Abstract
Motor symptoms of Parkinson's disease (PD) follow the degeneration of dopaminergic neurons in the substantia nigra pars compacta. Deep brain stimulation (DBS) treats some parkinsonian symptoms – tremor, rigidity, and bradykinesia – but may worsen certain medial motor symptoms, including hypokinetic dysarthria. The mechanisms by which DBS exacerbates dysarthria while improving other symptoms are unclear and difficult to study in human patients. In this work, we propose an animal model of DBS-exacerbated dysarthria. We used the unilateral, 6-hydroxydopamine rat model of PD to test the hypothesis that DBS exacerbates quantifiable aspects of vocalization. Mating calls were recorded from sexually-experienced male rats under healthy and parkinsonian conditions, and during DBS of the subthalamic nucleus. Relative to healthy rats, parkinsonian animals made fewer calls with shorter and less complex vocalizations. In the parkinsonian rats, putatively therapeutic DBS further reduced call frequency, duration, and complexity. Interestingly, the individual utterances of parkinsonian rats spanned a greater bandwidth than those of healthy rats, potentially reducing the effectiveness of the vocal signal. This utterance bandwidth was further increased by DBS. We propose that the parkinsonism-associated changes in call frequency, duration, complexity, and dynamic range combine to constitute a rat analog of parkinsonian dysarthria. Because DBS exacerbates the parkinsonism-associated changes in each of these metrics, the subthalamic stimulated 6-hydroxydopamine rat is a good model of DBS-induced hypokinetic dysarthria in PD. This model will help researchers examine how DBS alleviates many motor symptoms of PD, while exacerbating parkinsonian speech deficits that can greatly diminish patient quality of life.
Keywords: Parkinson's Disease, Dysarthria, Deep Brain Stimulation, Hypokinesia
Introduction
Parkinson's disease (PD) is a neurological disorder associated with motor and non-motor symptoms. Motor signs of PD can include tremor, rigidity, reduced movements manifesting as bradykinesia or akinesia, and speech disturbances. Non-motor symptoms of PD can include depression, apathy, anxiety, and executive dysfunction that may include bradyphrenia, or slowness of thought (Caballol et al., 2007; Jankovic, 2008). Speech described as “slurred, thick, indistinct” has been reported in PD patients for many decades (Kaplan et al., 1954), and classic studies found speech dysfunctions in as many as 90% of PD patients (Logemann et al., 1978). Most speech disturbances in PD patients are caused by reduced or disrupted motor activity of the vocal system, and categorized as hypokinetic dysarthria, a “multidimensional impairment leading to abnormalities in speech breathing, phonation, articulation, and prosody” (Skodda, 2011).
While treatments for PD motor signs exist, dysarthric symptoms do not always respond to these treatments as favorably as do motor symptoms. With the administration of dopaminergic medications, persons with PD typically experience improvements in speech intelligibility, comprehensibility, and variability of pitch and loudness (De Letter et al., 2005, 2007a, 2007b). However, late stage surgical interventions – pallidotomy, thalamotomy, and deep brain stimulation (DBS) – have been reported to worsen hypokinetic dysarthria (de Bie et al., 2002; Burghaus et al., 2006; Intemann et al., 2001; Kim et al., 1997; Romito et al., 2002; Troster et al., 2003; Umemura et al., 2011). With the increasing prevalence of DBS to alleviate motor symptoms of PD, the worsening of dysarthria will reduce quality of life for more and more patients (Wertheimer et al., 2014). Understanding how DBS therapy can exacerbate hypokinetic dysarthria may help us devise alternative strategies to treat motor and speech signs simultaneously.
Due to obvious ethical and experimental boundaries in human studies, progress regarding the mechanisms of parkinsonian dysarthria has been limited, despite more than 60 years of work. Recent observations that hypokinetic dysarthria may be worsened by otherwise therapeutic DBS are largely unexplored. We proposed to use the existing 6-hydroxydopamine (6-OHDA) rodent model of parkinsonism to study hypokinetic dysarthria and its exacerbation by DBS. Previous studies have demonstrated reductions in vocalizations and vocalization complexity in the 6-OHDA rat (Ciucci et al., 2008; Johnson et al., 2011), but to our knowledge, none have reported the effects of DBS in this model.
We advanced a vocalization protocol (Ciucci et al., 2009) to record and quantify the effects of PD and DBS in the 6-OHDA rat model. In normal (NAL), parkinsonian (PD), and parkinsonian with subthalamic stimulation (DBS) conditions, ultrasonic mating calls from sexually-frustrated males were recorded in the presence of an estrous female. Individual sounds were isolated in time and frequency from their neighbors, and sounds in rapid succession were grouped together as vocalization strings that we called phonological words. Sounds and words were separated into two frequency bands (Brudzynski, 2005, 2013). Rats use the low frequency 20–35 kHz band for aversive situations including social defeat (Litvin et al., 2007; Wohr and Schwarting, 2013), and the high frequency 35–100 kHz band for appetitive situations including social excitement (Sales, 1972; Willadsen et al., 2014). Male rats make calls in both bands during successful and/or frustrated female solicitation (Barfield et al., 1979; Seffer et al., 2014).
We found that the rich vocalization structure of male rats was substantially diminished by parkinsonian onset, for both short vocalizations and long vocalization strings, in both frequency bands. In the PD condition, rats generated fewer and shorter vocalizations that were less acoustically complex than in the normal condition. Supporting a model of DBS-exacerbated hypokinetic dysarthria, stimulation of the subthalamic nucleus worsened these parkinsonian vocalization symptoms. In the DBS condition, rats generated even fewer and shorter vocalizations that were less acoustically complex than those in the PD condition. Interestingly, the frequency bandwidth of individual sounds increased slightly with parkinsonian onset, and increased substantially more with DBS. These findings support the hypothesis that DBS in the 6-OHDA rat model of PD provides a viable platform for the study of hypokinetic dysarthria, allowing future work to explore the mechanisms of aggravated dysarthric symptoms caused by DBS.
Materials and Methods
All surgical and experimental procedures were performed in the Department of Bioengineering at the University of Utah, approved by the Institutional Animal Care and Use Committee of the University of Utah, and complied with U.S. Public Health Service policy on the care and use of laboratory animals.
Experimental Protocols
Vocalization data were collected from male rats in the presence of estrous female rats. The timeline of the protocol for eight adult Long-Evans male rats weighing 400–600 g was as follows. Each rat underwent stereotactic surgery to receive two chronic brain implants (Chang et al., 2003; Dorval and Grill, 2014): a cannula accessing the medial forebrain bundle, and an electrode array to stimulate the subthalamic nucleus. Beginning at least one week after surgery, ultrasonic recordings of each male calling to an estrous female were collected during fiveminute trials. After two weeks of these healthy-normal recordings, animals were injected intracranially with 6- OHDA through the implanted cannula to degenerate dopaminergic neurons in the substantia nigra pars compacta. Two weeks later, the rats were injected with 5 mg/kg of intraperitoneal amphetamine, and parkinsonism was diagnosed by the rapid rotations ipsilateral to 6-OHDA injection. Five rats developed a severe, unilateral parkinsonism: two of these were placed in the control group, and three were placed in the experimental group. The ultrasonic recording protocol in the presence of an estrous female was resumed for rats in both groups. On alternate recording sessions, rats in the experimental group received DBS through the subthalamic electrode array. Rats in the control group never received DBS, although they did have an electrode array in the subthalamic nucleus to control for the effects of surgery and neuronal damage. After 1–2 months of recordings, rats were euthanized.
Surgical Procedures
Male rats were provided 20 mg of carprofen (Bio-Serv, Flemington, NJ), and after consumption, anesthetized with 2–3% isoflurane. The surgical site was shaved and disinfected, and the rats were placed on a body-temperature heating pad in a stereotactic frame (Kopf Instruments, Tujunga, CA). The scalp was opened and cleaned to the skull, and craniotomy sites were measured and marked with respect to bregma (Paxinos and Watson, 2008). Seven titanium bone screws were driven through burr holes into the skull to anchor the eventual acrylic cap. To enable the eventual injection of 6-OHDA to the medial forebrain bundle, a 23-gauge stainless steel cannula was implanted through a burr hole 2.0 mm posterior and 2.0 mm lateral to bregma. For DBS of the subthalamic nucleus, a four-channel micro-stimulating array – 2×2 grid of 75 µm platinum-iridium electrodes (~100 kΩ @ 1 kHz) with 400 µm spacing (Microprobes for Life Science, Inc., Gaithersburg, MD) – was implanted through a burr hole 3.6 mm posterior and 2.6 mm lateral to bregma, at a ventral depth of 7.8 mm. Medial electrodes were 500 µm longer than their lateral counterparts to match the anatomy of the subthalamic nucleus. After each of the hardware elements was lowered to its target depth, a layer of dental acrylic was applied to attach it firmly to the anchor screws. Following these implantations, the screws, cannula, and array were encased in a thick dental acrylic cap, built smoothly over multiple layers. The incision was sutured around the acrylic cap and covered with topical antibiotics. Rats were returned to their home cages and given 7 days of recovery prior to experimental recordings.
Following healthy-normal recordings, each rat was injected with 6-OHDA to induce parkinsonism (Dorval and Grill, 2014; Fibiger et al., 1972). Rats were anesthetized with 2–3% isoflurane and returned to the stereotactic frame. A needle was inserted to 0.5 mm beyond target depth, left for 5 minutes to create a pocket, and then retracted by 0.5 mm. A bolus of 8.0 µg 6-OHDA dissolved in 8.0 µl of saline was injected at a rate of 1.0 µl/min. Ten minutes after the completion of the injection, the needle was withdrawn at a speed not exceeding 1.0 mm/min. Rats were returned to their home cages to develop parkinsonism over the next two weeks. To verify symptoms following 14 days of rest, rats were injected with 5 mg/kg amphetamine intraperitoneally, and placed in a cylindrical chamber (Castall et al., 1977; Pieri et al., 1975). Parkinsonism was confirmed in the five rats that turned ipsilateral to the 6-OHDA injection at a rate exceeding six complete rotations per minute. Data from those five rats are included in the following analyses.
Deep Brain Stimulation
For trials that included DBS, rats were briefly anesthetized with 2–3% isoflurane, and a four-channel tether was attached to the connector of the micro-stimulating array on the head. Rats were quickly moved to their cages before awaking, and the other end of the tether was connected to an analog current stimulator (Grass Technologies, Warwick, RI). Current amplitudes ranged from 100 to 500 µA, varying by animal; bipolar stimulation was delivered through the micro-stimulating array, with both medial contacts set as anodes and both lateral contacts set as cathodes. The stimulation waveform consisted of symmetric 100 µs biphasic current pulses repeating at 100 Hz. Stimulation was applied for 5 minutes prior to recordings and during 5 minute recordings for a total duration of 10 minutes per trial. Two weeks following 6-OHDA injection, stimulation thresholds were determined by gradually increasing the current amplitude until the appearance of physical side effects, including atypical whisker twitching, other dystonic facial contractions, or repetitive rotations contralateral to stimulation. Therapeutic DBS amplitude was then set to 90% of the minimum current that yielded side effects. Although stimulation was applied only during DBS trials, a tether was connected during some healthy-control and PD trials to ensure that it did not induce significant changes in behavior. Unilateral behavioral deficits following 6-OHDA injection and improvements with the application of DBS were observed and qualitatively determined as matching of those observed in previous work from our group (Anderson et al., 2015; Dorval and Grill, 2014). Specifically, 6-OHDA induced a rotational movement ipsilateral to the lesion that was partially reversed by DBS.
Behavioral Task
Ultrasonic male rat mating calls were recorded in the presence of an estrous female in each of three conditions: healthy-normal (NAL), parkinsonian (PD), and parkinsonian with high frequency stimulation of the subthalamic nucleus (DBS). Other than connecting the DBS tether as described above, the experimental configuration was identical for all conditions and lasted 5 minutes per male rat. Multiple males were recorded in succession on each day to take full advantage of the female being in heat. Prior to experimental recording sessions, estrus was induced in one of the four females used in this study. Beginning 48 hours before the session, a female rat was injected intraperitoneally with 10 mg/kg β-estradiol diacetate in 0.2 ml corn oil. Four hours prior to the session, the female was injected intraperitoneally with 10 mg/kg progesterone in 0.2 ml corn oil. Posture and movement were observed to determine whether the female displayed ear wiggling and lordosis, characteristics of being in heat. Once the female displayed these signs, experimental recording could begin.
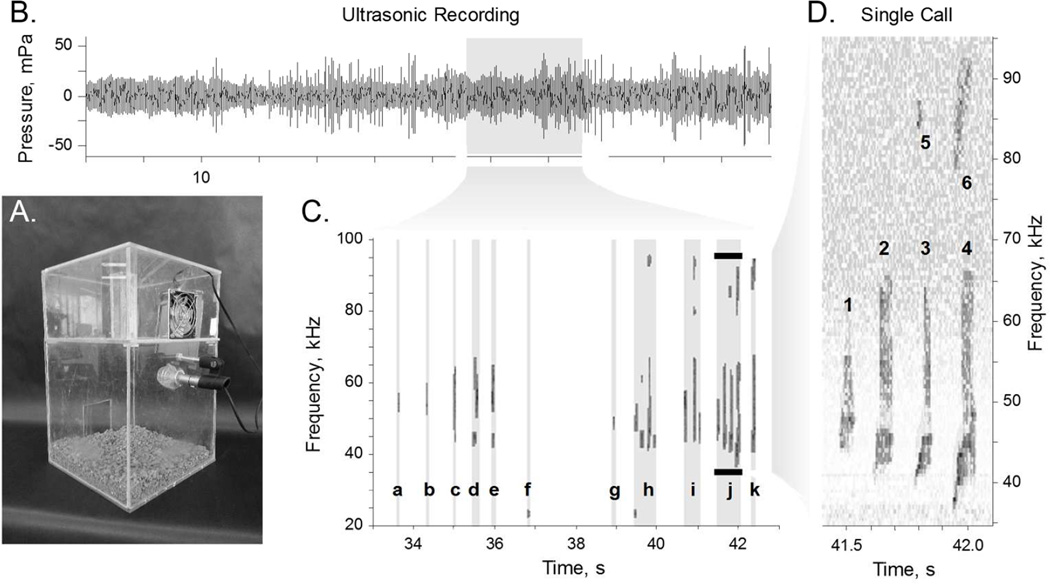
The male rat was placed in the lower chamber of the behavioral configuration (Fig. 1A) to acclimate to the environment. After 5 minutes, the estrous female was placed in the same chamber. Whenever the male attempted to mount the female, the experimenter physically removed him from her back. After three separations, the female was moved to the upper chamber (Fig. 1A). A fan in the upper chamber blew the female scent down through ten 1.0 mm diameter holes between the chambers. The male reared and vocalized in apparent attempts to attract the attention of the female. Mating calls were recorded using an ultrasonic microphone (CM16, Avisoft Bioacoustics, Glienicke, Germany). The microphone output (Fig. 1B) was recorded at 200 kHz on a standard Windows personal computer (LabView, National Instruments, Austin, TX).
Figure 1. Experimental Design.
A. Behavioral configuration, in which an estrous female rat was placed in the top chamber, and a male rat was placed in the bottom chamber. A fan in the top chamber blew the scent of the female through holes down into the bottom chamber. An ultrasonic microphone in the bottom chamber collected calls made by the male rat. B. One minute example of sound recording, converted into pressure. C. Time-frequency spectrogram from ten seconds of the example recording. Sounds were isolated from the background and collected into words (a–k) with at least 250 ms spacings. Calls labeled a–c, f, & g are single sound words; calls labeled d, e, & h–k are words with more than one sound. D. Expanded view shows that word j comprises six unique sounds isolated in time-frequency space. Note that sounds 5 & 6 (at ~86 Hz) are the first harmonics of the loud fundamental sounds 3 & 4 (at ~43 kHz).
Data Analyses
Time-frequency spectrograms were developed for each 5 minute trial via the following algorithm. Raw data at 200 kHz were convolved with a 10 ms wide Hamming kernel. The Goertzel algorithm was used to calculate a frequency-space representation of the signal, for 10 ms windows overlapping by 5 ms, in frequency bins 500 Hz wide. The resulting spectrograms were evaluated over their entire duration, between 20 and 100 kHz. Spectrograms were transformed into z-score representations by dividing each time-frequency value by the standard deviation of the signal in that frequency bin (Fig. 1D).
Vocalization Identification
Individual sounds were identified in time-frequency space (10 ms × 500 Hz pixels) by generating a binary mask and then removing small clusters and combining neighboring clusters iteratively through larger and larger cluster sizes. To begin, binary masks were made by thresholding: pixels with a z-score below 2.0 were set to zero. Mask clusters of fewer than four pixels were set to zero. The remaining clusters were inflated and then deflated by one time-frequency pixel in all eight directions. Mask clusters of fewer than eight pixels were set to zero. The remaining clusters were inflated and then deflated by 2.5 kHz toward higher and lower frequencies. Mask clusters of fewer than 12 pixels were set to zero. The remaining clusters were inflated and then deflated by two time-frequency pixels, in all eight directions. Mask clusters of fewer than 16 pixels were set to zero. To smooth the boundaries, the remaining clusters were filtered such that adjacent pixels were added to the cluster if at least four of their eight neighboring pixels were in the cluster, and pixels were removed if at most two of their neighboring eight pixels were in the cluster. This smoothing process was performed twice before mask clusters of fewer than 20 pixels were also set to zero. All clusters remaining after this process were taken as true sounds generated by the male rat. The analyses were performed with many variations on this isolation procedure, and all yielded similar results.
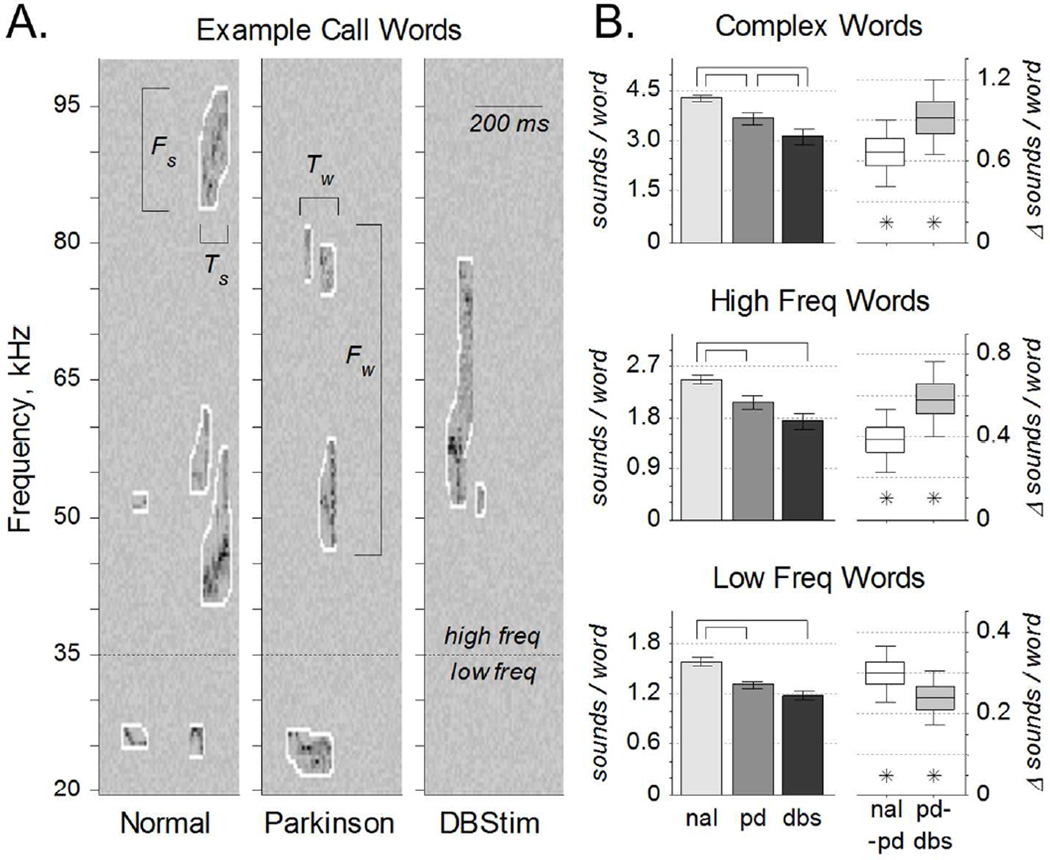
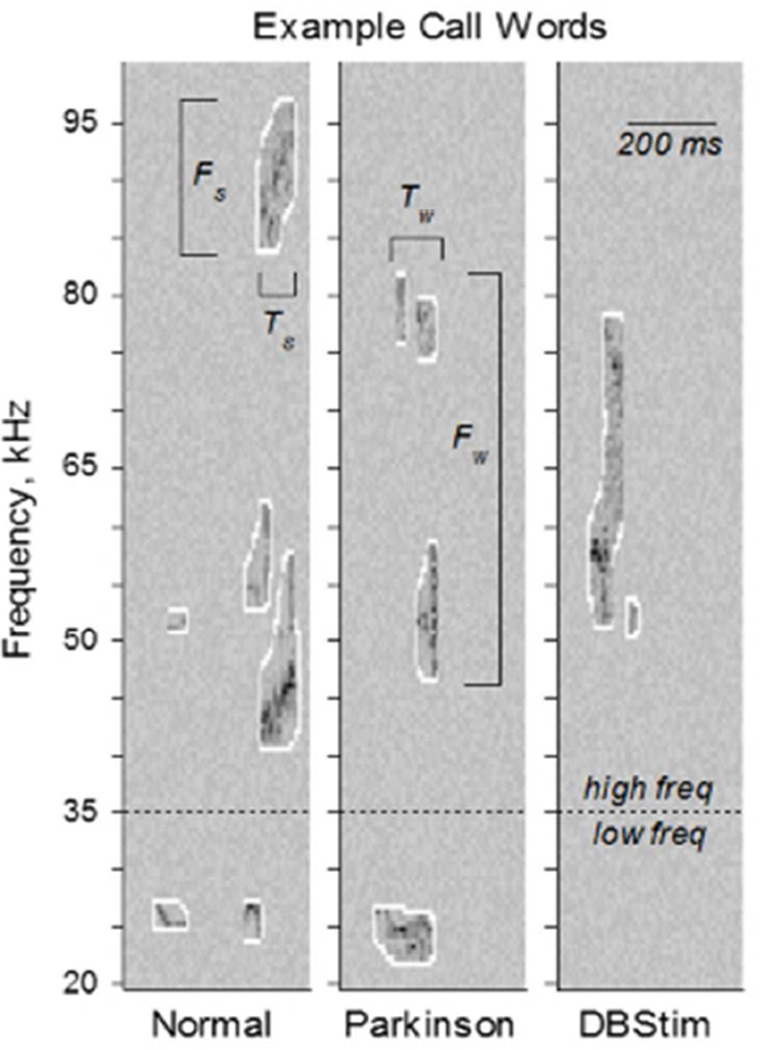
Sounds separated by less than 250 ms were grouped together into vocalization strings and identified as phonological words (Fig. 1C). Analyses repeated for vocalization strings with maximum sound separations of 100 to 500 ms yielded similar results. Sounds and words were also separated by frequency band into three call types: low, high, and complex. The division of low and high frequency bands was done to separate putatively aversive from putatively appetitive calls (Brudzynski, 2013; Willadsen et al., 2014; Wohr and Schwarting, 2013). To protect against the possibility that our sound filtering and isolating processes generated spurious single sound words, we included a category of complex sounds typical of rat vocalizations. Sound and call types are illustrated via examples in figure 2A, where the sounds are outlined in white. Low frequency calls included sounds from 20- 35 kHz. For example in figure 2A, the two-sound word below the dashed line in the left panel and the one-sound word below the dashed line in the middle panel are both low frequency calls. High frequency calls included sounds from 35–100 kHz. For example in figure 2A, high frequency calls include the four-sound, three-sound, and two-sound words above the dashed line in the left, middle, and right panels respectively. Complex calls could occur anywhere in the 20–100 kHz range, but had to include more than one sound. In figure 2A for example, each panel depicts a single complex word with 6, 4, and 2 sounds respectively, from left to right.
Figure 2. Vocalization Complexity.
A. Representative time-frequency spectrograms of three calls from the same rat in the normal (NAL), PD, and DBS conditions. Bandwidth (Fs) and duration (Ts) were calculated for each sound. Three call types were examined: low frequency calls included words with sounds between 20–35 kHz; high frequency calls included words with sounds between 35–100 kHz; and complex calls included all words with more than one sound in the 20–100 kHz range. Bandwidth (Fw) and duration (Tw) were also calculated for each word. B. Sounds per word, for each call type. Left: Complexity of words across the entire population (mean ±s.e.), quantified as the number of sounds per word. Word complexity was significantly reduced from normal to PD for all call types, and from PD to DBS for complex calls (over-bars, pHSD < 0.05). Right: Within-rat word complexity differences (median ±50% ±95% c.i.) from normal to PD (nal-pd) and from PD to DBS (pd-dbs). Word complexity was significantly higher in normal than PD, and in PD than DBS (asterisks, c95% > 0).
Statistical Compilation
Sound and word duration (Ts & Tw) and bandwidth (Fs & Fw) were calculated for all calls. Averages of those values, plus the mean number of calls per minute and the mean number of sounds per word, were found for each trial of each animal in each condition. The average values of each of those measures in a given trial were taken as a population for statistical analysis. All animals included in the analysis were recorded during at least seven trials in each condition (mean 12.9 ± std 4.6). Population analyses included recordings from five rats in normal (NAL), five rats in PD, and three rats in DBS conditions. Population changes are denoted with over-bars when a one-way ANOVA confirmed significant changes between group means that were subsequently verified with Tukey's honest significant difference test at the α = 0.05 level (i.e., pHSD < 0.05); detailed statistics for Tukey tests are listed in Table 1. Within-rat analyses include five rats in the NAL-to-PD transition, and three rats in the PD-to-DBS transition. Within-rat differences are denoted with an asterisk when the bootstrapped 95% confidence interval of the change between conditions did not include the zero value (i.e., c95% > 0 or c95% < 0).
Table 1.
Tukey's Honest Significant Difference Statistics.
| Low Band | High Band | Complex Band | ||||
|---|---|---|---|---|---|---|
| q | p | q | p | q | p | |
| Fig. 2B | panova = 3×10−7 | panova = 3×10−4 | panova = 1×10−4 | |||
| nal-vs-pd | 3.49 | 4×10−4 | 2.26 | 0.013 | 2.33 | 0.011 |
| pd-vs-dbs | 1.38 | 0.086 | 1.60 | 0.058 | 1.74 | 0.044 |
| nal-vs-dbs | 4.12 | 7×10−5 | 3.33 | 8×10−4 | 3.53 | 5×10−4 |
| Fig. 3B, top | panova = 1×10−7 | panova = 6×10− | panova = 1×10− | |||
| nal-vs-pd | 3.42 | 5×10−4 | 4.43 | 2×10−5 | 4.47 | 2×10−5 |
| pd-vs-dbs | 1.67 | 0.051 | 2.36 | 0.011 | 2.07 | 0.022 |
| nal-vs-dbs | 4.34 | 4×10−5 | 5.81 | 2×10−7 | 5.56 | 6×10−7 |
| Fig. 3B, bot | panova = 7×10−8 | panova = 2×10− | panova = 2×10− | |||
| nal-vs-pd | 3.52 | 4×10−4 | 4.05 | 7×10−5 | 3.96 | 9×10−5 |
| pd-vs-dbs | 1.60 | 0.058 | 2.14 | 0.018 | 1.96 | 0.028 |
| nal-vs-dbs | 4.35 | 4×10−5 | 5.29 | 1×10−6 | 5.04 | 4×10−6 |
| Fig. 4A, top | panova = 8×10−6 | panova = 7×10−5 | panova = 3×10−5 | |||
| nal-vs-pd | 2.99 | 0.002 | 2.52 | 0.007 | 2.19 | 0.016 |
| pd-vs-dbs | 1.46 | 0.075 | 1.59 | 0.059 | 2.27 | 0.014 |
| nal-vs-dbs | 3.79 | 2×10−4 | 3.54 | 5×10−4 | 3.91 | 1×10−4 |
| Fig. 4A, bot | panova = 0.009 | panova = 0.229 | panova = 0.118 | |||
| nal-vs-pd | 1.67 | 0.050 | 1.15 | 0.128 | 0.53 | 0.298 |
| pd-vs-dbs | 1.31 | 0.098 | 0.43 | 0.333 | 1.50 | 0.070 |
| nal-vs-dbs | 2.59 | 0.006 | 1.34 | 0.094 | 1.85 | 0.035 |
| Fig. 4B, top | panova = 1×10−4 | panova = 0.159 | panova = 0.751 | |||
| nal-vs-pd | 1.77 | 0.041 | 1.49 | 0.071 | 0.43 | 0.333 |
| pd-vs-dbs | 2.41 | 0.010 | 1.24 | 0.111 | 0.68 | 0.250 |
| nal-vs-dbs | 3.70 | 3×10−4 | 0.03 | 0.487 | 0.29 | 0.387 |
| Fig. 4B, bot | panova = 4×10−7 | panova = 0.001 | panova = 6×10−4 | |||
| nal-vs-pd | 2.76 | 0.004 | 0.67 | 0.252 | 1.42 | 0.080 |
| pd-vs-dbs | 2.38 | 0.010 | 2.81 | 0.003 | 2.37 | 0.011 |
| nal-vs-dbs | 4.48 | 2×10−5 | 3.19 | 0.001 | 3.39 | 7×10−4 |
Sample sizes for comparisons: Nnal=76, Npd=65, Ndbs=27.
Crossed out values were not significant at the 0.05 level.
Results
Eight male rats were implanted with a cannula to the right MFB and a 4-channel stimulating electrode array to the right subthalamic nucleus. For 5 min trials, each rat was placed in the bottom of a pair of stacked behavioral chambers, with an estrous female in the top chamber (Fig. 1A). Mating calls were recorded from an ultrasonic microphone in the bottom chamber with the male rat (Fig. 1B). Following sufficient healthy recordings, the neurotoxin 6-OHDA was injected through the cannula to the MFB to induce parkinsonism. Five male rats survived surgical implantation and healthy recordings, and had developed parkinsonian by two weeks following 6-OHDA injection. Of those five, three were placed in experimental group and recorded from with and without subthalamic DBS in separate trials. The other two were placed in the control group and never received DBS.
Vocalization Prevalence
Sounds recorded from an ultrasonic microphone between 20 kHz and 100 kHz were filtered and isolated from background noise as described above (Fig 1C). A word was defined as a vocalization string that comprised one or multiple sounds that were temporally isolated by at least 250 ms from other sounds (Fig. 1D) of the same call type: high frequency (35–100 kHz), low frequency (20–35 kHz), and complex for words with at least 2 sounds total in the 20–100 kHz range (Fig 2A).
Word complexity, defined as the average number of sounds per word (Fig. 2B), varied as a function of condition for each call type: low, F(2,154) = 16.5, p = 3×10−7; high, F(2,159) = 8.57, p= 3×10−4; complex, F(2,155) = 9.58, p = 1×10−7. Average word complexity was significantly reduced in the PD condition from the healthy condition for all call types. Similarly, within-rat differences between the healthy and the PD condition were greater than zero for all call types, meaning that rat vocalizations were significantly more complex before 6- OHDA injection than after. Across the population, word complexity was further reduced in the DBS condition for complex calls. Within-rat differences between the PD and DBS conditions were highly significant for all call types: parkinsonian rat vocalizations were significantly more complex without DBS than with it. In summary, the number of sounds per word was reduced by parkinsonian onset, and further reduced by DBS.
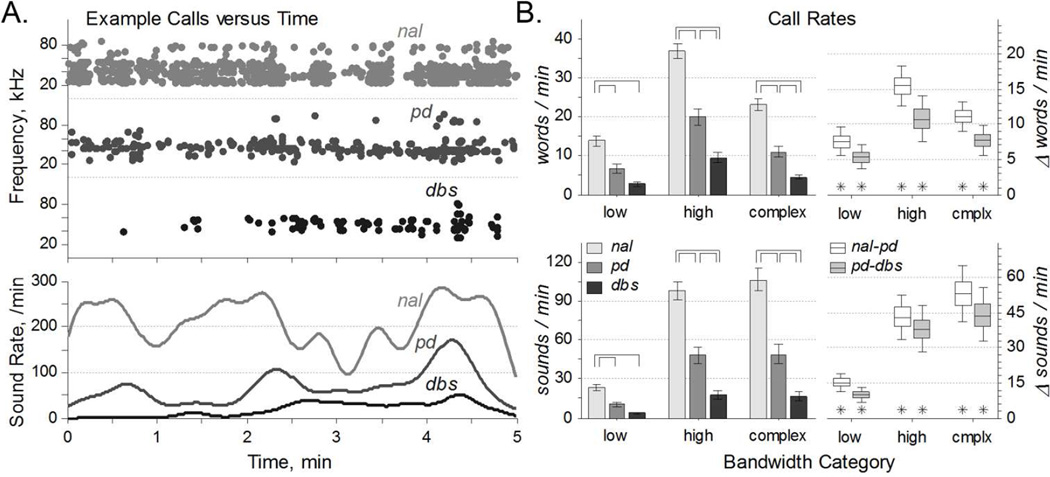
Call rates – in words per minute and sounds per minute – were computed for each condition and call type. Example data, all from the same animal, illustrate the statistically significant changes between conditions. Word rate varied as a function of condition for all call types: low, F(2,165) = 17.7, p = 1×10−7; high, F(2,165) = 36.9, p= 5×10−14; complex, F(2,165) = 34.3, p = 3×10−13. Sound rate also varied as a function of condition for all call types: low, F(2,165) = 18.2, p = 7×10−8; high, F(2,165) = 28.5, p= 2×10−11; complex, F(2,165) = 25.7, p = 2×10−10. Across the population, both word rate and sound rate were reduced from the normal to PD condition. Similarly, within-rat differences between the normal and PD conditions were always greater than zero, meaning that rats had higher call rates before 6-OHDA injection than after. Further, DBS reduced both word and sound rates from the PD condition, though the population decrease trend for low frequency calls did not reach statistical significance. However, within-rat differences between the PD and DBS conditions were all significantly greater than zero: parkinsonian rats had higher call rates without DBS, for all call types. In summary, DBS exacerbated all the reductions of word rates and sound rates associated with parkinsonian onset.
Vocalization Dynamics
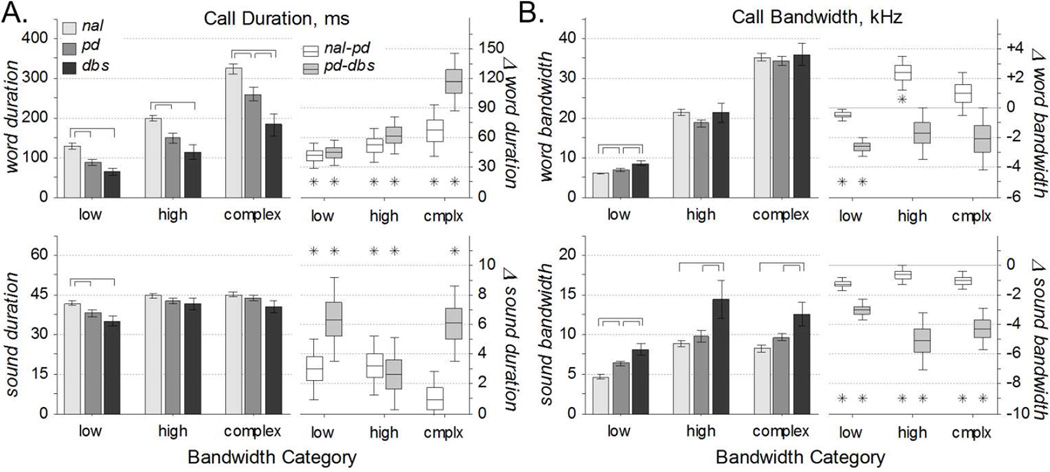
The time and frequency span of each word and sound were calculated for all call types (Fig. 2A). Note that these measures are independent of the call rate. Further, the time and frequency span of individual sounds (Ts and Fs, respectively) are independent of call complexity as defined above. However, the time and frequency span of individual words (Tw and Fw, respectively) do depend on the call complexity: words with fewer sounds are more likely to have smaller time and frequency spans.
Across the population, word duration (Tw) varied with condition for all call types: low, F(2,154) = 12.7, p = 8×10−6; high, F(2,159) = 10.1, p= 7×10−5; complex, F(2,155) = 11.3, p = 3×10−5. In particular, word duration was significantly reduced from the normal to PD condition (Fig. 4A, top). Within-rat differences supported that words were longer before 6-OHDA injection than after. Between groups, word duration was further reduced from the PD to DBS condition, though this universal trend was significant only for complex calls. However, within-rat differences between the PD and DBS conditions were significantly greater than zero for all call types, meaning that the average rat vocalized shorter words in the presence of DBS, for all call types. Thus, reductions in word durations brought on by parkinsonism were exacerbated by DBS.
Figure 4. Vocalization Statistics.
A. Call durations of words (top) and sounds (bottom) for all call types. Left: Population statistics (mean ±s.e.) reveal that word durations were decreased in the PD condition relative to control, and for complex calls further decreased in DBS relative to PD (over-bars, pHSD < 0.05). Sound durations were not largely affected by condition. Right: Within-rat comparisons (median ±50% ±95% c.i.) reveal that words and sounds were shorter in the PD condition relative to normal, and mostly shorter still in the DBS condition relative to PD (asterisks, c95% > 0). B. Frequency bandwidths of words (top) and sounds (bottom) for all call types. Left: Population statistics (mean ±s.e.) reveal that sound durations were increased in the DBS condition relative to PD and normal (over-bars, pHSD < 0.05). Word bandwidths were not largely affected by condition. Right: Within-rat comparisons (median ±50% ±95% c.i.) reveal that frequency bandwidth was less in the normal condition relative to PD, and less still in the PD condition relative to DBS (asterisks, c95% < 0). Changes in word bandwidth were less consistent.
Relative to the large changes in word duration, changes in sound duration (Ts) were proportionally much smaller (Fig. 4A, bottom) and less significant: low, F(2,154) = 4.82, p = 0.009; high, F(2,159) = 1.49, p= 0.229; complex, F(2,155) = 2.17, p = 0.118. Across the population, the only significant changes were that sounds in the low frequency range were significantly shorter following 6-OHDA injection with and without DBS than in the control condition. Despite these small changes, within-rat differences from the control to PD conditions revealed that sounds were significantly longer (~3 ms) for high and low frequency calls. Further, the within-rat differences were longer still (3–6 ms) from the PD to DBS conditions, for all call types. Thus, sound duration was decreased by parkinsonian onset, and decreased further by DBS, but the effects were too small and variable to manifest in the population measures.
Similarly, changes between conditions in word frequency span, or bandwidth (Fw), were small and mostly insignificant: low, F(2,154) = 9.64, p = 1×10−4; high, F(2,159) = 1.86, p= 0.159; complex, F(2,155) = 0.29, p = 0.751. The only population effects were slightly higher bandwidths of low frequency calls in the DBS condition relative to the PD condition, and in the PD condition relative to healthy. The within-rat analysis supported this finding, and added that the word bandwidth was slightly greater (~0.4 kHz) in the PD condition than in the healthy. In contrast to those low frequency results, high frequency word bandwidth was reduced in the PD condition relative to healthy. These conflicting results are clarified by quantifying the sound bandwidth.
In contrast to small changes in sound duration and muddled changes in word bandwidth, the bandwidth of individual sounds (Fs) varied substantially with condition (Fig. 4B, bottom): low, F(2,154) = 16.2, p = 4×10−7; high, F(2,159) = 7.04, p= 0.001; complex, F(2,155) = 7.81, p = 6×10−4. Across the population, the bandwidth of low frequency calls was greater in the PD condition than in the healthy condition. This change was more evident from the within-rat differences, where calls spanned greater bandwidth (~1 kHz) in the PD condition relative to control, for all call types. The larger population effect was that the bandwidth of each call type was increased in the DBS condition relative to PD. Further, within-rat differences show a significant bandwidth increase (3–5 kHz), supporting that increases in sound bandwidth associated with PD were exacerbated by DBS.
Discussion
High frequency stimulation of the subthalamic nucleus or globus pallidus internus can alleviate motor symptoms of PD, including tremor, rigidity, and bradykinesia (Limousin-Dowsey et al., 1999; Lyons and Pahwa, 2004). Effective DBS modifies neural firing activity within the basal ganglia (Benazzouz et al., 2000; Boraud et al., 1996; Da Cunha et al., 2015) and motor thalamus (Hershey et al., 2003). Regarding this neural activity however, DBS neither restores it to healthy-control levels (Anderson et al., 2003; Dorval and Grill, 2014; Hashimoto et al., 2003) nor modulates it in the same manner as dopamine-based drug therapies (Gilmour et al., 2011; Hilker et al., 2002; Hutchinson et al., 1997; Papa et al., 1999; Weick et al., 1990). Given their differential effects on neural activity, it is not surprising that electrical neuromodulation and medical therapy have disparate effects on various motor symptoms.
Despite decades of reports on hypokinetic dysarthria associated with PD, its origins are not fully understood (Kaplan et al., 1954; Logemann et al., 1978; Skodda, 2011). Further obscured are the mechanisms by which DBS may exacerbate dysarthria while simultaneously alleviating other parkinsonian motor symptoms (Burghaus et al., 2006; Romito et al., 2002; Umemura et al., 2011). The development of an animal model of these phenomena will facilitate the study of dysarthria in a high-throughput, patient-free manner, enabling new insights into both parkinsonian dysarthria and the maladaptive effects of DBS.
We built on a relatively recent rat model of parkinsonian dysarthria (Ciucci et al., 2009), and differences between our studies are worth mentioning. First, they identified vocalizations manually based on the quality of the acoustic signal, whereas we identified vocalizations algorithmically from z-score-transformed spectrograms. Second, they analyzed only 10% of the identified vocalizations, whereas we analyzed all vocalizations. Third, they categorized calls into simple, frequency modulated, and harmonic types, whereas we separated them by frequency band (low, high, and complex) and looked at single vocalizations separately from vocalization strings.
These differences may account for minor contrasting results between two studies. In the 6-OHDA condition relative to control, Ciucci and colleagues (2009) found that simple calls had smaller bandwidths, whereas we report that simple sounds had larger bandwidths, particularly in the 20–35 kHz range (Fig. 4B, bottom). That difference may be attributed to selection bias. More in line with the present findings, they reported that frequency-modulated calls had smaller bandwidths in the 6-OHDA condition, similar to our tentative finding that high-frequency words had smaller bandwidths in the 6-OHDA condition (Fig. 4B, top). However, DBS did not exacerbate that effect, but rather significantly increased sound-bandwidth in all frequency ranges, suggesting a potential DBS side-effect that is not merely an exacerbation of existing dysarthric symptoms.
In general however, the present findings confirm previous results associated with 6-OHDA-induced lesion (Ciucci et al., 2009). For example, they reported no significant change in vocalization duration with PD onset, but with a trend toward shorter calls. We report similarly small decreases in mean sound duration that were significant in within-animal paired tests but merely trends across the population (Fig. 4A, bottom). Finally, they report a reduction in the fraction of frequency-modulated calls, and we report a reduction in the number of sounds per vocalization string in all frequency ranges (Fig. 2B).
Although precise parallels between human speech and rat vocalizations are difficult to draw, many aspects of parkinsonian hypokinetic dysarthria are qualitatively similar to 6-OHDA-induced changes in the rats. Persons with PD can exhibit variable speech rates that slow down with disease progression (Martinez-Sanchez et al., 2015; Skodda et al., 2009). Rats in the PD condition produced many fewer vocalizations per unit time, for both individual sounds and more complex calls, and this symptom was exacerbated by DBS (Fig. 3). Persons with PD produce speech with less syntactic complexity by several measures (Illes et al., 1988; Walsh and Smith, 2011). Rats in the PD condition produced vocalization strings comprising fewer sounds, and this symptom was exacerbated by DBS (Fig. 2). Persons with PD produce words that are more variable in duration and shorter on average (Ackermann et al., 1997), though individual syllable duration may remain unchanged (Duez, 2006). Rats in the PD condition made shorter word calls on average, and this symptom was exacerbated by DBS (Fig. 4A, top), although the duration of individual sounds was largely unchanged (Fig. 4A, bottom). Together, these changes in vocalization constitute a reasonable quantification of parkinsonian hypokinetic dysarthria in a rat model.
Figure 3. Vocalization Rates.
A. Representative call data of sounds versus time from the same rat under each of the three conditions. Top: Each sound is depicted as a single dot at its center frequency. Bottom: Sound rates, calculated by convolving the events from above with a gaussian kernel with a 10 second standard deviation. Note the substantial variability in sound rate over each five minute interval. B. Call rates vary with condition. Left: Call rates (mean ±s.e.) of words (top) and sounds (bottom) were reduced from normal to PD, and typically reduced from PD to DBS conditions (over-bars, pHSD < 0.05). Right: Within-rat word and sound rate differences (median ±50% ±95% c.i.) revealed a higher call rates in normal relative to PD, and in PD relative to DBS (asterisks, c95% > 0).
One caveat of the model may be the increased bandwidth of single sounds in the DBS relative to PD conditions (Fig. 4B, bottom). Because human vocalizations are so much more intricate than those of rats, a reasonable human correlate of this measure is difficult to identify. Persons with PD produce vowel sounds that are less dynamic than their healthy counterparts. For example, reductions in vowel space area (Rusz et al., 2013) and articulatory-acoustic vowel space (Whitfield and Goberman, 2014) metrics are both associated with PD. However, by measuring simultaneous changes in two formants, those metrics evaluate two simultaneous sounds as defined in the present study, and are thus more analogous to word bandwidth. It may be that the increasing sound bandwidth is the rodent manifestation of speech slurring in human PD patients. Regardless, although sound bandwidths increase with PD and increase further with DBS, the decreased word complexity balances these effects to create only minimal (and generally insignificant) changes in word bandwidth (Fig. 4B, top).
While we did not measure electroneurographic or electromyographic activity to study the mechanisms of hypokinetic dysarthria, our model provides a framework for such studies. An obvious candidate for examination is the differential responses of neural activity to pharmacological versus neuromodulatory therapies. In particular, to ascertain how dopamine-based therapies alleviate dysarthria (De Letter et al., 2005, 2007a, 2007b), whereas DBS can worsen it (Burghaus et al., 2006; Romito et al., 2002; Umemura et al., 2011). Such studies might reveal changes in neural activity that would alleviate dysarthria and enable researchers to attempt appropriate neuromodulatory interventions in an ethical manner.
In this study, we aimed to develop a rodent model of DBS-exacerbated, parkinsonian hypokinetic dysarthria. However, dysarthria is not the only PD symptom poorly treated by DBS. For example, parkinsonian eyelid apraxia (Dewey and Maraganore, 1994; Yamada et al., 2004) has also been reported to worsen in response to DBS (Bologna et al., 2012; Shields et al., 2006; Tommasi et al., 2012). One possible hypothesis is that increased hypokinetic dysarthria and eyelid apraxia follow from stimulating the wrong target. The basal ganglia are somatotopically organized (Joint et al., 2013; Nambu, 2011), and the pathways responsible for orofacial and oculomotor control are likely specialized. Thus, stimulation of other basal nuclei or even other targets within subthalamic nucleus may be more therapeutic, as has been demonstrated in a few patients (Dietz et al., 2013; Fuss et al., 2004; Mikos et al., 2011). Hence, a useful future direction would involve DBS during vocalization protocols with subsequent histology to map which subregions of neural tissue are most responsible for dysarthria and potentially other side effects of, or symptoms exacerbated by, DBS.
In conclusion, deep brain stimulation of the subthalamic nucleus in the rodent 6-OHDA model of PD worsens parkinsonian symptoms related to hypokinetic dysarthria. Detrimental changes in vocalization complexity, vocalization rates, word durations, and individual sound bandwidths were all exacerbated by DBS. This study provides a framework for explorations into the electrophysiological mechanisms of parkinsonian hypokinetic dysarthria and its exacerbation by otherwise therapeutic DBS. Such explorations are essential to better understand, and henceforth minimize, this side effect of stimulation that greatly diminishes quality of life.
Time-frequency spectrogram depictions of male rat vocalizations while trying to attract the attention of an estrous female. The same rat made each of these calls when he was healthy, parkinsonian, and parkinsonian but receiving therapeutic deep brain stimulation. Shorter and less complex calls in the parkinsonian condition constitute a rodent model of parkinsonian dysarthria. By all measures explored in this paper, dysarthria was exacerbated by deep brain stimulation. 105×141mm (72 × 72 DPI)
Significance.
Parkinsonian hypokinetic dysarthria impairs quality of life, but with few animal models of parkinsonian speech the symptom has been difficult to study. In many cases, dysarthria is not alleviated and can be exacerbated by the increasingly popular therapy of deep brain stimulation. We present a rodent model of parkinsonian dysarthria that is worsened by deep brain stimulation, to provide a platform for future research.
Acknowledgments
Support Information: This work was supported by funding from the Utah Science, Technology, and Research Initiative, and by grants from: the National Science Foundation, CAREER 1351112 (A.D.D.); and the NIH, National Institute of Neurological Disorders and Stroke, K25 NS053544 (A.D.D.)
Footnotes
Conflicts of Interest
The authors declare no competing financial interests.
Author's Roles
N. King initiated the study, assembled the hardware configurations, contributed to the data collection software development, assisted in surgical implantations and neurotoxin injections, collected all behavioral data, and contributed to data analysis and manuscript preparation. C. Anderson performed all surgical implantations and neurotoxin injections, contributed to data collection software development, assisted in data collection and analysis, and was primarily responsible for manuscript preparation. A. Dorval secured funding for the study, contributed to data collection software development, guided data analysis, and oversaw manuscript preparation.
Literature Cited
- Ackermann H, Konczak J, Hertrich I. The Temporal Control of Repetitive Articulatory Movements in Parkinson’s Disease. Brain Lang. 1997;56:312–319. doi: 10.1006/brln.1997.1851. [DOI] [PubMed] [Google Scholar]
- Anderson CJ, Sheppard DT, Huynh R, Anderson DN, Polar CA, Dorval AD. Subthalamic deep brain stimulation reduces pathological information transmission to the thalamus in a rat model of parkinsonism. Front. Neural Circuits. 2015;9:31. doi: 10.3389/fncir.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J. Neurophysiol. 2003;89:1150–1160. doi: 10.1152/jn.00475.2002. [DOI] [PubMed] [Google Scholar]
- Barfield RJ, Auerbach P, Geyer LA, Mcintosh TK. Ultrasonic vocalizations in rat sexual behavior. Integr. Comp. Biol. 1979;19:469–480. [Google Scholar]
- Benazzouz A, Gao DM, Ni ZG, Piallat B, Bouali-Benazzouz R, Benabid AL. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience. 2000;99:289–295. doi: 10.1016/s0306-4522(00)00199-8. [DOI] [PubMed] [Google Scholar]
- De Bie RMA, de Haan RJ, Schuurman PR, Esselink RaJ, Bosch DA, Speelman JD. Morbidity and mortality following pallidotomy in Parkinson’s disease: a systematic review. Neurology. 2002;58:1008–1012. doi: 10.1212/wnl.58.7.1008. [DOI] [PubMed] [Google Scholar]
- Bologna M, Fasano A, Modugno N, Fabbrini G, Berardelli A. Effects of subthalamic nucleus deep brain stimulation and L-DOPA on blinking in Parkinson’s disease. Exp. Neurol. 2012;235:265–272. doi: 10.1016/j.expneurol.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci. Lett. 1996;215:17–20. doi: 10.1016/s0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav. Genet. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr. Opin. Neurobiol. 2013;23:310–317. doi: 10.1016/j.conb.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Burghaus L, Hilker R, Thiel A, Galldiks N, Lehnhardt FG, Zaro-Weber O, Sturm V, Heiss W-D. Deep brain stimulation of the subthalamic nucleus reversibly deteriorates stuttering in advanced Parkinson’s disease. J. Neural Transm. Vienna Austria 1996. 2006;113:625–631. doi: 10.1007/s00702-005-0341-1. [DOI] [PubMed] [Google Scholar]
- Caballol N, Martí MJ, Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Mov. Disord. 2007;22:S358–S366. doi: 10.1002/mds.21677. [DOI] [PubMed] [Google Scholar]
- Castall B, Marsden CD, Naylor RJ, Pycock CJ. Stereotyped behaviour patterns and hyperactivity induced by amphetamine and apomorphine after discrete 6-hydroxydopamine lesions of extrapyramidal and mesolimbic nuclei. Brain Res. 1977;123:89–111. doi: 10.1016/0006-8993(77)90645-x. [DOI] [PubMed] [Google Scholar]
- Chang J-Y, Shi L-H, Luo F, Woodward DJ. High frequency stimulation of the subthalamic nucleus improves treadmill locomotion in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 2003;983:174–184. doi: 10.1016/s0006-8993(03)03053-1. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Kane JR, Ahrens AM, Schallert T. Limb use and complex ultrasonic vocalization in a rat model of Parkinson’s disease: deficit-targeted training. Parkinsonism Relat. Disord. 2008;14(Suppl 2):S172–S175. doi: 10.1016/j.parkreldis.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: degradation of 50-kHz ultrasonic vocalization in rats. Behav. Neurosci. 2009;123:328–336. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cunha C, Boschen SL, Gómez-A A, Ross EK, Gibson WSJ, Min H-K, Lee KH, Blaha CD. Toward sophisticated basal ganglia neuromodulation: Review on basal ganglia deep brain stimulation. Neurosci. Biobehav. Rev. 2015 doi: 10.1016/j.neubiorev.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey RB, Maraganore DM. Isolated eyelid-opening apraxia: report of a new levodoparesponsive syndrome. Neurology. 1994;44:1752–1754. doi: 10.1212/wnl.44.9.1752. [DOI] [PubMed] [Google Scholar]
- Dietz J, Noecker AM, McIntyre CC, Mikos A, Bowers D, Foote KD, Okun MS. Stimulation region within the globus pallidus does not affect verbal fluency performance. Brain Stimulat. 2013;6:248–253. doi: 10.1016/j.brs.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval AD, Grill WM. Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. J. Neurophysiol. 2014;111:1949–1959. doi: 10.1152/jn.00713.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez D. Syllable structure, syllable duration and final lengthening in Parkinsonian French speech. J. Multiling. Commun. Disord. 2006;4:45–57. [Google Scholar]
- Fibiger HC, Pudritz RE, McGeer PL, McGeer EG. Axonal transport in nigro-striatal and nigrothalamic neurons: effects of medial forebrain bundle lesions and 6-hydroxydopamine. J. Neurochem. 1972;19:1697–1708. doi: 10.1111/j.1471-4159.1972.tb06214.x. [DOI] [PubMed] [Google Scholar]
- Fuss G, Spiegel J, Magnus T, Moringlane JR, Becker G, Dillmann U. Improvement of apraxia of lid opening by STN-stimulation in a 70-year-old patient with Parkinson’s disease. A case report. Minim. Invasive Neurosurg. MIN. 2004;47:58–60. doi: 10.1055/s-2003-812466. [DOI] [PubMed] [Google Scholar]
- Gilmour TP, Piallat B, Lieu CA, Venkiteswaran K, Ramachandra R, Rao AN, Petticoffer AC, Berk MA, Subramanian T. The effect of striatal dopaminergic grafts on the neuronal activity in the substantia nigra pars reticulata and subthalamic nucleus in hemiparkinsonian rats. Brain J. Neurol. 2011;134:3276–3289. doi: 10.1093/brain/awr226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Thiel A, Ghaemi M, Herholz K, Sturm V, Heiss W-D. Deep brain stimulation of the subthalamic nucleus versus levodopa challenge in Parkinson’s disease: measuring the on- and off-conditions with FDG-PET. J. Neural Transm. Vienna Austria 1996. 2002;109:1257–1264. doi: 10.1007/s00702-002-0696-5. [DOI] [PubMed] [Google Scholar]
- Hutchinson WD, Levy R, Dostrovsky JO, Lozano AM, Lang AE. Effects of apomorphine on globus pallidus neurons in parkinsonian patients. Ann. Neurol. 1997;42:767–775. doi: 10.1002/ana.410420513. [DOI] [PubMed] [Google Scholar]
- Illes J, Metter EJ, Hanson WR, Iritani S. Language production in Parkinson’s disease: acoustic and linguistic considerations. Brain Lang. 1988;33:146–160. doi: 10.1016/0093-934x(88)90059-4. [DOI] [PubMed] [Google Scholar]
- Intemann PM, Masterman D, Subramanian I, DeSalles A, Behnke E, Frysinger R, Bronstein JM. Staged bilateral pallidotomy for treatment of Parkinson disease. J. Neurosurg. 2001;94:437–444. doi: 10.3171/jns.2001.94.3.0437. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Johnson AM, Doll EJ, Grant LM, Ringel L, Shier JN, Ciucci MR. Targeted training of ultrasonic vocalizations in aged and Parkinsonian rats. J. Vis. Exp. JoVE. 2011 doi: 10.3791/2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint C, Thevathasan W, Green AL, Aziz T. Pallidal somatotopy suggested by deep brain stimulation in a patient with dystonia. Neurology. 2013;80:685–686. doi: 10.1212/WNL.0b013e318281cbfe. [DOI] [PubMed] [Google Scholar]
- Kaplan HA, Machover S, Rabiner A. A study of the effectiveness of drug therapy in parkinsonism. J. Nerv. Ment. Dis. 1954;119:398–411. doi: 10.1097/00005053-195405000-00003. [DOI] [PubMed] [Google Scholar]
- Kim R, Alterman R, Kelly PJ, Fazzini E, Eidelberg D, Beric A, Sterio D. Efficacy of bilateral pallidotomy. Neurosurg. Focus. 1997;2:e8. doi: 10.3171/foc.1997.2.6.9. [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, Van Borsel J. The effects of levodopa on word intelligibility in Parkinson’s disease. J. Commun. Disord. 2005;38:187–196. doi: 10.1016/j.jcomdis.2004.09.001. [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, De Bodt M, Van Maele G, Van Borsel J, Boon P. The effect of levodopa on respiration and word intelligibility in people with advanced Parkinson’s disease. Clin. Neurol. Neurosurg. 2007a;109:495–500. doi: 10.1016/j.clineuro.2007.04.003. [DOI] [PubMed] [Google Scholar]
- De Letter M, Santens P, Estercam I, Van Maele G, De Bodt M, Boon P, Van Borsel J. Levodopa-induced modifications of prosody and comprehensibility in advanced Parkinson’s disease as perceived by professional listeners. Clin. Linguist. Phon. 2007b;21:783–791. doi: 10.1080/02699200701538181. [DOI] [PubMed] [Google Scholar]
- Limousin-Dowsey P, Pollak P, Van Blercom N, Krack P, Benazzouz A, Benabid A. Thalamic, subthalamic nucleus and internal pallidum stimulation in Parkinson’s disease. J. Neurol. 1999;246(Suppl 2):II42–II45. doi: 10.1007/BF03161080. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Blanchard DC, Blanchard RJ. Rat 22kHz ultrasonic vocalizations as alarm cries. Behav. Brain Res. 2007;182:166–172. doi: 10.1016/j.bbr.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and Cooccurrence of Vocal Tract Dysfunctions in the Speech of a Large Sample of Parkinson Patients. J. Speech Hear. Disord. 1978;43:47. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- Lyons KE, Pahwa R. Deep brain stimulation in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2004;4:290–295. doi: 10.1007/s11910-004-0054-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Sánchez F, Meilán JJG, Carro J, Gómez Íñiguez C, Millian-Morell L, Pujante Valverde IM, López-Alburquerque T, López DE. Speech rate in Parkinson’s disease: A controlled study. Neurol. Barc. Spain. 2015 doi: 10.1016/j.nrl.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Mikos A, Bowers D, Noecker AM, McIntyre CC, Won M, Chaturvedi A, Foote KD, Okun MS. Patient-specific analysis of the relationship between the volume of tissue activated during DBS and verbal fluency. NeuroImage. 2011;54:S238–S246. doi: 10.1016/j.neuroimage.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A. Somatotopic organization of the primate Basal Ganglia. Front. Neuroanat. 2011;5:26. doi: 10.3389/fnana.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa SM, Desimone R, Fiorani M, Oldfield EH. Internal globus pallidus discharge is nearly suppressed during levodopa-induced dyskinesias. Ann. Neurol. 1999;46:732–738. doi: 10.1002/1531-8249(199911)46:5<732::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: Compact. Sixth Edition. Academic Press; 2008. [Google Scholar]
- Pieri M, Pieri L, Saner A, Da Prada M, Haefely W. A comparison of drug-induced rotation in rats lesioned in the medial forebrain bundle with 5,6-dihydroxytryptamine or 6-hydroxydopamine. Arch. Int. Pharmacodyn. Thérapie. 1975;217:118–130. [PubMed] [Google Scholar]
- Romito LMA, Scerrati M, Contarino MF, Bentivoglio AR, Tonali P, Albanese A. Long-term follow up of subthalamic nucleus stimulation in Parkinson’s disease. Neurology. 2002;58:1546–1550. doi: 10.1212/wnl.58.10.1546. [DOI] [PubMed] [Google Scholar]
- Rusz J, Cmejla R, Tykalova T, Ruzickova H, Klempir J, Majerova V, Picmausova J, Roth J, Ruzicka E. Imprecise vowel articulation as a potential early marker of Parkinson’s disease: effect of speaking task. J. Acoust. Soc. Am. 2013;134:2171–2181. doi: 10.1121/1.4816541. [DOI] [PubMed] [Google Scholar]
- Sales GD. Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. J Zool. 1972;168:149–164. [Google Scholar]
- Seffer D, Schwarting RKW, Wöhr M. Pro-social ultrasonic communication in rats: Insights from playback studies. J. Neurosci. Methods. 2014;234:73–81. doi: 10.1016/j.jneumeth.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Shields DC, Lam S, Gorgulho A, Emerson J, Krahl SE, Malkasian D, DeSalles AaF. Eyelid apraxia associated with subthalamic nucleus deep brain stimulation. Neurology. 2006;66:1451–1452. doi: 10.1212/01.wnl.0000210693.13093.c8. [DOI] [PubMed] [Google Scholar]
- Skodda S. Aspects of speech rate and regularity in Parkinson’s disease. J. Neurol. Sci. 2011;310:231–236. doi: 10.1016/j.jns.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Skodda S, Rinsche H, Schlegel U. Progression of dysprosody in Parkinson’s disease over time--a longitudinal study. Mov. Disord. Off. J. Mov. Disord. Soc. 2009;24:716–722. doi: 10.1002/mds.22430. [DOI] [PubMed] [Google Scholar]
- Tommasi G, Krack P, Fraix V, Pollak P. Effects of varying subthalamic nucleus stimulation on apraxia of lid opening in Parkinson’s disease. J. Neurol. 2012;259:1944–1950. doi: 10.1007/s00415-012-6447-0. [DOI] [PubMed] [Google Scholar]
- Tröster AI, Woods SP, Fields JA. Verbal fluency declines after pallidotomy: an interaction between task and lesion laterality. Appl. Neuropsychol. 2003;10:69–75. doi: 10.1207/S15324826AN1002_02. [DOI] [PubMed] [Google Scholar]
- Umemura A, Oka Y, Yamamoto K, Okita K, Matsukawa N, Yamada K. Complications of Subthalamic Nucleus Stimulation in Parkinson’s Disease. Neurol. Med. Chir. (Tokyo) 2011;51:749–755. doi: 10.2176/nmc.51.749. [DOI] [PubMed] [Google Scholar]
- Walsh B, Smith A. Linguistic complexity, speech production, and comprehension in Parkinson’s disease: behavioral and physiological indices. J. Speech Lang. Hear. Res. JSLHR. 2011;54:787–802. doi: 10.1044/1092-4388(2010/09-0085). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick BG, Engber TM, Susel Z, Chase TN, Walters JR. Responses of substantia nigra pars reticulata neurons to GABA and SKF 38393 in 6-hydroxydopamine-lesioned rats are differentially affected by continuous and intermittent levodopa administration. Brain Res. 1990;523:16–22. doi: 10.1016/0006-8993(90)91631-p. [DOI] [PubMed] [Google Scholar]
- Wertheimer J, Gottuso AY, Nuno M, Walton C, Duboille A, Tuchman M, Ramig L. The impact of STN deep brain stimulation on speech in individuals with Parkinson’s disease: the patient’s perspective. Parkinsonism Relat. Disord. 2014;20:1065–1070. doi: 10.1016/j.parkreldis.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Whitfield JA, Goberman AM. Articulatory-acoustic vowel space: application to clear speech in individuals with Parkinson’s disease. J. Commun. Disord. 2014;51:19–28. doi: 10.1016/j.jcomdis.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Willadsen M, Seffer D, Schwarting RKW, Wöhr M. Rodent ultrasonic communication: Male prosocial 50-khz ultrasonic vocalizations elicit social approach behavior in female rats (rattus norvegicus) J. Comp. Psychol. 2014;128:56–64. doi: 10.1037/a0034778. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Schwarting RKW. Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013;354:81–97. doi: 10.1007/s00441-013-1607-9. [DOI] [PubMed] [Google Scholar]
- Yamada S, Matsuo K, Hirayama M, Sobue G. The effects of levodopa on apraxia of lid opening A case report. Neurology. 2004;62:830–831. doi: 10.1212/01.wnl.0000113751.19247.84. [DOI] [PubMed] [Google Scholar]