Abstract
Although necrosis in the acetaminophen (APAP) model is known to be regulated by c-Jun NH2- terminal kinase, (JNK) through interaction with mitochondria, the role of necroptosis through receptor interacting proteins 1 and 3 (RIPK1 and RIPK3) has also been suggested. Our aim was to determine the relationship between these two mechanisms of cell death. To verify the participation of RIPK1, we used antisense knockdown and confirmed protection comparable to the RIPK1 inhibitor, necrostatin in vivo and in vitro. However, we found no evidence that RIPK3 is expressed in primary mouse hepatocytes under basal conditions or after APAP and RIPK3−/− mice were not protected. RIPK3 was exclusively expressed in nonparenchymal cells. RIPK1 knockdown protected RIPK3−/− mice to the same extent as wild type mice, underscoring the independent role of RIPK1. We confirmed necroptosis is not involved in APAP toxicity by using mixed lineage kinase domain-like protein (MLKL) −/− mice, which were not protected from APAP. Next we addressed if there is interplay between RIPK1 and JNK. RIPK1 knockdown decreased the level of JNK activation and translocation to mitochondria and abrogated subsequent translocation of dynamin-related protein-1 (Drp1). Interestingly, APAP induced the translocation of RIPK1 to mitochondria which was unaffected by the knockdown of the mitochondrial JNK docking protein, Sh3 homology3 binding protein5 (Sab).
Conclusion
RIPK1 participates in APAP induced necrosis upstream of JNK activation while RIPK3 and MLKL are dispensable, indicating that necroptosis does not contribute to APAP-induced necrosis and RIPK1 has a unique, independent role.
Keywords: Necrostatin, mitochondria, JNK, RIPK3, MLKL
Acetaminophen (APAP) is the leading cause of acute liver failure in the United States (1). The mode of cell death in APAP toxicity is thought to be largely necrosis and non-apoptotic (2–5). Prior research has shown that the JNK pathway, involving mitochondria via the JNK binding protein Src homology 3-domain binding protein-5 (Sh3bp5 or Sab), mediates APAP induced hepatocyte necrosis. Inhibition of this signaling cascade by JNK inhibition or its binding to Sab protects against APAP (6–10). Since interfering with this signal transduction pathway abrogates cell death, APAP toxicity may be viewed as a type of “regulated” necrosis.
Recently much interest has been directed towards elucidating the molecular mechanisms of necroptosis (11, 12). The receptor interacting proteins are serine-threonine protein kinases that have important roles in this pathway. The functions of receptor interacting protein kinase-1 (RIPK1) are complex as it participates in inflammatory pathways and apoptosis as well as necroptosis and has pro-survival functions (13). In the presence of caspase 8 inhibition, TNF binding to its receptor results in the formation of a structure containing RIPK1 and RIPK3, termed the necrosome which requires the kinase activity of RIPK1. RIPK3 then activates a pseudokinase, MLKL, which oligomerizes and breaches the cell membrane and perhaps intracellular organelles (14, 15). Furthermore, it has been recently shown that in certain cell types such as intestinal epithelial cells, RIPK1 paradoxically promotes cell survival indicating the intricacies of survival and death pathways and potentially alternative and kinase independent functions for RIPK1 (16, 17). The first studies showing the role of RIPK1 in necroptosis were carried out with the kinase inhibitor necrostatin-1 (nec-1) which protects mice against cardiac myocyte necrosis, renal ischemia reperfusion injury, retinal photoreceptor necrosis and TNF induced systemic inflammation among other disease models (18–23). Nec-1 has also been shown to inhibit APAP induced hepatotoxicity in mice both in vitro and in vivo, resulting in suggestions that necroptosis plays a role (24–27).
While RIPK1 is ubiquitously expressed and RIPK1 deficient mice die soon after birth, the presence of RIPK3 in all tissues has not been confirmed and RIPK3−/− animals are viable without any obvious phenotype. In fact RIPK3 presence in hepatocytes has been controversial with early studies not detecting RIPK3 in liver under basal conditions and more recent papers suggesting low expression in liver with rapid induction after APAP and alcohol feeding (25, 28–30).
We provide in vivo evidence that RIPK1 participates in APAP toxicity and that chemical inhibition of RIPK1, as well as, knockdown, abrogated APAP induced necrosis upstream of JNK activation. In contrast RIPK3−/− mice were not protected against APAP toxicity. However both nec-1 in vitro and RIPK1 knockdown in vivo protected RIPK3−/− mice from acetaminophen hepatotoxicity indicating a necroptosis independent mechanism for RIPK1. Additionally we observed no protection against APAP in MLKL −/− mice, which definitively rules out any role for necroptosis in this model. RIPK1 knockdown also decreased JNK activation and DRP1 translocation to mitochondria. Knockdown of Sab inhibited APAP-induced JNK activation and necrosis but did not prevent RIPK1 translocation to mitochondria. Thus, our work demonstrates a role for RIPK1 upstream of JNK, but no role for RIPK3, MLKL or necroptosis in murine APAP toxicity.
Experimental Procedures
Reagents
For Western blot analyses we used antisera to RIPK1, JNK, P-JNK, β actin and PHB1 from Cell Signaling (Danvers, MD). RIPK1, DLP1/DRP1 antisera from BD biosciences and RIPK3 antibody from Imgenex (San Diego, CA), ProSci (Loveland CO) and Abgent (San Diego, CA) and MLKL antisera from Millipore (Darmstadt, Germany) were also used. Monoclonal RIPK3 antibody was provided by Dr. Kim Newton of Genentech (San Francisco, CA). BALB/3T3 lysate was obtained from Abcam (Cambridge, MA). Acetaminophen and Mdivi were purchased from Sigma Aldrich (St. Louis, MO). NAPQI adduct antisera was provided by Laura James at the University of Arkansas. Necrostatin-1 (Nec-1) and inactive necrostatin (Nec-1i) were from Calbiochem (San Diego, CA). Antisense oligonucleotide was provided by William Gaarde, (Isis pharmaceuticals, Carlsbad, CA). Refer to Supplemental Methods for ASO treatment protocol.
Animals
Male C57BL/6n mice (6–8 weeks of age) were obtained from Harlan Bioproducts for Science Inc. (Indianapolis, IN). RIPK3−/− mice on a C57BL/6n background were provided by Dr. Vishva Dixit at Genentech (San Francisco, CA). MLKL −/− mice (on C57BL/6j background) were provided by Professor Warren Alexander at Walter and Eliza Hall Institute of Medical Research (Parkville, Australia). Male C57/BL6j mice (6–8 weeks old) were obtained from Jackson (Bar Harbor, Maine) as controls for the MLKL −/− mice. All animals were housed in an environmentally controlled room with 12 h light/dark cycle and allowed free access to food and water. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Southern California and followed the criteria of the National Research Council for the care and use of laboratory animals in research. APAP was dissolved in warm phosphate buffered saline (PBS) (55° C) and cooled to 37° C before intraperitoneal injection of overnight fasted mice at a dose of 300 mg/kg (for RIPK1 ASO or RIPK3−/− and MLKL−/− experiments) or 500 mg/kg (for survival experiments and in vivo experiments with inhibitors dissolved in DMSO). In in vivo experiments nec-1 was dissolved in DMSO (8.3%) and PBS (1 mg in 125 μl of DMSO diluted with 1375 μl of PBS). Nec-1 (10 mg/kg) was injected intraperitoneally, 45 minutes prior to APAP injection (500 mg/kg). The same volume of inactive necrostatin in DMSO/PBS was injected into control animals. Adenoviral shRNA knockdown of Sab was done as previously described (4). Serum alanine aminotransferase (ALT) was measured at the University of Southern California Pathology Reference Laboratory or using kit from Teco diagnostics (Anaheim, CA).
Hepatocyte isolation and culture
Primary mouse hepatocytes (PMH) were isolated and cultured as previously described (31). Quantitation of total and necrotic cells (Sytox Green positive) was performed by counting a minimum of 1,000 cells in 10 different fields using image J, as previously described (32). Cell culture experiments were performed in two different ways with inhibitors being added before or after APAP treatment. In the pre-APAP treatment model, hepatocytes were first treated with various inhibitors and one hour later, APAP dissolved in medium was added in the continual presence of an inhibitor. Cell viability was assessed at 24 or 48 hrs after exposure. In the post-APAP treatment protocol, hepatocytes were first exposed to different doses of APAP in media. After 2 h exposure to APAP, the culture media was changed and the APAP was removed; then hepatocytes were treated for 24–48 hrs with Nec-1, Mdivi, or inactive necrostatin and DMSO as controls.
Nonparenchymal cell isolation
For Kupffer cell isolation, mice were treated with PBS or APAP for 3 hours, subsequently anesthetized and after collagenase perfusion of liver, cells were placed on ice in Hank’s balanced salt solution for 15 mins, for the heavier hepatocytes to precipitate. The supernatant containing the non-parenchymal fraction was centrifuged at 1500 g at 4° for 10 mins. The pellet was resuspended in 8 ml of GBSS-B (Sigma Aldrich, St. Louis, MO) and mixed with 12 mls of 30% Histiodenz solution in GBSS-A (Sigma Aldrich, St. Louis, MO). The mixture was separated into two 15 ml tubes (10 mls each). Another 2 ml of GBSS-B was gently added to the top of each tube and again centrifuged (4°) at 1500 g for 20 mins. The thin brown layer below the top 2 mls was carefully collected into a clean 50 ml tube and washed with 50 ml of washing buffer. The solution was again centrifuged at 1500 g for 5 mins and supernatant discarded. The pellet, which is highly enriched Kupffer cells fraction was re-suspended. We confirmed the enrichment of F4/80 positive cells (over 65%) by FACS. In order to ensure high macrophage purity we repeated the experiments with plating of isolated KC on non-collagen coated cell culture plates and exchanged media at 3, 12 and 24 hours. We confirmed high purity of KC by imaging. These confirmatory KC isolation experiments were done at the Southern California APLD Non Parenchymal Core. Liver sinusoidal endothelial cells were isolated by elutriation as previously described (33, 34). For liver leukocyte isolation see Supplemental Methods.
Isolation of liver mitochondria and cytoplasm
Mitochondria were isolated by differential centrifugation as previously described (35). See Supplemental Methods for more detail.
Results
Role of RIPK1 in APAP induced hepatotoxicity
During the past few years, a number of investigators have demonstrated that nec-1 protects against APAP toxicity. We confirmed this in cell culture models in which nec-1 was added for one hour before APAP or first APAP was given for 2 hours, then media was changed and nec-1 or vehicle control was added to fresh media (Fig. S1A and S1B). Since nec-1 requires DMSO to solubilize, the latter design obviated the concern about DMSO inhibiting APAP metabolism. Similarly we confirmed that nec-1 protected in vivo, but the need for DMSO, though also given to controls, blunted the severity of the injury despite a high dose of APAP, 500 mg/kg (Fig. S2A and S2B).
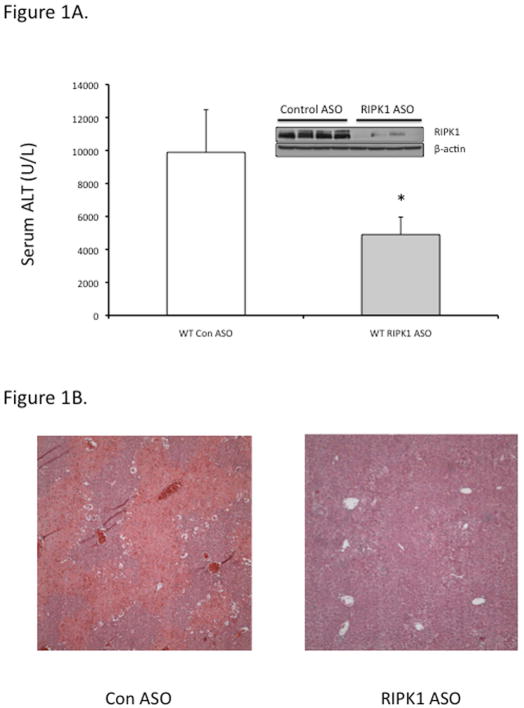
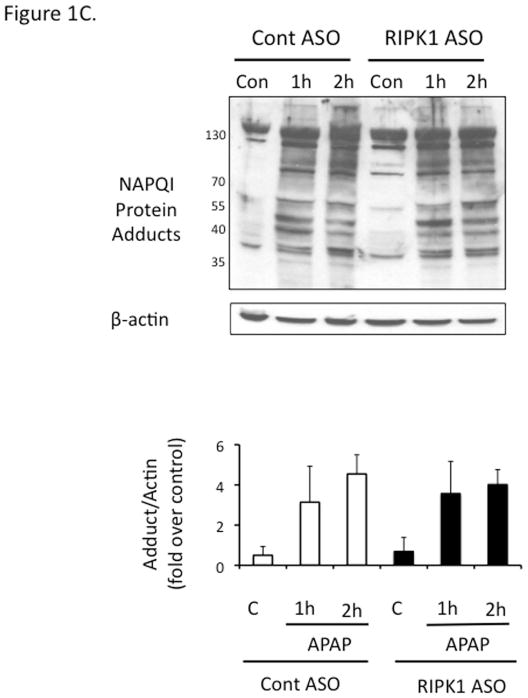
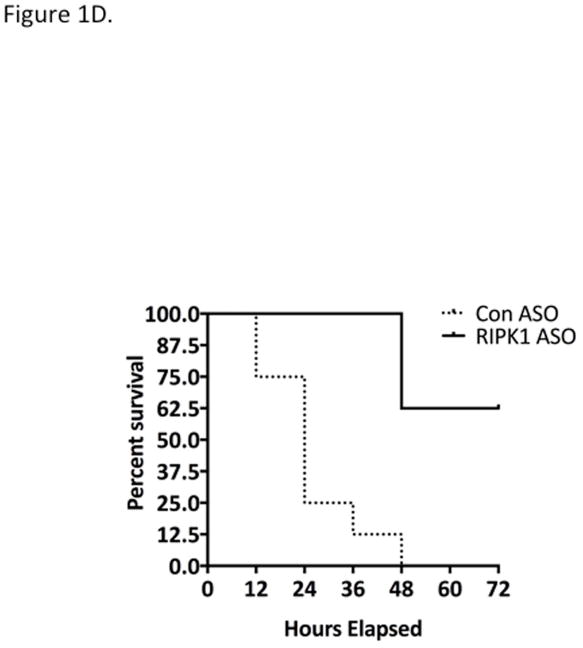
In view of the effects of nec-1 vehicle, DMSO, on APAP metabolism (36), and the possibility that the protection by nec-1 is due to increasingly recognized off target effects (37), we for the first time used an independent approach to avoid these concerns. Using antisense oligonucleotide (MOE-ASO), we efficiently knocked down RIPK1 and found significant protection in vivo against APAP 300 mg/kg compared to scrambled control ASO (Fig. 1A and 1B). The RIPK1 knockdown did not alter toxic metabolite exposure as reflected in protein adducts (covalent binding) (Fig. 1C) or GSH depletion (Fig. S3). To assess the extent of protection afforded by RIPK1 knockdown, we performed survival experiments with a lethal dose of APAP (500 mg/kg). Mice treated with RIPK1 ASO had a significantly higher survival than those treated with scrambled ASO, (62.5 % vs 0%) (Fig. 1D).
Figure 1. Effect of RIPK1 knockdown on APAP toxicity in vivo.
Mice were treated with RIPK1 or Control ASO (50 mg/kg) five times, subsequently injected with APAP 300 mg/kg and euthanized at 24 hrs. (A) Serum ALT (U/L), (inset, Western blot showing protein knockdown). N=12 per group; * p value ≤ 0.05 RIPK1 vs control ASO treated mice. (B) Representative histology H&E (10x); (C) Western blot of NAPQI adducts and densitometry. N=3; (D) Mice were treated with RIPK1 or control ASO and subsequently injected with 500 mg/kg APAP and followed for 72 hrs to assess survival. Results of two independent experiments N=8 per group; RIPK1 Log Rank P. value <0.001.
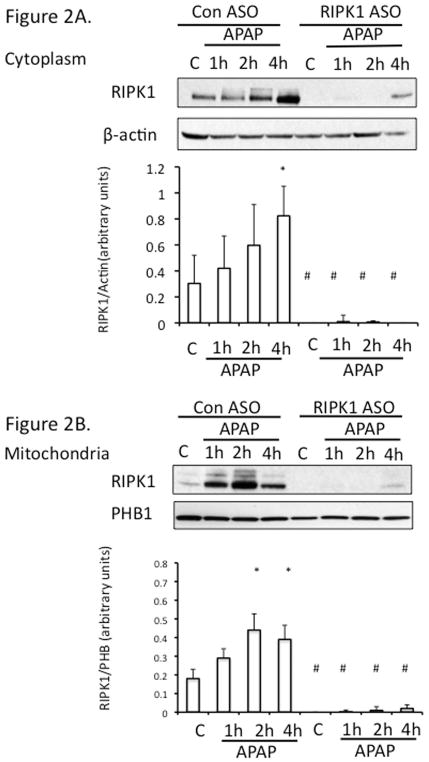
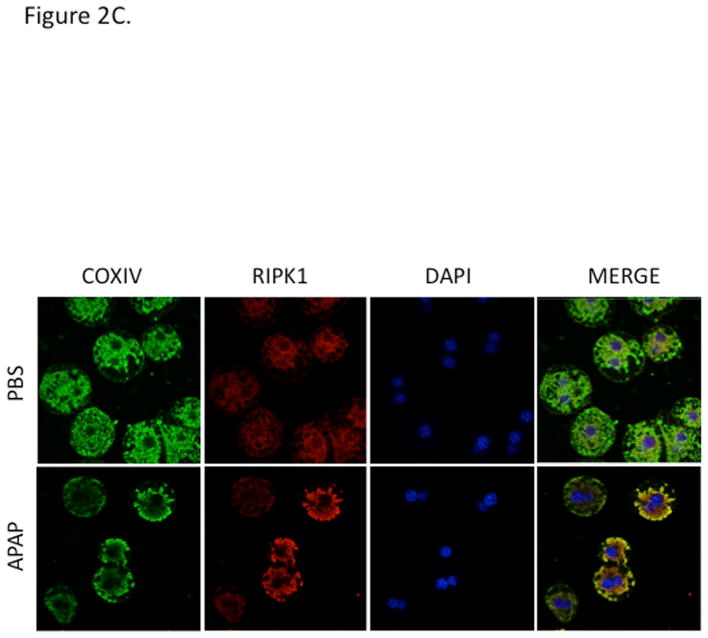
RIPK1 protein levels in cytoplasm increased over time up to 4 h after APAP in vivo (Fig. 2A) without an increase in mRNA (qPCR, not shown). Both in Western blots of mitochondrial extracts from in vivo experiments (Fig. 2B) and co-localization in cultured hepatocytes (Fig. 2C) increased association of RIPK1 with mitochondria was observed after exposure to APAP for 2 hr compared to controls.
Figure 2. RIPK1 translocates to mitochondria.
(A) Mice were treated with RIPK1 or control ASO and subsequently injected with 300 mg/kg of APAP and euthanized at the indicated time points. Western blot of cytoplasmic fraction for RIPK1 and densitometry; (B) Western blot of mitochondrial fraction for RIPK1 and densitometry; N= 3 mice per time point per group. * p value ≤ 0.05 APAP vs control #, p value ≤ 0.05 RIPK1 ASO vs control ASO. (C) Co-localization of RIPK1 and COXIV in PMH after PBS vs APAP10 mM for 2 hrs (64X magnification). Green: cytochrome oxidase IV, red: RIPK1, yellow: co-localization, and blue: DAPI nuclear stain.
Role of RIPK3
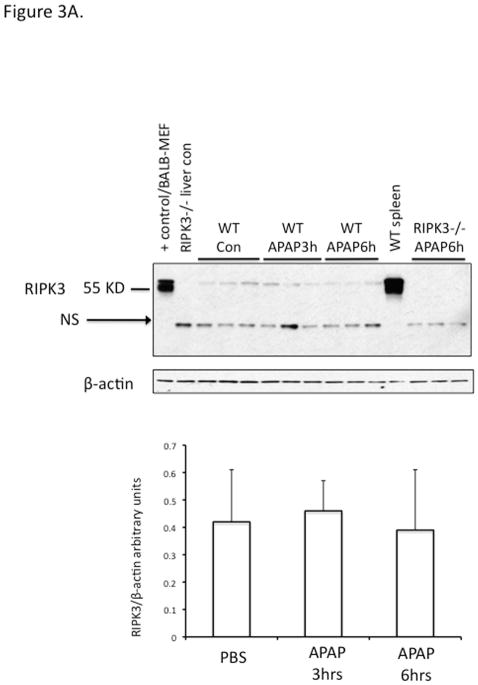
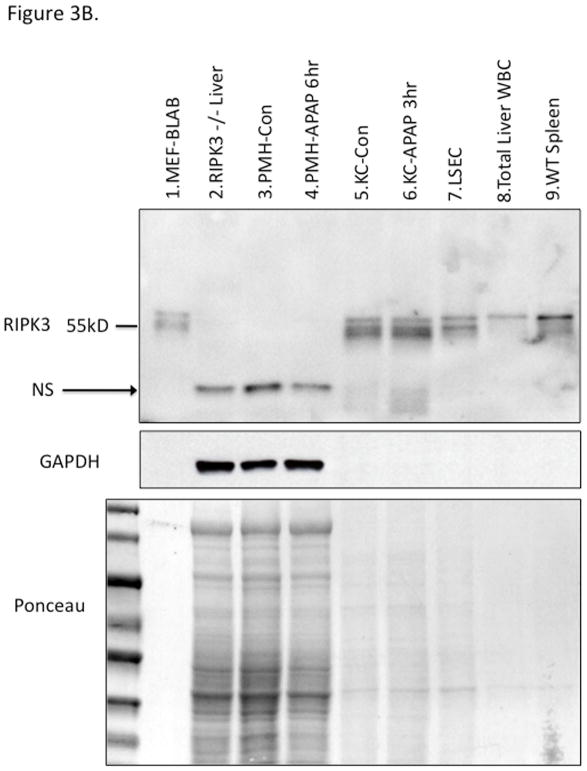
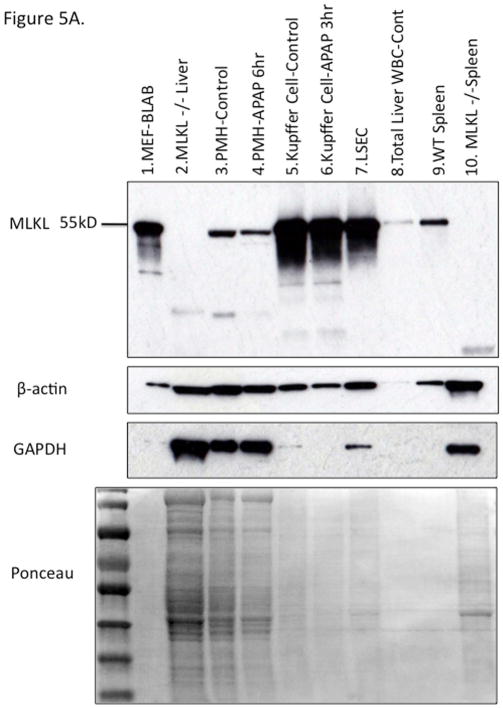
There has been a suggestion that RIPK3 plays a role in early APAP injury (25). Since RIPK1 and 3 form a complex which has been suggested to translocate to mitochondria to mediate necroptosis, we considered the possibility that the translocation of RIPK1 to mitochondria was indicative of co-translocation with RIPK3. Therefore we made a concerted effort to clarify this issue along with the role of RIPK3. First we assessed the levels of RIPK3 in liver in vivo before and after APAP. We screened nine different commercially available anti-sera, using positive (BALB and spleen) and negative (RIPK3−/− liver) controls. All showed a large number of nonspecific bands and several identified RIPK3 in the liver, but revealed no increase after APAP (Fig. S4A, S4B and S4C). Furthermore their use in IHC revealed artifactual identification of staining in zone 3 after APAP in RIPK3−/− liver in the area of necrosis, despite adequate peroxidase blocking (Fig. S4D). Therefore we focused on the use of a highly specific monoclonal antibody to RIPK3 provided by Kim Newton of Genentech. This antibody identified low levels of RIPK3 in liver under basal conditions which did not change after APAP (Fig. 3A). Cell fractionation of the liver showed that RIPK3 was completely absent in primary mouse hepatocytes (PMH) and did not increase after APAP (Fig. 3B). On the other hand isolated Kupffer cells (KC) were rich in RIPK3 but no change in protein level was seen after APAP in KC either. Isolated total liver leukocytes and sinusoidal endothelial cells also expressed high levels of RIPK3. In fact the NPC fraction expressed such high levels of protein that on depicted immunoblots it was necessary to load 1/4th or less the amount of protein as with hepatocytes (Ponceau Fig. 3B). Furthermore, we confirmed absence of RIPK3 in C57B6j hepatocytes (Fig. S4E). Therefore we conclude that RIPK3 is not directly relevant to hepatocytes in the APAP model.
Figure 3. Expression of RIPK3 in liver cells.
WT mice were treated with PBS or APAP 300 mg/kg for 3 and 6 hours and RIPK3 −/− mice were treated with APAP for 6 hrs. (A) Immunoblot of 30 ug of whole liver lysate using Genentech RIPK3 monoclonal antibody at indicated time points and positive and negative controls and densitometry normalized to β-actin. (B) Immunoblot depicting liver cell fractions and positive (lane 1) and negative controls (lane2) for RIPK3. 50 ug of freshly isolated untreated PMH (lane 3) or treated with APAP 10 mM for 6 hr prior to Western blot (lane 4), 15 ug of Kupffer cells isolated from control (lane 5) or APAP (300 mg/kg) treated mice for 3 hr (lane 6), 15 ug of LSECs isolated from control mice using elutriation (lane 7), 10 ug total liver leukocyte (lane 8) and 10 ug of wild type spleen (lane 9). Ponceau stain for protein loading shown for comparison, GAPDH is loading for PMH Con vs APAP 6 hr. NS: nonspecific.
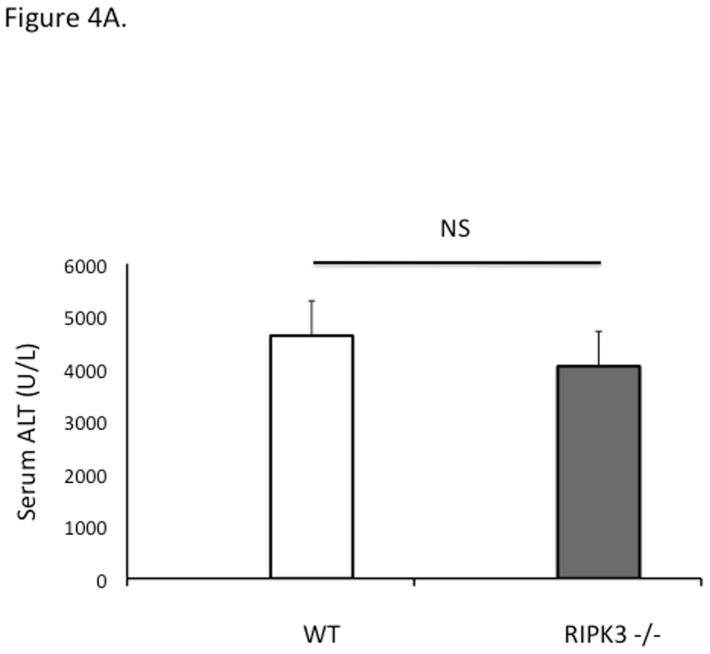
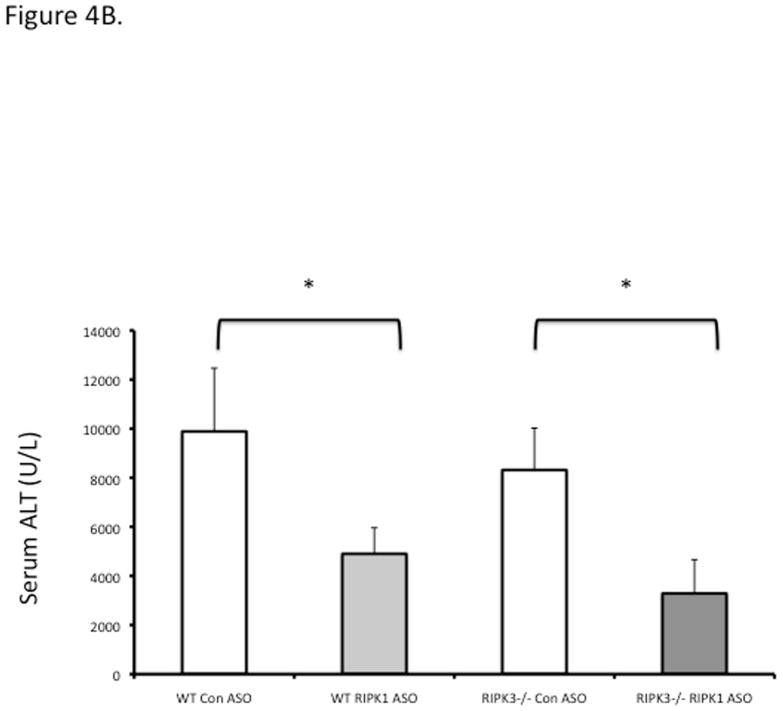
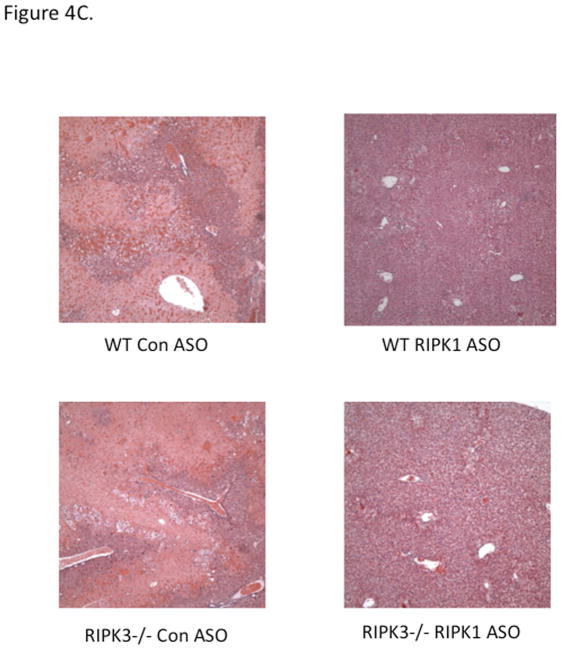
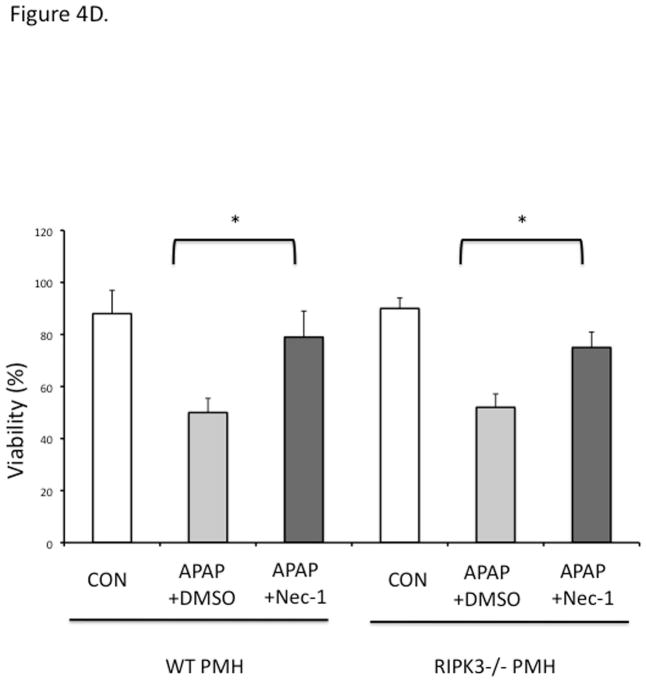
RIPK3−/− mice were not protected against APAP at early (6 h) or late (24 h) time points (Fig 4A, 4B). To prove that RIPK1 is playing a role in APAP toxicity independent of RIPK3, we assessed the effect of knockdown of RIPK1 in wild type and RIPK3 −/− mice after APAP. RIPK1 knockdown protected both wild type and RIPK3−/− mice against APAP (Fig. 4B and 4C). Isolated PMH from RIPK3 −/− mice were not protected against APAP 20 mM, however we observed a similar protection against APAP when nec-1 was added to RIPK3−/− PMH, 2 hours after continuous exposure to APAP 20 mM (Fig. 4D), consistent with the in vivo data. Therefore, RIPK1 inhibition or knockdown protects RIPK3−/− mice from APAP toxicity.
Figure 4. Effect of RIPK1 knockdown and inhibition on RIPK3 −/− mice and hepatocytes.
(A) Overnight fasted WT and RIPK3 −/− mice were treated with APAP 300 mg/kg and euthanized after 6 hr. N= 12 per group, ALT (U/L); (B) RIPK3−/− and WT controls were given Control ASO or RIPK1 ASO for five doses and then injected with 300 mg/kg of APAP and euthanized at 24 hrs, ALT (U/L), *P<0.05 RIPK1 vs Control ASO; (C) Representative histology (H&E) at 24 hr time point (10x). Results are mean ± S.D, For B&C N=12 at least per group; (D) Viability of PMH from WT vs RIPK3 −/− mice treated with APAP 20 mM for 2 hours. Subsequently media was exchanged and Nec-1 or DMSO was added. Results are mean ± S.D, N=3 *P<0.05.
Role of MLKL
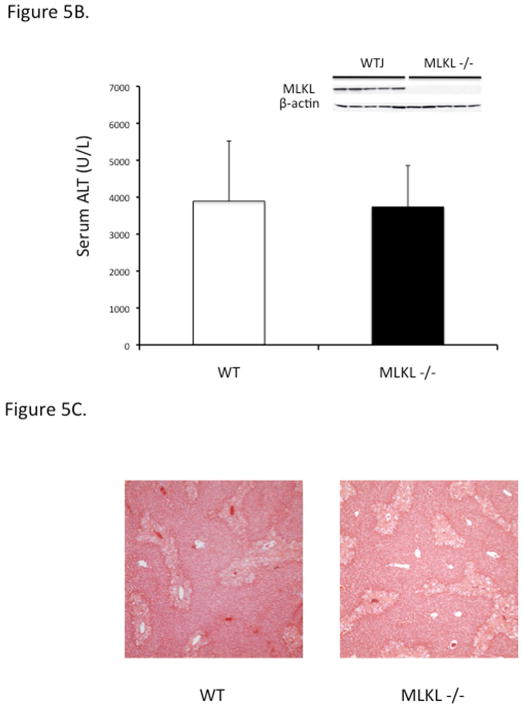
Nec-1 has been shown to protect against APAP in mice raising the possibility that necroptosis may be involved in this hepatotoxicity model. However RIPK3 −/− mice are not protected against APAP. Sincewe did not find any evidence of RIPK3 in PMH we next looked to see if MLKL is present in liver cells. Indeed, hepatocytes do express MLKL though to a lesser extent than nonparenchymal cells (Fig 5A). In order to definitively evaluate if APAP toxicity involves necroptosis we treated MLKL−/− mice with APAP and found no protection in ALT or histology compared to strain matched WT controls (Fig 5B and 5C). Note, the MLKL−/− mice are C57B6j substrain which are less sensitive to APAP toxicity compared to C57B6n substrain, accounting for somewhat lower ALT levels and extent of necrosis (38). Therefore, necroptosis is not contributing to cell death in the murine APAP hepatotoxicity model.
Figure 5. MLKL expression in liver cells and APAP toxicity in MLKL −/−.
(A) Immunoblot of MLKL, β-actin and GAPDH of liver cell fractions and BALB fibroblast cell lysate with variable loading. Positive control (lane 1, 10 ug) and negative control (lane2, 50 ug) for MLKL. Freshly isolated untreated PMH (lane 3, 30 ug) or treated with APAP 10 mM for 6 hr in vitro prior to Western blot (lane 4), Kupffer cells isolated from control (lane 5, 10 ug) or APAP (300 mg/kg) treated mice for 3 hr (lane 6, 10 ug), LSECs isolated from control mice using elutriation (lane 7, 10 ug), total liver leukocyte (lane 8, 5 ug), wild type spleen (lane 9, 5 ug) and MLKL −/− spleen (lane 10, 15 ug). Ponceau stain for protein loading shown for comparison among different cells. (B) Overnight fasted WT and MLKL −/− mice were treated with APAP 300 mg/kg and euthanized at 24 hrs, ALT (U/L) (inset, Western blot showing protein knockout) Results are mean ± S.D, N= 5 per group; (C) Representative histology (H&E) at 24 hrs (10X).
Role of RIPK1 in relation to JNK
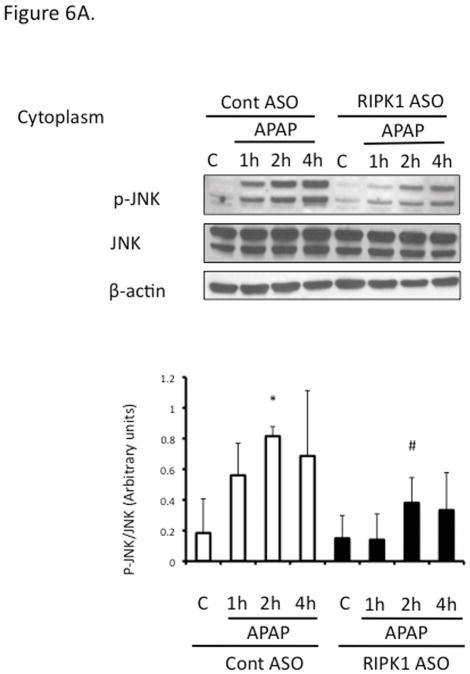
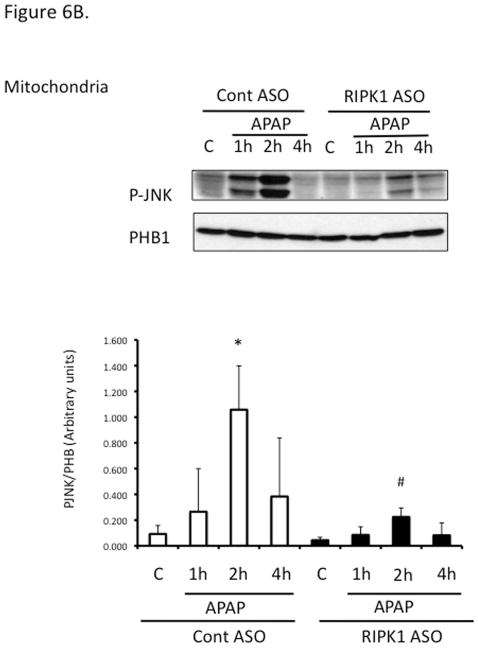
Since sustained JNK activation and translocation to mitochondria has been implicated in APAP toxicity, to begin to understand the mechanism of the necroptosis-independent role of RIPK1, we assessed the effect of RIPK1 knockdown on the time course of JNK. Sustained JNK activation and translocation to mitochondria were decreased after RIPK1 knockdown (Fig. 6A and 6B).
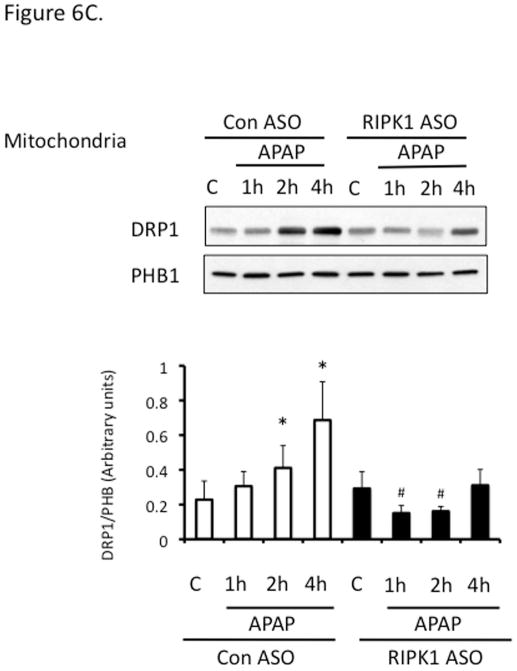
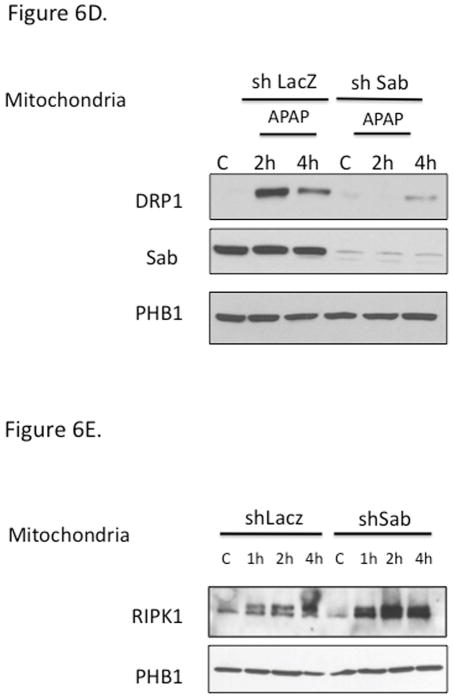
Figure 6. Relation of RIPK1 with the activation of JNK and tranlocation of DRP1.
Mice were treated with RIPK1 or control ASO and subsequently injected with 300 mg/kg of APAP and euthanized at the indicated time points; (A) Western blot of cytoplasm for P-JNK, total JNK, and β-actin and densitometry; (B) Western blot of mitochondrial fraction for p-JNK and PHB1 and densitometry; (C) Western blot of mitochondrial fraction of Control and RIPK1 ASO treated mice for Drp1 and densitometry; N= 3 mice per time point per group.* p value < 0.05 APAP vs control, # p value < 0.05 RIPK1 ASO vs control ASO; (D) Western blot of mitochondrial fraction of adenovirus shSab vs shLacZ treated mice for Drp1. (E) Western blot of mitochondrial fraction of adenovirus shSab vs shLacZ treated mice for RIPK1.
Drp1 translocation to mitochondria has been suggested to be downstream of JNK activation and mediate mitochondrial fission (25). First, we confirmed the protective effect of mdivi, a Drp1 inhibitor on APAP hepatotoxicity in PMH (Fig. S5). Interestingly, Drp1 translocation was suppressed after RIPK1 knockdown (Fig. 6C).
In further support for the role of JNK downstream of RIPK1 and upstream of Drp1 translocation, knockdown of Sab, the mitochondrial target of p-JNK, which is necessary for sustained JNK activation, abrogated Drp1 translocation (Fig. 6D). However, knockdown of Sab did not prevent APAP-induced RIPK1 translocation to mitochondria, which was somewhat enhanced after Sab knockdown (Fig. 6E). Therefore RIPK1 is not binding to Sab and its translocation to mitochondria is likely not secondary to toxicity as knockdown of Sab abrogates toxicity in the presence of RIPK1 translocation.
Discussion
Acetaminophen induced hepatotoxicity remains a leading cause of acute liver failure in the U.S. (1). The murine APAP toxicity model closely mimics human pathophysiology and is an excellent model to examine the mechanisms of necrotic liver cell death. APAP is metabolized in hepatocytes to a highly reactive metabolite (NAPQI) which covalently binds with intracellular and mitochondrial proteins(39). APAP necrosis is regulated by a series of signaling events that have been elucidated over the past decade. Inhibition, knockout and/or silencing of ASK1, MLK3, GSK3β, PKCα, JNK, Sab and Cyclophilin D have all been shown to be protective against APAP, indicating that APAP cytotoxicity is a model of regulated necrosis as its course can be altered by pharmacologic and genetic interventions (6, 7, 9, 26, 40–44). It is important to point out that APAP induced cell death is TNF and caspase independent (3–5). Recently much attention has been directed to the necroptosis pathway and it has been suggested that RIPK3 mediated necroptosis may play a role in liver cell death from APAP (24, 25). RIPK3−/− mice have been reported to be protected in the early phase of APAP toxicity and RIPK3 protein, though absent at baseline, was suggested to be induced in liver as early as three hours after APAP(25).
We had previously observed in preliminary studies that necrostatin protects against APAP both in vivo and in vitro (27), and this has subsequently been shown by others (24–26). However nec-1 has off target effects (37) and requires a vehicle (DMSO) which interferes with APAP metabolism (36). Therefore, a more definitive confirmation was required regarding the role of RIPK1 as well as RIPK3. In contrast to an earlier report, we found no evidence that RIPK3−/− mice are protected in the early phase of toxicity but our findings are in agreement that there is no protection in the late phase. However, we could find no evidence of induction of RIPK3 in whole liver or isolated PMH and RIPK3 in liver was found exclusively in the nonparenchymal cells. The reason for the discrepancies between others and our work are not entirely clear but could be due to mismatch of mouse strains or inaccuracies of commercial antisera. We examined many commercially available antibodies to RIPK3 but focused on the ones most used by other investigators. In liver homogenates, all antibodies identified many nonspecific bands, especially in the 55KD region where RIPK3 is seen. Most importantly all commercially available antibodies reacted with RIPK3−/− liver indicating the non-specificity of the 55KD band seen with these antisera (Fig. S4A–E). In contrast, the monoclonal Genentech antibody we used was highly sensitive and specific.
The ultimate mediator of necroptosis is MLKL (45–48). In order to conclusively rule out the involvement of necroptosis, we treated MLKL−/− mice with APAP and found no protection in knockout animals compared to strain matched controls (C57B6j). Since C57B6 mice sub-strains can vary in their susceptibility to APAP (38) we ensured accuracy by strain matching controls and knockout animals. The fact that MLKL−/− mice are not protected from APAP excludes necroptosis as a contributor to cell death in this model.
We next confirmed that RIPK1 was a key contributor to APAP hepatotoxicity. Knockdown of RIPK1 conferred a statistically significant survival benefit to WT mice when given a lethal dose of APAP.. Interestingly, we observed that the induction of RIPK1 after APAP occurred in conjunction with RIPK1 translocation to mitochondria. Therefore, since others have suggested that the RIPK1/RIPK3 complex translocates to mitochondria it was particularly noteworthy that we found no RIPK3 in hepatocytes (49). Thus RIPK1 plays a role independent of RIPK3, MLKL and the necrosome in this context. This conclusion was further strengthened by the fact that knockdown of RIPK1 in vivo protected RIPK3−/− mice as well as wild type mice.
In the first 4 hr after APAP RIPK1 appears as a doublet suggesting that a slower migrating band may be a modified form (phosphorylated or ubiquinated). At present we do not know what signal activates RIPK1 and what this modification may be or how RIPK1 activates JNK. Work in other contexts has shown associations of RIPK1 with ASK1 and IRE1 and ASK1 and ER stress have been implicated in the APAP necrosis model as well (41, 50–52). JNK activation is downstream of RIPK1 indicating a signaling pathway to the MAP kinases. Since RIPK1 translocates to the mitochondria, it is conceivable that the interplay between RIPK1 and MAP kinases is occurring at the outer membrane of mitochondria. We have found no evidence of direct interaction between RIPK1 and Sab or JNK in IP or proteomic analysis of mitochondrial extracts (unpublished, Dara et al). Interestingly, RIPK1 translocation to the mitochondria was not inhibited by Sab knockdown so the binding target of RIPK1 on mitochondria remains to be identified. Thus, our findings suggest a role for RIPK1 upstream of JNK activation. It is uncertain if the translocation of RIPK1 to mitochondria is required for its effect on the activation pathway of JNK (e.g. proximity of signaling molecules).
Although we conclude that RIPK3 and MLKL are dispensable for APAP induced acute liver injury, it is conceivable that necroptotic death of nonparenchymal cells such as LSECs and KCs contribute to microvascular injury and or inflammation in other types of liver disease and indirectly lead to hepatocyte death. Interestingly, in contrast to RIPK3, hepatocytes do express MLKL, which raises the question of its physiological role and significance especially in the absence of RIPK3.
Supplementary Material
Acknowledgments
This work was supported by NIH grant RO1-DK067215 (NK), the USC research Center for Liver Diseases P30-DK048522 cell culture, analytical/metabolic/instrumentation and cell and tissue imaging S10-RR022508 core facilities.
The assistance and advice of USC colleagues L Mesropyan, M Ybanez, E Zandi, B Saberi, L DeLeve and ZX Liu is greatly appreciated.
Abbreviations
- APAP
acetaminophen
- RIPK1
receptor interacting protein kinase 1
- RIPK3
receptor interacting protein kinase 3
- MLKL
mixed lineage kinase domain-like protein
- JNK
c-Jun NH2- terminal kinase
- Sab
Sh3 homology3 binding protein5
- Drp1
dynamin relatedprotein-1
- GSH
glutathione
- NAPQI
N-acetyl-p-benzoquinone imine
- ROS
reactive oxygen species
- MPT
mitochondrial permeability transition pore
- TNF
tumor necrosis factor
- PMH
primary mouse hepatocytes
- Nec-1
Necrostatin-1
- Nec-1i
inactive/control necrostatin
- ALT
alanine aminotransferase
- ASO
antisense
- CypD
Cyclophilin D
- COXIV
cytochrome oxidase IV
- PHB1
Prohibitin-1
- LSEC
liver sinusoidal endothelial cell
- KC
Kupffer cell
- Con
control
- WT
wild type
References
- 1.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Boess F, Bopst M, Althaus R, Polsky S, Cohen SD, Eugster HP, Boelsterli UA. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology. 1998;27:1021–1029. doi: 10.1002/hep.510270418. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaeschke H, Williams CD, Farhood A. No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology. 2011;53:718–719. doi: 10.1002/hep.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, et al. Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure. Gut. 2007;56:982–990. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jouan-Lanhouet S, Riquet F, Duprez L, Vanden Berghe T, Takahashi N, Vandenabeele P. Necroptosis, in vivo detection in experimental disease models. Semin Cell Dev Biol. 2014;35C:2–13. doi: 10.1016/j.semcdb.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35C:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 14.Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci U S A. 2014;111:15072–15077. doi: 10.1073/pnas.1408987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, Goncalves A, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 18.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 20.Lim SY, Davidson SM, Mocanu MM, Yellon DM, Smith CC. The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther. 2007;21:467–469. doi: 10.1007/s10557-007-6067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 23.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, Hisatomi T, et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A. 2010;107:21695–21700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An J, Mehrhof F, Harms C, Lattig-Tunnemann G, Lee SL, Endres M, Li M, et al. ARC is a novel therapeutic approach against acetaminophen-induced hepatocellular necrosis. J Hepatol. 2013;58:297–305. doi: 10.1016/j.jhep.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. The receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013 doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derick Han MY, Mesropyan Lusine, Kaplowitz Neil. Receptor Interacting Protein 1 works in parallele with JNK in mediating acetaminophen induced liver injury [Abstract] Hepatology. 2011:515A. [Google Scholar]
- 28.Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC. The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 2000;473:285–291. doi: 10.1016/s0014-5793(00)01473-3. [DOI] [PubMed] [Google Scholar]
- 29.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- 31.Han D, Hanawa N, Saberi B, Kaplowitz N. Hydrogen peroxide and redox modulation sensitize primary mouse hepatocytes to TNF-induced apoptosis. Free Radic Biol Med. 2006;41:627–639. doi: 10.1016/j.freeradbiomed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Nagai H, Matsumaru K, Feng G, Kaplowitz N. Reduced glutathione depletion causes necrosis and sensitization to tumor necrosis factor-alpha-induced apoptosis in cultured mouse hepatocytes. Hepatology. 2002;36:55–64. doi: 10.1053/jhep.2002.33995. [DOI] [PubMed] [Google Scholar]
- 33.Deleve LD. Dacarbazine toxicity in murine liver cells: a model of hepatic endothelial injury and glutathione defense. J Pharmacol Exp Ther. 1994;268:1261–1270. [PubMed] [Google Scholar]
- 34.DeLeve LD, Wang X, McCuskey MK, McCuskey RS. Rat liver endothelial cells isolated by anti-CD31 immunomagnetic separation lack fenestrae and sieve plates. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1187–1189. doi: 10.1152/ajpgi.00229.2006. [DOI] [PubMed] [Google Scholar]
- 35.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon MY, Kim SJ, Lee BH, Chung JH, Kim YC. Effects of dimethylsulfoxide on metabolism and toxicity of acetaminophen in mice. Biol Pharm Bull. 2006;29:1618–1624. doi: 10.1248/bpb.29.1618. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, Goossens V, et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourdi M, Davies JS, Pohl LR. Mispairing C57BL/6 substrains of genetically engineered mice and wild-type controls can lead to confounding results as it did in studies of JNK2 in acetaminophen and concanavalin A liver injury. Chem Res Toxicol. 2011;24:794–796. doi: 10.1021/tx200143x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts DW, Pumford NR, Potter DW, Benson RW, Hinson JA. A sensitive immunochemical assay for acetaminophen-protein adducts. J Pharmacol Exp Ther. 1987;241:527–533. [PubMed] [Google Scholar]
- 40.Loguidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin d-regulated permeability transition. Hepatology. 2011;54:969–978. doi: 10.1002/hep.24464. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, et al. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285:8244–8255. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saberi B, Ybanez MD, Johnson HS, Gaarde WA, Han D, Kaplowitz N. Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and -independent signaling pathways. Hepatology. 2014;59:1543–1554. doi: 10.1002/hep.26625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Murphy JM, Silke J. Ars Moriendi; the art of dying well - new insights into the molecular pathways of necroptotic cell death. EMBO Rep. 2014;15:155–164. doi: 10.1002/embr.201337970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 50.Estornes Y, Aguileta MA, Dubuisson C, De Keyser J, Goossens V, Kersse K, Samali A, et al. RIPK1 promotes death receptor-independent caspase-8-mediated apoptosis under unresolved ER stress conditions. Cell Death Dis. 2014;5:e1555. doi: 10.1038/cddis.2014.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uzi D, Barda L, Scaiewicz V, Mills M, Mueller T, Gonzalez-Rodriguez A, Valverde AM, et al. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol. 2013;59:495–503. doi: 10.1016/j.jhep.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Zhang H, Lin Y, Li J, Pober JS, Min W. RIP1-mediated AIP1 phosphorylation at a 14-3-3-binding site is critical for tumor necrosis factor-induced ASK1-JNK/p38 activation. J Biol Chem. 2007;282:14788–14796. doi: 10.1074/jbc.M701148200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.