Abstract
African-Americans (AA) have increased carotid artery intima-media thickness and decreased vascular function compared to their Caucasian (CA) peers. Aerobic exercise prevents and potentially reverses arterial dysfunction.
Purpose
The purpose of this study was to examine the effect of 8 weeks of moderate-high intensity aerobic training in young healthy sedentary AA and CA men and women.
Methods
Sixty-four healthy volunteers (men = 28, women = 36) with mean age = 24 underwent measures of arterial structure, function and blood pressure variables at baseline, post-4 week control period and 8 weeks post-training.
Results
There was a significant increase in VO2peak amongst both groups post exercise training. Brachial systolic blood pressure decreased significantly following control period in both groups but not following exercise training. Carotid pulse pressure decreased significantly in both groups post exercise training as compared to baseline. There was no change in any of the other blood pressure variables. AAs had a higher intima-media thickness at baseline and post-control period, but significantly decreased following exercise training compared to CAs. AAs had significantly lower baseline forearm blood flow and RH compared to CAs, but exercise training had no effect on these variables. There was no significant difference in arterial stiffness (cPWV) and wave-reflection (AIx) between the two groups at any time point.
Conclusions
This is the first study to show that, 8 weeks of aerobic exercise training causes significant improvement in the arterial structure in young, healthy AAs, making it comparable to the CAs and with minimal effects on blood pressure variables.
Keywords: Exercise training, Race and Ethnicity, Arterial function, Intima-media thickness, Arterial structure
Introduction
African-Americans (AA) have the highest prevalence of hypertension in the world, and develop hypertension and higher rate of other heart diseases at an earlier age compared to Caucasians (CA)(15, 20). Pathophysiological factors of the increased cardiovascular disease burden even in young AA adults include increased vascular resistance, reduced microvascular vasodilatory capacity (38), increased arterial stiffness (14), increased carotid intima-media thickness (IMT) (35) and decreased endothelial dependent and independent vasodilatation (9). This may contribute or cause the higher blood pressure (BP) reported even in young AA adults (47). Furthermore, young otherwise healthy AA men exhibit higher central BP compared to their CA peers even though brachial BP were similar (24). This is important as central BP is a better predictor of clinical outcomes and target organ damage (36). Young AA men have higher aortic stiffness compared to age and fitness matched CA men (46). Conversely, Arena et. al. found that differences in the aortic stiffness between AA and Caucasian participants were partially related to differences in aerobic fitness (2). Thus, whether or not improving aerobic fitness may help attenuate these racial differences in vascular function in young men and women is still not clear.
Endurance exercise can reduce both blood pressure and arterial stiffness coupled with an increase in endothelial function in healthy CA adults (8, 13). Short term (3 months or less) regular aerobic exercise can increase endothelial function by 30% (13), and central arterial compliance by 25% while producing a ∼ 20% decrease in the carotid β-stiffness index (39) in healthy previously sedentary CA adults. In addition, recent studies have reported an improvement in both arterial stiffness and endothelial function following endurance exercise training in young healthy adults (3, 4, 12, 33, 37). l Hence, there is general agreement that aerobic training is beneficial for arterial health in young, middle-aged and older CA; however, AA young adults may exhibit differential responses. Information on the effect of endurance exercise training is notably lacking in the AA population, and data regarding exercise training in younger AA women are particularly sparse in existing literature due to small sample sizes and a lack of control groups or control periods (6, 7, 21). Nevertheless, there is also no information suggesting that AA women would exhibit differential changes in response to training compared to AA men.
Hence, our aim was to examine the effect of 8 weeks of moderate-high intensity aerobic training on young healthy sedentary AA men and women compared to their CA peers. We hypothesized that AA would show significantly greater improvement in blood pressure, arterial stiffness, resting forearm blood flow, reactive hyperemia and IMT measures compared to CA.
Methods
Subjects
Healthy volunteers (men and women) between 18 - 35 years of age (mean age = 24 years) were recruited and screened via phone interview. 62 participants completed the study (Figure 1 – CONSORT diagram). All subjects were free of acute cardiovascular or respiratory disease and non-smoking. Exclusion criteria included participants with hypertension, stroke, or myocardial infarction, metabolic disease (diabetes mellitus), inflammatory diseases (rheumatoid arthritis and systemic lupus erythematosus), bleeding disorders, or were taking any medications on a regular basis other than oral contraceptives. Participants taking allergy medications, and participants who were taking over the counter pain/anti-inflammatory medications were asked to come in for testing only 72 hours after their last dose. Participants who had suffered from the common cold, influenza or upper respiratory tract infection 2 months preceding enrollment were also excluded. All subjects were recruited from the local community and provided written informed consent prior to participation. This study was approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign. The clinical trials identifier for the study is NCT01024634.
Figure 1.
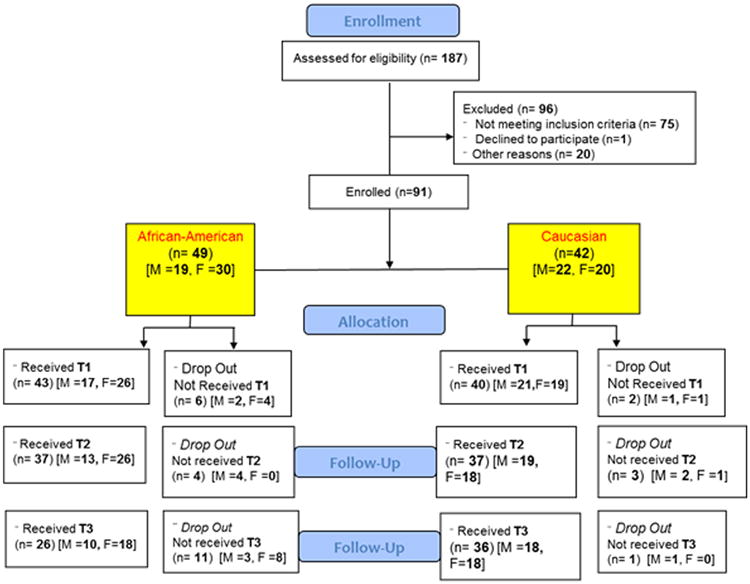
CONSORT diagram for the experimental study.
Study Design
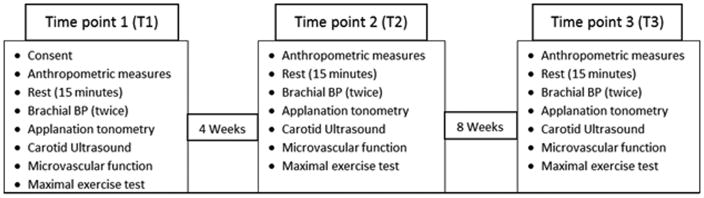
We used a longitudinal experimental design, with all subjects undergoing all the conditions. The first 4 weeks of the study following the baseline visit (T1) served as a reference/control time period for each participant. During this control period all the participants were asked to continue their regular lifestyle and refrain from making changes to their diet. They were also asked to refrain from starting any exercise protocols on their own. Following the 4-week control period, subjects visited the laboratory for a follow-up visit (T2). After that visit, all subjects were enrolled in an 8 week endurance exercise program. They then returned for a final visit after completion of the 8 week exercise program (T3). Subjects were asked to maintain their normal lifestyle and diet during the entire study period. All participants underwent 3 testing sessions and 24 exercise training sessions. The study design and visit details are represented in Figure 2.
Figure 2.
Experimental timeline for the study. All the measurements were performed in the chronological order.
To control for diurnal variation, all the measurements were performed during the same time of the day for each participant, with the exception of fasting blood draws, which were obtained on a separate day. Women were tested during the early follicular phase of their menstrual cycle or during the placebo phase if they were taking oral contraceptives. Prior to each testing session all participants were asked to fast for minimum of 4 hours. They were also asked to refrain from heavy physical activity for 24 hours prior to the testing session and anti-inflammatory medication for at least 3 days.
Anthropometric
Height and weight was measured using a stadiometer and a beam balance platform scale, respectively. BMI was calculated as weight (kg) divided by height (m) squared.
Assessment of hemodynamics and vascular structure and, function
Resting blood pressure
Resting blood pressure was measured in the supine position using an automated oscillometric cuff (HEM-907XL, Omron Corporation, Japan) following 15 minutes of rest period. The coefficient of variation (CV) for blood pressure measurements in our lab is between 2-3.3%.
Pulse Wave Analysis
Radial artery pressure waveforms were attained in the supine position from a 10-second epoch using applanation tonometry and using a generalized validated transfer function (10) the augmentation index (AIx) and AIx normalized to a heart rate of 75 bpm (AIx@75) were calculated. The augmentation index (AIx) which typically expressed in percent and used as an index of systemic arterial stiffness was calculated as the ratio of amplitude of the pressure wave above its systolic shoulder (i.e. the difference between the early and late systolic peaks of the arterial waveform), to the total PP. Because AIx is influenced by heart rate (HR), AIx values were normalized to a HR of 75 bpm (AIx@75). Only high-quality recordings i.e. recordings with a quality index > 80% were included in the analysis (44). All measurements were made in duplicate, and the mean value used for subsequent analysis. Reproducibility of measures attained from this technique has previously been shown to be high (45). The CV for AIx is <5.5% in our lab.
Pulse Wave Velocity
A high-fidelity strain gauge transducer (Millar Instruments, Houston, TX) was used to obtain the pressure waveform from: 1) the left common carotid artery and the left femoral artery, and 2) the left femoral artery and the ipsilateral superior dorsalis pedis artery. All measurements were conducted following guidelines of the Clinical Application of Arterial Stiffness Task Force III (42). This value was used as an index of central stiffness. Only those PWV values with a standard deviation < 10% as assessed by the integral software (SphygmoCor, AtCor Medical, Sydney, Australia) are included in subsequent analysis. All measurements were made in duplicate, and the mean value used for subsequent analysis. This technique is highly reproducible (45). The CV for PWV is 3.2% in our lab.
Carotid Artery Compliance and β-stiffness
The cephalic portion of carotid artery was imaged in longitudinal section, 1-2 cm proximal to the bifurcation, via ultrasonography (Aloka alpha-10, Tokyo, Japan), using a high frequency (7.5 MHz) linear array probe. Simultaneous blood pressure of the contralateral carotid artery was determined using applantion tonometry. Image analysis and calculation of arterial compliance (AC) and β-stiffness index (β) were carried out using an automated wall detection echo-tracking software system. The CV for β-stiffness in our lab is ∼5%.
Carotid artery Intima-media Thickness (IMT)
All measurements were made at end diastole measured via ultrasonography (Aloka alpha-10, Tokyo, Japan). The IMT of the common carotid artery was determined from an average of 5 measurements obtained 20 mm proximal to the carotid bifurcation. The CV for IMT in our lab is ∼1.4%.
Forearm resistance artery vasodilatory capacity
With participant in supine position, vasodilatory function of forearm resistance arteries was assessed using reactive hyperemia (RH) and strain-gauge plethysmography (EC-6, DE Hokonson, Inc, Bellevue, Wash). A wrist cuff was placed right at the wrist level to exclude hand circulation. Upper arm cuff was placed above the elbow on the upper arm, while strain gauge was placed on the widest area of the forearm. Forearm Blood Flow (FBF) was measured using strain-gauge plethysmography (EC-4, D.D. Hokanson, Inc., Bellevue, Wash.). Reactive hyperemia of the forearm vessels was evaluated immediately following FBF. Following 5 minutes of upper arm occlusion and last minute of wrist cuff occlusion, changes in the forearm volume was measured using rapid release of the upper arm cuff. 13 readings (3 minutes) were taken with a 15 seconds cycle. Peak forearm blood flow was recorded as the highest reading. Area under the curve was used as a measure of total RH by plotting all 13 measurements against time. The CV for RH in our lab is ∼9%. FBF was expressed as ml·min-1·100-1 ml of forearm tissue and also as flow per unit pressure (conductance) using the following equation:
Forearm vascular resistance was calculated using the equation:
Maximal Exercise
Peak oxygen consumption was evaluated using cycle ergometry to exhaustion as we have previously described (23). We used a graded protocol, starting at 50 watts followed by 30 watt increments every 2 minutes until exhaustion. The test was terminated when the subject could no longer continue and peak effort was determined based on meeting 3 of the following criteria: 1) Inability to maintain a 60 rpm pedal rate; 2) A respiratory exchange ratio of 1.1; 3) Achievement of ± 10 beats per min of predicted maximal HR; 4) A plateau in HR with an increase in work rate; 5) A plateau in VO2 with an increase in work rate (an increase of less than 150 ml/min); 6) A final rating of perceived exertion of ≥ 17 on the Borg scale (6-20).
Endurance Training Intervention
Both groups underwent a supervised endurance training program in accordance with established guidelines (1). Sessions were carried out 3 times per week. During each session, participants completed a 5 min warm-up followed by 30-45 min of endurance exercise. During the first 2 weeks, exercise was performed at an initial intensity equivalent to 65-75% of VO2peak measured from the peak cycle ergometry test. After the initial two weeks, intensity was increased to 75-85% of VO2peak in order to increase the training stimulus. This intensity range is considered moderate to high intensity and is designed to improve VO2peak and produce changes in arterial function. Intensity was monitored using heart rate monitors during each exercise session, and each participant received an exercise prescription with a heart range equivalent to 65-85% of their VO2peak. Participants were asked to complete 30 min of exercise during each session in week 1, 35 min in week 2 and 40-45 min from week 3 through week 8. Each exercise session was concluded with a 5 min cool down period.
Blood analysis
Following an overnight fast, on a separate day, venous blood samples were collected and analyzed for plasma concentrations of C-reactive protein (CRP) and Interleukin-6 (IL-6). Samples were collected into 10 ml tubes containing EDTA (anticoagulant and chelating agent). Samples were separated by centrifugation at 4° C for 15 min at 1100g and were stored at -80° C until analyzed. were measured to assess systemic inflammation. Separate Quantikine enzyme-linked immunosorbent assay (ELISA) kits (R&D systems, Minneapolis, MN) were used to measure plasma IL-6 and CRP. Sensitivities for the ELISA kits were 0.010ng/mL and 0.039pg/mL for CRP and IL-6, respectively.
Statistical analysis
All inferential statistics were conducted using Stata IC (12.0) statistical software and employed two-tail alpha to reject the null hypothesis of no-effect at 0.05. Each of our dependent variables were analyzed separately. All analyses were conducted in concert with our mixed-effects experimental design consisting of one independent measures factor (race: African American vs. not) and one longitudinal/repeated-measures factors (Time: pre control, post control, and after training). We analyzed our outcomes with mixed-effects linear regression models, also known as multilevel modeling (MLM). These are recent extensions of OLS regression/ANOVA methods that are particularly favorable for repeated-measures studies like ours because they enable us to incorporate each subjects' unique Y-intercept term (random effect for subject) to accommodate the within-subject correlation structure, yet not having the strict ANOVA requirement that all subjects have all data from all time points. That is, each subject contributes data from all available time periods for which they remained a participant, but occasional missed data acquisitions will not eliminate the participant from the entire analysis. Due to unplanned attrition, some subjects dropped out of the study prior to some data acquisitions, however their available observations remain in our analyses and helped inform our statistical models where possible. Each of our models included dummy-coded Time indicators comparing times 2 and 3 to the pre-control period, and thus forced no assumptions of linearity or non-linear change over time.
Results
All the values are reported as means ± SEM. AA had a significantly higher BMI (p<0.05) as compared to CA at all the three time points (Table 1). There was no significant difference between VO2 peak at baseline (T1) and the end of the control period (T2) for either group. There was a significant increase in VO2 peak (p<0.05) from and the end of the control period (T2) to end of exercise training (T3).
Table 1. Participant characteristics.
| Caucasian | African-Americans | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| T1 | T2 | T3 | T1 | T2 | T3 | |
|
| ||||||
| Age | 24.25 ± 0.8 | 24.25 ± 0.8 | 24.25 ± 0.8 | 24.46 ± 0.7 | 24.46 ± 0.7 | 24.46 ± 0.7 |
| BMI$ | 24.73 ± 0.61 | 24.85 ± 0.65 | 24.56 ± 0.67 | 29.41 ± 1.30 | 29.15 ± 1.43 | 29.90 ± 2.05 |
| VO2peak | 36.72 ± 8.1 | 35.80 ± 7.7 | 38.76 ± 7.7* | 30.97 ± 7.9 | 30.65 ± 7.9 | 32.96 ± 7.9* |
Note: Values are means ± SEM;
p <0.05 between groups at all-time points.
p<0.05 from T1. Body mass index, BMI; VO2peak, Maximal aerobic capacity.
Blood pressure
Baseline (T1)
There was no significant difference in brachial SBP, DBP and MAP between CA and AA at baseline (Table 2). PP was significantly higher in CA as compared to AA (p<0.05). However, aortic and carotid SBP, DBP, MAP or PP and aortic DBP or PP were not significantly different between CAs and AAs at baseline.
Table 2.
Blood pressure variables.
| Caucasian | African-Americans | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| T1 | T2 | T3 | T1 | T2 | T3 | |
|
| ||||||
| SBP (mmHg) Brachial | 122 ± 2 | 120 ± 2* | 120 ± 2* | 119 ± 2 | 117 ± 2* | 115 ± 2* |
| Carotid | 115 ± 2 | 111 ± 2 | 111 ± 2 | 112 ± 2 | 112 ± 3 | 110 ± 3 |
| Aortic | 103 ± 1 | 101 ± 2 | 101 ± 2 | 102 ± 1 | 102 ± 2 | 100 ± 2 |
|
| ||||||
| DBP (mmHg) Brachial | 69 ± 2 | 68 ± 1 | 68 ± 2 | 71 ± 1 | 68 ± 1 | 68 ± 2 |
| Carotid | 69 ± 1 | 68 ± 0 | 68 ± 2 | 70 ± 2 | 68 ± 1 | 69 ± 2 |
| Aortic | 70 ± 1 | 69 ± 1 | 69 ± 2 | 70 ± 1 | 68 ± 1 | 69 ± 2 |
|
| ||||||
| MAP (mmHg) Brachial | 87 ± 1 | 85 ± 1 | 85 ± 2 | 86 ± 1 | 85 ± 1 | 83 ± 2 |
| Carotid | 86 ± 1 | 85 ± 1 | 85 ± 2 | 86 ± 1 | 85 ± 1 | 85 ± 1 |
| Aortic | 85 ± 1 | 84 ± 1 | 84 ± 2 | 85 ± 1 | 84 ± 1 | 83 ± 2 |
|
| ||||||
| PP (mmHg) Brachia# | 53 ± 1 | 51 ± 1 | 51 ± 2 | 48 ± 2 | 48 ± 2 | 47 ± 2 |
| Carotid | 46 ± 2 | 43 ± 1 | 42 ± 2* | 41 ± 2 | 41 ± 2 | 39 ± 3* |
| Aortic | 33 ± 1 | 31 ± 1 | 32 ± 1 | 31 ± 1 | 33 ± 1 | 31 ± 1 |
Note: Values are mean ± SEM.
race versus time interaction.
p<0.05 time effect from T1. Systolic blood pressure, SBP; Diastolic blood pressure, DBP; Mean arterial pressure, MAP; Pulse pressure, PP; Baseline, T1; Post-control period, T2; Post-exercise, T3
Control period (T2)
There was a decrease in brachial SBP (p<0.05), but no change in DBP following the control period in both groups (Table 2). There were also no significant changes in carotid SBP, DBP and aortic SBP and DBP during the control period. There was a significant group by time interaction (p<0.05) in aortic PP at T2 (4-week control time period). This was attributed to the rise in aortic PP in AA by ∼2 mmHg at T2 while it decreased by ∼2mmHg in CA. Similarly, there was a significant interaction (p<0.05) in carotid PP at T2 (4-week control time period). Again, this is due to a drop in carotid PP by ∼3mmHg in CA while there was no change in the AA group.
Post-training period (T3)
There was a significant decrease in brachial SBP (p<0.05) following the aerobic exercise training period, compared to baseline, across groups (Table 2). Also, carotid PP decreased significantly following exercise training compared to baseline across groups. There was no exercise effect on any of the other blood pressure variables.
Blood variables
There were no differences in the levels of IL-6 and CRP between the two groups. There were no exercise effects for IL-6 or CRP in CAs or AAs.
Forearm blood flow
AA had significantly lower baseline FBF (P<0.05) compared to CA. Also, AA had significantly lower RH and AUC than CA (Table 3). However, there was no main-effect of exercise training and no interaction effects on resting forearm blood flow, RH or AUC. In addition, there were no differences at baseline, main-effect of exercise training and no interaction effects in forearm vascular conductance or resistance between AA and CA groups.
Table 3. Variables of arterial structure and function.
| Caucasian | African-Americans | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| T1 | T2 | T3 | T1 | T2 | T3 | |
|
| ||||||
| RH | 23.64 ± 1.5 | 21.30 ± 1.4 | 23.26 ± 1.6 | 18.18 ± 1.6 | 17.97 ± 1.6 | 19.83 ± 1.8 |
| AUC | 77.7 ± 4.6 | 80.5 ± 4.4 | 79.2 ± 4.5 | 55.6 ± 3.2 | 56.8 ± 3.5 | 55.5 ± 4.3 |
| FVC | 3.63 ± 0.32 | 3.5 ± 0.25 | 3.37 ± 0.24 | 2.89 ± 0.19 | 3.23 ± 0.25 | 3.1 ± 0.3 |
| FVR | 35.6 ± 3 | 33.9 ± 2.6 | 34.6 ± 2.9 | 41.74 ± 3 | 38.98 ± 3.4 | 40.98 ± 4.6 |
| PWV central (m/sec) | 5.84 ± 0.2 | 5.65 ± 0.16 | 5.53 ± 0.19 | 5.83 ± 0.24 | 5.41 ± 0.19 | 5.57 ± 0.14 |
| AIx (%) | 4.3± 1.9 | 5.3 ± 2.4 | 3.9 ± 2.1 | 7.2 ± 2.2 | 10.7 ± 2.9 | 8.2 ± 2.5 |
| β-Stiffness | 5.5 ± 0.2 | 5.8 ± 0.3 | 5.9 ± 0.3 | 5.5 ± 0.3 | 5.6 ± 0.3 | 5.8 ± 0.4 |
| D-Min (mm) | 6.58 ± 0.01 | 6.57 ± 0.12 | 6.86 ± 1 | 6.9 ± 0.12 | 6.83 ± 0.12 | 6.91 ± 0.16 |
| D-Max (mm) | 7.21 ± 0.1 | 7.22 ± 0.11 | 7.46 ± 0.11 | 7.51 ± 0.12 | 7.44 ± 0.12 | 7.49 ± 0.17 |
Note: Values are mean ± SEM. Reactive Hyperemia, RH; Area under curve, AUC; Forearm vascular conductance, FVC; Forearm vascular resistance, FVR; Pulse wave velocity, PWV; Augmentation index, AIx; Beta-stiffness, β-Stiffness; Diameter minimum, D-Min; Diameter maximum, D-Max
Arterial function and structure
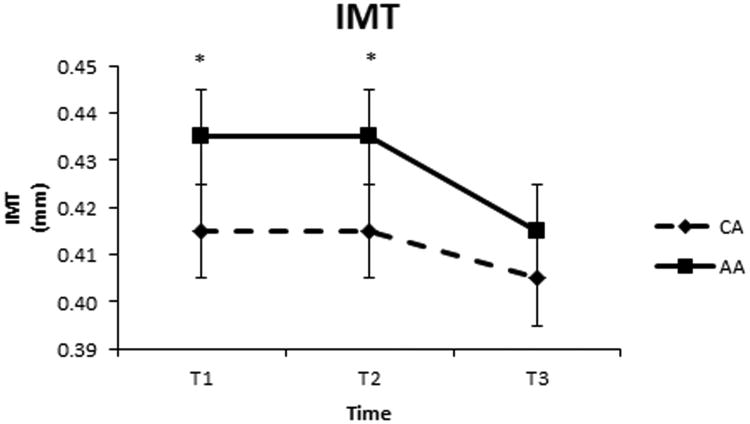
There was no significant baseline difference in central arterial stiffness (cPWV), wave-reflection (AIx) or carotid β-stiffness between the two groups (Table 3). Exercise training had no effect of cPWV, AIx or β-stiffness in either group. AAs had a higher IMT at baseline (p<0.05) compared to CAs (Figure 3). While both groups decreased IMT from T2 to T3, we observed a significant race by time interaction effect, with greater decreases in the AA group. In addition, there was no significant difference in the carotid diastolic diameter (D-min) at baseline between CAs and AAs, however, exercise training increased carotid diameter in CAs, but not in AAs (p=0.048). Because changes in IMT can be influenced by changes in arterial lumen diameter, we also conducted a follow-up mixed effects analysis controlling for the change in arterial diameter. This analysis showed a significant race by training interaction, where AA exhibited a significant decrease in IMT after controlling for changes in arterial diameter, whereas CA did not (Adjusted means ± SE of 0.421 ± 0.011 to 0.401 ± 0.011 mm for AA vs 0.407 ± 0.01 to 0.400 ± 0.01 mm for CA).
Figure 3.
Change in carotid artery IMT over a period of 12 weeks. T1 refers to time point 1 (Baseline), T2 refers to time point 2 (time control period-pre exercise training) and T3 refers to time point 3 (post-exercise training). * p<0.05 for AAs having a significantly higher IMT than CAs.
Discussion
This is the first study to examine the effects of race on vascular and hemodynamic adaptations following aerobic training in young men and women. Our primary finding was that 8 weeks of aerobic exercise training improved IMT profile in AA, and blood pressure changes do not explain the changes in IMT. While endurance exercise training did not influence FBF and RH in AA or CA our findings corroborate previous data (28) that even healthy young AAs have lower FBF and RH as compared to their CA counterparts. Interestingly, carotid PP decreased with exercise training across groups, suggesting exercise training affected carotid PP similarly in both AA and CA. While brachial SBP decreased following the control period and remained lower than baseline following exercise training, exercise training did not significantly affect SBP in either group, consistent with previous findings in young healthy adults (18, 19).
Intima-media thickness
Similar to previous work, we found AAs have an increased carotid-IMT as compared to their CA peers (14, 35). Even a 0.1mm increase in the carotid-IMT has been associated with increased risk of myocardial infarction after adjusting for age and sex (30). In addition, carotid-IMT provided risk stratification similar to that of Framingham Risk Score (31), and low physical fitness independently correlates with increased carotid-IMT in middle aged and older adults (34). In contrast to our study in younger (18-35 years) adults, longitudinal studies evaluating the effects of aerobic exercise training (8-12 weeks) on IMT in middle-aged and older adults found that training was unable to alter the carotid artery IMT (40). Conversely, Thijssen et. al. observed a significant reduction in wall thickness in the carotid and femoral artery following 8 weeks of exercise training in healthy young adults (41), consistent with our findings. Others have also found structural changes following a brief intervention period (8-12 weeks) in peripheral arteries (17). To our knowledge, the present study is the first to show a reduction in IMT with exercise training in young healthy AA. Furthermore, our time-control study design ascertained that our findings were not a result of potential pre-existing differences between groups. One of the potential reasons for this beneficial change in carotid artery IMT in AA may be due to the positive effect of exercise training on carotid PP. Increased PP has been associated with increased carotid artery wall thickness (16). Additionally, increased pressure also causes a shift in arterial endothelial cell phenotype by reducing eNOS and increasing VCAM-1, ICAM-1 and ET-1, which promote atherosclerotic process (29). In addition, vascular wall hypertrophy may depend more on the PP rather than mean pressure or wall tension (26). Hence, the reduction in PP seems to be consistent with the reduction in carotid artery IMT in AA, although similar changes in PP in CA had no effect on IMT when controlled for changes in carotid artery diameter. Thus, endurance exercise training seems to affect carotid artery remodeling differently in AA and CA young healthy adults.
Interestingly, internal carotid artery diameter increased significantly in CAs but not in AA following exercise training, even though there was no significant difference in the carotid diastolic diameter (D-min) at baseline between CAs and AAs. It is possible that a decrease in wall thickness is accompanied by lumen dilation, thus the change in IMT may simply be function of changes in lumen diameter. Our data support this notion in CAs as the change in IMT was not significant when controlled for changes in carotid diameter, whereas the training induced change in IMT was unaffected by carotid diameter changes in AAs. Since reductions in IMT with exercise training has more commonly been observed in obese populations (43)(32), it is possible that the higher BMI in our AA participants may have contributed to the greater reduction in IMT controlled for changes in arterial size in our AA group.
Blood flow and vascular responsiveness
Young healthy normotensive AAs have lower vascular responsiveness to nitric oxide (NO) compared to CA (9). Our current findings suggest that AAs not only have a reduced baseline blood flow in the forearm microvasculature but also exhibit significantly reduced reactivity of this vasculature compared to CAs. Contrary to our hypothesis, 8 weeks of aerobic training did not abolish the difference in microvascular circulation between AAs and CAs.
AAs have a higher minimum forearm vascular resistance compared to age, BMI, sex and blood pressure matched CAs (25). This reinforces our finding that the baseline measures of FBF and RH were significantly lower in AAs. This is of particular importance because the increase in forearm resistance can be a precursor for hypertension due to its effect on vascular remodeling and metabolic factors (25). However, in the present study, we did not find any significant difference in baseline forearm vascular resistance or conductance in AAs as compared to CAs.
Blood pressure
Brachial, carotid and aortic systolic, diastolic and mean blood pressure was not significant different between groups at baseline nor was there a significant change following exercise training. However, it should be noted that systolic brachial pressure was significantly higher in CA as compared to AA. Importantly, average blood pressure was in the normal range and both the groups had almost similar baseline pressures. Unexpectedly, there was a decrease in brachial SBP (p<0.05) following the control period in both groups. The decrease was ∼2mmHg in both the groups, and it is difficult to determine the exact reason for the change during the control period as opposed to following exercise training. Even though there was a significant reduction following exercise training compared to baseline, there was no change in brachial SBP at T3 as compared to the control period (T2). The lack of change in blood pressure following exercise training could be attributed to the fact that both the groups recruited were young healthy normotensive participants. Previous literature suggests that even patients with average fitness and mild hypertension fail to show significant reduction in BP following endurance exercise training without dietary changes (5).
Ceiling effect with vascular function
In the present study, we did not find any significant changes in the measures of vascular function despite an effect of exercise training on arterial structure (IMT). Interestingly, Green et. al. have reported that in world class athletes, there was an inverse relation between the diameter and flow-mediated dilation (FMD) in both femoral and brachial arteries (22). This suggests that adaptation of arterial function and arterial structure may not always coexist. One of the primary reasons for this lack of change could be that, the values at baseline and pre training period in our younger, healthy cohort were normal and hence there was a little scope for improvement with 8 weeks of exercise training. This ceiling effect may be challenged by either increasing the intensity of exercise or changing the mode or duration of exercise.
Limitations
One of the limitations for the study was that, we did not control the mode of aerobic training (bike, treadmill, or elliptical machine) and allowed participants to self-select intensity from the range normally used to induce exercise training effects (65-85% max HR). While we address this as a limitation, we also believe this improves the applicability of our study to real-world scenarios. Secondly, we did not control for regular individual activity of an individual by maintaining activity records. Additionally, AA have higher BMI as compared to the CA group and there was no significant decrease in BMI post training period. Hence, BMI one of the limitations is that BMI could have been a confounding variable for differences in IMT at baseline. However, as BMI did not change but there were changes seen in IMT, it is plausible that these vascular changes were not due to weight loss. Thirdly, we acknowledge that NO has been shown to play limited role in peak vasodilation during reactive hyperemia and other factors (endothelial dependent or independent) may be playing a role. Lastly, the study included only young healthy individuals from both ethnicities and hence there was lack of differences at baseline in vascular function and blood pressure. Therefore, these results cannot necessarily be extrapolated to clinical populations. However, as we reported in this study, even young healthy AAs have a significantly higher carotid-IMT and lower microvascular function as compared to CAs.
Perspectives
This is the first study to show that, 8 weeks of aerobic training significantly improves arterial structure (carotid artery IMT) in young, healthy AAs. In addition, the study shows that young healthy AAs have lower FBF and RH as compared to CA counterparts, which was not improved by 8 weeks of aerobic training.
Acknowledgments
source of funding: The present study was funded by National Institute of Health (NIH) - NHLBI 1R01HL093249-01A1. The results of the present study do not constitute endorsement by ACSM.
Footnotes
Conflict of interest: None.
References
- 1.American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975–91. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 2.Arena R, Fei DY, Arrowood JA, Kraft KA. Influence on aerobic fitness on aortic stiffness in apparently healthy Caucasian and African-American subjects. Int J Cardiol. 2007;122(3):202–6. doi: 10.1016/j.ijcard.2006.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck DT, Casey DP, Martin JS, Emerson BD, Braith RW. Exercise training improves endothelial function in young prehypertensives. Exp Biol Med (Maywood) 2013;238(4):433–41. doi: 10.1177/1535370213477600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens. 2013;26(9):1093–102. doi: 10.1093/ajh/hpt080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal JA, Siegel WC, Appelbaum M. Failure of exercise to reduce blood pressure in patients with mild hypertension. Results of a randomized controlled trial. Jama. 1991;266(15):2098–104. [PubMed] [Google Scholar]
- 6.Bond V, Millis RM, Adams RG, Oke LM, Enweze L, Blakely R, Banks M, Thompson T, Obisesan T, Sween JC. Attenuation of exaggerated exercise blood pressure response in African-American women by regular aerobic physical activity. Ethn Dis. 2005;15(4 Suppl 5):S5–10. 3. [PMC free article] [PubMed] [Google Scholar]
- 7.Bond V, Stephens Q, Adams RG, Vaccaro P, Demeersman R, Williams D, Obisesan TO, Franks BD, Oke LM, Coleman B, Blakely R, Millis RM. Aerobic exercise attenuates an exaggerated exercise blood pressure response in normotensive young adult African-American men. Blood Press. 2002;11(4):229–34. doi: 10.1080/08037050213765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol. 1994;266(2 Pt 2):H693–701. doi: 10.1152/ajpheart.1994.266.2.H693. [DOI] [PubMed] [Google Scholar]
- 9.Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol. 2002;40(4):754–60. doi: 10.1016/s0735-1097(02)02015-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95(7):1827–36. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 11.Cook MD, Heffernan KS, Ranadive S, Woods JA, Fernhall B. Effect of resistance training on biomarkers of vascular function and oxidative stress in young African-American and Caucasian men. J Hum Hypertens. 2013;27(6):388–92. doi: 10.1038/jhh.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currie KD, Thomas SG, Goodman JM. Effects of short-term endurance exercise training on vascular function in young males. Eur J Appl Physiol. 2009;107(2):211–8. doi: 10.1007/s00421-009-1116-4. [DOI] [PubMed] [Google Scholar]
- 13.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–7. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 14.Din-Dzietham R, Couper D, Evans G, Arnett DK, Jones DW. Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, ARIC cohort. Am J Hypertens. 2004;17(4):304–13. doi: 10.1016/j.amjhyper.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116(13):1488–96. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 16.Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278(4):H1205–10. doi: 10.1152/ajpheart.2000.278.4.H1205. [DOI] [PubMed] [Google Scholar]
- 17.Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534(Pt 1):287–95. doi: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan JJ, Gordon NF, Scott CB. Women walking for health and fitness. How much is enough? Jama. 1991;266(23):3295–9. [PubMed] [Google Scholar]
- 19.Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;33(6 Suppl):S484–92. doi: 10.1097/00005768-200106001-00018. discussion S93-4. [DOI] [PubMed] [Google Scholar]
- 20.Ferdinand KC, Armani AM. The management of hypertension in African Americans. Crit Pathw Cardiol. 2007;6(2):67–71. doi: 10.1097/HPC.0b013e318053da59. [DOI] [PubMed] [Google Scholar]
- 21.Glass JN, Miller WC, Szymanski LM, Fernhall B, Durstine JL. Physiological responses to weight-loss intervention in inactive obese African-American and Caucasian women. J Sports Med Phys Fitness. 2002;42(1):56–64. [PubMed] [Google Scholar]
- 22.Green DJ, Rowley N, Spence A, Carter H, Whyte G, George K, Naylor LH, Cable NT, Dawson EA, DH JT. Why isn't flow-mediated dilation enhanced in athletes? Med Sci Sports Exerc. 2013;45(1):75–82. doi: 10.1249/MSS.0b013e318269affe. [DOI] [PubMed] [Google Scholar]
- 23.Heffernan KS, Jae SY, Fernhall B. Racial differences in arterial stiffness after exercise in young men. Am J Hypertens. 2007;20(8):840–5. doi: 10.1016/j.amjhyper.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Heffernan KS, Jae SY, Wilund KR, Woods JA, Fernhall B. Racial differences in central blood pressure and vascular function in young men. Am J Physiol Heart Circ Physiol. 2008;295(6):H2380–7. doi: 10.1152/ajpheart.00902.2008. [DOI] [PubMed] [Google Scholar]
- 25.Hinderliter AL, Sager AR, Sherwood A, Light KC, Girdler SS, Willis PWt. Ethnic differences in forearm vasodilator capacity. Am J Cardiol. 1996;78(2):208–11. doi: 10.1016/s0002-9149(96)90397-5. [DOI] [PubMed] [Google Scholar]
- 26.DAS Joseph L Izzo, Jr, Black Henry R. Hypertension Primer: The essentials of high blood pressure. Philadelphia: Lippincott Williams & Wilkins; 2008. Mechanisms of vascular remodeling; p. 195. [Google Scholar]
- 27.Kalra L, Rambaran C, Chowienczyk P, Goss D, Hambleton I, Ritter J, Shah A, Wilks R, Forrester T. Ethnic differences in arterial responses and inflammatory markers in Afro-Caribbean and Caucasian subjects. Arterioscler Thromb Vasc Biol. 2005;25(11):2362–7. doi: 10.1161/01.ATV.0000183887.76087.6a. [DOI] [PubMed] [Google Scholar]
- 28.Lang CC, Stein CM, Brown RM, Deegan R, Nelson R, He HB, Wood M, Wood AJ. Attenuation of isoproterenol-mediated vasodilatation in blacks. N Engl J Med. 1995;333(3):155–60. doi: 10.1056/NEJM199507203330304. [DOI] [PubMed] [Google Scholar]
- 29.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol (1985) 2008;104(3):588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz MW, Schaefer C, Steinmetz H, Sitzer M. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten-year results from the Carotid Atherosclerosis Progression Study (CAPS) Eur Heart J. 2010;31(16):2041–8. doi: 10.1093/eurheartj/ehq189. [DOI] [PubMed] [Google Scholar]
- 32.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol. 2006;48(9):1865–70. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 33.Montero D, Walther G, Diaz-Canestro C, Pyke KE, Padilla J. Microvascular Dilator Function in Athletes: A Systematic Review and Meta-analysis. Med Sci Sports Exerc. 2015;47(7):1485–94. doi: 10.1249/MSS.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 34.Rauramaa R, Rankinen T, Tuomainen P, Vaisanen S, Mercuri M. Inverse relationship between cardiorespiratory fitness and carotid atherosclerosis. Atherosclerosis. 1995;112(2):213–21. doi: 10.1016/0021-9150(94)05416-g. [DOI] [PubMed] [Google Scholar]
- 35.Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW. Variation of common carotid artery elasticity with intimal-medial thickness: the ARIC Study. Atherosclerosis Risk in Communities. Ultrasound Med Biol. 1997;23(2):157–64. doi: 10.1016/s0301-5629(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 36.Roman MJ, Devereux RB. Association of central and peripheral blood pressures with intermediate cardiovascular phenotypes. Hypertension. 2014;63(6):1148–53. doi: 10.1161/HYPERTENSIONAHA.114.03361. [DOI] [PubMed] [Google Scholar]
- 37.Spence AL, Carter HH, Naylor LH, Green DJ. A prospective randomized longitudinal study involving 6 months of endurance or resistance exercise. Conduit artery adaptation in humans. J Physiol. 2013;591(Pt 5):1265–75. doi: 10.1113/jphysiol.2012.247387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36(6):945–51. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102(11):1270–5. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase in carotid artery intima-media thickness in healthy men. J Appl Physiol (1985) 2002;92(4):1458–64. doi: 10.1152/japplphysiol.00824.2001. [DOI] [PubMed] [Google Scholar]
- 41.Thijssen DH, Dawson EA, van den Munckhof IC, Birk GK, Timothy Cable N, Green DJ. Local and systemic effects of leg cycling training on arterial wall thickness in healthy humans. Atherosclerosis. 2013;229(2):282–6. doi: 10.1016/j.atherosclerosis.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens. 2002;15(5):445–52. doi: 10.1016/s0895-7061(01)02326-3. [DOI] [PubMed] [Google Scholar]
- 43.Vinet A, Karpoff L, Walther G, Startun A, Obert P, Goret L, Dauzat M, Perez-Martin A. Vascular reactivity at rest and during exercise in middle-aged obese men: effects of short-term, low-intensity, exercise training. Int J Obes (Lond) 2011;35(6):820–8. doi: 10.1038/ijo.2010.206. [DOI] [PubMed] [Google Scholar]
- 44.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109(2):184–9. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16(12 Pt 2):2079–84. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 46.Yan H, Ranadive SM, Heffernan KS, Lane AD, Kappus RM, Cook MD, Wu PT, Sun P, Harvey IS, Woods JA, Wilund KR, Fernhall B. Hemodynamic and arterial stiffness differences between African-Americans and Caucasians after maximal exercise. Am J Physiol Heart Circ Physiol. 2014;306(1):H60–8. doi: 10.1152/ajpheart.00710.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zion AS, Bond V, Adams RG, Williams D, Fullilove RE, Sloan RP, Bartels MN, Downey JA, De Meersman RE. Low arterial compliance in young African-American males. Am J Physiol Heart Circ Physiol. 2003;285(2):H457–62. doi: 10.1152/ajpheart.00497.2002. [DOI] [PubMed] [Google Scholar]