/ALN.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 18.Karlin A, Akabas MH. Substituted-cysteine accessibility method. Meth Enzymol. 1998;293:123–45. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- 19.Bali M, Akabas MH. Defining the propofol binding site location on the GABAA receptor. Mol Pharmacol. 2004;65:68–76. doi: 10.1124/mol.65.1.68. [DOI] [PubMed] [Google Scholar]
- 20.Stewart DS, Hotta M, Li GD, Desai R, Chiara DC, Olsen RW, Forman SA. Cysteine Substitutions Define Etomidate Binding and Gating Linkages in the alpha-M1 Domain of gamma-Aminobutyric Acid Type A (GABAA) Receptors. J Biol Chem. 2013;288:30373–86. doi: 10.1074/jbc.M113.494583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart DS, Hotta M, Desai R, Forman SA. State-Dependent Etomidate Occupancy of Its Allosteric Agonist Sites Measured in a Cysteine-Substituted GABAA Receptor. Mol Pharmacol. 2013;83:1200–8. doi: 10.1124/mol.112.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart DS, Pierce DW, Hotta M, Stern AT, Forman SA. Beta N265 in Gamma-Aminobutyric Acid Type A Receptors is Both a Binding and Efficacy Determinant for Etomidate and Propofol. PLoS One. 2014 Oct 27;9(10):e111470. doi: 10.1371/journal.pone.0111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCracken ML, Borghese CM, Trudell JR, Harris RA. A transmembrane amino acid in the GABAA receptor beta2 subunit critical for the actions of alcohols and anesthetics. J Pharmacol Exp Ther. 2010;335:600–6. doi: 10.1124/jpet.110.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 25.Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the beta3 subunit of the GABA(A) receptor. Mol Pharmacol. 2012;82:408–19. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rüsch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on alpha1beta2gamma2L GABA-A receptors accounts for both direct activation and agonist modulation. J Biol Chem. 2004;279:20982–92. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- 27.Stewart DS, Desai R, Cheng Q, Liu A, Forman SA. Tryptophan mutations at azi-etomidate photo-incorporation sites on α1 or β2 subunits enhance GABAA receptor gating and reduce etomidate modulation. Mol Pharmacol. 2008;74:1687–95. doi: 10.1124/mol.108.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rüsch D, Forman SA. Classic benzodiazepines modulate the open-close equilibrium in alpha1beta2gamma2L gamma-aminobutyric acid type A receptors. Anesthesiology. 2005;102:783–92. doi: 10.1097/00000542-200504000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Desai R, Rüsch D, Forman SA. Gamma-amino butyric acid type A receptor mutations at beta2N265 alter etomidate efficacy while preserving basal and agonist-dependent activity. Anesthesiology. 2009;111:774–84. doi: 10.1097/ALN.0b013e3181b55fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rüsch D, Neumann E, Wulf H, Forman SA. An Allosteric Coagonist Model for Propofol Effects on alpha1beta2gamma2L gamma-Aminobutyric Acid Type A Receptors. Anesthesiology. 2012;116:47–55. doi: 10.1097/ALN.0b013e31823d0c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro. an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012;4:17. doi: 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. Mapping general anesthetic binding site(s) in human alpha1beta3 gamma-aminobutyric acid type A receptors with [(3)H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 2012;51:836–47. doi: 10.1021/bi201772m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wooltorton JRA, McDonald BJ, Moss SJ, Smart TG. Identification of a Zn2+ binding site on the murine GABA-A receptor complex. dependence on the second transmembrane domain of beta subunits. J Physiol. 1997;505:633–40. doi: 10.1111/j.1469-7793.1997.633ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunne EL, Hosie AM, Wooltorton JR, Duguid IC, Harvey K, Moss SJ, Harvey RJ, Smart TG. An N-terminal histidine regulates Zn(2+) inhibition on the murine GABA(A) receptor beta3 subunit. Br J Pharmacol. 2002;137:29–38. doi: 10.1038/sj.bjp.0704835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkins ME, Hosie AM, Smart TG. Identification of a beta subunit TM2 residue mediating proton modulation of GABA type A receptors. J Neurosci. 2002;22:5328–33. doi: 10.1523/JNEUROSCI.22-13-05328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goren EN, Reeves DC, Akabas MH. Loose protein packing around the extracellular half of the GABA(A) receptor beta1 subunit M2 channel-lining segment. J Biol Chem. 2004;279:11198–205. doi: 10.1074/jbc.M314050200. [DOI] [PubMed] [Google Scholar]
- 37.Parikh RB, Bali M, Akabas MH. Structure of the M2 transmembrane segment of GLIC, a prokaryotic Cys loop receptor homologue from Gloeobacter violaceus, probed by substituted cysteine accessibility. J Biol Chem. 2011;286:14098–109. doi: 10.1074/jbc.M111.221895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velisetty P, Chalamalasetti SV, Chakrapani S. Conformational transitions underlying pore opening and desensitization in membrane-embedded Gloeobacter violaceus ligand-gated ion channel (GLIC) J Biol Chem. 2012;287:36864–72. doi: 10.1074/jbc.M112.401067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krasowski MD, Hong X, Hopfinger AJ, Harrison NL. 4D-QSAR analysis of a set of propofol analogues. mapping binding sites for an anesthetic phenol on the GABA(A) receptor. Journal of Medicinal Chemistry. 2002;45:3210–21. doi: 10.1021/jm010461a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai D, Pennefather PS, MacDonald JF, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABA(A) receptors. J Neurosci. 1999;19:10635–46. doi: 10.1523/JNEUROSCI.19-24-10635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eaton MM, Cao LQ, Chen ZW, Franks NP, Evers AS, Akk G. Mutational analysis of the putative high-affinity propofol binding site in human β3 homomeric GABAA receptors. Mol. Pharm. 2015;88:736–45. doi: 10.1124/mol.115.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–9. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 43.Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9305–10. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]