Abstract
The hallmark of primary biliary cirrhosis (PBC) is the presence of autoreactive T and B cell responses that target biliary epithelial cells (BEC). We have previously demonstrated that biliary cell cytotoxicity is dependent upon the initiation of innate immune responses followed by chronic adaptive as well as bystander mechanisms. Critical to these mechanisms are the interactions between natural killer (NK) cells and BEC. We have taken advantage of our ability to isolate relatively pure viable preparations of liver-derived NK cells, BEC, and endothelial cells, and studied the interactions between NK cells and BEC and focused on the mechanisms that activate autoreactive T cells, their dependence on IFN-γ, and the expression of BEC MHC class I and class II molecules. Importantly, we demonstrate herein that at a high NK/BEC ratio, NK cells are cytotoxic for autologous BECs, but are not dependent on autoantigen, but yet still activate autoreactive CD4+ T cells in the presence of antigen presenting cells (APC). In contrast, at a low NK/BEC ratio, BECs are not lysed, but IFN-γ production is induced, which facilitates expression of MHC class I and class II molecules on BEC and, interestingly, protects them from lysis upon subsequent exposure to autoreactive NK cells. Furthermore, IFN-γ secreted from NK cells after exposure to autologous BECs is essential for this protective function and enables autoreactive CD4+ T cells to become cytopathic. In conclusion, our data reveal that NK cell mediated innate immune responses are likely critical at the initial stage of PBC, but also facilitate and maintain the chronic cytopathic effect of autoantigen-specific T cells, essential for progression of disease.
Keywords: NK cells, innate immunity, adaptive immunity
Introduction
There have been considerable efforts to define the effector mechanisms that lead to biliary cell destruction in PBC (1-4). Indeed, such work has defined the epitopes recognized by autoimmune CD4+ and CD8+ T cells and autoantibody and has led to the thesis that a multi-lineage response against PDC-E2, the immunodominant autoantigen in PBC, is an essential component of disease pathogenesis (5-11). Our laboratory has suggested, both from work in human PBC and animal models of autoimmune cholangitis, that innate immune responses shape acquired immune responses in the pathogenesis of cholangitis (12-16). Indeed, there is increasing recognition that an adaptive immune response requires significant contributions from innate immunity, both in a normal immune response and in a breach of tolerance (13, 17-21).
PBC is an organ-specific autoimmune disease without evidence of global defect in either innate or adaptive responses (22, 23). It is particularly noteworthy that the liver, which normally functions to restore and maintain tolerance, itself becomes the victim of an autoimmune response, leading directly to portal inflammation and destruction of biliary epithelial cells (BEC) (24-26). Moreover, PBC reoccurs following liver transplantation and recent data suggests that although biliary epithelial cells express toll-like receptors (TLRs) and can function as antigen presenting cells (APC), that the biliary epithelial cell is itself only a victim of an immune attack based upon its own unique apoptotic properties, namely that the major mitochondrial autoantigens of PBC remain immunologically intact within an apoptotic bleb (24, 27-30). Our laboratory has demonstrated the ability to isolate BEC and phenotypically defined individually isolated liver infiltrating mononuclear cells (13, 14, 31). We have taken advantage of this technology and the availability of human liver to study in detail the role of activated natural killer (NK) cells in the induction of autologous biliary epithelium cytotoxicity. We propose, based on our data, that the critical bridge that links the innate immune response with the adaptive effector autoimmune responses characteristic of PBC is activation of NK cells. Indeed, we demonstrate herein that appropriate pre-treatment of NK cells can modulate BEC destruction. Further, NK cells directly influence the ability of autoreactive CD4+ T cells to destroy PDC-E2 peptide antigen pulsed BEC. Finally, IFN-γ appears to play a central role in this switch from innate to acquired immunity, highlighting the important function of Th1 cytokines in PBC. We submit that further studies on the switch mechanisms between innate and adaptive immunity have the potential to alter the effector mechanisms that lead to the continued portal inflammation.
Materials and Methods
Subjects and Protocol
Explanted liver and spleen from a total of 8 patients were used throughout this study and, in all cases, their liver derived mononuclear cell (LMC) populations and intrahepatic biliary epithelial cells were isolated and defined as described below. These 8 explanted organs included 3 livers with PBC and 5 livers with hepatitis C virus infection. We should emphasize, as described below, that there were no significant differences in the results derived from the liver mononuclear cell or the BEC population from either PBC or hepatitis C and ultimately all data were combined. Further, the observation that liver samples from PBC and hepatitis C can be used for the current study is consistent with our thesis that the BEC is a victim as well as the established data that PBC reoccurs following transplantation. All 8 livers were derived from patients with end-stage cirrhosis, but in whom there was no evidence of an acute liver process nor any unrelated disease. The 3 patients with PBC were known to have high titer anti-mitochondrial antibodies and the diagnosis was based on established criteria (32). In all cases, the livers were obtained after informed consent and all protocols were approved by the Research Ethics Committee of Kyushu University.
Isolation of intrahepatic biliary epithelial cells (BEC) and endothelial cells (EC) from liver
The LMC populations were isolated as previously described (7, 12). Briefly, liver specimens were first digested with 1 mg/ml of collagenase type I. Cells from the digested tissue were purified to obtain LMCs as described (8). The adherent cells within the LMCs were isolated following incubation of the cells overnight in tissue culture plates. The adherent cell population was maintained in tissue culture until the cells reached full confluence, usually by day 14, and the non-adherent cell population aspirated, washed and cryopreserved in medium containing 7.5% DMSO and stored in liquid nitrogen. BEC were separated from adherent cells using CD326 (EpCAM) conjugated MicroBeads (Miltenyi Biotec) specific for epithelial cells. Cells were then re-suspended in medium consisting of a 1:1 mixture of Ham’s F12 and DMEM, supplemented with 5% FCS, epithelial growth factor (10 ng/ml), cholera toxin (10 ng/ml), hydrocortisone (0.4 μg/ml), tri-iodothyronine (1.3 μg/l), transferrin (5 μg/ml), insulin (5 μg/ml), adenine (24.3 μg/ml), and 10 ng/ml hepatocyte growth factor (R&D systems, Minneapolis, MN) and cultured (12, 31). The purity of the cells was verified by immunohistochemistry using antibodies against cytokeratins 7 and 19 (Dako, Glostrup, Denmark) and only cultures that were > 90% positive for these cytokeratins and > 95% viable (as determined by trypan blue) utilized for the studies reported herein. EC were separated from BEC and adherent cells using CD31 microbeads specific for endothelial cells. The cultures used in the studies herein were between 4 to 6 passages to exclude the potential loss of phenotype after prolonged in vitro culture. The methods used herein have all been previously described (13, 14, 16, 31).
Cytotoxicity of NK cells against autologous BEC and EC
All assays were performed with autologous cell populations; the ability of NK cells to lyse BEC or EC was assessed using a previously described 8 hour 51Cr release assay against autologous BEC or EC (12, 32). Briefly, the detached BEC or EC were labeled with 2 μCi/well 51Cr (Amersham) overnight, washed X3 in medium and 5 × 103 cells dispensed into individual wells of a 96 well round-bottom plate. To prepare effector NK cells, spleen was mechanically disrupted and the dissociated cells were filtered through a 150-μm mesh and separated by Ficoll centrifugation to obtain SpMC (33). As described (7, 14), the SpMC used for the assay were stimulated for 3 days with the TLR3 ligand poly (I:C) and TLR4 ligand lipopolysaccharide (LPS) each at an optimal concentration of 10 μg/ml. Activated spleen NK cells were purified using an NK cell isolation kit (Miltenyi Biotec). The purity of the isolated NK cell population was >90% as determined by flow cytometry with anti-CD56 mAb (Miltenyi Biotec) and viability >95%. The isolated activated NK cells were added to triplicate wells with BEC or EC target cells at an effector to target cell ratio of 50:1, 10:1, 2:1 and 0.5:1 in a total volume of 200 μl in complete RPMI medium. Controls consisted of triplicate wells containing target cells cultured alone and target cells incubated with 10% triton X-100 to determine spontaneous and maximal 51Cr release, respectively. Following incubation of the co-cultures of the effector with target cells for 8 hr. 100 μl of supernatant fluid was collected from each well and counted and the percentage of specific 51Cr release calculated as (cpm of experimental release − cpm of spontaneous release) / (cpm of maximal release − cpm of spontaneous release) × 100 (%). In a modified cytotoxicity assay, BEC were incubated with or without autologous NK cells at an NK to BEC ratio of 0.5 for 24 hours in the presence or absence of either IFN-γ (final concentration: 0.4, 2.0 or 10ng/ml) or mAb to NKG2D (final concentration: 25 μg/ml) (BioLegend, San Diego, CA), IFN-γ or HLA class I (final concentration: 50 μg/ml) (R&D systems). Cytotoxicity was quantitated as described above.
Analysis of cellular debris released from the cytotoxicity assay
To analyze the contents of the cellular debris following NK cell-mediated lysis of BEC or EC, we first seeded BEC or EC at a concentration of 1×105 cells/well in 6-well plates in complete BEC medium, a 1:1 mixture of Ham’s F12 and DMEM, supplemented with 5% FCS, epithelial growth factor (10ng/ml), Cholera toxin (10ng/ml), hydrocortisone (0.4μg/ml), tri-iodo-thyronine (1.3μg/l), transferrin (5μg/ml), insulin (5μg/ml), adenine (24.3μg/ml) (all from Sigma) and hepatocyte growth factor (10ng/ml) (R&D systems), or endothelial specific medium (HuMedia-EG2) that included cell growth factors (Kurabo, Osaka, Japan). Activated NK cells were added to each well at 5×106 cells/well (E:T ratio=50) for BEC and EC, and 1×106 cells/well (E:T ratio=10) for BEC, then cultured for 24 hours. The subcellular fragments of BEC or EC were isolated by filtration and ultracentrifugation. Briefly, the cell culture supernatant fluid was collected and two additional centrifugation steps (500g for 5 minutes) were performed to remove remaining cells and cell fragments. The supernatant fluid was then passed through a 1.2μm-pyrogenic, hydrophilic syringe filter. BEC fluid from an E:T ratio of 50 were also used unfiltered as a positive control. After centrifugation at 100,000g for 30 minutes, the pellet containing micro particles was re-suspended in lysis buffer containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Lysis was performed for 30 minutes on ice. The protein content of the samples was determined by BCA (bicinconic acid assay) using a Nanodrop ND-1000 ultraviolet-visible Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Each sample (20 μg) was diluted in loading buffer and subjected to standard sodium dodecyl sulfate polyvinylidene fluoride membrane separation. The E2 component of pyruvate dehydrogenase (PDC-E2) was detected using our standardized monoclonal antibody, clone 2H-4C8 as previously described (34).
Proliferation assays of T cell clones that respond to PDC-E2 163-176
We took advantage of our previously well described and well characterized T cell clones that respond to PDC-E2 163-176. Firstly, the PDC-E2 163-176 peptide GDLLAEIETDKATI was synthesized by F-moc chemistry using a peptide synthesizer (Model Synergy; Applied Biosystems Inc., Foster City, CA). The peptide was purified by reverse phase high-pressure liquid chromatography (HPLC) to a degree of >90% as determined by HPLC. Cryopreserved T cell clone TCC independent 1 that is specific for PDC-E2 163-176 was thawed, tested for viability, and our standardized proliferation assays performed (35).
For antigen presentation, we used monocyte-derived dendritic cells (DC) prepared from HLA-DR53 positive healthy donors as described previously, with minor modifications (36). Briefly, peripheral blood mononuclear cells (PBMC) isolated using Ficoll-Hypaque were allowed to adhere overnight. After removing the non-adherent cell fraction by gentle washing with phosphate-buffered saline (PBS), the remaining adherent cells were maintained in complete RPMI 1640 medium with 1000 U/ml human recombinant GM-CSF and 500 U/ml human recombinant IL-4 (R&D systems) for 7 days. At the end of the 7 day incubation period, non-adherent cells were recovered as DC after gentle washing. DC as antigen presenting cells (APC) were seeded at 2×106 cells/well in 24-well round-bottomed plates and incubated in the presence or absence of the micro particles from BEC or EC (250μg/ml at final concentration) for 24 hours. For positive control DC were pulsed with PDC-E2 163-176 peptide (10 μg/ml at final concentration). Thence, DC were harvested, washed and irradiated (3000 rad). The CD4 T cell clone was seeded at 5×104 cells/well in 96-well round-bottomed plates with irradiated HLA-DR53 matched DC (5×104 cells/well) that were pre-pulsed or not pulsed with the micro particles or peptide for 72 hours in the presence of 1 μCi/well of 3H-thymidine during the final 12 hours. Cells were then harvested and 3H-thymidine incorporation measured in a beta scintillation counter.
Cytokine analysis
For the analysis of IFN-γ secreted by the T cell clone, the T cells were seeded at 5×105 cells/well in 48-well plates with irradiated HLA-DR53 matched DC (5×105 cells/well) as APC with PDC-E2 163-176 peptide (10 μg/ml at final concentration) or the micro particles from BEC or EC (250 μg/ml at final concentration) for 24 hours, and the supernatants harvested. For the analysis of cytokines secreted from NK cells, activated NK cells were co-cultured with BEC in 6-well plates with serial numbers of NK cells and BEC in complete BEC medium for 24 hours, and the supernatants harvested. The concentration of cytokines in the harvested supernatants were evaluated by a sandwich ELISA, using a combination of unlabeled and biotin- or enzyme-coupled monoclonal antibodies to IFN-γ, TNF-α, or LT-β (R&D systems).
Cytotoxic assay of autoreactive T cells
The cytotoxic activity of PDC-E2-specific CD4 T cells was assessed using the T cell clone TCC independent 1 as effector cells and HLA DR53-matched BEC as target cells. BEC were pre-incubated with activated autologous NK cells at an NK/BEC ratio of 0.5 for 24 hours. NK cells were removed by washing three times with PBS. The adherent BEC population was harvested and dispensed into a 96 well round-bottom plate at 5 × 103 cells/well. The BEC were incubated with either PDC-E2 163-176 peptide or a control peptide at 10 μg/mL overnight. T cell clones were then added at T/BEC ratios of 50:1, 10:1, 2:1 and 0.5:1 and the cytotoxicity assay described above was performed.
Flow Cytometry
To analyze surface expression of HLA molecules, BEC were stained by indirect immunofluorescence using optimal concentrations of mAb to HLA-ABC, E and DR (Biolegend) before or after incubation with NK cells at an NK/BEC ratio of 0.5, in the presence or absence of IFN-γ at serial final concentrations, for 24 hours. The stained cells were analyzed by flow cytometry.
Statistical analysis
All experiments were performed in triplicate and results are presented as mean ± standard deviation (SD). Comparison between groups were performed by Student’s t test. All analyses were two-tailed and p values <0.05 were considered significant. Statistical analyses were performed using Intercooled Stata 8.0 (Stata Corp, College Station, TX).
Results
Activated NK cells induced release of PDC-E2 from autologous BEC
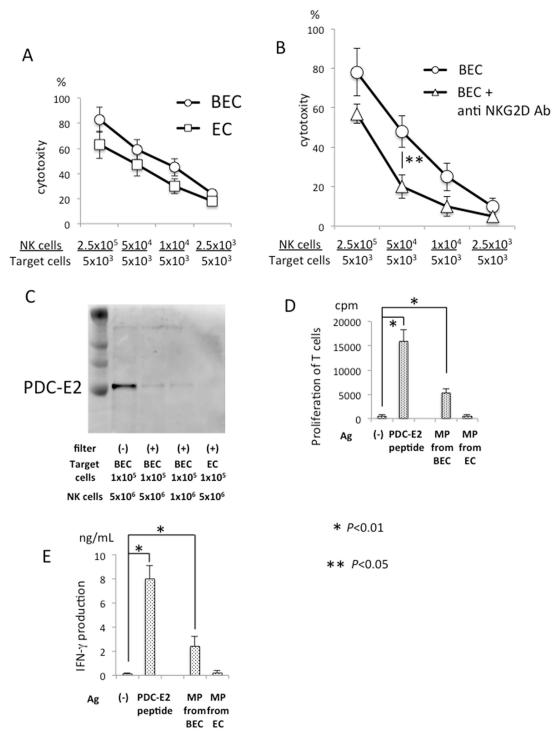
We first examined the cytotoxicity of activated NK cells on autologous BEC or EC. The NK cells isolated from TLR3-L and TLR4-L stimulated SpMC produced significant cytotoxicity when co-cultured with autologous BEC or EC (Figure 1A); this lysis was regulated by NKG2D and its receptors (Figure 1B). Importantly, intact PDC-E2 was detected in the unfiltered or filtered lysates obtained from BEC, but not in the filtered lysates of EC (Figure 1C). Of note, the filtration removed large cell debris resulting in isolation of microparticles. These results indicate that undegraded PDC-E2 is presented in the microparticles released from NK-lysed BEC but not in the lysed EC. A PDC-E2-specific CD4 T cell clone underwent proliferation in response to these BEC-derived microparticles (Figure 1D) and further, IFN-γ production of these T cells correlated with their proliferation (Figure 1E). Hence, although both BEC and EC were destroyed by NK cells, the microparticles from the lysed BEC differ from those from EC in terms of their content of the autoantigen PDC-E2 and their ability to activate PDC-E2-specific CD4 T cells.
Figure 1.
Activated NK cells cause damage of autologous BEC and release of autoantigen-containing microparticles. A. Cytotoxicity of activated NK cells against autologous BEC and EC. SpMC isolated from 2 PBC patients and 5 non-PBC patients were cultured in vitro with a mixture of TLR3-L and TLR4-L for 3 days, washed and then used for isolation of NK cells, which was assayed for cytotoxicity against autologous BEC or EC target cells at serial effector/target (E/T) ratios in a standard 51Cr release assay. The assay was performed in triplicate and results expressed as mean +/− S.D. Representative data from one PBC patient are shown. B. Activated NK cells were added to the co-cultures, in triplicate, to bring the NK/BEC ratio to the indicated levels for a standard 51Cr release assay, in the presence or absence of NKG2D Ab. C. Western blot analysis for detecting PDC-E2 using a mAb against PDC-E2. The whole lysate of NK-lysed BEC, or microparticles (MP) isolated by filtration and ultracentrifugation from cell lysates recovered after induction of cytolysis with E/T ratio of 50 or 10, were analyzed. D. Isolated microparticles (MP) were re-suspended in PBS and sterilized by filtration, then used as stimulating antigen in a standard 3H-TdR proliferation assay for the PDC-E2 specific CD4+ T cell clone TCC independent 1 (31). For the purpose of control, the T cell clone was incubated with the PDC-E2 163-176 peptide (P) or without the peptide (−). HLA DR53 matched irradiated monocyte derived dendritic cells were included in the assay as APC. E. Production of IFN-γ by the PDC-E2 specific CD4+ T cell clone stimulated with microparticles from BEC or EC as described in C. Results are presented as mean +/− S.D. *, significant differences (p<0.01) between Ag (−) versus PDC-E2 peptide or MP from BEC; **, significant differences (p<0.05) between anti NKG2D Ab treatment is present or absent.
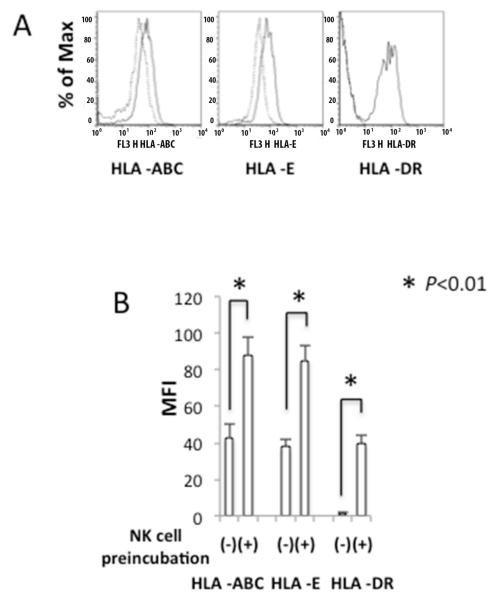
Phenotypic changes of BEC induced by NK cells
Next we examined the influence of NK cells on the co-cultured BEC. For BEC not incubated with NK cells, we detected expression of HLA-A, B, C and E, but not HLA-DR (Figure 2A). After incubation of BEC with NK cells at an NK/BEC ratio of 0.5, the majority of BEC were not lysed and the levels of these MHC class I molecules and HLA-DR increased significantly (Figure 2A and 2B), indicating that NK cells induced or enhanced the expression of the HLA class I and class II molecules on co-cultured BEC at low NK/BEC ratio.
Figure 2.
Effect of NK cells on the expression of MHC molecules on BEC. BEC were co-cultured with or without activated NK cells for 24 hours at an NK/BEC ratio of 0.5. Levels of cell surface HLA-ABC, -E and HLA-DR were assessed by flow cytometry. A. Representative FACS plots. B. Mean fluorescence intensity (MFI) of staining for MHC molecules on BECs with (+) or without (−) pre-incubation with NK cells.
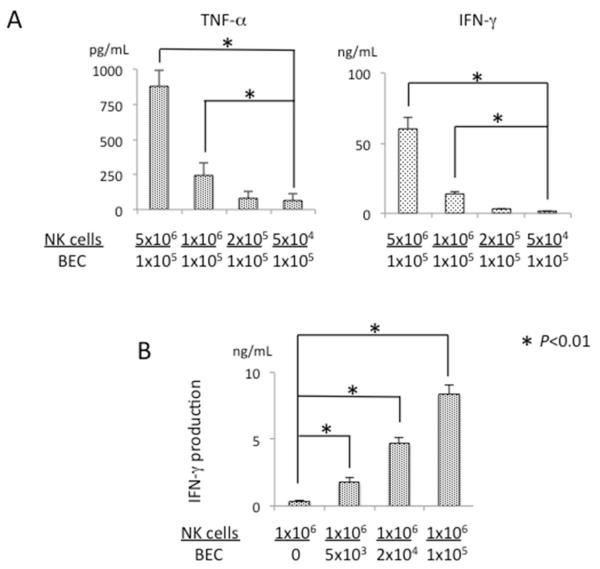
Cytokine expression of NK cells induced by BEC
We next evaluated the production of cytokines by activated NK cells co-cultured with BEC. In agreement with a previous report (37), activated NK cells produce TNF-α and IFN-γ at different NK/BEC ratios (Figure 3A) but did not produce LT-β (data not shown). The level of secreted IFN-γ increased with the number of BEC (Figure 3B). When activated NK cells were not co-cultured with BEC, the amount of secreted IFN-γ was negligible (Figure 3B). These results indicate that BEC induced production of IFN-γ from NK cells.
Figure 3.
BEC induced TNF-α and IFN-γ production from activated NK cells. A. Activated NK cells were co-cultured with autologous BEC (1×105 per well) in triplicate at serial NK/BEC ratios for 24 hours. Results are presented as mean +/− S.D. *, significant differences (p<0.01) between NK cells: 5×104 versus 1×106 or 5×106. B. Activated NK cells (1×106 per well) were co-incubated with BEC for 24 hours. The concentrations of TNF-α and IFN-γ in the conditioned medium were measured by ELISA. Results are presented as mean +/− S.D. *, significant differences (p<0.01) between BEC: 0 versus 5×103, 2×104 or 1×105.
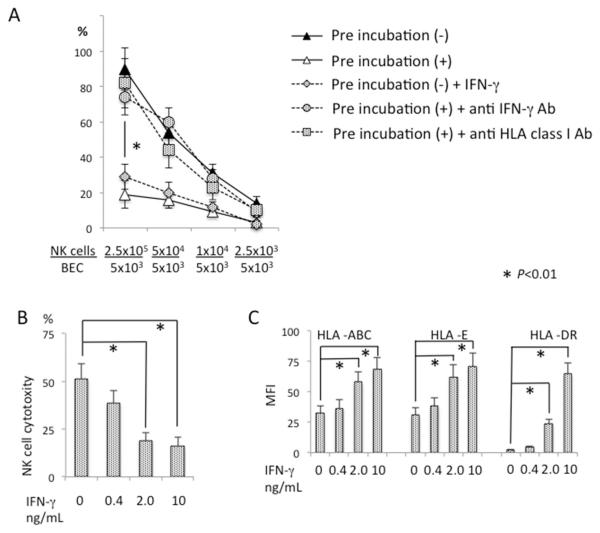
NK cell-derived IFN-γ modulates HLA-dependent NK cytotoxicity to BEC
As shown in Figure 1A, NK cells lysed autologous BEC efficiently at higher NK/BEC ratios. Reduction of this ratio from 50 to 0.5 in the co-culture cytotoxicity assay resulted in diminished BEC lysis. If BEC were pre-treated with small numbers of NK cells at an NK/BEC ratio of 0.5, subsequent addition of NK cells to higher E/T ratios did not result in the lysis of BEC under the same assay conditions (Figure 4A). To examine if the protective effect of NK pre-treatment is mediated by cytokines produced by NK cells in the presence of autologous BEC, IFN-γ or TNF-α were added to BEC that were not pre-treated with NK cells, followed by the NK/BEC co-culture cytotoxicity assay. Addition of TNF-α at a concentration of 10 ng/mL did not affect NK cell cytotoxicity against autologous BEC (data not shown). In contrast, addition of IFN-γ significantly suppressed lysis of BEC, which was reduced with an IFN-γ concentration of 0.4ng/ml and further reduced increased amounts of IFN-γ (Figure 4A and 4B). Addition of IFN-γ also resulted in induction of HLA-DR and increased levels of MHC class I molecules on the surface of BEC (Figure 4C). Addition of anti-IFN-γ mAb to BEC pretreated with NK cells abolished the protection effect of NK pretreatment (Figure 4A). In addition, blocking HLA class I by adding class I specific mAb to the NK-pretreated BEC also abolished the protective effect of NK pretreatment (Figure 4A). Taken together, these results suggest that exposure of NK cells to autologous BEC induced IFN-γ production from NK cells, which in turn enhanced the expression of class I MHC molecules on BEC and protected the latter from the cytotoxicity of NK cells.
Figure 4.
IFN-γ and HLA class I modulated NK cell cytotoxicity against BEC. BEC were mixed with activated autologous NK cells at an NK/BEC ratio of 0.5 and incubated for 12 hours during pre-incubation (+). After pre-incubation, activated NK cells were added to the co-cultures, in triplicate, to bring the NK/BEC ratio to the indicated levels for a standard 51Cr release assay, in the presence or absence of anti-IFN-γ Ab or anti-HLA class I Ab. BEC without NK cell pre-incubation (−) were also used as target cells with or without addition of IFN-γ. Results are presented as mean +/− S.D. *, significant difference (p<0.01) between pre-incubation (+) versus pre-incubation (−), pre-incubation (−) versus pre-incubation (−) + IFN-γ, pre-incubation (+) versus pre-incubation (+) + anti-IFN-γ Ab, or pre-incubation (+) versus pre-incubation (+) + anti-HLA Ab. B. BEC not pre-incubated with NK cells were co-cultured with autologous activated NK cells at an NK/BEC ratio of 50 for a standard 51Cr release assay in the presence of IFN-γ at the indicated concentrations. Results are presented as mean +/− S.D. *, significant differences (p<0.01) between IFN-γ 0 versus 2.0 or 10. C. BEC not pre-incubated with NK cells were co-cultured for 24 hours in the presence of IFN-γ at the indicated concentrations, then analyzed for the expression of MHC class I and class II molecules by flow cytometry. Results are presented as mean +/− S.D. *, significant differences (p<0.01) between IFN-γ 0 versus 2.0 or 10.
Cytotoxicity of autoreactive CD4+ T cell clone against BEC is enhanced by NK cells
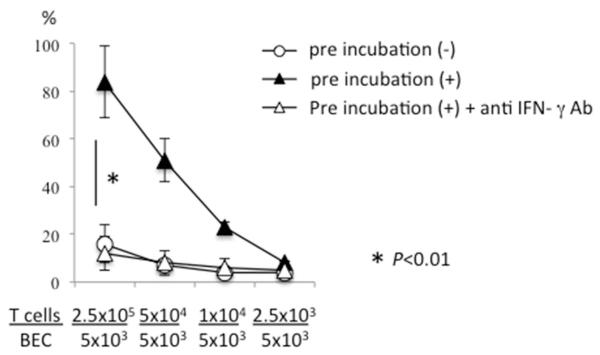
Our previous work demonstrated that PDC-E2 peptide specific CD4+ T cell clones were cytotoxic against HLA-matched BEC pulsed with PDC-E2 peptide in the presence of IFN-γ (38). Herein we note that in the absence of exogenous IFN-γ, PDC-E2-specific CD4+ T cells were unable to lyse PDC-E2 peptide pulsed BEC, even at high T/BEC ratios (Figure 5). However, when the PDC-E2 peptide-pulsed BEC were pre-incubated with NK cells at NK/BEC ratio of 0.5, the T cell clones were capable of lysing the target BEC at T/BEC ratios ranging from 50 to 2 (Figure 5). The effect of NK pretreatment was abolished by the addition of mAb to IFN-γ at all these T/BEC ratios (Figure 5). These data indicate that NK cells were capable of enhancing the cytotoxicity of autoantigen-specific CD4+ T cells against autologous BEC.
Figure 5.
NK cell pretreatment up-regulated cytotoxicity of autoreactive CD4+ T cells against BEC. BEC, pulsed with the PDC-E2 163-176 peptide, were mixed with (+) or without (−) activated autologous NK cells at an NK/BEC ratio of 0.5 and incubated for 24 hours. The PDC-E2 specific CD4+ T cell clone TCC independent 1 was then added as an effector cell at the indicated E/T ratios to the BEC in triplicate, followed by a standard 51Cr release assay in the presence or absence of anti-IFN-γ Ab. Results are presented as mean +/− S.D. *, significant differences (p<0.01) between pre-incubation (+) versus pre-incubation (−) or pre-incubation (+) + anti-IFN-γ Ab.
Discussion
In organ specific autoimmune diseases, target cells modify immunological responses and contribute to pathology. BEC in PBC express cell surface markers including HLA class II (39), CD40 (40) and DR5 (41) in addition to CX3CL1 (42) that serves as an adhesion molecule; in general, these molecules enhance the immunological response. In contrast, BEC in PBC also produce high levels of PG-E2 that suppress immunological responses (12). In our previous work, we reported that the cytotoxic activity of hepatic NK cells from patients with PBC was increased compared to NK cells from liver diseased controls against autologous BEC (31). In the work herein, there were no statistically significant cytotoxic differences between PBC and HCV patients, but the sample size was relatively small and, hence, we expanded our investigation of the interactions between NK cells and BEC in more detail. We isolated NK cells from splenic mononuclear cells following activation by TLR ligands and IL-2 stimulation; the number of activated NK cells in the groups herein were similar (data not shown). More importantly, we demonstrate that at high NK/BEC ratios, NK cells attacked BEC, resulting in release of autoantigens from injured BEC, which in turn activate autoreactive T cells in the presence of APC (Figure 1); at low NK/BEC ratios, NK cells do did not directly damage BEC, whereas IFN-γ secreted from NK cells induced the expression of HLA class II on BEC (Figure 4C), which leads to the destruction of BEC by autoreactive CD4+ T cells (Figure 5). In contrast, we confirmed the destruction of EC by autoreactive CD4+ T cells after exposure at low NK/autologous EC ratios (data not shown). We speculate that TLR ligands, NK cells and of course autoreactive CD4+ T cells surround BEC but not EC within the microenvironment of a PBC liver. Importantly, we demonstrate that at low NK/BEC ratios, IFN-γ produced from NK cells inhibits the direct cytotoxicity of NK cells toward autologous BEC (Figure 4A and 4B). Of particular interest, the destruction of BEC and EC by NK cells is neither PBC specific nor PDC-E2-specific (Figure 1A), suggesting that autoreactive T cell-mediated acquired immunity becomes the dominant immunological process only in established PBC that follows the initial non-specific innate immune attack that leads to breakdown of self-tolerance. This is supported by the finding that in established PBC, not only Th17 cells but also Th1 cells infiltrate into the lesions of chronic non-suppurative destructive cholangitis (CNSDC) with high levels of IFN-γ production derived from Th1 cells (43).
In addition to the IFN-γ that regulates the expression of HLA class I and class II and plays a role in the interaction between innate immunity and acquired immunity as reflected herein, it is well known that IFN-α primarily produced by plasmacytoid DC (pDC), leads to enhanced self-reactivity in several autoimmune diseases (44). Previously we demonstrated that IFN-α is necessary for activation of NK cells in their destruction of BEC (31). Like IFN-γ, IFN-α is also known to influence HLA expression (45, 46). However, pre-incubation of BEC with IFN-α did not affect damage of BEC mediated by NK cells (data not shown). As IFN-α promotes the activation and maturation of cDC, it is likely that type I IFN will enhance the antigen specific autoreactive T cell response. The role of type 1 IFNs in the pathogenic acquired immunity in PBC should be studied in the future.
The hallmark of acquired immunity in PBC are the T and B cell responses targeted to PDC-E2. As glutathiolation does not occur in apoptotic BEC, PDC-E2 is not degraded by caspases in PBC (29). As shown in the current work, PDC-E2 in apoptotic BEC is presented in an immunologically intact form in microparticles, which can be internalized by APC by the process of endocytosis (27, 30) and presented to antigen-specific T and B cells, resulting in the activation of these acquired immune cells after the initial attack of BEC by autologous NK cells in the presence of professional APC. It is currently not known if other parenchymal cells such as hepatocytes can also be damaged by NK cells. This should also be examined in future studies and, indeed, it is possible that following the action of NK cells on autologous EC, that autoantigens released from apoptotic ECs will be immediately digested and therefore not presented by APCs. In such a scenario, autoreactive responses would be reduced. Finally, we note that a weakness of the study herein is the small numbers of PBC livers and the surrogate use of HCV livers. However, our thesis, as discussed above, is that the BECs are victims of an effector response, a hypothesis supported by our previous data and by the recurrence of PBC following liver transplants.
Memory-like NK cells induced by viral infection or cytokine stimulation have been reported (47, 48). In many cases such memory-like NK cells become antigen specific in their reactivation. As the majority of memory NK cells reside in the liver (49), we speculate that memory NK cells may participate in the inflammatory process of PBC in an antigen specific way. The potential role of memory NK cells in PBC in terms of their interaction with and modulation of T cells and acquired immune responses either directly or indirectly (50, 51) should also be explored.
Acknowledgments
Financial support provided by Grant-in-Aid for Scientific Research (C) (Kakenhi 26461012) and National Institutes of Health grant, DK39588.
List of Abbreviations
- APC
antigen presenting cells
- BEC
biliary epithelial cells
- CNSDC
chronic non-suppurative destructive cholangitis
- DC
dendritic cells
- EC
endothelial cells
- ELISA
enzyme-linked immunosorbent assay
- HPLC
high pressure liquid chromatography
- IFN
interferon
- LMC
liver derived mononuclear cell
- mAb
monoclonal antibody
- NK cells
natural killer cells
- PBC
primary biliary cirrhosis
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate buffered saline
- pDC
plasmacytoid dendritic cells
- PDC-E2
E2 component of pyruvate dehydrogenase complex
- SD
standard deviation
- SpMC
spleen mononuclear cells
- TLR
toll like receptor
References
- 1.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 2.Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol. 2013;8:303–330. doi: 10.1146/annurev-pathol-020712-164014. [DOI] [PubMed] [Google Scholar]
- 3.Nakanuma Y, Sasaski M, Harada K. Autophagy and senescence in fibrosing cholangiopathies. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Selmi C, Zuin M, Gershwin ME. The unfinished business of primary biliary cirrhosis. J Hepatol. 2008;49:451–460. doi: 10.1016/j.jhep.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, et al. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui M, Nakamura M, Ishibashi H, Koike K, Kudo J, Niho Y. Human monoclonal antibodies from a patient with primary biliary cirrhosis that recognize two distinct autoepitopes in the E2 component of the pyruvate dehydrogenase complex. Hepatology. 1993;18:1069–1077. [PubMed] [Google Scholar]
- 7.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, et al. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lleo A, Zhang W, McDonald WH, Seeley EH, Leung PS, Coppel RL, Ansari AA, et al. Shotgun proteomics: identification of unique protein profiles of apoptotic bodies from biliary epithelial cells. Hepatology. 2014;60:1314–1323. doi: 10.1002/hep.27230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang CY, Ma X, Tsuneyama K, Huang S, Takahashi T, Chalasani NP, Bowlus CL, et al. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology. 2014;59:1944–1953. doi: 10.1002/hep.26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Zhang W, Leung PS, Bowlus CL, Dhaliwal S, Coppel RL, Ansari AA, et al. Ongoing activation of autoantigen-specific B cells in primary biliary cirrhosis. Hepatology. 2014;60:1708–1716. doi: 10.1002/hep.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Qiu J, Yu L, Hu X, Zhao P, Jiang Y. Increased numbers of CD5+CD19+CD1dhighIL-10+ Bregs, CD4+Foxp3+ Tregs, CD4+CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC patients. J Transl Med. 2014;12:251. doi: 10.1186/s12967-014-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamihira T, Shimoda S, Nakamura M, Yokoyama T, Takii Y, Kawano A, Handa M, et al. Biliary epithelial cells regulate autoreactive T cells: implications for biliary-specific diseases. Hepatology. 2005;41:151–159. doi: 10.1002/hep.20494. [DOI] [PubMed] [Google Scholar]
- 13.Shimoda S, Harada K, Niiro H, Shirabe K, Taketomi A, Maehara Y, Tsuneyama K, et al. Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology. 2011;53:1270–1281. doi: 10.1002/hep.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimoda S, Harada K, Niiro H, Taketomi A, Maehara Y, Tsuneyama K, Kikuchi K, et al. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51:567–575. doi: 10.1002/hep.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835–1845. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimoda S, Tsuneyama K, Kikuchi K, Harada K, Nakanuma Y, Nakamura M, Ishibashi H, et al. The role of natural killer (NK) and NK T cells in the loss of tolerance in murine primary biliary cirrhosis. Clin Exp Immunol. 2012;168:279–284. doi: 10.1111/j.1365-2249.2012.04581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudspeth K, Pontarini E, Tentorio P, Cimino M, Donadon M, Torzilli G, Lugli E, et al. The role of natural killer cells in autoimmune liver disease: a comprehensive review. J Autoimmun. 2013;46:55–65. doi: 10.1016/j.jaut.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Chuang YH, Lian ZX, Tsuneyama K, Chiang BL, Ansari AA, Coppel RL, Gershwin ME. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232–240. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Mao TK, Lian ZX, Selmi C, Ichiki Y, Ashwood P, Ansari AA, Coppel RL, et al. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42:802–808. doi: 10.1002/hep.20859. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, Miyakawa H, et al. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–312. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 22.Floreani A, Franceschet I, Cazzagon N. Primary biliary cirrhosis: overlaps with other autoimmune disorders. Semin Liver Dis. 2014;34:352–360. doi: 10.1055/s-0034-1383734. [DOI] [PubMed] [Google Scholar]
- 23.Selmi C, Lleo A, Pasini S, Zuin M, Gershwin ME. Innate immunity and primary biliary cirrhosis. Curr Mol Med. 2009;9:45–51. doi: 10.2174/156652409787314525. [DOI] [PubMed] [Google Scholar]
- 24.Allina J, Hu B, Sullivan DM, Fiel MI, Thung SN, Bronk SF, Huebert RC, et al. T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis. J Autoimmun. 2006;27:232–241. doi: 10.1016/j.jaut.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lleo A, Maroni L, Glaser S, Alpini G, Marzioni M. Role of cholangiocytes in primary biliary cirrhosis. Semin Liver Dis. 2014;34:273–284. doi: 10.1055/s-0034-1383727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selmi C, Meroni PL, Gershwin ME. Primary biliary cirrhosis and Sjogren’s syndrome: autoimmune epithelitis. J Autoimmun. 2012;39:34–42. doi: 10.1016/j.jaut.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuneyama K, Harada K, Kono N, Sasaki M, Saito T, Gershwin ME, Ikemoto M, et al. Damaged interlobular bile ducts in primary biliary cirrhosis show reduced expression of glutathione-S-transferase-pi and aberrant expression of 4-hydroxynonenal. J Hepatol. 2002;37:176–183. doi: 10.1016/s0168-8278(02)00105-8. [DOI] [PubMed] [Google Scholar]
- 29.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223–232. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimoda S, Harada K, Niiro H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y, et al. Biliary epithelial cells and primary biliary cirrhosis: the role of liver-infiltrating mononuclear cells. Hepatology. 2008;47:958–965. doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto N, Shimoda S, Kawanaka H, Tsuneyama K, Uehara H, Akahoshi T, Kinjo N, et al. Modulation of CD4(+) T cell responses following splenectomy in hepatitis C virus-related liver cirrhosis. Clin Exp Immunol. 2011;165:243–250. doi: 10.1111/j.1365-2249.2011.04393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Migliaccio C, Nishio A, Van de Water J, Ansari AA, Leung PS, Nakanuma Y, Coppel RL, et al. Monoclonal antibodies to mitochondrial E2 components define autoepitopes in primary biliary cirrhosis. J Immunol. 1998;161:5157–5163. [PubMed] [Google Scholar]
- 35.Shimoda S, Ishikawa F, Kamihira T, Komori A, Niiro H, Baba E, Harada K, et al. Autoreactive T-cell responses in primary biliary cirrhosis are proinflammatory whereas those of controls are regulatory. Gastroenterology. 2006;131:606–618. doi: 10.1053/j.gastro.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maio M, Altomonte M, Tatake R, Zeff RA, Ferrone S. Reduction in susceptibility to natural killer cell-mediated lysis of human FO-1 melanoma cells after induction of HLA class I antigen expression by transfection with B2m gene. J Clin Invest. 1991;88:282–289. doi: 10.1172/JCI115289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamihira T, Shimoda S, Harada K, Kawano A, Handa M, Baba E, Tsuneyama K, et al. Distinct costimulation dependent and independent autoreactive T-cell clones in primary biliary cirrhosis. Gastroenterology. 2003;125:1379–1387. doi: 10.1016/j.gastro.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Tsuneyama K, Van de Water J, Leung PS, Cha S, Nakanuma Y, Kaplan M, De Lellis R, et al. Abnormal expression of the E2 component of the pyruvate dehydrogenase complex on the luminal surface of biliary epithelium occurs before major histocompatibility complex class II and BB1/B7 expression. Hepatology. 1995;21:1031–1037. [PubMed] [Google Scholar]
- 40.Afford SC, Ahmed-Choudhury J, Randhawa S, Russell C, Youster J, Crosby HA, Eliopoulos A, et al. CD40 activation-induced, Fas-dependent apoptosis and NF-kappaB/AP-1 signaling in human intrahepatic biliary epithelial cells. FASEB J. 2001;15:2345–2354. doi: 10.1096/fj.01-0088com. [DOI] [PubMed] [Google Scholar]
- 41.Takeda K, Kojima Y, Ikejima K, Harada K, Yamashina S, Okumura K, Aoyama T, et al. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci U S A. 2008;105:10895–10900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isse K, Harada K, Zen Y, Kamihira T, Shimoda S, Harada M, Nakanuma Y. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41:506–516. doi: 10.1002/hep.20582. [DOI] [PubMed] [Google Scholar]
- 43.Harada K, Van de Water J, Leung PS, Coppel RL, Ansari A, Nakanuma Y, Gershwin ME. In situ nucleic acid hybridization of cytokines in primary biliary cirrhosis: predominance of the Th1 subset. Hepatology. 1997;25:791–796. doi: 10.1002/hep.510250402. [DOI] [PubMed] [Google Scholar]
- 44.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutterman JU. Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci U S A. 1994;91:1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayres RC, Neuberger JM, Shaw J, Joplin R, Adams DH. Intercellular adhesion molecule-1 and MHC antigens on human intrahepatic bile duct cells: effect of pro-inflammatory cytokines. Gut. 1993;34:1245–1249. doi: 10.1136/gut.34.9.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leavenworth JW, Wang X, Wenander CS, Spee P, Cantor H. Mobilization of natural killer cells inhibits development of collagen-induced arthritis. Proc Natl Acad Sci U S A. 2011;108:14584–14589. doi: 10.1073/pnas.1112188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen N, Odum N, Urso B, Lanier LL, Spee P. Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS One. 2012;7:e31959. doi: 10.1371/journal.pone.0031959. [DOI] [PMC free article] [PubMed] [Google Scholar]