Abstract
The pathophysiology of schizophrenia involves disturbances of information processing across brain regions, possibly reflecting, at least in part, a disruption in the underlying axonal connectivity. This disruption is thought to be a consequence of the pathology of myelin ensheathment, the integrity of which is tightly regulated by oligodendrocytes. In order to gain insight into the possible neurobiological mechanisms of myelin deficit, we determined the messenger RNA (mRNA) expression profile of laser captured cells that were immunoreactive for 2’, 3’-cyclic nucleotide 3’-phosphodiesterase (CNPase), a marker for oligodendrocyte progenitor cells (OPCs) in addition to differentiating and myelinating oligodendrocytes, in the white matter of the prefrontal cortex in schizophrenia subjects. Our findings pointed to the hypothesis that OPC differentiation might be impaired in schizophrenia. To address this hypothesis, we quantified cells that were immunoreactive for neural/glial antigen 2 (NG2), a selective marker for OPCs, and those that were immunoreactive for oligodendrocyte transcription factor 2 (OLIG2), an oligodendrocyte lineage marker that is expressed by OPCs and maturing oligodendrocytes. We found that the density of NG2-immunoreactive cells was unaltered, but the density of OLIG2-immunoreactive cells was significantly decreased in subjects with schizophrenia, consistent with the notion that OPC differentiation impairment may contribute to oligodendrocyte disturbances and thereby myelin deficits in schizophrenia.
INTRODUCTION
Schizophrenia can be conceptualized as the clinical manifestation of the dysfunction of information processing and integration of neural networks across brain regions, including the different areas of the cerebral cortex and subcortical domains, such as the thalamus and medial temporal structures. It is well established that neuronal circuits within these regions are functionally disturbed in schizophrenia. Furthermore, impaired myelin ensheathment of the axons that connect these neuronal circuits via the white matter can further exacerbate network dysfunction by disturbing the timing of the arrival of action potential, compromising spike-timing dependent potentiation and hence disrupting the precise temporal coherence of neuronal circuit output that is necessary for synchronized network activation (Fields, 2005; Fields et al., 2014; Pajevic et al., 2013) which, in turn, may underlie many of the cognitive and perceptual deficits that are characteristic of schizophrenia (Cho et al., 2006; Lisman and Buzsaki, 2008; Spencer et al., 2004; Uhlhaas et al., 2008; Uhlhaas and Singer, 2010).
Structural magnetic resonance imaging (MRI) investigations have converged upon the conclusion that the volume of the white matter is decreased in patients with schizophrenia (Bora et al., 2011; Breier et al., 1992; Buchanan et al., 1998; Sigmundsson et al., 2001), consistent with the notion that the integrity of myelin is compromised. Furthermore, diffusion tensor imaging (DTI) has revealed that fractional anisotropy, which is a measure of a combination of factors, including myelin sheath integrity, is also decreased in schizophrenia patients (Holleran et al., 2014). More direct evidence of myelin deficit in this illness has come from postmortem studies. Specifically, the density of oligodendrocytes has been found to be decreased by 25-31% in both the gray and white matter of the prefrontal cortex in schizophrenia subjects (Hof et al., 2003; Uranova et al., 2004; Uranova et al., 2007; Vostrikov and Uranova, 2011). Furthermore, ultrastructural examination of oligodendrocytes has revealed morphological changes associated with cell degeneration and death (Uranova et al., 2001; Uranova et al., 2007), in addition to disturbances in the integrity of myelinated axonal fibers (Uranova et al., 2011). Finally, gene expression profiling of RNA extracted from homogenized gray and white matter of the cerebral cortex have revealed that many oligodendrocyte- and myelin-associated genes appear to be differentially expressed in subjects with schizophrenia (Aston et al., 2004; Hakak et al., 2001; Haroutunian et al., 2007; Hof et al., 2002; Katsel et al., 2005; Mitkus et al., 2008; Sequeira et al., 2012; Sugai et al., 2004; Tkachev et al., 2003).
Myelin is actively maintained by a complex series of events that tightly regulate the generation, differentiation, survival and death of oligodendrocytes. In the adult rat brain, roughly 5-10% of cells are oligodendrocyte progenitors (OPCs) (Dawson, 2003; Polito and Reynolds, 2005), whereas approximately 20% of the cells in the white matter in humans may be OPCs (Lojewski et al., 2014). It is further estimated that up to 80% of OPCs are actively replicating or differentiating (Trotter et al., 2010; Young et al., 2013). Disturbances of any of the events that underlie the differentiation and mediate the integrity of oligodendrocytes could lead to myelin and myelination deficits.
In an attempt to gain insight into the possible molecular mechanisms specifically associated with the dysfunction of cells that belong to the oligodendrocyte lineage and thereby myelin and myelination deficits in the white matter, we profiled the expression of mRNA in these cells obtained by laser-capture microdissection (LCM). CNPase (2’,3’-cyclic nucleotide 3’-phosphodiesterase) was chosen as a marker, because it is expressed in OPCs in addition to differentiating and myelinating oligodendrocytes (Belachew et al., 2001; Dawson, 2003; Levine et al., 1993; Polito and Reynolds, 2005; Reynolds et al., 2002; Scherer et al., 1994; Sprinkle, 1989; Tomassy and Fossati, 2014). Together with immunohistochemical validation of findings of our gene expression profiling experiment using oligodendrocyte lineage markers including neural/glial antigen 2 (NG2), a marker for OPCs, and oligodendrocyte transcription factor 2 (OLIG2), which is present in maturing and mature, myelinating oligodendrocytes, the preponderance of evidence derived from the present study appears to favor the interpretation that impairment in the differentiation of OPCs may contribute to the pathophysiology of white matter deficits of schizophrenia.
MATERIALS AND METHODS
Postmortem human brain tissue
Fresh-frozen tissue blocks containing the prefrontal cortex (Brodmann's area 9) from 9 schizophrenia and 9 normal control subjects, matched for age, sex and postmortem interval (PMI), were obtained from the Harvard Brain Tissue Resource Center (Table 1). A detailed methodology for tissue preparation, LCM and RNA processing has been described in detail elsewhere (Pietersen et al., 2011; Pietersen et al., 2009; Pietersen et al., 2014a; Pietersen et al., 2014b; Simunovic et al., 2009). Postmortem human brain collection procedures have been approved by the Partners Human Research Committee. Written informed consent for use of each of the brains for research has been obtained by the legal next-of-kin. The diagnosis of schizophrenia was made by two Board-certified psychiatrists by reviewing medical records and an extensive family questionnaire that included medical, psychiatric and social history. All of the brains included in this study were also examined by a Board-certified neuropathologist to rule out any neurological conditions. In addition, none of the subjects had any history of active substance abuse or dependence, as confirmed by toxicological analysis.
Table 1.
Cases used in the present study
| Diagnosis | Age | Sex | PMI | aRNA concentration ng/ul |
|---|---|---|---|---|
| Schizophrenia | 69 | F | 23.08 | 923.13 |
| Schizophrenia | 75 | F | 18.66 | 457.45 |
| Schizophrenia | 58 | M | 25.33 | 519.9 |
| Schizophrenia | 77 | M | 25.33 | 1159.7 |
| Schizophrenia | 66 | M | 21.75 | 845.9 |
| Schizophrenia | 63 | M | 26.16 | 450.07 |
| Schizophrenia | 60 | F | 17.13 | 706.1 |
| Schizophrenia | 60 | M | 22.17 | 1091.2 |
| Schizophrenia | 55 | F | 22 | 772.6 |
| 64.8 ± 7.6 | 22.4 ± 3.0 | 769.6 ± 262.4 | ||
| Control | 69 | F | 18.65 | 1700 |
| Control | 78 | F | 18.7 | 295.16 |
| Control | 58 | M | 20.75 | 665.7 |
| Control | 76 | M | 23.92 | 684.6 |
| Control | 58 | M | 19.28 | 1437.88 |
| Control | 63 | M | 17.92 | 968.2 |
| Control | 60 | F | 21.67 | 645 |
| Control | 61 | M | 21 | 644.6 |
| Control | 63 | F | 23.5 | 800.4 |
| 65.1 ± 8.4 | 20.1 ± 2.1 | 871.3 ± 437.9 | ||
Laser-Capture Microdissection
Sections of 8 μm were cut on a cryostat, mounted on slides and stored at −80°C until use. Cells immunoreactive for CNPase, a myelin-associated enzyme that recognizes OPCs and both mature and immature oligodendrocytes, from the white matter (within 50 μm from the gray matter border) in the PFC were isolated using the Arcturus XT™ system (Life Technologies, Grand Island, CA). Approximately 400 cells per subject were captured onto a CapSure HS™ LCM cap (Life Technologies, Grand Island, CA). To avoid systematic biases, samples from schizophrenia and normal control subjects were processed for LCM in a random order.
Affymetrix platform-based microarray gene expression profiling
RNA processing
RNA isolation was performed using the Picopure™ RNA Isolation kit (Life Technologies, Grand Island, NY), with a DNase step (Qiagen, Valencia, CA). This typically resulted in approximately 1-25 ng of total RNA (Pietersen et al., 2011; Pietersen et al., 2009). The extracted RNA underwent two rounds of linear amplification using the RiboAmp® kit (Life Technologies, Grand Island, NY). A dilution of the resulting products (approximately 250 pg/μl) was used to determine the distribution of transcript lengths with the Experion StdSens Labchip (Bio-Rad Laboratories, Hercules, CA; Supplementary Figure S1). The concentration and purity of these samples were determined by absorbance measurements at the optical density of A260 and A280, using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA).
Data analysis
Each array was scanned twice and the Affymetrix Microarray Suite 5.1 software averaged the two images to compute an intensity value for each probe cell within each probe set. For the quality control step, we employed the Partek® software's built-in function (Partek, St. Louis, MO). We then normalized the data with Partek's standard normalization method (i.e. data has a mean of zero and a variance of one, and each column for each sample was divided by the average of all control samples). Differentially expressed genes were visualized by performing unsupervised hierarchical clustering as stringency of the filtering criteria (i.e. fold-change and FDR-adjusted p-value) was varied to determine a representative gene list for pathway analyses.
Pathway analyses were performed with two web-based algorithms, Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA) and MetaCore (GeneGo, New York, NY), to map the differentially expressed genes onto biological functions and canonical pathways (Pietersen et al., 2014a; Pietersen et al., 2014b). With Ingenuity, the significance for each of the identified pathways was determined via a Fisher's exact test, whereas GeneGo Metacore makes use of their algorithm for hypergeometric distribution, identifying pathways overrepresented with significant genes. Literature mining was then performed to elucidate the pathways or gene families that were particularly pertinent for oligodendrocyte functions.
Microarray and qRT-PCR validation
The TURBO Biotin labeling™ kit (end-labeling; Life Technologies, Grand Island, NY) was used to label the aRNA obtained from amplified samples. Gene expression profiling was performed using the Affymetrix Human X3P GeneChip®, which possesses an extreme 3’ bias in its probe design and hence is particularly suitable for samples that are prone to RNA degradation, such as postmortem human brain tissue. This chip has also been shown to be superior to the more commonly used Affymetrix human U133 plus 2.0 chip in terms of data reproducibility (Caretti et al., 2008). The hybridization and scanning procedures were performed at the Partners HealthCare Center for Personalized Genetic Medicine, Cambridge, MA.
For validation of microarray data, cDNA was reverse transcribed from aRNA (200 ng input) using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Carlsbad, CA). TaqMan®-based qRT-PCR (Applied Biosystems, Carlsbad, CA) was subsequently performed on selected differentially expressed genes within signaling pathways identified by pathway analysis as dysregulated in schizophrenia and randomly chosen genes (Supplementary Table S1). Due to the small amount of RNA available from laser-captured samples, only a relatively small number of genes were included. Samples were normalized with respect to the housekeeping gene, hypoxanthine guanine phosphoribosyltransferase (HPRT), which has been shown to produce reliable results in human brain tissue as its expression has been shown not to be altered in disease states (Radonic et al., 2004). Normalization was performed against only one gene because of the limited quantity of RNA available from laser-captured materials. Negative controls (negative reverse transcription and no template controls) were performed to detect any contamination of the samples, such as genomic (g)DNA. Where necessary, samples were treated with Turbo DNA-free™ kit (Applied Biosystems, Carlsbad, CA) and the negative controls repeated to confirm gDNA removal before qRT-PCR was performed. Samples were run in duplicate and the average of the duplicates was taken as input for quantification using the 2^-ΔCt method (Livak and Schmittgen, 2001) or the relative expression software tool (REST) for group-wise comparisons of expression ratios (Pfaffl et al., 2002). A Spearman Rho's correlation analysis was then performed on the fold-changes determined by microarray and qRT-PCR. In accordance with Morey et al.(Morey et al., 2006), a correlation of >0.8 with a significance of p<0.05 between qRT-PCR and microarray expression changes was considered validation of the microarray result.
NG2 and OLIG2 Immunohistochemistry
Tissue blocks were sectioned at 20 μm, mounted on gelatin-subbed slides, and post-fixed in 4% paraformaldehyde for 20 minutes at room temperature. Sections were then incubated in endogenous enzyme block (1% H2O2, 10% MeOH) for 15 minutes and, additionally, blocked using 2% bovine serum albumin (BSA) in 10% goat serum (Life Technologies, 16210-064, Grand Island, NY) at room temperature for 1 hour, followed by incubation in an anti-NG2 (1:200, Sigma-Aldrich, St. Louis, MO) or anti-OLIG2 (1:1,000, Phosphosolutions, Aurora, CO) antibody produced in rabbit (Sigma-Aldrich, St. Louis, MO) at 4°C overnight. Sections were then incubated at room temperature in biotinylated anti-rabbit IgG antibody produced in goat (1:500, Vector Labs, Burlingame, CA), followed by a 2-hour incubation in horseradish peroxidase-conjugated streptavidin (1:5,000, Zymed, San Francisco, CA) made in 1 Mol/L of phosphate buffer (PB) at room temperature, and finally in nickel-enhanced diaminobenzidine/peroxidase reaction (0.02% diaminobenzidine, 0.08% nickel-sulphate, 0.006% hydrogen peroxide in 1 Mol/L PB). Sections were finally dehydrated and coverslipped.
The number of NG2- or OLIG2-immunoreactive cells within three 100 μm × 100 μm squares that were 500 μm apart from one another were quantified by the same investigator (SAM) in a blind fashion using a StereoInvestigator system (MBF Bioscience, Williston, VT). Cell densities were compared between subjects with schizophrenia and normal control subjects using unpaired t-test. We also used correlation analysis to evaluate any potential confounding effect of each of the numerical covariates (i.e. age, PMI, antipsychotic exposure in terms of chlorpromazine equivalent) on the cell density measures. The possible confounding effect of sex was evaluated by comparing cell densities between the two sexes within each of the two subject groups.
RESULTS
Gene expression profile of CNPase-immunoreactive cells in schizophrenia
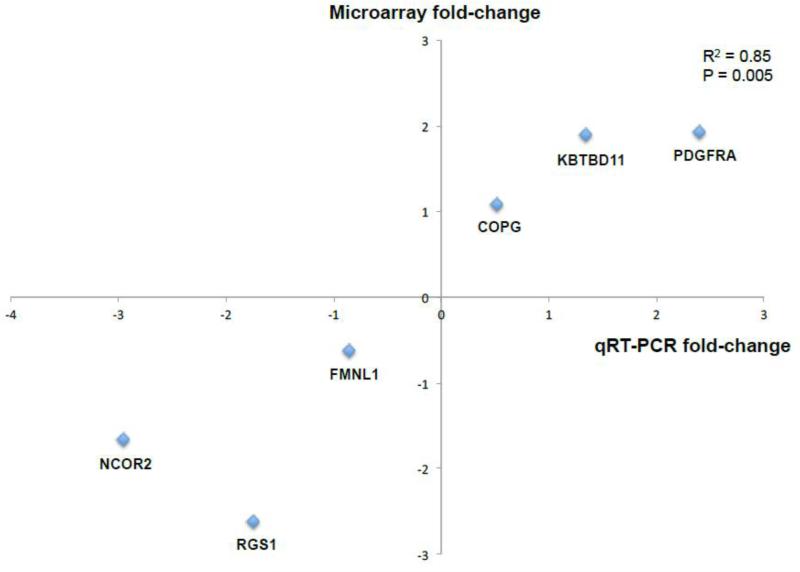
We identified 497 genes that were differentially expressed (i.e. fold-change >1.5 and p<0.05) in the CNPase-immunoreactive cells in subjects with schizophrenia (Supplementary Table S2), the majority of which (56%) were downregulated. Pathway analysis revealed that many of the signaling cascades related to oligodendrocyte proliferation and differentiation appeared to be differentially regulated in schizophrenia subjects (Table 2). Validation of microarray findings were performed by statistically comparing gene expression changes detected by microarray and qRT-PCR (Morey et al., 2006), which were found to be highly significantly correlated (R2=0.85, p=0.005; Figure 1).
Table 2.
Differentially regulated signaling pathways associated with oligodendrocyte differentiation in schizophrenia
| Pathway | FDR-adjusted p value |
|---|---|
| Normal and pathological TGF-beta-mediated regulation of cell proliferation | 1.395e-6 |
| Signal transduction_NOTCH signaling | 2.674e-5 |
| Development_PDGF signaling via MAPK cascades | 1.611e-4 |
| Development_Thromboxane A2 signaling pathway | 8.202e-4 |
| Development_Growth factors in regulation of oligodendrocyte precursor cell proliferation | 8.515e-4 |
| Cell cycle_G1-S Growth factor regulation | 2.412e-4 |
| Development_Hedgehog signaling | 2.477e-3 |
| Apoptosis_Anti-Apoptosis mediated by external signals via MAPK and JAK/STAT | 5.738e-3 |
| Signal transduction_WNT signaling | 1.647e-2 |
Figure 1.
Gene expression changes detected by microarray and qRT-PCR are highly significantly correlated (R2=0.85, p=0.005).
Immunohistochemical quantification of OPC differentiation
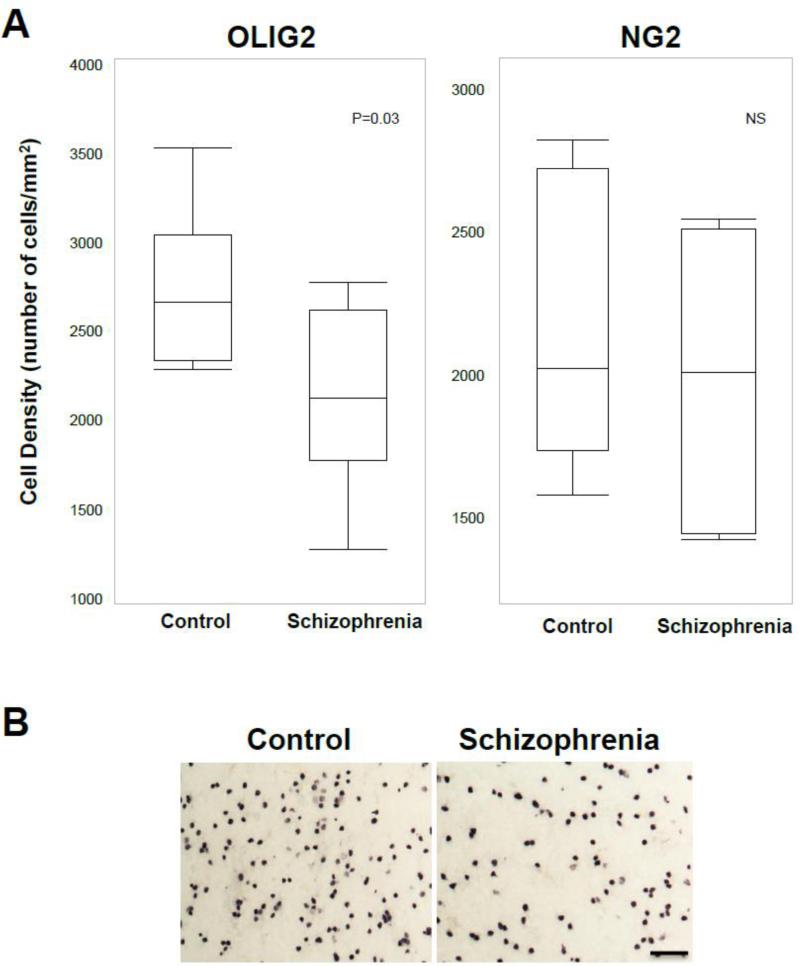
In order to address the hypothesis that OPC differentiation might be altered in schizophrenia, we quantified cells that were immunoreactive for NG2, a marker for OPCs, and those that were immunoreactive for OLIG2, an oligodendrocyte lineage marker for OPCs, maturing and possibly also mature oligodendrocytes (Ligon et al., 2004; Ligon et al., 2006). We found that the density of NG2-immunoreactive cells was unaltered in schizophrenia (1,973.3 ± 447.6 cells/mm2) compared to normal control (2,124.0 ± 462.6 cells/mm2) subjects, but the density of OLIG2-immunoreactive cells was significantly (p=0.032) decreased by 21.3% in schizophrenia (2,140.5 ± 517.6 cells/mm2) compared to normal control (2,721.9 ± 440.0 cells/mm2) subjects (Figure 2). Our statistical analyses indicate that these findings were not affected by any of the potential confounding variables.
Figure 2.
A. The density of OLIG2-immunoreactive cells is significantly decreased whereas the density of NG2-immunoreactive cells is unaltered in subjects with schizophrenia. B. Photomicrographs showing OLIG2-immunoreactive cells in a schizophrenia (right) and a normal control (left) subjects. Scale bar=50 μm.
DISCUSSION
Findings of the gene expression profiling experiment reported here suggest that dysregulation of the differentiation of OPCs may contribute to oligodendrocyte and myelin deficits of schizophrenia. To address this hypothesis, we immunohistochemically visualized cells that expressed NG2, a marker for OPCs, and OLIG2, an oligodendrocyte lineage marker for OPCs, maturing oligodendrocytes and some mature, myelinating oligodendrocytes. We found that the density of NG2-immunoreactive cells was unaltered, but the density of OLIG2-immunoreactive cells was significantly decreased in subjects with schizophrenia. Hence, even though the sample size of our gene expression profiling experiment was relatively small, a key hypothesis derived from findings of this experiment is in fact substantiated by our immunohistochemical findings. It should be noted, however, that these findings by themselves do not necessarily allow us to definitively conclude that oligodendrocyte differentiation is impaired, as the observed decrease in OLIG2-immunoreactive cells could reflect a deficit in the mature but not the maturing oligodendrocytes. Nevertheless, the interpretation of these findings in the context of the microarray observations does appear to favor the argument that impairment in the differentiation of OPCs contributes to the pathophysiology of schizophrenia.
Using LCM in combination with the Affymetrix platform of microarray, we identified signaling pathways that appear to be differentially regulated in cells of oligodendrocyte lineage in the white matter in the prefrontal cortex in schizophrenia. One of the most noticeable observations of this study is the dysregulation of platelet-derived growth factor (PDGF) signaling in subjects with schizophrenia (Table 2). For instance, PDGF receptor alpha (PDGFRA), which is specifically expressed in OPCs (Armati and Mathey, 2010; Baracskay et al., 2007; Wilson et al., 2006), was found to be upregulated by 3.8-fold. Previous studies have found that in the absence of PDGF, all OPCs stop dividing and instead differentiate into oligodendrocytes (Durand and Raff, 2000), which leads to the downregulation of PDGFRA (Hart et al., 1989). In this context, the observation that PDGFRA is upregulated in schizophrenia is consistent with the interpretation of impaired differentiation of OPCs into mature oligodendrocytes.
The substrate of PDGFRA, PDGF, regulates the survival, proliferation and differentiation of OPCs in part via phosphoinositide 3-kinase (PI3K) signaling. In fact, PIK3CA was also found to be upregulated by 3.4-fold in subjects with schizophrenia. Furthermore, in light of the fact that sonic hedgehog (SHH) appears to promote the OPC fate (Armati and Mathey, 2010; Mitew et al., 2013; Tomassy and Fossati, 2014), we found that SHH signaling was differentially regulated in subjects with schizophrenia. Other signaling pathways found to be differentially regulated in this study (Table 2), such as NOTCH signaling, are known to inhibit the oligodendrocyte differentiation process (Wang et al., 1998), whereas pathways such as the thromboxane A2 signaling cascade have been shown to promote OPC proliferation and oligodendrocyte survival (Lin et al., 2005; Mir and Le Breton, 2008). WNT signaling, on the other hand, appears to either promote or inhibit oligodendrocyte differentiation in a context-dependent fashion (Emery, 2010; Guo et al., 2015; Mitew et al., 2013). Similarly, TGF-beta signaling has been shown to play an important role in regulating the balance between OPC proliferation and oligodendrocyte differentiation (Zhang et al., 2010). Finally, cell cycle G1-S growth factor regulation pathway also appears to be affected. Altogether, the preponderance of evidence derived from this study is consistent with the hypothesis that mechanisms that regulate the generation and differentiation of olgiodendrocytes are involved in the pathophysiology of this illness.
To more directly address the hypothesis that oligodendrocyte differentiation may be dysregulated in schizophrenia, we compared the density of NG2- and OLIG2-immunoreactive cells between schizophrenia and normal control subjects. Because NG2 is a specific OPC marker, our finding that the density of NG2-immunoreactive neurons was unchanged in schizophrenia suggests that OPCs are numerically unaltered. In this context, because OLIG2 is expressed in both OPCs and maturing oligodendrocytes in addition to some mature oligodendrocytes, our observation of decreased density of OLIG2-immunoreactive cells in schizophrenia subjects, together with a previous study showing reduced immunoreactivity for CNPase (Flynn et al., 2003), a marker for the entire oligodendrocyte lineage, is consistent with the notion that oligodendrocyte differentiation is impaired in schizophrenia. Future studies should focus on further delineating the specific stage(s) during oligodendrocyte differentiation and maturation that are affected in schizophrenia by employing stage-specific markers.
Our data do not appear to implicate genes directly associated with myelination and myelin synthesis as differentially regulated in schizophrenia. Perhaps myelin deficit in the white matter in schizophrenia may be more of a consequence of an “inadequate” supply of myelinating oligodendrocyte instead of a deficit in the synthesis of myelin by oligodendrocytes, although these two possibilities need not be mutually exclusive. In fact, previous studies have found that some of the myelin-associated genes appear to be differentially expressed in schizophrenia, although findings from these studies have not always been consistent (Aston et al., 2004; Hakak et al., 2001; Katsel et al., 2005; Mitkus et al., 2008; Sequeira et al., 2012; Sugai et al., 2004; Tkachev et al., 2003). Our findings are in line with previous studies showing that the density of oligodendrocytes is decreased in both the gray and white matter of the prefrontal cortex in schizophrenia subjects (Hof et al., 2003; Uranova et al., 2007; Vostrikov and Uranova, 2011), although a lack of significant change in the density of these cells has also been reported (Hercher et al., 2014; Uranova et al., 2004). Insofar as the number of oligodendrocytes is in fact decreased in schizophrenia, this reduction has been speculated to represent a deficit in the normal age-dependent increase in the number of oligodendrocytes (Vostrikov and Uranova, 2011), perhaps reflecting an intrinsic deficit in cell lineage programming and differentiation. The decrease in the number of oligodendrocytes together with the resultant myelin deficit may together contribute to the observed reduction in white matter volume in various brain regions, including the prefrontal cortex, in patients with schizophrenia (Bora et al., 2011; Breier et al., 1992; Buchanan et al., 1998; Sigmundsson et al., 2001).
Oxidative stress is increasingly recognized as an important mechanism that may lead to the cellular insults characteristic of the pathophysiology of schizophrenia (Bitanihirwe and Woo, 2011; Do et al., 2009; Do et al., 2015; Fournier et al., 2014; Kulak et al., 2013). For instance, animal studies have shown that the inhibitory neurons that contain the calcium-binding protein parvalbumin, which are strongly implicated in contributing to the functional deficits of the cerebral cortex in schizophrenia, are particularly vulnerable to oxidative insult (Cabungcal et al., 2013a; Cabungcal et al., 2013b; Morishita et al., 2015). Likewise, oligodendrocytes are also especially susceptible to oxidative injury (Monin et al., 2014), which may in part contribute to the apparent decrease in the number of oligodendrocytes in schizophrenia (Hof et al., 2003; Uranova et al., 2004). The fact that there is an apparent failure in OPC differentiation to replenish the oligodendrocyte pool is likely to magnify the pathophysiological consequence of oxidative insult to oligodendrocytes. In this context, it is of interest to point out that haloperidol has previously been shown to potentially promote oligodendrocyte differentiation (Fang et al., 2013; Wang et al., 2010) and quetiapine has been found to directly facilitate myelin regeneration by replenishing mature oligodendrocytes in animals (Zhang et al., 2012). Hence, the efficacy of antipsychotics in the treatment of schizophrenia may, at least in part, be mediated by regulating oligodendrocyte lineage.
Supplementary Material
ACKNOWLEDGEMENT
This study was supported by grants P50MH080272 (Boston CIDAR: Vulnerability to Progression in Schizophrenia) and R01MH076060 from the National Institutes of Health.
ROLE OF THE FUNDING SOURCE
The NIH played no role in the design and execution of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS’ CONTRIBUTIONS
SAM and CYP carried out LCM and RNA processing for microarray experiments. SAM performed qRTPCR validation and immunohistochemistry, participated in the microarray data analysis and drafted the manuscript. KCS participated in data analysis and manuscript preparation. TUWW conceived of the study, participated in its design and data interpretation, and prepared the manuscript.
CONFLICTS OF INTEREST AND FINANCIAL DISCLOSURE
None.
REFERENCES
- Armati PJ, Mathey EK. The biology of oligodendrocytes. Cambridge University Press; Cambridge ; New York: 2010. [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77(6):858–866. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- Baracskay KL, Kidd GJ, Miller RH, Trapp BD. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia. 2007;55(10):1001–1010. doi: 10.1002/glia.20519. [DOI] [PubMed] [Google Scholar]
- Belachew S, Yuan X, Gallo V. Unraveling oligodendrocyte origin and function by cell-specific transgenesis. Dev Neurosci. 2001;23(4-5):287–298. doi: 10.1159/000048712. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Woo T-UW. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev. 2011;35(3):878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yucel M, Velakoulis D, Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127(1-3):46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry. 1992;49(12):921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Vladar K, Barta PE, Pearlson GD. Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry. 1998;155(8):1049–1055. doi: 10.1176/ajp.155.8.1049. [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-Life Insults Impair Parvalbumin Interneurons via Oxidative Stress: Reversal by N-Acetylcysteine. Biol Psychiatry. 2013a;73(6):574–582. doi: 10.1016/j.biopsych.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, Do KQ. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013b;110(22):9130–9135. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretti E, Devarajan K, Coudry R, Ross E, Clapper ML, Cooper HS, Bellacosa A. Comparison of RNA amplification methods and chip platforms for microarray analysis of samples processed by laser capture microdissection. J Cell Biochem. 2008;103(2):556–563. doi: 10.1002/jcb.21426. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical {gamma} synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103(52):19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Molecular and Cellular Neuroscience. 2003;24(2):476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19(2):220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cuenod M, Hensch TK. Targeting Oxidative Stress and Aberrant Critical Period Plasticity in the Developmental Trajectory to Schizophrenia. Schizophr Bull. 2015;41(4):835–846. doi: 10.1093/schbul/sbv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand B, Raff M. A cell-intrinsic timer that operates during oligodendrocyte development. BioEssays : news and reviews in molecular, cellular and developmental biology. 2000;22(1):64–71. doi: 10.1002/(SICI)1521-1878(200001)22:1<64::AID-BIES11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330(6005):779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Fang F, Zhang H, Zhang Y, Xu H, Huang Q, Adilijiang A, Wang J, Zhang Z, Zhang D, Tan Q, He J, Kong L, Liu Y, Li XM. Antipsychotics promote the differentiation of oligodendrocyte progenitor cells by regulating oligodendrocyte lineage transcription factors 1 and 2. Life Sci. 2013;93(12-14):429–434. doi: 10.1016/j.lfs.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11(6):528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Araque A, Johansen-Berg H, Lim SS, Lynch G, Nave KA, Nedergaard M, Perez R, Sejnowski T, Wake H. Glial biology in learning and cognition. Neuroscientist. 2014;20(5):426–431. doi: 10.1177/1073858413504465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, Smith GN, Arango V, Mann JJ, Dwork AJ, Falkai P, Honer WG. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8(9):811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- Fournier M, Ferrari C, Baumann PS, Polari A, Monin A, Bellier-Teichmann T, Wulff J, Pappan KL, Cuenod M, Conus P, Do KQ. Impaired Metabolic Reactivity to Oxidative Stress in Early Psychosis Patients. Schizophr Bull. 2014;40(5):973–983. doi: 10.1093/schbul/sbu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Lang J, Sohn J, Hammond E, Chang M, Pleasure D. Canonical Wnt signaling in the oligodendroglial lineage-puzzles remain. Glia. 2015;63(10):1671–1693. doi: 10.1002/glia.22813. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Dracheva S, Stewart DG, Davis KL. Variations in oligodendrocyte-related gene expression across multiple cortical regions: implications for the pathophysiology of schizophrenia. Int J Neuropsychopharmacol. 2007;10(4):565–573. doi: 10.1017/S1461145706007310. [DOI] [PubMed] [Google Scholar]
- Hart IK, Richardson WD, Bolsover SR, Raff MC. PDGF and intracellular signaling in the timing of oligodendrocyte differentiation. J Cell Biol. 1989;109(6 Pt 2):3411–3417. doi: 10.1083/jcb.109.6.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercher C, Chopra V, Beasley CL. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci. 2014;39(6):376–385. doi: 10.1503/jpn.130277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res. 2002;27(10):1193–1200. doi: 10.1023/a:1020981510759. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biological Psychiatry. 2003;53(12):1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Holleran L, Ahmed M, Anderson-Schmidt H, McFarland J, Emsell L, Leemans A, Scanlon C, Dockery P, McCarthy P, Barker GJ, McDonald C, Cannon DM. Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology. 2014;39(4):944–954. doi: 10.1038/npp.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res. 2005;79(2-3):157–173. doi: 10.1016/j.schres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Kulak A, Steullet P, Cabungcal JH, Werge T, Ingason A, Cuenod M, Do KQ. Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models. Antioxidants & redox signaling. 2013;18(12):1428–1443. doi: 10.1089/ars.2012.4858. [DOI] [PubMed] [Google Scholar]
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7(4):307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63(5):499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Olig gene function in CNS development and disease. Glia. 2006;54(1):1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- Lin X, Ramamurthy SK, Le Breton GC. Thromboxane A receptor-mediated cell proliferation, survival and gene expression in oligodendrocytes. J Neurochem. 2005;93(2):257–268. doi: 10.1111/j.1471-4159.2004.02969.x. [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34(5):974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lojewski X, Hermann A, Wegner F, Arauzo-Bravo MJ, Hallmeyer-Elgner S, Kirsch M, Schwarz J, Scholer HR, Storch A. Human adult white matter progenitor cells are multipotent neuroprogenitors similar to adult hippocampal progenitors. Stem cells translational medicine. 2014;3(4):458–469. doi: 10.5966/sctm.2013-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir F, Le Breton GC. A novel nuclear signaling pathway for thromboxane A2 receptors in oligodendrocytes: evidence for signaling compartmentalization during differentiation. Mol Cell Biol. 2008;28(20):6329–6341. doi: 10.1128/MCB.00482-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitew S, Hay CM, Peckham H, Xiao J, Koenning M, Emery B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Mitkus SN, Hyde TM, Vakkalanka R, Kolachana B, Weinberger DR, Kleinman JE, Lipska BK. Expression of oligodendrocyte-associated genes in dorsolateral prefrontal cortex of patients with schizophrenia. Schizophr Res. 2008;98(1-3):129–138. doi: 10.1016/j.schres.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin A, Baumann PS, Griffa A, Xin L, Mekle R, Fournier M, Butticaz C, Klaey M, Cabungcal JH, Steullet P, Ferrari C, Cuenod M, Gruetter R, Thiran JP, Hagmann P, Conus P, Do KQ. Glutathione deficit impairs myelin maturation: relevance for white matter integrity in schizophrenia patients. Mol Psychiatry. 2014;20(7):827–838. doi: 10.1038/mp.2014.88. [DOI] [PubMed] [Google Scholar]
- Morey JS, Ryan JC, Van Dolah FM. Microarray validation: factors influencing correlation between oligonucleotide microarrays and real-time PCR. Biol Proced Online. 2006;8:175–193. doi: 10.1251/bpo126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Cabungcal JH, Chen Y, Do KQ, Hensch TK. Prolonged Period of Cortical Plasticity upon Redox Dysregulation in Fast-Spiking Interneurons. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajevic S, Basser PJ, Fields RD. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience. 2013;276:135–147. doi: 10.1016/j.neuroscience.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen CY, Lim MP, Macey L, Woo T-UW, Sonntag KC. Neuronal type-specific gene expression profiling and laser-capture microdissection. Methods Mol Biol. 2011;755:327–343. doi: 10.1007/978-1-61779-163-5_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen CY, Lim MP, Woo T-UW. Obtaining high quality RNA from single cell populations in human postmortem brain tissue. J Vis Exp. 2009;(30) doi: 10.3791/1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen CY, Mauney SA, Kim SS, Lim MP, Rooney RJ, Goldstein JM, Petryshen TL, Seidman LJ, Shenton ME, McCarley RW, Sonntag KC, Woo TU. Molecular profiles of pyramidal neurons in the superior temporal cortex in schizophrenia. Journal of neurogenetics. 2014a;28(1-2):53–69. doi: 10.3109/01677063.2014.882918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen CY, Mauney SA, Kim SS, Passeri E, Lim MP, Rooney RJ, Goldstein JM, Petreyshen TL, Seidman LJ, Shenton ME, McCarley RW, Sonntag KC, Woo TU. Molecular profiles of parvalbumin-immunoreactive neurons in the superior temporal cortex in schizophrenia. Journal of neurogenetics. 2014b;28(1-2):70–85. doi: 10.3109/01677063.2013.878339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207(6):707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313(4):856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31(6-7):523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Braun PE, Grinspan J, Collarini E, Wang DY, Kamholz J. Differential regulation of the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene during oligodendrocyte development. Neuron. 1994;12(6):1363–1375. doi: 10.1016/0896-6273(94)90451-0. [DOI] [PubMed] [Google Scholar]
- Sequeira PA, Martin MV, Vawter MP. The first decade and beyond of transcriptional profiling in schizophrenia. Neurobiol Dis. 2012;45(1):23–36. doi: 10.1016/j.nbd.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158(2):234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Simunovic F, Yi M, Wang Y, Macey L, Brown LT, Krichevsky AM, Andersen SL, Stephens RM, Benes FM, Sonntag KC. Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson's disease pathology. Brain. 2009;132(7):1795–1809. doi: 10.1093/brain/awn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101(49):17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinkle TJ. 2′,3′-cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol. 1989;4(3):235–301. [PubMed] [Google Scholar]
- Sugai T, Kawamura M, Iritani S, Araki K, Makifuchi T, Imai C, Nakamura R, Kakita A, Takahashi H, Nawa H. Prefrontal abnormality of schizophrenia revealed by DNA microarray: impact on glial and neurotrophic gene expression. Ann N Y Acad Sci. 2004;1025:84–91. doi: 10.1196/annals.1316.011. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362(9386):798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, Fossati V. How big is the myelinating orchestra? Cellular diversity within the oligodendrocyte lineage: facts and hypotheses. Front Cell Neurosci. 2014;8:201. doi: 10.3389/fncel.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J, Karram K, Nishiyama A. NG2 cells: Properties, progeny and origin. Brain Res Rev. 2010;63(1-2):72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34(5):927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55(5):597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophrenia Research and Treatment. 2011;2011:325789. doi: 10.1155/2011/325789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67(2-3):269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Vikhreva OV, Zimina IS, Kolomeets NS, Orlovskaya DD. The role of oligodendrocyte pathology in schizophrenia. Int J Neuropsychopharmacol. 2007;10(4):537–545. doi: 10.1017/S1461145707007626. [DOI] [PubMed] [Google Scholar]
- Vostrikov V, Uranova N. Age-related increase in the number of oligodendrocytes is dysregulated in schizophrenia and mood disorders. Schizophrenia Research and Treatment. 2011;2011:174689. doi: 10.1155/2011/174689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xu H, Niu J, Mei F, Li X, Kong J, Cai W, Xiao L. Haloperidol activates quiescent oligodendroglia precursor cells in the adult mouse brain. Schizophr Res. 2010;119(1-3):164–174. doi: 10.1016/j.schres.2010.02.1068. [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21(1):63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176(1-2):162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77(5):873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Wang L, Jiang W, Xu H, Xiao L, Bi X, Wang J, Zhu S, Zhang R, He J, Tan Q, Zhang D, Kong J, Li XM. Quetiapine enhances oligodendrocyte regeneration and myelin repair after cuprizone-induced demyelination. Schizophr Res. 2012;138(1):8–17. doi: 10.1016/j.schres.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Navrazhina K, Argaw AT, Zameer A, Gurfein BT, Brosnan CF, John GR. TGFbeta1 induces Jagged1 expression in astrocytes via ALK5 and Smad3 and regulates the balance between oligodendrocyte progenitor proliferation and differentiation. Glia. 2010;58(8):964–974. doi: 10.1002/glia.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.