Abstract
Bipolar disorder (BD) is associated with mood episodes and low amplitude circadian rhythms. Previously, we demonstrated that fibroblasts grown from BD patients show weaker amplification of circadian rhythms by lithium compared to control cells. Since calcium signals impact upon the circadian clock, and L-type calcium channels (LTCC) have emerged as genetic risk factors for BD, we examined whether loss of function in LTCCs accounts for the attenuated response to lithium in BD cells. We used fluorescent dyes to measure Ca2+ changes in BD and control fibroblasts after lithium treatment, and bioluminescent reporters to measure Per2∷luc rhythms in fibroblasts from BD patients, human controls, and mice while pharmacologically or genetically manipulating calcium channels. Longitudinal expression of LTCC genes (CACNA1C, CACNA1D and CACNB3) was then measured over 12-24 hr in BD and control cells. Our results indicate that independently of LTCCs, lithium stimulated intracellular Ca2+ less effectively in BD vs. control fibroblasts. In longitudinal studies, pharmacological inhibition of LTCCs or knockdown of CACNA1A, CACNA1C, CACNA1D and CACNB3 altered circadian rhythm amplitude. Diltiazem and knockdown of CACNA1C or CACNA1D eliminated lithium's ability to amplify rhythms. Knockdown of CACNA1A or CACNB3 altered baseline rhythms, but did not affect rhythm amplification by lithium. In human fibroblasts, CACNA1C genotype predicted the amplitude response to lithium, and the expression profiles of CACNA1C, CACNA1D and CACNB3 were altered in BD vs. controls. We conclude that in cells from BD patients, calcium signaling is abnormal, and that LTCCs underlie the failure of lithium to amplify circadian rhythms.
Keywords: Circadian rhythms, calcium, lithium, bipolar disorder, gene, cells
Introduction
Bipolar Disorder (BD) is a common mood disorder defined by alternating periods of depression and mania/hypomania, leading to disability and elevated risk of suicide (Oquendo et al., 2010). It is estimated that BD is 70-85% heritable, suggesting a genetic basis for the disorder (McGuffin et al., 2003). Other hallmarks of BD include disrupted behavioral rhythms, with inappropriate timing of activity, sleep, and appetite, indicating that circadian rhythm abnormalities may underlie BD (McCarthy and Welsh, 2012). Additional evidence linking circadian rhythms to BD comes from lithium. Lithium corrects the mood, activity and sleep disturbances in BD, and alters circadian rhythms. In humans (Kripke et al., 1979) and animals (Kripke and Wyborney, 1980; Welsh and Moore-Ede, 1990), lithium lengthens the rhythm period; and in cells, increases rhythm amplitude (Li et al., 2012). A set of ∼20 “clock genes” (e.g. PER1/2, CLOCK and ARNTL) form the core of the circadian clock (Partch et al., 2014). Since BD is largely attributed to genetic factors, clock genes have been studied as susceptibility loci for BD. We previously identified circadian rhythm abnormalities in skin fibroblasts from BD patients. In particular, BD cells showed diminished capacity to amplify cellular circadian rhythms in response to lithium, with individual variation partly explained by a polymorphism in GSK3B (McCarthy et al., 2013). However, the mechanism by which lithium differentially increases amplitude in BD remains incompletely characterized. Since low rhythm amplitude may be one of the key rhythm abnormalities associated with BD (Jones et al., 2005; Gonzalez et al., 2014; McKenna et al., 2014), the cellular basis of amplitude modulation warrants investigation.
Ca2+ and the circadian clock have bidirectional interactions in neurons and peripheral cells (Noguchi et al., 2012; Ikeda, 2014; Schmutz et al., 2014). Hence, failure of lithium to amplify circadian rhythms in BD could be caused by altered Ca2+ clock inputs, or by clock outputs that perturb Ca2+ rhythms. Acutely, lithium activates intracellular Ca2+ release by inhibiting inositol monophophatases, and stimulating inositol 1,4,5 tris-phosphate (IP3). Chronic lithium has the opposite effect, decreasing basal and stimulated intracellular Ca2+, possibly by depleting cells of myo-inositol (Chen and Hertz, 1996; Yan et al., 2013). Lithium also inhibits glycogen synthase kinase β (GSK3B), a post-translational regulator of both LTCCs (Li and Sarna, 2011) and the circadian clock (Harada et al., 2005; Iitaka et al., 2005; Yin et al., 2006; Sahar et al., 2010).
Genome wide association studies indicate that clock gene variants are only weakly associated with BD (McCarthy et al., 2012; Byrne et al., 2014). In contrast, L-type Ca2+ channels (LTCCs) have been identified as important BD susceptibility genes (PGC-BD, 2011). Among Ca2+ channel genes, CACNA1C is most strongly implicated, with the C allele at the G/C polymorphism rs4765913 conferring risk for BD. Suggestive associations in CACNA1D, CACNB3 have also been identified (PGC-BD, 2011; Nurnberger et al., 2014). Calcium channels are classified according to their pore-forming α1 subunit, while accessory β and α2δ subunits are shared across multiple channels. CACNA1C and CACNA1D encode the α1 subunits for two LTCCs, Cav1.2 and Cav1.3, whereas CACNB3 encodes a β3 subunit. Previous studies have examined Ca2+ signaling in BD cells (Hahn et al., 2005; Chen et al., 2014), but few have linked abnormalities to particular LTCCs identified as genetic risk factors. Among investigations of CACNA1C, there is conflicting evidence as to whether BD-associated variation causes gain or loss of function. A recent study using human induced neurons homozygous for the variant risk allele reported that CACNA1C expression was increased, with corresponding increases in electrical activity (Yoshimizu et al., 2015). A previous post-mortem brain study came to the opposite conclusion, reporting decreased CACNA1C expression associated with the BD risk allele (Gershon et al., 2014). Therefore, BD-associated variants in LTCCs remain incompletely characterized in cellular models of BD.
We examined the hypothesis that the weak amplitude response to lithium in BD cells could be explained by loss of function in LTCCs, resulting in weak Ca2+ inputs to the circadian clock. We found that fibroblasts from BD patients were sufficient to model some aspects of LTCC function as they relate to circadian rhythms. Using molecular reporters to study Ca2+ and circadian rhythms in these cells, we investigated genetic and molecular connections among LTCCs, lithium and the clock, and determined that lithium engages Ca2+. Over the short term, the intracellular Ca2+ response is blunted in BD cells independently of LTCCs. Over hours-days, LTCCs regulate cellular circadian rhythms, and CACNA1C is involved with the lithium-induced amplification of rhythms. Finally, some LTCCs appear to be clock controlled genes, with altered features of expression in BD.
Methods
Human Subjects
Punch biopsies were obtained from the skin over the deltoid from BD (type I) patients who consented to research while hospitalized, or participating in a lithium clinical trial. Demographic characteristics of donors are shown in Table S1. Controls and BD donors did not differ in mean age or sex distribution. The majority of donors were Caucasian, and most BD patients (94%) were on medication at the time of biopsy. Use of human subjects was conducted in accordance with all pertinent regulations and approved by the VASDHS IRB.
Cell Culture
Human fibroblasts were grown from frozen cryovials to confluence in 100 mm plates in standard culture media [DMEM with 10% fetal boving serum (FBS), glutamine 2mM and antibiotics (penicillin, streptomycin, and amphotericin)]. In order to control for differences in donors' medication history, all cells were passaged a minimum of four times before use. Per2 expression reflects network activity across the circadian clock (Welsh et al., 2005). Therefore, human fibroblasts were transduced with the Per2∷luc lentiviral reporter gene to assess circadian rhythms as described previously (McCarthy et al., 2013). In order to facilitate screening of drugs and siRNAs in a cell type comparable to human fibroblasts, we developed a mouse fibroblast line that stably expressed the Per2∷luc reporter (NIH3T3P2L), using a construct described previously (Meng et al., 2008). NIH3T3P2L cells were grown under hygromycin selection to enrich Per2∷luc expression. Luminometer studies of human cells were conducted using ∼1.2 × 106 cells in 35mm plates. For NIH3T3P2L luminometer studies, cells were dispersed into 24 well plates at ∼2 × 105 cells / well.
Drugs
Diltiazem, verapamil, and 2-aminoethoxydiphenylborane (2-APB), were purchased from Tocris Biosciences. Lithium chloride was purchased from Sigma. Drugs were dissolved in sterile water or DMSO. Prior to drug studies, cells were distributed into multiple smaller plates, and treated in parallel, under identical conditions, allowing for within sample matching. To ensure even application, concentrated lithium (1000×) was added to growth media and distributed from a common drug solution into each culture plate. Additional drugs were handled in a similar manner, alone or with lithium. Vehicle controls for solvents were used when indicated.
Calcium Fluorescence Imaging
Ca2+ imaging was performed using a Fluo-4 NW calcium assay kit (Life Technologies) following the manufacturer's protocol. In brief, fibroblasts were distributed into 96 well plates and loaded with cell permeable fluo-4 for 30 min at 37°C, followed by incubation at 25°C for 30 min. Baseline fluoresc ence level was measured over 5 sec using a BioTek Cytation3 reader. Fluorescence was then measured at 0.25 sec intervals for 90 sec. Background was determined in wells containing no cells. Individual wells were analyzed singly in series with lithium added to cells to a final concentration of 1mM. Change in fluorescence were read immediately afterwards. Stimulation was determined by % increase from baseline to the smoothed peak. Relative increases in signal were determined by subtracting the background fluorescence, and calculating the fold change from baseline. Experiments were conducted over four runs (n= 4-8 cell lines / experiment) using control (N=9) and BD (N=10) cells. Data were normalized to reduce variability across experiments, and analyzed using a two-tailed T-test.
Luminometry
Human fibroblast rhythms were recorded from 35 mm plates over 5 days with a luminometer (Actimetrics) as described previously (McCarthy et al., 2013). NIH3T3P2L rhythms were measured using 4×24 well plate format luminometer (Actimetrics) over 5-7 days. Both luminometers were housed in a dry incubator and maintained in room air at 35°C. Immediately before rhythm recording, media was replaced with HEPES-buffered, serum-free recording media containing 1 mM luciferin (Biosyth International). To model therapeutic conditions, in some experiments drugs were added 48 hr before, and again at the addition of recording media, to maintain constant concentrations throughout the experiment.
Rhythm Analysis
Photoemissions (counts per second) from each sample were recorded every 10 minutes over the duration of the experiment and logged automatically for subsequent analyses. To reduce variability, the first 14 hr (human) or 24 hr (mouse) were excluded. Background subtracted luminometry data were fit to a damped sine curve by the least squares method using commercial software (Lumicycle Analysis). Rhythm parameters (period, amplitude) were estimated for each trace and averaged across replicates. For analyses of drug and siRNA effects, two-way ANOVA with post-hoc t-tests were conducted separately for amplitude and period. All analyses were completed using GraphPad Prism version 5.0 (San Diego, CA) with α <0.05.
SiRNA Transfection
Knockdown experiments in NIH3T3P2L cells were performed using a kit according to the manufacturer's protocol (GE Healthcare). Briefly, 2 × 104 cells were distributed into 24 well plates. After 24 hr, siRNA and transfection reagent were mixed with media and incubated for 20 min at room temperature. The transfection mixture was then added to the cells with media, and incubated 48 hrs at 37°C. To maximi ze knockdown, siRNA pools (SMARTpool), that bind four distinct sites within a transcript were used. The siRNA pools used were Arntl (M-040483-01-0005), Cacna1a (M-043179-01-0005), Cacna1c (M-040723-01-0005), Cacna1d (M-051142-01-0005), Cacnb3 (M-043190-01-0005), and negative control that has no interactions with known transcripts (D-001206-14-05).
DNA Preparation and Genotyping
DNA was obtained from 55 human cell lines (23 control, 32 BD) using a Qiagen DNeasy Kit following the manufacturer's protocol. SNPs in CACNA1C (rs4765913), CACNA1D (rs3774609) and CACNB3 (rs2070615) were selected from the literature (PGC-BD, 2011) based on association with BD, and minor allele frequency (>0.20). SNPs were genotyped with pre-designed Taqman assays (Applied Biosystems) using real time polymerase chain reaction (PCR). Due to scarcity of homozygotes for the minor alleles, heterozygotes and minor allele homozygotes were combined for the analyses and termed “carriers” for each allele.
Genetic Association Study of Amplitude
In human cells, Per2∷luc gene expression rhythms were recorded under baseline and lithium-treated conditions. Amplitude was measured simultaneously under both conditions for each individual. The average change in amplitude was then compared between two groups: homozygotes for the common allele and minor allele carriers for the CACNA1C variant. A two-way ANOVA with α <0.05 was used to determine statistical significance.
Gene Expression
For the time course analyses, fibroblasts (N=3 control, n=4 BD) were grown in parallel in six-well plates. Among risk allele carriers, three were heterozygous for rs4765913, one was homozygous for the BD-associated C allele. The latter cell line was selected for the rare CACNA1C genotype, but the donor's diagnosis of BD was uncertain, with reported symptoms (clinically significant depression), and positive family history of BD in multiple 1st degree relatives. Serum shock (DMEM + 50% FBS) was used to synchronize cells 18 hr before the first collection (Balsalobre et al., 1998). Plates were collected at 4 hr intervals over 12 or 24 hr. Upon collection, cells were rinsed with ice-cold PBS, plates sealed and frozen at -80°C. RNA was prepared using a Qiagen RNeasy kit, following the manufacturer's instructions. cDNA was synthesized using a kit (Applied Biosystems). Taqman RT-PCR was conducted using a BioRad CFX384 thermocycler with pre-made primers for each transcript (Applied Biosystems). CLOCK, and PER1 were used as positive controls and phase markers. GAPDH was used as a non-rhythmic housekeeping control (Kosir et al., 2010), against which gene expression was measured (Schmittgen and Livak, 2008). In order to test for the presence of rhythms, 24 hr expression data were fit to a sine wave using specialized software (CircWave, version 1.4). Rhythms were determined to be present when the resulting curve explained a significant portion of the variance (p<0.05). To evaluate differences in the strength of gene expression rhythms, a Fisher r to z transformation was used to compare the variance explained by the best fit sine wave in each group. For microarray studies, human fibroblast RNA was analyzed by the UCSD microarray core facility using the Affymetrix Human 1.0 Array. Expression levels of selected Ca2+ channel genes were extracted from the full data set for quantification.
Results
Calcium channel genes are expressed in fibroblasts
Ca2+ channel expression was measured in human fibroblasts. Expression of nineteen Ca2+ channel genes was detected, including three LTCC α1 subunits (CACNA1C, CACNA1D, CACNA1F), as well as α2δ, β and γ subunits (Figure S1). With multiple pore forming and regulatory subunits expressed, fibroblasts are theoretically capable of forming multiple LTCC combinations. T-type (TTCC, e.g. CACNA1G) and P/Q-type (PTCC, e.g. CACNA1A) channels were also readily detectable. N-and R-type channels were absent. Therefore, we determined that fibroblasts are a reasonable model to examine selected interactions between Ca2+ channels and cellular rhythms.
Acute lithium stimulates intracellular Ca2+ in human fibroblasts
To evaluate potential input signals to the clock, the acute effects of lithium on intracellular Ca2+ were examined in fibroblasts from BD patients and controls. We found that lithium (1mM) evokes an increase in intracellular Ca2+, with peak intensity 15-20 sec after administration. After background subtraction, lithium caused a 2.9 fold increase in Ca2+ in control cells, compared to a 1.8 fold increase in BD cells. After normalization, this corresponds to a significant (p<0.05) 26% reduction of lithium-induced Ca2+ stimulation in the BD cells compared to controls (Figure 1A, 1B). Pre-incubation of the cells with diltiazem had only a modest effect on lithium-induced Ca2+ stimulation (86% vs. vehicle, p=0.07 NS). The IP3 receptor antagonist APB had no effect on lithium-induced Ca2+ stimulation (not shown). Applying potassium (50mM) under the same conditions did not increase Ca2+, suggesting that the acute lithium response is unrelated to depolarization (not shown).
Figure 1.
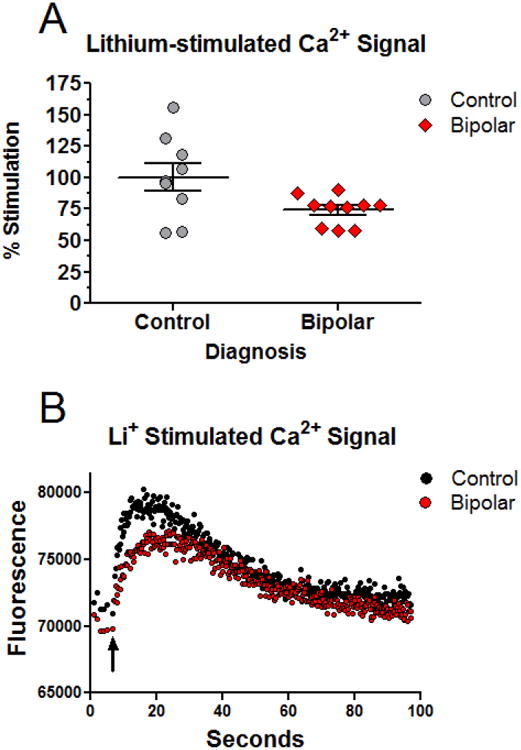
The intracellular Ca2+ response to acute lithium is attenuated in BD fibroblasts. A) Normalized increase in Ca2+ fluorescence observed in cells from controls (N=9) and BD patients (N=10). The Ca2+ increase following lithium in controls was significantly higher than in the BD group. Experiment was run four times. A two-tailed T-test comparing normalized increase in intracellular Ca2+ in BD and control cells indicated a significant (p<0.05) group difference. B) Representative traces showing the absolute increase in Ca2+ fluorescence for a control and BD cell line. Black arrow indicates the time of lithium application.
LTCC antagonists block lithium's amplification of circadian rhythms in fibroblasts
Neuronal LTCCs respond slowly, contributing to changes in gene expression after sustained activity (Mermelstein et al., 2000). We set out to determine if LTCCs play a role in the cellular response to lithium over longer times relevant to circadian rhythms. In long-term (5-7 day) studies of NIH3T3P2L cells, lithium increased circadian rhythm amplitude by ∼30% and lengthened period by ∼0.5 hr (Figure 2A-C), effects similar to what we found previously in human cells (McCarthy et al., 2013), but differing in the lithium requirement to increase amplitude (1 mM in human vs. 10mM in mouse). Depolarization of NIH3T3P2L cells with potassium chloride (45mM) transiently increased rhythm amplitude, an effect that was attenuated by verapamil, an LTCC antagonist (Figure S2). However, depolarization was not essential for the effects of LTCC antagonists on rhythms. Under baseline conditions a chemically distinct LTCC antagonist, diltiazem (1-20 μM) reduced rhythm amplitude in a concentration dependent manner (Figure 2D). Similar effects were observed in NIH3T3P2L cells with verapamil (Figure S3A). Neither antagonist had effects on period. When diltiazem or verapamil were co-administered with lithium to NIH3T3P2L cells, the amplitude increasing effect of lithium was attenuated in a concentration dependent manner (Figure 2E, S3B). Using matched human samples, cells from the same donor were treated in parallel with different drugs (n=4/group). Lithium increased amplitude by ∼36% at therapeutically relevant concentrations (1mM) in cells from controls, whereas diltiazem pre-treatment attenuated the amplitude increase (Figure 2F). In BD, rhythms largely fail to amplify in response to lithium, confounding the diltiazem studies. Therefore, BD samples were not considered in this experiment. Nonetheless, the control data support a link between LTCCs and lithium that emerges over hours to days to affect circadian rhythms.
Figure 2.
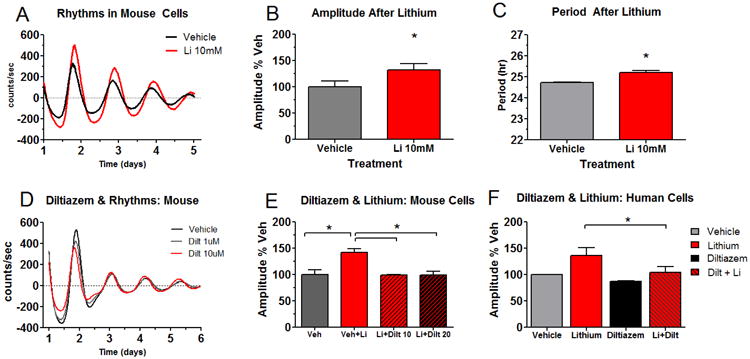
Blocking Ca2+ channels attenuates lithium's amplification of circadian rhythms. Circadian rhythms of mouse NIH3T3P2L fibroblasts (A) respond to lithium in a manner similar to human cells: increasing amplitude (B), and lengthening period (C). The Ca2+ channel antagonist diltiazem decreases rhythm amplitude in NIH3T3P2L cells (D) and blocks the rhythm amplifying effect of lithium (E). Similar effects of diltiazem on rhythm amplitude are observed in parallel cell cultures from human controls (n=4 / group). Because BD patients had more variable amplitude responses to lithium, they were not considered for this experiment (F). Values are means ± SEM; *p < 0.05 using ANOVA with post-hoc T-tests.
Knockdown of CACNA1C and CACNA1D
In order to examine the specific contributions of individual Ca2+ channel genes to rhythms, we used siRNA to reduce the expression of two BD associated LTCC α1 subunits, CACNA1C (Figure 3A-C) and CACNA1D (Figure 3D-F). Knockdown of ARNTL, an essential component of the clock served as a positive control. After siRNA treatment, we measured rhythms in NIH3T3P2L cells in the presence or absence of lithium. As expected, ARNTL knockdown led to a near total loss of rhythms in NIH3T3P2L cells (Figure S4). In the absence of lithium, knockdown of both CACNA1C and CACNA1D led to rhythm amplitude increases (Figure 3B, 3E). The increases were observed in the mean expression, and amplitude, suggesting increases in both constitutive and rhythmic expression of Per2∷luc. The effects were especially strong early in the recording period, and more pronounced for CACNA1C vs. CACNA1D. In response to lithium treatment of the cells, CACNA1C and CACNA1D knockdown attenuated the expected increase in rhythm amplitude. CACNA1D knockdown with lithium treatment led no change in amplitude, while CACNA1C knockdown caused an amplitude decrease after lithium (Figure 3B, 3E). Loss of CACNA1C had no effect on period by itself but attenuated the period lengthening effects of lithium (Figure 3C). Knockdown of CACNA1D, despite its lower abundance, had significant effects on period, shortening it by ∼1 hr at baseline, and enhancing the period lengthening properties of lithium (p<0.01 post-hoc T-test, 0.3 hr longer after lithium in negative control vs. 0.9 hr longer after CACNA1D siRNA) (Figure 3F).
Figure 3.
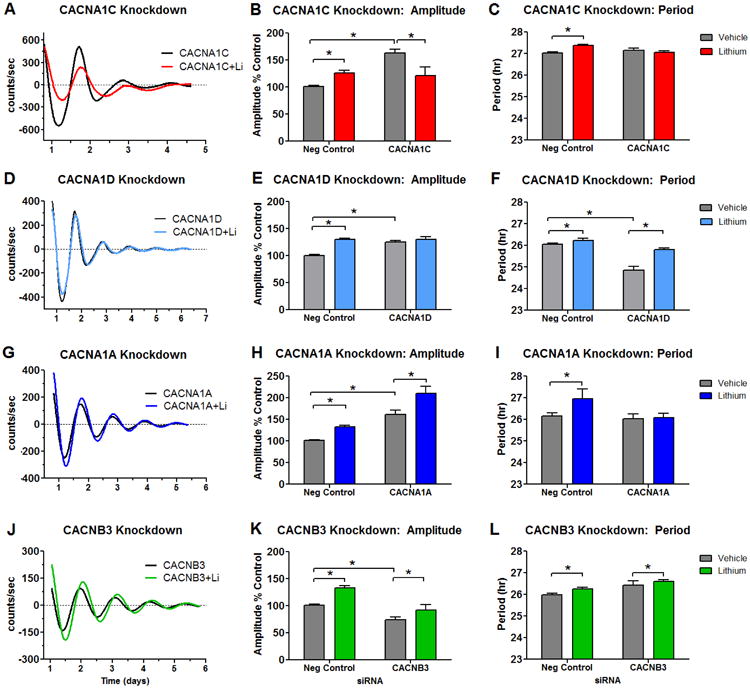
Effects of calcium channel knockdown on rhythms in NIH3T3P2L cells. Knockdown of CACNA1C (A-C) caused increases in amplitude at baseline, and reversed lithium's effect on rhythms, causing an amplitude decrease. CACNA1C knockdown alone had no effect on period, but attenuated the period lengthening action of lithium. CACNA1D (D-F) also caused increases in amplitude at baseline, and blocked lithium's ability to increase amplitude. Knockdown of CACNA1D shortened period, but had no effect on the period lengthening action of lithium. CACNA1A (G-I) caused increases in amplitude at baseline, but had no effect on lithium's ability to increase amplitude. CACNA1A knockdown alone had no effect on period, but attenuated the period lengthening action of lithium. Knockdown of CACNB3 (J-L) decreased baseline amplitude, but had no effect on lithium's amplification of rhythms. CACNB3 knockdown had no statistically significant impact on period at baseline or after lithium. Sample sizes are as follows: CACNA1C N= 12-15/ group, CACNA1D N= 3-6/ group, CACNA1A N= 4-6/ group, CACNB3 N= 9/ group. Normalized amplitude values for negative controls ± lithium were combined across experiments (total N=25 siRNA alone, N=31 siRNA+lithium). Values are means ± SEM; Analyses performed by one-way ANOVA, *p < 0.05 in post-hoc tests.
Knockdown of CACNA1A
To distinguish between effects that are specific to LTCCs and those related to general alterations in Ca2+, we performed knockdown of CACNA1A, a PTCC α1 subunit gene that is highly expressed in fibroblasts, but not strongly associated with BD (Figure 3G). As with the LTCC α1 genes, CACNA1A knockdown in NIH3T3P2L cells lead to increased rhythm amplitude (Figure 3H). However, unlike the LTCC α1 genes, CACNA1A knockdown did not alter lithium's effect on amplitude (Figure 3H). CACNA1A knockdown had no effect on period at baseline, but blocked the ability of lithium to lengthen period (Figure 3I).
Knockdown of CACNB3
Finally, to investigate the impact of β subunits on rhythms, we performed CACNB3 knockdown in NIH3T3P2L cells (Figure 3J-L). Unlike knockdown of pore forming α1 subunits, amplitude and mean expression decreased after knockdown of the regulatory CACNB3 subunit. Despite being a BD associated channel, lithium maintained its amplitude increasing effect under CACNB3 knockdown conditions (Figure 3K). CACNB3 knockdown had no effect on period at baseline or on the period lengthening effect of lithium (Figure 3L).
CACNA1C genotype is associated with the amplitude response to lithium
We conducted a rhythm analysis of 12 new fibroblast cell lines (9 BD, 3 controls) under baseline and lithium treated conditions. Data were combined with past results (McCarthy et al., 2013) and used for additional genetic analyses of CACNA1C. As before, there were individual differences in lithium-evoked amplitude increases, but these differences were explained by CACNA1C genotype (p<0.05). Regardless of diagnosis, cells homozygous for the common rs4765913 G allele increased amplitude by ∼35%, while in carriers of the BD-associated C allele, amplitude failed to increase (Figure 4A-C). Baseline amplitude did not differ between BD cases and controls (McCarthy et al., 2013) or as a function of CACNA1C genotype (Figure 4C).
Figure 4.
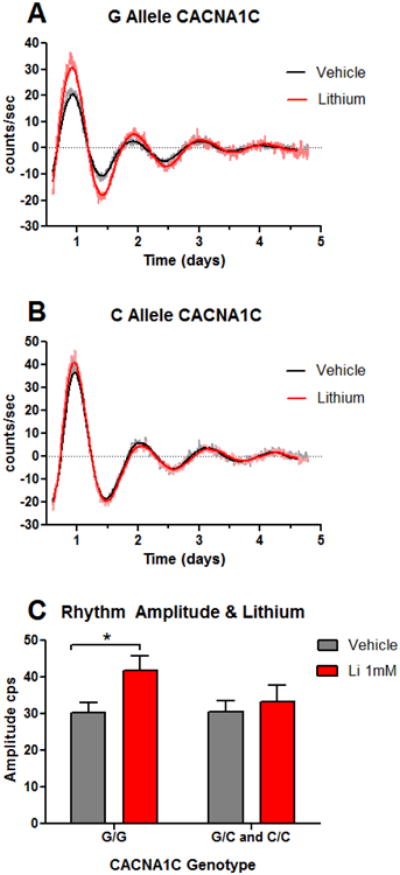
Lithium's amplification of circadian rhythms depends on CACNA1C genotype. Per2∷luc circadian rhythms were recorded in parallel at baseline and during treatment with 1 mM lithium in 55 human cell lines (32 BD, 23 control). Rhythms from two representative cell lines are shown ± lithium (A) one homozygous for the common G allele and (B) one carrier of the BD-associated C allele at CACNA1C rs4765913. Raw data and best fit damped sine curves are shown for each. (C) Lithium increased amplitude differentially according to genotype [two-way ANOVA indicates effect of drug (p<0.001) and drug × genotype interaction (p<0.05)]. N=38 GG genotype, N=17 GC/CC genotypes, values are means ± SEM; *p < 0.05 by post-hoc T-test.
Calcium channel gene expression is altered in BD
BD is associated with variants that regulate the expression of CACNA1C (Gershon et al., 2014; Yoshimizu et al., 2015), and several other genes pertinent to Ca2+ signaling (Ament et al., 2015). Therefore, we examined CACNA1C, CACNA1D and CACNB3 in fibroblasts to determine if the temporal control of these Ca2+ channels is altered in BD. Expression of CACNA1C in control cells was rhythmic (r2 best fit sine = 0.71, p<0.001, Figure 5A). In BD cells, expression was still rhythmic (r2 best fit sine = 0.25, p<0.05, Figure 5A), but the rhythm was significantly attenuated compared to controls (Fisher r-to-z test, p<0.05). To examine CACNA1C expression in the context of other Ca2+ channels and circadian clock genes, an experiment was conducted targeting four additional genes over 12 hr. In accordance with the 24 hr data, CACNA1C expression significantly differed by time in control cells (p<0.05), and was found to be in phase with PER1, and in anti-phase to CLOCK (Fig 5B, 5E). As before, CACNA1C expression varied weakly over 12 hr in BD cells (effect of time was not significant, p=0.5, Fig 5B). CACNA1D expression did not vary significantly by time in either group (Fig 5C), but was more highly expressed (p<0.01) in BD cells compared to controls. In both control and BD cells, CACNB3 expression varied across time, in phase with control CACNA1C expression (Fig 5D), but with reduced amplitude in BD cells (p<0.005).
Figure 5.
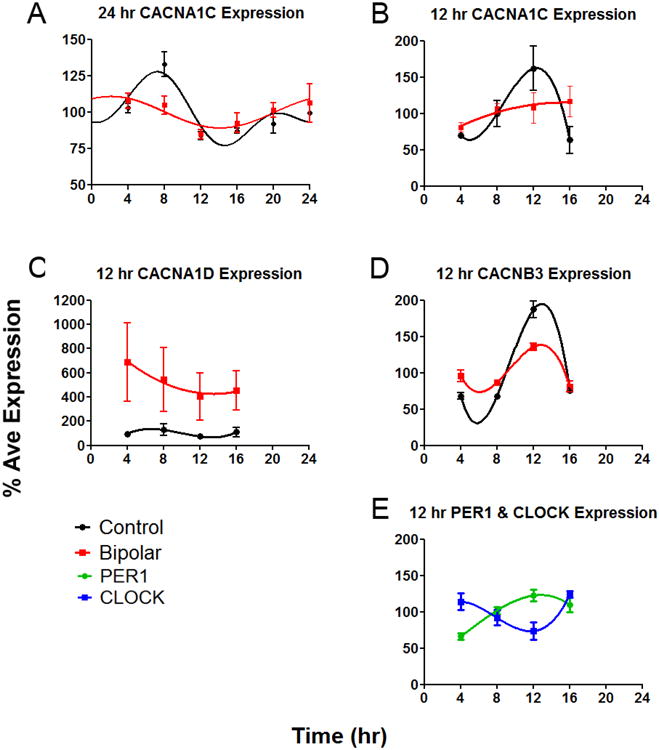
Gene expression profiles using fibroblasts from BD patients (n=4) and controls (N=3) A) Expression profiles and best fit curves indicate that expression of CACNA1C is rhythmic over 24 hr, but more so in controls compared to BD cells. Phase in 24 hr (A) and 12 hr (B-E) experiments may differ due to minor technical differences between experiments. To allow direct comparison to other genes, B) the 12 hr profile of CACNA1C is shown. C) CACNA1D expression over 12 hr was higher in BD compared to controls. D) Amplitude of CACNB3 expression over 12 hr was lower in BD compared to controls. E) PER1 and CLOCK were used as phase markers after combining BD and control samples. As expected, expression of these genes varied over time with opposite phases.
Discussion
We have shown that lithium engages Ca2+ signaling in a temporally complex manner that is altered in BD and/or by BD-associated genetic variation. Our main finding is that CACNA1C modulates the cellular rhythm amplitude response to lithium, providing a specific link between LTCCs and circadian rhythms in the context of BD and lithium. Our results validate past reports linking LTCCs to rhythms (Pennartz et al., 2002; Noguchi et al., 2012; Schmutz et al., 2014), and extend this work to identify subunit specific roles in rhythm regulation. We conclude that LTCC α1 subunit genes (CACNA1C, and to a lesser extent CACNA1D) selectively influence lithium's effects on rhythm amplitude, while β subunits (CACNB3) and PTCCs (CACNA1A) do not. While we did not focus on circadian period, each of the channels studied had specific effects on this parameter, indicating specialized roles of Ca2+ channels in modulating circadian rhythms, and suggesting that period and amplitude are independently regulated by distinct systems. Our data imply that lithium triggers a Ca2+ signaling response that initially does not rely on LTCCs, but recruits LTCCs to an important role over subsequent hours-days. We initially hypothesized that in BD cells, loss of LTCC function and weak Ca2+ inputs to the circadian clock could underlie the attenuated response of circadian rhythms to lithium. Support for this hypothesis is mixed. We found that the acute Ca2+ response to lithium is indeed weaker in BD cells, but not because of LTCCs. While we did not identify differences in overall CACNA1C expression, we did find attenuation of its rhythmic expression pattern. Speaking against weak Ca2+ inputs in BD cells, we found overall increases in CACNA1D expression. Our work suggests that the connection between LTCCs and the circadian clock may also involve clock outputs, with aberrant expression of CACNA1C, CACNA1D, and CACNB3 in BD cells, revealing possible instances of weak circadian control over clock-controlled genes. The latter suggests the presence of a feedback loop whereby altered outputs (Ca2+ channel gene expression) could affect the temporal control or intensity of subsequent Ca2+ inputs to back into the clock.
Strengths and limitations of the study
Fibroblasts contain cell autonomous circadian clocks (Welsh et al., 2004), and are readily available from human subjects, allowing longitudinal study of cellular circadian rhythms in BD patients. Our approach circumvents the problems affecting animal models that fail to recapitulate the BD phenotype in full. In parallel, we studied NIH3T3 cells, a mouse fibroblast line commonly used in circadian biology. Compared to human cells, this cell type is more readily manipulated, and offers an efficient and reproducible platform for siRNA and drug screening, while remaining similar enough to the human cells that preliminary findings can be readily pursued in clinical samples. Nonetheless, there are limitations to both cell models. We cannot fully exclude the possibility that psychotropic drugs given to BD patient donors had lasting effects on the fibroblasts grown from them. With respect to Ca2+ signaling, LTCCs are expressed in fibroblasts, but these cells are distinct from neurons which use Ca2+ to generate action potentials and release neurotransmitters. Therefore, we cannot connect our observations of Ca2+ and circadian rhythms to these essential neuronal functions. There may also be differences between neurons and fibroblasts in lithium sensitivity: Peripheral cells commonly require higher concentrations of lithium (i.e. >10 mM) to induce biological changes than would be therapeutically relevant (i.e. 1 mM). For these reasons, our work must be extrapolated to the brain with caution.
Genetic variation in CACNA1C and rhythms
The BD-associated CACNA1C haplotype containing rs4765913 is located in intron 2, and presumably affects regulatory sequence. However, it is unclear if there is a loss or gain of function. Previous studies have linked this haplotype to changes in CACNA1C expression, with decreases reported in brain (Gershon et al., 2014) and increases reported in cultured neurons (Yoshimizu et al., 2015). Brain imaging studies of CACNA1C show a variety of results that vary with the specific measure and brain region (Ou et al., 2015). The lack of consensus in findings is not necessarily surprising. Given the many roles of Ca2+ in various cell types, there may be many consequences of CACNA1C variation, some of which could be cell specific. Therefore, our association of CACNA1C with attenuated rhythm amplification by lithium, is most consistent with a loss of channel function in BD, but may not apply universally. It is known that CACNA1C is a clock controlled gene, regulated by ROR elements in the 5′ promoter that confer a rhythmic expression profile in the suprachiasmatic nucleus (SCN) in the brain (Schmutz et al., 2014). Our data extend this previous work to suggest that the BD-associated haplotype may affect this rhythmic expression of CACNA1C in fibroblasts. If variation in CACNA1C causes a similar loss of rhythms in neurons, it may result in abnormal expression patterns, affecting temporal control over Ca2+ inputs to brain clocks. Our preliminary investigation of intron 2 of CACNA1C reveals a number of potentially clock controlled transcriptional elements (ROREs and E-Boxes) that remain to be validated.
Loss of temporal regulation may alter LTCC subunit composition
CACNA1C expression was less rhythmic in BD cells. The CACNB3 expression profile showed a similar pattern, with a lower peak amplitude over 12 hr in BD cells compared to controls. This pattern of lower amplitude expression may indicate weakened circadian control over some LTCCs in BD cells. At the same time, CACNA1D expression was persistently elevated in BD cells compared to controls. Since Cav1.2 and Cav1.3 compete in some brain regions to form functional LTCCs (Giordano et al., 2010), changes in relative abundance of gene expression could have implications for LTCC composition, possibly favoring the formation of Cav1.3 channels in BD by flattening the temporal profile of Cav1.2 and β3 subunits. While our data cannot address the issue in detail, we speculate that disrupted LTCC composition in the brain could be one mechanism by which genetic variation in LTCCs predisposes to BD.
The acute Ca2+ response to lithium
Variability in LTCC genes could affect rhythm amplitude by altering the strength of Ca2+ signals shortly after lithium administration. We found little support for this hypothesis. BD cells did show a reduced Ca2+ response to lithium in the seconds following administration. However, the role for LTCCs in this process was modest, showing minimal attenuation with diltiazem. Lithium also inhibits IP3 catabolism, thereby stimulating short-term intracellular Ca2+ release. However, our studies indicated that IP3 receptors were not involved either. Collectively, these results indicate that BD is characterized by multiple deficits in Ca2+ signaling that extend beyond LTCCs. PTCCs, TTCCs and ryanodine receptors were detected in fibroblasts, and could also be engaged by lithium. Others have identified genetic variants in non-selective cation channels in BD, with corresponding functional differences in patient cells (Roedding et al., 2012). Our data indicate that in BD one or more of these non-LTCC channels is also abnormal. Therefore, future experiments on them are warranted to better understand the Ca2+ landscape in BD.
Pharmacological and genetic disruption of LTCCs
We observed long-lasting effects of diltiazem and LTCC knockdown on rhythm amplitude, but with important differences. Pharmacological inhibition decreased rhythm amplitude, while CACNA1C/CACNA1D knockdown increased amplitude. These data agree on the notion that LTCCs regulate the clock, but diverge in direction, suggesting the regulatory mechanisms involved are complex. CACNA1C and CACNA1D are translated into two proteins (Cav1.2 and Cav1.3) with distinct signaling properties (Zhang et al., 2006; Giordano et al., 2010; Wheeler et al., 2012), while the majority of LTCC antagonists, including diltiazem and verapamil are non-selective, binding Cav1.2 and Cav1.3, as well as TTCCs (De Paoli et al., 2002). Therefore, dual antagonism of Cav1.2 and Cav1.3, and/or antagonism of TTCCs are possible explanations for the amplitude decreasing effects of these drugs. One implication of this hypothesis is that more precise targeting of LTCCs may have distinct effects on the circadian system, possibly conferring advantages in regulating sleep or activity in BD. Indeed, clinical trials with isradipine, a selective Cav1.3 antagonist have shown potential in treating bipolar depression (Ostacher et al., 2014). The amplitude enhancing effects of LTCC knockdown are more difficult to explain. Calcium signals typically facilitate the expression of clock genes and so this finding is counterintuitive. Knockdown of CACNA1C/CACNA1D greatly reduces the amount of α1 protein available to form the corresponding LTCC pore, while leaving intact accessory subunits (e.g. β and α2γ) that are shared across multiple Ca2+ channel types. Changing the balance of channel subunits through knockdown appears to result in greater Ca2+ input to the clock and higher amplitude rhythms through an unknown compensatory mechanism. Compensatory changes could include substitution of low activity for high activity α1 subunits, the shunting of Ca2+ to other channels, or shifts in ion balance driven by electrochemical membrane potential. Given the likelihood of extensive compensation, LTCC knockdown phenotypes may not have a correlate in normal physiology. Nonetheless, after adjusting for the elevated baseline amplitude, they do afford insight into the role of LTCC α1 subunits in response to lithium, and suggest a direct rather than additive/indirect role of LTCCs in linking lithium to circadian rhythm amplitude modulation.
Incorporating LTCCs into the GSK3B model of lithium action
Lithium inhibits GSK3B at therapeutic concentrations (Klein and Melton, 1996). Accordingly, both lithium and selective pharmacological inhibition of GSK3B increase rhythm amplitude in a similar manner (Li et al., 2012); and our previous work demonstrated that genetic variation in GSK3B predicted lithium's ability to amplify circadian rhythms in BD cells (McCarthy et al., 2013). Therefore, considered with the present findings, we conclude that GSK3B and Ca2+ are both involved in the amplitude enhancing effect of lithium, with inhibition of GSK3B and stimulation of Ca2+ favoring amplitude increase. Given the complexity of Ca2+ signaling, the means by which GSK3B and Ca2+ interact to modulate amplitude are unclear. Calcium affects protein kinase A (PKA), protein kinase C (PKC) and mitogen activated protein kinase (MAPK) pathways (Manji and Lenox, 1999; Stork and Schmitt, 2002). Therefore upstream direction of these systems by Ca2+ could converge on, or work in concert with GSK3B to affect the circadian clock. Alternatively, lithium inhibition of GSK3B stabilizes Cav1.2 by reducing protein turnover (Li and Sarna, 2011), suggesting another way that lithium could amplify Ca2+ signals. In this case, the effects of Ca2+ would lie downstream of GSK3B. These mechanisms are not mutually exclusive and could co-occur.
Clinical implications for BD
The master clock in the SCN plays a central role in regulating circadian rhythms (Welsh et al., 2010). However, the loss of gene expression rhythms in additional, non-SCN brain regions has been reported in post-mortem patients with major depression (Li et al., 2013). These data suggest that loss of rhythms may be a feature of mood disorders that widely affects brain function beyond the SCN, affecting multiple brain oscillators (McCarthy and Welsh, 2012). If this is true, mood symptoms in BD could reflect episodes of low amplitude rhythms in one or more brain areas. We conclude from our past and current results that BD is characterized by rhythms that amplify weakly in response to lithium. This conceptualization has implications for how lithium may be therapeutic in BD. At therapeutic concentrations (1 mM), lithium had no effect on period in BD or control fibroblasts, suggesting this particular effect of lithium, well described in many systems (but often at higher concentrations) is not the basis of its therapeutic effect in BD patients. Instead, the effect on amplitude may be more important. Although the amplitude-enhancing effects of lithium are generally stronger in control than in BD cells, we demonstrated previously that when rhythms are damped, lithium bolsters amplitude equally well in BD and control cells (McCarthy et al., 2013). Therefore, it may be that lithium is therapeutic specifically in the context of a failing clock, working by improving weak rhythms. In this way, the failure of lithium to bolster high amplitude rhythms in BD may reflect a narrowing of the dynamic range, with maintained action on low amplitude rhythms that could be therapeutically important, but relatively weak effects on higher amplitude rhythms. Because the clinical treatment of our BD subjects was heterogeneous and the therapeutic outcomes are unknown in many cases, we cannot address the issue of amplitude modulation as it relates to specific treatment outcomes. Nonetheless, our data indicate that amplitude modulation by lithium distinguishes BD and control cells, and may have potential use as a diagnostic biomarker to distinguish BD patients from healthy subjects. The role of rhythms in mood regulation, and the range of amplitude responsiveness in cells are not well studied, and our speculations remain to be tested in future studies. The relationship between rhythm modulation and clinical outcomes in BD is a focus of our ongoing work and will be further elucidated in the future.
Supplementary Material
Highlights.
Calcium channel antagonists attenuate the effect of lithium on circadian rhythm amplitude.
Knockdown of calcium channel genes blocks the effect of lithium on circadian rhythm amplitude.
CACNA1C genotype is associated with the degree of rhythm amplification by lithium.
CACNA1C expression in fibroblasts is rhythmic in controls but less so in bipolar disorder.
Acknowledgments
The authors wish to thank Tanya Shekhtman, Dominic Landgraf and members of the UCSD Center for Circadian Biology for their collegial support and helpful comments.
Funding: Study funding was provided to MJM by a Veterans Affairs Career Development Award (1IK2BX001275). DKW receives support from a Veterans Affairs Merit Award (1I01BX001146). JRK is supported by a Veterans Affairs Merit Award (I01CX000363) and NIMH (MH92758, MH094483). The funders had no role in the analysis, decision to publish, or preparation of the manuscript. None of the authors have competing financial interests related to the work described.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ament SA, et al. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc Natl Acad Sci U S A. 2015;112:3576–3581. doi: 10.1073/pnas.1424958112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Byrne EM, Heath AC, Madden PA, Pergadia ML, Hickie IB, Montgomery GW, Martin NG, Wray NR. Testing the role of circadian genes in conferring risk for psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:254–260. doi: 10.1002/ajmg.b.32230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HM, DeLong CJ, Bame M, Rajapakse I, Herron TJ, McInnis MG, O'Shea KS. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl Psychiatry. 2014;4:e375. doi: 10.1038/tp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hertz L. Inhibition of noradrenaline stimulated increase in [Ca2+]i in cultured astrocytes by chronic treatment with a therapeutically relevant lithium concentration. Brain Res. 1996;711:245–248. doi: 10.1016/0006-8993(95)01199-4. [DOI] [PubMed] [Google Scholar]
- De Paoli P, Cerbai E, Koidl B, Kirchengast M, Sartiani L, Mugelli A. Selectivity of different calcium antagonists on T- and L-type calcium currents in guinea-pig ventricular myocytes. Pharmacol Res. 2002;46:491–497. doi: 10.1016/s1043661802002360. [DOI] [PubMed] [Google Scholar]
- Gershon ES, Grennan K, Busnello J, Badner JA, Ovsiew F, Memon S, Alliey-Rodriguez N, Cooper J, Romanos B, Liu C. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol Psychiatry. 2014;19:890–894. doi: 10.1038/mp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Tropea TF, Satpute SS, Sinnegger-Brauns MJ, Striessnig J, Kosofsky BE, Rajadhyaksha AM. Molecular switch from L-type Ca v 1.3 to Ca v 1.2 Ca2+ channel signaling underlies long-term psychostimulant-induced behavioral and molecular plasticity. J Neurosci. 2010;30:17051–17062. doi: 10.1523/JNEUROSCI.2255-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Tamminga CA, Tohen M, Suppes T. The relationship between affective state and the rhythmicity of activity in bipolar disorder. J Clin Psychiatry. 2014;75:e317–322. doi: 10.4088/JCP.13m08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Gomez G, Restrepo D, Friedman E, Josiassen R, Pribitkin EA, Lowry LD, Gallop RJ, Rawson NE. Aberrant intracellular calcium signaling in olfactory neurons from patients with bipolar disorder. Am J Psychiatry. 2005;162:616–618. doi: 10.1176/appi.ajp.162.3.616. [DOI] [PubMed] [Google Scholar]
- Harada Y, Sakai M, Kurabayashi N, Hirota T, Fukada Y. Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3 beta. J Biol Chem. 2005;280:31714–31721. doi: 10.1074/jbc.M506225200. [DOI] [PubMed] [Google Scholar]
- Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- Ikeda M. Bmal1 is an essential regulator for circadian cytosolic Ca2+ rhythms in suprachiasmatic nucleus neurons. J Neurosci. 2014;34:12029–12038. doi: 10.1523/JNEUROSCI.5158-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7:176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosir R, Acimovic J, Golicnik M, Perse M, Majdic G, Fink M, Rozman D. Determination of reference genes for circadian studies in different tissues and mouse strains. BMC Mol Biol. 2010;11:60. doi: 10.1186/1471-2199-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Wyborney VG. Lithium slows rat circadian activity rhythms. Life Sci. 1980;26:1319–1321. doi: 10.1016/0024-3205(80)90091-0. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Judd LL, Hubbard B, Janowsky DS, Huey LY. The effect of lithium carbonate on the circadian rhythm of sleep in normal human subjects. Biol Psychiatry. 1979;14:545–548. [PubMed] [Google Scholar]
- Li J, Lu WQ, Beesley S, Loudon AS, Meng QJ. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS One. 2012;7:e33292. doi: 10.1371/journal.pone.0033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Evans SJ, Choudary PV, Cartagena P, Barchas JD, Schatzberg AF, Jones EG, Myers RM, Watson SJ, Jr, Akil H, Bunney WE. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 2013;110:9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sarna SK. Chronic stress targets posttranscriptional mechanisms to rapidly upregulate alpha1C-subunit of Cav1.2b calcium channels in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G154–163. doi: 10.1152/ajpgi.00393.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27:339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Nievergelt CM, Kelsoe JR, Welsh DK. A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PLoS One. 2012;7:e32091. doi: 10.1371/journal.pone.0032091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Wei H, Marnoy Z, Darvish RM, McPhie DL, Cohen BM, Welsh DK. Genetic and clinical factors predict lithium's effects on PER2 gene expression rhythms in cells from bipolar disorder patients. Transl Psychiatry. 2013;3:e318. doi: 10.1038/tp.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- McKenna BS, Drummond SP, Eyler LT. Associations between circadian activity rhythms and functional brain abnormalities among euthymic bipolar patients: a preliminary study. J Affect Disord. 2014;164:101–106. doi: 10.1016/j.jad.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, McMaster A, Beesley S, Lu WQ, Gibbs J, Parks D, Collins J, Farrow S, Donn R, Ray D, Loudon A. Ligand modulation of REV-ERBalpha function resets the peripheral circadian clock in a phasic manner. J Cell Sci. 2008;121:3629–3635. doi: 10.1242/jcs.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Wang CW, Pan H, Welsh DK. Fibroblast circadian rhythms of PER2 expression depend on membrane potential and intracellular calcium. Chronobiol Int. 2012;29:653–664. doi: 10.3109/07420528.2012.679330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Koller DL, Jung J, Edenberg HJ, Foroud T, Guella I, Vawter MP, Kelsoe JR. Identification of pathways for bipolar disorder: a meta-analysis. JAMA Psychiatry. 2014;71:657–664. doi: 10.1001/jamapsychiatry.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Currier D, Liu SM, Hasin DS, Grant BF, Blanco C. Increased risk for suicidal behavior in comorbid bipolar disorder and alcohol use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) J Clin Psychiatry. 2010;71:902–909. doi: 10.4088/JCP.09m05198gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostacher MJ, Iosifescu DV, Hay A, Blumenthal SR, Sklar P, Perlis RH. Pilot investigation of isradipine in the treatment of bipolar depression motivated by genome-wide association. Bipolar Disord. 2014;16:199–203. doi: 10.1111/bdi.12143. [DOI] [PubMed] [Google Scholar]
- Ou X, Crane DE, MacIntosh BJ, Young LT, Arnold P, Ameis S, Goldstein BI. CACNA1C rs1006737 genotype and bipolar disorder: Focus on intermediate phenotypes and cardiovascular comorbidity. Neurosci Biobehav Rev. 2015;55:198–210. doi: 10.1016/j.neubiorev.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- PGC-BD. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roedding AS, Gao AF, Au-Yeung W, Scarcelli T, Li PP, Warsh JJ. Effect of oxidative stress on TRPM2 and TRPC3 channels in B lymphoblast cells in bipolar disorder. Bipolar Disord. 2012;14:151–161. doi: 10.1111/j.1399-5618.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One. 2010;5:e8561. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schmutz I, Chavan R, Ripperger JA, Maywood ES, Langwieser N, Jurik A, Stauffer A, Delorme JE, Moosmang S, Hastings MH, Hofmann F, Albrecht U. A Specific Role for the REV-ERBalpha-Controlled L-Type Voltage-Gated Calcium Channel CaV1.2 in Resetting the Circadian Clock in the Late Night. J Biol Rhythms. 2014;29:288–298. doi: 10.1177/0748730414540453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Moore-Ede MC. Lithium lengthens circadian period in a diurnal primate, Saimiri sciureus. Biol Psychiatry. 1990;28:117–126. doi: 10.1016/0006-3223(90)90629-g. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Imaizumi T, Kay SA. Real-time reporting of circadian-regulated gene expression by luciferase imaging in plants and mammalian cells. Methods Enzymol. 2005;393:269–288. doi: 10.1016/S0076-6879(05)93011-5. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012;149:1112–1124. doi: 10.1016/j.cell.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan E, Li B, Gu L, Hertz L, Peng L. Mechanisms for L-channel-mediated increase in [Ca(2+)]i and its reduction by anti-bipolar drugs in cultured astrocytes combined with its mRNA expression in freshly isolated cells support the importance of astrocytic L-channels. Cell Calcium. 2013;54:335–342. doi: 10.1016/j.ceca.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Pan JQ, Mungenast AE, Madison JM, Su S, Ketterman J, Ongur D, McPhie D, Cohen B, Perlis R, Tsai LH. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry. 2015;20:162–169. doi: 10.1038/mp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Fu Y, Altier C, Platzer J, Surmeier DJ, Bezprozvanny I. Ca1.2 and CaV1.3 neuronal L-type calcium channels: differential targeting and signaling to pCREB. Eur J Neurosci. 2006;23:2297–2310. doi: 10.1111/j.1460-9568.2006.04734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


