Abstract
Mucosal and barrier tissues are unique in that they mediate crosstalk between the host and the surrounding environment, which contains many potentially harmful factors. Therefore, it is critical that cell types present at barrier and mucosal surfaces are equipped with mechanisms to sense changes in the environment and to calibrate their responses accordingly. Aryl Hydrocarbon Receptor (AHR) is a ligand dependent transcription factor well known to generate biological responses to environmental pollutants, such as benzo{a}pyrene and halogenated dioxins. Surprisingly, in the last few years a large body of evidence has shown that AHR is also involved in maintaining homeostasis or in triggering pathology by modulating the biological responses of critical cell types at the barrier and mucosal interfaces. Here, we will review progresses in this field and discuss how targeting AHR activation may impact disease.
Keywords: Aryl Hydrocarbon Receptor, IL-22, Mucosal tissues, Innate Lymphoid Cells
1. Introduction
Organs at barrier surfaces such as lung, skin, gut, oral and genital mucosae and eyes are critical to maintain the integrity of the host. They must constantly survey for surrounding signals and carefully discriminate between harmless and harmful events. This discrimination is vital to allow the host to grow, extract nutrients from the environment, control and take advantage of symbiotic organisms and, at the same time, mount proper defenses to the challenge of threatening occurrences.
The Pern-Arnt-Sim (PAS) superfamily of transcription factors is an ancient and highly conserved pathway that regulates communications between the host and the environment and promotes “environmental adaptation” [1, 2]. The PAS superfamily contains proteins that are involved in chemical sensing (AHR), in regulation of circadian rhythm due to light-dark cycles (BMAL1 and BMAL2) and in the detection of variations in oxygen tension or redox potentials (HIF-1α, HIF-2α and HIF-3α). PAS superfamily members, such as the Drosophila SIM, a regulator of midline cell lineage [3], are also involved in organ development suggesting that developmental signals may be perceived similarly to environmental stresses.
2. AHR and its ligands
AHR is one of the initial PAS transcription factors identified [4]. AHR is normally present in the cytoplasm of cells bound to chaperones, such as Hsp90, which maintain it in an inactive state. Upon ligand binding, chaperones are released and AHR translocates to the nucleus. Here, it dimerizes with its homolog, AHR-Nuclear Translocator (AHRNT) and the resulting heterodimer binds to Dioxin Responsive Elements (DRE) on promoters to drive the transcription of target genes [5]. These target genes include Xenobiotic Metabolizing Enzymes (XME); among them, and most prominently, the microsomal cytochrome P450-dependent monooxygenases CYP1A1 and CYP1A2 [2]. These enzymes are induced in the attempt to metabolize and inactivate toxic pollutants that bind AHR, such as the polyphenols benzo{a}pyrene, 3-methylcolantrene and the infamous 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), which in 1976 was released following an industrial accident in the small city of Seveso, Italy, and led to many cases of chloracne [6].
While pollutants are well-established ligands of AHR, the precise identity of other physiological exogenous and endogenous ligands is still matter of debate [7]. Dietary ligands of AHR have been reported. Among them is the glucobrassicin derivative Indole-3-Carbinol (IC3), a chemical found in high concentrations in vegetables of the Brassica genus, including broccoli, cauliflower, Brussel sprouts and cabbages [8]. IC3 in the acidic environment of the stomach undergo dimerization and generates diindolylmethane (DIM) as well as other metabolites, such as indolylcarbazole (ICZ), that activate AHR. Other described dietary ligands of AHR are natural flavonoids present in fruits and vegetables, such as galangin, genystein, chrysin, apigenin and quercetin [9]. Resveratrol, which is abundant in red wine, has also been reported to bind AHR [7]. Ginsenoides extracted from ginseng, a perennial plant frequently utilized by traditional Chinese medicine, can also bind and activate AHR [10]. However, it is still unclear which, if any, dietary or naturally occurring ligands of AHR have direct agonistic activity or whether these compounds compete with environmental polyphenols, thus mediating an antagonist effect.
Known endogenous ligands of AHR are derivatives of the essential amino acid tryptophan. UV light-mediated degradation of tryptophan generates 6-formylindolo{3,2-b}carbazole (FICZ), which is a potent activator of AHR [11]. L-kynurenine, a catabolic metabolite of tryptophan formed along the pathway to generate niacin, is also a high-affinity AHR ligand [12]. Kynurenine can be generated by the enzyme Trypthophan2,3-dioxigenase (TDO) or the enzymes Indoleamine2,3-dyoxigenase (IDO1 and IDO2), and these enzymes have different roles in different biological scenarios, as discussed later.
Interestingly, bacteria, including commensals such as B. subtilis, also produce tryptophan and can regulate tryptophan synthesis by sensing tryptophan concentrations due to dietary intake [13]. Bacteria and fungi can also metabolize tryptophan into ligands that can activate AHR. Malassezia furfur, a fungus common causative agent of pytiriasis versicolor of the skin, secretes several ligands including malassezin, ICZ and FICZ that engage AHR [14]. Several species of lactobacilli, including Lactobacillus bulgaricus [15] and Lactobacillus reuteri [16] produce AHR ligands, such as indole-3-aldehyde (IAid), and modulate mucosal immune response. Importantly, pathogens such as Mycobacterium tuberculosis (Mtb) and Pseudomonas aeruginosa (P. aeruginosa) produce pigmented virulence factors that activate AHR [17]. Specifically, phenazines from P. aeruginosa and naphthoquinone phthiocol (Pht) from Mtb bind AHR and induce the detoxification enzymes CYP1A1 and CYP1B1 that inactivate these compounds, while instructing an innate immune defense pathway that counteracts and contains infection in hematopoietic and epithelial cells [17]. These findings highlight a new role for AHR as an unexpected pattern recognition receptor (PRR). As phenazynes and naphtoquinones have a broad distribution among prokaryotes present at mucosal barriers and in the environment, recognition of these products by AHR, once again, emphasizes that AHR represents a central node for chemical communication between the environment and the host.
3. AHR in immune responses: cellular targets and cell type specific effects
AHR is expressed by many cell types in the body and therefore exerts pleiotropic effects by integrating with other signaling pathways, such as sex hormones receptors and the Wnt-β catenin network [18]. For instance, AHR in keratinocytes, but not hematopoietic cells, curbs inflammation in a model of imiquimod-induced psoriasis [19]. The initial generation of AHR null mice by two distinct groups also highlighted a role for AHR in normal development. AHR deficient mice frequently died after birth or displayed a slower growth rate in the first few weeks of life [20, 21]. In addition, they developed hepatic anomalies, such as bile duct fibrosis, transient hepatic steatosis and abnormal retinoic acid metabolism in the liver [20-22]. One group also reported reduced spleen and lymph node cellularity [20], which was not noticed by the other group that generated AHR null mice [21]. AHR-deficient animals were resistant to TCDD, but their immune responses were not examined in detail. Emerging findings in the last few years have indicated that AHR signaling shapes a number of immune responses from different cell types and this has profound influences on host-commensal and host-pathogen interactions. AHR plays an especially critical role at mucosal interfaces where the host deals with other living entities and environmental agents, which possess the ability to engage AHR.
4. AHR and Dendritic Cells
It was originally shown that the AHR agonist VAF347 promotes in vivo allograft tolerance in a model of islet transplantation via a DC-mediated mechanism [23]. The same compound inhibited the production of inflammatory cytokines and the upregulation of costimulatory molecules on human monocyte-derived DCs stimulated by anti-CD40 and TNF-α [24] and reduced differentiation of CD34+ hematopoietic precursor cells to DCs and Langerhans cells (LCs) in vitro [25]. Accordingly, a small molecule purine derivative that acts as AHR antagonist, StemRegenin 1, greatly increased expansion of human CD34+ cells and enhanced their engraft into immune deficient mice in vivo [26]. StemRegenin 1 also supported differentiation of CD34+ precursors to myeloid and plasmacytoid DCs [27, 28]. AHR deficient LCs exhibited impaired maturation in vivo, resulting in diminished contact hypersensitivity [29]. In addition, AHR activation in DCs induced expression of the enzymes IDO1 and IDO2 [30] and consequent production of the tryptophan degradation product kynurenine [31], which skews T cell differentiation from Th17 to Foxp3+Treg, promoting immune tolerance. Similarly, the natural AHR ligand 2-(1'H-indole-3'-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), originally purified from lung [32], directly acted on DCs inducing tolerogenic properties that promoted Treg differentiation in a retinoic acid-dependent process and, ultimately, suppressed autoimmunity in EAE, a mouse model of human Multiple Sclerosis [33]. TLR agonists, such as LPS, upregulate AHR expression in DCs and other myeloid cells via a RelA/p50-mediated activation of the AHR promoter [34]. Moreover, AHR is involved in LPS response [35] and LPS tolerance [36]. The primary response to LPS is mitigated by activation of AHR upon generation of the endogenous ligand kynurenine by TDO. Accordingly, AHR and TDO2 deficient mice are highly susceptible to primary LPS challenge. However, LPS tolerance induction upon secondary challenge requires the combined action of AHR, IDO1 and TGFβ. In this context, AHR activation results in Src-mediated phosphorylation of IDO1 followed by TGFβ production by IDO1 competent DCs, a sequence of events that eventually prevents systemic inflammation and immunopathology [36]. Importantly, kynurenine is a very abundant AHR ligand generated by tumor cells via the TDO pathway [37]. In tumors kinurenyne promotes clonogenic tumor cell survival but also acts on infiltrating immune cells to prevent immune rejection. Moreover, TDO blockade restores immune–mediated tumor rejection [38], indicating that targeting the TDO pathway may be an effective strategy for cancer treatment.
Altogether, these findings suggest that AHR and its ligands act in a highly integrated “disease tolerance system” that may prevent autoimmunity and excessive immune responses to pathogens, but may be maliciously hijacked by tumor cells to their own advantage.
5. AHR and T cells
It was long known that dioxin exerts an immunosuppressive effects and a single treatment of laboratory mice with low amount of TCDD causes profound defects in humoral and cellular responses. However, the exact mechanisms that led to this immunosuppression were poorly understood. In 2005 Funatake and colleagues showed that TCDD induces CD4+ T cells with regulatory potential, which effectively suppress an acute graft-versus-host response [39]. In spite of this, it was only in 2008 that AHR finally took a central stage in T cell biology and T cell helper differentiation. Two ground-breaking studies showed that AHR plays a critical role in Th17 biology and controls IL-22 production by Th17 T cells [40, 41], suggesting that environmental clues can modulate autoimmune diseases and immune pathology. Interestingly, these studies pointed out that AHR ligands of different origin and, most likely, different affinities differentially impact the outcome of Th17-mediated autoimmune diseases. While TCDD and ITE are potent inducers of Treg differentiation and suppress EAE [41], FICZ enhances Th17 differentiation and increases severity of disease [40, 41]. Moreover, it became evident that the presence of AHR ligands in culture media strongly affects Th17 differentiation and IL-22 production in vitro [42, 43]. AHR signaling is particularly relevant for IL-22 production when TGFβ is present [44]. Notch signaling in CD4+ T cells also induces AHR ligands that boost IL-22 expression [45]. Paradoxically, however, in vivo AHR-deficient mice have exaggerated responses to Segmented Filamentous Bacteria (SFB) [46, 47], which selectively drive an antigen-specific Th17 response in small intestinal lamina propria (LP) [48-50].
In addition to having a central role in Th17 differentiation, AHR is also critical for generation of mouse Tr1 T cells, which suppress autoimmunity by secreting IL-10 [51, 52]. AHR is induced by IL-27 and binds to the transcription factor c-Maf. AHR and c-Maf cooperatively transactivate the IL-10 and IL-21 promoters [51]. Similarly, AHR is necessary for generation of human Tr1 T cells [53]. On human CD4+ T cells AHR ligands promote induction of Foxp3− Tr1-like cells that suppress via granzyme B and secrete IL-10. In addition, in the presence of TGFβ, AHR ligands induce Foxp3 Treg that suppress via the ectonucleoside triphosphate diphosphohydrolase CD39, which hydrolyzes ATP to AMP. Mechanistically, AHR ligands and TGFβ induce SMAD1, which binds to Foxp3 enhancer and promotes Foxp3 expression. AHR ligands and TGFβ also induce expression of Aiolos [54], which binds to Foxp3 to silence IL-2 expression [53]. AHR is further involved in Th17 to Tr1 trans-differentiation, as AHR agonists promote this process [55]. Moreover, AHR controls the metabolism of Tr1 cells at late stages, when Hif-1α is not longer expressed [56].
In addition to Th17 and Tr1 cells, AHR has also a critical role in the generation of IL-6-dependent Th22 cells, which produce IL-22 and mediate protection from entheropathogenic bacteria [57, 58].
Beyond CD4 T cells, AHR modulates antigen specific CD8 T cell responses. During influenza virus infection, AHR deficiency impacts the primary CD8 T cell response in a cell-extrinsic manner [59]. In the absence of AHR, antigen-specific CD8 T cells display altered DNA methylation patterns and a transcriptional profile reminiscent of exhausted CD8 T cells [60]. Similarly, AHR controls retention and/or survival of tissue resident memory CD8 T cells in the skin [61].
AHR is central to γδT cell biology, as well. Innate-like γδ T cells that produce IL-17 [62, 63] express AHR, and AHR is key for IL-22 production by these cells [64]. AHR signaling is crucially required to maintain skin-resident Vγ3+ γδ T cells, also known as Dendritic Epidermal γδ T cells (DECT), which derive from thymic precursors that migrate to the skin early on during life [65, 66]. Moreover, AHR ligands present in the diet are necessary for survival and maintenance of intraepithelial lymphocytes in small intestinal LP, including both Vγ5+ γδ T cells and CD8αα+ TCRαβ+ cells [65].
Altogether these findings corroborate the idea that AHR is uniquely positioned to control the function of many T cell subsets that constantly deal with environmental triggers at mucosal sites and that may generate pathological responses when inappropriately instructed.
6. AHR and Innate Lymphoid Cells
Innate lymphoid Cells (ILCs) are an emerging family of lymphocytes that fulfill the definition of “cytokine-responding by cytokine-producing” lymphocytes [67-71]. ILCs are preferentially distributed at mucosal sites, where they can sense changes in the surrounding microenvironment primarily through cytokine receptors signaling. ILCs produce signature cytokines that mirror adaptive Th1, Th2 and Th17/Th22 Thelper cells. ILC3s, which are the innate counterpart of Th17/Th22 cells, express high levels of AHR and are exquisitely sensitive to AHR signaling. AHR is not only required for IL-22 production by ILC3s, but is necessary for their development and/or maintenance [72-74]. AHR deficient mice have highly decreased numbers of ILC3s in small intestine LP and in Peyer's patches (PP) and rapidly succumb to C. rodentium infection. In addition, AHR-null mice lack “postnatally imprinted” cryptopatches (CPs) and isolated lymphoid follicles (ILFs) while “prenatally imprinted” PP are conserved. Mechanistically, AHR can promote expression of Notch, which is also required for NKp46+ ILC3 development [72], or can stabilize c-kit expression, as the c-kit promoter contains XRE binding sites [73]. Lack of AHR in ILC3s results in reduced innate-driven IL-22 production that may favor expansion of SFB leading to exaggerated Th17 responses that cause colitis [46, 47]. In human, AHR antagonism promotes differentiation of IL-22-producing ILC3s to IFN-γ-producing NK cells [75], further implying that AHR plays a key role in ILC3 biology and modulation of their plasticity [76].
Conceivably, AHR ligands present in the diet may contribute to ongoing AHR signaling and regulate the size of small intestinal LP ILC3s and other IELs [65, 73]. However and alternatively, endogenous AHR ligands, such as kynurenine and other tryptophan derivatives, may be more important than diet-derived ligands or may compete with them. On the contrary, AHR ligands generated by bacteria seem to be dispensable, as ILC3s are conserved in germ free mice [72], and germ free mice have normal CPs, despite reduced size of ILFs [77]. Nevertheless, under dietary conditions that provide unrestricted tryptophan availability, microbial-derived AHR ligands may become important to boost ILC3 responses and restrict the colonization and expansion of certain pathogens, such as C. albicans [16].
In addition to regulating ILC3 function, some reports suggest that AHR signaling is relevant to conventional NK cells function, the prototype of ILC1 cells. AHR ligands promote NK cytotoxic activity and IFNγ production; thus AHR deficient mice have defective NK cell-mediated antitumor activity in vivo [78]. Moreover, AHR in conventional NK cells is important to trigger IL-10 production during infection with Toxoplasma gondii (T. gondii). IL-12 and AHR are required for optimal IL-10 production, with IL-12 increasing AHR expression. AHR null mice have defective NK cell-derived IL-10 production during T. gondii infection and are more resistant to this pathogen, as IFNγ responses in the absence of IL-10 promote clearance of T. gondii [79].
Altogether these findings draw attention to the central role played by AHR in instructing ILC responses at mucosal interfaces. Moreover, they indicate that AHR signaling has a broad impact on several innate lymphocytes, as it modulates some aspects of conventional NK cells, which affect their responses to tumors or pathogens.
7. Concluding remarks
Despite the fact that AHR was cloned over 20 years ago there is still much that we need to learn about its function in health and diseases. Work done in the last years clearly indicates that AHR has a fundamental role in immune responses that goes well beyond simple recognition of pollutants. However, the crystal structure of AHR remains to be solved, and the interplay between environmental, dietary, bacterial-derived and endogenous ligands is still poorly understood.
Although multiple lines of evidence indicate that the AHR pathway could be an effective targeting strategy for diseases such as multiple sclerosis, inflammatory bowel diseases, psoriasis, cancer and stem cell transplantation, caution is necessary. Most likely, AHR activation must be tightly controlled in order to obtain the desired effects. For example, AHR-mediated IL-22 production from adaptive and innate cells normally drives protection from bacterial infections and wound healing. However, sustained and dysregulated IL-22 production becomes pathogenic and induce colitis and cancer [80, 81]. Similar exquisite regulation is likely important in many biological processes controlled by AHR.
Overall, the AHR pathway seems to integrate signals from different sources to ensure that host responses carefully reflect and adjust to continuous changes in the environment, a process that is integral to adaptation.
Highlights.
■ AHR links environmental clues to host immune responses.
■ AHR is activated by exogenous and endogenous ligands; their interplay is still poorly understood.
■ Different AHR ligands may differentially influence autoimmune diseases.
■ AHR signaling affects multiple immune cell types, including dendritic cells, T cells and Innate Lymphoid Cells.
Fig. 1.
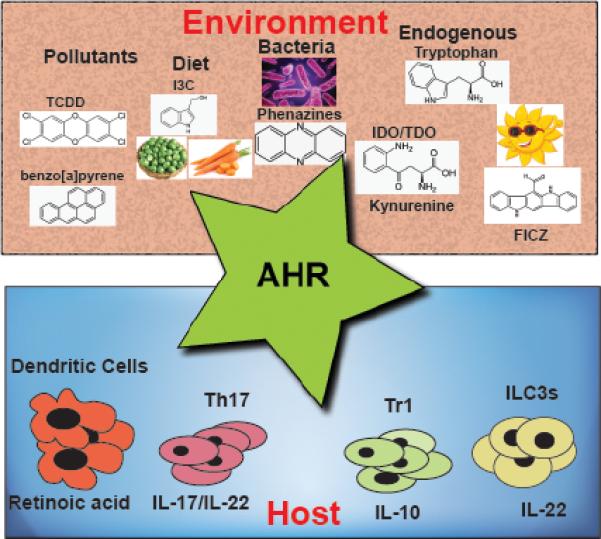
AHR integrates responses from environmental and endogenous ligands to mount appropriate immune responses at barrier organs.
Acknowlegments
The authors thank Michelle Robinette for critical reading of the manuscript. This work was supported by grants from the National Institutes of Health (1U01AI095542, R01DE021255, and R21CA16719) to M. Co.; grant RG4687A1 from National Multiple Sclerosis Society and RO1 CA176695 to M. Ce.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 2.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Ann. Rev. Pharmacol. Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 3.Nambu JR, Lewis JO, Wharton KA, Jr., Crews ST. The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell. 1991;67:1157–1167. doi: 10.1016/0092-8674(91)90292-7. [DOI] [PubMed] [Google Scholar]
- 4.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc. Natl. Acad. Sci. U. S. A. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Ann. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 6.Warner M, Mocarelli P, Brambilla P, Wesselink A, Patterson DG, Jr., Turner WE, et al. Serum TCDD and TEQ concentrations among Seveso women, 20 years after the explosion. J. Exp. Sci. Env. Epidem. 2014;24:588–594. doi: 10.1038/jes.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. U. S. A. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Env. Health Persp. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Q, He G, Zhao J, Soshilov A, Denison MS, Zhang A, et al. Ginsenosides are novel naturally-occurring aryl hydrocarbon receptor ligands. PloS ONE. 2013;8:e66258. doi: 10.1371/journal.pone.0066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rannug U, Rannug A, Sjoberg U, Li H, Westerholm R, Bergman J. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem & Biol. 1995;2:841–845. doi: 10.1016/1074-5521(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 12.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarsero JP, Merino E, Yanofsky C. A Bacillus subtilis operon containing genes of unknown function senses tRNATrp charging and regulates expression of the genes of tryptophan biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2656–2661. doi: 10.1073/pnas.050578997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaitanis G, Magiatis P, Stathopoulou K, Bassukas ID, Alexopoulos EC, Velegraki A, et al. AhR ligands, malassezin, and indolo[3,2-b]carbazole are selectively produced by Malassezia furfur strains isolated from seborrheic dermatitis. J. Inv. Dermatol. 2008;128:1620–1625. doi: 10.1038/sj.jid.5701252. [DOI] [PubMed] [Google Scholar]
- 15.Takamura T, Harama D, Fukumoto S, Nakamura Y, Shimokawa N, Ishimaru K, et al. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol. Cell Biol. 2011;89:817–822. doi: 10.1038/icb.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Moura-Alves P, Fae K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512:387–392. doi: 10.1038/nature13684. [DOI] [PubMed] [Google Scholar]
- 18.Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015;67:259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 19.Di Meglio P, Duarte JH, Ahlfors H, Owens ND, Li Y, Villanova F, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989–1001. doi: 10.1016/j.immuni.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreola F, Fernandez-Salguero PM, Chiantore MV, Petkovich MP, Gonzalez FJ, De Luca LM. Aryl hydrocarbon receptor knockout mice (AHR−/−) exhibit liver retinoid accumulation and reduced retinoic acid metabolism. Cancer Res. 1997;57:2835–2838. [PubMed] [Google Scholar]
- 23.Hauben E, Gregori S, Draghici E, Migliavacca B, Olivieri S, Woisetschlager M, et al. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008;112:1214–1222. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence BP, Denison MS, Novak H, Vorderstrasse BA, Harrer N, Neruda W, et al. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112:1158–1165. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platzer B, Richter S, Kneidinger D, Waltenberger D, Woisetschlager M, Strobl H. Aryl hydrocarbon receptor activation inhibits in vitro differentiation of human monocytes and Langerhans dendritic cells. J. Immunol. 2009;183:66–74. doi: 10.4049/jimmunol.0802997. [DOI] [PubMed] [Google Scholar]
- 26.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Ramachandran I, Gabrilovich DI. Regulation of plasmacytoid dendritic cell development in mice by aryl hydrocarbon receptor. Immunol. Cell Biol. 2014;92:200–203. doi: 10.1038/icb.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thordardottir S, Hangalapura BN, Hutten T, Cossu M, Spanholtz J, Schaap N, et al. The aryl hydrocarbon receptor antagonist StemRegenin 1 promotes human plasmacytoid and myeloid dendritic cell development from CD34+ hematopoietic progenitor cells. Stem Cell Develop. 2014;23:955–967. doi: 10.1089/scd.2013.0521. [DOI] [PubMed] [Google Scholar]
- 29.Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J. Immunol. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 30.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Bioph. Res. Comm. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, et al. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14694–14699. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel CF, Khan EM, Leung PS, Gershwin ME, Chang WL, Wu D, et al. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. J. Biol. Chem. 2014;289:1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J. Exp. Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 38.Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2497–2502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 2005;175:4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 40.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 41.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 42.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, et al. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nat. Immunol. 2011;12:1238–1245. doi: 10.1038/ni.2134. [DOI] [PubMed] [Google Scholar]
- 45.Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5943–5948. doi: 10.1073/pnas.0911755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo X, Liang Y, Zhang Y, Lasorella A, Kee BL, Fu YX. Innate Lymphoid Cells Control Early Colonization Resistance against Intestinal Pathogens through ID2-Dependent Regulation of the Microbiota. Immunity. 2015;42:731–743. doi: 10.1016/j.immuni.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu HY, Quintana FJ, da Cunha AP, Dake BT, Koeglsperger T, Starossom SC, et al. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PloS ONE. 2011;6:e23618. doi: 10.1371/journal.pone.0023618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat. Immunol. 2010;11:846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat. Immunol. 2012;13:770–777. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gagliani N, Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat. Med. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, Dayer JM, et al. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur. J. Immunol. 2010;40:2450–2459. doi: 10.1002/eji.201040461. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence BP, Roberts AD, Neumiller JJ, Cundiff JA, Woodland DL. Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8+ T cells in the lung. J. Immunol. 2006;177:5819–5828. doi: 10.4049/jimmunol.177.9.5819. [DOI] [PubMed] [Google Scholar]
- 60.Winans B, Nagari A, Chae M, Post CM, Ko CI, Puga A, et al. Linking the aryl hydrocarbon receptor with altered DNA methylation patterns and developmentally induced aberrant antiviral CD8+ T cell responses. J. Immunol. 2015;194:4446–4457. doi: 10.4049/jimmunol.1402044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl. Acad. Sci. U. S. A. 2014;111:5307–5312. doi: 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 63.Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe. 2010;7:140–150. doi: 10.1016/j.chom.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 66.Kadow S, Jux B, Zahner SP, Wingerath B, Chmill S, Clausen BE, et al. Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. J. Immunol. 2011;187:3104–3110. doi: 10.4049/jimmunol.1100912. [DOI] [PubMed] [Google Scholar]
- 67.Guo L, Junttila IS, Paul WE. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 2012;33:598–606. doi: 10.1016/j.it.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cella M, Miller H, Song C. Beyond NK cells: the expanding universe of innate lymphoid cells. Front. Immunol. 2014;5:282–292. doi: 10.3389/fimmu.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–365. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 72.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 74.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hughes T, Briercheck EL, Freud AG, Trotta R, McClory S, Scoville SD, et al. The transcription Factor AHR prevents the differentiation of a stage 3 innate lymphoid cell subset to natural killer cells. Cell Rep. 2014;8:150–162. doi: 10.1016/j.celrep.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 78.Shin JH, Zhang L, Murillo-Sauca O, Kim J, Kohrt HE, Bui JD, et al. Modulation of natural killer cell antitumor activity by the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12391–12396. doi: 10.1073/pnas.1302856110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wagage S, John B, Krock BL, Hall AO, Randall LM, Karp CL, et al. The aryl hydrocarbon receptor promotes IL-10 production by NK cells. J. Immunol. 2014;192:1661–1670. doi: 10.4049/jimmunol.1300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O'Connor W, Jr., et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J. Exp. Med. 2011;208:1027–1040. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 2013;210:917–931. doi: 10.1084/jem.20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]


