Abstract
Smooth pursuit eye tracking deficits are a promising intermediate phenotype for schizophrenia and possibly for psychotic disorders more broadly. The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium investigated the severity and familiality of different pursuit parameters across psychotic disorders. Probands with schizophrenia (N=265), schizoaffective disorder (N=178), psychotic bipolar disorder (N=231), their first-degree relatives (N=306, N=217, N=273, respectively) and healthy controls (N=305) performed pursuit tracking tasks designed to evaluate sensorimotor and cognitive/predictive aspects of pursuit. Probands from all diagnostic groups were impaired on all pursuit measures of interest compared to controls (p<0.001). Schizophrenia probands were more impaired than other proband groups on both early pursuit gain and predictive gain. Relatives with and without enhanced psychosis spectrum personality traits were impaired on initial eye acceleration, the most direct sensorimotor pursuit measure, but not on pursuit gain measures. This suggests that alterations in early sensorimotor function may track susceptibility to psychosis even in the absence of psychosis related personality traits. There were no differences in pursuit measures between relatives of the three proband groups. Familiality estimates of pursuit deficits indicate that early pursuit gain was more familial than predictive gain, which has been the most widely used measure in previous family studies of psychotic disorders. Thus, while disease-related factors may induce significant impairments of pursuit gain, especially in schizophrenia, the pattern of deficits in relatives and their familiality estimates suggest that alterations in sensorimotor function at pursuit onset may indicate increased susceptibility across psychotic disorders.
Keywords: schizophrenia, schizoaffective disorder, bipolar disorder, sensorimotor processing, predictive pursuit eye movements, familiality
1. Introduction
Pursuit eye tracking deficits represent a well-established intermediate phenotype for schizophrenia reflecting an impaired ability to visually track slowly moving objects (Diefendorf and Dodge, 1908; Holzman, 1992; Thaker, 2008). Available evidence from small sample studies indicates that pursuit deficits are also present in schizoaffective and psychotic bipolar disorder, suggesting that they may represent a common neurophysiological intermediate phenotype across psychotic disorders (Blackwood et al., 2007; Flechtner et al., 2002; Ivleva et al., 2014; Kathmann et al., 2003; Lencer et al., 2011; Lencer et al., 2004b; Sweeney et al., 1999). The model of pursuit deficits as intermediate phenotypes for psychosis is supported by preliminary studies indicating that pursuit deficits are observed in unaffected relatives of both patients with schizophrenia and psychotic affective disorders (Blackwood et al., 1996; Calkins et al., 2008; Clementz et al., 1990; Kathmann et al., 2003; Lencer et al., 2003; Rosenberg et al., 1997).
Pursuit deficits can result from a range of disturbances in neural circuitry throughout the brain involving motion sensitive visual area V5, parietal and frontal areas supporting sensorimotor transformation, and subcortical areas involved in motor control (Berman et al., 1999; Ilg and Thier, 2008; Lencer et al., 2004a; Sharpe, 2008). In psychotic disorders, impairments in sensorimotor systems that provide the transformation of visual motion signals into oculomotor commands have been suggested to underlie pursuit disturbances (Chen et al., 1999; Clementz and McDowell, 1994; Lencer et al., 2010; Slaghuis et al., 2007; Sweeney et al., 1999). Sensorimotor measures include initial eye acceleration in response to the onset of a visual target movement, and early integration of visual feedback about performance accuracy before predictive pursuit maintenance is established.
Deficits of sustained pursuit maintenance when tracking targets moving back and forth across the field of view have been most widely investigated in psychotic disorders, especially in genetic association and family studies (Arolt et al., 1996; Calkins et al., 2008; Haraldsson et al., 2009; Rybakowski et al., 2001; Wonodi et al., 2011). During sustained pursuit maintenance, cognitive factors including prediction of target motion become more prominent components of pursuit drive relative to sensorimotor processes (Barnes, 2008; Becker and Fuchs, 1985). Sustained pursuit maintenance deficits in schizophrenia have therefore been considered to represent impaired integration of higher-order predictive mechanisms (Levy et al., 2010; Thaker et al., 1999; Thaker et al., 1998).
Recent studies measuring both sensorimotor components and predictive pursuit indicate that sensorimotor deficits may be more pronounced than predictive pursuit deficits in patients with psychotic disorders (Lencer et al., 2011; Lencer et al., 2010; Lencer et al., 2008). Further, we recently demonstrated different associations of sensorimotor and sustained pursuit maintenance impairments with genes regulating dopamine and glutamate systems in psychotic disorders (Lencer et al., 2014).
To date, the relative impairment of different pursuit measures and their utility as intermediate phenotypes across psychotic disorders is unclear. Previous large family studies of psychotic disorders have focused on antisaccades, another eye movement measure that is independent from pursuit (Radant et al., 2010; Reilly et al., 2014). The present study addressed the question of whether different pursuit deficits and their familiality are generalized across psychotic disorders or specific to schizophrenia. Secondly, we evaluated the severity and familiality of impairments in sensorimotor function and predictive maintenance pursuit.
2. Material and methods
2.1 Subjects
Smooth pursuit measures were assessed in probands with schizophrenia (N=265), schizoaffective disorder (N=178), bipolar disorder with psychotic features (N=231), their first-degree relatives (N=306, N=217, N=273, respectively) and healthy controls (N=305) studied by the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium (Table 1). Details of recruitment and evaluation procedures have been described previously (Tamminga et al., 2013). DNA genotypes (GWAS) are currently available in 80% of cases. The PREST program (http://utstat.toronto.edu/sun/Software/Prest/) was used to identify and exclude individuals who were not related in the manner reported by study participants. In all participants, diagnoses were made by a consensus process at each consortium site using all available clinical information and the Structured Clinical Interview for DSM IV (First et al., 1995). Current symptoms and cognitive status were assessed using standard scales, see Table 1 (and Table 1S in supplemental material). Relatives without a psychotic disorder and healthy controls were administered the Structured Interview for DSM-IV Personality (SID-P) (Pfohl, 1997) to assess personality traits and disorders. A diagnosis of elevated psychosis spectrum personality traits was defined as meeting full or within one criteria of a Cluster A (psychosis spectrum) Axis-II diagnosis.
Table 1. Demographic and Clinical Characteristics.
| PROBANDS | RELATIVES | |||||||
|---|---|---|---|---|---|---|---|---|
| Controls N=305 | SZ N=265 | SZAFF N=178 | BP N=231 | SZ N=314 | SZAFF N=227 | BP N=274 | Statistics | |
| Age, Mean (SD) | 36.5 (12.4) | 34.5 (12.5) | 36.3 (11.6) | 36 (13.0) | 43 (15.5) | 40.2 (16.1) | 40.9 (15.7) | F(6 1787)=13.7; p<0.001 |
| Sex (% Male) | 45% | 67% | 40% | 35% | 29% | 31% | 36% | X2(6)=112.1; p<0.001 |
| Race | ||||||||
| % Caucasia n | 63% | 46% | 55% | 73% | 55% | 61% | 80% | X2(6)=92.4; p<0.001 |
| % African American | 27% | 45% | 40% | 22% | 40% | 24% | 15% | X2(6)=85.7; p<0.001 |
| % Other | 10% | 9% | 5% | 5% | 5% | 5% | 5% | X2(6)=15.7; p=0.02 |
| Cognitive Assessments, Mean (SD) | ||||||||
| WRAT 41 | 103.8 (14) | 94.9 (16) | 96.7 (14.9) | 101.6 (13.6) | 97.5 (14.7) | 98.9 (16.1) | 103 (14.1) | F(6 1738)=13.6; p<0.001 |
| BACS2 | 0.0 (1.0) | -1.8 (1.4) | -1.5 (1.3) | -0.9 (1.3) | -0.6 (1.2) | -0.6 (1.4) | -0.2 (1.2) | F(6 1682)=73.5; p<0.001 |
| Relatives' Psychotic Disorders and Psychosis Spectrum Personality Traits, N (%) | ||||||||
| Psychotic Disorder | 22 (7%) | 27 (12%) | 21 (8%) | n.s. | ||||
| Psychosis Spectrum Personality Traits3 | 47 (15%) | 32 (14%) | 36 (13%) | n.s. | ||||
| No Psychosis Spectrum Personality Traits | 238 (78%) | 165 (74%) | 214 (79%) | n.s. | ||||
Wide Range Achievement Test 4th - Edition: Reading (Wilkinson et al., 2006)
Brief Assessment of Cognition in Schizophrenia (Keefe et al., 2008), z-scores are given
as defined by meeting full or within one criteria of a Cluster A personality disorder diagnosis (SID-P)
Inclusion criteria for all subjects included (1) age 15-65; (2) WRAT reading score ≥ 60 (Wilkinson et al., 2006); (3) no history of neurologic disorder; (4) minimum of 20/40 acuity (with our without correction), (5) no history of substance abuse within the last month or substance dependence within the last three months, and negative urine toxicology on assessment day. Inclusion criteria for control subjects additionally included: (1) no personal or family history (first-degree) of psychotic or bipolar disorders; (2) no history of recurrent mood disorder; and (3) no history of psychosis spectrum personality traits as defined above. The study was approved by institutional review boards at each study site, and written informed consent was obtained prior to study participation.
2.2 Eye movement testing
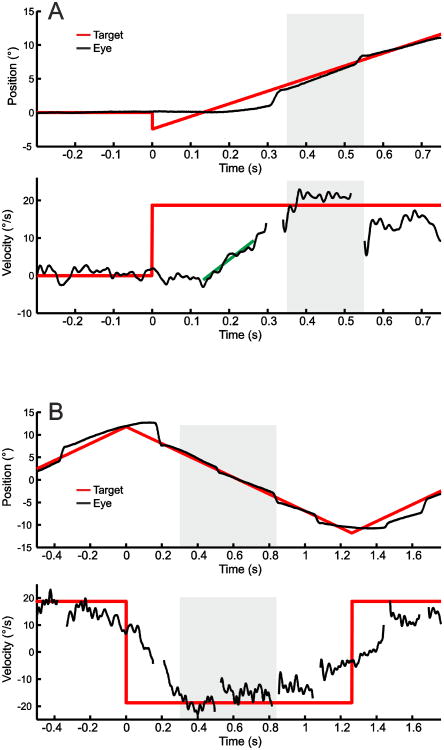
Smooth pursuit testing took place in a darkened room (∼2 Lux) using identical stimuli and recording devices across sites (Eyelink II, SR Research Ltd., Ontario/Canada, sampling rate 500 Hz). Participants were seated 60 cm from a 22-inch CRT monitor (1360 × 768 resolution; 150 Hz refresh rate) with their heads stabilized with a chin and forehead restraint. Participants were instructed to follow the target, a red cross in a box covering 0.5°, with their eyes as precisely as possible. To assess initial eye acceleration and early eye velocity under feedback control which both reflect visual sensorimotor function during pursuit, 32 foveo-petal step-ramps (Rashbass, 1961) starting from central position were presented (Figure 1A, see also supplemental material). Step size was 2.4° to either right or left appearing in randomized order followed immediately by a target sweep moving at a constant velocity of 18.7°/s in the opposite direction, designed so that the target crossed the central position after 133ms, close to the time of pursuit initiation, without eliciting an initial catch-up saccade. To assess predictive pursuit maintenance we used a triangular waveform with target sweeps, also at a constant velocity of 18.7°/s in the horizontal plane (+/- 12°), Figure 1B. Forty-eight sweeps with continuously visible targets were used for analyses. Additionally, blocks of either 9.7°/s or 26.6°/s sweeps (30% of trials) and sweeps with intervals of target blanking (100-500ms) were interspersed occasionally to enhance engagement but were not included in analyses. Calibration trials were presented between blocks of trials for offline recalibration.
Figure 1. Examples of smooth pursuit stimuli with eye position and eye velocity traces.
(A) Foveo-petal step-ramp task for assessment of sensorimotor measures: step size was 2.4° followed immediately by a target sweep in opposite direction moving at a constant velocity of 18.7°/s. Pursuit initiation started without a catch-up saccade. The slope of the linear regression line on eye velocity (green line) was used to calculate initial eye acceleration. (B) Triangular wave task for assessment of predictive pursuit. Gray areas in A and B refer to the intervals used for calculating eye velocity gain. Note, that saccade were removed from velocity traces.
An automated program using MatLab (The MathWorks Inc., Natick, USA) was developed for analyses. Eye position data was filtered (30 Hz Gaussian filter) before eye velocity was calculated with central median differentiation of 9ms (Sprenger et al., 2011). Saccades occurring during pursuit, e.g. catch-up saccades, and blinks were removed and treated as missing values before conducting pursuit measurements. Individual position and overlaid velocity traces were checked by visual inspection. In step-ramp tasks, initial eye acceleration was computed by linear regression (RobustFit® in MatLab) of eye velocity in a 100ms time window beginning after eye velocity exceeded a noise threshold defined as 3.2 standard deviations above mean resting eye velocity from 200ms before to 100ms after ramp-onset (Carl and Gellman, 1987; Lencer et al., 2004b). To assess early maintenance pursuit gain under visual feedback control, median eye velocity relative to target velocity was computed in an interval 350-550ms after ramp onset in the step-ramp condition (Figure 1A). In the repetitive triangular wave target sweeps, median eye velocity was determined in intervals 300 to 840ms after reversal of target direction to compute predictive maintenance pursuit gain, Figure 1B (also see supplemental material).
2.3 Statistical analyses
Prior to analysis, each eye movement measure was standardized using a normative regression approach and z-score transformation using age, race, and sex as covariates (Table 1). This was done to remove variance in data related to demographic parameters from all groups in a similar way, and to facilitate comparison of the magnitude of deficits across the different groups and pursuit measures. Extremely low scores (i.e. outliers) were truncated to z-score = −4.0 before statistical analysis (16 datasets were truncated for predictive maintenance gain (3%), 2 datasets for eye acceleration and 3 datasets for early maintenance gain). There were no significant interactions between subject group and B-SNIP site for any pursuit measure.
We used fixed effects one-way analyses of variance (ANOVA) and the least significant difference post-hoc procedure for pairwise group comparisons. Additionally, Pearson's correlations of pursuit measures with clinical ratings, BACS z-scores, exposure to different types of medication and chlorpromazine equivalents were determined.
Familiality was estimated using a maximum likelihood method in the Sequential Oligogenic Linkage Analysis Routines (SOLAR) software (v4.3.1; (Almasy and Blangero, 1998)) using an ascertainment bias correction since families were recruited through the identification of a psychotic proband rather than as a representative community sample (Beaty and Liang, 1987). Familiality was determined using a maximum likelihood ratio test of a model in which phenotypic variation explained by family membership was compared to one in which it was not. Here, the term familiality indicates the degree of within family correlation in the pursuit phenotypes, while heritability remains to be established in genetic association studies.
3. Results
3.1 Pursuit alterations across all proband and relative groups
Groups differed on all three pursuit measures. Initial eye acceleration was decreased in all three proband groups (F(3,963)=23.6, p<0.001) and all three relative groups (F(3,1092)=5.79, p=0.001) compared to controls. While initial eye acceleration was more reduced in probands than relatives (F(5,1447)=9.4, p<0.001) there were no differences of initial eye acceleration between the three proband groups or between the three relative groups (Figure 2A).
Figure 2. Measures of smooth pursuit tracking in probands and first-degree relatives.
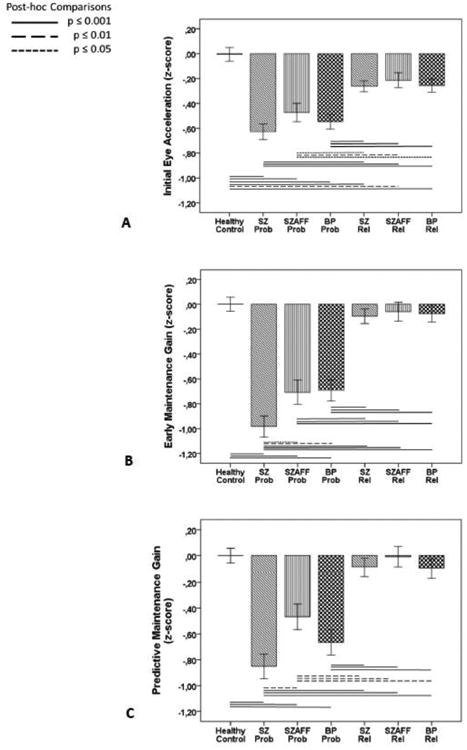
Measures of initial eye acceleration (A), early maintenance gain (B), and predictive maintenance gain (C) in probands (Prob) with schizophrenia (SZ), schizoaffective disorder (SZAFF) and bipolar disorder (BP) and their first-degree relatives (Rel) compared to healthy controls are given as effect sizes relative to performance in healthy controls corrected for differences in age, sex and race. For raw values of sensorimotor measures see Table 3S in supplemental material.
Early maintenance gain (F(3,970)=33.1, p<0.001) and predictive maintenance gain (F(3,975)=21.3, p<0.001) were decreased in all three proband groups compared to healthy controls. Early maintenance gain was lower in schizophrenia than both schizoaffective and bipolar disorder and predictive maintenance gain was lower in schizophrenia than schizoaffective but not bipolar disorder (Figures 2B and 2C). Neither gain measure differed from healthy controls in any relative group.
As expected, there was a gain increase from early to predictive maintenance pursuit in all groups (task: F(6,1760)=61.1, p<0.001) with a larger gain improvement in all proband groups than in both healthy controls and all relative groups which did not differ (taskxgroup: F(6,1760)=18.8, p<0.001), see Table 3S and 4S in supplemental material.
Neither initial acceleration nor any gain measure was robustly associated with clinical ratings, exposure to different types of medication or chlorpromazine equivalents except for schizoaffective disorder. In this group, the number of psychotropic medications and chlorpromazine equivalents accounted for approximately 10% of variance in pursuit measures (Table 2S in supplemental material for full presentation of clinical correlations). Furthermore, none of pursuit measures accounted for more than 10% of the variance in BACS z-scores (r<0.31) in any group, indicating a relative independence of pursuit deficits from general cognitive deficits.
3.2 Pursuit alterations in subgroups of relatives and familiality of pursuit measures
For planned follow-up analyses, relatives were divided into subgroups: (1) those with a psychotic disorder, (2) those with elevated psychosis spectrum personality traits, and (3) those without either of these traits. The proportions of each relative subgroup did not differ between diagnostic groups (Table 1), and relative subgroups were first examined pooled across diagnostic groups. Initial eye acceleration was comparably decreased in all relative subgroups compared to healthy controls (F(3,1079)=6.4, p=0.001, Figure 3). There were no differences between relative subgroups and controls for early maintenance gain and predictive maintenance gain.
Figure 3. Sensorimotor function during smooth pursuit tracking in relative subgroups.
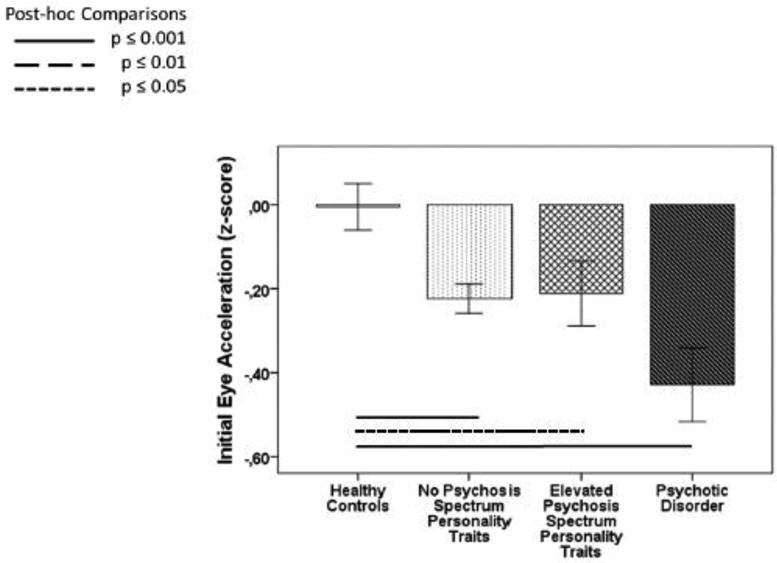
Initial eye acceleration in subgroups of first-degree relatives (1) without psychosis spectrum personality traits, (2) with elevated psychosis spectrum personality traits and (3) with psychotic disorders compared to healthy controls is shown as effect sizes relative to performance in healthy controls corrected for differences in age, sex and race. For raw values of pursuit measures see Table 3S in supplemental material.
Familiality estimates of early pursuit maintenance gain across diagnostic groups were higher than for predictive pursuit maintenance gain, for more details see Table 2. Early pursuit maintenance gain was also the only measure that was significantly familial in all three groups.
Table 2. h2 Estimates (Standard Error) in Individual Diagnostic Groups of the B-SNIP Sample.
| Initial Eye Acceleration | Early Maintenance Gain | Predictive Maintenance Gain | |
|---|---|---|---|
| SZ Families | h2= 0.32 (0.09), p = 4.0*10-4 CI 0.14 - 0.50 |
h2 = 0.32 (0.09), p = 2.0*10-4 CI 0.14 - 0.50 |
h2 = 0.17 (0.10), p = 0.04 CI -0.16 - 0.50 |
| SZAFF Families | h2 = 0.23 (0.13), p = 0.04 CI -0.03 - 0.49 |
h2 = 0.45 (0.12), p = 1.0*10-4 CI 0.21 - 0.67 |
h2 = 0.27 (0.13),p = 0.02 CI 0.02 - 0.53 |
| BP Families | h2 = 0.27 (0.12), p = 0.01 CI 0.04 - 0.51 |
h2 = 0.44 (0.10), p =6.0*10-6 CI 0.24 - 0.64 |
h2 = 0.25 (0.10), p = 7.0*10-3 CI 0.05 - 0.45 |
| Combined Families | h2 = 0.28 (0.07), p = 9.0*10-6 CI 0.14 – 0.42 |
h2 = 0.40 (0.06), p = 2.0*10-11 CI 0.28 – 0.52 |
h2 = 0.23 (0.06), p = 9.0*10-5 CI 0.11 – 0.35 |
4. Discussion
There were several novel findings from this large family study. First, smooth pursuit performance was impaired in probands and their first-degree relatives across psychotic disorders. Second, alterations of both sensorimotor and predictive pursuit measures were present in all three proband groups, with more severe impairments in schizophrenia than in both other proband groups for pursuit gain. This suggests that these traits have a relative diagnostic specificity in affected individuals. Third, among first-degree relatives, initial eye acceleration, the most direct indicator of sensorimotor function, was the only pursuit measure that was impaired compared to healthy controls. Further, this pattern was noted irrespective of whether elevated psychosis spectrum personality traits were present or not. Additionally, early pursuit gain deficits were more familial than predictive pursuit deficits. Thus, reduced maintenance gain appears to be a useful biomarker for discriminating psychotic illnesses and patients from controls, while sensorimotor measures obtained near the point of pursuit initiation may have advantages by virtue of greater familiality and significant abnormality in unaffected relatives.
4.1 Pursuit impairments in probands
The findings of reduced initial eye acceleration and pursuit gain in all proband groups that were relatively independent of clinical ratings, cognitive impairment and medication status are in line with previous reports from smaller sample studies indicating pursuit deficits as trait markers across psychotic disorders (Flechtner et al., 2002; Ivleva et al., 2014; Lencer et al., 2010; Lencer et al., 2004b; Sweeney et al., 1999). The observation of more severe impairments of pursuit maintenance in schizophrenia than in schizoaffective and psychotic bipolar disorders has not been reported previously. In our previous study on untreated first-episode psychosis, psychotic bipolar patients had poorer pursuit gain than schizophrenia patients (Lencer et al., 2010). This implies that group differences in gain impairment may increase over the course of illness, perhaps due to specific illness-related factors in schizophrenia. Also, the greater statistical power in the present large-sample study may have revealed more subtle differences between disorders that were not detected with previous samples.
The current finding of reduced initial eye acceleration across proband groups is in line with the previous report of reduced initial eye velocity in first-episode patients across psychotic disorders (Lencer et al., 2010). Similar impairments of pursuit initiation in first-episode and chronically ill patients, and their impairments in relatives, are consistent with the interpretation that impaired pursuit initiation is a relatively robust intermediate phenotype.
4.2 Pursuit impairments in relatives and their familiality
The examination of relatives along with their index probands provides an approach for separating familial and illness-related biomarkers (Gottesman and Gould, 2003). The majority of earlier small sample family studies showing impaired pursuit maintenance in relatives have not differentiated between relatives with and without psychosis spectrum traits (Calkins et al., 2008). Only a very few studies reported impaired pursuit maintenance compared to controls not only in spectrum-relatives of schizophrenia probands but also in non-spectrum relatives (Clementz et al., 1990; Hong et al., 2006; Lencer et al., 2003; Ross et al., 2002). Despite our large study sample, we did not find pursuit maintenance impairments in relative subgroups which might be due to a greater representativeness of our sample or due to specific characteristics of the pursuit tasks we used (see below).
For the first time, we report here altered initial eye acceleration in relatives of psychotic probands. This effects was similar across relatives from different diagnostic categories (Figure 2A). More detailed analysis in relative subgroups showed that this measure of sensorimotor function was even impaired in relatives without psychosis spectrum traits suggesting that pursuit initiation abnormalities may track increased vulnerability to psychosis compared to controls independently of the expression of subthreshold psychosis spectrum traits (Figure 3).
The findings of higher familiality estimates for sensorimotor measures, notably early maintenance gain, than for predictive maintenance gain, which has been most commonly used in previous family studies of pursuit tracking in psychotic disorders, underline the potential value of deficits in sensorimotor function as an intermediate phenotype. A relatively low familiality estimate for sustained maintenance pursuit has previously been reported in schizophrenia (Hong et al., 2006). Lower familiality estimates for predictive than early maintenance gain highlight the potential of sensorimotor vs. predictive pursuit impairments for family genetic research. Further, the pattern of findings suggest that preserved predictive components of pursuit in relatives may support pursuit maintenance despite their impairments in sensorimotor aspects of pursuit.
4.3 Implications for alterations in sensorimotor networks
Initial eye acceleration represents the most direct indicator of the ability to use visual motion information for early pursuit drive as processing of visual feedback and cognitive predictive mechanisms play a less important role in immediate pursuit initiation. Impaired use of early visual motion information for sensorimotor transformation in all proband and relative subgroups together with high familiality of early pursuit gain deficits suggest specific abnormalities in the processing of visual motion information in extrastriate cortex or in its visuo-motor transformation in parietal or frontal association cortex. In line with this hypothesis, fMRI and EEG studies have suggested an altered transfer of visual motion information from extrastriate cortex to parietal and frontal eye fields in patients with psychotic disorders (Chen et al., 2008; Lencer et al., 2011; Wang et al., 2010). These findings are broadly consistent with models proposing that visual information processing deficits represent a core feature of psychotic disorders (Butler et al., 2007; Yoon et al., 2013).
The observation that relatives did not show deficits of pursuit gain in contrast to probands suggests that with our tasks relatives were able to compensate for dysfunctions seen with initial eye acceleration when visual feedback and cognitive components, i.e. mechanisms of prediction and anticipation, were integrated to pursuit drive. This is consistent with our recent study showing that even patients can track very fast moving targets up to 32°/s as well as controls in conditions optimizing predictive influences and minimizing demands for ongoing sensorimotor transformation, while pursuit maintenance was severely impaired in patients with less predictable ramp targets (Lencer et al., 2010). There is evidence from functional imaging studies in schizophrenia for increased activation of dorsolateral prefrontal cortex and frontal eye fields that are known for coding predictive signals (Lencer et al., 2011; Nagel et al., 2007). This is consistent with a capacity in patients to recruit resources needed to compensate for sensorimotor deficits.
4.4 Limitations
There are limitations to this study that need to be considered. First, the representativeness of our sample may be limited by the inclusion criteria for probands such as no recent or significant lifetime substance dependence and the presence of a family member willing and able to participate. Second, there are other tasks that can be used to assess different components of pursuit responses than those used here. Their potential with regard to transdiagnostic and familial effects remains to be explored. Third, despite our finding of a relative independence of pursuit deficits from current medications, effects of chronic medication treatment are potential confounds on performance measures that we cannot fully exclude. Fourth, we focused on familiality estimates across rather than between diagnostic groups, which reflect familial similarity that may be genetic or environmental in origin. Future genetic association studies are needed to assess heritability of the identified phenotypic traits.
The findings from this large family sample offer a promising approach for advancing pathophysiological models and understanding discrete components of the complex multifactorial risk for psychosis across diagnostic categories. While disease related factors may induce more severe impairments of pursuit maintenance in probands with schizophrenia compared to other psychotic disorders, findings in relatives and familiality estimates suggest that measures of sensorimotor function may be promising indicators for indexing susceptibility to psychosis in future genetic studies.
Supplementary Material
Acknowledgments
The authors thank the patients and their family members who contributed their time and effort to participate in this study. We also thank Gunvant Thaker, MD, for his many scientific contributions to the B-SNIP consortium, especially his role in supporting and initiating the pursuit studies reported here, as well as collecting data.
Role of funding source: This work was supported by the National Institute of Mental Health [grant numbers: MH077851 (CAT), MH078113 (MSK), MH077945 (GDP), MH077852 (GKT) and MH077862 (JAS)]. The NIMH has no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or the decision to submit the manuscript for publication. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number TL1TR001104. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Contributors: Authors Tamminga, Keshavan, Pearlson and Sweeney designed the study and wrote the protocol, then collected the data. Authors Lencer and Sweeney managed the literature searches and worked with Sprenger who conducted eye tracking data analyses. Authors Lencer, Reilly and Rubin undertook the statistical analysis. Lencer, Sprenger and Sweeney wrote the draft of the manuscript and all co-authors reviewed and suggested revisions for the final version. All authors contributed to and have approved the final manuscript.
Conflict of interests: All authors have declared no conflicts of interest in relation to the subject of this study.
Rebekka Lencer, James L. Reilly, Andreas Sprenger, Jennifer E. McDowell, Brett A. Clementz, Godfrey D. Pearlson report no biomedical financial interests or potential conflicts of interest.
Matcheri S. Keshavan reports the following financial disclosure: Sunovion
John A. Sweeney reports the following financial disclosures: Eli Lilly Pharmaceuticals – Ad Hoc Consultant, F. Hoffmann-La Roche, Ltd. – Ad Hoc Consultant
Carol A. Tamminga reports the following financial disclosures:
American Psychiatric Association – Deputy Editor; Astellas – Ad Hoc Consultant; Autifony – Ad Hoc Consultant; The Brain and Behavior Foundation – Council Member; Eli Lilly Pharmaceuticals – Ad Hoc Consultant; Intra-cellular Therapies (ITI, Inc.) – Advisory Board, drug development; Institute of Medicine – Council Member; National Academy of Medicine – Council Member; Pfizer – Ad Hoc Consultant; Sunovion -- Investigator Initiated grant funding
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arolt V, Lencer R, Nolte A, Muller-Myhsok B, Purmann S, Schurmann M, Leutelt J, Pinnow M, Schwinger E. Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. Am J Med Genet. 1996;67(6):564–579. doi: 10.1002/(SICI)1096-8628(19961122)67:6<564::AID-AJMG10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Barnes GR. Cognitive processes involved in smooth pursuit eye movements. Brain Cogn. 2008;68(3):309–326. doi: 10.1016/j.bandc.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Beaty TH, Liang KY. Robust inference for variance components models in families ascertained through probands: I. Conditioning on proband's phenotype. Genet Epidemiol. 1987;4(3):203–210. doi: 10.1002/gepi.1370040305. [DOI] [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Prediction in the oculomotor system: smooth pursuit during transient disappearance of a visual target. Exp Brain Res. 1985;57(3):562–575. doi: 10.1007/BF00237843. [DOI] [PubMed] [Google Scholar]
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA. Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum Brain Map. 1999;8(4):209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, Pickard BJ, Thomson PA, Evans KL, Porteous DJ, Muir WJ. Are some genetic risk factors common to schizophrenia, bipolar disorder and depression? Evidence from DISC1, GRIK4 and NRG1. Neurotox Res. 2007;11(1):73–83. doi: 10.1007/BF03033484. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Sharp CW, Walker MT, Doody GA, Glabus MF, Muir WJ. Implications of comorbidity for genetic studies of bipolar disorder: P300 and eye tracking as biological markers for illness. Br J Psychiatry Suppl. 1996;(30):85–92. [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130(Pt 2):417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Iacono WG, Ones DS. Eye movement dysfunction in first-degree relatives of patients with schizophrenia: a meta-analytic evaluation of candidate endophenotypes. Brain Cogn. 2008;68(3):436–461. doi: 10.1016/j.bandc.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl JR, Gellman RS. Human smooth pursuit: stimulus-dependent responses. J Neurophysiol. 1987;57(5):1446–1463. doi: 10.1152/jn.1987.57.5.1446. [DOI] [PubMed] [Google Scholar]
- Chen Y, Grossman ED, Bidwell LC, Yurgelun-Todd D, Gruber SA, Levy DL, Nakayama K, Holzman PS. Differential activation patterns of occipital and prefrontal cortices during motion processing: evidence from normal and schizophrenic brains. Cogn Affect Behav Neurosci. 2008;8(3):293–303. doi: 10.3758/cabn.8.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Nakayama K, Matthysse S, Palafox G, Holzman PS. Dependence of impaired eye tracking on deficient velocity discrimination in schizophrenia. Arch Gen Psychiatry. 1999;56(2):155–161. doi: 10.1001/archpsyc.56.2.155. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE. Smooth pursuit in schizophrenia: abnormalities of open- and closed-loop responses. Psychophysiology. 1994;31(1):79–86. doi: 10.1111/j.1469-8986.1994.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hirt M, Haas G. Pursuit gain and saccadic intrusions in first-degree relatives of probands with schizophrenia. J Abnorm Psychol. 1990;99(4):327–335. doi: 10.1037//0021-843x.99.4.327. [DOI] [PubMed] [Google Scholar]
- Diefendorf AR, Dodge R. An experimental study of the ocular reactions on the insane from photographic records. Brain. 1908;31:451–489. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Flechtner KM, Steinacher B, Sauer R, Mackert A. Smooth pursuit eye movements of patients with schizophrenia and affective disorder during clinical treatment. Eur Arch Psychiatry Clin Neurosci. 2002;252(2):49–53. doi: 10.1007/s004060200011. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Haraldsson HM, Ettinger U, Magnusdottir BB, Sigmundsson T, Sigurdsson E, Ingason A, Petursson H. COMT val(158)met genotype and smooth pursuit eye movements in schizophrenia. Psychiatry Res. 2009;169(2):173–175. doi: 10.1016/j.psychres.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Holzman PS. Behavioral markers of schizophrenia useful for genetic studies. J Psychiatr Res. 1992;26(4):427–445. doi: 10.1016/0022-3956(92)90044-o. [DOI] [PubMed] [Google Scholar]
- Hong LE, Mitchell BD, Avila MT, Adami H, McMahon RP, Thaker GK. Familial aggregation of eye-tracking endophenotypes in families of schizophrenic patients. Arch Gen Psychiatry. 2006;63(3):259–264. doi: 10.1001/archpsyc.63.3.259. [DOI] [PubMed] [Google Scholar]
- Ilg UJ, Thier P. The neural basis of smooth pursuit eye movements in the rhesus monkey brain. Brain Cogn. 2008;68(3):229–240. doi: 10.1016/j.bandc.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Moates AF, Hamm JP, Bernstein IH, O'Neill HB, Cole D, Clementz BA, Thaker GK, Tamminga CA. Smooth pursuit eye movement, prepulse inhibition, and auditory paired stimuli processing endophenotypes across the schizophrenia-bipolar disorder psychosis dimension. Schizophr Bull. 2014;40(3):642–652. doi: 10.1093/schbul/sbt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathmann N, Hochrein A, Uwer R, Bondy B. Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. Am J Psychiatry. 2003;160(4):696–702. doi: 10.1176/appi.ajp.160.4.696. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, Hawkins K. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS) Schizophr Res. 2008;102(1-3):108–115. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Lencer R, Bishop JR, Harris MS, Reilly JL, Patel S, Kittles R, Prasad KM, Nimgaonkar VL, Keshavan MS, Sweeney JA. Association of variants in DRD2 and GRM3 with motor and cognitive function in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci. 2014;264(4):345–355. doi: 10.1007/s00406-013-0464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer R, Keedy SK, Reilly JL, McDonough BE, Harris MS, Sprenger A, Sweeney JA. Altered transfer of visual motion information to parietal association cortex in untreated first-episode psychosis: Implications for pursuit eye tracking. Psychiatry Res Neuroimaging. 2011;194(1):30–38. doi: 10.1016/j.pscychresns.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer R, Nagel M, Sprenger A, Zapf S, Erdmann C, Heide W, Binkofski F. Cortical mechanisms of smooth pursuit eye movements with target blanking. An fMRI study. Eur J Neurosci. 2004a;19(5):1430–1436. doi: 10.1111/j.1460-9568.2004.03229.x. [DOI] [PubMed] [Google Scholar]
- Lencer R, Reilly JL, Harris MS, Sprenger A, Keshavan MS, Sweeney JA. Sensorimotor transformation deficits for smooth pursuit in first-episode affective psychoses and schizophrenia. Biol Psychiatry. 2010;67(3):217–223. doi: 10.1016/j.biopsych.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer R, Sprenger A, Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Effects of second-generation antipsychotic medication on smooth pursuit performance in antipsychotic-naive schizophrenia. Arch Gen Psychiatry. 2008;65(10):1146–1154. doi: 10.1001/archpsyc.65.10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer R, Trillenberg-Krecker K, Schwinger E, Arolt V. Schizophrenia spectrum disorders and eye tracking dysfunction in singleton and multiplex schizophrenia families. Schizophr Res. 2003;60(1):33–45. doi: 10.1016/s0920-9964(02)00165-2. [DOI] [PubMed] [Google Scholar]
- Lencer R, Trillenberg P, Trillenberg-Krecker K, Junghanns K, Kordon A, Broocks A, Hohagen F, Heide W, Arolt V. Smooth pursuit deficits in schizophrenia, affective disorder and obsessive-compulsive disorder. Psychol Med. 2004b;34(3):451–460. doi: 10.1017/s0033291703001314. [DOI] [PubMed] [Google Scholar]
- Levy DL, Sereno AB, Gooding DC, O'Driscoll GA. Eye tracking dysfunction in schizophrenia: characterization and pathophysiology. Curr Top Behav Neurosci. 2010;4:311–347. doi: 10.1007/7854_2010_60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Sprenger A, Nitschke M, Zapf S, Heide W, Binkofski F, Lencer R. Different extraretinal neuronal mechanisms of smooth pursuit eye movements in schizophrenia: An fMRI study. Neuroimage. 2007;34(1):300–309. doi: 10.1016/j.neuroimage.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Pfohl B, B N, Zimmerman M. Structured Interview for DSM-IV Personality: SIDP-IV. Washington, DC: 1997. [Google Scholar]
- Radant AD, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, Freedman R, Green MF, Greenwood TA, Gur RE, Gur RC, Light GA, Meichle SP, Millard SP, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang MT, Turetsky BI, Tsuang DW. Antisaccade performance in schizophrenia patients, their first-degree biological relatives, and community comparison subjects: data from the COGS study. Psychophysiology. 2010;47(5):846–856. doi: 10.1111/j.1469-8986.2010.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Frankovich K, Hill S, Gershon ES, Keefe RS, Keshavan MS, Pearlson GD, Tamminga CA, Sweeney JA. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr Bull. 2014;40(5):1011–1021. doi: 10.1093/schbul/sbt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Sweeney JA, Squires-Wheeler E, Keshavan MS, Cornblatt BA, Erlenmeyer-Kimling L. Eye-tracking dysfunction in offspring from the New York High-Risk Project: diagnostic specificity and the role of attention. Psychiatry Res. 1997;66(2-3):121–130. doi: 10.1016/s0165-1781(96)02975-7. [DOI] [PubMed] [Google Scholar]
- Ross RG, Olincy A, Mikulich SK, Radant AD, Harris JG, Waldo M, Compagnon N, Heinlein S, Leonard S, Zerbe GO, Adler L, Freedman R. Admixture analysis of smooth pursuit eye movements in probands with schizophrenia and their relatives suggests gain and leading saccades are potential endophenotypes. Psychophysiology. 2002;39(6):809–819. doi: 10.1111/1469-8986.3960809. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Hauser J. Dopamine D3 receptor (DRD3) gene polymorphism is associated with the intensity of eye movement disturbances in schizophrenic patients and healthy subjects. Mol Psychiatry. 2001;6(6):718–724. doi: 10.1038/sj.mp.4000927. [DOI] [PubMed] [Google Scholar]
- Sharpe JA. Neurophysiology and neuroanatomy of smooth pursuit: lesion studies. Brain Cogn. 2008;68(3):241–254. doi: 10.1016/j.bandc.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Hawkes A, Holthouse T, Bruno R. Eye movement and visual motion perception in schizophrenia I: Apparent motion evoked smooth pursuit eye movement reveals a hidden dysfunction in smooth pursuit eye movement in schizophrenia. Exp Brain Res. 2007;182(3):399–413. doi: 10.1007/s00221-007-1000-6. [DOI] [PubMed] [Google Scholar]
- Sprenger A, Trillenberg P, Pohlmann J, Herold K, Lencer R, Helmchen C. The role of prediction and anticipation on age-related effects on smooth pursuit eye movements. Ann N Y Acad Sci. 2011;1233:168–176. doi: 10.1111/j.1749-6632.2011.06114.x. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME. Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry. 1999;46(5):671–680. doi: 10.1016/s0006-3223(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170(11):1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull. 2008;34(4):760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Buchanan RW, Adami HM, Medoff DR. Smooth pursuit eye movements to extra-retinal motion signals: deficits in patients with schizophrenia. Psychiatry Res. 1999;88(3):209–219. doi: 10.1016/s0165-1781(99)00084-0. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, Lahti A. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55(9):830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- Wang J, Brown R, Dobkins KR, McDowell JE, Clementz BA. Diminished parietal cortex activity associated with poor motion direction discrimination performance in schizophrenia. Cereb Cortex. 2010;20(7):1749–1755. doi: 10.1093/cercor/bhp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ, Psychological Assessment, Resources I. WRAT 4 : Wide Range Achievement Test. (4th) 2006 [Google Scholar]
- Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, Kajii Y, Thaker GK, Schwarcz R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry. 2011;68(7):665–674. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Sheremata SL, Rokem A, Silver MA. Windows to the soul: vision science as a tool for studying biological mechanisms of information processing deficits in schizophrenia. Frontiers in Psychology. 2013;4:681. doi: 10.3389/fpsyg.2013.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.