Abstract
Oncolytic viruses including oncolytic herpes simplex virus (oHSV) have produced provocative therapeutic responses in patients with glioblastoma (GB), the most aggressive brain tumor. Paradoxically, innate immune responses mediated by natural killer (NK) cells and macrophages/microglia appear to limit oHSV efficacy. Therefore, we investigated whether pretreatment with an immunosuppressive cytokine, TGF-β, might reverse these effects and thereby potentiate oHSV efficacy. TGF-β treatment of NK cells rendered them less cytolytic against oHSV-infected GB cells and stem-like cells in vitro. Further, TGF-β treatment of NK cells, macrophages or microglia increased viral titers of oHSV in co-cultures with GB cells. In a syngeneic mouse model of GB, administering TGF-β prior to oHSV injection inhibited intracranial infiltration and activation of NK cells and macrophages. Notably, a single administration of TGF-β prior to oHSV therapy was sufficient to phenocopy NK cell depletion and suppress tumor growth and prolong survival in both orthograft and syngeneic models of GB. Collectively, our findings show how administering a single dose of TGF-β prior to oncolytic virus treatment of GB can transiently inhibit innate immune cells that limit efficacy, thereby improving therapeutic responses and survival outcomes.
Keywords: TGF-β, glioblastoma, NK cells, oncolytic viruses, innate immune response, Oncolytic Herpes Simplex Virus
INTRODUCTION
Glioblastoma (GB) is the most common and aggressive primary brain tumor in adults (1). The current standard treatment for GB consists of surgical resection followed by radiotherapy and chemotherapy. However, even with this multipronged approach, the median overall survival of GB patients is only 14.6 months due to the highly infiltrative nature of GB that prevents effective resection (2). Therefore, there is an urgent need to develop novel and effective therapies for this devastating malignancy. Oncolytic viruses (OVs) are viruses genetically engineered to selectively replicate in tumor cells and trigger tumor cell lysis while sparing normal cells (3). Importantly, this activity is also associated with the enhancement of anti-tumor immune responses, introducing the potential for extended disease control (4,5). Oncolytic herpes simplex viruses (oHSV) have been shown to be effective for the treatment of various cancers especially when combined with other reagents, and an oHSV expressing GM-CSF has demonstrated improvement in durable response rate with a tolerable safety profile in phase III malignant melanoma trials (6). OVs have attracted particular attention as distinctive anti-GB biological agents, due not only to the relatively restricted localization of GB in the brain, but also to the fact that the surrounding normal cells are post-mitotic and thus less susceptible to non-selective viral infection and lysis (7).
However, the host innate immune response to oHSV has been shown to impair efficient virus replication and spread within tumor tissues following initial infection, which results in compromised therapeutic efficacy of oHSV against GB (8,9). We previously demonstrated that administration of oHSV in the brain induced rapid recruitment and activation of natural killer (NK) cells, which accounted for obvious viral clearance and limited anti-tumor efficacy of oHSV in both athymic and immunocompetent mouse models (10). In addition, oHSV-activated NK cells coordinated macrophage and microglia activation within tumors, thereby facilitating their viral clearance properties. NK cell depletion prolonged overall survival of GB-bearing mice in a xenograft U87DeltaEGFR (U87dEGFR) model and a syngeneic 4C8 model (10). Suppression of initial innate antiviral defense responses is thus predicted to augment virus replication and tumor lysis, prior to eventual tumor clearance by multiple mechanisms including later-stage host antitumor immune responses. Therefore, we hypothesized that temporary or transient inhibition of innate immune responses would enhance the efficacy of oHSV in the treatment of GB.
The cytokine transforming growth factor (TGF)-β is secreted by a variety of cells and can exert multiple effects, but in general it produces cell growth inhibition and apoptosis via transcriptional induction of genes such as the cyclin-dependent kinase inhibitors p15 and p21 (11,12). Importantly, it plays a critical role in dampening innate immune responses. The immunosuppressive properties of TGF-β motivated us to explore whether a single pretreatment dose prior to oHSV treatment can temporarily inhibit the anti-oHSV innate immune responses in order to augment anti-GB efficacy. In the present study, we found that pretreatment of GB-bearing mice with a single dose of TGF-β prior to oHSV administration created a temporary immunosuppressive window that allowed oHSV to replicate and propagate efficiently in the GB cells, maximizing anti-GB therapy efficacy and prolonging mouse survival.
MATERIALS AND METHODS
Cell culture
Vero, Gli36dEGFR, U251 cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). Monkey kidney epithelial derived Vero cells were obtained in April 2005 from Dr. E Antonio Chiocca [Ohio State University (OSU), Columbus, Ohio]. GB30, GB1123, GB84, and GB157 neurospheres, also from Dr. Chiocca (received 2012), were maintained as tumor spheres in Neurobasal Medium supplemented with 2% B27, human EGF (20 ng/ml), and bFGF (20 ng/ml) in low-attachment cell culture flasks.
Vero cells have not been authenticated since receipt. Gli36dEGFR, GB30, and U251 cells were authenticated by the University of Arizona Genetics Core via STR profiling on January 2015. Murine BV2 microglia were maintained in DMEM supplemented with 2% FBS. BV2 cells were obtained in January 2009 from J. Godbout (OSU). Murine RAW264.7 macrophages, received in June 2010 from Dr. Susheela Tridandapani (OSU), were cultured in RPMI supplemented with 10% FBS. Murine BV2 and RAW264.7 cells have not been authenticated since receipt. Human NK cell line NK-92 was purchased from ATCC and has been authenticated with STR profiling. Human primary NK cells were isolated from peripheral blood leukopacks of healthy donors (American Red Cross, Columbus, Ohio) as described previously (13). Human NK cells were maintained in RPMI-1640 supplemented with 20% FBS and 150 IU/ml recombinant human (rh) IL-2 (Hoffman-La Roche Inc, Nutley, NJ). All cells were incubated at 37°C in an atmosphere with 5% carbon dioxide and maintained with penicillin (100 U/ml) and streptomycin (100 μg/ml). All cells are routinely monitored for changes in morphology and growth rate. All cells are negative for mycoplasma.
All above antibiotics and cytokines were purchased from Invitrogen (Grand Island, NY). TGF-β1 was purchased from Peprotech (Rocky Hill, NJ). Pan-TGF-β neutralizing antibody 1D11 was purchased from R&D Systems (Minneapolis, MN).
Mice
Six to eight-week-old nude mice, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice, and B6D2F1 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animal work was approved by The Ohio State University Animal Care and Use Committee and carried out according to an approved protocol. For survival studies, mice were monitored frequently for GB disease progression, and sacrificed when they became moribund with neurologic impairments and obvious weight loss. For flow cytometry analysis of immune regulation in the setting of oHSV, unless specified, mice were all sacrificed two days after oHSV injection, and tissues were harvested for immune cell isolation. oHSV used in vivo and in vitro is rQNestin34.5, which was previously described (14).
Cytotoxicity assay
A standard 51Cr release assay was performed as described previously (15). Briefly, target cells were labeled with 51Cr and co-cultured with the NK-92 cell line or freshly isolated primary NK cells pretreated with or without TGF-β1 at 20 ng/ml for 24 h at various effector:target (E:T) ratios in the wells of 96-well V-bottom plates at 37°C for 8 h. GB cell lines or patient-derived GB stem-like cells as target cells were pre-incubated with or without oHSV for 30 min at 37°C (multiplicity of infection, MOI=3). Supernatants were harvested and transferred into scintillation vials containing a liquid scintillation cocktail (Fisher Scientific, Waltham, MA), and the release of 51Cr was measured on Beckman Liquid Scintillation Counter LS-6500 (Beckman Coulter, Fullerton, CA). Target cells incubated in complete medium or 1% SDS were used to determine spontaneous or maximal 51Cr release, respectively. Percentage of specific cell lysis was calculated using the standard formula: 100 × (cpm experimental release – cpm spontaneous release) / (cpm maximal release – cpm spontaneous release).
Real-time reverse transcription PCR
To detect TNF-α, IFN-γ, and NOS2 mRNA expression in brain tissues, RNA was extracted from brain samples with RNeasy Mini Kit (Qiagen, Valencia, CA) and quantified with NanoDrop (Thermo Fisher, Wilmington, DE). Reverse transcripts were produced using M-MLV reverse transcriptase (Invitrogen), and real-time PCR was conducted with SYBR Green PCR Master Mix (Life Technologies, Grand Island, NY). The PCR primers were described previously (10). PCR reaction parameters were 95°C 10 min, 40 cycles at 95°C 10 s and 60°C 60 s, followed by 72°C 10 min for final extension.
Co-culture virus replication assay
5 × 105 U251 cells were seeded in wells of six-well plates with 2% FBS medium and incubated at 37°C overnight. 3 ml DMEM media supplemented with 0.05% FBS containing oHSV at an MOI of 3 were added to the wells after media aspiration, and were incubated at 37°C for 20 minutes. After removal of supernatants and washing with DMEM media, the cells (BV2, RAW264.7 or NK-92 cells) pretreated with TGF-β1 or untreated control cells were added (1 × 106 cells per well). After incubation for 12 h at 37°C, both media and cells were collected for virus titer assays as reported previously (16).
In vivo testing of TGF-β1 effects on oHSV therapy using the orthotopic human GB30 xenograft model and the 4C8 syngeneic model
GB30 human glioma stem-like cells were retrovirally transduced with Pinco-pGL3-luc/GFP virus expressing firefly luciferase (FFL). GFP positive cells were sorted using a FACSAria II cell sorter (BD Biosciences, San Diego, CA) and were designated “GB30-FFL” cells. On day 0, 40 nude mice were anesthetized and fixed in a stereotactic apparatus, a burr hole was drilled 2 mm lateral and 1 mm anterior to the bregma to a depth of 3.25 mm, and 5×104 GB30-FFL cells in 2 μl Hank’s buffered salt solution (HBSS) were implanted. On day 7, the mice were divided into 4 groups. Mice from the TGF-β1 and oHSV combination group were intravenously injected with TGF-β1 (1 μg in 200 μl PBS per mouse). Mice from the combination of 1D11 and oHSV group were intravenously injected with 1D11 pan-TGF-β neutralizing antibody (5 μg in 200 μl PBS per mouse), and mice from the oHSV-only group and the HBSS group were intravenously injected with 200 μl PBS. On day 8, all mice from the TGF-β1 and oHSV combination, the 1D11 and oHSV combination, and the oHSV-only groups were intracranially injected with 2×105 pfu oHSV in 3 μl HBSS, and mice from HBSS group were intracranially injected with 3μl HBSS. Mice were monitored daily and euthanized when moribund. 10 days after inoculation of GB30-FFL cells, the mice were intraperitoneally infused with D-luciferin (150 mg/kg body weight; Gold Biotechnology, St. Louis, MO), anesthetized with isoflurane, and imaged using In Vivo Imaging System (IVIS-100, PerkinElmer, Waltham, MA) and analyzed with live image software (PerkinElmer). For the syngeneic model, 105 4C8 murine glioma cells were intracranially injected to each B6D2F1 mouse in the same way as in the GB30-FFL model.
NK cell depletion in GB30-bearing athymic nude mice and 4C8-bearing B6D2F1 mice
25 nude mice were intracranially implanted with 5×104 GB30-FFL cells, as described above, on day 0. On day 8, the mice in the NK cell depletion groups (asialo+oHSV and asialo+TGF-β+oHSV) were intraperitoneally injected with 50 μl anti-asialo GM1 antibody (Wako, Osaka, Japan) diluted in 50 μl distilled water. The mice in other groups were intraperitoneally injected with 100 μl distilled water. On day 9, mice in the TGF-β+oHSV group and the asialo+TGF-β+oHSV group were intravenously injected with TGF-β1 (1 μg in 200 μl PBS per mouse). Mice in other groups were intravenously injected with 200 μl PBS. On day 10, mice in the HBSS group were intracranially injected with 3μl HBSS, the other mice were intracranially injected with 2×105 pfu oHSV in 3μl HBSS. Mice were monitored daily and euthanized when moribund. 14 days after inoculation of GB30-FFL cells, the mice were imaged and analyzed as aforementioned. Survival data were analyzed only after all mice were euthanized. For the syngeneic model, 4C8 murine glioma cells were intracranially injected to each B6D2F1 mouse in the same way as in the GB30-FFL model.
Flow cytometry
Murine mononuclear cells from oHSV-infected brains were isolated as previously described (17). To obtain splenocytes, spleens were collected and homogenized through a 70 mm strainer. Erythrocytes were lysed using RBC lysis buffer (Biolegend, San Diego, CA). Cells isolated from either brains or spleens were treated with Fc Block antibody (anti CD16/32, BD Biosciences). Cells were stained with mouse-specific immune cell surface markers for 30 min at 4°C. The following anti-mouse antibodies were used at a dilution of 1:200: CD3-APC, NK1.1-PE, CD69-FITC, CD27-V450, CD11b-PE, CD45-APC, CD3-PE-Cy7, CD107-APC, CD11b-PerCP-Cy5.5, and IFN-γ-FITC (Biolegend, San Diego, CA). For CD107a staining, mononuclear cells were cultured in 10% RPMI media with monensin (eBioscience, San Diego, CA) for 4 h before cell-surface staining. For staining of IFN-γ, we treated the cells with Cytofix/Cytoperm (BD) following initial cell-surface staining and then performed intracellular staining.
Statistics
Unpaired Student’s t tests were utilized to compare two independent groups for continuous endpoints if normally distributed. For non-normally distributed endpoints, such as the in vivo bioluminescence intensity, a Kruskal-Wallis test was utilized to compare the median of experimental groups. For survival analysis, Kaplan-Meier curves were plotted and compared using a log-rank test. All tests were two-sided. P values were adjusted for multiple comparisons using Bonferroni method. P values less than 0.05 were considered statistically significant.
Results
TGF-β1 inhibits NK-92 and primary NK cell cytotoxicity against oHSV-infected GB cells
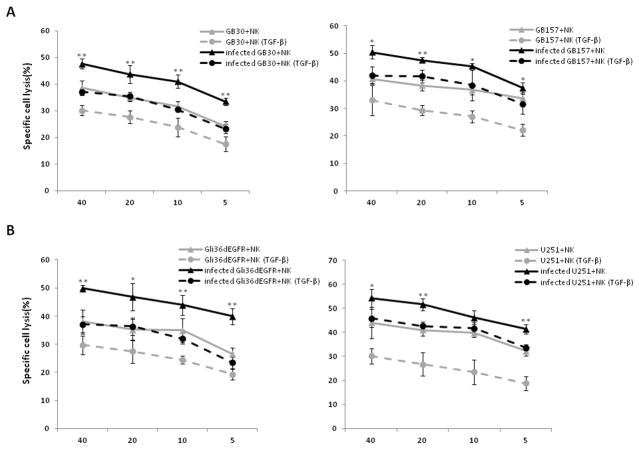
Our previous study showed that activated NK cells were recruited to oHSV-treated tumors within hours of infection, resulting in substantially compromised oHSV-mediated anti-tumor efficacy (10). Given that TGF-β1 is a well-characterized immune-suppressive factor, we first assessed in vitro whether TGF-β1 treatment affects the cytotoxic activity of NK cells against oHSV-infected GB cells. We pre-incubated the NK-92 cell line or primary NK cells with or without TGF-β1 for 24 h, and infected Cr51-labeled patient-derived GB stem-like cells or GB cell lines with or without oHSV for 30 minutes at 37°C. We then combined these cells and assessed target (oHSV-infected or un-infected GB cell) lysis by a standard chromium release assay. TGF-β1 treatment significantly impaired the cytotoxic activity of primary NK cells against oHSV-infected or uninfected patient-derived GB mesenchymal (MES) stem-like cells, GB30, and GB157 GB proneural (PN) stem-like cells (Fig. 1A), as well as two oHSV-infected or uninfected GB cell lines, Gli36dEGFR and U251 (Fig. 1B). Similarly, after being pretreated with TGF-β1, NK-92 cells also showed decreased cytotoxic activity against the oHSV-infected GB stem-like cells, GB30, GB1123, GB84 and GB157 (Supplementary Fig. 1A) and the oHSV-infected GB cell lines, Gli36dEGFR and U251 (Supplementary Fig. 1B).
Figure 1. TGF-β1 pretreatment decreases NK cell cytotoxicity against GB cell lines and patient-derived GB stem-like cells.
A) Cytotoxic activity of primary NK cells or TGF-β1-pretreated primary NK cells against oHSV infected or uninfected patient-derived GB stem-like cells (GB30 and GB157) using chromium-51 release assay. B) Cytotoxic activity of primary NK cells or TGF-β1-pretreated primary NK cells against oHSV infected or uninfected GB cell lines (Gli36dEGFR and U251) using chromium-51 release assay. Representative data from three independent experiments are shown. *P < 0.05; **P < 0.01( infected GB cells + NK vs. infected GB cells + TGF-β-pretreated NK).
TGF-β1 treatment enhances oHSV titers in vitro and in vivo in the presence of GB cells and innate immune cells
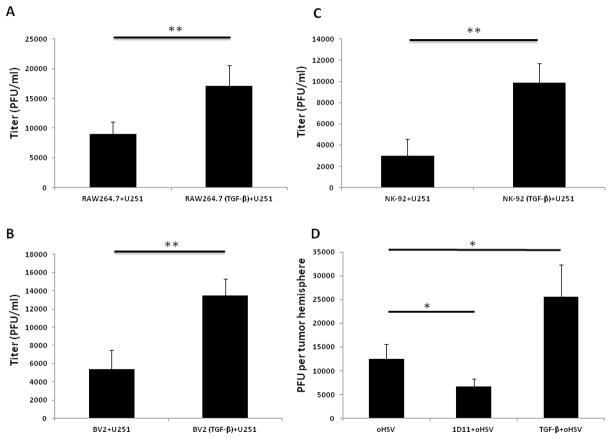
We previously reported that not only NK cells, but also macrophages or microglia, are recruited to oHSV infection sites to launch an immune response against oHSV and decrease oHSV replication (10,16,18). We then tested if TGF-β1 pretreatment could inhibit the anti-viral effects mediated by macrophages, microglia and/or NK cells in vitro and in vivo. The macrophage cell line RAW 264.7 was pre-incubated with or without TGF-β1 for 24 h and then co-cultured with oHSV-infected U251 cells for 12 h, followed by determination of oHSV titers. In these experiments, the titer of oHSV was significantly increased with TGF-β1 pretreatment (Fig. 2A). Similar results were observed using the microglia cell line BV2 (Fig. 2B) or the NK cell line NK-92 (Fig. 2C). We next intracranially implanted U87dEGFR cells into nude mice on day 0. On day 7, the mice were treated with TGF-β1 or 1D11 (TGF-β neutralizing antibody) by intravenous injection, and on day 8, oHSV was administered intracranially. On day 10, the mice were sacrificed and oHSV titers in the inoculated hemispheres were measured as described previously (16). Compared to the oHSV-only group virus titers in the hemispheres from the combination of TGF-β1 and oHSV group increased significantly, but titers in the 1D11 plus oHSV combination group decreased (Fig. 2D).
Figure 2. TGF-β1 pretreatment increases oHSV titer in both in vitro co-culture system and GB-bearing nude mice.
A) oHSV-infected U251 cells were co-cultured with TGF-β1 pretreated or untreated macrophages (RAW 264.7) for 12 h, and oHSV titer was measured by plaque assay. B) oHSV-infected U251 cells were co-cultured with TGF-β1 pretreated or untreated microglia (BV2) for 12 h, followed by oHSV titer measurement by plaque assay. C) oHSV-infected U251 cells co-cultured with TGF-β1 treated or untreated NK-92 cells for 12 h, followed by oHSV titer measurement by plaque assay. D) 24 h prior to oHSV injection to the hemisphere of GB bearing mice, TGF-β1 or 1D11 was administered to the mice through i.v. injection. Two days later, the hemisphere was harvested and lysed. The lysates were serially diluted and added to Vero cells seeded in 96-well plates to determine viral plaques. Representative data of three independent experiments are shown in A, B and C. D is representative of two independent experiments with three mice in each group in each experiment. * P < 0.05; **P < 0.01
Pretreatment with a single dose of TGF-β1 decreases NK cell intracranial infiltration and activation in an immunocompetent syngeneic GB mouse model treated with oHSV
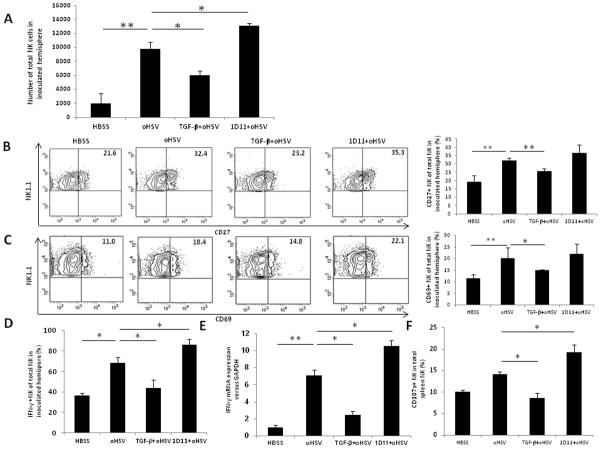
Our previous study showed that NK cells were recruited to the brain and became activated following oHSV therapy (10). We next investigated if there was any change in NK cell intracranial infiltration and activation after modulation of TGF-β signaling in a syngeneic GB model. Here, 4C8 murine GB cells were implanted into B6D2F1 mice on day 0, followed by an i.v. injection of TGF-β1 or 1D11 on day 7 and an intracranial injection of oHSV or vehicle (HBSS) on day 8 post tumor cell implantation. Mice receiving oHSV showed a significant increase in infiltrating NK cells in the brain relative to mice receiving vehicle (Fig. 3A). However, pretreatment with TGF-β1 resulted in a significant decrease in the total number of intracranially infiltrating NK cells, whereas pretreatment with 1D11 produced a significant increase. We also performed a time-course analysis of TGF-β-mediated inhibition of NK cell infiltration after oHSV injection. The results showed that a single dose of TGF-β led to a significant inhibition of NK cell intracranial infiltration at days 1 and 2 after oHSV infection. On day 3 TGF-β still produced a moderate, but not statistically significant, inhibitory effect. No inhibition was observed on and after day 5 (Supplementary Fig. 2A). We next characterized the activation status of the intracranially recruited NK cells by evaluating NK cell activation markers. As expected, oHSV treatment resulted in an increased percentage of CD27+ NK cells in the brain compared to the HBSS control group, while TGF-β1 pretreatment dampened CD27 surface expression induced by oHSV treatment (Fig. 3B). The percentage of CD69-expressing NK cells was also significantly decreased with TGF-β1 pretreatment in the setting of oHSV therapy (Fig. 3C). Compared to oHSV treatment alone, expression of CD27 and CD69 appeared to be increased following 1D11 pretreatment, although this increase did not reach statistical significance. IFN-γ expressing NK cells in inoculated hemispheres significantly decreased after TGF-β1 pretreatment compared to treatment with oHSV alone, and increased significantly following 1D11 pretreatment compared to treatment with oHSV alone (Fig. 3D). At the mRNA level, IFN-γ gene expression in brain tissues was significantly increased with oHSV treatment (Fig. 3E). This increase was effectively blocked by TGF-β1 pretreatment, whereas 1D11 pretreatment enhanced it. We then checked the status of NK cells in the spleens to determine whether the systemic response paralleled the local brain response. We found that both the percentage of splenic NK cells and expression of the activation/degranulation marker CD107a were decreased after TGF-β1 pretreatment (Supplementary Fig. 3A and Fig. 3F). Percentages of CD27 and CD69 positive NK cells in the spleens also decreased significantly with TGF-β1 pretreatment compared with the oHSV-only group (Supplementary Fig. 3B). Together, these in vivo assays demonstrate that NK cell numbers and activity are compromised by TGF-β1 treatment prior to oHSV therapy, resulting in attenuated NK cell clearance of oHSV-infected GB cells.
Figure 3. TGF-β1 pretreatment decreases NK cell infiltration and activity in oHSV-inoculated hemispheres in the 4C8 syngeneic mouse model.
A) Flow cytometric quantification of NK cells in tumor hemispheres of 4C8 bearing mice 48 h after inoculation with oHSV. B) Percentage of NK cells expressing activation markers CD27 48 h after oHSV inoculation, assessed by flow cytometry. C) Percentage of NK cells expressing activation markers CD69 48 h after oHSV inoculation, assessed by flow cytometry. D) Percentage of IFN-γ+ NK cells 48 h after oHSV inoculation, assessed by flow cytometry. E) IFN-γ mRNA expression was determined in oHSV inoculated hemisphere in the presence or absence of TGF-β1 or the neutralizing antibody 1D11 by real-time RT PCR. F) Percentage of splenic NK cells expressing the degranulation marker, CD107a, following TGF-β1 treatment, quantified by flow cytometry. The data are representative of two independent experiments with three mice in each group per experiment. * P < 0.05. ** P < 0.01
Macrophage/microglia are suppressed by TGF-β1 treatment in vivo
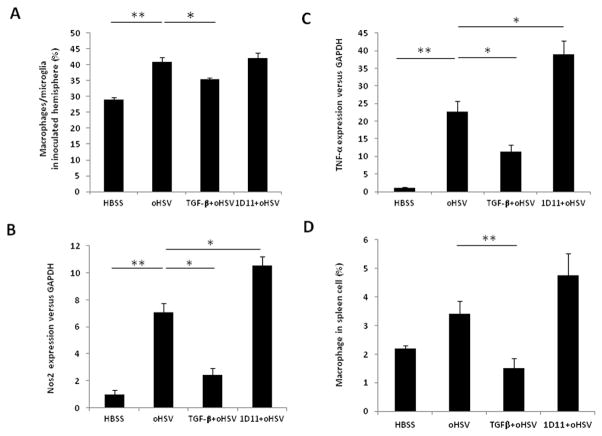
We then investigated whether TGF-β1 pretreatment affected macrophage/microglia intracranial infiltration in the 4C8 syngeneic GB mouse model treated with oHSV. Indeed, a significant decrease in the percentage of macrophage/microglia (CD45+CD11b+) was observed in the brain tissues of mice receiving TGF-β1 in conjunction with OV therapy (Fig. 4A). Consistent with this result, the mRNA expression levels of two macrophage/microglia activation markers, Nos2 and TNF-α (19), were decreased in tumor-bearing hemispheres after TGF-β1 treatment, while the opposite effect was observed in mice receiving 1D11 (Figs. 4B and 4C). A similar decrease in the percentage of macrophages was also detected in spleens from TGF-β1 pretreated mice (Fig. 4D). The time-course analysis of TGF-β1-mediated inhibition of macrophage/microglia intracranial infiltration in the presence of oHSV was similar to that of NK cells (Supplementary Fig. 2B)
Figure 4. TGF-β1 pretreatment decreases macrophage/microglia infiltration and activity in brains and spleens of the 4C8 syngeneic immunocompetent GB mouse model.
A) Percentage of CD45+CD11b+ macrophage/microglia in tumor-inoculated hemispheres after TGF-β1 or 1D11 pretreatment in oHSV therapy by flow cytometry. B) Nos2 mRNA expression in CD45+CD11b+ macrophage/microglia in tumor-inoculated hemispheres after TGF-β1 or 1D11 pretreatment in oHSV therapy by real-time RT-PCR. C) TNF-α mRNA expression in CD45+CD11b+ macrophage/microglia in 4C8 tumor-inoculated hemispheres after TGF-β1 or 1D11 pretreatment in oHSV therapy by real-time RT-PCR. D) Percentage of CD45+CD11b+ macrophages in spleens of 4C8 syngeneic GB mice after TGF-β1 or 1D11 treatment prior to oHSV therapy, by flow cytometry. The data are representative of two independent experiments with three mice in each group in every experiment. * P < 0.05, * * P < 0.01
TGF-β1 pretreatment prior to oHSV administration inhibits GB tumor growth and prolongs survival of tumor-bearing mice in both xenograft and syngeneic models
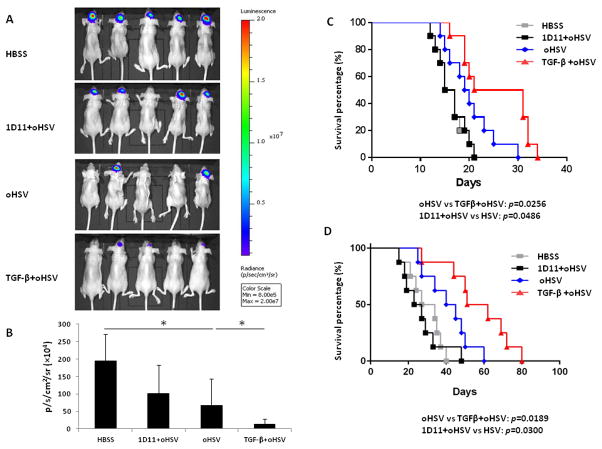
To further address the potential application of TGF-β1 treatment in oHSV therapy, we first utilized the GB30 xenograft GB model. Patient-derived GB30 stem-like cells expressing firefly luciferase were intracranially implanted into the brains of nude mice. TGF-β1 or 1D11 was administered on day 7 and oHSV or vehicle (HBSS) was injected on day 8 after the tumor cell implantation. Compared to control mice receiving HBSS without oHSV or 1D11 prior to oHSV, mice that received either oHSV alone or TGF-β1 prior to oHSV had significantly reduced tumor growth as determined by bioluminescence imaging (Fig. 5A). This reduction in tumor growth was significantly greater in mice pretreated with TGF-β1 compared to those receiving oHSV alone (Fig. 5B). In agreement with these data, mice treated with TGF-β1 and oHSV survived significantly longer than those treated with oHSV alone (median survival of 26 vs. 19.5 days between the TGF-β1 plus oHSV combination group and the oHSV-only group, p < 0.05) (Fig. 5C). Additionally, mice receiving oHSV alone survived significantly longer than those pretreated with the TGF-β blocking antibody 1D11 (median survival of 19.5 vs. 16 days between the oHSV-only and the 1D11+oHSV combination group, p < 0.05; median survival was 16 days for the HBSS group). Results from the 4C8 syngeneic mouse model, shown in Fig 5D, were consistent with those from the GB30 xenograft model. In the 4C8 model, mice that were pretreated with TGF-β1 before receiving oHSV survived longer than those receiving oHSV alone, while survival of mice pretreated with 1D11 was significantly shorter (median survival of 56.5 vs. 42.5 days between the TGF-β1+oHSV combination group vs. the oHSV-only group, p < 0.05; median survival of 42.5 vs. 25 days between the oHSV-only group and the 1D11+TGF-β1 combination group, p < 0.05; median survival of HBSS group is 30.5 days).
Figure 5. TGF-β1 pretreatment suppresses growth of orthotopic GB and prolongs the survival of GB-bearing mice in the presence of oHSV treatment.
A) Brain bioluminescence imaging of GB30-bearing mice, which are treated with vehicle (HBSS), 1D11 plus oHSV, oHSV alone, or TGF-β1 plus oHSV. B) Quantification summary of units of photons per second per centimeter squared per steradian from (A). C) Survival analysis of GB30-bearing mice treated with HBSS, 1D11 plus oHSV, oHSV alone, or TGF-β1 plus oHSV (n = 10 for each group). D) Survival analysis of 4C8-bearing mice treated with HBSS, 1D11 plus oHSV, oHSV alone, or TGF-β1 plus oHSV (n = 8 for each group). * P < 0.05
TGF-β pretreatment achieved effects similar to NK cell depletion in oHSV therapy
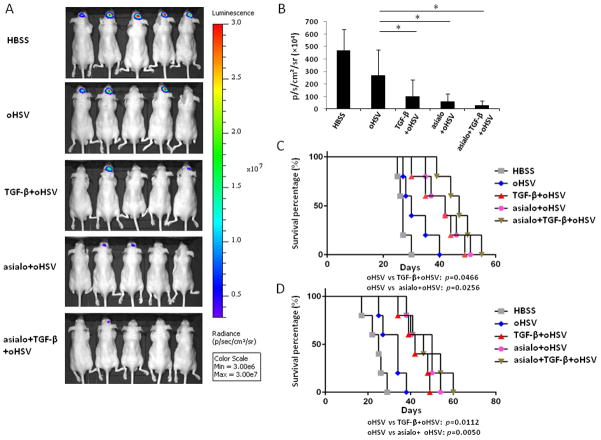
To further explore if the augmented oHSV efficacy resulting from TGF-β1 treatment was associated with dampened NK cell innate immune responses, an asialo-GM1 antibody was injected into athymic mice or B6D2F1 mice to deplete NK cells prior to TGF-β1 treatment, both in our oHSV-treated GB30-luciferase model and our 4C8 model. As shown in Figure 6A and quantified in 6B, bioluminescence imaging data indicated that TGF-β1 pretreatment showed effects similar to asialo-mediated NK cell depletion. Both NK cell depletion and TGF-β1 treatment resulted in significant improvements in survival in the presence of oHSV therapy. However, there were no significant differences among TGF-β1 treatment, NK cell depletion, and their combination in either the GB30 xenograft model (Fig. 6C) or the 4C8 syngeneic model (Fig. 6D). Also, virus titers in the hemispheres had no differences among TGF-β1 treatment, NK cell depletion, and their combination (Supplementary Fig. 4A). Similar data were observed for macrophage/microglia intracranial infiltration (Supplementary Fig. 4B).
Figure 6. TGF-β pretreatment achieved effects similar to NK cell depletion in oHSV therapy.
A) Brain bioluminescence imaging of GB30-bearing mice treated with vehicle (HBSS), oHSV alone, TGF-β1 plus oHSV, asialo plus oHSV, and the combination of asialo, TGF-β1, and oHSV. B) Quantification summary in units of photons per second per centimeter squared per steradian from (A). C) Survival analysis of GB30-bearing mice treated with HBSS, oHSV alone, TGF-β1 plus oHSV, asialo plus oHSV, and the combination of asialo, TGF-β1, and oHSV (n = 5 for each group). D) Survival analysis of 4C8-bearing mice treated with HBSS, oHSV alone, TGF-β1 plus oHSV, asialo plus oHSV, and the combination of asialo, TGF-β1, and oHSV (n = 5 for each group). * P < 0.05
Discussion
In this study, we determined the effect of TGF-β in innate immune responses to oHSV infection. We also characterized the potential use of TGF-β to inhibit innate immune responses and enhance oHSV therapy for GB (9,10,16). Our in vitro and in vivo studies demonstrate that TGF-β inhibits NK cell and macrophage/microglia intracranial recruitment, activation, and function, thereby permitting enhanced oHSV replication. We demonstrate that the combination of TGF-β with oHSV significantly increases the survival of mice in both syngeneic and xenograft GB models. The evidence in this study strongly supports that TGF-β regulates innate immune responses in the setting of oHSV therapy and that TGF-β levels can be therapeutically modulated to enhance oHSV efficacy.
OV can selectively infect and reproduce in cancer cells, resulting in cell lysis and virus spread. However, the recruitment and activation of innate immune cells following OV infection are detrimental to anti-tumor efficacy. NK cells, macrophages, and microglia have all been shown to be deleterious to oHSV replication and spread in tumor cells, as reported by our group and others (10,16,20). Using the potently immunosuppressive cytokine TGF-β, here we demonstrate that innate immune responses can be temporarily suppressed to facilitate oHSV replication and spread, thus enhancing antitumor efficacy of the viruses.
While the host innate immune response can be detrimental to OV efficacy, this response also possesses an anti-tumor capacity which can be harnessed for anti-GB therapy. NK cells can recognize and eliminate tumor cells while sparing normal “self” cells. Many tumor cells down-regulate class I major histocompatibility complex (MHC) molecules and/or up-regulate NKG2D ligands, both of which can promote NK cell cytotoxicity to these cells (21). Therefore, it is important to balance the anti-virus and anti-tumor arms of the host innate immune response. There is a consensus that TGF-β acts as an immunosuppressive agent in the case of GB(22). TGF-β not only down-regulates the receptor NKG2D on NK cells and on CD8+ T cells in glioma patients, but it also suppresses expression of the ligands for NKG2D on glioma cells (23,24). Based on this, we pretreated GB-bearing mice with a single dose TGF-β before oHSV administration to create a temporary immunosuppressive window that allowed oHSV to replicate and propagate efficiently in GB cells and maximize oHSV anti-GB activity. Our data suggest that TGF-β, utilized in a proper manner, has the potential to enhance oHSV efficacy against GB in the clinical setting.
In addition to tumor cells themselves, tumor-associated macrophage/microglia in the glioma microenvironment also secrete high levels of TGF-β (25). Our data indicate that these levels of TGF-β are insufficient to allow effective viral propagation, although they likely have a minor positive effect. We found that the inhibition of basal levels of TGF-β in the tumor microenvironment with a pan-TGF-β neutralizing antibody prior to oHSV injection reduced the efficacy of oHSV therapy. This further supports TGF-β as an important modulator in oHSV efficacy, and is consistent with our data showing that transiently increasing TGF-β levels in the tumor microenvironment enhances oHSV-mediated tumor eradication and prolongs survival of GB-bearing mice.
Innate immunity is the first line of viral defense, and early oHSV expansion is thought to be important for anti-tumor efficacy. Of note, in our orthotopic GB model, we observed significant numbers of NK cells and macrophages but few T and B cells in the brain after the intratumoral injection of oHSV (10). These results suggest that early immune suppression of NK cells and macrophages in particular may improve oHSV therapy. TGF-β has the capacity to inhibit multiple types of innate immune cells, including NK cells, macrophages and microglia, presenting an ideal cytokine approach to limit innate immune response in the setting of oHSV therapy for GB. In the GB30 xenograft athymic mouse model, which lacks an adaptive immunity arm, we found that the administration of TGF-β enhanced oHSV therapy to GB. However, the role of the adaptive immune response in oHSV therapy for GB cannot be examined in this model. It has been reported that in certain circumstances, an adaptive immune response to OV in the treatment of GB can occur (26). Importantly, in the immune-competent 4C8 GB mouse model, we still observed enhanced survival of oHSV and TGF-β treated mice. These data strengthen our original hypothesis that TGF-β is an excellent agent to enhance oHSV efficacy in the treatment of GB.
As with most cancers, a single agent is unlikely to achieve optimal therapeutic efficacy in GB. This might be true when considering the transient suppression of immune responses to oHSV therapy, where interactions are occurring among tumor cells, immune cells, and oHSV. Furthermore, as mentioned above, an adaptive immune response may be also involved in this setting, especially at later time points. In addition, defining optimal doses of TGF-β1 will also be important, as doses other than 1 μg per mouse also provide an improved but not optimal efficacy (data not shown). Thus, refinement of TGF-β1 doses and combinations with other immunosuppressive agents may achieve an optimal window of immune suppression, and further maximize the efficacy of oHSV therapy.
In this study, we utilized TGF-β’s potent immunosuppressive properties to enhance the efficacy of oHSV therapy for GB. However, a number of studies have indicated that endogenous TGF-β in tumor microenvironment has a tumor-promoting role (27,28). We did not observe a significant tumor promoting role when TGF-β was administered alone (Supplementary Fig. 5). Our speculation for this paradox is that the tumor-promoting role of TGF-β may only be derived from long-term exposure in the tumor microenvironment, rather than a short-term exposure with exogenous TGF-β, as performed here.
In conclusion, we demonstrate that TGF-β plays a role in the interactions among tumor cells, OV, and host immune responses, and that modulation of TGF-β levels can be harnessed to improve the efficacy of OV therapy. Further investigation is warranted to explore the clinical application of this novel strategy to improve the efficacy of oncolytic viral therapy for patients with GB and potentially other types of cancer.
Supplementary Material
Acknowledgments
Grant Support
This project was supported in part by grants from US National Institutes of Health (CA155521 to J.Y., CA185301 to M.A.C and J.Y, CA163205 to M.A.C and E.A.C, R01CA150153 and R01NS064607 to BK). J.Y was also supported by an American Cancer Society Research Scholar Grant (RSG-14-243-01-LIB), and a grant from Gabrielle’s Angel Foundation for Cancer Research.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. The New England journal of medicine. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2006–2010. Neuro-oncology. 2013;15(Suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer immunology research. 2014;2(4):295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woller N, Gurlevik E, Ureche CI, Schumacher A, Kuhnel F. Oncolytic viruses as anticancer vaccines. Frontiers in oncology. 2014;4:188. doi: 10.3389/fonc.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atherton MJ, Lichty BD. Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy. 2013;5(11):1191–206. doi: 10.2217/imt.13.123. [DOI] [PubMed] [Google Scholar]
- 6.Ingemar Robert Hans, Andtbacka FAC, Amatruda Thomas, et al. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol. 2013;31(suppl):abstr LBA9008. [Google Scholar]
- 7.Wollmann G, Ozduman K, van den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer journal. 2012;18(1):69–81. doi: 10.1097/PPO.0b013e31824671c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. Journal of the National Cancer Institute. 2007;99(23):1768–81. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nature medicine. 1999;5(8):881–7. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Breckenridge CA, Yu J, Price R, Wojton J, Pradarelli J, Mao H, et al. NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nature medicine. 2012;18(12):1827–34. doi: 10.1038/nm.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rich JN, Zhang M, Datto MB, Bigner DD, Wang XF. Transforming growth factor-beta-mediated p15(INK4B) induction and growth inhibition in astrocytes is SMAD3-dependent and a pathway prominently altered in human glioma cell lines. J Biol Chem. 1999;274(49):35053–8. doi: 10.1074/jbc.274.49.35053. [DOI] [PubMed] [Google Scholar]
- 12.Robson CN, Gnanapragasam V, Byrne RL, Collins AT, Neal DE. Transforming growth factor-beta1 up-regulates p15, p21 and p27 and blocks cell cycling in G1 in human prostate epithelium. J Endocrinol. 1999;160(2):257–66. doi: 10.1677/joe.0.1600257. [DOI] [PubMed] [Google Scholar]
- 13.He S, Chu J, Wu LC, Mao H, Peng Y, Alvarez-Breckenridge CA, et al. MicroRNAs activate natural killer cells through Toll-like receptor signaling. Blood. 2013;121(23):4663–71. doi: 10.1182/blood-2012-07-441360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34. 5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer research. 2005;65(7):2832–9. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115(2):274–81. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulci G, Dmitrieva N, Gianni D, Fontana EJ, Pan X, Lu Y, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer research. 2007;67(19):9398–406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. Journal of immunology. 2008;181(9):6417–26. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne AH, Meisen WH, Russell L, Yoo JY, Bolyard CM, Lathia JD, et al. Role of cysteine-rich 61 protein (CCN1) in macrophage-mediated oncolytic herpes simplex virus clearance. Mol Ther. 2014;22(9):1678–87. doi: 10.1038/mt.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meisen WH, Wohleb ES, Jaime-Ramirez AC, Bolyard C, Yoo JY, Russell L, et al. The Impact of Macrophage- and Microglia-Secreted TNFalpha on Oncolytic HSV-1 Therapy in the Glioblastoma Tumor Microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21(14):3274–85. doi: 10.1158/1078-0432.CCR-14-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(34):12873–8. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diefenbach A, Raulet DH. The innate immune response to tumors and its role in the induction of T-cell immunity. Immunological reviews. 2002;188:9–21. doi: 10.1034/j.1600-065x.2002.18802.x. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Alvarez-Breckenridge CA, Wang QE, Yu J. TGF-beta signaling and its targeting for glioma treatment. American journal of cancer research. 2015;5(3):945–55. [PMC free article] [PubMed] [Google Scholar]
- 23.Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro-oncology. 2010;12(1):7–13. doi: 10.1093/neuonc/nop009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, et al. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain : a journal of neurology. 2006;129(Pt 9):2416–25. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 25.Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. Journal of immunology. 2012;189(1):444–53. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 26.Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Human gene therapy. 2009;20(10):1119–32. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy LO, Poirier MB, Fortin D. Transforming growth factor-beta and its implication in the malignancy of gliomas. Targeted oncology. 2014 doi: 10.1007/s11523-014-0308-y. [DOI] [PubMed] [Google Scholar]
- 28.Katz LH, Li Y, Chen JS, Munoz NM, Majumdar A, Chen J, et al. Targeting TGF-beta signaling in cancer. Expert opinion on therapeutic targets. 2013;17(7):743–60. doi: 10.1517/14728222.2013.782287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.