Abstract
The enhancement of GABAergic and monoaminergic neurotransmission has been the mainstay of pharmacotherapy and the focus of drug-discovery for anxiety and depressive disorders for several decades. However, the significant limitations of drugs used for these disorders underscores the need for novel therapeutic targets. Neuronal nicotinic acetylcholine receptors (nAChRs) may represent one such target. For example, mecamylamine, a non-competitive antagonist of nAChRs, displays positive effects in preclinical tests for anxiolytic and antidepressant activity in rodents. In addition, nicotine elicits similar effects in rodent models, possibly by receptor desensitization. Previous studies (Xiao et al., 2001) have identified two metabolites of methadone, EMDP (2-ethyl-5-methyl-3,3-diphenyl-1-pyrroline) and EDDP (2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine), which are considered to be inactive at opiate receptors, as relatively potent noncompetitive channel blockers of rat α3β4 nAChRs. Here, we show that these compounds are likewise highly effective blockers of human α3β4 and α4β2 nAChRs. Moreover, we show that they display relatively low affinity for opiate binding sites labeled by [3H]-naloxone. We then evaluated these compounds in rats and mice in preclinical behavioral models predictive of potential anxiolytic and antidepressant efficacy. We found that EMDP, but not EDDP, displayed robust effects predictive of anxiolytic and antidepressant efficacy without significant effects on locomotor activity. Moreover, EMDP at behaviorally active doses, unlike mecamylamine, did not produce eyelid ptosis, suggesting it may produce fewer autonomic side effects than mecamylamine. Thus, the methadone metabolite EMDP may represent a novel therapeutic avenue for the treatment of some affective disorders.
Keywords: anxiety, depression, nicotinic acetylcholine receptor, behavior, rodent, opiate
1. Introduction
Anxiety and depression are the two most common mental health disorders, affecting ~25% of the US population in any given year (Kessler et al., 2005). While the treatment of affective disorders has improved substantially since the introduction of drugs such as selective monoaminergic reuptake inhibitors, one-third of individuals with these disorders remain treatment resistant (Trivedi et al., 2006). Furthermore, adverse side effects of these drugs limit their use in some patients. These issues with conventional pharmacotherapy for affective disorders underscore the need for drugs aimed at new targets for the treatment of anxiety and depression. Neuronal nicotinic acetylcholine receptors (nAChRs) may be one such target because they potentially have widespread influence on CNS functions of neurotransmitters.
Anxiety and depression are both highly co-morbid with nicotine dependence (Glassman et al., 1990; Paperwalla et al., 2004; Bertrand, 2005; Zvolensky et al., 2008; Mineur and Picciotto, 2010), suggesting that nicotine use may in part be an attempt at self-medication in these conditions. Indeed, nicotine produces effects consistent with anxiolytic (File et al., 1998; Turner et al., 2010, 2011; McGranahan et al., 2011; Anderson and Brunzell, 2012; Hussmann et al., 2014) and antidepressant activity (Tizabi et al., 1999; Ferguson et al., 2000; Vázquez-Palacios et al., 2005) in preclinical rodent models. Nicotine also increases the rate of adult neurogenesis (Mudò et al., 2007; Belluardo et al., 2008), a proposed biomarker of antidepressant efficacy common to clinically used antidepressant drugs (Eisch and Petrik, 2012).
nAChRs are pentameric ligand-gated ion channels comprised from nine α and three β subunits, which are widely expressed throughout the nervous system. While peripheral nAChRs have a direct, fast excitatory signaling role at ganglia, in the brain these receptors are frequently associated with modulation of the release of several neurotransmitters, including GABA (Lena et al., 1993; Lu et al., 1998; McClure-Begley et al., 2014), norepinephrine (Clarke and Reuben, 1996; Kulak et al., 2001; Leslie et al., 2002; Grille et al., 2005), dopamine (Rowell et al., 1987; Rapier et al., 1988; Grady et al., 1992; Nissell et al., 1994), and acetylcholine (Marchi and Raiteri, 1996; Grady et al., 2001; Grille et al., 2005). These effects, together with their potential to activate intracellular signaling mechanisms, can induce long-lasting changes in neuroplasticity (McKay et al., 2007).
Thus, nAChRs have the potential to modulate multiple brain pathways, including those involved in complex behaviors and affect; they are therefore attractive potential therapeutic targets. Indeed, nAChR ligands have recently been examined as potential antidepressant and/or anxiolytic compounds (Picciotto et al., 2002; Mineur et al., 2007, 2009, 2011; Rollema et al., 2009; Turner et al., 2010, 2011; Caldarone et al., 2011; Anderson and Brunzell, 2012). Interestingly, mecamylamine, a noncompetitive blocker of nAChR channels, also displays positive effects in preclinical tests for anxiolytic and antidepressant activity in rodents (Rabenstein et al., 2006; Lippiello et al., 2008; Nickell et al., 2013), and it was initially reported to have antidepressant and anxiolytic effects in preliminary human trials (Shytle et al., 2002; Bacher et al., 2009; but see Ledford, 2011). While seemingly paradoxical, both nicotine (and other nicotinic agonists) as well as nicotinic antagonists (such as mecamylamine) appears to exert anxiolytic/antidepressant effects. This may be explained by activation of GABAergic, noradrenergic, or serotonergic neurotransmission by agonists, or by disinhibition of these systems by antagonists. We favor the latter explanation, as most agonists of nAChRs induce rapid and long-lasting receptor desensitization.
Two compounds of interest are the methadone primary N-demethylated metabolite, EDDP, and its trace secondary N-demethylated derivative, EMDP. Both compounds are believed to be inactive at opiate receptors (Pohland et al., 1971), but both potently block rat α3β4 nAChR channels (Xiao et al., 2001). The effects of EDDP and EMDP on affective behaviors in vivo are unknown. Therefore, to evaluate whether EDDP and/or EMDP exert anxiolytic-like and/or antidepressant-like activities in vivo, we tested their efficacy in preclinical paradigms sensitive to anxiolytic or antidepressant drugs. We found that EMDP and, to a lesser degree, EDDP displayed anxiolytic-like activity. Furthermore, EMDP also displayed antidepressant-like activity. Interestingly, eyelid ptosis, an indicator of ganglionic side effects, was present following behaviorally active doses of mecamylamine, but not EMDP, suggesting that EMDP may produce fewer autonomic side effects compared to mecamylamine.
2. Materials and Methods
2.1 Stable cell lines and 86Rb+ efflux Assay
The cell lines expressing human α4β2 (YXα4β2H1) and α3β4 (YXα3β4H1) nAChRs were established recently (Xiao et al., 2012). These cell lines were maintained in minimum essential medium (MEM) supplemented with 10% fetal bovine serum, 100 units/ml penicillin G, 100 mg/ml streptomycin and selective antibiotics at 37°C with 5% CO2 in a humidified incubator. Tissue culture medium and antibiotics were obtained from Invitrogen Corporation (Carlsbad, CA), unless otherwise stated. Fetal bovine serum was obtained from Gemini Bio-Products (Woodland, CA).
Functional properties of compounds at nAChRs expressed in the transfected cells were measured using 86Rb+ efflux assays as described previously (Xiao et al., 1998, 2001). In brief, cells were plated into 24-well plates coated with poly-D-lysine. The plated cells were grown at 37° C for 18 to 24 hour to reach 85 - 95% confluence. The cells were then incubated in growth medium (0.5 ml/well) containing 86Rb+ (2 μCi/ml) for 4 hour at 37° C. The loading mixture was then aspirated, and the cells were washed four times with 1 ml buffer (15 mM HEPES, 140 mM NaCl, 2 mM KCl, 1 mM MgSO4, 1.8 mM CaCl2, 11 mM Glucose, pH 7.4). One ml of buffer with or without compounds to be tested was then added to each well. After incubation for 2 min, the assay buffer was collected for measurements of 86Rb+ released from the cells. Cells were then lysed by adding 1 ml of 100 mM NaOH to each well, and the lysate was collected for determination of the amount of 86Rb+ that was in the cells at the end of the efflux assay. Radioactivity of assay samples and lysates was measured by liquid scintillation counting. Total loading (cpm) was calculated as the sum of the assay sample and the lysate of each well, and the amount of 86Rb+ efflux was expressed as a percentage of 86Rb+ loaded. Stimulated 86Rb+ efflux was defined as the difference between efflux in the presence and absence of nicotine. To obtain IC50 values, inhibition curves were constructed in which increasing concentrations of a compound were included in the assay to inhibit efflux stimulated by 100 μM nicotine. IC50 values were determined by nonlinear least-squares regression analyses (GraphPad, San Diego, CA).
2.2 [3H]-Naloxone binding assay
Rat forebrain (anterior to the colliculi) was weighed, suspended in cold 50 mM Tris-HCl buffer (pH 7.4) containing 100 mM NaCl and homogenized with a polytron homogenizer. The homogenate was centrifuged at 35,000 × g for 10 min, the pellet was resuspended in fresh buffer, centrifuged again, and the final pellet resuspended in buffer. Aliquots equivalent to 10 mg of original tissue weight (~500 μg protein) were added to tubes containing 2 nM [3H]-naloxone (53.7 Ci/mmol; Perkin Elmer, Inc. Boston, MA) and a range of concentrations of methadone, EMDP or EDDP in a final volume of 1 ml. The tubes were incubated for 1 h at 24°C and then filtered over GF/C filters pre-wet with polyethyleneimine and mounted on a Brandel cell harvester. The filters were washed 3 times with buffer, placed in vials and then counted in a Beckman scintillation counter. Non-specific binding was measured in the presence of 10 μM unlabeled naloxone or 100 μM methadone (which gave similar values). Specific binding was defined as the difference between total and nonspecific binding. Competition curves for each added drug were analyzed with GraphPad Prism 5 (GraphPad Software, Inc. San Diego, CA).
2.3 Rats
Adult, male Sprague-Dawley rats (225-300 g at the start of the study; Harlan Labs) were housed two per cage in a temperature-controlled vivarium (22°C) at Georgetown University Medical Center and maintained on a standard 12-hr, light–dark cycle (lights on from 6 a.m. – 6 p.m.), with food and water available ad libitum. All experimental manipulations were performed during the light phase. All procedures were completed with approval from the Georgetown University Animal Care and Use Committee and in accordance with AALAC recommendations and the Guide for Care and Use of Laboratory Animals (National Research Council (U.S.) et al., 2011). Behavioral tests were conducted in separate groups of rats.
2.4 Mice
Adult male 129SvEv;C57Bl/6J F1 hybrid mice (7-10 weeks of age; 25-35g; Taconic) were group-housed (5/cage) and maintained on a 12h light/dark cycle with food and water available ad libitum. This mouse strain was selected as it has previously been used for examining anxiety and depression-like behaviors in mice (e.g., Turner et al., 2010, 2011, 2013; Hussmann et al., 2014). All experimental testing sessions were conducted between 9:00 A.M. and 3:00 P.M., with animals randomly assigned to treatment conditions and tested in counterbalanced order. All procedures with mice were completed with approval from the University of Pennsylvania Animal Care and Use Committee.
2.5 Drugs and Treatments
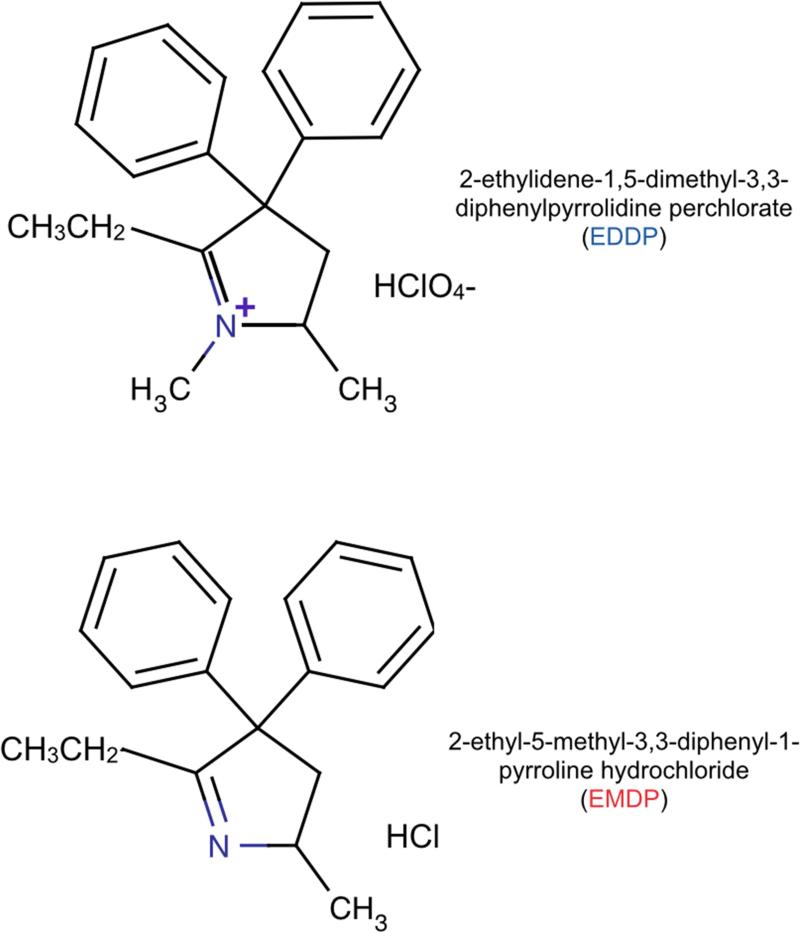
The following compounds were generously provided by Research Triangle Institute (Research Triangle Park, NC) through the National Institute on Drug Abuse: (+)-EDDP, (−)-EDDP and racemic EDDP [2-ethylidene-1,5,dimethyl-3,3-diphenylpyrrolidine perchlorate], and (+)-EMDP, (−)-EMDP and racemic EMDP [2-ethyl-5-methyl-3,3,-diphenyl-1-pyrroline hydrochloride], The structures of EDDP and EMDP are shown in Figure 1. Note that EDDP carries a positive charge.
Figure 1. Chemical structures of EDDP and EMDP.
Note that the structural difference between the two compounds is a single methyl group and the charge on the associated quaternary nitrogen.
Mecamylamine HCl, sertraline HCl, chlordiazepoxide HCl (CDP) and (−)-nicotine hydrogen tartrate were purchased from Sigma-Aldrich (St. Louis, MO).
All drug doses in animal studies are expressed as milligrams per kilogram of the salt form. Racemic EMDP and EDDP were used for behavioral studies. For rat studies, drugs were dissolved in 1:1 DMSO:saline and administered subcutaneously (s.c.). For mouse studies, drugs were dissolved in 0.9% saline and injected intraperitoneally (i.p.). We believe these different parenteral routes of administration, while not pre-planned, broaden the interpretability of our findings.
The dose of mecamylamine, sertraline, and chlordiazepoxide fall within the dose-range previously found effective for these drugs (e.g., Cervo et al., 1991; Turner et al., 2010) . Due to the relatively few previous studies with EMDP and EDDP and the similar in vitro potencies of EMDP, EDDP, and mecamylamine, we selected starting doses (5-10 mg/kg), which fall within the range previously reported to be anxiolytic for mecamylamine. In some cases, when an effect was detected, we increased the dose of EMDP or EDDP to determine if we reached a maximal response. In the case of ptosis, we selected a dose 4× the minimal effective dose for EMDP to better test the hypothesis that EMDP was without effect on ptosis.
2.6 Behavioral Assays
Animals were transported from the animal facility to testing rooms, where they were allowed to acclimate in their home cage for a minimum of 30 minutes prior to the onset of behavioral testing. All behavioral testing was conducted and scored while blind to treatment conditions. We included standard reference compounds for at least one behavioral test in each area of interest as a measure of assay sensitivity; but standard reference compounds were not included for all behavioral tests because the positive effects detected in each assay enabled us to omit these groups from some experiments, reducing the number of animals needed for this study (i.e., the reference compounds would be needed to interpret a null finding, but not a positive finding). For example, the increased open-arm exploration in the elevated plus maze with rats (Fig 5) allowed us to omit chlordiazepoxide as a control. Likewise, the decreased immobility with EMDP in the mouse forced swim test (Fig 8) allowed us to omit sertraline as a positive control for those experiments.
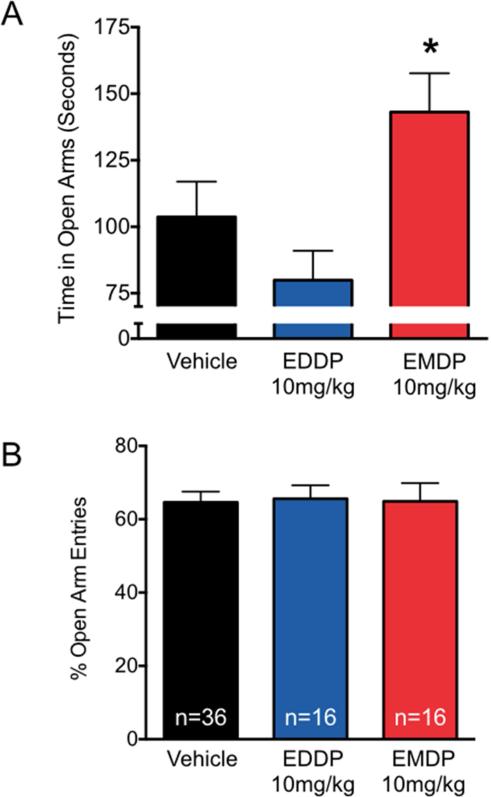
Figure 5. Anxiolytic-like effect of EMDP, but not EDDP, measured using the elevated plus maze in rats.
(A) Time that the rats spent in the open arms of the elevated plus maze. (B) Percent of total entries that were made into the open arms of the plus maze. n = number of animals per group. * = Significantly different from control (p<0.05; Kruskal-Wallis test with Dunn's post-hoc).
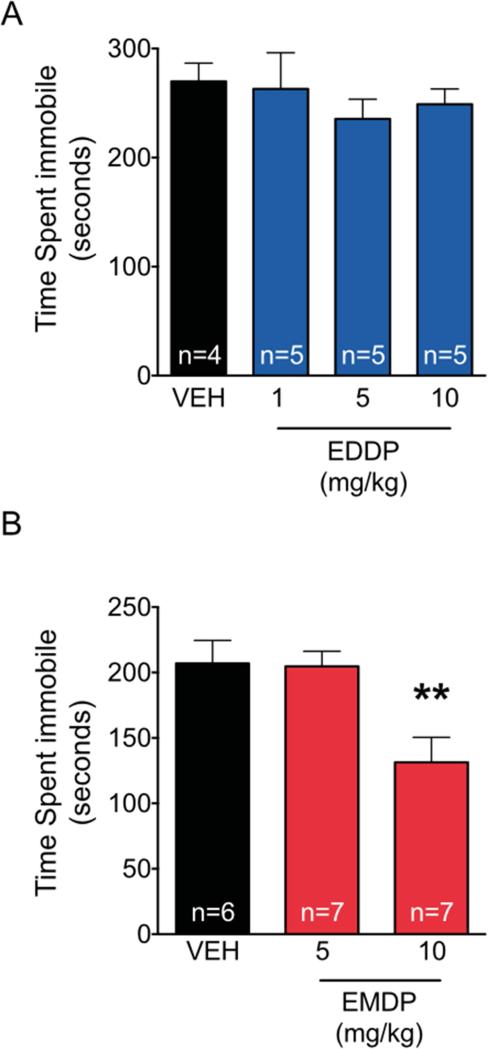
Figure 8. Antidepressant-like effect of EMDP, but not EDDP, in the mouse forced swim task.
Time spent immobile during the 360 sec forced swim test. (A) EDDP at the doses shown did not decrease time spent immobile. (B) EMDP at a dose of 10mg/kg did decrease time spent immobile. ** p<0.01, analysis of variance with Holm-Sidak post-hoc test.
2.6.1 Locomotor Activity (Rats)
Locomotor activity was measured 30 minutes after sc injection of test compound. Animals were placed into a novel Plexiglass enclosure (16”×16”×16”, TruScan Arena, Coulbourn Instruments, Whitehall, PA) with 770 lux illumination over the center of the arena. Animals were allowed to explore for 10 min, during which total distance traveled was recorded using ANYmaze software (Stoelting Co., Wood Dale, IL), as previously described (Forcelli et al., 2012).
2.6.2 Locomotor Activity (Mice)
Locomotor activity in response to i.p. drug administration was analyzed in a “home cage” activity monitoring system (Med Associates, St. Albans, VT), as previously described (Walters and Blendy, 2001; Mackler et al., 2008; Isiegas et al., 2009). Briefly, a novel cage identical to the home cage (28.9 cm × 17.8 cm × 12 cm) was placed in a photo-beam frame (30 cm × 24 cm × 8 cm) with sensors arranged in an 8-beam array strip. For dose studies, mice were injected i.p. with saline or drug. Immediately following drug administration, the mice were placed individually in the cages with 4000 lux illumination, identical to their normal housing conditions. Beam break data was monitored and recorded for 60 min.
2.6.3 Elevated Plus Maze
(Rats) Plus maze testing was performed and scored as previously described (Forcelli and Heinrichs, 2008; Forcelli et al., 2012), in a standard grey rat elevated plus maze (50cm arms, elevated 40cm off the ground [Stoelting Co., Wood Dale, IL]). Testing was conducted under 20 lux red light, 30 min after drug administration. The test lasted 300 sec. The number of arm entries and time spent in open and closed arms were recorded using ANYmaze (Stoelting Co, Wood Dale, IL).
2.6.4 Marble Burying
(Mice) After a 1 h period of acclimation to the testing room, the mice (n=6-10 per group) were injected i.p. with saline, EDDP, EMDP, or CDP at the doses indicated. Ten minutes later, the mice were placed individually in small cages (26×20×14 cm), in which twenty marbles had been equally distributed on top of mouse bedding (5-cm deep), and a wire lid was placed on top of the cage. Mice were left undisturbed for 15 min, after which time the number of buried marbles (i.e., those covered by bedding three-quarters or more) was counted.
2.6.5 Forced Swim Test
(FST; Rats) The FST in rats was conducted as previously described (Porsolt et al., 2001). On day 1, rats were placed in a cylindrical chamber (20 cm in diameter, 46 cm deep) filled to a height of 38 cm with room temperature water for 900 sec. After the completion of this pre-exposure test, animals were removed from the apparatus, dried with towels and placed under a heat lamp for ~30 min. Immediately prior to returning the animals to their home cage, they were treated with either EMDP (10 or 20 mg/kg), EDDP (10 mg/kg), sertraline (20 mg/kg), mecamylamine (5 mg/kg) or vehicle. Animals were treated with these same doses again 20h and 23h after the initial dose. This dosing paradigm is commonly used in forced swim experiments in rats (Porsolt et al., 2001). Twenty-four h after the pre-exposure, animals were re-introduced to the apparatus and video-recorded for 300 sec. Immobility was hand-scored for each video record by a treatment blind observer (P.A.F.) using ANYmaze (Stoelting) to record the data.
2.6.6 Forced Swim Test
(FST; Mice) The FST in mice was conducted as previously described (Turner et al., 2011). Briefly, 10 min following i.p. injection of saline, EDDP or EMDP, mice were placed in Plexiglas cylinders filled with water (25°C) for 6 min while being videotaped. The forced swim score for the entire 360 sec test was assessed using the Cleversystems videotracking system (CleverSys Inc, Reston, VA) and confirmed with visual scoring by a trained observer. A mouse was judged to be immobile when making only those movements necessary to keep its head above water.
2.6.7 Ptosis Assessment
(Rats) Eyelid ptosis in rats was assessed 15 min prior to and 15, 30, 45, and 180 min after administration of either mecamylamine (5mg/kg) or EMDP (20mg/kg). Ptosis was assessed as previously described (Rubin et al., 1957). Each animal was photographed while gently restrained by hand. Photographs were analyzed by a treatment blind observer (P.A.F.) and assigned a score of 0 (no ptosis, eye completely open), 1 (mild, eye partially closed), 2 (moderate, eye half closed), 3 (severe, eye ¾ closed), or 4 (complete, eye completely closed).
2.6.8 Statistical Analysis
Data were analyzed using SPSS (IBM, Somers, NY) and GraphPad Prism (GraphPad Software, La Jolla, CA). Normally distributed data (open field, home cage locomotor behavior, marble burying, and forced swim) were analyzed by analysis of variance followed by Holms-Sidak post-hoc test. Nonparametric data (elevated plus maze, ptosis scores) were analyzed using the Kruskal-Wallis test followed by Dunn's post-hoc test. The threshold for statistical significance was set as p<0.05.
3. Results
3.1 EDDP and EMDP block nicotine-stimulated 86Rb+ efflux mediated by α3β4 and α4β2 nAChRs
We compared the potencies of EDDP and EMDP in blocking human α3β4 and α4β2 nAChR channel function stimulated by nicotine using 86Rb+ efflux assays. We previously showed that each of the two enantiomers of these two methadone metabolites are equipotent in blocking rat α3β4 nAChR channels (Xiao et al., 2001), and as shown in Table 1, their potencies in blocking the channel function of human α3β4 and α4β2 nAChRs were also similar. Both compounds were 2.5 to 3 times more potent at α3β4 than at α4β2 nAChRs, which was the opposite order of potency for mecamylamine, a widely used nAChR channel blocker. EDDP was 10- to 13-times more potent than EMDP at both receptor subtypes in these assays.
Table 1.
Inhibitory properties of enantiomers of EDDP and EMDP on function of α4β2 and α3β4 nAChR subtypes.
| Drug | IC50 (μM) | |
|---|---|---|
| α4β2 nAChRsa | α3β4 nAChRsa | |
| (+)-EDDP | 1.8 ± 0.6 | 0.9 ± 0.09 |
| (-)-EDDP | 1.4 ± 0.2 | 0.7 ± 0.06 |
| (+)-EMDP | 24 ± 5 | 7.4 ± 2.1 |
| (-)-EMDP | 19 ± 4 | 7.0 ± 1.2 |
| Mecamylamine | 1.0 ± 0.3 | 3.7 ± 1.2 |
The defined human α4β2 and α3β4 nAChRs are stably expressed in HEK cells designated YXα4β2H1 and YXα3β4H1, respectively. See Materials and Methods for details.
IC50 values were calculated from inhibition curves in which 86Rb+ efflux was stimulated by 100 μM nicotine, as described under Materials and Methods. Mecamylamine, a noncompetitive nAChR antagonist was included for comparison. Data shown are mean ± standard error of three to six independent measurements.
Because (+) and (−) enantiomers of both EDDP and EMDP demonstrated nearly equal potencies at inhibiting α3β4 and α4β2 nAChR channel function in these 86Rb+ efflux assays, the subsequent assays were carried out with racemic mixtures of the two compounds.
3.2 Binding of methadone, EDDP and EMDP to opiate receptors labeled by [3H]-naloxone
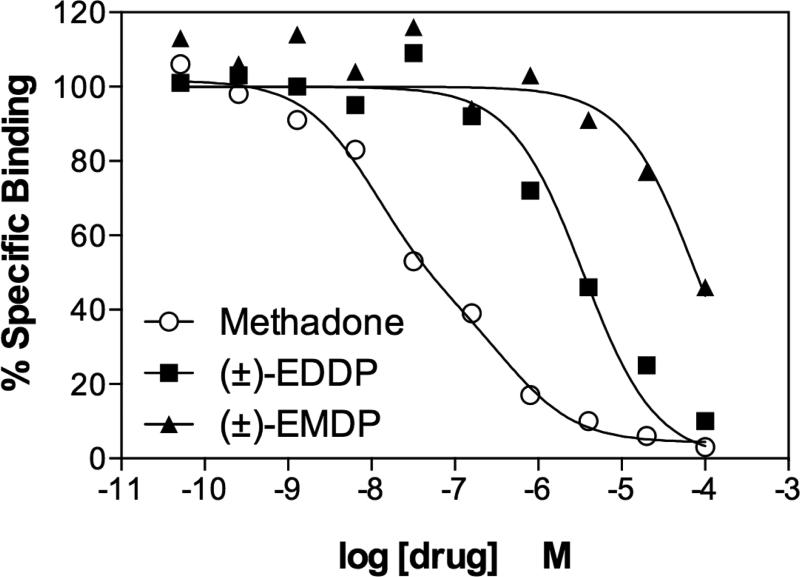
Methadone is a μ-opiate receptor agonist. EDDP, which is the N-demethylated primary metabolite of methadone, and EMDP, the secondary N-demethylated trace derivative of EDDP, are considered to be inactive at opiate receptors (Pohland et al., 1971). Consistent with this assessment, as shown in Figure 2, binding competition assays demonstrate that methadone competes for the majority of [3H]-naloxone binding sites in rat forebrain with high affinity (Ki ~11 nM), while EDDP and EMDP compete with 84-fold and 1900-fold lower affinities, respectively.
Figure 2. Methadone, EDDP and EMDP competition for opiate receptor binding sites labeled by [3H]-naloxone in rat forebrain membranes.
Opiate receptors in rat forebrain (colliculi forward) were labeled by 2 nM [3H]-naloxone (Kd is ~ 0.7 nM) in the absence or presence of (−)-methadone, (±)-EDDP or (±)-EMDP at the concentrations shown. Methadone competition curves fit a model for two sites, with the largest fraction having a high binding affinity, Ki ~ 11 nM. EDDP and EMDP competition curves fit a model for a single binding site. EDDP competed for nearly all of the binding sites but with an apparent affinity ~83-fold lower than that of methadone. EMDP competed for only 60% of the sites even at a concentration of 100 μM, and its apparent affinity was ~1900-fold lower than that of methadone. The Ki values for EDDP and EMDP were 918 nM and 21 μM, respectively. The results shown are representative of two independent assays.
3.3 Effects of EDDP and EMDP on Locomotor Activity in Rats
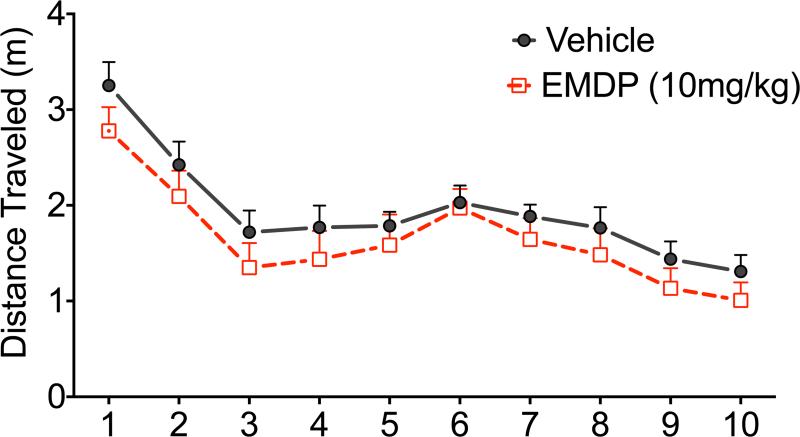
Locomotor activity provides an overall measure of spontaneous motor activity, as well as a measure of exploratory drive, as indicated by greater degree of exploration of the center of the maze (Crawley, 1985). As shown in Figure 3, although analysis of variance (ANOVA) showed a significant main effect of time (F9,189=15.4, p<0.0001), both control and treated rats habituated to the open field at similar rates and traveled a similar distance over the course of the test. There was no main effect of treatment (F1,25=0.54, p=0.54) nor a treatment-by-time interaction (F9,225=0.56, p=0.83). Thus, neither EDDP nor EMDP significantly altered open field locomotor activity in rats. The size of the open field, and the lack of optimization of the apparatus for testing anxiety-like behavior precluded a meaningful analysis of exploration of the center of the maze in the present study.
Figure 3. EDDP and EMDP do not change rat locomotor behavior in the open field.
(A) Time-course of locomotion in vehicle treated (solid line) and 10mg/kg EDDP (broken line). (B) Total distance traveled in the 10 min test period. (C) Time-course of locomotion in vehicle treated (solid line) and 5mg/kg EMDP (broken line). (D) Total distance traveled in the 10 min test period. n = number of animals per group.
3.4 Effects of EMDP on Locomotor Activity in Mice
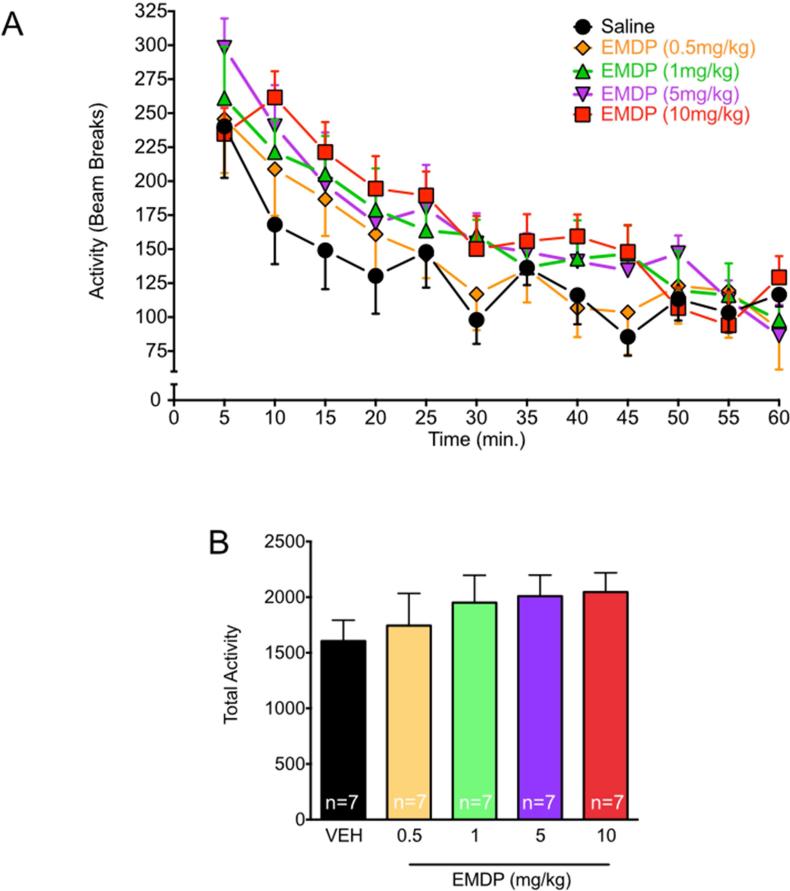
Consistent with our results in rats, ANOVA indicated that the general locomotor activity of mice placed in the novel cage decreased with time (Fig. 4A; F11,330 =38.79, p<0.0001). Again, EMDP did not significantly affect overall locomotor activity (Fig 4B, F4,,30=0.73, p=0.58), nor was there a treatment-by-time interaction (F44,330=1.08, p=0.34). EDDP was not tested in for locomotor activity in mice.
Figure 4. EMDP does not change home cage locomotor activity in mice.
(A) Time-course of locomotor activity after vehicle (circle) or EMDP: 0.5mg/kg = diamond, 1mg/kg = upward triangle, 5mg/kg = downward triangle, 10mg/kg = square. (B) Total activity. n = number of animals per group.
3.5 Anxiolytic-like effects in rats: Elevated Plus Maze
To examine the potential anxiolytic effects of EDDP and EMDP, we used the elevated plus maze, a standard test for anxiety-like behavior in rodents (Carobrez and Bertoglio, 2005). This test exploits the natural exploratory drive of rodents by pitting the relative safety of the dimly lighted closed arms of the maze against the open, unenclosed, elevated spaces, which are presumed to be anxiety-inducing (Pellow et al., 1985). The rats were tested in the EPM 30 min after drug treatment.
As shown in Figure 5A, vehicle-treated control rats spent a mean duration of 104 s in the open arms of the elevated plus maze; whereas rats treated with EDDP (10 mg/kg) spent a mean duration of 80 s in the open arms, which was not statistically different from controls. In contrast, rats treated with EMDP spent a mean duration of 143 s in the open arms, which was statistically different from controls (Kruskal-Wallis test, H(2)=7.1, p<0.05; p<0.05, Dunn's multiple comparison test), suggesting that EMDP has an anxiolytic effect in this test. As shown in Figure 5B, the groups did not differ with respect to percentage of entries made into the open arms of the plus maze. Finally, the latency to first entry in the open arms did not differ as a function of treatment group (data not shown).
3.6 Anxiolytic-like effects in mice: Marble-burying test
Marble burying is a natural tendency of mice and rats (Poling et al., 1981; Njung'e and Handley, 1991). Although this behavior itself does not necessarily reflect a state of anxiety in rodents (Njung'e and Handley, 1991; Thomas et al., 2009; Wurzman et al., 2014), inhibition of this behavior does appear to reliably distinguish antianxiety drugs (Broekkamp et al., 1986; Njung'e and Handley, 1991; Nicolas et al., 2006). Therefore, we examined the effects of EDDP and EMDP on this behavior.
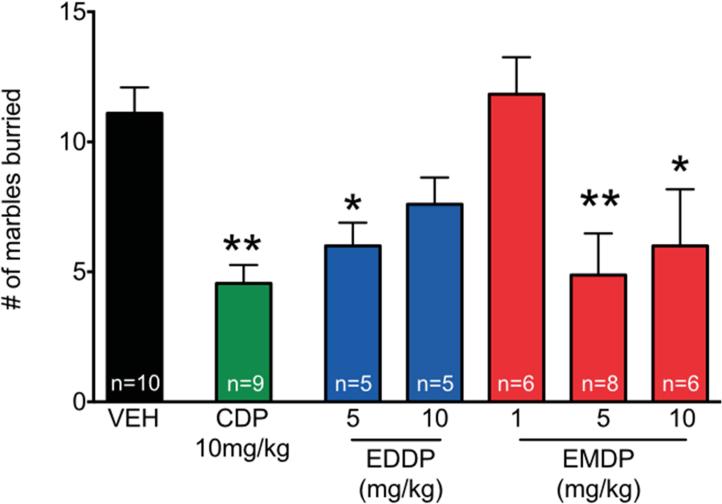
Mice were injected i.p. with saline (vehicle), CDP (positive control), EDDP, or EMDP at the doses shown. Ten minutes after injection, the mice were placed individually in small cages with 20 marbles on top of the bedding, and 15 min later the number of marbles buried was counted. As shown in Figure 6, vehicle-treated control mice buried a mean of 11 marbles during the 15 min test. As expected, animals treated with the positive control CDP (10mg/kg) buried significantly fewer marbles than vehicle treated animals (p<0.01). Mice treated with EDDP at a dose of 5 mg/kg also buried significantly fewer marbles than did controls (p<0.05), but at the higher dose of 10 mg/kg, this did not reach statistical significance. Mice treated with EMDP buried significantly fewer marbles at both the 5 mg/kg (p<0.01) and 10 mg/kg dose (p<0.05), but not at the 1 mg/kg dose. These effects were analyzed by post-hoc tests (Holm-Sidak corrected) after analysis of variance revealed a significant main effect of drug treatment (F6,,42=5.49, p=0.0005).
Figure 6. Anxiolytic-like effects of EDDP and EMDP measured using the marble-burying task in mice.
The number of marbles buried out of 20 in the 15-minute test after injection with chlordiazepoxide (CDP), EDDP or EMDP at the doses shown. n = number of animals per group. Significantly different from control (* p<0.05; ** p<0.01; analysis of variance with Holm-Sidak post-hoc test).
3.7 Antidepressant-like effects in rats: Forced swim test
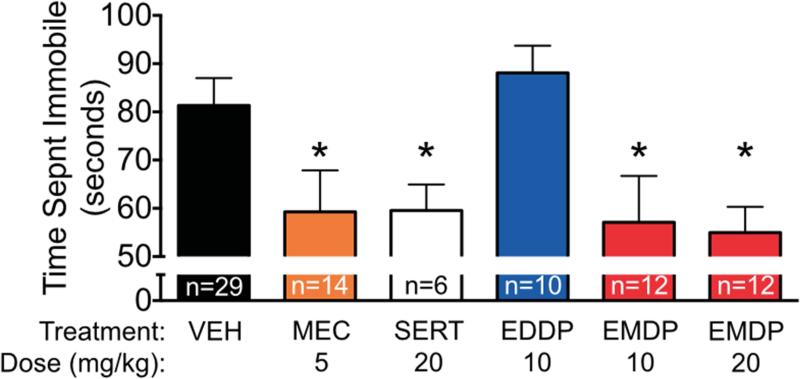
The FST (Porsolt et al., 1977) is a well-established assay with predictive utility for antidepressant drug efficacy. As shown in Figure 7, vehicle-treated rats spent a mean duration of 81 s immobile during the 5 min forced swim test. Treatment of rats with sertraline (20 mg/kg), an antidepressant used here as a positive control, significantly reduced immobile time (p<0.05). Similarly, treatment of rats with the nAChR channel blocker mecamylamine (5 mg/kg), which has previously been found to reduce immobile time in this test (Caldarone et al., 2004; Andreasen and Redrobe, 2009), reduced immobile time (p<0.05). Treatment with EDDP (10 mg/kg) was without effect in this test, but treatment with EMDP at both 10 and 20 mg/kg reduced immobile time (p<0.05) to the same extent as sertraline (Holm-Sidak corrected post-hoc tests after ANOVA showed a significant main effect of treatment (F5,76=3.489, p<0.001).
Figure 7. Antidepressant-like effect of EMDP, but not EDDP, in the rat forced swim task.
Time spent immobile during the 300 sec forced swim test after injection with mecamylamine (MEC), sertraline (SERT), EDDP or EMDP at the doses shown. Significantly different from control (* p<0.05; analysis of variance with Holm-Sidak post-hoc test).
3.8 Antidepressant-like effects in mice: Forced Swim Test
As shown in Fig. 8A, vehicle treated control mice spent a mean duration of 270 s immobile during the 6 min forced swim test. This is similar to the immobility time we have previously reported for this mouse strain under these test conditions (Turner et al., JPET 2010; Gundersen et al., 2013; Gundersen and Blendy, 2009; Cleck et al, 2008; Conti et al, 2004; Conti et al, 2002), and is optimal for avoiding floor effects. Treatment of mice with 1, 5, or 10 mg/kg EDDP did not produce a significant effect on duration of immobility (F3,15=0.45, p=0.72). In contrast, in separate experiments using different groups of mice, EMDP significantly reduced immobility time. Thus as shown in figure 8B, in these experiments vehicle treated mice spent a mean duration of 207 s immobile during this 6 min forced swim test. EMDP at a dose of 5 mg/kg did not affect immobility time, but at a dose of 10 mg/kg it significantly reduced it (ANOVA and one-tailed Holm-Sidak corrected post-hoc tests (F2,17=7.128, p<0.01).
3.9 Comparison of ganglionic effects of mecamylamine and EMDP in rats
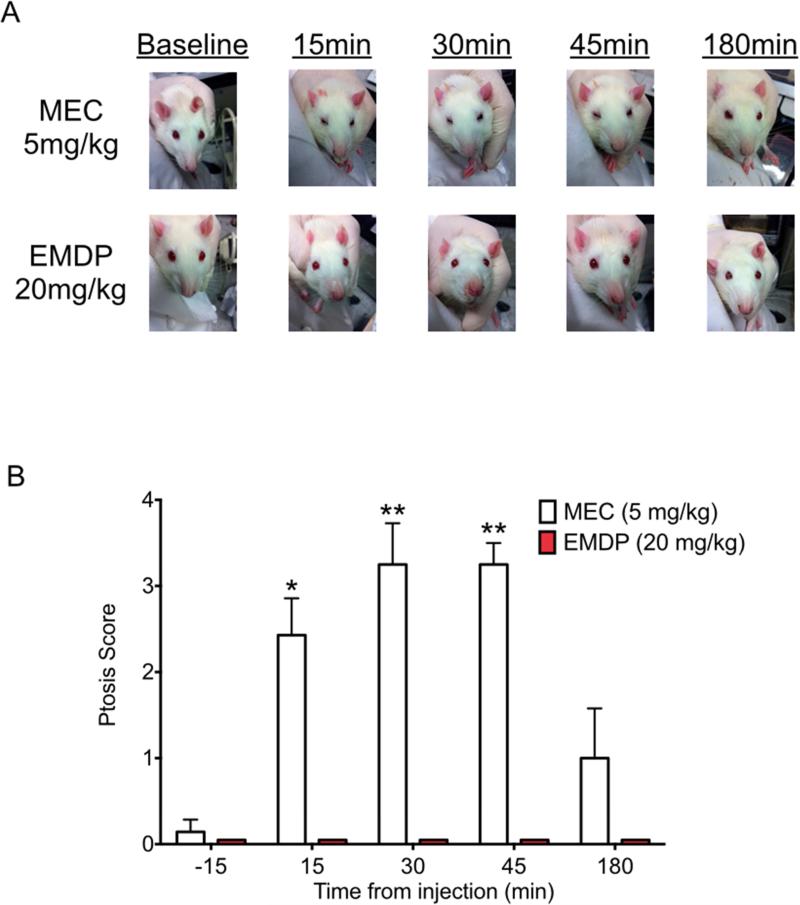
Ganglionic blockade can cause eyelid ptosis; and indeed, during forced swim tests in rats, we observed obvious ptosis in response to mecamylamine (5 mg/kg). But interestingly, EMDP at doses (10 and 20 mg/kg) equally efficacious in the FST did not produce ptosis. To examine this difference in more detail, an additional group of rats was treated with either mecamylamine (5 mg/kg) or EMDP (20 mg/kg) and ptosis was observed and quantified over the course of 3h. Mecamylamine produced ptosis in a time-dependent manner (Kruskal-Wallis test, H(4)=16.63, p<0.005), with onset within 15 min after injection, and statistically significant effects still apparent at 30 and 45 min, but not at 3 h after injection (Fig. 9). In contrast, ptosis was not apparent at any time during the 3h observation period in animals treated with EMDP (Fig. 9).
Figure 9. Mecamylamine, but not EMDP results in ptosis in the rat.
(A) Representative photographs of animals treated with mecamylamine (5mg/kg) or EMDP (20mg/kg), at each observation time (B) Quantification of ptosis severity at 15 min intervals. n=7 in both groups. * p<0.05, ** p<0.01; Kruskal-Wallis test with Dunn's post-hoc.
4. Discussion
Previous studies found that both EDDP and EMDP blocked rat α3β4 nAChR channels (Xiao et al., 2001), and here we show that they do the same at human α3β4 and α4β2 nAChR channels. Our evaluations of these two compounds in three behavioral tests predictive of anxiolytic and/or antidepressant activity in rodents indicate that EMDP, in particular, shows positive effects in each of these tests in both rats and mice. The pattern of behavioral responses to EMDP was consistent across tasks and species. Moreover, the efficacy of EMDP in these behavioral tests was similar to chlordiazepoxide and sertraline, standard positive control anxiolytic and antidepressant compounds, respectively. In contrast, EDDP produced a significant effect only in the marble burying test in mice, and reached the level of statistical significance for only the lower dose.
The obvious difference in efficacy between EDDP and EMDP in these behavioral assays is opposite to the potencies of these two compounds in blocking both α3β4 and α4β2 nAChR channel function in the 86Rb+ assays in vitro. A possible explanation for this is that EDDP carries a permanent positive charge, which may limit its ability to cross the blood-brain-barrier and reach behaviorally active concentrations in brain. Interestingly, however, a positive charge may allow it to more readily enter the nAChR channel pore and thus be a potent channel blocker in vitro. Future examination of higher doses of EDDP may be warranted in order to overcome any pharmacokinetic limitations.
Importantly, while EMDP produced a robust effect in these preclinical tests for anxiolytic and antidepressant activity in both rats and mice, it did not significantly affect locomotor activity in either species. Moreover, EMDP even at the highest dose used in these studies in the rat did not produce eyelid ptosis, which is a common feature of drugs such as mecamylamine that block nAChRs in autonomic ganglia. This may be because EMDP is only half as potent as mecamylamine in blocking human α3β4 nAChRs and only one-sixth as potent in blocking rat α3β4 nAChRs (Xiao et al, 2001), which are important for ganglionic function (Xu et al., 1999; Mao et al., 2006).
There is a substantial literature supporting nAChRs as targets for treatment of affective disorders (File et al., 1998; Mineur and Picciotto, 2010). Indeed, studies in rodents demonstrate bimodal effects of nicotinic agonists on anxiety-related behaviors, with low doses of agonists eliciting anxiolytic-like behaviors and higher doses evoking anxiogenic-like behaviors (File et al., 1998; Anderson and Brunzell, 2012; Varani et al., 2012). In fact, low doses of nicotine and the nAChR competitive antagonist dihydro-β-erythroidine produced similar effects in these behavioral studies (Anderson and Brunzell, 2012; 2015), suggesting that desensitization of nAChRs by low (possibly sub-activating) doses of nicotine are anxiolytic, while higher doses, which initially activate the receptors, elicit an anxiogenic response, perhaps involving downstream GABA-B receptors (Varani et al., 2012).
Hyperactivity of the cholinergic system has long been hypothesized to be a marker for affective disorders (Janowsky et al., 1972, 1974, 1994; Mineur and Picciotto, 2010). Furthermore, it has been suggested that nicotine use (i.e., smoking) may be, in part, an attempt at self-medication of these disorders (Mineur and Picciotto, 2010). Consistent with this suggestion, it is estimated that nearly one-half of all cigarettes smoked in the US are consumed by persons suffering from some degree of a neuropsychiatric disorder (Dani and Harris, 2005). However, the results from our studies here do not allow us to determine whether the effects of EMDP in these preclinical tests for anxiolytic and antidepressant activity involve primarily α3β4* or α4β2* nAChRs, or both. Although EMDP is more potent blocking α3β4 than α4β2 nAChRs, previous studies have implicated β2-containing nAChRs in antidepressant (Tizabi et al., 1999; Rabenstein et al., 2006; Mineur et al., 2007) and anxiolytic actions (Turner et al., 2010, 2011, 2013; Anderson and Brunzell, 2012) of nicotinic agonists and antagonists. In fact, both of these receptors could be involved, as well as other nAChR subtypes, including α7 (Rabenstein et al., 2006). Moreover, our data do not exclude an effect of EMDP on opiate receptors, some of which appear to acutely mediate anxiolytic and/or anxiogenic effects of some opiates (Vant Veer and Carlezon, 2013; Rorick-Kehn et al., 2014). Importantly, however, while both EMDP and EDDP were able to compete with methadone for binding to opiate receptors within the μM range in the present study, prior examinations of these compounds failed to detect in vivo opiate activity. Indeed, neither compound produces analgesia in the tail-flick assay at a dose of 100 mg/kg, sc. Moreover, neither compound antagonized the increase of tail-flick latency produced by methadone or morphine when given at a dose of 100 mg/kg (Pohland et al., 1971). The absence of analgesia effects in these prior studies is consistent with our binding data, which indicate that these methadone metabolites have low affinity for opiate receptors. Moreover, we failed to observe any Straub tail effects with EMDP or EDDP, providing further support to the notion that these metabolites do not activate opiate receptors. Finally, given the fact that the doses of EMDP and EDDP that we used here were much lower than those used by Pohland and colleagues, who failed to detect any opiate-mediated action, suggests a large separation (at least 10×) between any opiate effects and the functionally effective anxiolytic or antidepressant dose.
The seeming discrepancy between our data here, which indicate that a methadone metabolite that blocks nAChRs may have potential as an antianxiety and/or antidepressant drug, and the use of the quintessential nAChR agonist nicotine (e.g., smoking) to relieve anxiety and/or depression is more apparent than real, because in virtually all cases where it has been studied, administration of nicotine rapidly and potently desensitizes nAChRs, both in vivo (Balfour, 1980; Sharp and Beyer, 1986; Hulihan-Giblin et al., 1990; for reviews, see Buccafusco et al., 2009) and in vitro (Grady et al., 1994; Marks et al., 1994; Lester and Dani, 1995; Pidoplichko et al., 1997; Lu et al., 1999; Paradiso and Steinbach, 2003). In fact, nicotine is much more potent in desensitizing these receptors than in activating them (Hulihan-Giblin et al., 1990; Lu et al., 1999). Moreover, the desensitizing effects of nicotine last so much longer than its brief agonist effects that nicotine can reasonably be considered a time-averaged antagonist (Hulihan-Giblin et al., 1990).
The behavioral assays we have employed have a long history of use in the screening of anti-anxiety and anti-depressant compounds; indeed the EPM and FST are the most common preclinical tests of anxiolytic and antidepressant efficacy, respectively. These tests show excellent predictive validity, for example, more than 90% of antidepressants show efficacy in the FST (Petit-Demouliere et al., 2005). Similarly, the EPM shows bidirectional sensitivity: known anxiolytic compounds produce increases in open arm exploration, while known anxiogenic compounds reduce open arm exploration (Carobrez and Bertoglio, 2005). However, an important caveat to consider is the lack of construct validity of these tests. Exploring the efficacy of these compounds in additional models, such as animals with genetic predisposition to anxiety-like or depression-like behavior, and/or models in which these behaviors are evoked is an important future direction. This may be particularly useful as some models, such as the Wistar-Kyoto rat show resistance to SSRI therapy in the FST model (Lopez-Rubalcava and Lucki, 2000).
Methadone has a long record of use in humans (>50 years) as an analgesic and as an orally effective replacement to treat heroin addiction. It has an apparently excellent safety record, so presumably its metabolites EDDP and EMDP are also relatively safe. Indeed, after an oral maintenance dose of methadone (50-100 mg), up to milligram amounts of both EMDP and EDDP are recovered in urine over a 24 hour period (Sullivan and Due, 1973). Indeed, ng/ml concentrations of EDDP are detected after single doses of methadone in humans, with T1/2 of approximately 40-50 hours (Dale et al., 2002); comparable data for EMDP are not available. Our data, in this broader clinical framework, suggest that EMDP through blockade of nAChRs and/or even previously unrecognized actions at opiate receptors may have therapeutic potential as an anxiolytic and/or antidepressant agent meriting further clinical evaluation.
5. Conclusions
In summary, our findings demonstrate that the methadone metabolites EMDP and EDDP are pharmacologically active at nAChRs in vitro and that EMDP displayed behavioral activity in in vivo preclinical tests for anxiolytic and antidepressant action. Thus, our studies build upon an existing literature demonstrating anxiolytic- and antidepressant-like effects of drugs that decrease nAChR activity, whether by desensitization (e.g., nicotine) or receptor blockade (e.g., EMDP, mecamylamine, dihydro-β-erythroidine). Finally, these studies provide preclinical support for and suggest the use of EMDP, in particular, as a potential treatment for affective disorders. Importantly, since humans have been exposed both acutely and chronically to EMDP as a metabolite of methadone for more than 50 years, a trial of its clinical utility when repurposed as an anxiolytic and/or antidepressant is warranted and should be relatively straightforward.
Highlights.
❖ EMDP and EDDP block α3β4 and α4β2 nicotinic receptors
❖ EMDP and EDDP displayed anxiolytic-like effects in the marble burying tests
❖ EMDP displayed anxiolytic-like effects in the elevated plus maze
❖ EMDP displayed antidepressant-like effects in mouse and rat forced swim tests
❖ EMDP, unlike mecamylamine, did not cause eyelid ptosis
Acknowledgments
This research was supported by grants from the National Cancer Institute and National Institute on Drug Abuse, P50-CA143187 (JAB), T32-GM008076 (BGL) and K99-DA032681 (JRT), T32-HD046388 (PAF) and U19 DA027990 (KJK).
Nonstandard abbreviations
- FST
forced swim task
- nAChR
nicotinic acetylcholine receptor
- EMDP
2-ethyl-5-methyl-3,3-diphenyl-1-pyrroline
- EDDP
2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
Participated in research design: PAF, JRT, JAB, KJK
Conducted experiments: PAF, JRT, BGL, TTO, TX
Performed data analysis: PAF, JRT, BGL, YX, KJK
Contributed new reagents or analytic tools: YX, KJK
Contributed to the writing of the manuscript: PAF, JRT, BGL, JAB, YX, KJK
- 2-ethyl-5-methyl-3,3-diphenyl-1-pyrroline (EMDP, PubChem CID: 69492930, 69492891)
- 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP; PubChem CID: 115159)
- Sertraline (PubChem CID: 68617)
- Chlordiazepoxide (PubChem CID: 2712)
- Mecamylamine (PubChem CID: 4032)
References
- Anderson SM, Brunzell DH. Low dose nicotine and antagonism of β2 subunit containing nicotinic acetylcholine receptors have similar effects on affective behavior in mice. PloS One. 2012;7:e48665. doi: 10.1371/journal.pone.0048665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Brunzell DH. Anxiolytic-like and anxiogenic-like effects of nicotine are regulated via diverse action at β2*nicotinic acetylcholine receptors. Br J Pharmacol. 2015 Jan 27; doi: 10.1111/bph.13090. doi: 10.1111/bph.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher I, Wu B, Shytle DR, George TP. Mecamylamine - a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders. Expert Opin Pharmacother. 2009;10:2709–2721. doi: 10.1517/14656560903329102. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. Studies on the biochemical and behavioral effects of oral nicotine. Arch Int Pharmacodyn Ther. 1980;245:95–103. [PubMed] [Google Scholar]
- Belluardo N, Mudo’ G, Bonomo A, Di Liberto V, Frinchi M, Fuxe K. Nicotine-induced fibroblast growth factor-2 restores the age-related decline of precursor cell proliferation in the subventricular zone of rat brain. Brain Res. 2008;1193:12–24. doi: 10.1016/j.brainres.2007.11.069. [DOI] [PubMed] [Google Scholar]
- Bertrand D. The possible contribution of neuronal nicotinic acetylcholine receptors in depression. Dialogues Clin Neurosci. 2005;7:207–216. doi: 10.31887/DCNS.2005.7.3/dbertrand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol. 1986;126:223–229. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach JW, Terry AV. Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol. Exp Ther. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone BJ, Wang D, Paterson NE, Manzano M, Fedolak A, Cavino K, Kwan M, Hanania T, Chellappan SK, Kozikowski AP, Olivier B, Picciotto MR, Ghavami A. Dissociation between duration of action in the forced swim test in mice and nicotinic acetylcholine receptor occupancy with sazetidine, varenicline, and 5-I-A85380. Psychopharmacology (Berl) 2011;217:199–210. doi: 10.1007/s00213-011-2271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cervo L, Grignaschi G, Rossi C, Samanin R. Role of central serotonergic neurons in the effect of sertraline in rats in the forced swimming test. Eur J Pharmcol. 1991;196:217–22. doi: 10.1016/0014-2999(91)90433-q. [DOI] [PubMed] [Google Scholar]
- Clark PB, Reuben M. Release of [3H]-noradrenaline from rat hippocampal synaptosomes by nicotine: mediation by different nicotinic receptor subtypes from striatal [3H]-dopamine release. Br J Pharmacol. 1996;117(4):595–606. doi: 10.1111/j.1476-5381.1996.tb15232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleck JN, Ecke LE, Blendy JA. Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice. Psychopharmacology (Berl) 2008 Nov;201(1):15–28. doi: 10.1007/s00213-008-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AC, Kuo YC, Valentino RJ, Blendy JA. Inducible cAMP early repressor regulates corticosterone suppression after tricyclic antidepressant treatment. J Neurosci. 2004 Feb 25;24(8):1967–75. doi: 10.1523/JNEUROSCI.4804-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002 Apr 15;22(8):3262–8. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Dale O, Hoffer C, Sheffels P. Disposition of nasal, intravenous, and oral methadone in healthy volunteers. Clin Pharmacol Ther. 2002;72(5):536–45. doi: 10.1067/mcp.2002.128386. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Petrik D. Depression and Hippocampal Neurogenesis: A Road to Remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F. Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacology (Berl) 2000;152:295–303. doi: 10.1007/s002130000531. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav Neurosci. 1998;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- Forcelli PA, Heinrichs SC. Teratogenic effects of maternal antidepressant exposure on neural substrates of drug-seeking behavior in offspring. Addict Biol. 2008;13:52–62. doi: 10.1111/j.1369-1600.2007.00078.x. [DOI] [PubMed] [Google Scholar]
- Forcelli PA, Kozlowski R, Snyder C, Kondratyev A, Gale K. Effects of neonatal antiepileptic drug exposure on cognitive, emotional, and motor function in adult rats. J Pharmacol Exp Ther. 2012;340:558–566. doi: 10.1124/jpet.111.188862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Grady SR, Marks MJ, Collins AC. Desensitization of nicotine-stimulated [3H]dopamine release from mouse striatal synaptosomes. J Neurochem. 1994;62:1390–1398. doi: 10.1046/j.1471-4159.1994.62041390.x. [DOI] [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3HDopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59(3):848–56. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Grady SR, Meinerz NM, Cao J, Reynolds AM, Picciotto MR, Changeux JP, McIntosh JM, Marks MJ, Collins AC. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. J Neurochem. 2001;76(1):258–68. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- Grilli M, Parodi M, Raiteri M, Marchi M. Chronic nicotine differentially affects the function of nicotinic receptor subtypes regulating neurotransmitter release. J Neurochem. 2005;93:1353–60. doi: 10.1111/j.1471-4159.2005.03126.x. [DOI] [PubMed] [Google Scholar]
- Gundersen BB, Briand LA, Onksen JL, Lelay J, Kaestner KH, Blendy JA. Increased hippocampal neurogenesis and accelerated response to antidepressants in mice with specific deletion of CREB in the hippocampus: role of cAMP response-element modulator τ. J Neurosci. 2013;33(34):13673–85. doi: 10.1523/JNEUROSCI.1669-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009;57(1):67–74. doi: 10.1016/j.neuropharm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulihan-Giblin BA, Lumpkin MD, Kellar KJ. Acute effects of nicotine on prolactin release in the rat: agonist and antagonist effects of a single injection of nicotine. J Pharmacol Exp Ther. 1990;252:15–20. [PubMed] [Google Scholar]
- Hussmann GP, DeDominicis KE, Turner JR, Yasuda RP, Klehm J, Forcelli PA, Xiao Y, Richardson JR, Sahibzada N, Wolfe BB, Lindstrom J, Blendy JA, Kellar KJ. Chronic sazetidine-A maintains anxiolytic effects and slower weight gain following chronic nicotine without maintaining increased density of nicotinic receptors in rodent brain. J Neurochem. 2014;129:721–731. doi: 10.1111/jnc.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isiegas C, Mague SD, Blendy JA. Sex differences in response to nicotine in C57Bl/6:129SvEv mice. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2009;11:851–858. doi: 10.1093/ntr/ntp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Overstreet DH, Nurnberger JI. Is cholinergic sensitivity a genetic marker for the affective disorders? Am J Med Genet. 1994;54:335–344. doi: 10.1002/ajmg.1320540412. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak JM, McIntosh JM, Yoshikami D, Olivera BM. Nicotine-evoked transmitter release from synaptosomes: functional association of specific presynaptic acetylcholine receptors and voltage-gated calcium channels. J Neurochem. 2001;77:1581–89. doi: 10.1046/j.1471-4159.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- Ledford H. Depression drug disappoints. Nature. 2011;479:278. doi: 10.1038/479278a. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP, Mulle C. Evidene for “preterminal” nicotinic receptos on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993;13(6):2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Dani JA. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. J Neurophysiol. 1995;74:195–206. doi: 10.1152/jn.1995.74.1.195. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Gallardo KA, Park MK. Nicotinic acetylcholine receptor-mediated release of [3H]norepinephrine from developing and adult rat hippocampus: direct and indirect mechanisms. Neuropharmacology. 2002;42:653–661. doi: 10.1016/s0028-3908(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Lippiello PM, Beaver JS, Gatto GJ, James JW, Jordan KG, Traina VM, Xie J, Bencherif M. TC-5214 (S-(+)-mecamylamine): a neuronal nicotinic receptor modulator with antidepressant activity. CNS Neurosci Ther. 2008;14:266–277. doi: 10.1111/j.1755-5949.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22(2):191–9. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Lu Y, Marks MJ, Collins AC. Desensitization of nicotinic agonist-induced [3H]-ϒ-aminobutyric acid release from mouse brain synaptosomes is produced by subactivating concentrations of agonists. 1999 [PubMed] [Google Scholar]
- Lu Y, Grady S, Marks MJ, Picciotto M, Changeux JP, Collins AC. Pharmacological characterization of nicotinic receptor-stimulated GABA release from mouse brain synaptosomes. J Pharmacol Exp Ther. 1998;287(2):648–57. [PubMed] [Google Scholar]
- Mackler S, Pacchioni A, Degnan R, Homan Y, Conti AC, Kalivas P, Blendy JA. Requirement for the POZ/BTB protein NAC1 in acute but not chronic psychomotor stimulant response. Behav Brain Res. 2008;187:48–55. doi: 10.1016/j.bbr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Yasuda RP, Fan H, Wolfe BB, Kellar KJ. Heterogeneity of nicotinic cholinergic receptors in rat superior cervical and nodose Ganglia. Mol Pharmacol. 2006;70:1693–1699. doi: 10.1124/mol.106.027458. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Grady SR, Yang JM, Lippiello PM, Collins AC. Desensitization of nicotine-stimulated 86Rb+ efflux from mouse brain synaptosomes. J Neurochem. 1994;63:2125–2135. doi: 10.1046/j.1471-4159.1994.63062125.x. [DOI] [PubMed] [Google Scholar]
- Marchi M, Raiteri M. Nicotinic autoreceptors mediating enhancement of acetylcholine release become operative in conditions of “impaired” cholinergic presynaptic function. J Neurochem. 1996;67(5):1974–81. doi: 10.1046/j.1471-4159.1996.67051974.x. [DOI] [PubMed] [Google Scholar]
- McClure-Bengley TD, Grady SR, Marks MJ, Collins AC, Stitzel JA. Presynaptic GABAB autoreceptor regulation of nicotinic acetylcholine receptor mediated [(3)H]-GABA release from mouse synaptosomes. Biochem Pharmacol. 2014;91(1):87–96. doi: 10.1016/j.bcp.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. α4β2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci Off J Soc Neurosci. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1120–1133. doi: 10.1016/j.bcp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Eibl C, Young G, Kochevar C, Papke RL, Gündisch D, Picciotto MR. Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Ther. 2009;329:377–386. doi: 10.1124/jpet.108.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Einstein EB, Seymour PA, Coe JW, O'neill BT, Rollema H, Picciotto MR. α4β2 nicotinic acetylcholine receptor partial agonists with low intrinsic efficacy have antidepressant-like properties. Behav Pharmacol. 2011;22:291–299. doi: 10.1097/FBP.0b013e328347546d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudò G, Belluardo N, Mauro A, Fuxe K. Acute intermittent nicotine treatment induces fibroblast growth factor-2 in the subventricular zone of the adult rat brain and enhances neuronal precursor cell proliferation. Neuroscience. 2007;145:470–483. doi: 10.1016/j.neuroscience.2006.12.012. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.), Institute for Laboratory Animal Research (U.S.), and National Academies Press (U.S.) Guide for the care and use of laboratory animals. 8th ed National Academies Press; Washington, D.C.: 2011. [Google Scholar]
- Nickell JR, Grinevich VP, Siripurapu KB, Smith AM, Dwoskin LP. Potential therapeutic uses of mecamylamine and its stereoisomers. Pharmacol Biochem Behav. 2013;108:28–43. doi: 10.1016/j.pbb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas LB, Kolb Y, Prinssen EPM. A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol. 2006;547:106–115. doi: 10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16(1):36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Njung'e K, Handley SL. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice; a putative test for anxiolytic agents. Br J Pharmacol. 1991;104:105–112. doi: 10.1111/j.1476-5381.1991.tb12392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paperwalla KN, Levin TT, Weiner J, Saravay SM. Smoking and depression. Med Clin North Am. 2004;88:1483–1494. x–xi. doi: 10.1016/j.mcna.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Paradiso KG, Steinbach JH. Nicotine is highly effective at producing desensitization of rat alpha4beta2 neuronal nicotinic receptors. J Physiol. 2003;553:857–871. doi: 10.1113/jphysiol.2003.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacol (Berl) 2005;177(3):245–55. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pohland A, Boaz HE, Sullivan HR. Synthesis and identification of metabolites resulting from the biotransformation of DL-methadone in man and in the rat. J Med Chem. 1971;14:194–197. doi: 10.1021/jm00285a004. [DOI] [PubMed] [Google Scholar]
- Poling A, Cleary J, Monaghan M. Burying by rats in response to aversive and nonaversive stimuli. J Exp Anal Behav. 1981;35:31–44. doi: 10.1901/jeab.1981.35-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci Editor Board Jacqueline N Crawley Al. 2001 doi: 10.1002/0471142301.ns0810as14. Chapter 8:Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology (Berl) 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- Rapier C, Lunt GG, Wonnacott S. Stereoselective nicotine-induced release of dopamine from striatal synaptosomes concentration dependence and repetitive stimulation. J Neurochem. 1988;50(4):1123–30. doi: 10.1111/j.1471-4159.1988.tb10582.x. [DOI] [PubMed] [Google Scholar]
- Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, Picciotto MR. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline's effect. Eur J Pharmacol. 2009;605:114–116. doi: 10.1016/j.ejphar.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle E, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Xia L, Crile RS, et al. LY2456302 is a novel, potent, orally-bioavailable small molecule kapp-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014;77:131–144. doi: 10.1016/j.neuropharm.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Rowell PP, Carr LA, Garner AC. Stimulation of [3H]dopamine release by nicotine in rat nucleus accumbens. J Neurochem. 1987;49(5):1449–54. doi: 10.1111/j.1471-4159.1987.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Rubin B, Malone MH, Waugh MH, Burke JC. Bioassay of Rauwolfia roots and alkaloids. J Pharmacol Exp Ther. 1957;120:125–136. [PubMed] [Google Scholar]
- Sharp BM, Beyer HS. Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. J Pharmacol Exp Ther. 1986;238:486–491. [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Sheehan KH, Sheehan DV, Sanberg PR. Neuronal nicotinic receptor inhibition for treating mood disorders: preliminary controlled evidence with mecamylamine. Depress Anxiety. 2002;16:89–92. doi: 10.1002/da.10035. [DOI] [PubMed] [Google Scholar]
- Sullivan HR, Due SL. Urinary metabolites of dl-methadone in maintenance subjects. J Med Chem. Aug. 1973;16(8):909–13. doi: 10.1021/jm00266a009. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, STAR*D Study Team Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. J Pharmacol Exp Ther. 2010;334:665–672. doi: 10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2011;13:41–46. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Wilkinson DS, Poole RL, Gould TJ, Carlson GC, Blendy JA. Divergent functional effects of sazetidine-a and varenicline during nicotine withdrawal. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2013;38:2035–2047. doi: 10.1038/npp.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vant Veer A, Carlezon Wm A. Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani AP, Moutinho LM, Balerio GN. Acute behavioural responses to nicotine and nicotine withdrawal syndrome are modified in GABA(B1) knockout mice. Neuropharmacology. 63:863–872. doi: 10.1016/j.neuropharm.2012.06.006. 20120. [DOI] [PubMed] [Google Scholar]
- Vázquez-Palacios G, Bonilla-Jaime H, Velázquez-Moctezuma J. Antidepressant effects of nicotine and fluoxetine in an animal model of depression induced by neonatal treatment with clomipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:39–46. doi: 10.1016/j.pnpbp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Walters CL, Blendy JA. Different requirements for cAMP response element binding protein in positive and negative reinforcing properties of drugs of abuse. J Neurosci Off J Soc Neurosci. 2001;21:9438–9444. doi: 10.1523/JNEUROSCI.21-23-09438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzman R, Forcelli PA, Griffey CJ, Kromer LF. Repetitive grooming and sensorimotor abnormalities in an ephrin-A knockout model for Autism Spectrum Disorders. Behav Brain Res. 2014 doi: 10.1016/j.bbr.2014.09.012. doi: 10.1016/j.bbr.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol. 1998;54:322–333. doi: 10.1124/mol.54.2.322. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Smith RD, Caruso FS, Kellar KJ. Blockade of rat alpha3beta4 nicotinic receptor function by methadone, its metabolites, and structural analogs. J Pharmacol Exp Ther. 2001;299:366–371. [PubMed] [Google Scholar]
- Xiao Y, Tuan E, Sahibzada N, Tran T, Al-Muhtasib N, Yasuda R, Liu Y, Yenugonda V, Paige M, Brown M, Kellar K. Comparison of pharmacological properties of human versus rat alpha4beta2 and alpha3betat4 nicotinic acetylcholine receptors stabely expressed in HEK293 cells. New Orleans. 2012 [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, Biasi MD. Multiorgan Autonomic Dysfunction in Mice Lacking the β2 and the β4 Subunits of Neuronal Nicotinic Acetylcholine Receptors. J Neurosci. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Gonzalez A, Bonn-Miller MO, Bernstein A, Goodwin RD. Negative reinforcement/negative affect reduction cigarette smoking outcome expectancies: incremental validity for anxiety focused on bodily sensations and panic attack symptoms among daily smokers. Exp Clin Psychopharmacol. 2008;16:66–76. doi: 10.1037/1064-1297.16.1.66. [DOI] [PubMed] [Google Scholar]