Abstract
OBJECTIVE
Deficits in cognitive functioning are related to functional disability in people with serious mental illness. Measures of functional capacity are commonly used as a proxy for functional disabilities for cognitive remediation programs, and robust linear relationships between functional capacity and cognitive deficits are frequently observed. This study aimed to determine whether a curvilinear relationship better approximates the association between cognitive functioning and functional capacity.
METHOD
Two independent samples were studied. Study 1: Participants with schizophrenia (n=435) and bipolar disorder (n=390) aged 18–83 completed a neuropsychological battery and a performance-based measure of functional capacity. Study 2: 205 participants with schizophrenia (age range=39–72) completed a brief neuropsychological screening battery and a performance-based measure of functional capacity. For both studies, linear and quadratic curve estimations were conducted with cognitive performance predicting functional capacity scores.
RESULTS
Significant linear and quadratic trends were observed for both studies. Study 1: In both the schizophrenia and bipolar participants, when cognitive composite z-scores were >0 (indicating normal to above normal performance), cognition was not related to functional capacity. Study 2: When neuropsychological screening battery z-scores were >−1 (indicating low average to average performance), cognition was not related to functional capacity.
CONCLUSIONS
These results illustrate that in cognitively normal adults with serious mental illness, the relationship between cognitive function and functional capacity is relatively weak. These findings may aid clinicians and researchers determine who may optimally benefit from cognitive remediation programs, with greater benefits possibly being achieved for individuals with cognitive deficits relative to individuals with normal cognition.
1. Introduction
Cognitive deficits are a prominent and stable feature of many patients with serious mental illness, and, in addition to mood and psychotic symptoms, account for high rates of disability and service utilization. Patients with schizophrenia average between one to three standard deviations below the mean of normal comparison subjects across a wide range of cognitive domains (Heinrichs and Zakzanis, 1998; Saykin et al., 1991). These cognitive deficits have been found to pre-date illness onset (Allen et al., 2003), thus suggesting impairment in cognition may be a core feature of the pathophysiology of the disorder irrespective of positive and negative symptoms typically attributed to illness severity.
Although cognitive and functional impairment appear to be key features of severe mental illnesses, they may not be universal. For example, although most patients experience neuropsychological deficits, approximately 20–25% of patients with schizophrenia have been found to have neuropsychological function within normal limits (i.e., not greater than 1 standard deviation from average performance) (Malhi et al., 2007; Palmer et al., 2009). This is even more characteristic of patients with bipolar disorder, with an estimated prevalence of 57% performing within normal limits on neuropsychological assessments (Gualtieri and Morgan, 2008). Similarly, whereas the vast majority of patients with schizophrenia and bipolar disorder show reduced rates of employment and independent living (Depp et al., 2012), other patients exhibit milder functional impairments. Given this variability in both cognitive and functional outcomes, it is imperative to determine how deficits in cognitive functioning relate to functional disability in people with serious mental illness.
Recent studies suggest that cognitive functioning may be differentially related to functional outcomes depending on the severity of the cognitive impairment. For example, one recent study of community dwelling adults with schizophrenia found that a supported employment + cognitive remediation intervention had significantly better employment outcomes than supported employment alone, but only among lower functioning participants (Bell et al., 2014). These results suggest that improvements in cognitive functioning are not associated with universal gains in functional outcomes, but rather that cognition may be more strongly related to functional outcomes among individuals with more severe cognitive impairment. In other words, a differential relationship on treatment outcome appears to exist as a result of the association between cognition and functioning.
The aim of the current study was to test whether cognitive function is more strongly related to functional capacity in persons with more severe cognitive impairment, a relationship which, to our knowledge, has not been previously tested. We tested linear and quadratic trends using two independent samples of patients with schizophrenia and a sample of patients with bipolar disorder. To assess functional capacity, we used the UCSD Performance-Based Skills Assessment-Brief (USPA-B)(Mausbach et al., 2007) as our primary outcome measure. We hypothesized that a linear relationship would provide valuable estimations of this relationship; however, we further postulated that a curvilinear would be more accurate in improving model fit. A curvilinear relationship suggests that as cognitive functioning improves, it’s relationship to functioning becomes weaker. If a curvilinear relationship between cognitive functioning and functional capacity is confirmed, this may indicate that there are ceiling effects for the UPSA-B and a more sensitive instrument of assessing functional capacity is needed for patients with higher levels of cognition. Additionally, a curvilinear relationship may indicate that cognitively normal patients have the capacity to be functionally normal as well.
2. Method: Study 1
The aim of this study was to examine the nature of the relationships between neurocognition and everyday functioning in a sample of adults with schizophrenia and a sample of adults with bipolar disorder. This study used a Neurocognitive Composite Score to measure neurocognitive ability and the UPSA-B to measure functional capacity.
2.1. Participants
Eight hundred and twenty-five individuals diagnosed with serious mental illnesses were included in this study, including 435 participants having a diagnosis of schizophrenia and 390 having a diagnosis of bipolar I disorder. All participants were enrolled in a parent study in the Epidemiology-Genetics Program in Psychiatry at the Johns Hopkins School of Medicine, as well as a subsequent study of clinical and neuropsychological factors associated with daily functional outcomes. Participants are of full or mixed Ashkenazi Jewish background, which was determined from ancestry of four grandparents. Participants were enrolled in the parent study between 1996 and 2006, at which time diagnosis was determined. Diagnosis was obtained by skilled clinicians (master’s or Ph.D. level) using the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994), as well as collateral review of medical records and informant reports by at least two clinicians (Ph.D. or psychiatrist). The procedures for the consensus diagnosis are described in detail elsewhere (Chen et al., 2009; Fallin et al., 2005; Fallin et al., 2003, 2004). The follow-up study of genetic study participants was conducted between 2007 and 2012 and focused on neurocognition and functional capacity. At this time, participants who were diagnosed with schizophrenia, schizoaffective disorder or bipolar disorder in the parent study were contacted regarding participation in the follow-up study. The study protocol was approved by the Johns Hopkins Medicine Institutional Review Board, and all participants provided written informed consent. The assessments were completed in participant’s homes or in residential outpatient treatment settings.
2.2. Measures
2.2.1. Neurocognitive Ability
Estimated premorbid intelligence was assessed with the Wide Range Achievement Test (WRAT; Jastak, 1993) Reading subtest. A Neurocognitive Composite Score (NCS) was used to assess neurocognitive ability. The NCS battery of tests was administered to measure executive functions, attention, verbal working memory, episodic learning, verbal fluency, and information processing speed. The NCS included the following: total learning from trials 1 to 5 on the Rey Auditory Verbal Learning Test (RAVLT; Schmidt, 1996), total time on Trail Making Test Parts A and B (Reitan and Wolfson, 1993), number of correct responses on WAIS-IV Letter Number Sequencing and Digit Symbol Coding (Wechsler, 2008), total unique correct responses on Animal Fluency (Spreen and Strauss, 1998), perseverative errors standard score from the 64-card computerized Wisconsin Card Sorting Test (Heaton et al., 1993), and Continuous Performance Test Identical Pairs Version, 4-digit condition, d-Prime (Cornblatt et al., 1988). Raw scores were adjusted for age, gender, and education on the basis of published normative data. Variables were transformed to equally weighted standardized scores (z-scores; Mean=0, SD=1) and averaged to obtain the NCS.
2.2.2. Functional Capacity
The UCSD Performance-Based Skills Assessment, Brief version (UPSA-B; Mausbach et al., 2007) was used to assess functional capacity. The UPSA-B is a measure of functional capacity that measures two domains of functioning: finances and communication (Mausbach et al., 2007; Mausbach et al., 2010; Patterson et al., 2001). During the Finance subtest, participants are required to role-play various financial tasks, such as counting change and paying a utility bill. The Communication subtest requires participants to role-play exercises using an unplugged phone (e.g., make an emergency call, dial a number from memory, call and reschedule a doctor’s appointment). The UPSA-B takes approximately 10–15 minutes to complete, and scaled scores range from 0–100, with higher scores indicating better functional capacity. Psychometric properties for the UPSA-B are very good (Mausbach et al., 2007; Mausbach et al., 2009; Velligan et al., 2013), including high test-retest reliability and correlations with the full-length version of the UPSA (Patterson et al., 2001).
2.2.3. Clinical Symptoms
The Positive and Negative Syndrome Scale (PANSS; Kay, 1987) was used to assess symptoms of psychopathology. The total Positive and Negative domain scores were used in this study, with higher scores indicating greater severity of symptoms. Depressive symptoms were assessed with the Beck Depression Inventory-II (BDI-II) (Dozois et al., 1998) and manic symptoms were assessed with the PANSS-Excitement subscale (Kay, 1987). The PANSS-Excitement subscale consists of four items: uncooperativeness, excitement, impulsivity, and hostility. Other studies using samples of bipolar patients have found strong correlations between these four items and clinician-rated scales to assess manic symptoms (e.g., the Young Mania Rating Scale (YMRS); Young et al., 1978). Higher scores on both the BDI-II and PANSS-Excitement subscale indicated greater symptomatology.
2.3. Data Analysis
SPSS Statistics Version 21 (SPSS, 2013) was used to carry out all analyses. Pearson correlations were conducted to analyze the relationship between the study variables. Variables of interest for the curve estimations were centered around their means. For both groups, we conducted a linear regression analysis including estimated premorbid intelligence and clinical symptoms in Step 1. The NCS was entered in Step 2 and the NCS squared was entered in Step 3 to create the quadratic model.
3. Results – Study 1
Summary statistics are presented in Table 1. NCS for the schizophrenia sample was in the below average range (Mean z-score=−1.1, SD=1.0). For the bipolar sample, NCS was considered average (Mean z-score=−0.3, SD=0.8). UPSA-B scores for both patient groups were above 60 (M schizophrenia group=77.3 (17.9); M bipolar group=87.9 (9.9)), a cut-off value which has been found to be predictive of residential independence (Mausbach et al., 2007). The correlation between NCS and functional capacity was higher in the schizophrenia group, yet significant in both groups (schizophrenia group: r=0.65, p<0.001; bipolar group r=0.39, p<0.001). See Table 2 for correlations between NCS, functional capacity, and clinical symptoms.
Table 1.
Demographic, Clinical, Cognitive, and Functioning Characteristics from Studies 1 and 2.
| Study 1: Hopkins Schizophrenia Sample (N=435) |
Study 1: Hopkins Bipolar Sample (N=390) |
Study 2:UCSD Schizophrenia Sample (N=205) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Range=21–78 | M=50 | SD=10 | Range=18–83 | M=48 | SD=13 | Range=38–72 | M=52 | SD=7 |
| Sex (% Female) | 35.8% | 52.3% | 39.5% | ||||||
| Education (Years) | Range=6–20 | M=14 | SD=2 | Range=9–20 | M=16 | SD=2 | Range=3–22 | M=12 | SD=2 |
| Race/Ethnicity (n, %)a | |||||||||
| White | 111 (54%) | ||||||||
| Black | 41 (20%) | ||||||||
| Hispanic | 31 (15%) | ||||||||
| Asian | 9 (4%) | ||||||||
| American Indian | 6 (3%) | ||||||||
| Other | 7 (3%) | ||||||||
| Living Situation (% Independent Living) | 50.5% | 83.6% | 20.5% | ||||||
| PANSS Positive Symptoms | Range=7–34 | M=15.4 | SD=6.2 | Range=7–26 | M=10.3 | SD=3.7 | Range=7–36 | M=15.4 | SD=6.1 |
| PANSS Negative Symptoms | Range=7–45 | M=15.5 | SD=7.3 | Range=7–25 | M=9.2 | SD=3.0 | Range=7–33 | M=15.8 | SD=5.2 |
| PANSS-Excitement Subscale | Range=4–22 | M=6.1 | SD=3.0 | Range=4–22 | M=6.4 | SD=3.1 | N/A | ||
| Beck Depression Inventory-II | Range=0–56 | M=11.3 | SD=9.2 | Range=0–48.3 | M=9.5 | SD=10.1 | N/A | ||
| WRAT Standard Score | Range=62–120 | M=102.9 | SD=11.5 | Range=68–120 | M=108 | SD=9 | N/A | ||
| RBANS Total Scoreb | N/A | N/A | Range=−3.93–0.40 | M=−2.4 | SD=0.9 | ||||
| RBANS Immediate Memoryc | N/A | N/A | Range=40–117 | M=61.5 | SD=16.4 | ||||
| RBANS Visuospatial/ Constructional Skillsc | N/A | N/A | Range=50–126 | M=70.5 | SD=15.0 | ||||
| RBANS Languagec | N/A | N/A | Range=40–113 | M=81.4 | SD=14.9 | ||||
| RBANS Attentionc | N/A | N/A | Range=40–112 | M=71.7 | SD=15.7 | ||||
| RBANS Delayed Memoryc | N/A | N/A | Range=40–112 | M=67.2 | SD=18.7 | ||||
| Neurocognitive Compositeb,d | Range=−3.6–1.2 | M= −1.1 | SD=1.0 | Range=−3.0–1.4 | M= −0.3 | SD=0.8 | N/A | ||
| UPSA-B Total Score | Range=14.7–100 | M=77.3 | SD=17.9 | Range=39.4–100 | M=87.9 | SD=9.9 | Range=0–94.4 | M=53.0 | SD=20.7 |
Note. PANSS: Positive and Negative Syndrome Scale; WRAT = Wide Range Achievement Test; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; UPSA-B = UCSD Performance-Based Skills Assessment, Brief version
All participants in Study 1 were Ashkenazi Jewish
Reported in z-scores
Reported in standard scores
Includes measures in the following domains: Attention, language, working memory, processing speed, memory, executive functions
Table 2.
Correlations between clinical, cognitive, and functional capacity variables in Studies 1 and 2.
| Study 1: Schizophrenia Participants | Cognitive Composite | UPSA-B Total Score |
|---|---|---|
| WRAT | 0.51** | 0.45** |
| PANSS Positive Symptoms | −0.29** | −0.32** |
| PANSS Negative Symptoms | −0.52** | −0.57** |
| PANSS Excitability | −0.12* | −0.14** |
| BDI | −0.08 | −0.02 |
| Study 1: Bipolar Participants | Cognitive Composite | UPSA-B Total Score |
|---|---|---|
| WRAT | 0.44** | 0.27** |
| PANSS Positive Symptoms | −0.29** | −0.15** |
| PANSS Negative Symptoms | −0.41** | −0.19** |
| PANSS Excitability | −0.23** | −0.13* |
| BDI | −0.14** | −0.05 |
| Study 2: Schizophrenia Participants | RBANS Total Score | UPSA-B Total Score |
|---|---|---|
| PANSS Positive Symptoms | −0.19** | −0.20** |
| PANSS Negative Symptoms | −0.34** | −0.44** |
Note.
p < 0.05;
p < 0.01
In this study, significant linear and quadratic trends were observed for both samples. For the schizophrenia participants, results of the linear model were t=8.74, df=397, p<0.001. The quadratic trend significantly improved overall model fit by 1.4% (Step 1: R2 = 0.360, ΔR2 = 0.360, ΔF = 44.78, df(5,398), Sig. ΔF < 0.001; Step 2: R2 = 0.466, ΔR2 = 0.106, ΔF = 78.87; df(1,397), Sig. ΔF < 0.001; Step 3: R2 = 0.480; ΔR2 = 0.014, ΔF = 10.96, df(1,396), Sig. ΔF = 0.001). Similar results were found for the bipolar participants. For the linear model, t=4.97, df=369, p<0.001, with the quadratic trend significantly improving the overall model fit by 1.3% (Step 1: R2 = 0.088, ΔR2 = 0.088, ΔF = 7.16, df(5,370), Sig. ΔF < 0.001; Step 2: R2 = 0.166, ΔR2 = 0.078, ΔF = 34.39; df(1,369), Sig. ΔF < 0.001; Step 3: R2 = 0.179; ΔR2 = 0.013, ΔF = 5.94, df(1,368), Sig. ΔF < 0.001). When neurocognitive composite z-scores were >0, cognition was not significantly related to functional capacity in either the schizophrenia or bipolar participants. See Table 3 for full regression results.
Table 3.
Regression Results from Study 1.
| Schizophrenia Participants (N=435)
| |||||
|---|---|---|---|---|---|
| B | SE | Beta | 95% Lower | 95% Upper | |
| WRAT | 0.17 | 0.06 | 0.12 | 0.05 | 0.30 |
| PANSS Positive | −0.28 | 0.12 | −0.10 | −0.52 | −0.04 |
| PANSS Negative | −0.52 | 0.11 | −0.21 | −0.72 | −0.31 |
| PANSS Excitability | 0.21 | 0.24 | 0.04 | −0.26 | 0.68 |
| BDI-II | 0.10 | 0.07 | 0.06 | −0.03 | 0.24 |
| Neurocognitive Composite | 7.34 | 0.84 | 0.42 | 5.69 | 8.99 |
| Squared Neurocognitive Composite | −1.91 | 0.58 | −0.12 | −3.04 | −0.78 |
| Bipolar Participants (N=390)
| |||||
|---|---|---|---|---|---|
| B | SE | Beta | 95% Lower | 95% Upper | |
| WRAT | 0.08 | 0.06 | 0.07 | −0.04 | 0.19 |
| PANSS Positive | −0.35 | 0.20 | −0.13 | −0.75 | 0.05 |
| PANSS Negative | 0.05 | 0.18 | 0.02 | −0.29 | 0.39 |
| PANSS Excitability Subscale | 0.30 | 0.25 | 0.09 | −0.19 | 0.78 |
| BDI-II | 0.03 | 0.05 | 0.03 | −0.07 | 0.12 |
| Neurocognitive Composite | 3.37 | 0.68 | 0.28 | 2.03 | 4.70 |
| Squared Neurocognitive Composite | −1.27 | 0.55 | −0.12 | −2.35 | −0.19 |
Note. PANSS: Positive and Negative Syndrome Scale; WRAT = Wide Range Achievement Test; BDI-II=Beck Depression Inventory-II. All variables were centered around their means. All variables were configured as z-scores, and therefore already centered around zero.
4. Method - Study 2
As a follow-up to Study 1, the aim of Study 2 was to see if the results of the first study could be replicated in an independent sample of patients with schizophrenia while using only abbreviated measures, which have more clinical utility than lengthier batteries. We used both the abbreviated measure of functional capacity used in the first study and a brief neurocognitive screening battery.
4.1. Participants
A total of 205 middle-aged and older adults with schizophrenia (mean age=52 years, range=38–72 years) enrolled in a randomized clinical trial examining the effectiveness of skills-based interventions for improving daily functioning were enrolled in this study. Data was obtained at baseline. Participants were included if they (a) were 40 years of age or older, (b) had a Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; American Psychiatric Association, 2000) chart diagnosis of schizophrenia or schizoaffective disorder (confirmed by patient’s psychiatrist), and (c) were psychiatrically stable (e.g., taking antipsychotic medications and not residing in an inpatient setting). Patients were excluded if they (a) had a primary DSM-IV Axis I diagnosis other than schizophrenia or schizoaffective disorder, (b) had a comorbid diagnosis of dementia, (c) were judged to be a suicide risk, (d) were unable to complete the assessment battery, or (e) were currently participating in any other psychosocial or pharmacological intervention at the time of intake.
The study protocol was approved by the University of California, San Diego Institutional Review Board. A formal assessment of decisional capacity (Jeste et al., 2007) was completed prior to enrollment, and participants who failed the decisional capacity assessment were not enrolled. All participants provided written, informed consent prior to participating. Assessments were completed in the San Diego community at Board and Care facilities, clubhouses or recovery-based day programs, and mental health clinics. Demographic and psychiatric histories were obtained by formal interview.
4.2. Measures
4.2.1. Neurocognitive Ability
Participants were administered the Repeatable Battery for the Assessment of Neuropsychological Status, Form A (RBANS; Randolph, 1998). The RBANS was created as a screening tool for use in neurologically impaired individuals and was standardized on a United States-Census-matched adult population between the ages of 20–89 years. It is a brief yet comprehensive neurocognitive screening battery that assesses neurocognitive status in the following domains: Immediate and Delayed Memory, Attention, Language, and Visuospatial/Constructional Skills. Among patients with schizophrenia, the RBANS has demonstrated good reliability, sensitivity to impairment, convergent validity with longer neuropsychological tests batteries, and relationships to functional outcomes (Gold et al., 1999; Ho et al., 2013; Hobart et al., 1999). The RBANS was scored according to the manual (Randolph, 1998), which provides age-adjusted standardized scores for each domain as well as a total global neurocognitive status standard score. Standard scores were converted to z-scores. Scores range from severely impaired to above average, with higher scores indicating better performance. Administration time is approximately 30 minutes. In this study, the RBANS demonstrated good internal consistency, with a Cronbach alpha of 0.77.
4.2.2. Functional Capacity
The UCSD Performance-Based Skills Assessment, Brief version (UPSA-B; Mausbach et al., 2007) was used to assess functional capacity, and was described in the methods section of Study 1.
4.2.3. Clinical Symptoms
Symptoms of schizophrenia were measured using the Positive and Negative Syndrome Scale (PANSS; Kay, 1987). As with Study 1, the total Positive and Negative domain scores were used.
4.3. Data Analysis
The same data analysis procedure was used in this study as in Study 1. Similar to Study 1, a linear regression analysis was performed which included education, PANSS Positive, and PANSS Negative symptoms in Step 1. RBANS total score was included in Step 2. To create the quadratic model, RBANS total score squared was included in Step 3. All of the covariates were centered around their means, with the exception of education which was centered at 12 years and RBANS which was centered at a score of 60. Additionally, the RBANS provides Index Scores for five specific cognitive domains (immediate memory, delayed memory, visuospatial/ constructional skills, language, and attention), and we conducted follow-up exploratory analyses to determine whether a quadratic relationship with functional capacity exists for all five domains or for only a subset.
5. Results – Study 2
Summary statistics of participant demographics, clinical variables, and performance-based measures are presented in Table 1. On average, RBANS z-scores were in the moderately-to-severely impaired range (M=−2.4, SD=0.9). Additionally, UPSA-B scores (M=53, SD=20.7) fell below the clinical cutoff of 60, which has been found to be predictive of residential independence (Mausbach et al., 2007). Almost identical to the correlation between cognition and UPSA-B performance in the schizophrenia sample in Study 1, the RBANS and UPSA-B were highly correlated in this study (r=0.68, p<0.001). Correlations between both the RBANS and UPSA-B and symptoms of psychopathology are presented in Table 2.
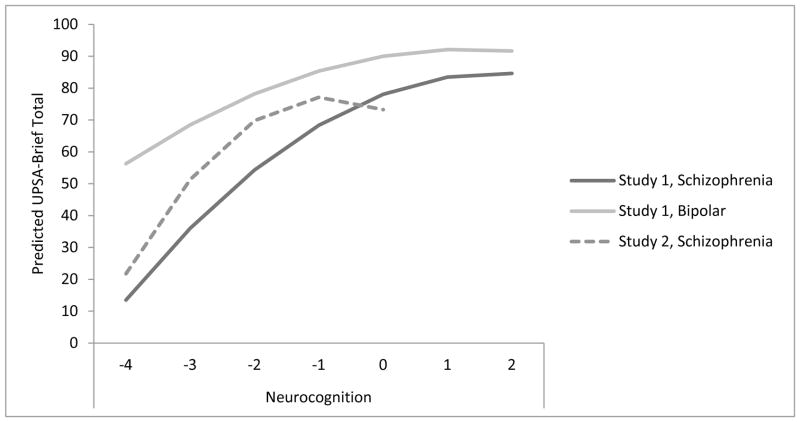
After statistically controlling for education and positive and negative symptoms, significant linear and quadratic trends were observed for cognitive functioning (see Table 4). We tested each of the covariates for quadratic trends; none of these variables had significant quadratic results and were therefore not included in the final regression model. In the final model, RBANS showed a significant quadratic (t=−4.89, df=198, p<0.001), but not linear (t=−1.90, df=199, p=0.06) relationship with UPSA-B scores. Addition of the quadratic trend significantly improved overall model fit by 5.3% (Step 1: R2 = 0.232, ΔR2 = 0.232, ΔF = 20.20, df(3,201), Sig. ΔF < 0.001; Step 2: R2 = 0.510, ΔR2 = 0.278, ΔF = 113.50; df(1,200), Sig. ΔF < 0.001; Step 3: R2 = 0.562; ΔR2 = 0.053, ΔF = 23.88, df(1,199), Sig. ΔF < 0.001). When RBANS z-scores were greater than −1 (indicating low average performance), cognition was not significantly related to UPSA-B performance (Figure 1).
Table 4.
Regression Results from Study 2 (N=205).
| B | SE | Beta | 95% Lower | 95% Upper | |
|---|---|---|---|---|---|
| Education | 0.51 | 0.42 | 0.06 | −0.32 | 1.34 |
| PANSS Positive | −0.03 | 0.17 | −0.01 | −0.35 | 0.30 |
| PANSS Negative | −0.78 | 0.21 | −0.20 | −1.18 | −0.37 |
| RBANS | −9.44 | 4.98 | −0.39 | −19.25 | 0.38 |
| Squared RBANS | −5.58 | 1.14 | −1.01 | −7.83 | −3.33 |
Note. PANSS positive, and PANSS negative were centered around their means. Education was centered at 12 years. RBANS was centered at 60.
Figure 1.
Linear and Quadratic Trend for Study 1, Schizophrenia Participants (N=435), Study 1, Bipolar Participants (N=390), and Schizophrenia Participants (N=205).
Note. UPSA-B = UCSD Performance-Based Skills Assessment-Brief; For Study 1, Neurocognition is the Neurocognitive Composite Score, comprised of tests designed to measure executive functions, attention, verbal working memory, episodic learning, verbal fluency, and information processing speed. For Study 2, Neurocognition is Repeatable Battery of the Assessment of Neuropsychological Status Total Scores. Z-scores are reported for Neurocognition. Z-scores are only included up to the z-value representative of the study.
Follow-up exploratory analyses with specific cognitive domains derived from the RBANS indicated significant linear and quadratic trends for immediate memory and visuospatial/constructional skills, but only significant linear trends for language (t=6.59, p<0.001), attention (t=8.17, p<0.001), and delayed memory (t=6.92, p<0.001). For immediate memory, results of the linear model were t=8.59, df=199, p<0.001. The quadratic trend significantly improved overall model fit by 2.5% (Step 1: R2 = 0.232, ΔR2 = 0.232, ΔF = 20.20, df(3,201), Sig. ΔF < 0.001; Step 2: R2 = 0.422, ΔR2 = 0.190, ΔF = 65.76; df(1,200), Sig. ΔF < 0.001; Step 3: R2 = 0.446; ΔR2 = 0.025, ΔF = 8.83, df(1,199), Sig. ΔF = 0.003). For visuospatial/constructional skills, results of the linear model were t=5.97, df=199, p<0.001. The quadratic trend significantly improved overall model fit by 2.9% (Step 1: R2 = 0.232, ΔR2 = 0.232, ΔF = 20.20, df(3,201), Sig. ΔF < 0.001; Step 2: R2 = 0.321, ΔR2 = 0.089, ΔF = 26.24; df(1,200), Sig. ΔF < 0.001; Step 3: R2 = 0.349; ΔR2 = 0.029, ΔF = 8.80, df(1,199), Sig. ΔF = 0.003).
6. Discussion
In large independent samples of outpatients with schizophrenia and bipolar disorder, we assessed linear and curvilinear relationships between neurocognitive ability and functional capacity. The participants varied greatly in regard to demographic and clinical characteristics as well as overall cognitive ability and functional capacity, with the schizophrenia participants in Study 2 having more impaired cognition and functional capacity than participants in Study 1. Despite these sampling differences, results of both studies illustrate that in subgroups of individuals with neurocognition in the low average to average range, the cognition-functional capacity relationship weakens. This pattern of findings persisted after controlling for markers of cognitive reserve and clinical symptoms.
The results of this study have demonstrated that the relationship between functional capacity and cognition in patients with serious mental illness is best understood in the context of quadratic relationships rather than the widely held current practice that attempts to capture the cognitive-functional link using linear models. Specifically, we demonstrated that the correlation between cognition and functional capacity was significantly weaker among individuals with cognition within normal limits or the mild impairment range than among those with moderate or severe cognitive impairment. As such, cognitive functioning may be limited in predicting functional capacity beyond a certain point. Our results were similar across the two independent studies using both a composite and a briefer measure of neurocognitive ability, and may help explain why a combination of cognitive remediation and supplemental skills are efficacious treatments for many, but not all, adults with serious mental illness (Bowie et al., 2012). Additionally, exploratory analyses conducted in Study 2 examining the linear and curvilinear relationships between specific domains of neurocognitive ability and functional capacity found curvilinear relationships for immediate memory and visuospatial/constructional skills, but only linear relationships for language, attention, and delayed memory. These findings suggest cognitive remediation strategies emphasizing immediate memory and visuospatial/constructional skill deficits among moderately to severely impaired middle-aged and older adults with schizophrenia may be more effective than these strategies would be among patients with only mild neurocognitive impairment.
There are limitations to both studies that must be acknowledged. In Study 1, all of the participants were of full or mixed Ashkenazi Jewish background and were relatively high functioning and more educated than most community-based samples of adults with serious mental illness. Therefore, these results may not be generalizable to more socioeconomically or ethnically diverse samples. Additionally, as a group, the bipolar participants reported minimal depression and excitability scores; the relative impact of symptoms on functional capacity may be greater among more symptomatic patients. Another limitation of Study 1 was that only one measure was included for each neurocognitive domain in the Neurocognitive Composite Score. It has been recommended to include two or more tests per domain to generate inferences regarding neurocognitive functioning in particular domains; therefore, follow-up exploratory analyses of specific domains using Study 1 data were not conducted.
In contrast to Study 1, the participants in Study 2 represented a diverse sample that included almost 50% racial/ethnic minorities. However, the age range of participants in this study was restricted to middle-aged and older adults. The UPSA-B is primarily focused on characterizing functional capacity within the impaired ranges, and a ceiling effect has previously been identified with the UPSA-B in a sample of patients with bipolar disorder (Mausbach et al., 2010). In the present sample of bipolar patients, a ceiling effect may have existed, as an asymptote occurred at UPSA scores above 90, which is near the tests ceiling. However, an asymptote for schizophrenia patients occurred in the range of 70–80 on the UPSA-B, well below the ceiling of 100 for the test, suggesting a ceiling effect did not occur. Similarly, the RBANS was originally designed as a dementia screening measure and has poor reliability in distinguishing among different levels of cognitive functioning including average, above average, and superior ranges. It could be that a linear relationship would still exist with tests more sensitive to differences in cognitive functioning (such as the neurocognitive composite used in Study 1) and functional capacity in the average and above average/superior ranges; future development of more challenging performance-based tasks that are representative of a wider variety of functional abilities appears warranted.
Despite these limitations, while there is a consensus in the literature that cognitive deficits have an adverse impact on functional and psychosocial outcomes in schizophrenia and, to a lesser degree, bipolar disorder (for e.g., Andreou and Bozikas, 2013; Bowie et al., 2008; Bowie et al., 2006), few studies have explicitly examined the role of normal cognitive functioning on a well-established measure of functional capacity. Factors other than cognitive impairment (e.g., motivation, self-efficacy, resilience) likely determine aspects of functional capacity in cognitively normal patients. There is some evidence that limited experiences with functional tasks in the community explain some variance in the relationship between neurocognition and functional capacity (Holshausen et al., 2014). In summary, future research and clinical intervention programs would benefit from a more individualized approach to intervention strategies for patients with serious mental illness, potentially based on level of cognitive functioning. Moreover, given that curvilinear relationships in our study significantly improved model fit, our findings lend support to the benefits of future studies to test not only linear, but also quadratic trends when examining the relationships between variables.
Acknowledgments
Role of Funding Source
Primary funding was provided by NIMH grant R01MH070784 to Dr. Pulver and R01MH084967 to Dr. Mausbach. Additional funding was provided by NIH grants T32 MH019934, P30MH066248 and NCRS UL1RR031980.
Footnotes
Contributors
Raeanne Moore carried out the statistical analyses and wrote the paper. Alexandrea Harmell, Philip Harvey, Christopher Bowie, Colin Depp, Thomas Patterson, Veronica Cardenas, and Dilip Jeste assisted with writing the paper. Ann Pulver helped with the design of the study, supervised data collection, and assisted with writing the paper. John McGrath, Paula Wolyniec, Mary Thornqueist, and James Luke collected data and assisted with writing the paper. Brent Mausbach helped with the conceptualization and design of the study, supervised data collection and statistical analyses, and assisted with writing the paper. All authors contributed to and have approved the final manuscript.
Statement of Conflicts of Interest
Dr. Bowie has served on a scientific advisory board and as a consultant for Abbott Pharmaceuticals. Dr. Harvey has served as a consultant to Abbott, Dainippon Sumitomo Pharma America, Eli Lilly, Johnson & Johnson, Merck, Shire Pharma, and Solvay and has received grant support from AstraZeneca. All other authors report no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Raeanne C. Moore, Email: r6moore@ucsd.edu.
Alexandrea L. Harmell, Email: aharmell@ucsd.edu.
Philip D. Harvey, Email: pharvey@med.miami.edu.
Christopher R. Bowie, Email: bowiec@queensu.ca.
Colin A. Depp, Email: cdepp@ucsd.edu.
Ann E. Pulver, Email: aepulver@jhmi.edu.
John A McGrath, Email: jmcgrath@jhmi.edu.
Thomas L. Patterson, Email: tpatterson@ucsd.edu.
Veronica Cardenas, Email: vcardenas@ucsd.edu.
Paula Wolyniec, Email: wolyniec@jhmi.edu.
Mary H. Thornquist, Email: thornquist@jhmi.edu.
James R. Luke, Email: jrluke@jhmi.edu.
Barton W. Palmer, Email: bpalmer@ucsd.edu.
Dilip V. Jeste, Email: djeste@ucsd.edu.
Brent T. Mausbach, Email: bmausbach@ucsd.edu.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) 4. American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- Allen DN, Goldstein G, Warnick E. A consideration of neuropsychologically normal schizophrenia. Journal of the International Neuropsychological Society. 2003;9(1):56–63. doi: 10.1017/s135561770391006x. [DOI] [PubMed] [Google Scholar]
- Andreou C, Bozikas VP. The predictive significance of neurocognitive factors for functional outcome in bipolar disorder. Current opinion in psychiatry. 2013;26(1):54–59. doi: 10.1097/YCO.0b013e32835a2acf. [DOI] [PubMed] [Google Scholar]
- Bell MD, Choi KH, Dyer C, Wexler BE. Benefits of cognitive remediation and supported employment for schizophrenia patients with poor community functioning. Psychiatric Services. 2014;65(4):469–475. doi: 10.1176/appi.ps.201200505. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biological Psychiatry. 2008;63(5):505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. American Journal of Psychiatry. 2012;169(7):710–718. doi: 10.1176/appi.ajp.2012.11091337. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: Correlations with cognition, functional capacity, and symptoms. American Journal of Psychiatry. 2006;163(3):418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- Chen PL, Avramopoulos D, Lasseter VK, McGrath JA, Fallin MD, Liang KY, Nestadt G, Feng N, Steel G, Cutting AS, Wolyniec P, Pulver AE, Valle D. Fine mapping on chromosome 10q22-q23 implicates Neuregulin 3 in schizophrenia. American Journal of Human Genetics. 2009;84(1):21–34. doi: 10.1016/j.ajhg.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Research. 1988;26(2):223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Depp CA, Mausbach BT, Bowie C, Wolyniec P, Thornquist MH, Luke JR, McGrath JA, Pulver AE, Harvey PD, Patterson TL. Determinants of occupational and residential functioning in bipolar disorder. Journal of Affective Disorders. 2012;136(3):812–818. doi: 10.1016/j.jad.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozois DJA, Dobson KS, et al. A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment. 1998;10(2):83–89. [Google Scholar]
- Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, Steel G, Nestadt G, Liang KY, Huganir RL, Valle D, Pulver AE. Bipolar I disorder and schizophrenia: A 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. American Journal of Human Genetics. 2005;77(6):918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Wolyniec PS, McGrath JA, Nestadt G, Valle D, Liang KY, Pulver AE. Genomewide linkage scan for schizophrenia susceptibility loci among Ashkenazi Jewish families shows evidence of linkage on chromosome 10q22. American Journal of Human Genetics. 2003;73(3):601–611. doi: 10.1086/378158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Wolyniec PS, McGrath JA, Nestadt G, Valle D, Liang KY, Pulver AE. Genomewide linkage scan for bipolar-disorder susceptibility loci among Ashkenazi Jewish families. American Journal of Human Genetics. 2004;75(2):204–219. doi: 10.1086/422474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable Battery for the Assessment of Neuropsychological Status as a screening test in schizophrenia, I: Sensitivity, reliability, and validity. American Journal of Psychiatry. 1999;156(12):1944–1950. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. The Journal of Clinical Psychiatry. 2008;69(7):1122–1130. doi: 10.4088/jcp.v69n0712. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chellune CJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual-Revised and expanded. Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Ho JS, Moore RC, Davine T, Cardenas V, Bowie CR, Patterson TL, Mausbach BT. Direct and mediated effects of cognitive function with multidimensional outcome measures in schizophrenia: the role of functional capacity. Journal of Clinical and Experimental Neuropsychology. 2013;35(8):882–895. doi: 10.1080/13803395.2013.828021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobart MP, Golberg R, Bartko JJ, Gold JM. Repeatable Battery for the Assessment of Neuropsychological Status as a screening test in schizophrenia, II: convergent/discriminant validity and diagnostic group comparisons. American Journal of Psychiatry. 1999;156(12):1951–1957. doi: 10.1176/ajp.156.12.1951. [DOI] [PubMed] [Google Scholar]
- Holshausen K, Bowie CR, Mausbach BT, Patterson TL, Harvey PD. Neurocognition, functional capacity, and functional outcomes: the cost of inexperience. Schizophrenia Research. 2014;152(2–3):430–434. doi: 10.1016/j.schres.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Jastak S. Wide range achievement test. Vol. 3. Wide Range Inc; San Antonio, TX: 1993. [Google Scholar]
- Jeste DV, Palmer BW, Appelbaum PS, Golshan S, Glorioso D, Dunn LB, Kim K, Meeks T, Kraemer HC. A new brief instrument for assessing decisional capacity for clinical research. Archives of General Psychiatry. 2007;64(8):966–974. doi: 10.1001/archpsyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Lewis OA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disorders. 2007;9(1–2):114–125. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- Mausbach Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophrenia Bulletin. 2007;33(6):1364–1372. doi: 10.1093/schbul/sbm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Pulver AE, Depp CA, Wolyniec P, Thornquist MH, Luke J, McGrath J, Bowie CR, Patterson TL. Relationship of the Brief UCSD Performance-Based Skills Assessment (UPSA-B) to multiple indicators of functioning in people with schizophrenia and bipolar disorder. Bipolar Disorders. 2010;12(1):45–55. doi: 10.1111/j.1399-5618.2009.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Moore RC, Bowie C, Cardenas V, Patterson TL. A review of instruments for measuring functional recovery in those diagnosed with psychosis. Schizophrenia Bulletin. 2009;35(2):307–318. doi: 10.1093/schbul/sbn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychology Review. 2009;19(3):365–384. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD performance-based skills assessment: Development of a new measure of everyday functioning for severely mentally ill adults. Schizophrenia Bulletin. 2001;27(2):235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. Psychological Corp; San Antonio: 1998. [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Neuropsychology Press; Tucson, AZ: 1993. [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Auditory and Verbal Learning Test: A handbook. Western Psychological Services; Los Angeles, CA: 1996. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests and norms. Oxford University Press; New York, NY: 1998. p. 2. [Google Scholar]
- SPSS. SPSS Statistics Base. 21.0. IBM Corporation; Chicago, IL: 2013. [Google Scholar]
- Velligan DI, Fredrick M, Mintz J, Li X, Rubin M, Dube S, Deshpande SN, Trivedi JK, Gautam S, Avasthi A, Kern RS, Marder SR. The reliability and validity of the MATRICS Functional Assessment Battery. Schizophrenia Bulletin. 2013;40(5):1047–1052. doi: 10.1093/schbul/sbt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-IV: Technical and Scoring Manual. Pearson; San Antonio, TX: 2008. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry : The Journal of Mental Science. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]