Abstract
Background
Mechanical ventilation worsens acute respiratory distress syndrome (ARDS), but this secondary ‘ventilator-associated’ injury is variable and difficult to predict. We aimed to visualize the propagation of such ventilator-induced injury, in the presence (and absence) of a primary underlying lung injury, and to determine the predictors of propagation.
Methods
Anesthetized rats (n=20) received acid aspiration (HCl) followed by ventilation with moderate tidal volume (VT). In animals surviving ventilation for at least two hours, propagation of injury was quantified using serial computed tomography (CT). Baseline lung status was assessed by oxygenation, lung weight, and lung strain (VT/expiratory lung volume). Separate groups of rats without HCl aspiration were ventilated with large (n=10) or moderate (n=6) VT.
Results
In 15 rats surviving longer than two hours, CT opacities spread outwards from the initial site of injury. Propagation was associated with higher baseline strain (propagation vs. no propagation, mean ± SD: 1.52 ± 0.13 vs. 1.16 ± 0.20, p<0.01), but similar oxygenation and lung weight. Propagation did not occur where baseline strain <1.29. In healthy animals, large VT caused injury that was propagated inwards from the lung periphery; in the absence of preexisting injury, propagation did not occur where strain was <2.0.
Conclusions
Compared with healthy lungs, underlying injury causes propagation to occur at a lower strain threshold and, it originates at the site of injury; this suggests that tissue around the primary lesion is more sensitive. Understanding how injury is propagated may ultimately facilitate a more individualized monitoring or management.
Introduction
Mechanical ventilation at lower tidal volume (VT) has been shown to improve survival in acute respiratory distress syndrome (ARDS) 1, suggesting that VT reduction protects against ventilator induced lung injury (VILI) by reducing inspiratory strain. To best limit damage, it might be important to detect early changes in vulnerable lung and to accurately determine the propensity for VILI. However, the onset of VILI is poorly characterized in the clinical setting, and it is almost always superimposed upon an underlying primary pulmonary lesion (e.g. aspiration, pneumonia, contusion) 2. Unfortunately, animal models with predictable (and short) courses of injury do not reproduce the variability observed in patients.
Computed tomography (CT) suggested that ARDS lung is composed of an atelectatic (dependent) region and a normally aerated (non-dependent) region 3. In this two-compartment model, the aerated ‘baby’ lung preferentially receives the bulk of each VT, but comprises a significantly smaller available volume than the lungs in a healthy subject. Because inflammation predominates in this ‘aerated’ region 4,5, measuring strain and visualizing the propagation of lung injury in the aerated lung could predict and characterize the trajectory of VILI.
However, in many patients with ARDS, the aerated and non-aerated lung is not clearly delineated into two distinct compartments. Instead, the radiologic pattern may be of diffusely distributed moderately aerated tissue 6. Though the volume of aerated lung may be appreciable in diffuse injury7, aerated and non-aerated lung are co-localized such that lung tissue interdependence 8,9 may be important. It is unlikely that the same relationship among inflation, deformation, and injury is at play when a given VT inflates a separate aerated lung compartment, compared with where aeration and non-aeration are diffusely intermingled. Heterogeneous tissue inflates in non-uniform fashion, which can increase strain in some microenvironments 10. In fact, recent CT findings indicate that variably aerated regions surrounding a primary locus of injury predict ARDS mortality 11. Thus, the distribution of aerated lung in ARDS could be a factor in determining how injury is propagated during mechanical ventilation. If true, this could help identify the most beneficial approaches and adjuncts to ventilation (e.g., very low VT, prone positioning) that are appropriate in any given patient.
In the current study, we used sequential CT to investigate the relationship between the spatial distribution of aerated/non-aerated lung vs. the tendency of injury to propagate during mechanical ventilation. In an in-vivo rat model of acid aspiration with variable injury trajectory, we sought predictors of its propagation. We hypothesized that secondary VILI originates adjacent to the primary lesions—due to the local diffuse intermingling of aerated and collapsed tissue—and propagates concentrically and in proportion to strain. Such a pattern was contrasted with observations in lungs that were free of pre-existing injury, where VILI has been shown to originate in the lung periphery 12 and, in the setting of the ‘baby lung’ appears to become generalized across the normally aerated lung tissue 4.
Methods
Male Sprague-Dawley rats were studied with approval by the Institutional Animal Care and Use Committee of the University of Pennsylvania (Philadelphia, PA, USA). The experimental protocol is described in full in the Supplemental Digital Content 1. General anesthesia and paralysis were induced and maintained with intraperitoneal pentobarbital and intravenous pancuronium bromide, the trachea was intubated, and peak inspiratory airway pressure (PIP), dynamic compliance, heart rate, blood pressure, and arterial blood gases were measured. Rats were ventilated in the supine position with a small animal ventilator. All animals received intraperitoneal and intravenous hydration. After euthanasia, lungs were fixed, sliced in the coronal plane, stained, and reviewed by a pathologist (CGD). 2 groups were studied:
Acid aspiration with moderate VT
We studied 20 ventilated rats (353±26 g) after hydrochlocic acid (HCl 2.5 mL•kg-1, pH 1.25) intratracheal. Because we aimed to investigate secondary progression of mild primary lung injury, no randomization was performed and animals were recruited sequentially: only rats that survived long enough to display the propagation were studied. HCl was injected in two aliquots with the animal in the right and left lateral positions and 45° head elevation. Rats were immediately returned to the supine position and allowed to stabilize while ventilated with PEEP 10 cm H2O and VT 6 ml/kg for 1 h. In pilot experiments with this HCl dose, we confirmed heterogenous distribution of HCl solution and variable course of injury in ventilated rats. After stabilization, ventilation continued for 3 h with moderate VT (12 mL•kg-1, PEEP 3 cmH2O, FiO2 1.0, f 53 min-1; Fig. 1). Six additional healthy rats (365 ± 28 g) were ventilated for three hours with these settings, to show the radiological and physiological effects of moderate VT ventilation in absence of underlying lung injury.
Figure 1.
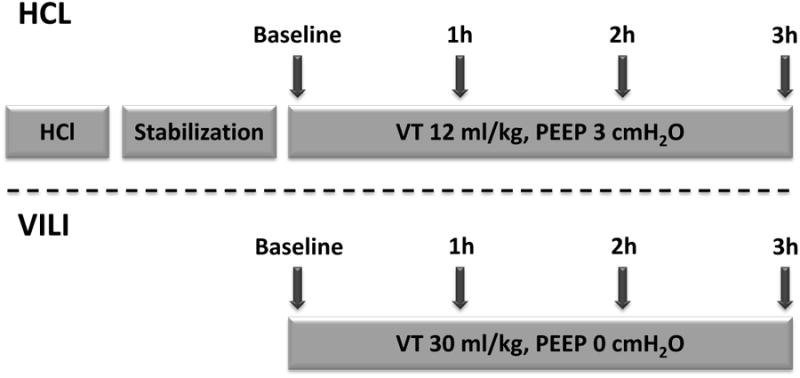
Experimental timeline in rats ventilated with moderate tidal volume (VT) after hydrochloric acid (HCl) aspiration (Top) and in previously normal rats with ventilator induced lung injury (VILI) by large VT ventilation (Bottom). In rats with aspiration, imaging was performed without interrupting moderate VT ventilation. In normal rats, high volume ventilation was suspended and VT was set at 12 ml/kg during each image acquisition, including baseline.
Normal animals with large VT
To compare the pattern of secondary propagation of preexisting lung injury with the evolution of VILI in the absence of primary injury, we ventilated a separate group of 10 healthy rats (VT 30 mL•kg-1, PEEP 0 cm H2O, FiO2 0.5, f 27 min-1) for up to 3 h or until PIP increased by 50% (Figure 1). A broad range of body weights (360 - 600 g) was used to ensure variable lung volumes.
Computed tomography
High-resolution whole-lung CT scans were acquired and reconstructed to three-dimensional whole lung maps with 200 μm isotropic resolution. Imaging was ventilator-gated and performed during 500-ms breath-holds. Following acid aspiration, inspiratory and expiratory images were obtained at baseline and repeated hourly (Figure 1) without changing VT. In healthy rats, imaging was repeated more often if PIP increased rapidly, and VT was reduced to 12 mL•kg-1 (f 53 min-1) during CT acquisitions to facilitate comparisons with the other group.
Image analysis
For each inspiratory image, three independent evaluators, blinded to group assignment, semi-quantitatively 13 rated (0 to 4) the spatial extension of high-density (ground-glass and consolidations) tissue in 4 central and in 4 peripheral sectors of 3 coronal (frontal-to-dorsal) and 3 axial (apical-to-basal) slices. Observers set image contrast to a fixed intensity window (2000 HU) and level (-1000). A global injury score was obtained at each time point; partial scores were also obtained in ventral and in dorsal areas. For quantitative CT analysis, 3-D whole-lung regions of interest were obtained by semi-automated, multi-landmark, registration-based lung segmentation methodology (the delineation of lung borders from surrounding structures) developed by the authors 14. Using established methods 15 of CT density analysis, each voxel was partitioned in air and tissue, allowing to quantify lung weight, end-inspiratory, and end-expiratory lung gas volumes (EILV, EELV); lung strain was defined as VT/EELV 16,17. The total weight of the lungs was partitioned between tissue compartments with different aeration (normo-aerated, poorly aerated, non-aerated, and hyperinflated tissue)18. Tidal recruitment (the weight of lung that collapses and reopens after each inspiration) was also calculated 18.
Statistical Analysis
The sample size was decided based on pilot studies where we noticed a survival rate of approximately 75% at two hours and of 50% at three hours, which allowed us to study propagation and to test whether baseline characteristics (chosen a priori) were predictive of such propagation. For semi-quantitative CT analysis, inter-observer agreement was expressed using the quadratic weighted kappa statistic, a test to compare two raters who use a categorically ordered measurement scale 19. Because there were three raters, kappa was calculated three times for each rat between the three possible pairs of data. Correlations between variables were tested by linear regression. Phi coefficient was calculated as a measure of association for binary variables. To study the relations between baseline variables and the propagation of injury in the lung, multiple ANOVA tests were performed separately for non-imaging and imaging markers followed by post hoc t-tests to specifically identify which means were significantly different from each other. Bonferroni adjustment was performed for multiple post-hoc comparisons. Repeated measurements two-way ANOVA was conducted to examine the main effects of time on CT-derived and physiological variables. Binary variables were tested using Fischer's exact test. P<0.05 (for two-tailed testing) was considered significant. Statistical analysis was performed using “R” (R Foundation for Statistical Computing; Vienna Austria, http://www.R-project.org) applications developed in the authors' laboratory.
Results
Underlying Injury Present
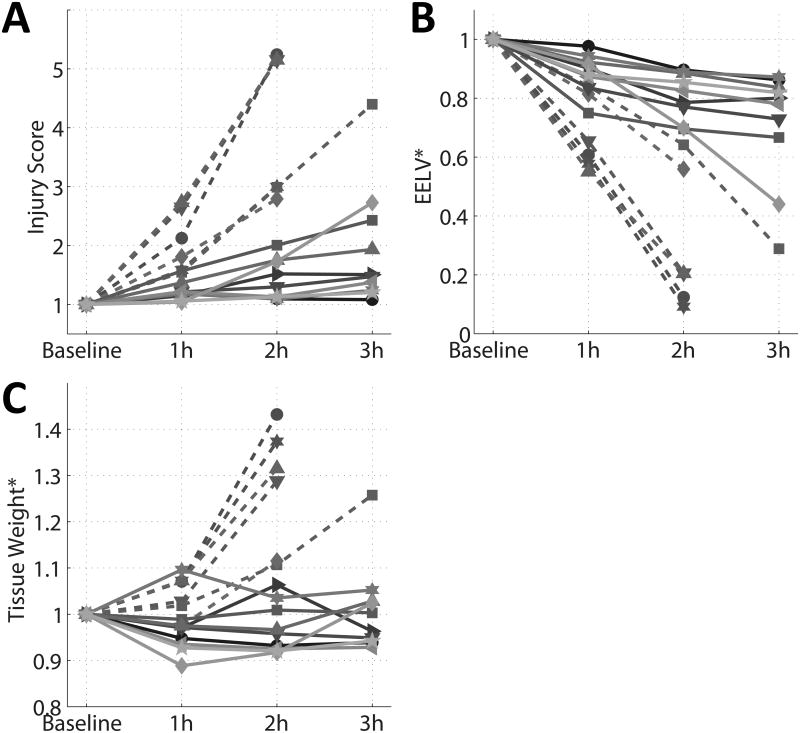
Of the 20 rats that received HCl, five were excluded from the analysis because of severe post-aspiration hypoxemia (PaO2/FiO2 <300 mmHg; n=2) or short survival <2h (n=3), precluding the quantification of injury propagation (baseline characteristics of this subgroup are shown in Supplemental Digital Content 2). In the 15 rats that survived longer than 2h, baseline (i.e. post-aspiration) CT demonstrated circumscribed lesions in the dorsal lung (Figure 2). Two radiological patterns of injury were observed in this cohort: ‘diffuse’ (Propagated; Figure 2, A), and Contained (Figure 2, B). The evolution of lung injury is also illustrated by animations accessible in Supplemental Digital Content 3 and 4. Both animations were obtained by combining sequential CT scans in the coronal slice in two animals after HCl: propagation was centrifugal in both animals. Injury progression—reflected in the radiologic injury score (Figure 3, A)—was accompanied by decreased EELV (Figure 3, B) and increased lung weight (Figure 3, C). Propagation of injury, defined by >100% increase in score after two hours of ventilation with moderate VT, occurred in six animals (of which five died before 3h).
Figure 2.

Radiological injury propagation in two representative rats. Images were obtained after acid aspiration (following a period of stabilization of one hour) and were repeated hourly. Coronal (A) and axial (B) images are shown. One animal (top panel) showed rapidly spreading radiological infiltrates and died after two hours of ventilation. The second animal (bottom panel) had limited injury propagation and survived until the end of the experiment.
Figure 3.
Individual trends of: A) radiological injury score, B) end-expiratory lung volume (EELV), and C) estimated lung weight after acid aspiration. All variables were measured by computed tomography during ventilation and are expressed as fraction of the baseline values obtained after acid aspiration. Dashed lines represent animals with radiological propagation of lung injury (score more than doubled within the first two hours).
Two approaches were taken to analyzing the HCl aspiration cohort. First, all 15 rats were considered as a single group. Second, animals were divided into two subgroups based on radiological injury propagation (i.e., Propagated vs. Contained).
Whole-group analysis
During the experiments, oxygenation, EELV, PIP, and compliance were all impaired, and estimated lung weight (edema) increased, consistent with progressive lung injury (Table 1). The radiologic injury score increased from 37.6±10.2 to 58.2±22.8 to 101.8±60.3 at baseline, 1h, and end-experiment, respectively (F2,42 = 11.35, P<0.001); these increases in injury score were closely correlated with increases in individual lung weights (R2 =0.75, P <0.001). The Kappa statistic for agreement among raters for the injury score was 0.91±0.05 (three observers).
Table 1.
Physiological parameters measured at baseline and at the end of a period of moderate tidal volume ventilation (12 ml/kg) following acid aspiration in rats (n=15).
| Baseline | End | P | |
|---|---|---|---|
|
| |||
| PaO2 (mmHg) | 439.3 ± 64.0 | 184.0 ± 169.4 | 0.00009 |
| PaCO2 (mmHg) | 44.8 ± 7.7 | 51.0 ± 8.2 | 0.04 |
| pH | 7.336 ± 0.047 | 7.285 ± 0.072 | 0.02 |
| Lactic acid (mEq/l) | 1.4±0.7 | 2.2±1.3 | 0.07 |
| ABP (mmHg) | 116.9±16.9 | 82.3±32.0 | 0.003 |
| PIP (cmH2O) | 22.1 ± 3.5 | 28.8 ± 5.2 | 0.000003 |
| Compliance(ml/cmH2O) | 0.24±0.04 | 0.18±0.04 | 0.000005 |
| EELV (ml) | 3.56 ± 0.81 | 2.05 ± 1.23 | 0.000008 |
| Lung weight (grams) | 4.30 ± 0.45 | 4.88 ± 1.11 | 0.04 |
ABP: arterial blood pressure; PIP: peak inpiratory pressure; EELV: end expiratory lung volume.
For comparison, healthy rats (no HCl) ventilated with identical settings had no radiological changes (at baseline and after 3h) and minimal worsening in mechanics and gas exchange at 3h (baseline and 3h data for these rats are shown in the table in Supplemental Digital Content 5).
Propagation vs. Containment - subgroup analysis
Five animals (5/6) died in the Propagation subgroup vs. none (0/9) in the Containment subgroup (Phi = 0.87, P=0.002). While the overall injury scores were similar in both groups at baseline, the injury was considerably increased (≈4-fold) in the Propagation group at 2 h and minimally increased in the Containment group (Table 2).
Table 2.
Markers of lung injury at baseline (after acid aspiration) and after two hours of moderate tidal volume ventilation. Rats with propagation of lung injury were analyzed separately from those with more contained injury. Vertical distribution of lung injury was quantified by dividing partial scores measured in central over peripheral areas of interest. C: compliance EELV: end expiratory lung volume.
| Propagation (n=6) | Containment (n=9) | |||
|---|---|---|---|---|
|
| ||||
| Baseline | 2 hrs | Baseline | 2 hrs | |
|
| ||||
| Body weight (g) | 361 ± 26 | - | 349 ± 26 g | - |
| PaO2 (mmHg) | 421.5 ± 72.1 | 80.2 ± 25.1 ‡ | 451.2 ± 59.4 | 278.5 ± 158.3 † |
| Lung Weight (grams) | 4.29 ± 0.21 | 5.73 ± 1.09 † | 4.30 ± 0.59 | 4.36 ± 1.04 § |
| C(ml/cmH2O) | 0.21 ± 0.03 | 0.14 ± 0.02 ‡ | 0.25± 0.03 | 0.21 ±0.03 § ‡ |
| EELV(ml) | 2.92 ± 0.30 | 0.90 ± 0.73 ‡ | 3.99 ± 0.78 * | 3.20 ± 0.44 § ‡ |
| Lung Strain | 1.52 ± 0.13 | - | 1.16 ± 0.20 § | - |
| Injury Score | 39.3±12.0 | 154.8 ± 45.1 ‡ | 36.5 ± 9.4 | 51.0 ± 16.0 § ‡ |
| Dorsal/ventral score | 3.6 ± 0.8 | 1.4 ± 0.6 ‡ | 4.8 ± 4.1 | 3.6 ± 3.2 |
: P<0.05 and
: P<0.01 between groups.
: P<0.05 and
: P< 0.01 vs Baseline.
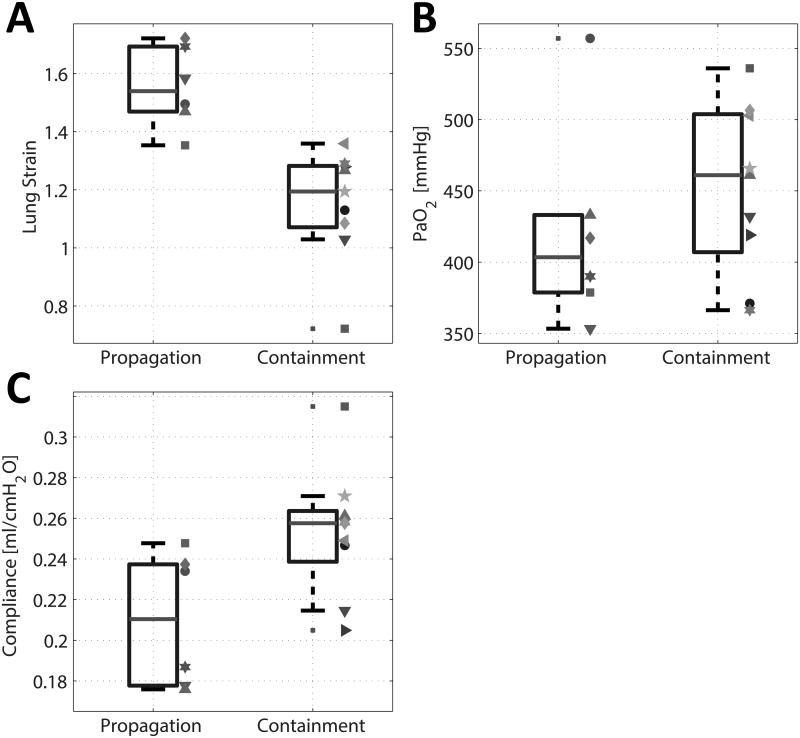
At baseline, gas exchange and hemodynamic variables (PaO2, PaCO2, lactate and arterial blood pressure) were not significantly different between Propagation and Containment subgroups (F1,13 = 1.44, P = 0.253) (Table 2). The two groups were significantly different (F1,13 = 6.67, P = 0.023) as judged by the baseline mechanical properties of the lung (strain, compliance and EELV) (Table 2). Further post hoc tests showed that, among these variables, lung strain was the major source of this significant difference (1.52±0.13 vs. 1.16±0.20; 31% difference; F1,13 = 19.02, PAdj=0.002), while the two groups did not show a significantly different compliance (F1,13 = 6.46, PAdj = 0.074). Finally, EELV was significantly lower in the propagation cohort (F1,13 = 9.80, PAdj = 0.024).At baseline, there was no overlap of the individual lung strain values between the two groups, but there was considerable overlap in PaO2 and compliance (Figure 4, A-C). We found no differences in tidal recruitment and in the aeration partitioning (by tissue weight), between Propagation and Containment subgroups (see table, Supplemental Digital Content 6, which shows the results of the quantitative analysis of CT density distributions).
Figure 4.
Individual baseline values and summary statistics (median, interquartile range, and extremes of the distribution) of: a) Lung Strain, b) Compliance, and c) PaO2 in rats that had progression vs. containment of lung injury while receiving moderate volume ventilation following acid aspiration. Individual subjects are indicated with symbols and colors matching those in Figure 3.
During ventilation with moderate VT, the decreases in PaO2, EELV, and compliance were greater in the Propagation vs. Containment groups. While the estimated lung weight increased by 30% in the Propagation group, it was unchanged in the Containment group (Table 2).
Injury scores at baseline were higher in the dorsal vs. ventral regions in both groups (no between-group differences); during ventilation the injury spread to the ventral regions in the Propagation group, but not the Containment group (Table 2).
Histological analysis in four rats (2 Propagation, 2 Containment) confirmed inflammatory injury, displaying edema, intra-alveolar and perivascular neutrophils, and hyaline membranes (Figure 5). These changes were ubiquitous in animals with radiological propagation, but they were spatially more limited in contained injury. By the end of the experiment, the topographic distribution of the histologic injury corresponded to the radiological injury scoring (Figure 5).
Figure 5.
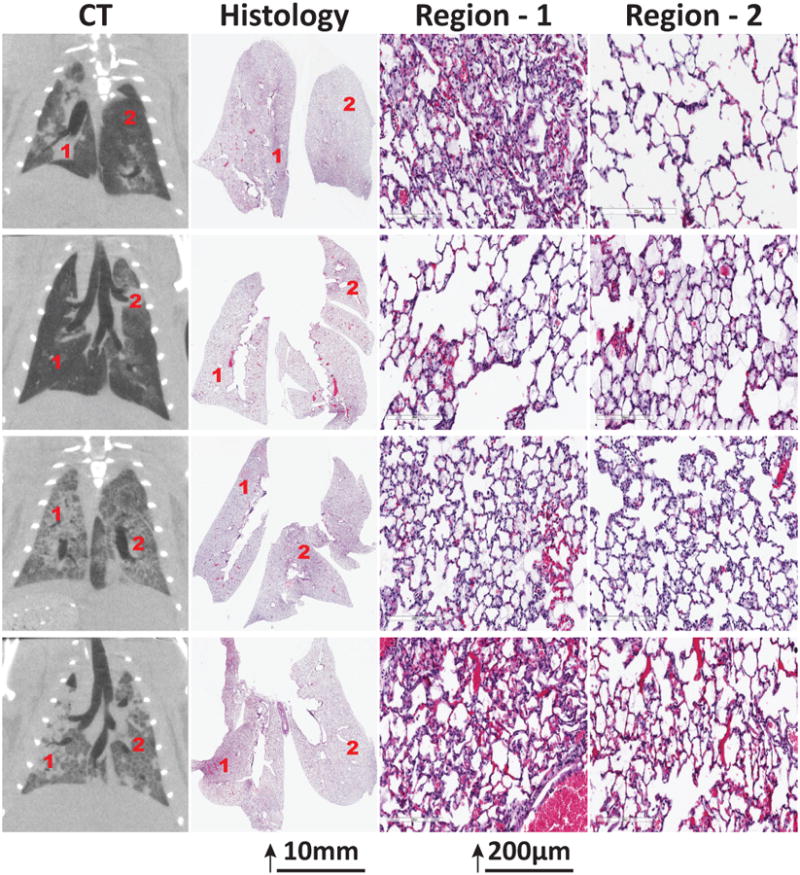
Computed tomography (CT) and histological appearance of rat lungs with acid aspiration after ventilation with moderate tidal volume. Changes compatible with inflammatory injury were more widely disseminated in animals with larger extension of CT abnormalities
Relationship between lung strain and injury propagation
To further examine the correlation between strain and injury propagation, we plotted the increase of injury score in the first hour of ventilation vs. baseline strain. This plot and regression line (P = 0.01) are shown in the figure in Supplemental Digital Content 7 and suggest minimal propagation at lower strain. Therefore, we divided the overall population of the HCl cohort into two subgroups with baseline strain > or ≤ the median value (1.3). In animals with baseline strain above the median, mortality was higher and propagation was more marked; in addition, injury scores and gain in lung weight were greater (Table 3).
Table 3.
Evolution of lung injury in the first two hours of moderate tidal volume ventilation in rats with higher vs. lower baseline strain (> or ≤ median) at baseline after acid aspiration.
| Baseline Strain >1.29 | Baseline Strain ≤ 1.29 | P | |
|---|---|---|---|
| Injury propagation/total | 6/7 | 0/8 | 0.0014 |
| Death/total | 5/7 | 0/8 | 0.007 |
| Change of injury score | 99.8 ± 55.9 | 15.6 ±12.0 | 0.001 |
| Change of lung weight (g) | 1.19 ± 1.22 | -0.12 ± 0.24 | 0.01 |
Underlying Injury Absent, large VT
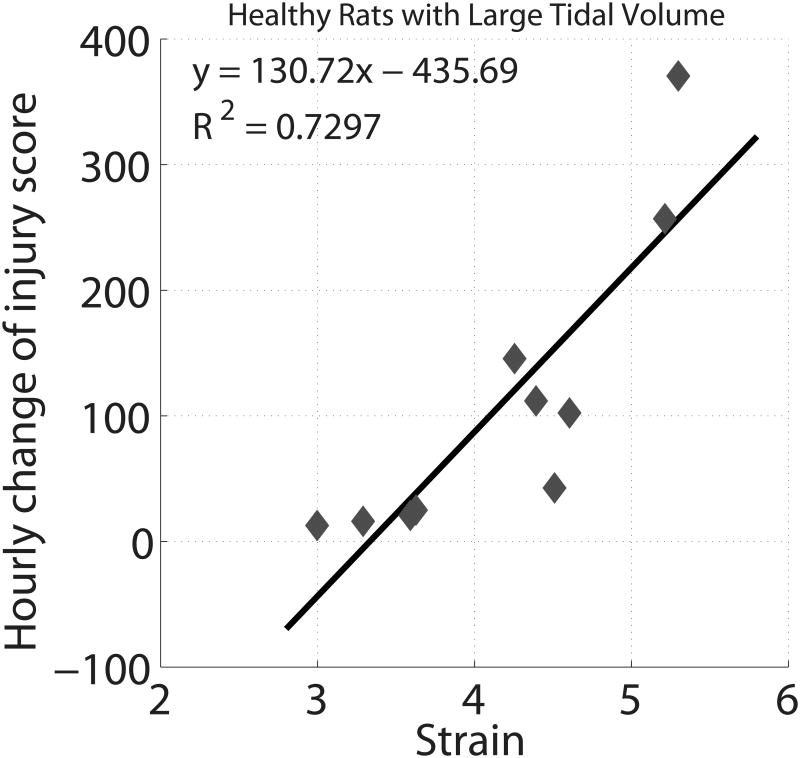
Ventilation with large VT caused injury in all animals within three hours and death before two hours in five animals. Injury started in the lung periphery and rapidly progressed towards the lung hilum (Figure 6, top panel). The animation illustrating the evolving injury is shown in Supplemental Digital Content 8. Lung weight increased from 3.7±0.4 to 6.9±1.3 g (P<0.00001), EELV decreased from 3.5±0.9 to 1.2±1.1 ml (P<0.0001), the lung injury score reached 162.5±37.1, and histological abnormalities were widespread (Figure 6, bottom panel). The baseline strain (4.2±0.8; range 3.0-5.3) was markedly higher than in the rats with acid aspiration (Table 2) and was correlated with the hourly increase of injury score (R2 =0.73, P<0.002; Figure 7).
Figure 6.
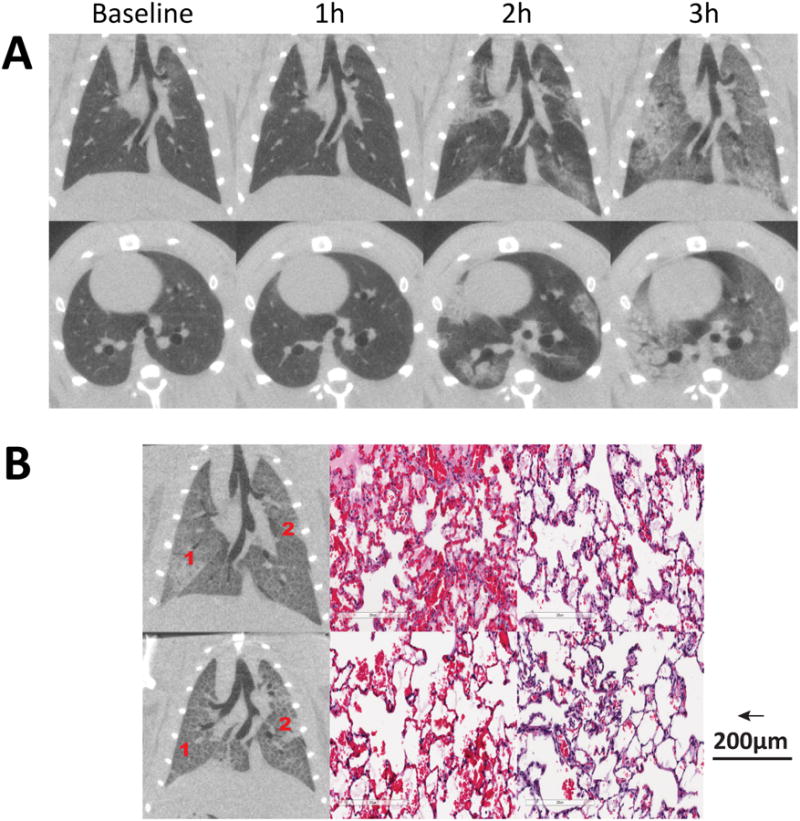
A) Radiological propagation of lung injury in healthy rats ventilated with high stretch ventilation: injury consistently started in the lung periphery and propagated towards the hila; B) Histological and radiological distribution of lesions in two rats of this group.
Figure 7.
Relationship between the rate of propagation of ventilator induced lung injury, expressed as the hourly increase in semiquantitative radiological score, and the pulmonary strain at baseline during ventilation with tidal volume 30 ml/kg in previously healthy rats.
Discussion
To the best of our knowledge, the current study provides the first reported visualization of the propagation of lung injury (see animations in the Supplemental Digital Content 3, 4, and 8). Our key finding is the nature of VILI propagation in the presence or absence of an underlying (primary) lesion. When VILI complicated a pre-existing lesion, it started at that lesion and was propagated concentrically toward the rest of the lung. By contrast, when VILI was induced as the primary injury in previously healthy lungs, it originated in peripheral lung regions and spread centrally towards the hilum. Furthermore, we found that while impaired oxygenation was not a predictor of injury propagation in pre-injured animals, strain predicted propagation in both cohorts. These findings suggest that it may ultimately be possible to predict (and perhaps prevent) propagation of injury and thereby minimize its generalization.
Our data on VILI progression in previously healthy lungs agree with the small body of previous work, including ours20, on this topic. 12,21-23: when VILI was induced as the primary injury, it originated in peripheral regions and rapidly disseminated centrally towards the hilum. Primary VILI was proportional to strain, also corroborating previous research 16. Dorso-basilar localization of unstable atelectasis 21 and strain 24 can partly explain this VILI distribution: in healthy supine rats, we found that dependent airspaces undergo larger dimensional changes during recruitment-derecruitment 25. In contrast, when VILI complicated a pre-existing lesion, it started at the underlying lesion and propagated concentrically to the rest of the lung; this secondary VILI was also strain dependent. Although VILI cannot be directly differentiated from aspiration, the worsening of pre-existing injury was less likely caused by direct extension of the initial insult. Rather, as predicted by the “baby lung” construct 3, it was more likely a consequence of subsequent ventilation at the indicated volumes. This interpretation is supported by studies comparing the effects of higher vs. lower VT in this model 26, which showed that moderate VT worsened the severity of injury following acid aspiration.
However, the baby lung model does not fully account for our observations here. Crucially, it cannot explain why secondary VILI spread locally outward from pre-injured tissue. According to the baby lung theory, the ‘healthy’ aerated parenchyma in the injured rats should have been smaller than in healthy animals but otherwise possessive of normal mechanical and biological characteristics; consequently, it should have displayed a centripetal propagation pattern suggestive of primary VILI. However, lung tissue at lesion margins had faster worsening of injury than the lung periphery. It is consequently evident that injured lung makes proximal tissue more sensitive to strain.
We suggest that the vulnerability of this peri-lesional tissue can best be explained by local discontinuities of aeration. Regions of intermediate CT attenuation (between -100 and -500 HU) concentric to the injury core were consistently visible in the initial CT scans (see Supplemental Digital Content 9, which shows binary maps of lung aeration). This radiological pattern indicates decreased but not absent gas content, compatible with sub-voxel co-dispersion of well-ventilated and non-ventilated airspaces. Functional CT studies have shown hyperventilation within poorly aerated lung regions 7, perhaps caused by reciprocal dilation of ventilated airspaces near collapsed (i.e. microatelectasis) 8 or fluid-filled alveoli 27; similarly, using hyperpolarized diffusion magnetic resonance we observed inspiratory dilatation of residual ventilated airspaces in poorly recruited lungs 28. Moderate strain may worsen injury beginning near initial lesions (cf. Figure 2) because tissue with discontinuous aeration is more exposed to local augmentation of inspiratory stress 10. Recent clinical findings have lent credence to this hypothesis by demonstrating that the presence of small-scale heterogeneity of CT density predicts ARDS mortality 11.
It is important to note, however, that other mechanisms may contribute to local injury propagation near a primary injury. For example, systemic inflammation could sensitize pulmonary cells to mechanical stress 29. But it is likely that circulating inflammatory mediators facilitate VILI globally rather than locally, since septic rats have faster VILI onset but identical dissemination compared to non-septic animals 20. In addition, endothelial gap junction communication has been implicated in the propagation of lung edema and inflammation 30 after focal lesions by HCl. Furthermore, regional hyperperfusion 31 could promote capillary stress-failure and edema during ventilation 32. Finally, dissemination of airway fluid facilitated by mechanical ventilation 33 can spread injury in the distal airways.
The results of this study have two potential implications for the eventual management of patients with early lung injury. First, studying the spatial propagation of lung injury might facilitate the investigation of containment strategies. In contrast, studying established ARDS clouds understanding about the condition's initial evolution. Moreover, clinical definitions 34 omit the evolving distribution of injury which may be especially important in ARDS that presents with non-diffuse injury 6.
Second, our work is consistent with other studies emphasizing that protecting injured lungs from excessive strain is essential to mitigating ARDS 26. To reduce strain, clinicians prescribe VT according to a patient's predicted body weight 1. However, recent studies have highlighted that severely ill patients have smaller lung capacities 35 and are at risk of VILI even with smaller VT 36. Global lung strain can be measured at bedside with non-radiological instruments 17, although regional stress distribution is missed 11. Our work extends the relevance of strain to less severe injury, where lung capacity is preserved. This may be relevant in low-VT ventilation in patients without established ARDS 37, though we note that generalized use of low VT can have undesired consequences such as atelectasis 38 and patient discomfort 39. While some data suggest no increase in sedation doses during low VT ventilation 40, this may change with the wider use of restricted sedation 41.
Risk of propagation after HCl was not related to oxygenation. This suggests that baseline PaO2/FiO2 measurement has only a limited ability to characterize early lung injury and to predict its evolution. The hypoxemia that reflects severity in ARDS 34 is perhaps the end-result of complex maldistributions of ventilation and blood flow rather than a marker of injury progression. Within this context, pulmonary strain may be a more pertinent measurement for characterizing individual risk of ARDS progression.
Our study has important limitations. The design is not randomized because we aimed to study only animals (with limited injury) that were able to display propagation, and to identify baseline predictors of such behavior. The exclusion of 5 rats with worse injury had probably minimal effects on our conclusions, because their baseline mechanics (shown in Supplemental Digital content 2) were also worse than in the main group. Since this was a short-term small animal experiment, the data are difficult to extrapolate to a clinical context. For example, highly compliant chest wall in rats may attenuate the binary dorsal/ventral distribution of gas and atelectasis; however, baseline HCl injury was more represented dorsally. The serial CT imaging provides topographical descriptors of regional edema and atelectasis, though histology corroborates the observed injury differences between propagated vs. contained responses to high VT (Figure 5). Because we used a semi-quantitative metric and a fixed grayscale to quantify injury propagation, subtle inter-subject variability in aeration (e.g. due to uneven acid delivery) could be under-detected. However, quantitative analysis (shown in Supplemental Digital Content 6) did not show differences between subgroups in lung density distribution. Methodology to measure strain is always complex, and overall lung deformation may not directly reflect the alveolar micro-environment 25. Correctly relating injury propagation to VILI necessitated a pragmatic approach, and we chose to measure strain as the ratio between VT and EELV 17 rather than index VT to functional residual capacity 42. This approach has been successfully used in patients with ARDS 4,17 but yields lower strain values where PEEP (and thus, EELV) are raised. Thus comparisons with animals in which PEEP was not used may result in bias. However, the low levels of PEEP were just sufficient to match EELV in injury vs. control groups at baseline (3.6±0.9 mL after HCl vs. 3.5 ± 0.7 mL in healthy rats). We did not correct strain for tidal recruitment 42 because this variable was not different between the Propagation vs. Containment subgroups (See table, Supplemental Digital Content 6, with the results of quantitative CT density analysis). All rats were ventilated with high FiO2 in order to optimize survival, but this (vs. ambient levels of FiO2) may potentiate VILI 43; however, the responses of VILI to VT and strain were similar to previously reported sham-operated animals ventilated with FiO2 1.0 20.
Conclusions
We provide visual evidence of the spatial propagation of VILI. In the presence of pre-existing injury, propagation begins in regions of primary injury and spreads concentrically; this is in contrast to the centripetal pattern of propagation –towards the hilum– observed in previously healthy lungs. In both cases, propagation is predicted by strain. Injury propagation in perilesional tissue may reflect the maldistribution of local lung inflation, which makes tissue more vulnerable to strain. Better knowledge of the process by which primary injury disseminates during ventilation could help quantify the risk of ARDS progression and thereby potentially optimize individual management.
Supplementary Material
What we already know about this topic
Mechanical ventilation can damage un-injured lungs, but more commonly it worsens previously injured lungs (e.g. aspiration, infection); however, secondary injury from mechanical ventilation is unpredictable and its propagation has never been visualized.
What this article tells us that is new
Sequential computed tomography illustrates how lung strain, but not hypoxemia, predicts the spatial propagation of lung injury following acid aspiration. Lung regions near the initial injury focus may be more vulnerable to injury propagation by mechanical ventilation.
Acknowledgments
Funding: This work was supported by NIH (Bethesda, MD, USA) grants R01-HL116342 and R01-HL124986. Dr. Cereda is supported by a grant from the Foundation for Anesthesia Education and Research (Schaumburg, IL) and from the Society of Critical Care Anesthesiologists (Park Ridge, IL, USA), and by the Transdisciplinary Awards Program in Translational Medicine and Therapeutics (Philadelphia, PA). BPK is supported by operating funds from the CIHR (Ottawa, ON, Canada) and holds the Dr. Geoffrey Barker Chair in Critical Care Medicine.
Footnotes
Conflicts of interest: The authors declare no competing interests
References
- 1.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England journal of medicine. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Frank JA, Parsons PE, Matthay MA. Pathogenetic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest. 2006;130:1906–1914. doi: 10.1378/chest.130.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. The American Review of Respiratory Disease. 1987;136:730–736. doi: 10.1164/ajrccm/136.3.730. [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, Messa C, Pesenti A. Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. American journal of respiratory and critical care medicine. 2011;183:1193–1199. doi: 10.1164/rccm.201008-1318OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, McKerlie C, Post M, McLoughlin P, Kavanagh BP. Atelectasis causes alveolar injury in nonatelectatic lung regions. American journal of respiratory and critical care medicine. 2006;174:279–289. doi: 10.1164/rccm.200506-1006OC. [DOI] [PubMed] [Google Scholar]
- 6.Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ Group CTSAS. Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2001;163:1444–1450. doi: 10.1164/ajrccm.163.6.2005001. [DOI] [PubMed] [Google Scholar]
- 7.Bayat S, Porra L, Albu G, Suhonen H, Strengell S, Suortti P, Sovijarvi A, Petak F, Habre W. Effect of positive end-expiratory pressure on regional ventilation distribution during mechanical ventilation after surfactant depletion. Anesthesiology. 2013;119:89–100. doi: 10.1097/ALN.0b013e318291c165. [DOI] [PubMed] [Google Scholar]
- 8.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. Journal of applied physiology. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 9.Bachofen H, Gehr P, Weibel ER. Alterations of mechanical properties and morphology in excised rabbit lungs rinsed with a detergent. Journal of applied physiology: respiratory, environmental and exercise physiology. 1979;47:1002–1010. doi: 10.1152/jappl.1979.47.5.1002. [DOI] [PubMed] [Google Scholar]
- 10.Mertens M, Tabuchi A, Meissner S, Krueger A, Schirrmann K, Kertzscher U, Pries AR, Slutsky AS, Koch E, Kuebler WM. Alveolar dynamics in acute lung injury: heterogeneous distension rather than cyclic opening and collapse. Critical Care Medicine. 2009;37:2604–2611. doi: 10.1097/CCM.0b013e3181a5544d. [DOI] [PubMed] [Google Scholar]
- 11.Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, Brioni M, Carlesso E, Chiumello D, Quintel M, Bugedo G, Gattinoni L. Lung inhomogeneity in patients with acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2014;189:149–158. doi: 10.1164/rccm.201308-1567OC. [DOI] [PubMed] [Google Scholar]
- 12.Broccard A, Shapiro RS, Schmitz LL, Adams AB, Nahum A, Marini JJ. Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Critical care medicine. 2000;28:295–303. doi: 10.1097/00003246-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Burnham EL, Hyzy RC, Paine R, 3rd, Coley C, 2nd, Kelly AM, Quint LE, Lynch D, Janssen WJ, Moss M, Standiford TJ. Chest CT features are associated with poorer quality of life in acute lung injury survivors. Crit Care Med. 2013;41:445–56. doi: 10.1097/CCM.0b013e31826a5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xin Y, Song G, Cereda M, Kadlecek S, Hamedani H, Jiang Y, Rajaei J, Clapp J, Profka H, Meeder N, Wu J, Tustison NJ, Gee JC, Rizi RR. Semiautomatic segmentation of longitudinal computed tomography images in a rat model of lung injury by surfactant depletion. J Appl Physiol (1985) 2015;118:377–85. doi: 10.1152/japplphysiol.00627.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denison DM, Morgan MD, Millar AB. Estimation of regional gas and tissue volumes of the lung in supine man using computed tomography. Thorax. 1986;41:620–628. doi: 10.1136/thx.41.8.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Protti A, Cressoni M, Santini A, Langer T, Mietto C, Febres D, Chierichetti M, Coppola S, Conte G, Gatti S, Leopardi O, Masson S, Lombardi L, Lazzerini M, Rampoldi E, Cadringher P, Gattinoni L. Lung stress and strain during mechanical ventilation: any safe threshold? American journal of respiratory and critical care medicine. 2011;183:1354–1362. doi: 10.1164/rccm.201010-1757OC. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Lopez A, Garcia-Prieto E, Batalla-Solis E, Amado-Rodriguez L, Avello N, Blanch L, Albaiceta GM. Lung strain and biological response in mechanically ventilated patients. Intensive care medicine. 2012;38:240–247. doi: 10.1007/s00134-011-2403-1. [DOI] [PubMed] [Google Scholar]
- 18.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. The New England journal of medicine. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 19.Ludbrook J. Statistical techniques for comparing measurers and methods of measurement: a critical review. Clin Exp Pharmacol Physiol. 2002;29:527–36. doi: 10.1046/j.1440-1681.2002.03686.x. [DOI] [PubMed] [Google Scholar]
- 20.Yehya N, Xin Y, Oquendo Y, Cereda M, Rizi RR, Margulies SS. Cecal ligation and puncture accelerates development of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L443–51. doi: 10.1152/ajplung.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair SE, Chi E, Lin HI, Altemeier WA. Positive end-expiratory pressure alters the severity and spatial heterogeneity of ventilator-induced lung injury: an argument for cyclical airway collapse. Journal of critical care. 2009;24:206–211. doi: 10.1016/j.jcrc.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura M, Honda O, Tomiyama N, Johkoh T, Kagawa K, Nishida T. Body position does not influence the location of ventilator-induced lung injury. Intensive Care Med. 2000;26:1664–9. doi: 10.1007/s001340000664. [DOI] [PubMed] [Google Scholar]
- 23.Cressoni M, Chiurazzi C, Gotti M, Amini M, Brioni M, Algieri I, Cammaroto A, Rovati C, Massari D, di Castiglione CB, Nikolla K, Montaruli C, Lazzerini M, Dondossola D, Colombo A, Gatti S, Valerio V, Gagliano N, Carlesso E, Gattinoni L. Lung Inhomogeneities and Time Course of Ventilator-induced Mechanical Injuries. Anesthesiology. 2015;123:618–27. doi: 10.1097/ALN.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 24.Hubmayr RD, Walters BJ, Chevalier PA, Rodarte JR, Olson LE. Topographical distribution of regional lung volume in anesthetized dogs. Journal of applied physiology: respiratory, environmental and exercise physiology. 1983;54:1048–1056. doi: 10.1152/jappl.1983.54.4.1048. [DOI] [PubMed] [Google Scholar]
- 25.Cereda M, Xin Y, Emami K, Huang J, Rajaei J, Profka H, Han B, Mongkolwisetwara P, Kadlecek S, Kuzma NN, Pickup S, Kavanagh BP, Deutschman CS, Rizi RR. Positive end-expiratory pressure increments during anesthesia in normal lung result in hysteresis and greater numbers of smaller aerated airspaces. Anesthesiology. 2013;119:1402–9. doi: 10.1097/ALN.0b013e3182a9b0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. American journal of respiratory and critical care medicine. 2002;165:242–249. doi: 10.1164/ajrccm.165.2.2108087. [DOI] [PubMed] [Google Scholar]
- 27.Perlman CE, Lederer DJ, Bhattacharya J. Micromechanics of alveolar edema. American journal of respiratory cell and molecular biology. 2011;44:34–39. doi: 10.1165/rcmb.2009-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cereda M, Emami K, Xin Y, Kadlecek S, Kuzma NN, Mongkolwisetwara P, Profka H, Pickup S, Ishii M, Kavanagh BP, Deutschman CS, Rizi RR. Imaging the interaction of atelectasis and overdistension in surfactant-depleted lungs. Critical Care Medicine. 2013;41:527–535. doi: 10.1097/CCM.0b013e31826ab1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine GK, Deutschman CS, Helfaer MA, Margulies SS. Sepsis-induced lung injury in rats increases alveolar epithelial vulnerability to stretch. Critical Care Medicine. 2006;34:1746–1751. doi: 10.1097/01.CCM.0000218813.77367.E2. [DOI] [PubMed] [Google Scholar]
- 30.Parthasarathi K, Bhattacharya J. Localized Acid instillation by a wedged-catheter method reveals a role for vascular gap junctions in spatial expansion of Acid injury. Anatomical record (Hoboken, N J: 2007) 2011;294:1585–1591. doi: 10.1002/ar.21460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richter T, Bergmann R, Knels L, Hofheinz F, Kasper M, Deile M, Pietzsch J, Ragaller M, Koch T. Pulmonary blood flow increases in damaged regions directly after acid aspiration in rats. Anesthesiology. 2013;119:890–900. doi: 10.1097/ALN.0b013e3182a17e5b. [DOI] [PubMed] [Google Scholar]
- 32.Broccard AF, Hotchkiss JR, Kuwayama N, Olson DA, Jamal S, Wangensteen DO, Marini JJ. Consequences of vascular flow on lung injury induced by mechanical ventilation. Am J Respir Crit Care Med. 1998;157:1935–42. doi: 10.1164/ajrccm.157.6.9612006. [DOI] [PubMed] [Google Scholar]
- 33.de Prost N, Roux D, Dreyfuss D, Ricard JD, Le Guludec D, Saumon G. Alveolar edema dispersion and alveolar protein permeability during high volume ventilation: effect of positive end-expiratory pressure. Intensive care medicine. 2007;33:711–717. doi: 10.1007/s00134-007-0575-5. [DOI] [PubMed] [Google Scholar]
- 34.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA: the journal of the American Medical Association. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 35.Mattingley JS, Holets SR, Oeckler RA, Stroetz RW, Buck CF, Hubmayr RD. Sizing the lung of mechanically ventilated patients. Critical Care (London, England) 2011;15:R60. doi: 10.1186/cc10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, Slutsky AS, Gattinoni L, Ranieri VM. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2007;175:160–166. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 37.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA: the journal of the American Medical Association. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 38.Richard JC, Maggiore SM, Jonson B, Mancebo J, Lemaire F, Brochard L. Influence of tidal volume on alveolar recruitment. Respective role of PEEP and a recruitment maneuver. American journal of respiratory and critical care medicine. 2001;163:1609–1613. doi: 10.1164/ajrccm.163.7.2004215. [DOI] [PubMed] [Google Scholar]
- 39.Kallet RH, Luce JM. Detection of patient-ventilator asynchrony during low tidal volume ventilation, using ventilator waveform graphics. Respiratory care. 2002;47:183–185. [PubMed] [Google Scholar]
- 40.Kahn JM, Andersson L, Karir V, Polissar NL, Neff MJ, Rubenfeld GD. Low tidal volume ventilation does not increase sedation use in patients with acute lung injury. Critical care medicine. 2005;33:766–771. doi: 10.1097/01.ccm.0000157786.41506.24. [DOI] [PubMed] [Google Scholar]
- 41.Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, McArthur C, Seppelt IM, Webb S, Weisbrodt L Sedation Practice in Intensive Care Evaluation Study I, Group ACT. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. American journal of respiratory and critical care medicine. 2012;186:724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 42.Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, Tallarini F, Cozzi P, Cressoni M, Colombo A, Marini JJ, Gattinoni L. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2008;178:346–355. doi: 10.1164/rccm.200710-1589OC. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. 2004;32:2496–501. doi: 10.1097/01.ccm.0000148231.04642.8d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.