Abstract
The strength of the blood-brain barrier (BBB) in providing protection to the central nervous system from exposure to circulating chemicals is maintained by tight junctions between endothelial cells and by a broad range of transporter proteins that regulate exchange between CNS and blood. The most important transporters that restrict the permeability of large number of toxins as well as therapeutic agents are the ABC transporters. Among them, P-gp, BCRP, MRP1 and MRP2 are the utmost studied. These efflux transporters are neuroprotective, limiting the brain entry of neurotoxins; however, they could also restrict the entry of many therapeutics and contribute to CNS pharmacoresistance. Characterization of several regulatory pathways that govern expression and activity of ABC efflux transporters in the endothelium of brain capillaries have led to an emerging consensus that these processes are complex and contain several cellular and molecular elements. Alterations in ABC efflux transporters expression and/or activity occur in several neurological diseases. Here, we review the signaling pathways that regulate expression and transport activity of P-gp, BCRP, MRP1 and MRP2 as well as how their expression/activity changes in neurological diseases.
Keywords: ABC efflux transporters, blood-brain barrier, neuroprotection, pharmacoresistance
Introduction
Since its discovery by Paul Ehrlich in 1885 and for more than 100 years of extensive research, blood-brain barrier (BBB) has been recognized as a dynamic and highly organized interface between brain and blood that prevents free passage of solutes and protects the CNS by isolating it from the periphery (Ribatti et al., 2006). The discovery of ABC efflux transporters in the early 1970s and subsequent demonstration of their expression within the BBB added an important element that contributes barrier function. ABC efflux transporters are a challenging research field because of their complex regulation at the BBB, both in physiological and pathological conditions. Moreover, the overlap in substrate recognition and function of these transporters adds an additional level of complexity to the understanding of their role and regulation (Miller, 2010). Here, we present an overview of the pathways that regulate the major ABC efflux transporters at BBB and their dysregulation in neurological disease.
The blood-brain barrier
The blood-brain barrier (BBB) is a selectively restrictive interface between the vascular system and the CNS that maintains brain homeostasis by regulating the chemical environment, immune cell transport, and the entry and disposition of xenobiotics (Hawkins and Davis, 2005). The layer of endothelial cells (ECs) that form the vessel/capillary walls defines the BBB (Ballabh et al., 2004). More recently, the concept of the neurovascular unit has been introduced to recognize the functional interactions among neurons and non-neuronal cells, such as vascular cells (ECs and pericytes) and glia (astrocytes, microglia, and oligodendroglia), in regulating the function of BBB (Abbott et al., 2010; Abbott, 2013; Hawkins and Davis, 2005). However, the EC layer lining the brain capillaries is the main infrastructure of the BBB that forms the physical barrier against free movement of drugs, xenobiotics, endogenous peptides and other circulating compounds (Abbott et al., 2006; Wong et al., 2013). In addition to the BBB, capillaries in the spinal cord form a blood-spinal cord barrier (BSCB) that is similar in structure and function to the brain ECs (Campos et al., 2012; Wang et al., 2014). Within CNS barriers, ECs are polarized cells with an apical membrane facing the blood (luminal membrane) and a basolateral membrane facing the brain tissue (abluminal membrane) and anchored to a continuous basement membrane; ECs are connected to each other by tight and adherens junctions (Wong et al., 2013). Tight junctions (TJ) between adjacent ECs contain transmembrane TJ proteins, occludin, claudins and junctional adhesion molecules (JAMs), and adaptor molecules that connect TJ proteins to the actin cytoskeleton. Similarly, adherens junctions (AJ) consist of transmembrane AJ proteins VE-cadherin and catenin, and adaptor molecules that link AJ proteins to the actin cytoskeleton (Abbott et al., 2010; Zlokovic, 2008). Although a sealed EC monolayer forms a restrictive barrier that precludes free movement of substances between blood and brain, these cells express a wide range of transport proteins and receptors that modulate the movement of solutes across the BBB (Cecchelli et al., 2007). It has been estimated that these transporters constitute 10–15% of all proteins in the neurovascular unit (Enerson and Drewes, 2006). Four types of transport protein are expressed at the BBB; ion channels, solute transporters, aquaporins, and ATP-powered pumps. ATP-powered pumps or ABC transporters play critical roles in neuroprotection and pharmacoresistance (Vasiliou et al., 2009).
ABC transporters at BBB
ATP-binding cassette (ABC) transporters comprise one of the largest protein superfamilies, with members found in all living organisms, from microbes to humans. In humans, 49 genes encode for ABC transporters; these are classified into seven subfamilies, designated A to G, on the basis of sequence homology and functional similarities (Moitra and Dean, 2011; Vasiliou et al., 2009). ABC transporters are ubiquitous membrane-bound proteins that utilize ATP hydrolysis to drive the transport of a vast array of lipophilic, amphipathic substrates across membranes (Loscher and Potschka, 2005b). ABC transporters are critically important in several physiological functions and defects in many of these transporters are associated with severe inherited disorders (Moitra and Dean, 2011). ABC transporters are expressed in most cells, but they are most highly expressed in barriers (blood-brain barrier, blood-spinal cord barrier, blood-cerebrospinal fluid barrier, blood-testis barrier, and blood-placental barrier) (Aye and Keelan, 2013; Campos et al., 2012; Hartz and Bauer, 2011; Leslie et al., 2005; Robillard et al., 2012), excretory tissues (liver and kidney) (Choi and Yu, 2014) and absorption tissues (intestine and colon) (Oude Elferink and de Waart, 2007). In human BBB, three ABC subfamilies, B, C, and G, contain efflux transporters that extrude metabolic waste products, foreign chemicals (xenobiotics), and a large number of drugs from the brain into the blood (Hartz and Bauer, 2011). Among these subfamilies, ABCB1 (multidrug resistance proteins; MDR1 or P-gp), ABCC (multidrug resistance-associated protein; MRPs), and ABCG2 (breast cancer resistance protein; BCRP) are the best-studied (Hartz and Bauer, 2011; Miller, 2015). We will focus our discussion on the mechanisms that regulate expression and activity of these three transporters in the endothelium of BBB, their dysregulation in neurological disease, and their contribution to neuroprotection and development of CNS pharmacoresistance.
ABCB1
ABCB1 or P-glycoprotein (P-gp) is the 170-kDa glycoprotein product of the MDR1 gene that is highly expressed on the luminal membrane of the endothelial cells at the BBB, and to a much lesser extent in brain parenchyma, neuronal, and glial cells (Loscher and Potschka, 2005b; Sharom, 2008). Several studies have reported that astrocyte and microglial cultures express low levels of P-gp (Decleves et al., 2000; Ronaldson et al., 2004), while other studies did not confirm these findings (Daood et al., 2008; Volk et al., 2005). These contradictions suggested that P-gp expression in brain parenchymal cells is nominal, although it may be significantly enhanced in pathologic conditions, for example, amyotrophic lateral sclerosis (ALS) (Jablonski et al., 2012), epileptic seizures (Bauer et al., 2008), focal cortical dysplasia (Ak et al., 2007), and brain tumors (Aronica et al., 2003; Haar et al., 2012; Lin et al., 2014).
P-gp is composed of two homologous and highly conserved six-transmembrane proteins interconnected by a central cytoplasmic linker domain with a large substrate-binding pocket within the plasma membrane (Aller et al., 2009; Hennessy and Spiers, 2007). In addition, P-gp is subjected to post-translational modifications, in particular glycosylation in the Golgi apparatus, to form the mature core glycosylated protein. Previous studies suggested that glycosylation protects P-gp from degradation and mislocalization (Fu and Arias, 2012; Perego et al., 2010). Strategic localization of P-gp at the luminal membrane of BBB endothelial cells and its wide substrate selectivity make it an effective defense against a wide spectrum of endogenous ligands, therapeutic drugs and xenobiotics (Miller et al., 2008). Therefore, P-gp is widely believed to be the most important ABC transporter in the CNS that plays a crucial role in both neuroprotection and pharmacoresistance. It provides brain neuroprotection by efficiently limiting the entry of exogenous molecules into brain, while it could limit the entry of therapeutics and contributes to the development of pharmacoresistance particularly in certain conditions where its expression at the BBB is increased significantly (Miller et al., 2008).
ABCC Family
In humans, the family of multidrug resistance-associated proteins (MRPs or ABCC) has nine members involved in drug transport (Dallas et al., 2006). Among them, MRP1, 2, 4 and 5 are the most studied. MRP1, MRP2 and MRP4 have definitive localization in brain capillaries (Miller, 2014). In the CNS, MRP1 is a landmark of the blood-CSF barrier (BCSFB) because it localizes at the basolateral membrane of choroid plexus epithelial cells; it is also detected at the luminal and abluminal membranes of capillary endothelium (Daood et al., 2008; Gazzin et al., 2008; Gazzin et al., 2011; Rao et al., 1999). Moreover, MRP1 expression has been reported in human astrocytoma and glioblastoma cells obtained from epileptic patients (Decleves et al., 2002; Hirrlinger et al., 2002). However, using immunofluorescence techniques, Nies et al could not detect MRP1 in neurons or glial cells in human cerebral cortex and subcortical white matter (Nies et al., 2004). MRP2 is expressed mainly on the apical side of cell membrane of capillary endothelium while, in contrast to MRP1, its expression in BCSFB is negligible (Dallas et al., 2006). Moreover, MRP2 expression was detected in rat astrocytes (Dallas et al., 2003) as well as pericytes in culture (Yousif et al., 2007). MRP2 expression data within the human brain parenchyma is currently lacking. Mrp4 seems to be expressed on both the luminal and abluminal membranes of the rat brain capillary endothelium. Similar to P-gp, MRPs are made of two homologous and highly conserved six-transmembrane proteins interconnected by a central cytoplasmic linker domain and they are subjected to glycosylation that protects MRPs from degradation and mislocalization (Chang, 2007; Perego et al., 2010). They also contain an additional membrane-spanning domain.
ABCG2
ABCG2 or breast cancer resistance protein (BCRP) can actively extrude a broad range of endogenous and exogenous substrates. It is predominantly expressed at the luminal membrane of the BBB cells and displays substrate overlap with P-gp (Aronica et al., 2005; Maliepaard et al., 2001). However, the unique structural feature that distinguishes BCRP from other ABC transporters, including P-gp, is that it is a 72-kD half ABC transporter with only one nucleotide-binding domain and one membrane-spanning domain. It works as homodimer, possibly as heterodimer with other ABCG half transporter isoforms (Mao and Unadkat, 2015). In addition, posttranslational modifications such as glycosylation do not appear to contribute to function or membrane localization of BCRP (Diop and Hrycyna, 2005). Interestingly, whereas expression of P-gp is higher than that of BCRP in the murine BBB, recent data showed that mRNA levels of BCRP are about eightfold higher than P-gp mRNA levels in human brain capillaries (Dauchy et al., 2008; Kamiie et al., 2008). Nevertheless, BCRP is thought to act in concert with P-gp to exert a synergistic effect in limiting BBB permeability (Agarwal et al., 2011). Since its discovery in 1998, expression of BCRP has been confirmed in endothelial cells of capillaries isolated from human brain (Aronica et al., 2005), in cultured human BMEC mouse brain capillaries, immortalized rat brain endothelial cells, primary cultured rat astrocytes and rat and mouse brain microglial cells (Eisenblatter et al., 2003; Lee et al., 2007; Zhang et al., 2003). BCRP localization in neurons is controversial. Although BCRP was not observed initially in neurons of normal subjects and epileptic patients (Aronica et al., 2005), it was detected later in neurons of stroke patients and refractory epileptic patients (Dazert et al., 2006; Lazarowski et al., 2007).
ABC efflux transporters substrates
The most remarkable feature of ABC efflux transporters is their broad substrate spectrum (Miller, 2015). Although less data are available for specific endogenous substrates, some hormones, phospholipids, cytokines, and prostaglandins are known to be effluxed. For instance, several phospholipids, sphingolipids, aldosterone, and amyloid-β (neuronal byproduct) are P-gp substrates; glutathione (GSH), GSH-conjugated leukotrienes and prostaglandins, glucoronide and sulfate conjugates are MRP1 substrates; estrones and bile acids are BCRP substrates (Sharom, 2008). In addition to endogenous molecules, ABC efflux transporters handle structurally diverse therapeutic agents that range from small drug molecules such as morphine (285 Da) to large drugs such as cyclosporine-A (1200 Da). However, it is worth noting that P-gp, BCRP and MRPs have, in part, overlapping substrates belonging to different chemical classes including chemotherapeutics, HIV protease inhibitors, opioids, antibiotics, and many more (Table 1) (Sharom, 2008). Despite their large substrate-binding pocket and wide range of substrate specificity, ABC efflux transporters show some preferences for molecules with certain physicochemical characteristics. For example, most of P-gp substrates are low molecular weight molecules (300-1000 Da) that are amphipathic, lipophilic, planar (have aromatic rings) and either neutral or cationic at physiological pH (Begley, 2004). MRPs prefer anionic substrates and conjugates, while BCRP transports both positively and negatively charged molecules and conjugated organic anions, particularly sulfated and glucuronide conjugates (Mao and Unadkat, 2015; Zhou et al., 2008). It is worth mentioning that MRP1 and MRP2 have similar substrate profiles but are distinguishable based on the degree of substrate affinity and transport kinetics, with MRP1 having higher affinity in several instances (Dallas et al., 2006; Kruh and Belinsky, 2003). Table 1 lists selected therapeutic agents that are used in the treatment of neurological disorders and are known to be substrates for efflux transporters.
Table 1.
List of selected clinically relevant ABC efflux transporters substrates
| Drug Class | P-gp | BCRP | MRPs |
|---|---|---|---|
| ALS drug | Riluzole | Riluzole | |
| Analgesics | Morphine | ||
| Methadone | |||
| Fentanyl | |||
| Antibiotics | Erythromycin | Ciprofloxacin | Azithromycin |
| Gramicidin A | Ofloxacin | Ciprofloxacin | |
| Rifampicin | Norfloxacin | Difloxacin | |
| Anticancer | Etoposide | Etoposide | Etoposide |
| Irinotecan | Irinotecan | Irinotecan | |
| Vinblastine | Methotrexate | Vinblastine | |
| Vincristine | Imatinib | Vincristine | |
| Temozolomide | Erlotinib | Methotrexate | |
| Imatinib | Gefitinib | Cisplatin | |
| Gefitinib | Mitoxantrone | Imatinib | |
| Gefitinib | |||
| Antidepressant | Amitryptiline | ||
| Nortryptiline | |||
| Doxepin | |||
| Venlafaxine | |||
| Paroxetine | |||
| Antidiarrheal | Loperamide | ||
| Antiemetic | Ondansetron | ||
| Domperidone | |||
| Antiepileptics | Felbamate | Levetiracetam | |
| Topiramate | Phenobarbital | ||
| Phenytoin | |||
| Phenobarbital | |||
| Lamotrigine | |||
| Levetiracetam | |||
| gabapentin | |||
| Antihelminthic | Ivermectin | ||
| Abamectin | |||
| Antihistamine | Fexofenadine | ||
| Cardiovascular agents | Reserpine | ||
| Propronalol | |||
| Verapamil | |||
| Nifedipine | |||
| Diltiazem | |||
| Digoxin | |||
| Quinidine | |||
| Amiodarone | |||
| Lovastatin | |||
| Simvastatin | |||
| Corticosteroids | Dexamethasone | ||
| Hydrocortisone | |||
| Corticosterone | |||
| Cortisol | |||
| Aldosterone | |||
| HIV drugs | Ritonavir | Zidovudine | Ritonavir |
| Indinavir | Lamivudine | Indinavir | |
| Saquinavir | Saquinavir | ||
| Amprenavir | Nelfinavir | ||
| Nelfinavir | Zidovudine | ||
| Lopinavir | |||
| Immunosuppressant | Cyclosporine-A | Cyclosporine-A | Cyclosporine-A |
| Tacrolimus | Tacrolimus | Tacrolimus | |
| Sirolimus | Sirolimus | Sirolimus | |
| Reserpine | |||
| Prazosin |
Regulation of ABC efflux transporters at BBB
Evidence from numerous studies indicates that regulation of the expression and activity of ABC efflux transporters is complex, involving pre- or post-transcriptional modifications (Miller, 2015). Most of these signaling pathways that regulate expression of ABC efflux transporters in brain endothelium are activated by either surface receptors or nuclear receptors (Miller, 2014). Studies of the regulatory mechanisms of ABC efflux transporters are important to understand how BBB respond to the changes in its environment (dynamics of BBB), what are the factors that urge specific response, and the extent and the rapidity of this response. Moreover, identifying signaling pathways that regulate ABC efflux transporters in health and in neurological disorders could provide promising therapeutic targets that could be modified to change disease progression or enhance the efficacy of therapeutic agents. In the following sections, we will discuss regulatory signaling pathways that control the expression and activity of ABC efflux transporters. These pathways will be classified according to the trigger or stressor that activates them.
Inflammatory stress
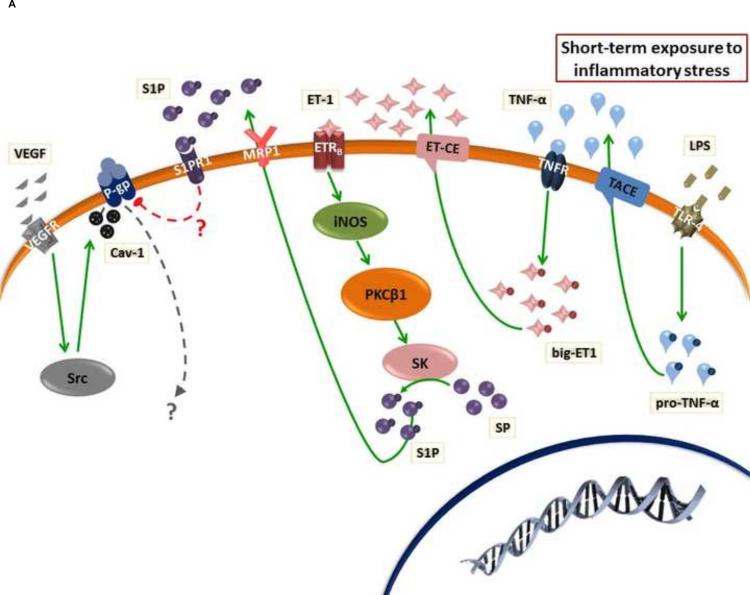
Many systemic and CNS diseases, such as infections, stroke, trauma, neurodegenerative diseases, and neuroinflammation, are associated with inflammatory responses that affect the BBB (Rosenberg, 2012; Zlokovic, 2008). Inflammatory responses are mediated by proinflammatory cytokines [e.g. tumor necrosis factor-α (TNF-α), interleukins (IL-1 and IL-6)] and inflammogens [e.g., lipopolysaccharide (LPS)] that bind to endothelial cell surface receptors and trigger a wide range of changes in endothelial cell function (Chakravarty and Herkenham, 2005; Chan et al., 2011; Heneka et al., 2015; Konsman et al., 2004; Nadeau and Rivest, 1999; Nguyen et al., 2002; Rivest, 2003; von Wedel-Parlow et al., 2009). Not surprisingly, these inflammatory mediators can alter the expression and activity of ABC efflux transporters (Bauer et al., 2007; Hartz et al., 2006; von Wedel-Parlow et al., 2009). ABC efflux transporter expression and activity in brain capillary endothelial cells showed complex responses to inflammatory stress based on the time course of exposure (Figure 1) (Bauer et al., 2007; Hartz et al., 2004; Hartz et al., 2006). One of the most important inflammatory mediators that participates in the regulation of ABC efflux transporters is proinflammatory cytokine, TNF-α (Bauer et al., 2007; Hartz et al., 2004; Hartz et al., 2006). Short-term exposure of isolated rat brain capillaries (~1h) to TNF-α decreased P-gp activity without affecting its expression while, expression and activity of MRP1 and BCRP were unchanged (Hartz et al., 2004; Hartz et al., 2006). In these capillaries, TNF-α induced release of ET-1 from endothelial cells and activation of endothelin receptor-B (ETRB) (Figure 1A) (Hartz et al., 2004). Activation of ETRB enhanced activity of inducible nitric oxide synthase (iNOS) and protein kinase C-β1 (PKCβ1) (Hartz et al., 2006; Rigor et al., 2010; Wang et al., 2011b). PKCβ1 activates sphingosine kinase (SK), which converts sphingosine into sphingosine-1-phosphate (SIP) (Cannon et al., 2012). S1P is effluxed to extracellular space by MRP1, which has been reported to be essential for the activity of this regulatory pathway (Cartwright et al., 2013). Extracellular S1P binds to S1P receptor-1 (S1PR-1) at the cell membrane of endothelial cells and activates a signaling cascade that involves a G-protein coupled receptor and results in a rapid reduction in P-gp activity (Cannon et al., 2012).
Figure 1.
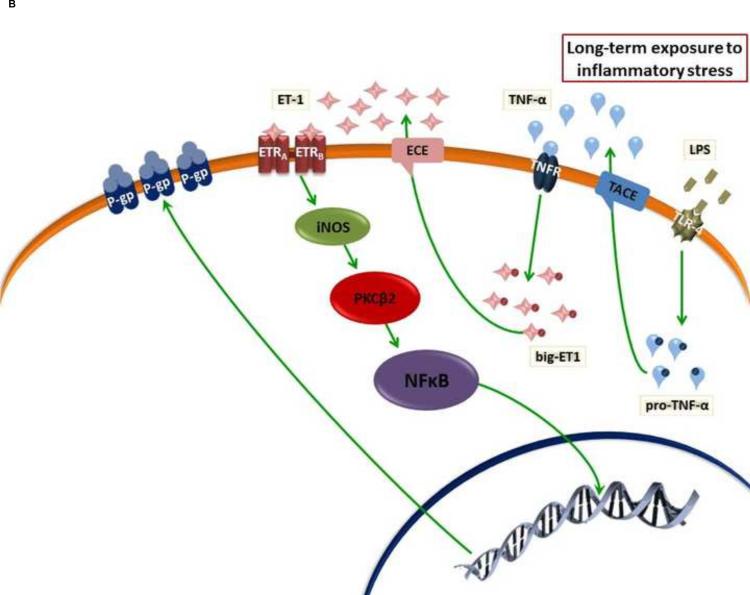
(A) Effect of short-term exposure to inflammatory stress on ABC efflux transporter activity and expression in the endothelium of BBB. ET-1 and VEGF enhance rapid reduction in the activity of P-gp through activation of PKCβ1 and Src kinase, respectively. (B) Long-term exposure to inflammatory stress enhances increased P-pg expression through PKCβ2 and NF-κB pathway. In both pathways, TNF-α or LPS could stimulate ET-1 release. While BCRP and MRP1 activity and expression did not change upon short-term exposure to inflammatory stress, only BCRP expression decreased after long-term exposure. However, the cascade of molecular steps that mediates reduction in BCRP expression has not been defined.
In accordance with ET-1 signaling pathway, it has been reported that TNF-α release under inflammatory stress activates TNF receptor-1 (TNF-R1) in the cell membrane of endothelial cells and enhances ET-1 release. ET-1 is produced in endothelial cells in a proform (big-ET), which is cleaved by endothelin-converting enzyme (ECE) to the soluble active ET-1 (Figure 1) (Hartz et al., 2006). In case of infection, it has been reported that LPS binds to toll-like receptor-4 (TLR4) in brain capillary endothelial cells and enhances rapid reduction in P-gp activity through the release of TNF-α [TNF-α is produced in endothelial cells in a proform (TNF-α precursor), which is cleaved by TNF-α converting enzyme (TACE) to the soluble active TNF-α] (Hartz et al., 2006). However, it is not yet clear whether pathogen (or microbe) associated particles other than LPS [e.g. double-stranded (viral) RNA that activates TLR3 and gram-positive cell wall constituents that activate TLR2)] directly or indirectly (through TNF-α) activate ETB receptor signaling and thereby rapidly alter P-gp activity (Bauer et al., 2007; Hartz et al., 2006; Schinelli, 2006).
Another endogenous polypeptide that could be released in response to different conditions including inflammatory stress is the vascular endothelial growth factors (VEGF) (Ferrara, 2002). Similar to ET-1, VEGF has been observed to mediate rapid, specific, and concentration-dependent reduction in P-gp transport activity in brain endothelial cells (Figure 1A) (Hawkins et al., 2010b). However, different pathways other than the PKCβ1 pathway mediate VEGF activity. In isolated rat capillaries VEGF reduces P-gp transport activity through activation of VEGF receptor-2 (VEGFR-2 or flk-1 receptor) and Src kinase (Hawkins et al., 2010b). Consistent with this, intracerebroventricular injection of low doses of VEGF in rat brain increases accumulation of the P-gp substrates, 3H-morphine, and 3H-verapamil. Systemic administration of a Src kinase inhibitor blocked the effects of VEGF on P-gp mediated transport (Hawkins et al., 2010b). In accordance with Src activation, it has been reported that inhibition of P-gp transport activity is associated with caveolin-1 (Cav-1) phosphorylation by Src kinase in caveolae, which provides a favorable environment for the regulation of P-gp (Figure 1A) (Jodoin et al., 2003). Moreover, transfection of rat brain endothelial cells (RBE4 cells) with Src inhibits the transport of 3H-verapamil or 3H-taxol by P-gp through phosphorylation of Cav-1 (Barakat et al., 2007).
Although it is not yet clear how this rapid reduction in P-gp function occurs, a recent study showed that signaling through VEGF/Src-kinase removed P-gp from the surface of the luminal plasma membrane and provided protection from proteolysis in an in vivo protease-K protection assay (Hawkins et al., 2010a). In the same study, activation of PKCβ1 provided no detectable protection, indicating no change in the cellular location of the P-gp. However, other possible mechanisms such as presence of PKCβ1 adaptor protein, covalent modification of P-gp or changes in its lipid or protein microenvironment may underlie the rapid loss of transport activity (Hawkins et al., 2010a).
In contrast to reduced P-gp activity observed after short-term exposure to inflammatory stress, several studies reported increased activity and expression of P-gp with long-term exposure (Bauer et al., 2007; Zhao et al., 2002). Bauer et al showed that long-term exposure (>6 h) of rat brain capillaries to TNF-α activates TNF-R1 and induces release of ET-1, which binds to endothelin receptor (ETR) (Figure 1B) (Bauer et al., 2007). It was suggested that both isoforms of ETR (ETRA and ETRB) are involved by forming a dimer, which stimulates NF-κB nuclear translocation through activation of inducible nitric oxide synthase (iNOS) and protein kinase C-β2 (PKCβ2) (Figure 1B) (Bauer et al., 2007). NF-κB is a stress-driven, master regulator of ABC efflux transporter expression at the blood–brain barrier. NF-κB is a homo- or hetero-dimer of RelA/p65, RelB, c-Rel, p50, and p52 subunits. In the cytoplasm, NF-κB dimers are held inactive by association with IκB proteins (Inhibitor of κB); this masks the nuclear localization signals of NF-κB. Phosphorylation of IκB by IκB kinase complex, leads to degradation of IκB and release of NF-κB dimer, which translocates to the nucleus, where it binds DNA consensus sequences, and alters expression of target genes (Hayden and Ghosh, 2012). In addition to TNF-α and ET-1, a wide range of soluble extracellular ligands, membrane-bound extracellular ligands as well as changes of endothelial intracellular environment (e.g. DNA damage and reactive oxygen species) appear to activate the NF-κB pathway in the brain endothelial cells.
ABC efflux transporter function and expression in endothelial cells is inhibited by the pro-inflammatory cytokines, IL-1β and IL-6. Von Wedel-Parlow et al showed that IL-1β rapidly decreases BCRP mRNA level within 6 h. However, after 24 and 48 h the mRNA levels returned to control values. On the other hand, IL-1β caused a continuous decrease in P-gp expression but did not affect MRP (von Wedel-Parlow et al., 2009). A previous study showed that treatment of the brain endothelial cell derived from various stages of development of the guinea pig with the pro-inflammatory cytokines, IL-1β, IL-6 and TNF-α has a different effect on expression of P-gp. At late gestational stage and at postnatal stage, IL-1β, IL-6 and TNF-α exposure resulted in a dose-dependent decrease in P-gp function (Iqbal et al., 2012). Cytokine-induced reductions in P-gp function were associated with decreased ABCB1 mRNA expression (Iqbal et al., 2012). Using human endothelial cells (hCMEC/D3), Poller et al showed that BCRP mRNA levels were significantly reduced by IL-1β, IL-6 and TNF-α, while P-gp mRNA levels were slightly reduced by IL-6, but significantly increased after TNF-α treatment (Poller et al., 2010). Unlike the effect of TNF-α, the cascade of molecular steps that is activated by IL-1β and IL-6 and mediates changes in expression of P-gp and BCRP has not been defined.
Consistent with the complexity of inflammatory response, effects of proinflammatory stimuli on ABC efflux transporter expression/activity at the BBB are complex, depending on the nature of the stimulus, the dose, the time of exposure and the transporter under investigation (Miller, 2015).
Oxidative stress
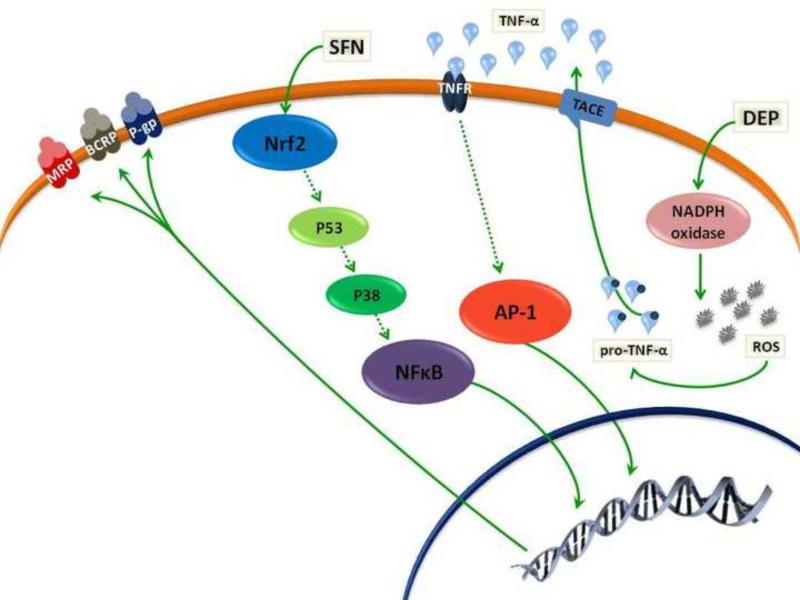
Another important stressor that mediates alteration in ABC efflux transporters is oxidative stress (Miller, 2014). Felix and Barrand provided evidence for the effect of oxidative stress on the expression of P-gp at BBB endothelium (Felix and Barrand, 2002). They exposed primary cultured rat brain endothelial cells with up to 500 μM H2O2, causing a concentration-dependent increase in expression and activity of P-gp but not of MRP1 (Felix and Barrand, 2002). A follow up study by the same group suggested involvement of several signaling effectors including ERK1/2, PKC, Akt, JNK, and NF-κB. However, the authors did not provide any evidence for the connections between these activated proteins, although they suggested that NF-κB could play the central role in P-gp modulation (Nwaozuzu et al., 2003). Another study showed that oxidative stress, induced by sulphoraphane (SFN) administration, increases overexpression of P-gp, BCRP and MRP2 at the rat BBB via a signaling pathway that includes activation of the nuclear factor (erythroid derived 2)-like 2 (Nrf2) (Figure 2). Nrf2 is a transcription factor that regulates the expression of proteins that protect against oxidative and electrophilic stress. At the rat BBB, SFN increased P-gp, BCRP and MRP2 expression by signaling indirectly through Nrf2, p53, p38 and NF-κB (Wang et al., 2014). In addition to Nrf2 pathway, it has been reported that oxidative stress induced by diesel exhaust particles (DEPs) stimulates TNF-α release and activate TNF-R1 (Figure 2). DEPs are ultrafine particles present in diesel exhaust, one of the most common urban air pollutant (Nel, 2005). Exposure to this environmental toxin has been correlated with increased risk of fatal stroke, cerebrovascular and oxidative stress that may trigger several neurological disorders (Block et al., 2004; Calderon- Garciduenas et al., 2004; Wellenius et al., 2005). Previous studies showed that, DEPs could enter the body and reach the brain, where they cause oxidative stress and up-regulate P-gp expression (Hartz et al., 2008). In this study, Hartz et al showed that 6 hours exposure of isolated rat brain capillaries to DEPs increased P-gp, BCRP, MRP1 and MRP2 expression in a concentration-dependent manner. This study demonstrated that exposure of isolated capillaries to DEPs activated NADPH oxidase and superoxide production. Release of reactive oxygen species (ROS) stimulates TNF-α release. Although DEP exposure caused TNF-α was released and TNF-R1 activated, there was no evidence for activation of ETR or PKC. Instead, TNF-R1 signaling activated the stress-activated protein kinase, c-Jun N-terminal kinase (JNK) (Figure 2) (Hartz et al., 2008). Consistent with Nwaozuzu et al., JNK phosphorylates and thus activates c-jun, a key component of the transcription factor activator protein-1 (AP-1), which in turn increases P-gp expression (Hartz et al., 2008; Nwaozuzu et al., 2003). Together, these findings suggest that downstream events for TNF-α release and TNF-R1 activation depend on the type of initial stressor.
Figure 2.
Oxidative stress induces ABC efflux transporter expression. SFN activates an Nrf2 signaling pathway that includes P53, P38 and NF-κB activation. DEP activates NADPH oxidase that releases ROS. ROS stimulates TNF-α release that activates AP-1 transcription factors through activation of TNF-R1. In DEP-driven oxidative stress, TNF-R1 activates pathways that do not involve NF-κB.
Xenobiotics
Xenobiotics activate nuclear receptors in the endothelial cells of BBB and increase expression of ABC efflux transporters (Miller, 2015). Nuclear receptors are the largest known family of transcription factors that function as modulators of tissue gene expression in response to exposure to xenobiotics (Novac and Heinzel, 2004). Several nuclear receptors have been shown to regulate ABC efflux transporters (Miller, 2014). Activation of pregnane X receptor (PXR) (Bauer et al., 2006), constitutive androstane receptor (CAR) (Wang et al., 2010), arylhydrocarbon receptor (AhR) (Wang et al., 2011a) and glucocorticoid receptors (GR) (Narang et al., 2008) increases P-gp, BCRP and MRP2 expression in the brain capillary endothelium. Wide range of substrates including, but not limiting to, therapeutic agents, dietary constituents, nutraceuticals and environmental toxicants can activate these receptors (Miller, 2014; Novac and Heinzel, 2004). In addition to xenobiotics, some nuclear receptors can be activated by endogenous ligands such as steroid hormones, bile salts, and vitamins (Novac and Heinzel, 2004). This broad substrate specificity is in accordance with the function of these nuclear receptors in the activation of the proteins that are involved in the detoxification of xenobiotics from the body such as metabolizing enzymes and efflux transporters.
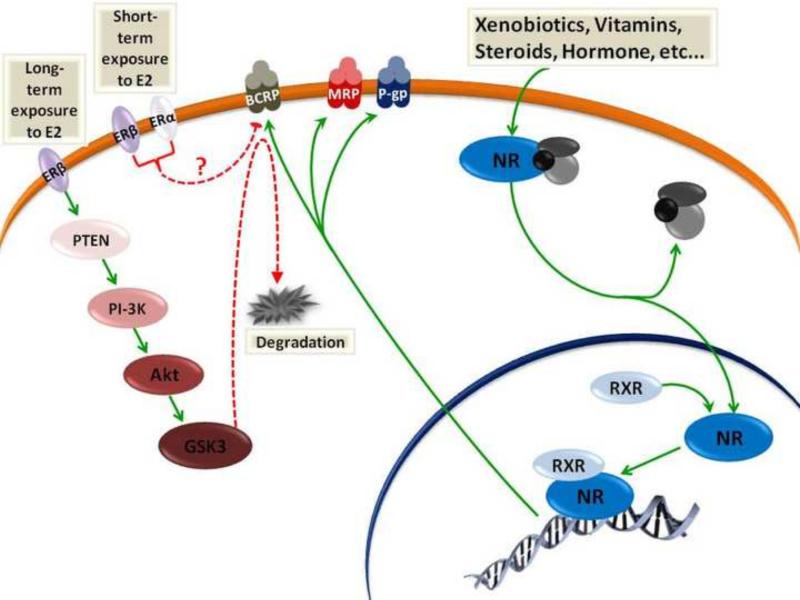
In their inactivated state, nuclear receptors typically reside in cytoplasm where they are bound to transcriptional corepressors that recruit specific histone deacetylase-containing complexes. The activation of nuclear receptors begins when xenobiotics permeate through the cell membrane either by selective transport or diffusion, bind to the nuclear receptor and stimulate its dissociation from the histone deacetylase-containing complex, translocating to the nucleus (Figure 3). Once in the nucleus, the ligand-receptor complex recruits coactivators and forms homodimers or, in many cases, heterodimerizes with the retinoid X receptor (RXR). These heterodimers bind to response elements in the promoter and enhancer regions of target genes and increase their transcription (Novac and Heinzel, 2004; Olefsky, 2001). To date, direct interaction of the ligand-bound nuclear receptor with promoter regions in the target transporter genes is the only known mechanism by which these receptors can activate ABC efflux transporters expression at BBB (Miller, 2015).
Figure 3.
Nuclear receptor activation by xenobiotics or other endogenous ligands enhances release of nuclear receptors from co-suppressors and translocation into the nucleus where they form homodimers or heterodimers with RXR to stimulate ABC efflux transporter expression. Short-term exposure to estradiol (E2) reduces BCRP activity rapidly through activation of ERα and ERβ, while long-term exposure to E2 activates ERβ, which stimulates BCRP degradation and through PTEN/PI-3K/Akt/GSK3 dependent pathway.
Although most of the nuclear receptors activate expression of P-gp, BCRP and MRP2 at BBB, there is one exception for this observation, which is related to the reported down regulation of BCRP by activation of estrogen receptor (Figure 3) (Hartz et al., 2010a; Hartz et al., 2010b; Mahringer and Fricker, 2010). Short-term exposure of male or female rat and mouse brain capillaries to estradiol (E2) rapidly and reversibly reduces BCRP transport activity without altering protein expression. Both ERα and ERβ are involved (Figure 3) (Hartz et al., 2010b). Extended exposure to E2 for more than 6 hours decreases BCRP expression. This reduction in BCRP expression is mediated by ERβ but not ERα. To decrease BCRP expression, ERβ signals through phosphatase and tensin homolog (PTEN), phosphatidylinositide-3-kinase (PI3-K), protein kinase B (Akt), and glycogen synthase kinase-3β (GSK-3β) (Figure 3) (Hartz et al., 2010a).
Glutamate
Glutamate is an excitatory neurotransmitter that contributes to excitotoxicity in several neurological diseases. For instance, excess glutamate release occurs in amyotrophic lateral sclerosis and epilepsy and its signaling via an ionotropic, N-Methyl-D-aspartate (NMDA) receptors is a major contributor to the pathophysiology of these disorders (Barnes and Slevin, 2003). Activation of glutamate receptor NMDA has been implicated in P-gp overexpression at the BBB of epileptic patients (Bauer et al., 2008). Responses to increased levels of glutamate in the brain include NF-κB activation and nuclear translocation and consequent alteration in ABC efflux transporter expression. Glutamate binds to NMDA receptor, increases calcium entry and activates phospholipase A2 (cPLA2), produceing arachidonic acid. This metabolite is converted to prostaglandin-E2 (PGE2) by cyclooxygenase-2 (COX-2) and released to extracellular space by efflux activity of MRP, likely MRP4 (Bauer et al., 2008). Extracellular PGE2 binds to a prostaglandin E receptor 1 (EP-1R), which then signals NF-κB activation and increased P-gp expression (Bauer et al., 2008; Pekcec et al., 2009; Zibell et al., 2009).
In contrast to a well-defined effect of glutamate on P-gp expression, BCRP expression at BBB after exposure to high levels of glutamate is controversial. While Yousif et al showed a significant increase in BCRP expression at rat BBB mediated by NMDA/COX-2 cascade (Yousif et al., 2012), a recent report by Salvamoser et al showed that glutamate exposure decreased BCRP expression and activity in isolated porcine capillaries (Salvamoser et al., 2015). These authors provided evidence for the contribution of NMDA/COX-2 cascade to the down regulation of BCRP expression and activity in porcine capillaries. Moreover, using human capillaries isolated from surgical specimens of epilepsy patients, they were able to confirm a glutamate-induced down-regulation of BCRP expression and activity in human capillaries, which argued against major species differences (Salvamoser et al., 2015). In similar study, the same group showed that MRP2 expression and activity increased significantly in porcine and human BBB models after exposure to glutamate. The NMDA/COX-2 cascade was shown to be involved (Luna-Munguia et al., 2015).
Dysregulation of ABC transporters in neurological disease
A compromised BBB is associated with several neurological diseases. Disease-induced BBB changes could manifest as mild disruption in tight junctions and enhanced BBB permeability or loss of chronic barrier integrity with altered transport of molecules across BBB, brain hypoperfusion and inflammatory responses (Zlokovic, 2008). Moreover, several lines of evidence implicate dysregulation of ABC efflux transporters to the malfunctioning of the BBB during neurological diseases and suggest that ABC efflux transporters dysfunction may exacerbate neurological disease or decrease the efficacy of CNS acting therapeutics (Abbott et al., 2010; Weiss et al., 2009; Wong et al., 2013). Table 2 summarizes changes in ABC efflux transporters that are associated with neurological disorders.
Table 2.
Summary of changes in ABC efflux transporters activity in neurological disorders
| Disease | P-gp | BCRP | MRP1 | MRP2 |
|---|---|---|---|---|
| Alzheimer's disease | ↓ | ↔ | ↓ | ? |
| Amyotrophic lateral sclerosis | ↑ | ↑ | ↔ | ↔ |
| Brain tumors | ↓ | ? | ↑ | ↔ |
| Creutzfeldt-Jakob disease | ↓ | ? | ? | ? |
| Depression | ↓ | ? | ? | ? |
| Epilepsy | ↑ | ↑ | ↑ | ↑ |
| HIV-encephalitis | ↔ | ↑ | ↑ | ? |
| Multiple sclerosis | ↓ | ↔ | ↔ | ↔ |
| Parkinson's disease | ↓ | ? | ? | ? |
| Schizophrenia | ↓ | ? | ? | ? |
| Stroke and ischemic brain injuries | ↑ | ↑ | ↑ | ? |
Alzheimer's disease (AD)
AD is the most common cause of irreversible dementia among the elderly with a rapidly increasing socioeconomic impact (Citron, 2010). It is a progressive neurodegenerative disorder with a complex pathogenesis that involves two well-defined pathologies, amyloid-β (Aβ) and tau-related neuropathologies (Selkoe, 2001). During the last decade, the role of ABC efflux transporters in the pathology of AD has been recognized (Cirrito et al., 2005; Qosa et al., 2014a; Rapposelli et al., 2009). Several studies have suggested ABC efflux transporters to play a pivotal role in the clearance of Aβ from the brain across BBB (Abuznait and Kaddoumi, 2012; Wolf et al., 2012). Consistent with its suggested role in Aβ clearance, a significant negative correlation exists between the densities of senile plaque lesions and P-gp levels in brain capillaries of patients with AD (Vogelgesang et al., 2002). In addition to this clinical study, P-gp was implicated to function as an Aβ efflux pump in several in vivo and in vitro studies (Cirrito et al., 2005; Kuhnke et al., 2007; Qosa et al., 2014a; Tai et al., 2009). Interestingly, using 11C-verapamil and positron emission tomography imaging, a clinical study showed significant reduction in P-gp activity in AD patients compared to cognitively normal subjects (van Assema et al., 2012). Another study detected 25% lower P-gp protein expression levels in hippocampal blood vessels in post-mortem brain samples from AD patients than in samples from age-matched non-demented patients (Wijesuriya et al., 2010). Moreover, isolated brain capillaries from transgenic hAPP-overexpressing mouse model (Tg2576 mice) showed a 70% decrease in P-gp transport activity and a 60% decrease in P-gp protein expression compared to age-matched wild-type mice (Hartz et al., 2010c). Although the molecular mechanism underlying this down regulation of P-gp is not clear, some studies pointed at Aβ accumulation as a causative factor (Carrano et al., 2011; Kania et al., 2011; Park et al., 2014; Qosa et al., 2014b). Park et al showed that Aβ42 mediates P-gp down regulation in murine brain endothelial cells (bEnd3 cells) by activation of receptor for advanced glycation end products (RAGE). The authors suggest that activation of RAGE by Aβ42 would enhance NF-κB activity that decreases P-gp expression (Park et al., 2014). Similarly, Kania et al showed that, exposure of immortalized human brain endothelial cell line (hCMEC/D3) to Aβ peptides reduced P-gp expression with more definitive and consistent effect produced by Aβ42 than Aβ40 (Kania et al., 2011). Authors of this study suggested reduction in the activity of the Wnt/β-catenin pathway by Aβ exposure in the brain endothelial cells (Kania et al., 2011). Consistent with this finding, recent study demonstrated that the expression of P-gp in human BBB cells is controlled by a cross-talk between the Wnt/GSK3 canonical pathway and the Wnt/RhoA/RhoAK non-canonical pathway. Increased activity of Wnt/GSK3 canonical pathway reduced GSK3-mediated phosphorylation and ubiquitination of β-catenin, decreasing its proteasomal degradation, enhances its nuclear translocation, and increases P-gp expression (Pinzon-Daza et al., 2014). Similarly, RhoA activation through non-canonical Wnt3/RhoA/RhoAK pathway reduced the activity of GSK3. The active RhoA increases the activity of RhoAK, which induces the phosphorylation of protein tyrosine phosphatase-1B (PTP1B). After this phosphorylation, PTP1B dephosphorylates GSK3 and inactivates it (Pinzon-Daza et al., 2014).
Similar to P-gp, several studies also confirm the role of BCRP in Aβ extrusion at the BBB. Optical imaging analyses of live animals showed that Bcrp-null mice significantly accumulated more Aβ in their brains than wild-type mice (Xiong et al., 2009). Moreover, in vitro studies utilizing BCRP overexpressing cells demonstrated BCRP to restrict the apical-to-basolateral permeability of Aβ isoforms (Candela et al., 2010; Do et al., 2012; Tai et al., 2009). However, unlike P-gp, studies of BCRP expression in the brain of AD patients showed conflicting results. Xiong et al showed that ABCG2 gene was significantly up-regulated in the brains of AD and CAA patients compared to age matched control group and the same applies to the Tg3x mouse model of AD against its matched littermate control (Xiong et al., 2009). On the other hand, Wijesuriya et al reported lack of difference in BCRP expression in the brain vasculature of AD compared to normal cases (Wijesuriya et al., 2010). Xiong et al have suggested BCRP up-regulation in the brains of AD patients as a protective mechanism during oxidative stress and inflammatory response by inhibiting the NF-κB signaling pathway, leading to decreased expression of interleukin-8 and growth-related oncogene (GRO) inflammatory genes (Xiong et al., 2009). Among the ABCC subfamily, only MRP1 has been linked to AD (Krohn et al., 2011). In a mouse model of AD that is null for the Abcc1 gene (APP/PS1×Abcc1−/−), cerebral Aβ levels increased without changes in the expression of most enzymes that would favor the production of Aβ from APP. Moreover, treatment of APP/PS1 mice with the Abcc1 gene inducer, thiethylperazine reduced brain Aβ levels (Krohn et al., 2011).
Amyotrophic lateral sclerosis (ALS)
ALS is a fatal, progressive neurodegenerative disorder that affects upper and lower motor neurons with poor prognosis. The pathogenesis of the disease is complex and involves different pathological lesions in the brain and spinal cord that are incompletely understood (Pratt et al., 2012). Recently, several reports showed evidence of BBB disturbances in the brain of ALS patients carrying the SOD1 mutation and in postmortem brains of sporadic ALS patients (Garbuzova-Davis and Sanberg, 2014). Our previous study showed a significant increase in the expression of P-gp and BCRP but not MRPs (1, 2, 4, and 5) in the spinal cords of patients and mouse models of ALS (mutant SOD1-G93A and mutant TDP43-A315T mice) (Jablonski et al., 2012). Interestingly, the increase in P-gp and BCRP expression was observed only in cerebral cortex and spinal cord while cerebellum and systemic expression of these transporters did not change (Jablonski et al., 2012). Highlighting the clinical significance of the overexpression of drug transporters in ALS, several reports confirmed that, riluzole, the only FDA-approved drug for the treatment of ALS, and other investigational ALS therapeutics are P-gp and BCRP substrates (Jablonski et al., 2014; Milane et al., 2007). Therefore, this ALS-acquired and CNS-specific overexpression of P-gp and BCRP could contribute significantly to the development of drug pharmacoresistance in ALS. In a proof-of-concept study using ALS mouse model, systemic administration of elacridar (P-gp/BCRP inhibitor) significantly increased riluzole concentrations in the brain and spinal cord and prolonged mouse survival (Jablonski et al., 2014). However, data about the regulation of P-gp and BCRP expression in ALS is still lacking and further investigations are needed to understand the mechanisms underlying this ALS-acquired and CNS-specific up-regulation of P-gp and BCRP.
Brain tumors
Brain tumors are devastating pathologies considered among the most lethal types of all cancers. Brain tumors can be divided into two categories: primary brain tumors and metastatic brain tumors. Primary brain tumors arise from the constituent cells of the brain or their meningeal covering, whereas metastatic brain tumors are secondary tumors that metastasize from a distant site (Agarwal et al., 2011; Kamar and Posner, 2010; Parrish et al., 2015). Despite extensive research, treatment of brain tumors has been largely ineffective and systemically administered chemotherapeutic drugs show poor efficacy. This low efficacy is at least partly due to overexpression of ABC efflux transporters P-gp, BCRP and MRPs in tumor cells (Deeken and Loscher, 2007; Parrish et al., 2015). Up-regulation of P-gp, BCRP, and MRP1 has been reported in a number of neuroblastomas, astrocytomas, and glioblastomas, suggesting their contribution in generating resistance toward a number of therapeutic substrates (Agarwal et al., 2011; Haber et al., 2006; Regina et al., 2001; Wang et al., 2012). In addition to tumor cells, several studies showed that the expression of P-gp, BCRP and MRPs is also altered in the endothelial cells that form the vasculature around tumors (blood-tumor barrier) compared with normal brain vasculature (Agarwal et al., 2011; Deeken and Loscher, 2007; Parrish et al., 2015; Regina et al., 2001). For instance, P-gp expression in the blood vessels supplying brain melanoma metastases and lung metastases (metastatic brain tumors) were only 5% and 40% of that seen in normal brain tissue (Regina et al., 2001). Similarly, in primary brain tumors, Becker et al showed diminished level of P-gp in the vasculature of malignant gliomas compared with normal brain vasculature, whereas Toth et al found no difference between the P-gp expression in glioma tumor vasculature and normal tissue (Becker et al., 1991; Toth et al., 1996). Concerning MRPs Haga et al found no difference in the expression level of MRP2 between normal brain and malignant glioma cells (Haga et al., 2001). However, they did find increased expression of MRP1 in the endothelial cells forming the vasculature around tumor sites. In contrast, Pilorget et al demonstrated that P-gp is up-regulated in brain EC isolated from brain tumors compared with normal brain EC (Pilorget et al., 2007). Moreover, in this study authors provided evidence for activation of SK-1 and its product S1P to modulate P-gp transport activity in the rat brain endothelial cell line RBE4. It is worth noting that, in addition to ABC efflux transporters alterations at blood-tumor barrier, the integrity of this barrier is also compromised. Thus, the BBB is less intact in the core of primary and metastatic brain tumors compared with the normal brain vasculature. However, this disruption was seen in the site of tumor, while the vasculature remains grossly intact in other brain regions (Deeken and Loscher, 2007; Parrish et al., 2015).
Creutzfeldt-Jakob's disease (prion disease)
Creutzfeldt-Jakob's disease (CJD) is a rare, neurodegenerative, invariably fatal brain disorder, also known as prion disease (Ironside and Head, 2008). This disease is characterized by the accumulation of an abnormal isoform (PrPSc) of the prion protein (PrP) which forms aggregates responsible for the pathological progress of this disease (Ironside and Head, 2008). Like HD, studies about the involvement of P-gp and other ABC efflux transporters are lacking in CJD. Only one report by Vogelgesang et al showed that P-gp expression within endothelial cells of brain vessels is significantly lower in CJD patients than in age-matched control cases (Vogelgesang et al., 2006). In this study, authors could not detect any immunohistochemically PrP in the blood vessels of CJD patients, which raises the question of whether reduction the P-gp expression in the endothelium is the cause or the consequence of the prion disease process.
Epilepsy
Epilepsy is a neurological disorder characterized by recurrent seizures. It is one of the most common neurological disorders and affects approximately 1-2% of the world's population (Ahmed, 2005; Kotsopoulos et al., 2005). Although several antiepileptic drugs (AEDs) have been developed and are in clinical use, about 40% of the patients failed to respond to AEDs treatment due to the development of pharmacoresistance (Loscher and Potschka, 2005c). Enhanced efflux of AEDs at the BBB due to overexpression of ABC efflux transporters is considered one of the major mechanisms underlying AEDs pharmacoresistance (Loscher and Potschka, 2005a). The first evidence for the role of ABC efflux transporter in AEDs pharmacoresistance was provided by Tishler et al who detected high expression of P-gp in the capillary endothelial cells isolated from the brain tissue of refractory epileptic patients (Tishler et al., 1995). Since then, overexpression of P-gp and other ABC efflux transporters such as BCRP and MRP have been confirmed by in vitro, in vivo and clinical studies (Aronica et al., 2012). Consistent with these findings, numerous in vivo and in vitro studies showed that many AEDs are substrates for ABC efflux transporters (Luna-Tortos et al., 2008). Moreover, co-administration of ABC efflux transporters inhibitors significantly enhanced brain penetration and efficacy of AEDs (Brandt et al., 2006; van Vliet et al., 2006). Over the past 10 years, numerous studies have been performed to understand the regulatory mechanisms that stand behind the development of pharmacoresistance against AEDs. One hypothesis that has been addressed by several studies suggested constitutive or inherited overexpression of P-gp. However, there is still substantial controversy about the validity of this hypothesis due to poor correlation between polymorphisms in the ABCB1 gene and drug-response in epileptic patients (Kim et al., 2009; Siddiqui et al., 2003; Sills et al., 2005; Tan et al., 2004).
Recent in vitro and in vivo studies have suggested that overexpression of ABC efflux transporters is complex and involves several elements of pathological alterations observed in epilepsy such as inflammatory responses, oxidative stress, ligand-activated nuclear receptors and glutamate (Potschka, 2010b). In accordance with high glutamate levels in epileptic brain, it has been shown that seizures induce overexpression of P-gp at the level of the BBB in endothelial cells through glutamate by an NMDA receptor and COX2-dependent mechanism (Bauer et al., 2008). Consistent with this sequence, the role of COX2 in regulating P-gp expression has been confirmed in different experimental models of epilepsy and inhibiting elements of NMDA/COX2 signaling pathway such as prostaglandin-E2 receptor (EP1) improves efficacy of certain AEDs (Pekcec et al., 2009; van Vliet et al., 2010).
Finally, treatment with AEDs could induce pharmacoresistance in epilepsy. Although several in vitro and in vivo studies showed the potential of AEDs to enhance expression of ABC efflux transporters, the data are still controversial (Aronica et al., 2012). In addition, because overexpression of ABC efflux transporters has been reported in the brain of epileptic patients before exposure to AEDs, it is not likely that treatment with AEDs per se is responsible for the observed pharmacoresistance (Sisodiya et al., 1999).
HIV-1 encaphelitis
Human immunodeficiency virus (HIV) is an enveloped retrovirus that causes the acquired immunodeficiency syndrome (AIDS) (Douek et al., 2009). HIV can penetrate the brain in early course of infection and causes HIV-encephalitis that is associated with chronic neuroinflammation (Gonzalez-Scarano and Martin-Garcia, 2005). Brain autopsy tissues from patients with HIV encaphilitis have increased P-gp expression in glial cells (Hayashi et al., 2005). This results is consistent with the findings that viral protein (gp120, Tat) enhance secreation of cytokines (IL-6, IL-1β and TNF-α), which in turn can enhance P-gp expression in the brain endothelium and astrocytes (Ronaldson and Bendayan, 2006). In contrast, some studies showed that HIV has downregulatory effect on P-gp expression mediated by IL-6 and NF-κB pathway (Ashraf et al., 2011). In addition to P-gp, increased expression of MRP1 has been detected in cultured astrocytes after treatment with Tat and gp120 proteins (Hayashi et al., 2006; Ronaldson and Bendayan, 2008). This increase in MRP1 expression was suggested to be stimulated by released TNF-α and mediated by NF-κB pathway (Ronaldson and Bendayan, 2008). In addition to viral proteins, it has been reported that antiretroviral drugs can also increase expression of P-gp and mediate the development of pharmacoresistance in HIV encaphelitis. Two HIV protease inhibitors, atazanavir and ritonavir, activate CAR and PXR nuclear translocation in human endothelial cells (hCMEC/D3) and in primary culture of human brain endothelial cells which in turn enhances P-gp expression (Chan et al., 2013; Zastre et al., 2009). Moreover, a recent study suggested that other antiretroviral drugs such as maraviroc, vicriviroc and elavitegravir can modulate P-gp and/or BCRP expression (Zembruski et al., 2011).
Mental disorders
Depression and schizophrenia are the most common mental disorders that inflict the life of more than 350 and 24 millions people worldwide, respectively (Battle, 2013). Depression is a mood disorder that causes a persistent feeling of sadness, loss of interest and interferes with the ability to work, sleep, study, eat, and enjoy life (Driessen and Hollon, 2010). Schizophrenia is a severe brain disorder characterized by abnormal social behavior, failure to recognize what is real, hallucinations, delusions, and extremely disordered thinking (van Os and Kapur, 2009). The causes of these mental disorders are still unclear with complex pathogenesis that triggered by several genetic, clinical, enviromental and social factors (Battle, 2013). Changes in the ABC efflux transporters at the BBB in mental disorders are understudied with only few studies that suggested local changes of P-gp function in the brain of depressive or schizophrenic patinets . (de Klerk et al., 2009; de Klerk et al., 2010). Using positron emission tomography, a significantly lowered 11C-verapamil uptake (indicating increased P-gp activity) in prefrontal cortex and temporal lobes was found in patients with a major depressive episode. Moreover, this significant decrease in 11C-verapamil uptake was found in a subset of patients diagnosed with treatment-resistant depressive disorder. In addition, the same group of researchers reported a similar increase in P-gp activity in the brains of schezophrenic patients (de Klerk et al., 2010). Using positron emission tomography, patients with chronic schizophrenia under treatment with antipsychotic drugs showed a significant decrease in 11C-verapamil uptake in the temporal cortex, the basal ganglia, and the amygdala, and a trend towards a significant decrease was seen throughout the brain compared with healthy controls (de Klerk et al., 2010). This decrease of 11C-verapamil uptake in the brain of depressive and schizophrenic patients may be correlated with an increased activity of the P-gp pump. These results suggested a local increase in the activity and or expression of P-gp at areas that are mostly affected by depression and schezophrenia. Moreover, clinical findings of these studies speculated that pharmacoresistance in depression and schezophrenia may in fact be associated with increased P-gp function that may cause low uptake of antipsychotics (de Klerk et al., 2009). However, it is still unclear wheather the observed increase in P-gp activity in depression and schezophrenia is related to specific pathological alterations in these diseases or to the drugs used in the treatment of these diseases (de Klerk et al., 2009; de Klerk et al., 2010).
Multiple sclerosis
Multiple sclerosis (MS) is chronic inflammatory disease in which the myelin covers of nerve axons in the brain and spinal cord are damaged. Neuropathologically multiple focal demyelinated lesions scattered throughout the CNS characterize MS. These lesions disrupt the ability of parts of the nervous system to communicate, resulting in a wide range of signs and symptoms including physical, mental, and sometimes psychiatric problems (Frohman et al., 2006). Recent studies show a decreased endothelial P-gp expression in MS, whereas endothelial BCRP, MRP1 and MRP2 were unchanged (Kooij et al., 2010; Kooij et al., 2011). Loss of vascular P-gp function during MS neuroinflammation may disturb brain homeostasis and thereby aggravate disease progression via exposure of vulnerable CNS cells to detrimental compounds. Authors of this study identified a crucial role for activated CD4+ T cells in endothelial P-gp down-regulation via intracellular adhesion molecule-1 and NF-κB signaling (Kooij et al., 2010). On the other hand, both active and inactive multiple sclerosis lesions were associated with an increase in astrocytic P-gp, MRP1 and MRP2 but not BCRP expression. The authors suggested that, TLR-3 activation of astrocytes by TNF-α led to increased P-gp and MRP1 activities (Kooij et al., 2011).
Parkinson's disease
Parkinson's disease (PD) is the second most common neurodegenerative disease that mainly affects the motor system. It is characterized by progressive loss of nigral dopaminergic neurons and formation of Lewy bodies. Although, most cases of PD are idiopathic, many studies have implicated the accumulation of environmental neurotoxins and lack of efficient brain detoxification to the development of PD (Kalia and Lang, 2015). Because impaired function of drug transporters may be associated with brain accumulation of neurotoxins, genetic polymorphism in MDR1 gene has been suggested as a risk factor for PD (Liu et al., 2013; Zschiedrich et al., 2009). Moreover, reduction in the expression of P-gp has been reported in the endothelial cells of postmortem PD patients (Westerlund et al., 2008). Consistent with that finding, positron emission tomography measurements of brain uptake of 11C-verapamil, which is normally extruded from the brain by P-gp, showed significantly elevated uptake of 11C-verapamil in the midbrain of PD patients relative to controls (Kortekaas et al., 2005). These studies confirmed the reduction in P-gp expression at the BBB of PD patients and loss of one important neuroprotective component of the barrier.
Stroke and ischemic brain injuries
Stroke is the third leading causes of death and disability in the world (Truelsen and Bonita, 2009). It occurs when blood flow to an area of brain is cut off due to a blocked blood vessel or bleeding in the brain. Approximately 85% of all strokes are ischaemic and 15% haemorrhagic. Ischaemic stroke is a complex pathophysiological process that triggers a cascade of biochemical and molecular events, finally causing an impairment of BBB integrity (Dirnagl et al., 1999). It has been reported that ABC efflux transporters at the BBB exhibit profound expressional changes in the brain capillaries of the ischaemic brain that have functional implications (Patak and Hermann, 2011). BBB P-gp expression and activity is elevated three hours following transient intraluminal middle cerebral artery occlusion (MCAO). The observed overexpression of P-gp was persistent up to 24 hours after reperfusion, independent of the severity of the ischaemic event and observed also in newly generated capillaries 14 days post-stroke (Cen et al., 2013; Dazert et al., 2006; Hermann et al., 2006; Ueno et al., 2009). In contrast only a few studies have investigated the role of other ABC efflux transporters at the BBB after brain ischaemia. In mice that underwent MCAO for 90 or 30 min, Kilic et al showed that the levels of MRP1 in the brain endothelium declined 3 hours after ischaemia and recovered partly within 72 hours (Kilic et al., 2008). Regarding BCRP, Dazert et al showed increased in BCRP mRNA and protein levels 3 to 14 days post-ischemic injury (Dazert et al., 2006).
Little is known about their mechanisms that regulate ABC transporter expression in cerebral ischaemia. Since hypoxia is a major factor contributing to ischemic injury, previous study investigated the role of hypoxia inducible factors (HIF)-dependent signaling in the ischemia-induced overexpression of P-gp and MRP1 in an in vitro model of human brain endothelial cells (hCMEC/D3) (Patak et al., 2011). However, no changes in P-gp or MRP1 levels were detected in this study, despite a strong induction of HIF in the hCMEC/D3 cells, suggesting that HIF might not be essential for ABC efflux transporters regulation in endothelial cells (Patak et al., 2011). Authors of this study suggested that the overexpression of both transporters seen in previous studies after cerebral ischaemia could depend on other factors than hypoxia alone that are associated with ischaemia and have been described to induce P-gp expression, such as glucose depletion (Ledoux et al., 2003), reactive oxygen species that are generated by reoxygenation following hypoxia as shown in rat brain endothelial cells (Li and Jackson, 2002; Robertson et al., 2009; Robertson et al., 2011) or glutamate that is released during brain ischaemia (Zhu and Liu, 2004). Moreover, recent studies provided evidence for P-gp and BCRP regulation by ApoE in response to ischemia (ElAli and Hermann, 2010). Using brain microvascular extracts, the authors indicated that P-gp mRNA increased in ischaemic cerebral microvessels of wild-type, but not ApoE−/− mice, whereas MRP1 mRNA was increased in the ischaemic brain capillaries of ApoE−/−, but not wild-type mice.
Conclusions
ABC drug efflux transporters are important elements of the BBB. Regulation of expression and activity of these transporters are complex processes that are affected by numerous pathophysiological stressors, such as inflammatory and oxidative stress and xenobiotics. A primary function of ABC efflux transporters at BBB is to move molecules, both endogenous and xenobiotics, out of the CNS (You and Morris, 2006). Although these transporters play an important role in providing neuroprotection, their wide range substrate specificities grants them the potential to recognize many therapeutic drugs targeted at the CNS (Miller, 2015). As a consequence, these ABC efflux transporters play a significant role in governing bioavailability, therapeutic efficacy, pharmacokinetics and most importantly brain disposition of a variety of drugs, thereby governing drug efficacy and significantly contributing to the development of pharmacoresistance (Loscher and Potschka, 2005b; Miller, 2015; Sharom, 2008). Therefore, ABC efflux transporters could have two contradictory effects on the development and progression of neurological diseases: On the one hand, they protect the CNS by promoting detoxification, but they also constitute an obstacle to brain penetration, diffusion and bioavailability of CNS therapeutics.
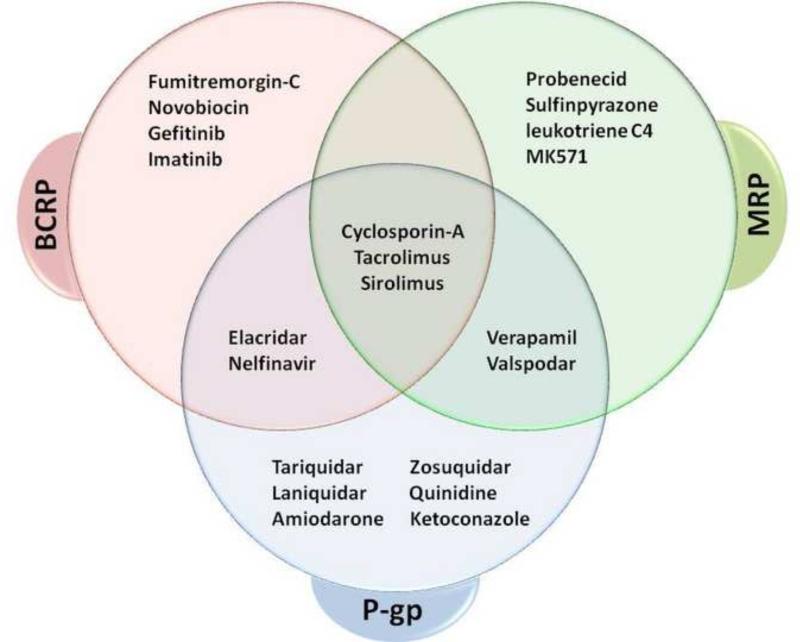
Several approaches have been used to decrease the activity of ABC efflux transporters, mainly P-gp, in neurological disorders such as epilepsy (Potschka, 2010a). Transient inhibition of ABC efflux transporters by using direct inhibitors may be a useful strategy to reverse or prevent pharmacoresistance due to overexpression of such transporters. Various inhibitors of P-gp, BCRP and MRPs have been developed, however, most of these inhibitors lack specificity and inhibit several ABC efflux transporters; they also exhibit side effects at high concentrations (Figure 4). Moreover, according to the fact that P-gp and BCRP act synergistically in limiting drug entry to the brain, the use of dual inhibitor that could inhibit both P-gp and BCRP has the potential to dramatically increase the brain accumulation of drugs that are dual substrates. In this regard, previous studies reported about 70-fold increase in the brain distribution of gefitinib (anticancer agent) in Mdr1(−/−) Bcrp(−/−) mice (Agarwal et al., 2010). Therefore, direct inhibitors (such as elacridar or tariquidar) could enhance significantly brain levels of centrally acting drugs.
Figure 4.
Venn diagram shows common inhibitors for P-gp, BCRP, MRP1 and MRP2.
In contrast, results from several reports showed that increasing expression of P-gp could significantly enhance the extrusion of Aβ from the brain across BBB (Abuznait et al., 2013; Brenn et al., 2014; Hartz et al., 2010c; Qosa et al., 2012). However, further pre-clinical and clinical studies are needed to prove the validity of this approach and whether it could be generalized for other ABC efflux transporters and other diseases.
When targeting ABC efflux transporters by inducers or inhibitors, one should take into account the effects of long-term modulation on the transporters’ protective function as well as the potential of development of pharmacoresistance. One must be mindful of not impairing brain clearance of potentially toxic metabolites and proteins when inhibiting efflux transporters and of not developing pharmacoresistance with inducers or with disease targeting drugs that also act as inducers (PXR and CAR activators). Finally, changes in the expression and activity of ABC efflux transporters in neurological diseases might result from complex interacting factors, such as progression of disease, alterations in target sites and changes in the expression of other proteins. Therefore, successful targeting of ABC efflux transporters in the treatment of neurological diseases requires further studies to understand the specific mechanisms that regulate individual ABC efflux transporters.
Highlights.
ABC efflux transporters are important element of BBB.
ABC efflux transporters provide neuroprotection against several neurotoxins.
ABC efflux transporters contribute to the pharmacoresistance against CNS drugs.
Several stressors modulate the activity of ABC efflux transporters at BBB.
Pathological alterations of ABC efflux transporters are associated with many CNS disorders.
Acknowledgment
This work was supported by the National Institute of Health grant RO1-NS074886 (to DT), and by a program grant from Target ALS (to DT, PP and DSM) and the Intramural Research program NIH/NIEHS. The Weinberg Unit for ALS research is also supported by the Farber Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–49. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- Abuznait AH, Kaddoumi A. Role of ABC transporters in the pathogenesis of Alzheimer's disease. ACS Chem Neurosci. 2012;3:820–31. doi: 10.1021/cn300077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuznait AH, Qosa H, Busnena BA, El Sayed KA, Kaddoumi A. Olive-oil-derived oleocanthal enhances beta-amyloid clearance as a potential neuroprotective mechanism against Alzheimer's disease: in vitro and in vivo studies. ACS Chem Neurosci. 2013;4:973–82. doi: 10.1021/cn400024q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 2010;334:147–55. doi: 10.1124/jpet.110.167601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Hartz AM, Elmquist WF, Bauer B. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des. 2011;17:2793–802. doi: 10.2174/138161211797440186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SN. Epileptic seizures and epilepsy. Epilepsia. 2005;46:1700–1. doi: 10.1111/j.1528-1167.2005.00273_3.x. author reply 1701-2. [DOI] [PubMed] [Google Scholar]
- Ak H, Ay B, Tanriverdi T, Sanus GZ, Is M, Sar M, Oz B, Ozkara C, Ozyurt E, Uzan M. Expression and cellular distribution of multidrug resistance-related proteins in patients with focal cortical dysplasia. Seizure. 2007;16:493–503. doi: 10.1016/j.seizure.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–22. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Jansen GH, van Veelen CW, van Rijen PC, Leenstra S, Ramkema M, Scheffer GL, Scheper RJ, Troost D. Expression and cellular distribution of multidrug transporter proteins in two major causes of medically intractable epilepsy: focal cortical dysplasia and glioneuronal tumors. Neuroscience. 2003;118:417–29. doi: 10.1016/s0306-4522(02)00992-2. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Redeker S, van Vliet EA, Ramkema M, Scheffer GL, Scheper RJ, van der Valk P, Leenstra S, Baayen JC, Spliet WG, Troost D. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 2005;46:849–57. doi: 10.1111/j.1528-1167.2005.66604.x. [DOI] [PubMed] [Google Scholar]
- Aronica E, Sisodiya SM, Gorter JA. Cerebral expression of drug transporters in epilepsy. Adv Drug Deliv Rev. 2012;64:919–29. doi: 10.1016/j.addr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Ashraf T, Ronaldson PT, Persidsky Y, Bendayan R. Regulation of P-glycoprotein by human immunodeficiency virus-1 in primary cultures of human fetal astrocytes. J Neurosci Res. 2011;89:1773–82. doi: 10.1002/jnr.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aye IL, Keelan JA. Placental ABC transporters, cellular toxicity and stress in pregnancy. Chem Biol Interact. 2013;203:456–66. doi: 10.1016/j.cbi.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Barakat S, Demeule M, Pilorget A, Regina A, Gingras D, Baggetto LG, Beliveau R. Modulation of p-glycoprotein function by caveolin-1 phosphorylation. J Neurochem. 2007;101:1–8. doi: 10.1111/j.1471-4159.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- Barnes GN, Slevin JT. Ionotropic glutamate receptor biology: effect on synaptic connectivity and function in neurological disease. Curr Med Chem. 2003;10:2059–72. doi: 10.2174/0929867033456800. [DOI] [PubMed] [Google Scholar]
- Battle DE. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas. 2013;25:191–2. doi: 10.1590/s2317-17822013000200017. [DOI] [PubMed] [Google Scholar]
- Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–9. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–75. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Pekcec A, Toellner K, Miller DS, Potschka H. Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol. 2008;73:1444–53. doi: 10.1124/mol.107.041210. [DOI] [PubMed] [Google Scholar]
- Becker I, Becker KF, Meyermann R, Hollt V. The multidrug-resistance gene MDR1 is expressed in human glial tumors. Acta Neuropathol. 1991;82:516–9. doi: 10.1007/BF00293387. [DOI] [PubMed] [Google Scholar]
- Begley DJ. ABC transporters and the blood-brain barrier. Current Pharmaceutical Design. 2004;10:1295–312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18:1618–20. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- Brandt C, Bethmann K, Gastens AM, Loscher W. The multidrug transporter hypothesis of drug resistance in epilepsy: Proof-of-principle in a rat model of temporal lobe epilepsy. Neurobiol Dis. 2006;24:202–11. doi: 10.1016/j.nbd.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Brenn A, Grube M, Jedlitschky G, Fischer A, Strohmeier B, Eiden M, Keller M, Groschup MH, Vogelgesang S. St. John's Wort reduces beta-amyloid accumulation in a double transgenic Alzheimer's disease mouse model-role of P-glycoprotein. Brain Pathol. 2014;24:18–24. doi: 10.1111/bpa.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, Dragustinovis I, Franco-Lira M, Aragon-Flores M, Solt AC, Altenburg M, Torres-Jardon R, Swenberg JA. Brain inflammation and Alzheimer's-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–8. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- Campos CR, Schroter C, Wang X, Miller DS. ABC transporter function and regulation at the blood-spinal cord barrier. J Cereb Blood Flow Metab. 2012;32:1559–66. doi: 10.1038/jcbfm.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela P, Gosselet F, Saint-Pol J, Sevin E, Boucau MC, Boulanger E, Cecchelli R, Fenart L. Apical-to-basolateral transport of amyloid-beta peptides through blood-brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. J Alzheimers Dis. 2010;22:849–59. doi: 10.3233/JAD-2010-100462. [DOI] [PubMed] [Google Scholar]
- Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci U S A. 2012;109:15930–5. doi: 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano A, Hoozemans JJ, van der Vies SM, Rozemuller AJ, van Horssen J, de Vries HE. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal. 2011;15:1167–78. doi: 10.1089/ars.2011.3895. [DOI] [PubMed] [Google Scholar]
- Cartwright TA, Campos CR, Cannon RE, Miller DS. Mrp1 is essential for sphingolipid signaling to p-glycoprotein in mouse blood-brain and blood-spinal cord barriers. J Cereb Blood Flow Metab. 2013;33:381–8. doi: 10.1038/jcbfm.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck MP, Fenart L. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov. 2007;6:650–61. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- Cen J, Liu L, Li MS, He L, Wang LJ, Liu YQ, Liu M, Ji BS. Alteration in P-glycoprotein at the blood-brain barrier in the early period of MCAO in rats. J Pharm Pharmacol. 2013;65:665–72. doi: 10.1111/jphp.12033. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–96. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Gold R, von Ahsen N. ATP-binding cassette transporters in inflammatory brain disease. Curr Pharm Des. 2011;17:2803–7. doi: 10.2174/138161211797440131. [DOI] [PubMed] [Google Scholar]
- Chan GN, Patel R, Cummins CL, Bendayan R. Induction of P-glycoprotein by antiretroviral drugs in human brain microvessel endothelial cells. Antimicrob Agents Chemother. 2013;57:4481–8. doi: 10.1128/AAC.00486-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang XB. A molecular understanding of ATP-dependent solute transport by multidrug resistance-associated protein MRP1. Cancer Metastasis Rev. 2007;26:15–37. doi: 10.1007/s10555-007-9041-7. [DOI] [PubMed] [Google Scholar]
- Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20:793–807. doi: 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–90. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9:387–98. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- Dallas S, Zhu X, Baruchel S, Schlichter L, Bendayan R. Functional expression of the multidrug resistance protein 1 in microglia. J Pharmacol Exp Ther. 2003;307:282–90. doi: 10.1124/jpet.103.054304. [DOI] [PubMed] [Google Scholar]
- Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58:140–61. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- Daood M, Tsai C, Ahdab-Barmada M, Watchko JF. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics. 2008;39:211–8. doi: 10.1055/s-0028-1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, Scherrmann JM, De Waziers I, Decleves X. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem. 2008;107:1518–28. doi: 10.1111/j.1471-4159.2008.05720.x. [DOI] [PubMed] [Google Scholar]
- Dazert P, Suofu Y, Grube M, Popa-Wagner A, Kroemer HK, Jedlitschky G, Kessler C. Differential regulation of transport proteins in the periinfarct region following reversible middle cerebral artery occlusion in rats. Neuroscience. 2006;142:1071–9. doi: 10.1016/j.neuroscience.2006.07.056. [DOI] [PubMed] [Google Scholar]
- de Klerk OL, Willemsen AT, Roosink M, Bartels AL, Hendrikse NH, Bosker FJ, den Boer JA. Locally increased P-glycoprotein function in major depression: a PET study with [11C]verapamil as a probe for P-glycoprotein function in the blood-brain barrier. Int J Neuropsychopharmacol. 2009;12:895–904. doi: 10.1017/S1461145709009894. [DOI] [PubMed] [Google Scholar]
- de Klerk OL, Willemsen AT, Bosker FJ, Bartels AL, Hendrikse NH, den Boer JA, Dierckx RA. Regional increase in P-glycoprotein function in the blood-brain barrier of patients with chronic schizophrenia: a PET study with [(11)C]verapamil as a probe for P-glycoprotein function. Psychiatry Res. 2010;183:151–6. doi: 10.1016/j.pscychresns.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Decleves X, Regina A, Laplanche JL, Roux F, Boval B, Launay JM, Scherrmann JM. Functional expression of P-glycoprotein and multidrug resistance-associated protein (Mrp1) in primary cultures of rat astrocytes. J Neurosci Res. 2000;60:594–601. doi: 10.1002/(SICI)1097-4547(20000601)60:5<594::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Decleves X, Fajac A, Lehmann-Che J, Tardy M, Mercier C, Hurbain I, Laplanche JL, Bernaudin JF, Scherrmann JM. Molecular and functional MDR1-Pgp and MRPs expression in human glioblastoma multiforme cell lines. Int J Cancer. 2002;98:173–80. doi: 10.1002/ijc.10135. [DOI] [PubMed] [Google Scholar]
- Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–74. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- Diop NK, Hrycyna CA. N-Linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane. Biochemistry. 2005;44:5420–9. doi: 10.1021/bi0479858. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Do TM, Noel-Hudson MS, Ribes S, Besengez C, Smirnova M, Cisternino S, Buyse M, Calon F, Chimini G, Chacun H, Scherrmann JM, Farinotti R, Bourasset F. ABCG2- and ABCG4-mediated efflux of amyloid-beta peptide 1-40 at the mouse blood-brain barrier. J Alzheimers Dis. 2012;30:155–66. doi: 10.3233/JAD-2012-112189. [DOI] [PubMed] [Google Scholar]
- Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–84. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen E, Hollon SD. Cognitive behavioral therapy for mood disorders: efficacy, moderators and mediators. Psychiatr Clin North Am. 2010;33:537–55. doi: 10.1016/j.psc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenblatter T, Huwel S, Galla HJ. Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain Res. 2003;971:221–31. doi: 10.1016/s0006-8993(03)02401-6. [DOI] [PubMed] [Google Scholar]
- ElAli A, Hermann DM. Apolipoprotein E controls ATP-binding cassette transporters in the ischemic brain. Sci Signal. 2010;3:ra72. doi: 10.1126/scisignal.2001213. [DOI] [PubMed] [Google Scholar]
- Enerson BE, Drewes LR. The rat blood-brain barrier transcriptome. J Cereb Blood Flow Metab. 2006;26:959–73. doi: 10.1038/sj.jcbfm.9600249. [DOI] [PubMed] [Google Scholar]
- Felix RA, Barrand MA. P-glycoprotein expression in rat brain endothelial cells: evidence for regulation by transient oxidative stress. J Neurochem. 2002;80:64–72. doi: 10.1046/j.0022-3042.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–55. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Fu D, Arias IM. Intracellular trafficking of P-glycoprotein. Int J Biochem Cell Biol. 2012;44:461–4. doi: 10.1016/j.biocel.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Sanberg PR. Blood-CNS Barrier Impairment in ALS patients versus an animal model. Front Cell Neurosci. 2014;8:21. doi: 10.3389/fncel.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzin S, Strazielle N, Schmitt C, Fevre-Montange M, Ostrow JD, Tiribelli C, Ghersi-Egea JF. Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J Comp Neurol. 2008;510:497–507. doi: 10.1002/cne.21808. [DOI] [PubMed] [Google Scholar]
- Gazzin S, Berengeno AL, Strazielle N, Fazzari F, Raseni A, Ostrow JD, Wennberg R, Ghersi- Egea JF, Tiribelli C. Modulation of Mrp1 (ABCc1) and Pgp (ABCb1) by bilirubin at the blood-CSF and blood-brain barriers in the Gunn rat. PLoS One. 2011;6:e16165. doi: 10.1371/journal.pone.0016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Haar CP, Hebbar P, Wallace G.C.t., Das A, Vandergrift WA, 3rd, Smith JA, Giglio P, Patel SJ, Ray SK, Banik NL. Drug resistance in glioblastoma: a mini review. Neurochem Res. 2012;37:1192–200. doi: 10.1007/s11064-011-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M, Smith J, Bordow SB, Flemming C, Cohn SL, London WB, Marshall GM, Norris MD. Association of high-level MRP1 expression with poor clinical outcome in a large prospective study of primary neuroblastoma. J Clin Oncol. 2006;24:1546–53. doi: 10.1200/JCO.2005.01.6196. [DOI] [PubMed] [Google Scholar]
- Haga S, Hinoshita E, Ikezaki K, Fukui M, Scheffer GL, Uchiumi T, Kuwano M. Involvement of the multidrug resistance protein 3 in drug sensitivity and its expression in human glioma. Jpn J Cancer Res. 2001;92:211–9. doi: 10.1111/j.1349-7006.2001.tb01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol. 2004;66:387–94. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–70. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008;22:2723–33. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Madole EK, Miller DS, Bauer B. Estrogen receptor beta signaling through phosphatase and tensin homolog/phosphoinositide 3-kinase/Akt/glycogen synthase kinase 3 down-regulates blood-brain barrier breast cancer resistance protein. J Pharmacol Exp Ther. 2010a;334:467–76. doi: 10.1124/jpet.110.168930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Mahringer A, Miller DS, Bauer B. 17-beta-Estradiol: a powerful modulator of blood-brain barrier BCRP activity. J Cereb Blood Flow Metab. 2010b;30:1742–55. doi: 10.1038/jcbfm.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease. Mol Pharmacol. 2010c;77:715–23. doi: 10.1124/mol.109.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Bauer B. ABC transporters in the CNS - an inventory. Curr Pharm Biotechnol. 2011;12:656–73. doi: 10.2174/138920111795164020. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Rigor RR, Miller DS. Rapid loss of blood-brain barrier P-glycoprotein activity through transporter internalization demonstrated using a novel in situ proteolysis protection assay. J Cereb Blood Flow Metab. 2010a;30:1593–7. doi: 10.1038/jcbfm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Sykes DB, Miller DS. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010b;30:1417–25. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Pu H, Tian J, Andras IE, Lee YW, Hennig B, Toborek M. HIV-Tat protein induces P-glycoprotein expression in brain microvascular endothelial cells. J Neurochem. 2005;93:1231–41. doi: 10.1111/j.1471-4159.2005.03114.x. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Pu H, Andras IE, Eum SY, Yamauchi A, Hennig B, Toborek M. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J Cereb Blood Flow Metab. 2006;26:1052–65. doi: 10.1038/sj.jcbfm.9600254. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–34. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy M, Spiers JP. A primer on the mechanics of P-glycoprotein the multidrug transporter. Pharmacological Research. 2007;55:1–15. doi: 10.1016/j.phrs.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Kilic E, Spudich A, Kramer SD, Wunderli-Allenspach H, Bassetti CL. Role of drug efflux carriers in the healthy and diseased brain. Ann Neurol. 2006;60:489–98. doi: 10.1002/ana.21012. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Konig J, Dringen R. Expression of mRNAs of multidrug resistance proteins (Mrps) in cultured rat astrocytes, oligodendrocytes, microglial cells and neurones. J Neurochem. 2002;82:716–9. doi: 10.1046/j.1471-4159.2002.01082.x. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Ho HL, Petropoulos S, Moisiadis VG, Gibb W, Matthews SG. Pro-inflammatory cytokine regulation of P-glycoprotein in the developing blood-brain barrier. PLoS One. 2012;7:e43022. doi: 10.1371/journal.pone.0043022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside JW, Head MW. Biology and neuropathology of prion diseases. Handb Clin Neurol. 2008;89:779–97. doi: 10.1016/S0072-9752(07)01268-7. [DOI] [PubMed] [Google Scholar]
- Jablonski MR, Jacob DA, Campos C, Miller DS, Maragakis NJ, Pasinelli P, Trotti D. Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol Dis. 2012;47:194–200. doi: 10.1016/j.nbd.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski MR, Markandaiah SS, Jacob D, Meng NJ, Li K, Gennaro V, Lepore AC, Trotti D, Pasinelli P. Inhibiting drug efflux transporters improves efficacy of ALS therapeutics. Ann Clin Transl Neurol. 2014;1:996–1005. doi: 10.1002/acn3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodoin J, Demeule M, Fenart L, Cecchelli R, Farmer S, Linton KJ, Higgins CF, Beliveau R. P-glycoprotein in blood-brain barrier endothelial cells: interaction and oligomerization with caveolins. J Neurochem. 2003;87:1010–23. doi: 10.1046/j.1471-4159.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015 doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Kamar FG, Posner JB. Brain metastases. Semin Neurol. 2010;30:217–35. doi: 10.1055/s-0030-1255225. [DOI] [PubMed] [Google Scholar]
- Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, Sekine Y, Uchida Y, Ito S, Terasaki T. Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res. 2008;25:1469–83. doi: 10.1007/s11095-008-9532-4. [DOI] [PubMed] [Google Scholar]
- Kania KD, Wijesuriya HC, Hladky SB, Barrand MA. Beta amyloid effects on expression of multidrug efflux transporters in brain endothelial cells. Brain Res. 2011;1418:1–11. doi: 10.1016/j.brainres.2011.08.044. [DOI] [PubMed] [Google Scholar]
- Kilic E, Spudich A, Kilic U, Rentsch KM, Vig R, Matter CM, Wunderli-Allenspach H, Fritschy JM, Bassetti CL, Hermann DM. ABCC1: a gateway for pharmacological compounds to the ischaemic brain. Brain. 2008;131:2679–89. doi: 10.1093/brain/awn222. [DOI] [PubMed] [Google Scholar]
- Kim DW, Lee SK, Chu K, Jang IJ, Yu KS, Cho JY, Kim SJ. Lack of association between ABCB1, ABCG2, and ABCC2 genetic polymorphisms and multidrug resistance in partial epilepsy. Epilepsy Res. 2009;84:86–90. doi: 10.1016/j.eplepsyres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–29. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- Kooij G, van Horssen J, de Lange EC, Reijerkerk A, van der Pol SM, van Het Hof B, Drexhage J, Vennegoor A, Killestein J, Scheffer G, Oerlemans R, Scheper R, van der Valk P, Dijkstra CD, de Vries HE. T lymphocytes impair P-glycoprotein function during neuroinflammation. J Autoimmun. 2010;34:416–25. doi: 10.1016/j.jaut.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Kooij G, Mizee MR, van Horssen J, Reijerkerk A, Witte ME, Drexhage JA, van der Pol SM, van Het Hof B, Scheffer G, Scheper R, Dijkstra CD, van der Valk P, de Vries HE. Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain. 2011;134:555–70. doi: 10.1093/brain/awq330. [DOI] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–9. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos I, de Krom M, Kessels F, Lodder J, Troost J, Twellaar M, van Merode T, Knottnerus A. Incidence of epilepsy and predictive factors of epileptic and non-epileptic seizures. Seizure. 2005;14:175–82. doi: 10.1016/j.seizure.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, Schumacher T, Bruning T, Plath AS, Alfen F, Schmidt A, Winter F, Rateitschak K, Wree A, Gsponer J, Walker LC, Pahnke J. Cerebral amyloid-beta proteostasis is regulated by the membrane transport protein ABCC1 in mice. J Clin Invest. 2011;121:3924–31. doi: 10.1172/JCI57867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–52. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- Kuhnke D, Jedlitschky G, Grube M, Krohn M, Jucker M, Mosyagin I, Cascorbi I, Walker LC, Kroemer HK, Warzok RW, Vogelgesang S. MDR1-P-Glycoprotein (ABCB1) Mediates Transport of Alzheimer's amyloid-beta peptides--implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007;17:347–53. doi: 10.1111/j.1750-3639.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski A, Czornyj L, Lubienieki F, Girardi E, Vazquez S, D'Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 48 Suppl. 2007;5:140–9. doi: 10.1111/j.1528-1167.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- Ledoux S, Yang R, Friedlander G, Laouari D. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Res. 2003;63:7284–90. [PubMed] [Google Scholar]
- Lee G, Babakhanian K, Ramaswamy M, Prat A, Wosik K, Bendayan R. Expression of the ATP-binding cassette membrane transporter, ABCG2, in human and rodent brain microvessel endothelial and glial cell culture systems. Pharm Res. 2007;24:1262–74. doi: 10.1007/s11095-007-9244-1. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–37. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–41. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Lin F, de Gooijer MC, Roig EM, Buil LC, Christner SM, Beumer JH, Wurdinger T, Beijnen JH, van Tellingen O. ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clin Cancer Res. 2014;20:2703–13. doi: 10.1158/1078-0432.CCR-14-0084. [DOI] [PubMed] [Google Scholar]
- Liu X, Ma T, Qu B, Ji Y, Liu Z. Pesticide-induced gene mutations and Parkinson disease risk: a meta-analysis. Genet Test Mol Biomarkers. 2013;17:826–32. doi: 10.1089/gtmb.2013.0313. [DOI] [PubMed] [Google Scholar]
- Loscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005a;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005b;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005c;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- Luna-Munguia H, Salvamoser JD, Pascher B, Pieper T, Getzinger T, Kudernatsch M, Kluger G, Potschka H. Glutamate-mediated upregulation of the multidrug resistance protein 2 in porcine and human brain capillaries. J Pharmacol Exp Ther. 2015;352:368–78. doi: 10.1124/jpet.114.218180. [DOI] [PubMed] [Google Scholar]
- Luna-Tortos C, Fedrowitz M, Loscher W. Several major antiepileptic drugs are substrates for human P-glycoprotein. Neuropharmacology. 2008;55:1364–75. doi: 10.1016/j.neuropharm.2008.08.032. [DOI] [PubMed] [Google Scholar]
- Mahringer A, Fricker G. BCRP at the blood-brain barrier: genomic regulation by 17beta-estradiol. Mol Pharm. 2010;7:1835–47. doi: 10.1021/mp1001729. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64. [PubMed] [Google Scholar]
- Mao Q, Unadkat JD. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update. AAPS J. 2015;17:65–82. doi: 10.1208/s12248-014-9668-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milane A, Fernandez C, Vautier S, Bensimon G, Meininger V, Farinotti R. Minocycline and riluzole brain disposition: interactions with p-glycoprotein at the blood-brain barrier. J Neurochem. 2007;103:164–73. doi: 10.1111/j.1471-4159.2007.04772.x. [DOI] [PubMed] [Google Scholar]
- Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31:246–54. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS. ABC transporter regulation by signaling at the blood-brain barrier: relevance to pharmacology. Adv Pharmacol. 2014;71:1–24. doi: 10.1016/bs.apha.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Miller DS. Regulation of ABC transporters at the blood-brain barrier. Clin Pharmacol Ther. 2015;97:395–403. doi: 10.1002/cpt.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra K, Dean M. Evolution of ABC transporters by gene duplication and their role in human disease. Biol Chem. 2011;392:29–37. doi: 10.1515/BC.2011.006. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience. 1999;93:1449–64. doi: 10.1016/s0306-4522(99)00225-0. [DOI] [PubMed] [Google Scholar]
- Narang VS, Fraga C, Kumar N, Shen J, Throm S, Stewart CF, Waters CM. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood-brain barrier. Am J Physiol Cell Physiol. 2008;295:C440–50. doi: 10.1152/ajpcell.00491.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–6. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–27. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, Keppler D. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129:349–60. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Novac N, Heinzel T. Nuclear receptors: overview and classification. Curr Drug Targets Inflamm Allergy. 2004;3:335–46. doi: 10.2174/1568010042634541. [DOI] [PubMed] [Google Scholar]
- Nwaozuzu OM, Sellers LA, Barrand MA. Signalling pathways influencing basal and H(2)O(2)-induced P-glycoprotein expression in endothelial cells derived from the blood-brain barrier. J Neurochem. 2003;87:1043–51. doi: 10.1046/j.1471-4159.2003.02061.x. [DOI] [PubMed] [Google Scholar]
- Olefsky JM. Nuclear receptor minireview series. J Biol Chem. 2001;276:36863–4. doi: 10.1074/jbc.R100047200. [DOI] [PubMed] [Google Scholar]
- Oude Elferink RP, de Waart R. Transporters in the intestine limiting drug and toxin absorption. J Physiol Biochem. 2007;63:75–81. doi: 10.1007/BF03174087. [DOI] [PubMed] [Google Scholar]
- Park R, Kook SY, Park JC, Mook-Jung I. Abeta1-42 reduces P-glycoprotein in the blood-brain barrier through RAGE-NF-kappaB signaling. Cell Death Dis. 2014;5:e1299. doi: 10.1038/cddis.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish KE, Sarkaria JN, Elmquist WF. Improving drug delivery to primary and metastatic brain tumors: strategies to overcome the blood-brain barrier. Clin Pharmacol Ther. 2015;97:336–46. doi: 10.1002/cpt.71. [DOI] [PubMed] [Google Scholar]
- Patak P, Hermann DM. ATP-binding cassette transporters at the blood-brain barrier in ischaemic stroke. Curr Pharm Des. 2011;17:2787–92. doi: 10.2174/138161211797440195. [DOI] [PubMed] [Google Scholar]
- Patak P, Jin F, Schafer ST, Metzen E, Hermann DM. The ATP-binding cassette transporters ABCB1 and ABCC1 are not regulated by hypoxia in immortalised human brain microvascular endothelial cells. Exp Transl Stroke Med. 2011;3:12. doi: 10.1186/2040-7378-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekcec A, Unkruer B, Schlichtiger J, Soerensen J, Hartz AM, Bauer B, van Vliet EA, Gorter JA, Potschka H. Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J Pharmacol Exp Ther. 2009;330:939–47. doi: 10.1124/jpet.109.152520. [DOI] [PubMed] [Google Scholar]
- Perego P, Gatti L, Beretta GL. The ABC of glycosylation. Nat Rev Cancer. 2010;10:523. doi: 10.1038/nrc2789-c1. [DOI] [PubMed] [Google Scholar]
- Pilorget A, Demeule M, Barakat S, Marvaldi J, Luis J, Beliveau R. Modulation of P-glycoprotein function by sphingosine kinase-1 in brain endothelial cells. J Neurochem. 2007;100:1203–10. doi: 10.1111/j.1471-4159.2006.04295.x. [DOI] [PubMed] [Google Scholar]
- Pinzon-Daza ML, Salaroglio IC, Kopecka J, Garzon R, Couraud PO, Ghigo D, Riganti C. The cross-talk between canonical and non-canonical Wnt-dependent pathways regulates P-glycoprotein expression in human blood-brain barrier cells. J Cereb Blood Flow Metab. 2014;34:1258–69. doi: 10.1038/jcbfm.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poller B, Drewe J, Krahenbuhl S, Huwyler J, Gutmann H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell Mol Neurobiol. 2010;30:63–70. doi: 10.1007/s10571-009-9431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschka H. Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia. 2010a;51:1333–47. doi: 10.1111/j.1528-1167.2010.02585.x. [DOI] [PubMed] [Google Scholar]
- Potschka H. Targeting regulation of ABC efflux transporters in brain diseases: a novel therapeutic approach. Pharmacol Ther. 2010b;125:118–27. doi: 10.1016/j.pharmthera.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Pratt AJ, Getzoff ED, Perry JJ. Amyotrophic lateral sclerosis: update and new developments. Degener Neurol Neuromuscul Dis. 2012;2012:1–14. doi: 10.2147/DNND.S19803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Abuznait AH, Hill RA, Kaddoumi A. Enhanced brain amyloid-beta clearance by rifampicin and caffeine as a possible protective mechanism against Alzheimer's disease. J Alzheimers Dis. 2012;31:151–65. doi: 10.3233/JAD-2012-120319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Abuasal BS, Romero IA, Weksler B, Couraud PO, Keller JN, Kaddoumi A. Differences in amyloid-beta clearance across mouse and human blood-brain barrier models: Kinetic analysis and mechanistic modeling. Neuropharmacology. 2014a;79C:668–678. doi: 10.1016/j.neuropharm.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, LeVine H, 3rd, Keller JN, Kaddoumi A. Mixed oligomers and monomeric amyloid-beta disrupts endothelial cells integrity and reduces monomeric amyloid-beta transport across hCMEC/D3 cell line as an in vitro blood-brain barrier model. Biochim Biophys Acta. 2014b;1842:1806–15. doi: 10.1016/j.bbadis.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A. 1999;96:3900–5. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapposelli S, Digiacomo M, Balsamo A. P-gp transporter and its role in neurodegenerative diseases. Curr Top Med Chem. 2009;9:209–17. doi: 10.2174/156802609787521544. [DOI] [PubMed] [Google Scholar]
- Regina A, Demeule M, Laplante A, Jodoin J, Dagenais C, Berthelet F, Moghrabi A, Beliveau R. Multidrug resistance in brain tumors: roles of the blood-brain barrier. Cancer Metastasis Rev. 2001;20:13–25. doi: 10.1023/a:1013104423154. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Crivellato E, Artico M. Development of the blood-brain barrier: a historical point of view. Anat Rec B New Anat. 2006;289:3–8. doi: 10.1002/ar.b.20087. [DOI] [PubMed] [Google Scholar]
- Rigor RR, Hawkins BT, Miller DS. Activation of PKC isoform beta(I) at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J Cereb Blood Flow Metab. 2010;30:1373–83. doi: 10.1038/jcbfm.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–9. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Kania KD, Hladky SB, Barrand MA. P-glycoprotein expression in immortalised rat brain endothelial cells: comparisons following exogenously applied hydrogen peroxide and after hypoxia-reoxygenation. J Neurochem. 2009;111:132–41. doi: 10.1111/j.1471-4159.2009.06306.x. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Mokgokong R, Kania KD, Guedj AS, Hladky SB, Barrand MA. Nitric oxide contributes to hypoxia-reoxygenation-induced P-glycoprotein expression in rat brain endothelial cells. Cell Mol Neurobiol. 2011;31:1103–11. doi: 10.1007/s10571-011-9711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard KR, Hoque T, Bendayan R. Expression of ATP-binding cassette membrane transporters in rodent and human sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther. 2012;340:96–108. doi: 10.1124/jpet.111.186916. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan M, Gingras D, Piquette-Miller M, Bendayan R. Cellular localization and functional expression of P-glycoprotein in rat astrocyte cultures. J Neurochem. 2004;89:788–800. doi: 10.1111/j.1471-4159.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 triggers an inflammatory response in cultured rat astrocytes and regulates the functional expression of P-glycoprotein. Mol Pharmacol. 2006;70:1087–98. doi: 10.1124/mol.106.025973. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J Neurochem. 2008;106:1298–313. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1139–51. doi: 10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvamoser JD, Avemary J, Luna-Munguia H, Pascher B, Getzinger T, Pieper T, Kudernatsch M, Kluger G, Potschka H. Glutamate-Mediated Down-Regulation of the Multidrug-Resistance Protein BCRP/ABCG2 in Porcine and Human Brain Capillaries. Mol Pharm. 2015 doi: 10.1021/mp500841w. [DOI] [PubMed] [Google Scholar]
- Schinelli S. Pharmacology and physiopathology of the brain endothelin system: an overview. Curr Med Chem. 2006;13:627–38. doi: 10.2174/092986706776055652. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–27. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, Wood NW, Sisodiya SM. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348:1442–8. doi: 10.1056/NEJMoa021986. [DOI] [PubMed] [Google Scholar]
- Sills GJ, Mohanraj R, Butler E, McCrindle S, Collier L, Wilson EA, Brodie MJ. Lack of association between the C3435T polymorphism in the human multidrug resistance (MDR1) gene and response to antiepileptic drug treatment. Epilepsia. 2005;46:643–7. doi: 10.1111/j.1528-1167.2005.46304.x. [DOI] [PubMed] [Google Scholar]
- Sisodiya SM, Heffernan J, Squier MV. Over-expression of P-glycoprotein in malformations of cortical development. Neuroreport. 1999;10:3437–41. doi: 10.1097/00001756-199911080-00032. [DOI] [PubMed] [Google Scholar]
- Tai LM, Loughlin AJ, Male DK, Romero IA. P-glycoprotein and breast cancer resistance protein restrict apical-to-basolateral permeability of human brain endothelium to amyloid-beta. J Cereb Blood Flow Metab. 2009;29:1079–83. doi: 10.1038/jcbfm.2009.42. [DOI] [PubMed] [Google Scholar]
- Tan NC, Heron SE, Scheffer IE, Pelekanos JT, McMahon JM, Vears DF, Mulley JC, Berkovic SF. Failure to confirm association of a polymorphism in ABCB1 with multidrug-resistant epilepsy. Neurology. 2004;63:1090–2. doi: 10.1212/01.wnl.0000137051.33486.c7. [DOI] [PubMed] [Google Scholar]
- Tishler DM, Weinberg KI, Hinton DR, Barbaro N, Annett GM, Raffel C. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia. 1995;36:1–6. doi: 10.1111/j.1528-1157.1995.tb01657.x. [DOI] [PubMed] [Google Scholar]
- Toth K, Vaughan MM, Peress NS, Slocum HK, Rustum YM. MDR1 P-glycoprotein is expressed by endothelial cells of newly formed capillaries in human gliomas but is not expressed in the neovasculature of other primary tumors. Am J Pathol. 1996;149:853–8. [PMC free article] [PubMed] [Google Scholar]
- Truelsen T, Bonita R. The worldwide burden of stroke: current status and future projections. Handb Clin Neurol. 2009;92:327–36. doi: 10.1016/S0072-9752(08)01916-7. [DOI] [PubMed] [Google Scholar]
- Ueno M, Nakagawa T, Huang CL, Ueki M, Kusaka T, Hosomi N, Kanenishi K, Onodera M, Wu B, Sakamoto H. The expression of P-glycoprotein is increased in vessels with blood-brain barrier impairment in a stroke-prone hypertensive model. Neuropathol Appl Neurobiol. 2009;35:147–55. doi: 10.1111/j.1365-2990.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- van Assema DM, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, Comans EF, Hoetjes NJ, Tolboom N, Langer O, Muller M, Scheltens P, Lammertsma AA, van Berckel BN. Blood-brain barrier P-glycoprotein function in Alzheimer's disease. Brain. 2012;135:181–9. doi: 10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, van Schaik R, Edelbroek PM, Redeker S, Aronica E, Wadman WJ, Marchi N, Vezzani A, Gorter JA. Inhibition of the multidrug transporter P-glycoprotein improves seizure control in phenytoin-treated chronic epileptic rats. Epilepsia. 2006;47:672–80. doi: 10.1111/j.1528-1167.2006.00496.x. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Zibell G, Pekcec A, Schlichtiger J, Edelbroek PM, Holtman L, Aronica E, Gorter JA, Potschka H. COX-2 inhibition controls P-glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology. 2010;58:404–12. doi: 10.1016/j.neuropharm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3:281–90. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, Kunert-Keil C, Walker LC, Warzok RW. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–41. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Glatzel M, Walker LC, Kroemer HK, Aguzzi A, Warzok RW. Cerebrovascular P-glycoprotein expression is decreased in Creutzfeldt-Jakob disease. Acta Neuropathol. 2006;111:436–43. doi: 10.1007/s00401-006-0042-3. [DOI] [PubMed] [Google Scholar]
- Volk H, Potschka H, Loscher W. Immunohistochemical localization of P-glycoprotein in rat brain and detection of its increased expression by seizures are sensitive to fixation and staining variables. J Histochem Cytochem. 2005;53:517–31. doi: 10.1369/jhc.4A6451.2005. [DOI] [PubMed] [Google Scholar]
- von Wedel-Parlow M, Wolte P, Galla HJ. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem. 2009;111:111–8. doi: 10.1111/j.1471-4159.2009.06305.x. [DOI] [PubMed] [Google Scholar]
- Wang T, Agarwal S, Elmquist WF. Brain distribution of cediranib is limited by active efflux at the blood-brain barrier. J Pharmacol Exp Ther. 2012;341:386–95. doi: 10.1124/jpet.111.190488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sykes DB, Miller DS. Constitutive androstane receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol Pharmacol. 2010;78:376–83. doi: 10.1124/mol.110.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hawkins BT, Miller DS. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J. 2011a;25:644–52. doi: 10.1096/fj.10-169227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hawkins BT, Miller DS. Activating PKC-beta1 at the blood-brain barrier reverses induction of P-glycoprotein activity by dioxin and restores drug delivery to the CNS. J Cereb Blood Flow Metab. 2011b;31:1371–5. doi: 10.1038/jcbfm.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE, Miller DS. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J Neurosci. 2014;34:8585–93. doi: 10.1523/JNEUROSCI.2935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–57. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005;36:2549–53. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- Westerlund M, Belin AC, Olson L, Galter D. Expression of multi-drug resistance 1 mRNA in human and rodent tissues: reduced levels in Parkinson patients. Cell Tissue Res. 2008;334:179–85. doi: 10.1007/s00441-008-0686-5. [DOI] [PubMed] [Google Scholar]
- Wijesuriya HC, Bullock JY, Faull RL, Hladky SB, Barrand MA. ABC efflux transporters in brain vasculature of Alzheimer's subjects. Brain Res. 2010;1358:228–38. doi: 10.1016/j.brainres.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Wolf A, Bauer B, Hartz AM. ABC Transporters and the Alzheimer's Disease Enigma. Front Psychiatry. 2012;3:54. doi: 10.3389/fpsyt.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Callaghan D, Jones A, Bai J, Rasquinha I, Smith C, Pei K, Walker D, Lue LF, Stanimirovic D, Zhang W. ABCG2 is upregulated in Alzheimer's brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Abeta(1-40) peptides. J Neurosci. 2009;29:5463–75. doi: 10.1523/JNEUROSCI.5103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You G, Morris ME. Drug Transporters: Molecular Characterization and Role in Drug Disposition. John Wiley & Sons, Inc.; 2006. Overview of Drug Transporter Families. pp. 1–10. [Google Scholar]
- Yousif S, Marie-Claire C, Roux F, Scherrmann JM, Decleves X. Expression of drug transporters at the blood-brain barrier using an optimized isolated rat brain microvessel strategy. Brain Res. 2007;1134:1–11. doi: 10.1016/j.brainres.2006.11.089. [DOI] [PubMed] [Google Scholar]
- Yousif S, Chaves C, Potin S, Margaill I, Scherrmann JM, Decleves X. Induction of P-glycoprotein and Bcrp at the rat blood-brain barrier following a subchronic morphine treatment is mediated through NMDA/COX-2 activation. J Neurochem. 2012;123:491–503. doi: 10.1111/j.1471-4159.2012.07890.x. [DOI] [PubMed] [Google Scholar]
- Zastre JA, Chan GN, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA, Weksler B, Bendayan M, Bendayan R. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res. 2009;87:1023–36. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]
- Zembruski NC, Buchel G, Jodicke L, Herzog M, Haefeli WE, Weiss J. Potential of novel antiretrovirals to modulate expression and function of drug transporters in vitro. J Antimicrob Chemother. 2011;66:802–12. doi: 10.1093/jac/dkq501. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, Stanimirovic DB. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003;17:2085–7. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]
- Zhao YL, Du J, Kanazawa H, Cen XB, Takagi K, Kitaichi K, Tatsumi Y, Ohta M, Hasegawa T. Shiga-like toxin II modifies brain distribution of a P-glycoprotein substrate, doxorubicin, and P-glycoprotein expression in mice. Brain Res. 2002;956:246–53. doi: 10.1016/s0006-8993(02)03546-1. [DOI] [PubMed] [Google Scholar]
- Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Liu GQ. Glutamate up-regulates P-glycoprotein expression in rat brain microvessel endothelial cells by an NMDA receptor-mediated mechanism. Life Sci. 2004;75:1313–22. doi: 10.1016/j.lfs.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Zibell G, Unkruer B, Pekcec A, Hartz AM, Bauer B, Miller DS, Potschka H. Prevention of seizure-induced up-regulation of endothelial P-glycoprotein by COX-2 inhibition. Neuropharmacology. 2009;56:849–55. doi: 10.1016/j.neuropharm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zschiedrich K, Konig IR, Bruggemann N, Kock N, Kasten M, Leenders KL, Kostic V, Vieregge P, Ziegler A, Klein C, Lohmann K. MDR1 variants and risk of Parkinson disease. Association with pesticide exposure? J Neurol. 2009;256:115–20. doi: 10.1007/s00415-009-0089-x. [DOI] [PubMed] [Google Scholar]