Abstract
Purpose
Recent data from randomized clinical trials with oncolytic viral therapies and with cancer immunotherapies have finally recapitulated the promise these platforms demonstrated in pre-clinical models. Perhaps the greatest advance with oncolytic virotherapy has been the appreciation of the importance of activation of the immune response in therapeutic activity. Meanwhile, the understanding that blockade of immune checkpoints (with antibodies that block the binding of PD1 to PDL1 or CTLA4 to B7-2) is critical for an effective anti-tumor immune response has revitalized the field of immunotherapy. The combination of immune activation using an oncolytic virus and blockade of immune checkpoints is therefore a logical next step.
Experimental Design
Here we explore such combinations and demonstrate their potential to produce enhanced responses in mouse tumor models. Different combinations and regimens were explored in immunocompetent mouse models of renal and colorectal cancer. Bioluminescence imaging and immune assays were used to determine the mechanisms mediating synergistic or antagonistic combinations.
Results
Interaction between immune checkpoint inhibitors and oncolytic virotherapy was found to be complex, with correct selection of viral strain, antibody and timing of the combination being critical for synergistic effects. Indeed, some combinations produced antagonistic effects and loss of therapeutic activity. A period of oncolytic viral replication and directed targeting of the immune response against the tumor were required for the most beneficial effects, with CD8+ and NK, but not CD4+ cells mediating the effects.
Conclusions
These considerations will be critical in the design of the inevitable clinical translation of these combination approaches.
Keywords: Oncolytic Virus, Vaccinia, checkpoint inhibitor, CTLA4, immunotherapy
INTRODUCTION
The last 5 years have seen the emergence of antibody-mediated blockade of immune checkpoints as a key new weapon in the anti-cancer arsenal (1,2). The anti-CTLA4 inhibitor Ipilimumab has been approved for the treatment of melanoma(3,4), while a panel of monoclonal antibodies targeting the interaction of PD1 and PDL1 have also demonstrated promising responses in a succession of clinical trials (5,6). Together these trials have demonstrated the clinical need to overcome the tumor’s capacity to shut down the T-cell response in the creation of an effective cancer immunotherapy.
The field of oncolytic virotherapy has also recently demonstrated its potential to produce clinically effective cancer treatments, with data from several recent randomized trials resulting in impressive response rates (7,8). One factor that has united the most successful oncolytic vectors has been the expression of an immune activating transgene (GM-CSF), an indication that a key determinant of the activity of oncolytic viruses is their capacity to activate and target the immune response (9,10). This has since been confirmed in a multitude of pre-clinical studies (11–13), such that the oncolytic virus platform might best be considered an immunotherapeutic.
We have previously developed several oncolytic vectors, primarily focusing on vectors based on vaccinia virus (14–17). These provide several advantages as immunotherapies beyond their long historical use as vaccines; (i) they can induce an adaptive immune response raised against tumor antigens as a result of their selective replication within the tumor microenvironment (18,19). This in situ vaccination effect results in production of CTL targeting relevant tumor antigens without the need for any prior interrogation of the tumor; and (ii) viral replication within the tumor can at least transiently overcome localized immunosuppression, something that most traditional vaccine approaches fail to achieve. However in many cases, once the oncolytic virus is cleared by the host immune response, the immunosuppressive environment is apparently restored and the tumor relapses. The combination of oncolytic virus and the blockade of immune checkpoint inhibitor therefore is an appealing strategy.
Although there has been much interest in this combination, including the proposed clinical combinations of the oncolytic HSV T-Vec (Amgen) and Ipilimumab (Yervoy, Bristol Myers Squibb) in the treatment of melanoma (Clinical Trials.gov NCT01740297), there has been very little supportive data reported to date. Here we examine the combination of oncolytic vaccinia with several different immune-targeting monoclonal antibodies.
MATERIALS AND METHODS
Cell culture and viruses
Renca (murine renal adenocarcinoma) cell line was obtained from ATCC (Manassas, VA). MC38 cell line (murine colon adenocarcinoma) was a kind gift from Dr. David Bartlett (University of Pittsburgh Cancer Institute). Cell lines were maintained in recommended culture media containing 5–10% fetal bovine serum at 37°C, 5% CO2. Cell lines have not been authenticated by the authors beyond their ability to form tumors in syngeneic mouse models.
All recombinant vaccinia strains used in this work are derived from the Western Reserve (WR) strain (BEI Resources; Manassas, VA). The double-deleted strains vvDD and WR.B18R-.TK- (B18R- in short) have been described previously (15,20). These contain deletions in the tk gene and in the vgf or B18R viral genes, respectively. In addition, both strains express the firefly luciferase gene from the synthetic vaccinia promoter pE/L (21), which allows monitoring of luciferase expression as a surrogate indicator of viral replication (22). Viruses were titered, manufactured and purified as previously described (23).
Animal models
All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. C57/BL6 and BALB/c female mice (6–8 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME). Renca or MC38 tumor cell lines were implanted subcutaneously at 5×105 cells per mouse into BALB/c or C57/BL6 mice, respectively. Oncolytic Vaccinia viruses were injected intravenously (tail vein) at 2×108 pfu/mouse when tumors reached ~50–100 mm3.
Anti-mouse CTLA4 (9D9) and anti-mouse CD25 (PC-61.5.3) antibodies (BioXCell, West Lebanon, NH) were injected intraperitoneally at 100 or 200 μg/mouse/dose, respectively, with treatments consisting of 3 doses each 3 days apart. Mouse IgG2b κ Isotype Control (BioXCell) was used as a control. For depletion experiments, anti-mouse CD8 (2.43), anti-mouse CD4 (GK1.5), anti-mouse NK1.1 (PK136), and anti-mouse IFNγ (XMG1.2) were purchased from BioXCell, and mice were injected intraperitoneally with 500 μg at days -1 and 2 after tumor implantation, followed by 250 μg injection every 5 days till the end of the experiment.
Tumor volume was monitored by caliper measurement and defined by V(mm3)= π/6 X W2 X L, where W and L are the width and the length of the tumor, respectively. Data are expressed as tumor size relative to the beginning of the therapy (100%). For Kaplan-Meier survival curves, end point was established at ≥750 mm3. Animals whose tumor size never achieved the threshold were included as right-censored information.
Bioluminescence imaging
Viral gene expression was determined through bioluminescence imaging of luciferase expression in vivo. A dose of 4.5 mg of D-luciferin (GoldBio, St Louis, MO) was injected intraperitoneally per mouse before imaging on an IVIS2000 (PerkinElmer, Waltham, MA)(2% isoflurane). Images were analyzed using LivingImage software (PerkinElmer, Waltham, MA).
IFN-γ ELISPOTs
For ELISPOT assays, splenocytes were mixed with tumor cells or splenocytes from naïve mice infected with UV-inactivated Vaccinia virus at 5:1 ratio. Naïve splenocytes were used as control. 96-well membrane filter plates (EMD Millipore, Billerica, MA) coated with 15 μg/ml of monoclonal anti-mouse IFN-γ antibody AN18 (Mabtech, Inc., Cincinnati, OH) were used. Cells were maintained for 48 hours at 37 °C and spots were detected using 1 μg/ml of biotinylated anti-mouse INF-γ antibody R4-6A2-biotin (Mabtech). Plates were developed using an ABC kit and an AEC substrate kit for peroxidase (Vector Laboratories, Inc., Burlingame, CA). Specific spots were counted and analyzed using an ImmunoSpot Analyzer and ImmunoSpot software from CTL (Shaker Heights, OH).
Flow cytometry
Tumors were harvested from mice and mechanically disaggregated and digested with triple enzyme mixture (Collagenase type IV, DNase type IV, and Hyaluronidase type V (Sigma-Aldrich, St Louis, MO)). Cell surface and intracellular immunostaining analyses were performed using a Gallios Flow Cytometer (Beckman Coulter, Inc., Brea, CA). For intracellular staining, cells were fixed and permeabilized using a Foxp3 Fix/Perm Buffer Set (eBioscience, San Diego, CA). Tumor-disaggregated cells were stained using PE-Cy7 anti-mouse CD3 (BD Biosciences, San Jose, CA), eFlour450 anti-mouse NKp46, APC anti-mouse NKg2D, FITC anti-mouse CD4, PerCP-Cy5.5 anti-mouse CD8, PE anti-mouse CD25, and APC anti-mouse Foxp3 antibodies (eBioscience).
Statistical analysis
Standard Student’s t-tests (two-tailed) were used throughout this work, except for the comparison of survival curves, where a log-rank test was used. In all cases, significance was achieved if P<0.05.
RESULTS
Anti-CTLA4 antibody hinders Vaccinia virus replication in mice
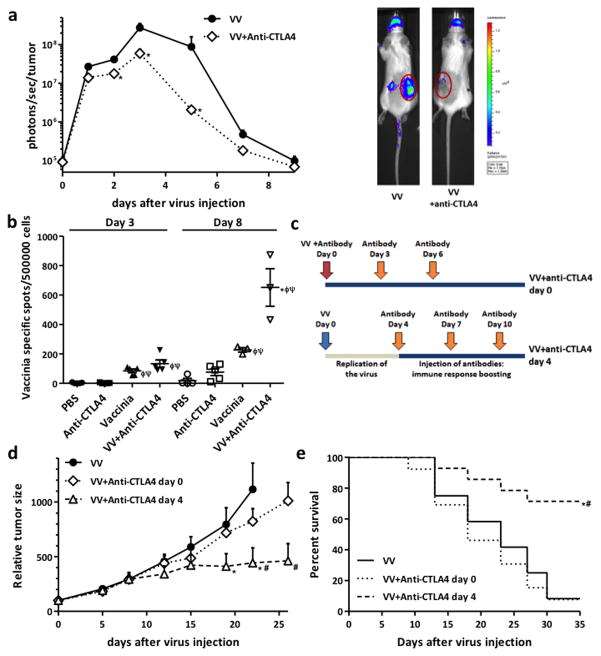
Mice harboring syngeneic subcutaneous mouse renal adenocarcinomas (Renca cells) were injected with a single intravenous dose of oncolytic Vaccinia virus and with three intraperitoneal doses of 100 μg of mouse anti-CTLA4 antibody at days 0, 3 and 6 after virus injection. The schedule and doses of anti-CTLA4 antibody used were determined based on previously published pre-clinical studies (24–26). Initially we looked to determine the safety of the combination and the effects of injection of anti-CTLA4 antibody on the replication of Vaccinia in the tumors. We monitored viral luciferase transgene expression with bioluminescence imaging as ourselves and others have shown this to directly correlate with viral replication (22,27). Anti-CTLA4 antibody significantly reduced viral luciferase expression from within the tumors (Fig 1a) (at days 3 and 5 after virus injection, >5- and 40-fold reduction was detected). A similar depletion in viral replication was also observed in a second tumor model (MC38 tumors implanted subcutaneously in C57/B6 mice, Figure S1b), demonstrating that this was not a cell line- or mouse strain-associated effect. Reduced viral replication did however correlate with enhanced immune activation, as see with the increased numbers of CTLs recognizing Vaccinia epitopes detected in the spleens of the mice (Figure 1B). Although increased anti-viral CTL appeared as early as day 3 post treatment it is likely that innate immune responses may also be enhanced with the combination as viral replication is reduced as early as 24h after treatment. This indicated a more robust immune response was raised in mice when anti-CTLA4 antibody was injected together with the viral therapy.
Figure 1. Combining Oncolytic Vaccinia Virus and anti-CTLA4 antibody therapies.
(a) Anti-CTLA4 antibody injection reduces Vaccinia Virus replication in the tumor in vivo. Balb/c mice with subcutaneous Renca tumors (renal adenocarcinoma) were randomized and injected with a single intravenous dose of 2×108 plaque-forming units (pfu) per mouse of oncolytic B18R- Vaccinia Virus (VV). In the combination group, 100 μg of mouse anti-CTLA4 antibody were injected intraperitoneally on days 0, 3 and 6 post-virus administration. Bioluminescence imaging was used to follow viral luciferase transgene expression from within the tumor. Mean values of 9–10 animals +SD are plotted. Representative luciferase signals from at day 3 post-injection are also depicted (tumors are circled). (b) Viral/anti-CTLA4 combiunation results in increased levels of Vaccinia-specific cytotoxic T cells (CTLs). Mice were treated as in (a), adding PBS and single therapy with anti-CTLA4 antibody as additional controls. At day 3 and 8 post-virus injection, spleens were harvested and quantified by IFN-γ ELISpot assay for Vaccinia-reacting T cells. Values of individual mice and means ± SEM of the different treatments are plotted. (c) Alternative schedule for Vaccinia Virus and anti-CTLA4 antibody combination. Anti-CTLA4 antibody doses were administrated at days 4, 7 and 10 after virus injection, in an approach designed to permit an initial period of viral replication. (d) Injection of anti-CTLA4 antibody after Vaccinia Virus replication improves therapeutic activity of combination therapy. Mice (Balb/c bearing Renca tumors) were treated as before or in combination with anti-CTLA4 antibody as depicted in (c). Relative tumor growth and (e) Kaplan-Meier survival curves are plotted. For survival curves, the end point was established at a tumor volume of ≥750 mm3. Mean values of 7–8 mice/group +SE are plotted. (* P<0.05 compared with VV group; φ P<0.05 compared with PBS group; ψ P<0.05 compared with anti-CTLA4 group; # P<0.05 compared with VV+anti-CTLA4 day 0 group).
Delayed administration of anti-CTLA4 antibody improves antitumor efficacy
A novel schedule for oncolytic Vaccinia and anti-CTLA4 antibody combination was therefore designed in order to permit an initial phase of viral oncolytic activity prior to anti-CTLA4 antibody administration (Figure 1c). Anti-CTLA4 doses were therefore injected at days 4, 7 and 10 after virus injection, allowing an initial phase of unhindered viral replication and spread within the tumor (Figure S1c). Whereas simultaneous injection of Vaccinia virus and anti-CTLA4 antibody resulted in no significant anti-tumor benefit (compared with mice treated with single Vaccinia therapy), when we delayed administration of the blocking antibody until after the peak of viral replication a greater than threefold reduction (P<0.04) in tumor volume was observed (Figure 1d) (isotype control antibody had no effect on tumor growth Figure S1a). In addition, this novel treatment schedule was able to significantly increase survival of mice relative to groups treated either with single Vaccinia therapy or Vaccinia injected concurrently with anti-CTLA4 (Figure 1e).
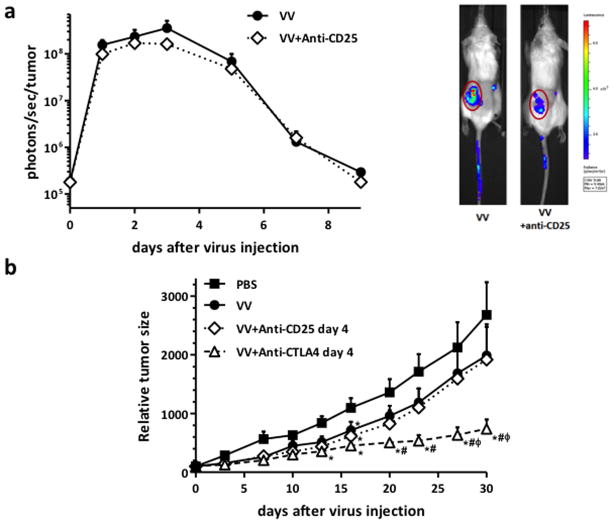
Combination of anti-CD25 antibody with Vaccinia provided no therapeutic benefit
As an alternative to immune checkpoint blockade therapy (anti-CTLA4), we looked to test whether other approaches that also target tumor immunosuppression (such as depletion of regulatory T-cells with anti-CD25 therapy) also synergized with oncolytic Vaccinia therapy. We again initially monitored virus replication in tumors via luciferase bioluminescence imaging after anti-CD25 administration. As with anti-CTLA4 combination, we observed a reduction in viral kinetics when antibody therapy began on the same day as viral treatment, however differences were not significant (Figure 2a). Further, when the anti-tumor effects of Vaccinia/anti-CD25 combination therapy were tested, neither regimen (injecting anti-CD25 antibody concurrently with virus or after viral replication peak) resulted in improved efficacy relative to single oncolytic Vaccinia therapy (Figure S2b&2b). As a further test, anti-CD25 antibody was also added before viral therapy (Figure S2c), however again no therapeutic advantage was seen (T-reg depletion with the anti-CD25 regimen used was also confirmed, Figure S2a). Finally, a direct comparison of the anticancer activity of Vaccinia/anti-CD25 versus Vaccinia/anti-CTLA4 combination therapies confirmed the enhanced efficacy of combining oncolytic virus with blockade of CTLA4 (Figure 2b).
Figure 2. Combination of Vaccinia Virus with anti-CD25 antibody did not provide any therapeutic advantage.
(a) Anti-CD25 antibody therapy effect on Vaccinia Virus replication. Balb/c mice bearing Renca tumors were injected intravenously with 2×108 PFU/mouse of oncolytic Vaccinia Virus (VV, strain B18R-). For the combination group, a dose of 200 μg of mouse anti-CD25 antibody was also injected intraperitoneally at days 0, 3 and 6 post-virus administration. Viral luciferase expression from within the tumor was quantified at indicated time points by bioluminescence imaging. Mean values of 7–8 animals +SD are plotted. Bioluminescence signals from one representative animal of each group at day 3 post-administration are also shown (tumors are circled). (b) Mice (Balb/c with subcutaneous Renca tumors) were treated IV with 2×108 pfu of VV (n=10–12 per group). For combination groups, anti-CTLA4 or anti-CD25 antibodies were injected with 100 or 200 μg/mouse, respectively, at days 4, 7 and 10 post-virus injection. PBS was injected intraperitoneally as a control. Tumor growth was followed by caliper measurements. Means +SE are plotted. (*P<0.05 compared with PBS group; # P<0.05 compared with VV group; φ P<0.05 compared with VV+anti-CD25 day 4 group).
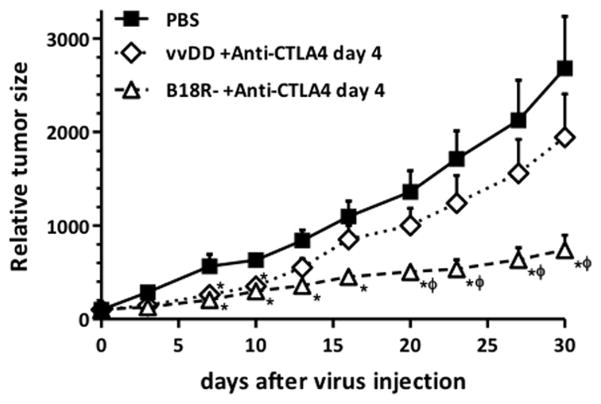
Immunogenicity-enhanced oncolytic Vaccinia vectors improve synergistic effects with anti-CTLA4 antibody
As a next step we looked to examine the importance of the viral vector used in these combination approaches. Two different double-deleted oncolytic Vaccinia viruses were compared in combination with anti-CTLA4 antibody therapy. vvDD (vgf and tk double-deleted Vaccinia virus) has demonstrated highly tumor-restricted replication (28) that is equivalent in level and selectivity to the B18R- strain. B18R- (B18R and tk double-deleted Vaccinia virus) also demonstrated highly tumor-restricted replication but this was coupled with enhanced immunogenicity relative to vvDD (including increased production of cytokines and chemokines within the tumor) (29). This is due to the loss of B18R, that encodes a secreted type I interferon-binding protein (14). When both viral strains were compared for anticancer effects in combination with anti-CTLA4 antibody (Figure 3), B18R-/anti-CTLA-4 treatment induced a more than 3.6-fold (P<0.009) reduction in tumor size at sacrifice compared to PBS treatment, while in this model vvDD/anti-CTLA4 combination only induced a 1.4-fold inhibition.
Figure 3. Therapeutic activity of oncolytic vaccinia in combination with anti-CTLA4 antibody is viral strain dependent.
2×108 pfu of oncolytic Vaccinia Virus (B18R- or vvDD) were administrated intravenously to Balb/c mice bearing subcutaneous Renca tumors. At days 4, 7 and 10 after virus injection, a dose of 100 μg of anti-CTLA4 antibody was injected intraperitoneally. B18R- displayed greater inhibition of tumor growth relative to vvDD. Relative tumor volume after virus administration is plotted (n=12–15 mice/group +SE). (* P<0.05 compared with PBS group; φ P<0.05 compared with vvDD+anti-CTLA4 day 4 group).
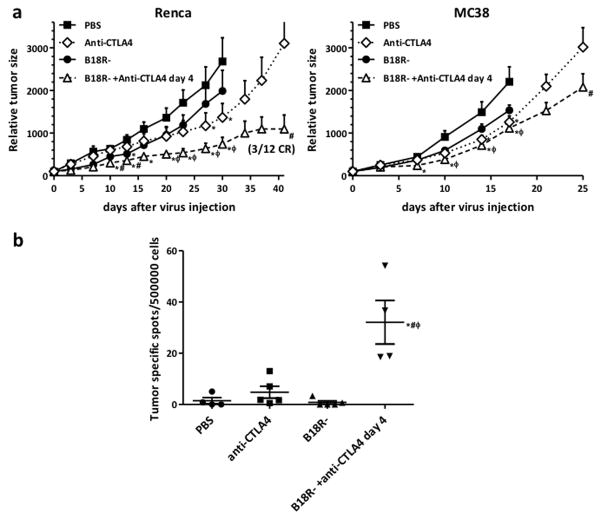
B18R- oncolytic Vaccinia virus exhibits potent antitumor efficacy in optimized combination with anti-CTLA4 antibody therapy
We next looked to test in more detail the most effective combination of viral vector (B18R-), antibody (anti-CTLA4) and regimen (antibody treatment beginning 4 days after viral therapy) determined from the previous studies.
Mice carrying either Renca (renal adenocarcinoma) or MC38 (colon adenocarcinoma) tumors were injected with a single intravenous dose of B18R- at 2×108 pfu per mouse. At days 4, 7 and 10 after virus injection, an intraperitoneal dose of 100 μg of mouse anti-CTLA4 antibody was administrated. PBS, or single therapy treatments were used as controls. At the time of sacrifice, combination therapy resulted in a reduction of more than 2.7- (P<0.035) and 1.3-fold (P<0.02) in Renca and MC38 tumor models respectively relative to single B18R- therapy (Figure 4a).
Figure 4. Optimized combination therapy results in synergistic anticancer activity.
(a) Renca (left) or MC38 (right) tumors were implanted into Balb/c or C57/Bl6 mice respectively. Mice were injected with PBS or 2×108 pfu of B18R- oncolytic Vaccinia Virus (VV) through the tail vein. For the anti-CTLA4 group, 100 μg of anti-CTLA4 antibody was injected intraperitoneally at days 0, 3 and 6. For the combination group, anti-CTLA4 antibody doses were administrated at days 4, 7 and 10 after virus injection. Tumor volumes were measured and relative tumor volume +SE of the 12–15 mice/group is plotted. (b) Combination therapy increases cytotoxic T cells recognizing tumor antigens. Cellular immune responses to tumor cells was evaluated by IFN-γ ELISpot assay. At day 11 post-virus administration spleens were harvested from Balb/c mice bearing Renca tumors and treated as in (a). Splenocytes were evaluated for CTLs recognizing Renca cells. Values of individual mice and means ± SEM are depicted. (*P<0.05 compared with PBS group; φ P<0.05 compared with VV group; # P<0.05 compared with anti-CTLA4 group)
The combination induced a reduction of more than 2.8-fold (P<0.04) in tumor volume compared to singe anti-CTLA4 therapy at day 42 after treatment in Renca models. Importantly, B18R-/anti-CTLA4 combination therapy induced 3 out of 12 complete responses in this model. For MC38 tumors, B18R-/anti-CTLA4 combination therapy did not produce as dramatic an effect, but still reduced tumor volume 1.5-fold (P<0.045) compared to single anti-CTLA4 therapy, a significant improvement by day 24 after treatment.
Vaccinia/anti-CTLA4 combination therapy resulted in enhanced systemic and tumor-specific cellular immune response
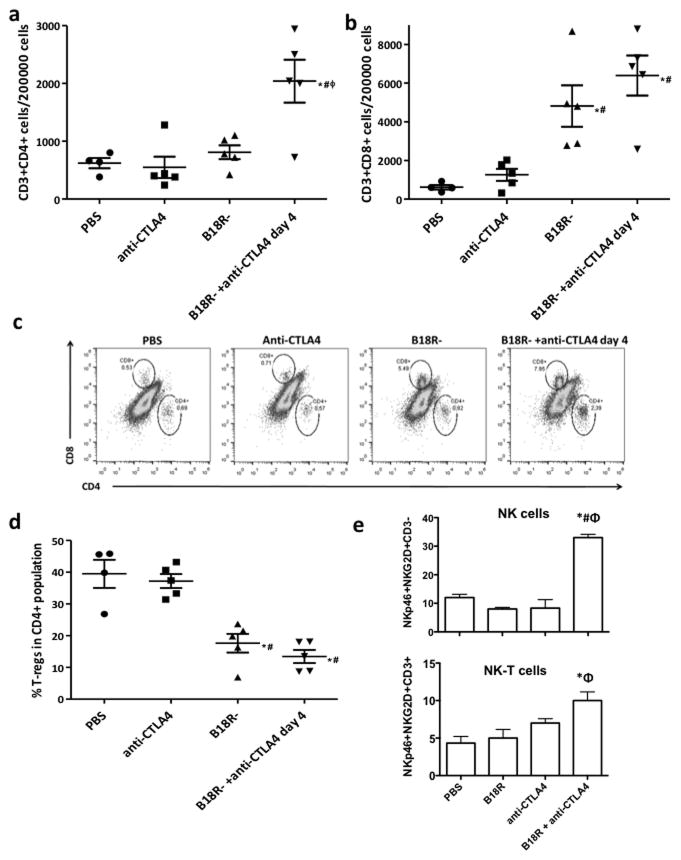
In order to evaluate the mechanisms driving the most effective combination of oncolytic vaccinia and anti-CTLA4 antibody, we examined the immune response raised against and within the tumor. Mice bearing Renca tumors were treated as before. Controls included PBS, single B18R- therapy, or single anti-CTLA4 therapy (injected at days 0, 3 and 6). Mice were sacrificed at day 11 after virus administration and evaluated for specific-CTLs in the spleen by ELISpot assay and for immune cell populations in tumors by flow cytometry. Combination therapy was able to significantly increase the numbers of CTLs recognizing tumor cell antigens compared to any of the controls (Fig 4b). When CD3+CD4+ populations in tumors were quantified, a significant percentage increase was observed after treatment with B18R-/anti-CTLA4 combination therapy relative to any other treatment (Figure 5a and 5c). An increase in the percentage of CD3+CD8+ cells infiltrating the tumor was also observed, but appeared to be more closely associated with replication of the virus in the tumor (Figure 5b and 5c), with both viral treated groups displaying high levels of these cells. Finally, in order to ensure that the increased CD3+CD4+ population infiltrating the tumors did not represent regulatory T-cells, additional staining for CD25 and FoxP3 was used (Figure 5d). We observed that in the control group, about 40% of the CD3+CD4+ cells present a regulatory T cell phenotype (CD25+Foxp3+). Anti-CTLA4 treatment barely reduced this percentage, but treatment with B18R- virus dropped amounts to 17%, and this improved further to only 13% when anti-CTLA4 was combined with oncolytic virus. Although very few NK or NK-T cells were detected in the tumor (<0.01% of cells), this number was also significantly increased only when the combination of B18R- virus and anti-CTLA4 antibody was used in combination (Fig 5e and S4).
Figure 5. Altered T-cell repertoire in the tumor after Vaccinia/anti-CTLA4 combination therapy.
Balb/c mice with subcutaneous Renca tumors were treated as before (Fig 4) and tumors were harvested at day 11 post-virus injection and evaluated for lymphocyte populations by flow cytometry. Number of (a) CD3+CD4+ and (b) CD3+CD8+ cells per 200000 total cells are plotted. (c) Representative distributions of CD4+ and CD8+ populations within CD3+ population within the tumor. (d) Percentage of regulatory T-cells (CD25+Foxp3+) within the CD3+CD4+ population of the tumor. Values for individual tumors and means +SEM are plotted. (e) Numbers of NK cells (NKp46+NKg2D+CD3−) and NK-T cells (NKp46+NKg2D+CD3+) per 200 000 events within the tumor. (* P<0.05 compared with PBS group; # P<0.05 compared with anti-CTLA4 group; φ P<0.05 compared with B18R- group).
Vaccinia/anti-CTLA4 combination therapy synergistic effects require CD8+ T-cells, NK cells and IFN-γ, but not CD4+ T-cells
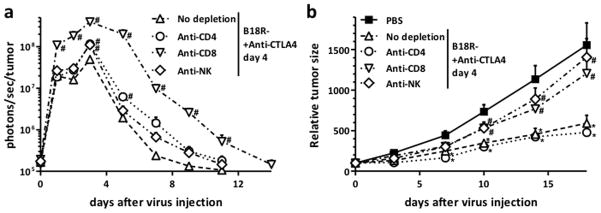
In order to define the host factors critical for the therapeutic advantage seen with the B18R-/anti-CTLA4 combination, viral replication and anti-tumor effect experiments were repeated in RENCA tumor-bearing mice depleted for CD4+ T-cells, CD8+ T-cells or NK cells (Figure 6). It was seen that both CD8+ T-cells and NK cells were required for the therapeutic advantage (while anti-tumor effects were maintained after depletion of CD4+ T-cells) (Figure 6b). Depletion of CD8+ T-cells but not NK cells or CD4+ T-cells also significantly enhanced viral replication, indicating this cell lineage was responsible for both reduced viral replication and enhanced anti-tumor effects during B18R-/anti-CTLA4 combination (Figure 6a). Interestingly CD8+ T-cells appeared responsible for reduced viral replication in the tumor, even at times as soon as 1 day after viral treatment. The importance of CD8+ T-cells was supported through depletion of IFN-γ, which also resulted in loss of therapeutic advantage and enhanced viral tumor-specific replication (Figure S5). NK cells appear to be required for the anti-tumor effect, but do not limit viral replication.
Figure 6. Depletion of different immune cell subsets alters Vaccinia Virus replication and anti-tumor activity of combination therapy.
(a) Replication of Vaccinia Virus (VV strain B18R-) is increased by depletion of CD4+, CD8+, and NK cells. Balb/C mice injected with CD4, CD8, and NK depleting antibodies were challenged with Renca tumors and treated as before (Fig 4). Viral luciferase expression from within the tumor was quantified at indicated time points by bioluminescence imaging. Mean values of 12–13 animals +SD are depicted. (b) CD8+ and NK cells are essential for the anti-tumor activity of Vaccinia Virus/anti-CTLA4 combination therapy. Mice were treated as in (a) and tumor growth was monitored (+SE, 12–15 mice/group) (*P<0.05 compared with PBS group; # P<0.05 compared with No depletion group).
DISCUSSION
It is evident that the blockade of immune checkpoint alone is rarely curative, but has the capacity to synergize with other therapies that selectively activate the immune response. As such, the realization that the immune response raised by oncolytic viral therapies is a critical mechanism mediating their therapeutic activity means that the combination of these two platforms would be logical and appealing (30). However, despite the fact that clinical trials have been proposed combining the oncolytic HSV T-Vec with Ipilimumab, little pre-clinical data has been reported on such combinations (31).
Here we examine approaches to combine oncolytic Vaccinia viruses with different monoclonal antibodies that target cancer mediated immunosuppression. Significantly improved anti-tumor responses were demonstrated in several mouse tumor models, providing strong support for the clinical translation of this approach. However, it was initially seen that careful consideration was needed to identify the correct combination of antibody, viral strain and especially in the timing of application of the different treatments. Incorrect combination resulted in a loss of benefit, and potentially antagonistic effects.
In initial studies combining anti-CTLA4 blocking antibody with Vaccinia virus, no therapeutic benefit was seen (Figure 1d). In these studies the antibody treatment was begun at the same time as viral inoculation, and imaging of viral luciferase transgene expression demonstrated that viral gene expression was reduced by >40-fold relative to virus used alone (Figure 1a). This indicated that a robust anti-viral immune response was being raised leading to premature clearance of the virus. Indeed, this combination was also shown to result in a significant increase in the level of anti-viral CTL (Figure 1b) and viral replication was restored after depletion of either CD8+ T-cells or IFN-γ (Figure 6a and S4a). This is potentially important as several groups are looking to express antibodies blocking immune checkpoints directly from oncolytic vectors (32). We have previously used exogenous regulation of cytokine transgene function to down regulate cytokine function for a period of around 4 days after initial treatment (22,33). This allowed an initial phase of viral oncolytic activity and unhindered replication within the tumor, prior to a secondary phase of immunotherapeutic activity that could be enhanced through subsequent stabilization of the cytokine function. Using a similar tactical approach, it was felt that addition of anti-CTLA4 antibody at later times after viral therapy could result in improved therapeutic activity.
This was indeed confirmed (Fig 1d), with initiation of anti-CTLA4 therapy 4 days after viral delivery found to result in significantly improved anti-tumor effects in mouse syngeneic tumor models.
Several different monoclonal antibody therapies target cancer-mediated immunosuppression, including both blockade of immune checkpoints as well as direct depletion of suppressive immune cell types that are known to accumulate within the tumor microenvironment (34). One example of the latter approach uses anti-CD25 antibody to deplete regulatory T-cells within the tumor. It was determined however, that independent of whether anti-CD25 was added prior to, at the same time as, or after viral therapy there was no therapeutic advantage seen with anti-CD25 antibody combined with oncolytic vaccinia (relative to vaccinia used alone) (Figure 2b& S2). This somewhat surprising result may be due to the fact that oncolytic vaccinia alone was actually found to be an effective means to reduce the levels of T-regs in the tumor microenvironment (Figure S3a). This also highlights the fact that the important contribution of the anti-CTLA-4 antibody to the synergistic combination with oncolytic vaccinia appears to be dependent on its activation of CD8+ T-cells rather than on depletion of T-regs.
We have also previously demonstrated that some viral mutations result in production of viral vectors with enhanced immune activation properties (29). For example, although vaccinia strains carrying the thymidine kinase (TK) deletion typically used to mediate tumor selectivity in oncolytic vaccinia vectors such as JX-594 (Pexa-Vec, Jennerex, now part of Sillajen)(10), vvDD (28) or GLV-1h68 (GL-ONC1, GeneLux)(35) did result in immune activation, this could be enhanced if the viral B18R gene (a secreted type I IFN binding protein)(36) was also deleted. When the oncolytic vaccinia strain vvDD was compared head to head with a comparable strain carrying the B18R gene deletion (WR.TK-.B18R-) in combinations with anti-CTLA4 antibody, it was found that the ‘immune activation enhanced’ virus (B18R-) was significantly more potent (Fig 3). Therefore viral strain and backbone are also important considerations when designing combination therapies with blockade of immune checkpoints.
The optimal combination of monoclonal antibody (anti-CTLA4), viral strain (B18R-) and treatment regimen (antibody therapy begun 4 days after viral delivery) resulted in significantly enhanced therapeutic responses in different mouse cancer models (Figure 4a), including renal cancer and colorectal cancer models in different mouse genetic backgrounds (BALB/c or C57/BL6). The combination was most effective against the Renca tumor model, and less effective against MC38 (but still significantly better than either therapy used alone). It is possible the MC38 model may be less immunogenic, and so enhanced induction of anti-tumor CTL may not be possible or may remain ineffective at enhancing therapeutic responses. The enhanced therapeutic activity of this combination is primarily immune mediated as (i) the combination resulted in significantly greater numbers of CTLs in the spleen that target tumor antigens (relative to either therapy used alone) (Fig 4b); (ii) the combination also resulted in significant increases in the number of CD3+CD4+ T-cells in the tumor relative to either therapy alone (coupled to a decrease in the relative amounts of T-regs), and an increase in CD3+CD8+ T-cells (although this was only significant relative to control or anti-CTLA4 used alone) (Fig 5); and (iii) depletion of either CD8+ T-cells, IFN-γ or NK cells (but not CD4+ T-cells) resulted in loss of the therapeutic advantage seen with the combination (Figure 6 & S4). It therefore appears that immune enhanced oncolytic vaccinia strains (such as WR.TK-.B18R-) can activate an adaptive immune response targeting tumor associated antigens that is dependent on CD8+ T-cells and requires NK cell involvement during early immune activation (Fig S3b) (although NK cells return to close to baseline levels in the tumor by day 10 after viral treatment). Under single viral therapy treatment this CD8+ T-cell immune response is blunted by premature shut down of T-cell proliferation, an effect that can be overcome by adding anti-CTLA-4 antibody. If the antibody is added too early, then the virus cannot induce the correct immune response before its immune-mediated removal and the benefits are lost.
Together this data demonstrates how the correct combination of oncolytic virus and anti-CTLA4 antibody results in robust induction of anti-tumor CTL coupled to targeting of localized immune suppression within the tumor. This combination therefore more efficiently activates the immune response to target the tumor as well as blocking the capacity of the local tumor microenvironment to suppress the resultant immune response, leading to significantly improved therapeutic effects. The clinical examination of these combinations is therefore an exciting prospect.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Two of the most promising novel therapeutic platforms for the treatment of cancer are blockade of immune checkpoints and oncolytic viral therapies. Here we look to combine these in pre-clinical mouse tumor models. The realization that inhibition of immune checkpoints is a critical need for successful immunotherapy and that the immune response activated by oncolytic viral therapies provide their most potent anti-tumor effects, means that the combination of these approaches is likely to result in significant clinical benefit. The enthusiasm in this combination is seen with the ongoing clinical combination of the oncolytic T-Vec with Ipilimumab. However there has been almost no pre-clinical data reported to support this combination to date. In this work, we not only demonstrate that clinically relevant combinations can produce significantly enhanced responses in mouse tumor models, but also provide mechanistic insight into why some combinations are synergistic and others resulted in complete loss of therapeutic advantage.
Acknowledgments
Financial Support: This work was supported by R01CA140215 & R01CA178766, while core facilities funded under the CCSG (P30CA047904) were used, including In Vivo Imaging, Small Animal and Flow Cytometry facilities.
Footnotes
Conflict of Interest: Dr. Steve Thorne holds equity in Western Oncolytics Ltd. None of the other authors have any conflict of interest.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11(11):805–12. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol. 2012;13(12):1129–32. doi: 10.1038/ni.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nature Medicine. 2013;19(3):329–36. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andtbacka RHI, Collichio FA, Amatruda T, Senzer N, Chesney J, Delman KA, et al. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol. 2013;31(Suppl):LBA9008. [Google Scholar]
- 9.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Annals of surgical oncology. 2010;17(3):718–30. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, et al. Systemic Armed Oncolytic and Immunologic Therapy for Cancer with JX-594, a Targeted Poxvirus Expressing GM-CSF. Mol Ther. 2006;14(3):361–70. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Thorne SH. Immunotherapeutic potential of oncolytic vaccinia virus. Immunol Res. 2011;50(2–3):286–93. doi: 10.1007/s12026-011-8211-4. [DOI] [PubMed] [Google Scholar]
- 12.Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15(13):4374–81. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rommelfanger DM, Wongthida P, Diaz RM, Kaluza KM, Thompson JM, Kottke TJ, et al. Systemic Combination Virotherapy for Melanoma with Tumor Antigen-Expressing Vesicular Stomatitis Virus and Adoptive T-Cell Transfer. Cancer Research. 2012 doi: 10.1158/0008-5472.CAN-12-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4(12):e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorne SH, Hwang TH, O’Gorman WE, Bartlett DL, Sei S, Kanji F, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117(11):3350–58. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirn DH, Wang Y, Liang W, Contag CH, Thorne SH. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 2008;68(7):2071–5. doi: 10.1158/0008-5472.CAN-07-6515. [DOI] [PubMed] [Google Scholar]
- 17.Li J, O’Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, et al. Chemokine Expression From Oncolytic Vaccinia Virus Enhances Vaccine Therapies of Cancer. Molecular therapy: the journal of the American Society of Gene Therapy. 2011 doi: 10.1038/mt.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne SH, Liang W, Sampath P, Schmidt T, Sikorski R, Beilhack A, et al. Targeting localized immune suppression within the tumor through repeat cycles of immune cell-oncolytic virus combination therapy. Molecular therapy: the journal of the American Society of Gene Therapy. 2010;18(9):1698–705. doi: 10.1038/mt.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang LC, Lynn RC, Cheng G, Alexander E, Kapoor V, Moon EK, et al. Treating Tumors With a Vaccinia Virus Expressing IFNbeta Illustrates the Complex Relationships Between Oncolytic Ability and Immunogenicity. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20(4):736–48. doi: 10.1038/mt.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang LC, Lynn RC, Cheng G, Alexander E, Kapoor V, Moon EK, et al. Treating tumors with a vaccinia virus expressing IFNbeta illustrates the complex relationships between oncolytic ability and immunogenicity. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20(4):736–48. doi: 10.1038/mt.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakrabarti S, Sisler JR, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23(6):1094–7. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Sampath P, Hou W, Thorne SH. Regulating cytokine function enhances safety and activity of genetic cancer therapies. Mol Ther. 2013;21(1):167–74. doi: 10.1038/mt.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampath P, Li J, Hou W, Chen H, Bartlett DL, Thorne SH. Crosstalk between immune cell and oncolytic vaccinia therapy enhances tumor trafficking and antitumor effects. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21(3):620–8. doi: 10.1038/mt.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. The Journal of clinical investigation. 2006;116(7):1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha A, Chatterjee SK. Combination of CTL-associated antigen-4 blockade and depletion of CD25 regulatory T cells enhance tumour immunity of dendritic cell-based vaccine in a mouse model of colon cancer. Scandinavian journal of immunology. 2010;71(2):70–82. doi: 10.1111/j.1365-3083.2009.02355.x. [DOI] [PubMed] [Google Scholar]
- 26.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. The Journal of experimental medicine. 1999;190(3):355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luker KE, Hutchens M, Schultz T, Pekosz A, Luker GD. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology. 2005;341(2):284–300. doi: 10.1016/j.virol.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 28.McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61(24):8751–7. [PubMed] [Google Scholar]
- 29.Wang LC, Lynn RC, Cheng G, Alexander E, Kapoor V, Moon EK, et al. Treating Tumors With a Vaccinia Virus Expressing IFNbeta Illustrates the Complex Relationships Between Oncolytic Ability and Immunogenicity. Molecular therapy: the journal of the American Society of Gene Therapy. 2011 doi: 10.1038/mt.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauzon M, Hermiston T. Armed Therapeutic Viruses - A Disruptive Therapy on the Horizon of Cancer Immunotherapy. Frontiers in immunology. 2014;5:74. doi: 10.3389/fimmu.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6(226):226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dias JD, Hemminki O, Diaconu I, Hirvinen M, Bonetti A, Guse K, et al. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012;19(10):988–98. doi: 10.1038/gt.2011.176. [DOI] [PubMed] [Google Scholar]
- 33.Banaszynski LA, Sellmyer MA, Contag CH, Wandless TJ, Thorne SH. Chemical control of protein stability and function in living mice. Nat Med. 2008 doi: 10.1038/nm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7(2):95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ, et al. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67(20):10038–46. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]
- 36.Colamonici OR, Domanski P, Sweitzer SM, Larner A, Buller RM. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J Biol Chem. 1995;270(27):15974–8. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.