Abstract
HIV infection affects approximately 1.2 million persons in the United States and 35 million worldwide. Progression to advanced liver disease remains a leading cause death among HIV-infected persons in the U.S. and elsewhere. Though mortality from HIV complications has been dramatically reduced wherever effective combination antiretroviral therapy is utilized, there has been little impact on liver related mortality. Causes of liver disease in the setting of HIV infection include viral hepatitis, NAFLD/NASH, drug-associated toxicities, and other metabolic/genetic disorders which interact in an environment modulated by persistent immune activation and altered cytokine display. Despite significant advances in treatment of HCV and suppression of HBV, treatment and management principles remain challenging. Limited resources, fragmented healthcare and high levels of injection drug use, alcohol use and depression remain relevant issues in the HIV-infected patient.
Keywords: HIV, HCV, HBV, Coinfection, Treatment, Diagnosis, Pathophysiology
Introduction
Liver disease remains an important contributor to morbidity and mortality among those with HIV infection. Many overlapping factors contribute to the development of liver injury and progressive hepatic fibrosis. Viral infections such as chronic hepatitis B and C are over-represented compared to the general population due to the commonality of risk factors for disease acquisition with HIV infection. These infections appear to generate increased rates of hepatic fibrosis with progression to cirrhosis and end-stage liver disease. There may be impairment of immune surveillance of hepatocellular carcinoma (HCC) that leads to earlier and more rapid cancer dissemination and growth. Antiretroviral therapies are life-saving in terms of HIV but some may modulate pathways involved with fat metabolism and stellate cell activation leading to chronic liver injury. Other viral infections such as hepatitis E virus (HEV) may exhibit chronicity with persistent injury in those with HIV, though this is rarely observed in the non-immunosuppressed hosts. Care models of liver disease are often inadequate for those with HIV with limited availability of liver transplantation and little understanding of complex antiretroviral regimens by consulting hepatologists. In 2006, the first biennial HIV and Liver Disease Forum was held to promote cross-disciplinary attention to the research, clinical, and psycho-social needs of HIV infected persons. This and subsequent meetings have been supported by multiple institutes of the NIH as well as industry partners to facilitate the identification of the research agenda in the field, and to fully characterize our understanding related the care and management of liver disease in the HIV-infected patient. To this end, the 5th Biennial HIV and Liver Disease conference was held in Jackson, Wyoming in September 2014. Speakers were charged with providing up to the moment updates of the field and to identify the research agenda for the next two years. This report provides a summation of the progress in the field and a guide to the research agenda going forward.
Epidemiology/Natural History/Assessment of Liver Disease in HIV
HIV infection remains a significant health problem within the U.S. and around the globe. In countries where combination antiretroviral therapy is widely available, those with HIV infection demonstrate a non-progressive chronic viral infection state. There is a stable incidence of approximately 50,000 new HIV infections/year in the U.S. and the prevalence of those living with HIV/AIDS is thought to now approach 1.2 million persons. The rate of new diagnoses is not evenly distributed in the general population and ranges from 1.9 cases/100,000 persons for white women to 103.9 cases/100,000 for African-American men. The largest number of new infections is among men who have sex with men (MSM). In terms of new HIV infections, cities in the Southern tier of the U.S. predominate, accounting for 11 of the top 15 metropolitan areas.
Antiretroviral therapy has revolutionized management. In the pre-cART (Combination Antiretroviral Therapy) era the expected survival of a 20 year old was an additional 8 years. Today, a treated 20 year old can have an expected survival of 55 more years which is close to that seen in an HIV uninfected individual. 1,2 Unfortunately the cascade of care in the U.S. is far from perfect. (Figure 1). The CDC estimates that while approximately 80% of those with HIV have been diagnosed, only about 60% of those infected are linked to care, 35% are on cART and 28% have suppressed HIV RNA ≤200 copies/ml.3 The implications of this observation, with regard to HCV coinfection are described below. Furthermore, the diagnosis of HIV tends to occur relatively late in the disease process with the median CD4 count at time of diagnosis at 182 cells/mm3 (http://www.cdc.gov/hiv/surveillance/resources/reports/2010supp_vol16no1/pdf/HIVAIDS_ssr_vol16_no1.pdf). Patients who are unaware of their HIV infection account for approximately 49% of new transmissions of HIV.4
Figure 1.
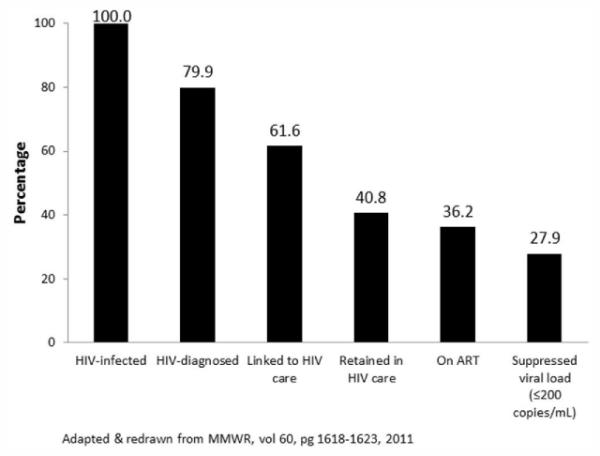
Percentage of HIV-infected persons engaged in selected stages of the continuum of HIV care in the United States
The diagnosis and assessment of liver disease in the HIV-infected patient mirrors that performed in those without HIV-infection, with some notable exceptions. Baseline physical examination and laboratory evaluation may be altered by the presence of HIV infection or the treatment of HIV with certain antiretroviral agents. In patients with AIDS (CD4< 200 cells/mm3) relative neutropenia is observed. In addition, it is not uncommon to have thrombocytopenia, either attributed to the antiretroviral therapy or to increased risk of HIV-associated thrombocytopenic purpura (TTP) and/or thrombotic microangiopathy (TMA).5 The presence of thrombocytopenia may lead to erroneous conclusions regarding the presence of cirrhosis, particularly since splenomegaly can be a feature of HIV infection as well.6 Non-cirrhotic portal hypertension is also more common, and may be associated with older nucleoside analogue agents.7,8 Non-invasive tests of hepatic fibrosis may also be less accurate in certain settings. Tests like FIB-4 and APRI utilize platelet count in the calculation and falsely low platelet counts will overestimate the degree of hepatic fibrosis leading to classification error unless corrections are made in the formulas used to calculate fibrosis scores.9 Since HIV infected persons can have thrombocytopenia from HIV itself, it is possible that tests that rely on the platelet count might overestimate the disease stage in select patients. However, to date, the validity of tests like FIB4 and APRI has been similar in HCV-infected persons with HIV as in those without HIV. Furthermore, the FIB-4 score appears to be a useful predictor of liver-related morbidity and mortality.10Antiretroviral agents including indinivir and atazanavir inhibit UTP-glucuronyl transferase activity and increase total and indirect bilirubin levels.11,12 Calculations of MELD and other prognostic tests can be altered by these measurements even if liver disease status is unaffected.13
Etiology of Liver Disease in HIV Infection
Liver disease in the HIV-infected patient is due to a complex mix of both unique and common etiologies. (Figure 2). Data from the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D.) clearly demonstrate the central role of chronic infection with hepatitis B and hepatitis C. Liver related death occurred 3.73 times as frequently among those with hepatitis B vs. without hepatitis B and 6.66 times more frequently in those with HCV than those who were not HCV infected. However, relative death rates were also highly associated with lower CD4 counts as well, demonstrating the linkage between extrinsic etiologies (e.g. viral) and host compromise.14 Viral infections like HEV that primarily cause acute and often subclinical infection among immunocompetent patients can become persistent in those with HIV infection and lead to progressive liver disease.15 Hepatitis D is increasingly recognized in HIV populations in Europe, but may also be more prevalent in the U.S. than previously suspected.16 Indeed, data discussed at this meeting suggests that 2% of HBV/HIV coinfected patients also have circulating HDV RNA detectable in serum.17 The clinical implications of this are not fully understood at this point.
Figure 2.
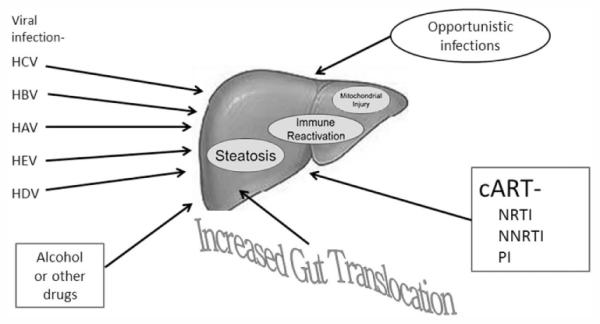
Etiology of liver disease in the HIV-infected patient
Hepatotoxicity related to cART and other agents appears to also be an important contributor to the overall risk hepatic fibrosis and disease progression. Some agents, including zidovudine, didanosine and stavudine are decreasing in use, but many patients remain on these drugs as part of their antiretroviral regimen. These drugs are more commonly used in resource-poor regions of the world due to low cost and high disease burden.18 Patients with HIV are often exposed to a wide variety of agents including sulfa drugs (e.g. trimethoprim-sulfamethoxazole), antifungals (e.g. fluconazole), antimycobacterials agents, statins, and cardiovascular medications that are associated with the potential for liver injury. Complex drug-drug interactions mediated by antiretroviral agents may increase risk of hepatotoxicity by modulation of pathways associated with drug metabolism. Some antiretroviral agents carry direct risk of drug-associated injury due to eosinophil-mediated hypersensitivity in certain individuals with specific HLA phenotype (e.g.abacavir, nevirapine).19,20
Fatty liver is also common in the setting of HIV-infection. Several etiologic factors come together in HIV that increases risk of NAFLD/NASH/ASH. Antiretroviral agents can cause lipodystrophy, insulin-resistance and increased oxidative stress.21 Alcohol use is highly prevalent in the setting of HIV, and high rates of HCVinfection can also influence fat metabolism. Male gender predominance may also be a factor when comparing rates to the general population. Overall prevalence rates of fatty liver have ranged from 15-53%.22-24
ESLD and HCC
Longitudinal studies which have evaluated long term outcomes continue to demonstrate increased risk of progressive disease in those with HIV infection. Lo Re and colleagues evaluated long-term outcomes in 4280 HCV/HIV coinfected patients vs. 6079 HCV-monoinfected controls, and have demonstrated that effective HIV control modulates but does not eliminate risk of disease progression in those with coinfection. Rates of hepatic decompensation remain higher among HIV-infected patients than those not HIV-infected.25 HCC rates in those with HIV appear to be increased and the risk is inversely related to the CD4 count.26,27
The management of patients with advanced liver disease and HIV is complex and often fragmented. Linkage between hepatology and HIV providers is not strong in many venues, and problems arise from the lack of recognition or access to staging methods for advanced liver fibrosis by the care provider.28,29 Hepatologists tend to be found in larger cities and methods to assess presence of advanced liver disease are not easily accessible to many HIV caregivers. Community gastroenterologists rarely consider HIV surveillance in those with colitis/inflammatory bowel disease even when recommended.30 Surveys to determine the comfort and interest of the community gastroenterologist in the management of those with HIV has not been reported. Research is needed in newer care models that can provide such linkage, including expansion of networks such as Project ECHO that have been highly effective in managing HCV in rural communities.31
Transplant of HIV infected patients remains a vexing problem. Data from the NIH HIV Solid Organ Transplant study clearly demonstrated the efficacy of transplantation in those with HBV/HIV coinfection but reported lower graft and patient survival rates in those with HCV/HIV coinfection.32,33 Selection of patients and donor organs may mitigate this issue, but needs to be studied prospectively. These data have led to a decrease in the number of centers performing liver transplantation in HIV-infected patients due to fear that poor outcomes could affect the perceived quality of the transplant program. This makes it more difficult to appropriately refer and manage patients with ESLD in the setting of HIV infection. Hopefully, application of all oral DAA regimens will restore patient/graft survival in HCV/HIV coinfected patients but this remains to be determined. Excellent treatment outcomes have been observed in the post-transplantation setting among HCV-monoinfected patients with recurrent HCV disease, including those with rapidly progressive fibrosing cholestatic hepatitis.34
Pathogenesis/Immunobiology of Liver Disease in HIV-infected patients
Direct hepatic effects of HIV and microbial translocation
Chemokine (C-C motif) receptor 5 (CCR5) and cysteine-X-cysteine receptor 4 (CXCR4), the two major co-receptors required for HIV entry into cells, are expressed on activated hepatic stellate cells (HSCs), the principle fibrogenic cell type in the liver. Through in vitro experiments, it has been shown that the laboratory-adapted viruses HIV-IIIB (CXCR4-tropic or X4) and HIV-BaL (CCR5-tropic or R5) and primary HIV isolates can infect both a human stellate cell line, LX-2 and primary human HSCs.35 Interestingly, HIV infection promotes HSC collagen I expression and secretion of the proinflammatory cytokine monocyte chemoattractant protein-1. These findings underscore the direct HIV effects on liver cells and their implications for fibrogenesis in the setting of uncontrolled HIV replication. More recently, first data has been presented from studies examining the impact of HIV infection on Kupffer cell biology.36 HIV causes a productive non-cytopathic infection of Kupffer cells which can demonstrate an exaggerated pro-inflammatory and pro-fibrogenic response to LPS. Even in the setting of undetectable plasma HIV, Kupffer cells from HIV-infected patients were shown to contain HIV transcripts and pro-viral DNA and to continue to hyper-respond to LPS.
Direct hepatic effects of HIV and microbial translocation in HCV coinfection
Patients coinfected with dual HIV and HCV infection develop more rapid fibrosis than those infected with HCV only. In HIV/HCV-coinfected patients, fibrosis progression correlates with HIV RNA levels, suggesting a direct role of HIV in liver fibrogenesis. Moreover, increased microbial translocation has been postulated as one mechanism for accelerated development of cirrhosis in HIV-coinfection.37 At the HIV and Liver Disease meeting additional data was presented exploring markers of enterocyte health and immune activation in HIV, HCV and HIV/HCV coinfected cirrhotics. Intestinal Fatty-Acid Binding Protein (I-FABP) levels, but not sCD14 levels were higher in HIV/HCV co-infection compared to HCV mono-infection whereas there was no difference noted in I-FABP or sCD14 levels between HIV/HCV co-infection and HIV mono-infection These findings support the hypothesis that intestinal epithelial integrity and microbial translocation may play a role in the accelerated development of cirrhosis even when HIV viral load is fully suppressed.
Immune dysfunction
In HIV the immune response to HCV is dysfunctional, presumably related to the depletion of CD4+ T cells.38 In acute HCV infection in patients already infected with HIV, there is impairment in HCV-specific IFN-γ responses compared to acute infection in HIV-negative patients.39 More recently, data has been presented which shows that a strong IFN-γ-mediated antiviral NK cell response was associated with a self-limited course of acute hepatitis C infection in HIV-coinfected patients.40 In chronic HCV infection, co-infected patients also depict CD4-level dependent reduced HCV-specific lymphoproliferative responses.41 Natural killer (NK) cells are stimulated by CD4+ T-cells in an IL-2 dependent fashion and can modulate liver fibrosis by killing activated hepatic stellate cells.42 Recent experiments also demonstrated that following incubation with CD4(+) T cell supernatants, NK cells displayed a significantly increased activity against primary hepatic stellate cells as compared to unstimulated NK cells.43 HCV/HIV co-infection was associated with an impaired IL-2 secretion of CD4(+) T cells resulting in an ineffective stimulation of anti-fibrotic NK cell function. These findings suggest that both quantitative and qualitative immune dysfunction contributes to accelerated fibrosis in HIV/HCV co-infection.
Host genetics
There is a host genetic basis to many HCV outcomes including spontaneous clearance of HCV which is diminished in HIV infected patients, response to interferon based HCV therapy and development of cirrhosis and hepatocellular cancer.44-47 Although, important prognostic genes have been discovered and confirmed, the biologic mechanism underlying the gene associations remains unclear. This underlines the need for future studies including full genome and epigenetic screening linked to mechanistically meaningful study of mutational variability.
HIV Treatment in Patients with Liver Disease
There is increasing evidence that cART induced immune reconstitution might attenuate the unfavorable accelerated course of hepatic fibrosis for hepatitis C in patients with severe HIV-associated immune deficiency and reduce the rate of hepatic decompensation.48,49 The risk of liver disease progression is highest in those with CD4 counts less than 200 cells/ul, suggesting that increases in CD4+ lymphocyte counts while on cART may reduce the risk of severe liver disease.50 Indeed numerous cohort analyses by now have demonstrated that HIV/HCV-coinfected individuals on cART have significantly lower liver-related mortality than patients receiving either suboptimal (one or two nucleoside reverse transcriptase inhibitors) or no antiretroviral therapy.51-53 These findings have been the basis upon which current HIV guidelines have strongly recommended early HIV treatment initiation in HIV patients with viral hepatitis coinfection. Interestingly, more recently a cohort study has compared rates of hepatic decompensation in HCV mono- vs HIV/HCV co-infected patients receiving antiretroviral therapy and still observed a higher rate of events in the coinfected patients. This effect was particularly enhanced in those patients with a CD4 count below 200/μl or who had detectable HIV RNA> 1000 copies/ml while on therapy, again underlining the importance of complete suppression of viral replication in these patients.25 In addition, a study has recently addressed the modulation of HCV replication after starting suppressive HIV therapy .54 Of note, starting cART resulted in increased HCV replication and increased alanine aminotransferase (ALT) in a subset of patients. Subjects with evidence of hepatic injury (increased ALT) were more likely to have HCV-specific immune responses directed against HCV epitopes. Over time however, HCV viral loads declined. Reproducible and biologically important gene expression changes occurred in co-infected patients who underwent successful cART. Indeed, early events involving down-regulation of interferon-stimulated genes may have led to the transiently increased viral replication and hepatic injury noticed in the study.
Short-term and long-term virologic success rates of cART in HIV/HCV coinfection are limited by an increased risk of hepatotoxicity. With regard to drug choice some of the more historically used drugs such as zidovudine, stavudine and didanosine have been associated with increased mitochondrial damage and increased risk for causing lactic acidosis and fatty liver disease.55 Also some boosted protease inhibitors have been hypothesized to potentially induce insulin resistance and/or dyslipidemia and to contribute to development of non-alcoholic fatty liver disease.56 Also idiosyncratic liver failure has been described after nevirapine treatment and also inhibition of liver metabolism enzymes by ritonavir and cobicistat need to be taken into consideration (see below paragraph on drug-drug interactions). As a consequence patients with dyslipidemia and/or insulin resistance should be started on, or switched to cART regimens, which are considered to be metabolically neutral (for example, rilpivirine or an integrase inhibitor as third agent within cART) and drugs with a well-known increased risk for hepatoxicity should be avoided.56
Treatment of hepatitis C in the HIV-infected patient
How to treat
The question of how to treat HCV or HIV in a person with both infections has increasingly become a consideration of interactions between some direct acting HCV agents and some HIV antiretroviral therapies (see below). Whereas sustained virologic response (SVR) rates in HIV/HCV coinfected persons taking peginterferon and ribavirin were lower than in HCV monoinfected persons, recent reports suggest similar SVR rates are achieved when using direct acting agents.57-61 Thus, current guidelines recommend essentially the same treatments in HIV infected patients as in those without HIV, after accounting for drug interactions (www.HCVGuidelines.org).62 One exception is that a person infected with HIV may require a longer duration of HCV treatment than the same person without HIV infection. This point has not been proven and the abundance of data suggest that baseline viral load is more important than HIV status. Nonetheless, until there are data from HIV/HCV coinfected persons, many experts do not recommend treatment abbreviation strategies.
Genotype 1
Recommended treatments for HIV/HCV coinfected persons are the same as for persons without HIV after accounting for drug interactions (www.hcvguidelines.org).62 The data for HIV/HCV coinfected patients come from several recently completed trials (Table 1). In one study (ION-4), 335 HCV treatment-naïve and -experienced HIV co-infected patients were enrolled and received the combined formulation of 90 mg of ledipasvir and 400 mg of sofosbuvir (LDV/SOF) once daily for 12 weeks.58 Patients were on tenofovir and emtricitabine with raltegravir (44%), efavirenz (48%), or rilpivirine (9%). Patients were all genotype 1a (75%), 1b (23%), or 4 (2%), 20% had cirrhosis, 34% were black, and 55% had not responded to prior HCV treatment. Overall, the SVR12 rate was 96% (320/335).; SVR12 was 94% (63/67) among cirrhotic and 97% (179/185) among treatment-experienced patients. Interestingly, all of the relapses occurred among African American/Black subjects which were highly correlated with non-CC IL28B genotype. No patients discontinued study drug due to an adverse event. Drug interaction studies (summarized below) suggested that some patients would have elevated tenofovir levels and all patients had glomerular filtration rates above 60 at study entry. There were 4 patients in whom serum creatinine rose ≥4mg/dl: two remained on tenofovir, one had tenofovir dose reduction, and the other stopped tenofovir.
Table 1.
Treatment of HCV in HIV-coinfected persons*
| Genotype | Patients | Study | Medications | Duration (weeks) |
N | SVR12 (%) | Ref |
|---|---|---|---|---|---|---|---|
| 1 or 41 | TN | ION4 | LDV/SOF | 12 | 335 | 96 | 56 |
| 1 | TN | NIH | LDV/SOF | 12 | 50 | 98 | 55 |
| 1 | TN + TE | TURQUOISE-1 | OPrD RBV | 12 | 31 | 94 | 57 |
| 1 | TN + TE | TURQUOISE-1 | OPrD RBV | 24 | 32 | 91 | 57 |
| 1 | TN | ALLY-2 | DCV/SOF | 12 | 83 | 96 | 59 |
| 1 | TN | ALLY-2 | DCV/SOF | 8 | 41 | 76 | 59 |
| 1 | TE | ALLY-2 | DCV/SOF | 12 | 44 | 98 | 59 |
| 2 | TN | PHOTON 1+2 | SOF + RBV | 12 | 45 | 89 | 58, 61 |
| 2 | TE | PHOTON 1+2 | SOF + RBV | 24 | 30 | 90 | 58, 61 |
| 3 | TN | PHOTON 1+2 | SOF + RBV | 12 | 42 | 67 | 58, 61 |
| 3 | TN | PHOTON 1+2 | SOF + RBV | 24 | 57 | 91 | 58, 61 |
| 3 | TE | PHOTON 1+2 | SOF + RBV | 24 | 66 | 88 | 58, 61 |
| 3 | TN + TE | ALLY-2 | DCV/SOF | 12 | 10 | 100 | 59 |
| 4 | TN | PHOTON 1+2 | SOF + RBV | 24 | 31 | 84 | 58, 61 |
TN = treatment naïve; TE = treatment experienced; LDV = ledipasvir 90mg; SOF= sofosbuvir 400 mg; OPrD = ombitasvir, paritaprevir, ritonavir, and dasabuvir; RBV=weight-based ribavirin; DCV = daclatasvir
8 persons had genotype 4 and all had SVR12
The data for the paritaprevir/ritonavir/ombitasvir, dasabuvir, and ribavirin regimen come from a multicenter study of 63 patients with HCV genotype 1 and HIV-1 co-infection who were HCV treatment-naive or had history of prior treatment failure with peginterferon plus ribavirin therapy.59 Among patients receiving 12 or 24 weeks of paritaprevir/ritonavir/ombitasvir, dasabuvir and ribavirin, SVR12 was achieved by 29 of 31 (94%) and 29 of 32 patients (91%), respectively. No adverse events were reported as serious or leading to discontinuation.
Non-1 genotypes
Regimens recommended for HIV/HCV coinfected persons with non-1 genotypes are also the same as with persons with HCV monoinfection. However, there are far fewer data in most instances. All-oral therapy with sofosbuvir (SOF) plus ribavirin for the treatment of HCV genotypes 2, 3 and 4 infection was reported in two studies in patients coinfected with HIV (PHOTON-1 and 2).60,63 With genotype 2, there were a combined 45 HIV/HCV coinfected treatment-naive patients who received 12 weeks of SOF and ribavirin, and 40 (89%) had SVR12. With genotype 3, there were a combined 42 treatment-naive patients who received 12 weeks and 28 (67%) had SVR12 while 57 received 24 weeks and 52 (91%) had SVR12. SVR12 rates were lower for patients with genotype 3 who were treatment-experienced or had cirrhosis. Recently, data were also presented on the use of daclatasvir (DCV) and SOF for HIV/HCV coinfected patients (ALLY-2).61 Adjustments in DCV doses were made based on predicted interactions with antiretroviral drugs, but most regimens were allowed. There were 10 genotype 3 infected patients treated for 12 weeks with DCV and SOF, all of whom achieved SVR 12. There were 8 genotype 4 infected patients treated with LDV/SOF for 12 weeks in ION 4 summarized above for genotype 1 and all had SVR12.60
Management of hepatitis B in the HIV-infected patient
Epidemiology, natural history, and whom to treat
Approximately 8% of HIV infected persons also has chronic hepatitis B virus (HBV) infection. As with HCV, HIV accelerates liver disease progression in HBV-infected persons. It is also possible that chronic HBV infection adversely affects HIV. HBV and HIV also have similar enough polymerase structure that some nucleoside(-tide) agents are active against both, and if used without a fully-suppressive regimen, could select for resistance. Thus, it is recommended that persons with HIV or HBV infection be tested for the other. ART is routinely recommended for HIV infected persons, while HBV treatment is typically targeted to those with ongoing necroinflammation and high HBV DNA levels. However, given the overlapping activity of treatments and potential that each infection could exacerbate or modulate the other, treatment of both HIV and HBV is recommended for persons with both infections.
How to treat
Tenofovir and emtricitabine have activity against both HIV and HBV and are first line agents for each. Thus, that combination (along with other ART) is usually recommended for HIV/HBV coinfected persons.64 There are studies that show the combination effectively suppresses both infections and improves liver fibrosis progression and probably formation of liver cancer.65 Treatment of HIV/HBV coinfected patients is more difficult when there is renal insufficiency, since current ART guidelines recommend stopping tenofovir when creatinine clearance drops below 50 mL/min/1.73m2. In that instance and when the HBV is not highly resistant to lamivudine, entecavir can be added to a fully-suppressive ART regimen to provide HBV suppression that does not have renal toxicity. Entecavir dose adjustment may be necessary depending on renal status. If there has been lamivudine exposure in the past or there is documented resistance the higher 1mg dose of entecavir has to be used (some recommend it always be used unless one can confidently eliminate the possibility of resistant HBV). In addition, if the HBV is highly resistant (2 or more lamivudine resistance mutations), then it may be necessary to use renal-dose adjusted tenofovir instead of entecavir, despite the potential for tenofovir to add renal toxicity. Since entecavir has some activity against HIV, it has to be used with a fully-suppressive HIV regimen. It appears that tenofovir alefenamide (TAF) offers noninferior antiretroviral activity with less renal (and bone) toxicity.66 If the HBV activity is also confirmed clinically, TAF may provide a solution to the treatment of HIV/HBV coinfected persons with renal insufficiency and resistant HBV.
In comparison to HIV and HCV, there have been few recent new drug approvals for HBV. Thus, additional research to develop new HBV drugs is needed. There is considerable interest in the role of anti-PD1 and anti-PDL1 antibodies to reverse immune exhaustion that occurs in both hepatitis B and in HIV which may increase rates of clearance leading to functional or complete cure. This principle has been demonstrated in vitro but in vivo studies are lacking.67 However, these agents have been shown to cause significant immune mediated liver injury in studies of cancer patients and initial exploration in the setting of HIV has been cautious. Other strategies to achieve functional cure are also under evaluation and may play a future role in those with HBV/HIV coinfection.68
HIV/HBV coinfected patients are monitored in much the same way as those with just HBV infection. HBV replication is assessed by following plasma HBV DNA levels, and liver disease stage is evaluated by a combination of clinical and laboratory findings including serum aminotransferase levels. Liver biopsy may be used to stage disease, and there is accumulating evidence that noninvasive blood testing and elastography can also stage HBV related liver fibrosis.69,70
Management of Drug-drug Interactions
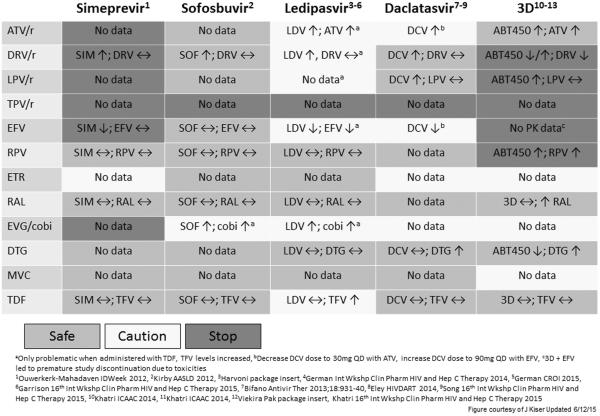
Drug-drug interactions are common when treating HCV in HIV dually infected subjects especially in DAA combinations including HCV protease inhibitors, which are metabolized through the cytochrome 450 pathways just like HIV nonnucleoside reverse-transcriptase inhibitors (NNRTIs) or protease inhibitors .71,72 Therefore, combinations with HIV drugs must be checked on drug interaction websites such as www.hep-druginteractions.org or http://www.hcvdruginfo.ca.
Potential drug–drug interactions with the nucleotide HCV polymerase inhibitor sofosbuvir, which is not metabolized by the cytochrome p450 pathway, are low. The only antiretroviral drug which should not be co-administered because of significant interactions is tipranavir.
Significant interactions exist with simeprevir and paritaprevir/r (component of the ombitasvir/paritaprevir/ritonavir and dasabuvir drug regimen), which both use metabolization pathways that are similar to those in HIV PIs and NNRTIs. Indeed no HIV PI is combinable with simeprevir. Efavirenz and cobicistat boosted ART regimens are also not recommended making raltegravir, dolutegravir, and rilpivirine the preferred 3rd agent which can be coadministered with a simeprevir containing HCV therapy. Lopinavir/r and tipranavir/r are also contraindicated with the paritaprevir/r/ombitasvir + dasabuvir (3-D) regimen. Atazanavir and darunavir are both combinable with this regimen according to the European drug label although Ctrough levels for darunavir decrease by 50% but Cmax and AUC remain unchanged (Viekirax EU prescribing information January 2015). The FDA does not recommend coadministration of darunavir and the paritaprevir/r/ombitasvir + dasabuvir (3-D) regimen . When using atazanavir or darunavir with the paritaprevir/r/ombitasvir + dasabuvir (3-D) regimen , it is recommended to give the respective HIV PI without supplemental ritonavir simultaneously with the paritaprevir/r/ombitasvir + dasabuvir 3-D regimen to allow for appropriate PK boosting of the HIV PI in the morning. Due to induction effects and decreases in HCV DAA levels efavirenz is contraindicated in the combination with ombitasvir/paritaprevir/ritonavir.
Daclatasvir is an NS5A inhibitor which is combinable with HIV PIs but in some cases needs dose adaptation (i.e. 30mg in combination with boosted atazanavir). Due to induction effects and decreases in HCV DAA levels when coadministering efavirenz with daclatasvir, the daclatasvir dose needs to be increased to 90mg qd.
Finally, pgp or transporter interactions may also become relevant. For example co-administration of ledipasvir/sofosbuvir together with tenofovir has been demonstrated to increase tenofovir levels. This is even more enhanced in patients on a boosted HIV PI and tenofovir which also already increases tenofovir exposure. Therefore the label of ledipasvir/sofosbuvir suggests considering alternative HCV or antiretroviral therapy in patients on a boosted PI and tenofovir to avoid increases in tenofovir exposures which may be nephrotoxic. If no alternative is possible more careful renal monitoring is warranted (Harvoni US Prescribing Information, Gilead Sciences, October 2014).
Biopsychosocial Issues Related to Liver Disease
As our understanding of pathophysiology improves and new more potent antiviral agents become available, concerns regarding individual and societal biopsychosocial issues are highlighted.
Issues in HIV Treatment
Depression, bipolar disease and substance abuse are all more common in those with HIV than the general population. These issues conspire synergistically to limit availability of care and to fully utilize the potential for disease cure and mitigation. HIV-infected patients with severe psychiatric disorders are less likely to access care for HIV and less likely to be treated for HCV and HBV.73 Limitation in the ability to manage these issues leads to poor control of HIV or even complete lack of antiretroviral therapies. As noted above, only a small fraction of persons living with HIV infection have achieved effective viral suppression despite the availability of highly effective regimens since 1996. Much of this failure can be laid at the feet of societal pressure to not focus resources on psychiatric care models, even when otherwise costly medications are provided through Ryan White and other governmental programs. Care teams that include psychological, psychiatric and social expertise appear to overcome many of these barriers, thus increasing adherence with HIV regimens.74
Issues in Treatment of HCV and other Liver Disease
Effective HIV treatment opens the door to dealing with other comorbidities including HCV, HCV, and NAFLD/NASH particularly in persons who inject drugs. Methodologies that have proven effective include directly observed therapy (DOT) for HCV and “Group Treatment”.75,76 The latter modality involves creating a collective with a group orientation with individual HCV workup. Then six to twelve patients form a cohort and initiate treatment together, allowing multiple cycles each year. Benefits accrue for both patients and providers. For patients, there is built-in social support and misconceptions related to care are quickly addressed. Reassurance within the group decreases fear of side effects. For providers, co-management enhances expertise and confidence and allows frequent contact between providers in multidisciplinary setting. The use of these methods and multidisciplinary teams appears to improve treatment completion and efficacy.77 Substance abuse treatment can serve as a bridge to HCV treatment if coordinated care models are implemented and made available. Sylvestre et al. described use of buprenorphine as the first step in bring HCV-monoinfected patients to HCV therapy.78 However, a retrospective chart review of HCV/HIV infected patients whose opioid dependence was bridged with buprenorphine demonstrated limited success in bringing patients to HCV therapy.79 It should be noted that this analysis preceded availability of all-oral regimens and further studies in this area are needed.
Prevention of new HCV, HBV and HIV infection through the development and implementation of needle exchange programs remains a hot topic of discussion and research. Mathematical models highlight the potential value of needle exchange programs in stable injection-drug using populations.80 High levels of community engagement lead to decreases in new cases and in test and treat scenarios combined with needle exchange, may extinguish infections like HCV. However, needle exchange requires persistent involvement of community organizers, health-care providers and the support of government officials. Further research in outcomes is needed to convince many community leaders that provision of clean needles does not encourage increased risk behavior in their communities. It is not clear if implementation strategies can be standardized, because local/regional differences in the nature of the epidemic and disease transmission may affect the structure of the intervention.
Future Research Needs
The last five years have seen a revolution in our understanding of liver disease pathogenesis and treatment in those with HIV infection. During this timeframe, we have witnessed the emergence of drugs that treat HCV in the setting of HIV with equivalent efficacy in terms of SVR. We are beginning to understand how HIV and HIV soluble proteins may modulate hepatic fibrosis, and there is a better understanding of how HIV suppression modulates HCV replication. However, significant questions remain unanswered—despite effective HIV suppression, hepatic decompensation appears to continue at a higher rate than non-HIV infected individuals. The mediators of this are partially characterized and include ongoing immune activation, altered responses to fatty infiltration and oxidative stress, direct effects of antiretroviral regimens and failure to completely restore an intact immune response. Continued research in all these areas remains key to our understanding and eventual control of liver disease. Though it appears that HCV can be readily treated with new all-oral DAA regimens, drug-drug interactions have not been fully explored. Differences in pK findings in healthy volunteer studies and those with HIV infection on cART have been described, and these are drug rather than class specific. Therefore, further research in those with HIV appears necessary. Hepatitis B remains an active problem with poor response to current vaccines in those with HIV. Treatment with the goal of HBV suppression appears feasible but functional cure is rare. Other viral infections such as HEV and HDV appear to pose unique problems in those with HIV. Health resource utilization research remains a priority. After 20 years of cART, not all patients with HIV in the U.S. are identified, let alone treated effectively. Though it may be possible to cure HCV in all HIV-infected patients, the systems to achieve this goal are sorely lacking and research into new models and best-practices remains needed. The NIH remains keenly interested in pursuing a variety of research agendas in this area and continues to issue programmatic requests for funding opportunities in several institutes.
Figure 3. Treatment recommendations related to HCV direct acting antivirals and HIV antiretroviral agent drug interactions.
Abbreviations: SIM, simeprevir; SOF, sofosbuvir; LDV, ledipasvir; DCV, daclatasvir; 3D, ombitasvir+paritaprevir+ dasabuvir +ritonavir; ABT450, paritaprevir; /r, ritonavir-boosted; ATV, atazanavir; DRV, darunavir; LPV, lopinavir; TPV, tipranavir; EFV, efavirenz; RPV, rilpivirine; ETR, etravirine; RAL, raltegravir ; EVG(ELV)/cobi, elvitegravir/cobicistat ; DTG, dolutegravir; MVC, maraviroc ; TDF, tenofovir disoproxil fumarate; TFV, tenofovir.
Acknowledgments
Funding for this conference was made possible [in part] by the National Institutes of Health under Award Number R13AI071925 from the National Institute of Allergy and Infectious Diseases (NIAID), with co-funding from the National Institute on Drug Abuse (NIDA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
Supported by educational grants from: Gilead Sciences, Inc, Janssen Therapeutics, Division of Janssen Products,LP, Kadmon Pharmaceuticals, LLC, MedImmune, LLC, Merck & Co.,Inc.
Sponsorship:AbbVie
Footnotes
Meeting Participants (Speakers whose lectures contributed to the content of this meeting summary.) Meena Bansal, MD, Mt. Sinai School of Medicine; John T. Brooks, MD, Centers for Disease Control and Prevention; Raymond T. Chung, MD, Harvard Medical School, Massachusetts General Hospital; Douglas Dieterich, MD, Mount Sinai School of Medicine; Judith Feinberg, MD, University of Cincinnati; Zachary Goodman, MD, PhD, Inova Pathology Institute; Jag Khalsa, PhD, NIH, NIDA; Arthur Kim, MD, Harvard Medical School, Massachusetts General Hospital; Jennifer Kiser, Pharm D, University of Colorado, Denver; Shyam Kottilil MD, PhD, Johns Hopkins University; Alain Litwin, MD, MPH, Montefiore Medical Center; Marion Peters, MD, University of California San Francisco; Jürgen Rockstroh, MD, University of Bonn, Germany;Michael Saag, MD, University of Alabama at Birmingham; Kenneth E. Sherman, MD, PhD, University of Cincinnati College of Medicine; Sreetha Sidharthan, Critical Care Medicine Department, National Institutes of Health Clinical Center, National Institutes of Health, Bethesda, Maryland., ; Richard Sterling, MD, Virginia Commonwealth University; Peter Stock, MD, University of California, San Francisco; Chloe Thio, MD, Johns Hopkins School of Medicine; David Thomas, MD, MPH, Johns Hopkins University School of Medicine; Kerry Townsend, Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, ;Glenn J. Treisman, MD, PhD, Johns Hopkins University School of Medicine; Alysse Wurcel, Tufts Medical Center, Boston, Massachusetts.
References
- 1.Lohse N, Hansen AB, Pedersen G, et al. [Survival in patients with HIV infection] Ugeskr Laeger. 2007;169:2529–32. [PubMed] [Google Scholar]
- 2.May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28:1193–202. doi: 10.1097/QAD.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen SM, Van Handel MM, Branson BM, et al. Vital Signs: HIV Prevention Through Care and Treatment-United States (Reprinted from MMWR, vol 60, pg 1618-1623, 2011) Jama-J Am Med Assoc. 2012;307:247–50. [Google Scholar]
- 4.Hall HI, Walker F, Shah D, Belle E. Trends in HIV diagnoses and testing among U.S. adolescents and young adults. AIDS Behav. 2012;16:36–43. doi: 10.1007/s10461-011-9944-8. [DOI] [PubMed] [Google Scholar]
- 5.Brecher ME, Hay SN, Park YA. Is it HIV TTP or HIV-associated thrombotic microangiopathy? Journal of clinical apheresis. 2008;23:186–90. doi: 10.1002/jca.20176. [DOI] [PubMed] [Google Scholar]
- 6.Goedert JJ, Second Multicenter Hemophilia Cohort S Prevalence of conditions associated with human immunodeficiency and hepatitis virus infections among persons with haemophilia, 2001-2003. Haemophilia. 2005;11:516–28. doi: 10.1111/j.1365-2516.2005.01138.x. [DOI] [PubMed] [Google Scholar]
- 7.Schiano TD, Kotler DP, Ferran E, Fiel MI. Hepatoportal sclerosis as a cause of noncirrhotic portal hypertension in patients with HIV. Am J Gastroenterol. 2007;102:2536–40. doi: 10.1111/j.1572-0241.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 8.Maida I, Garcia-Gasco P, Sotgiu G, et al. Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors and outcome. Antivir Ther. 2008;13:103–7. [PubMed] [Google Scholar]
- 9.Shire NJ, Rao MB, Succop P, et al. Improving noninvasive methods of assessing liver fibrosis in patients with hepatitis C virus/human immunodeficiency virus co-infection. Clin Gastroenterol Hepatol. 2009;7:471–80. doi: 10.1016/j.cgh.2008.12.016. 80 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berenguer J, Zamora FX, Aldamiz-Echevarria T, et al. Comparison of the prognostic value of liver biopsy and FIB-4 index in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2015;60:950–8. doi: 10.1093/cid/ciu939. [DOI] [PubMed] [Google Scholar]
- 11.Zucker SD, Qin X, Rouster SD, et al. Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci U S A. 2001;98:12671–6. doi: 10.1073/pnas.231140698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33:1729–39. doi: 10.1124/dmd.105.005447. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez JM, Hermida JM, Casado JL, et al. The use of atazanavir in HIV-infected patients with liver cirrhosis: lack of hepatotoxicity and no significant changes in bilirubin values or model for end-stage liver disease score. AIDS. 2011;25:1006–9. doi: 10.1097/QAD.0b013e3283466f85. [DOI] [PubMed] [Google Scholar]
- 14.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 15.Kamar N, Izopet J, Rostaing L. Hepatitis E virus infection. Curr Opin Gastroenterol. 2013;29:271–8. doi: 10.1097/MOG.0b013e32835ff238. [DOI] [PubMed] [Google Scholar]
- 16.Gish RG, Yi DH, Kane S, et al. Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern California. J Gastroenterol Hepatol. 2013;28:1521–5. doi: 10.1111/jgh.12217. [DOI] [PubMed] [Google Scholar]
- 17.SD Razeghi SR, Sherman KE. Prevalence of HDV in a Midwestern HIV-HBV Coinfected Population; Conference on Retroviruses and Opportunistic Infections (CROI); Seattle: Feb 23-26, 2015. 2015. Abstract #707. [Google Scholar]
- 18.Muhula SO, Peter M, Sibhatu B, Meshack N, Lennie K. Effects of highly active antiretroviral therapy on the survival of HIV-infected adult patients in urban slums of Kenya. The Pan African medical journal. 2015;20:63. doi: 10.11604/pamj.2015.20.63.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocroft A, Rockstroh J, Soriano V, et al. Are specific antiretrovirals associated with an increased risk of discontinuation due to toxicities or patient/physician choice in patients with hepatitis C virus coinfection? Antivir Ther. 2005;10:779–90. [PubMed] [Google Scholar]
- 20.Macias J, Gonzalez J, Tural C, et al. Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV-infected patients. AIDS. 2014;28:1279–87. doi: 10.1097/QAD.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 21.Ryan P, Berenguer J, Michelaud D, et al. Insulin resistance is associated with advanced liver fibrosis and high body mass index in HIV/HCV-coinfected patients. J Acquir Immune Defic Syndr. 2009;50:109–10. doi: 10.1097/QAI.0b013e318186ede8. [DOI] [PubMed] [Google Scholar]
- 22.Crum-Cianflone N, Dilay A, Collins G, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464–73. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macias J, Berenguer J, Japon MA, et al. Hepatic steatosis and steatohepatitis in human immunodeficiency virus/hepatitis C virus-coinfected patients. Hepatology. 2012;56:1261–70. doi: 10.1002/hep.25791. [DOI] [PubMed] [Google Scholar]
- 24.Li Vecchi V, Giannitrapani L, Di Carlo P, et al. Non-invasive assessment of liver steatosis and fibrosis in HIV/HCV- and HCV-infected patients. Ann Hepatol. 2013;12:740–8. [PubMed] [Google Scholar]
- 25.Lo Re V, 3rd, Kallan MJ, Tate JP, et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med. 2014;160:369–79. doi: 10.7326/M13-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer JR, Kowalkowski MA, Duan Z, Chiao EY. The Effect of HIV Viral Control on the Incidence of Hepatocellular Carcinoma in Veterans With Hepatitis C and HIV Coinfection. J Acquir Immune Defic Syndr. 2015;68:456–62. doi: 10.1097/QAI.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47:527–37. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Sebastiani G, Ghali P, Wong P, Klein MB, Deschenes M, Myers RP. Physicians' practices for diagnosing liver fibrosis in chronic liver diseases: a nationwide, Canadian survey. Canadian journal of gastroenterology & hepatology. 2014;28:23–30. doi: 10.1155/2014/675409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borgia SM, Borgia S, Kan V, Marquez Azalgara V, Ford J-A, et al. Patient preference and willingness to pay for transient elastography versus liver biopsy: A perspective from British Columbia. FibroScan(R) access in Canada: Time for reform, a call for universal access. Can J Gastroenterol Hepatol. Canadian journal of gastroenterology & hepatology. 2015;2015;2929:72–6. 221–2. doi: 10.1155/2015/169190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh AW, Burger D, et al. Implementing guidelines on the prevention of opportunistic infections in inflammatory bowel disease. Journal of Crohn's & colitis. 2013;7:e449–56. doi: 10.1016/j.crohns.2013.02.019. M. [DOI] [PubMed] [Google Scholar]
- 31.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terrault NA, Roland ME, Schiano T, et al. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl. 2012;18:716–26. doi: 10.1002/lt.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terrault NA, Carter JT, Carlson L, Roland ME, Stock PG. Outcome of patients with hepatitis B virus and human immunodeficiency virus infections referred for liver transplantation. Liver Transpl. 2006;12:801–7. doi: 10.1002/lt.20776. [DOI] [PubMed] [Google Scholar]
- 34.Leroy V, Dumortier J, Coilly A, et al. Efficacy of Sofosbuvir and Daclatasvir in Patients with Fibrosing Cholestatic Hepatitis C After Liver Transplantation. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Tuyama AC, Hong F, Saiman Y, et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology. 2010;52:612–22. doi: 10.1002/hep.23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosoian AH, Saiman Y, et al. HIV infection of Kupffer cells results in dysregulated response to LPS despite effective antiretroviral therapy. HEPATOLOGY. 2013:281A. F. Abstract 145. [Google Scholar]
- 37.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–33. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JY, Feeney ER, Chung RT. HCV and HIV co-infection: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2014;11:362–71. doi: 10.1038/nrgastro.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flynn JK, Dore GJ, Matthews G, et al. Impaired hepatitis C virus (HCV)-specific interferon-gamma responses in individuals with HIV who acquire HCV infection: correlation with CD4(+) T-cell counts. J Infect Dis. 2012;206:1568–76. doi: 10.1093/infdis/jis544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kokordelis P, Kramer B, Korner C, et al. An effective interferon-gamma-mediated inhibition of hepatitis C virus replication by natural killer cells is associated with spontaneous clearance of acute hepatitis C in human immunodeficiency virus-positive patients. Hepatology. 2014;59:814–27. doi: 10.1002/hep.26782. [DOI] [PubMed] [Google Scholar]
- 41.Kim AY, Schulze zur Wiesch J, Kuntzen T, et al. Impaired hepatitis C virus-specific T cell responses and recurrent hepatitis C virus in HIV coinfection. PLoS Med. 2006;3:e492. doi: 10.1371/journal.pmed.0030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morishima C, Paschal DM, Wang CC, et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–80. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 43.Glassner A, Eisenhardt M, Kokordelis P, et al. Impaired CD4(+) T cell stimulation of NK cell anti-fibrotic activity may contribute to accelerated liver fibrosis progression in HIV/HCV patients. J Hepatol. 2013;59:427–33. doi: 10.1016/j.jhep.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 44.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 45.Duggal P, Thio CL, Wojcik GL, et al. Genome-wide association study of spontaneous resolution of hepatitis C virus infection: data from multiple cohorts. Ann Intern Med. 2013;158:235–45. doi: 10.7326/0003-4819-158-4-201302190-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aka PV, Kuniholm MH, Pfeiffer RM, et al. Association of the IFNL4-DeltaG Allele With Impaired Spontaneous Clearance of Hepatitis C Virus. J Infect Dis. 2014;209:350–4. doi: 10.1093/infdis/jit433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trepo E, Nahon P, Bontempi G, et al. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: Evidence from a meta-analysis of individual participant data. Hepatology. 2014;59:2170–7. doi: 10.1002/hep.26767. [DOI] [PubMed] [Google Scholar]
- 48.Verma S. HAART attenuates liver fibrosis in patients with HIV/HCV co-infection: fact or fiction? J Antimicrob Chemother. 2006;58:496–501. doi: 10.1093/jac/dkl280. [DOI] [PubMed] [Google Scholar]
- 49.Anderson JP, Tchetgen Tchetgen EJ, Lo Re V, 3rd, et al. Antiretroviral therapy reduces the rate of hepatic decompensation among HIV- and hepatitis C virus-coinfected veterans. Clin Infect Dis. 2014;58:719–27. doi: 10.1093/cid/cit779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rockstroh JK, Spengler U, Sudhop T, et al. Immunosuppression may lead to progression of hepatitis C virus-associated liver disease in hemophiliacs coinfected with HIV. Am J Gastroenterol. 1996;91:2563–8. [PubMed] [Google Scholar]
- 51.Qurishi N, Kreuzberg C, Luchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–13. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 52.Brau N, Salvatore M, Rios-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Loko MA, Bani-Sadr F, Valantin MA, et al. Antiretroviral therapy and sustained virological response to HCV therapy are associated with slower liver fibrosis progression in HIV-HCV-coinfected patients: study from the ANRS CO 13 HEPAVIH cohort. Antivir Ther. 2012;17:1335–43. doi: 10.3851/IMP2419. [DOI] [PubMed] [Google Scholar]
- 54.Sherman KE, Guedj J, Shata MT, et al. Modulation of HCV replication after combination antiretroviral therapy in HCV/HIV co-infected patients. Sci Transl Med. 2014;6:246ra98. doi: 10.1126/scitranslmed.3008195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–7. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 56.Rockstroh JK, Mohr R, Behrens G, Spengler U. Liver fibrosis in HIV: which role does HIV itself, long-term drug toxicities and metabolic changes play? Curr Opin HIV AIDS. 2014;9:365–70. doi: 10.1097/COH.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 57.Osinusi A, Townsend K, Kohli A, et al. Virologic Response Following Combined Ledipasvir and Sofosbuvir Administration in Patients With HCV Genotype 1 and HIV Co-infection. JAMA. 2015 doi: 10.1001/jama.2015.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naggie SC, Saag MS, Yang J.c., et al. CROI 2015. Seattle, WA: 2015. Ledipasvir/Sofosbuvir for 12 Weeks in Patients Coinfected With HCV and HIV-1; p. 152LB. C. [Google Scholar]
- 59.Sulkowski MS, Eron JJ, Wyles D, et al. Ombitasvir, Paritaprevir Co-dosed With Ritonavir, Dasabuvir, and Ribavirin for Hepatitis C in Patients Co-infected With HIV-1: A Randomized Trial. JAMA. 2015 doi: 10.1001/jama.2015.1328. [DOI] [PubMed] [Google Scholar]
- 60.Molina JM, Orkin C, Iser DM, et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet. 2015 doi: 10.1016/S0140-6736(14)62483-1. [DOI] [PubMed] [Google Scholar]
- 61.Wyles DR, Sulkowski M, Dieterich D, Luetkemeyer A, Morgan T, Sherman KE, Liu Z, Noviello S, Ackerman P. CROI 2015. Seattle, WA: 2015. Daclatasvir in Combination With Sofosbuvir for HIV/HCV Coinfection: ALLY-2 Study; p. 151LB. P. [Google Scholar]
- 62.Panel AIHG Hepatitis C Guidance: AASLD-IDSA Recommendations for Testing, Managing, and Treating Adults Infected with Hepatitis C Virus. Hepatology. 2015 doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 63.Sulkowski MS, Naggie S, Lalezari J, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA. 2014;312:353–61. doi: 10.1001/jama.2014.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:410–25. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 65.Price H, Dunn D, Pillay D, et al. Suppression of HBV by tenofovir in HBV/HIV coinfected patients: a systematic review and meta-analysis. PLoS One. 2013;8:e68152. doi: 10.1371/journal.pone.0068152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wohl D, Pozniak A, Thompson M, et al. CROI 2015. Seattle, WA: 2015. Tenofovir Alafenamide (TAF) in a Single-Tablet Regimen in Initial HIV-1 Therapy; p. 113LB. [Google Scholar]
- 67.Sherman AC, Trehanpati N, Daucher M, et al. Augmentation of hepatitis B virus-specific cellular immunity with programmed death receptor-1/programmed death receptor-L1 blockade in hepatitis B virus and HIV/hepatitis B virus coinfected patients treated with adefovir. AIDS Res Hum Retroviruses. 2013;29:665–72. doi: 10.1089/aid.2012.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeisel MB, Lucifora J, Mason WS, et al. Towards an HBV cure: state-of-the-art and unresolved questions-report of the ANRS workshop on HBV cure. Gut. 2015;64:1314–26. doi: 10.1136/gutjnl-2014-308943. [DOI] [PubMed] [Google Scholar]
- 69.Hawkins C, Agbaji O, Ugoagwu P, et al. Assessment of liver fibrosis by transient elastography in patients with HIV and hepatitis B virus coinfection in Nigeria. Clin Infect Dis. 2013;57:e189–92. doi: 10.1093/cid/cit564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat. 2011;18:61–9. doi: 10.1111/j.1365-2893.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 71.Kiser JJ, Burton JR, Jr., Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10:596–606. doi: 10.1038/nrgastro.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Kanter CT, Drenth JP, Arends JE, et al. Viral hepatitis C therapy: pharmacokinetic and pharmacodynamic considerations. Clinical pharmacokinetics. 2014;53:409–27. doi: 10.1007/s40262-014-0142-5. [DOI] [PubMed] [Google Scholar]
- 73.Treisman G, Angelino A. Interrelation between psychiatric disorders and the prevention and treatment of HIV infection. Clin Infect Dis. 2007;45(Suppl 4):S313–7. doi: 10.1086/522556. [DOI] [PubMed] [Google Scholar]
- 74.Rausch DM, Grossman CI, Erbelding EJ. Integrating behavioral and biomedical research in HIV interventions: challenges and opportunities. J Acquir Immune Defic Syndr. 2013;63(Suppl 1):S6–11. doi: 10.1097/QAI.0b013e318292153b. [DOI] [PubMed] [Google Scholar]
- 75.Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis. 2013;57(Suppl 2):S56–61. doi: 10.1093/cid/cit271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stein MR, Soloway IJ, Jefferson KS, Roose RJ, Arnsten JH, Litwin AH. Concurrent group treatment for hepatitis C: implementation and outcomes in a methadone maintenance treatment program. Journal of substance abuse treatment. 2012;43:424–32. doi: 10.1016/j.jsat.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis. 2013;56:806–16. doi: 10.1093/cid/cis1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sylvestre DL, Zweben JE. Integrating HCV services for drug users: a model to improve engagement and outcomes. The International journal on drug policy. 2007;18:406–10. doi: 10.1016/j.drugpo.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 79.Taylor LE, Maynard MA, Friedmann PD, et al. Buprenorphine for human immunodeficiency virus/hepatitis C virus-coinfected patients: does it serve as a bridge to hepatitis C virus therapy? Journal of addiction medicine. 2012;6:179–85. doi: 10.1097/ADM.0b013e318257377f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(Suppl 2):S39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]