Abstract
Purpose
Amplification and high-levels of NOTCH ligand expression have been identified in several types of pediatric brain tumors. A phase I trial of weekly MK-0752, an oral inhibitor of gamma-secretase, was conducted in children with recurrent central nervous system (CNS) malignancies to estimate the maximum tolerated dose, dose-limiting toxicities (DLT), pharmacokinetics (PK), and pharmacodynamics of weekly MK-0752.
Methods
MK-0752 was administered once weekly at 1,000 mg/m2 and 1,400 mg/m2 using a rolling-6 design. PK analysis was performed during the first course. NOTCH and HES expression was assessed by immunohistochemistry and Western blot.
Results
Ten eligible patients were enrolled (median age 8.8 years; range 3.1–19.2) with diagnoses of brain stem glioma (n=3), ependymoma (n=2), anaplastic astrocytoma (n=1), choroid plexus carcinoma (n=2), medulloblastoma (n=1), and primitive neuroectodermal tumor (n=1). Nine were evaluable for toxicity. One DLT of fatigue occurred in the 6 evaluable patients enrolled at 1,000 mg/m2/dose. No DLTs were experienced by 3 patients treated at 1,400 mg/m2/dose. Non-dose-limiting grade 3 toxicities included lymphopenia, neutropenia, and anemia. Median number of treatment courses was 2 (range 1–10). Two patients continued on therapy for at least 6 months. The median (range) Cmax of MK-0752 was 88.2 μg/mL (40.6 to 109 μg/mL) and 60.3 μg/mL (59.2 to 91.9 μg/mL) in patients receiving 1,000 mg/m2/week and 1,400 mg/m2/week, respectively. NOTCH expression was decreased in 6 of 7 patients for whom tissue was available at 24 hours post-MK-0752.
Conclusion
MK-0752 is well-tolerated and exhibits target inhibition at 1,000 mg/m2/week and 1,400 mg/m2/week in children with recurrent CNS malignancies.
Keywords: Notch, recurrent, brain tumor, pediatric
Introduction
The NOTCH signaling pathway is a highly-conserved receptor-ligand system that plays an integral role in proliferation, survival, apoptosis, and differentiation [1, 2]. Binding of NOTCH ligands (Jagged and Delta) to one of four heterodimeric transmembrane NOTCH receptors induces proteolysis by gamma-secretase, releasing the NOTCH intracellular domain (NICD) [3]. The NICD binds the CBFI/Suppressor of hairless/LAG-1 and promotes transcription of pro-survival genes, such as the hairy enhancer of split (HES) gene families [4]. The Cancer Genome Atlas project recently identified NOTCH as one of the most potentially druggable targets in human cancer [5].
NOTCH signaling plays an important role in neural development through direct regulation of transcriptional effectors of neural stem cell proliferation and self-renewal, including SHH and Wnt [6–8]. NOTCH activation has been implicated in meningioma [9], choroid plexus carcinoma (CPC) [10], medulloblastoma [11], high-grade glioma [12], and ependymoma [13]. Specifically, recent genomic studies have identified NOTCH amplification in ~15% of embryonal tumors [6] and some posterior fossa ependymoma [13]; less commonly somatic mutations of NOTCH3 and NOTCH4 have been reported in medulloblastoma [14]. NOTCH1 mutation in conjunction with loss of P53 appears sufficient to induce formation of SHH-like medulloblastoma in mice [15]. Overexpression of NOTCH receptors and ligands is commonly observed in high-grade glioma [16]. NOTCH-mediated promotion of CD133+ glioblastoma stem cells has been demonstrated in vitro and in vivo, and importantly, gamma-secretase inhibition depletes this stem cell fraction and inhibits tumor growth [17].
MK-0752, a potent oral gamma-secretase inhibitor, prevents cleavage of gamma-secretase substrates with a 50% inhibitory concentration (IC50) of 50 nmol/L [18]. MK-0752 was initially developed as a treatment for Alzheimer’s disease and, in animal models, was shown to penetrate the blood-brain barrier and reduce amyloid peptide levels by 50%. The safety and tolerability of MK-0752 has been studied in refractory adult solid tumors [19] and in combination with docetaxel for advanced breast cancer [20]. MK-0752 toxicities are highly schedule-dependent [19]. Weekly administration was most tolerable and achieved target inhibition at 3,200 mg, providing rationale to study this schedule in children.
Our group previously published a phase I study of MK-0752 in children with recurrent CNS tumors using a 3-days-on/4-days-off dosing schedule, which was well-tolerated at 260 mg/m2 [21]. We now report results of a phase I study of weekly MK-0752. The primary objective was to estimate the maximum tolerated dose (MTD) of weekly MK-0752. The secondary objectives were to describe the dose-limiting toxicities (DLTs) and plasma pharmacokinetics (PK) of weekly MK-0752, to describe NOTCH pathway protein expression in archival tumor tissue, and to assess the pharmacodynamic effects of MK-0752 on peripheral blood mononuclear cells (PBMCs).
Materials and Methods
Patient Eligibility
Eligible patients were > 3 and ≤ 21 years with a Lansky or Karnofsky score ≥ 60 and a histologically verified recurrent malignant CNS tumor. Histology was waived for diffuse intrinsic brainstem glioma patients. Other eligibility criteria included: recovery from acute toxic effects of prior therapy; ≥ 3 weeks since myelosuppressive therapy; ≥ 7 days since growth factor (14 days if long-acting) or biologic agents; ≥ 6 months since craniospinal irradiation; ≥ 2 weeks since local palliative radiotherapy; ≥ 3 months since autologous bone marrow transplant; adequate bone marrow (absolute neutrophil count ≥ 1,000/μL, platelet count ≥ 100,000/μL, hemoglobin ≥ 8.0 g/dL), renal (normal serum creatinine for age or glomerular filtration rate ≥ 70 mL/min/1.73 m2), and liver (total bilirubin ≤ 1.5x and ALT ≤ 2.5x institutional upper limit of normal for age and albumin ≥ 2.5 g/dL) function. Patients on corticosteroids had to be on a stable or decreasing dose for ≥ 2 weeks. Pregnant or lactating females and patients on enzyme-inducing anticonvulsants or systemic anticoagulation were excluded. Patients of childbearing or child fathering potential had to agree to use birth control, including abstinence, while on study. The institutional review boards of participating institutions approved the protocol. Informed consent and assent were obtained according to local institutional guidelines.
Drug Administration and Study Design
MK-0752 (Merck, West Point, PA), supplied as 10-, 50-, and 200-mg dry filled, hard gelatin capsules, was administered orally one time each week. Each course was 28 days. The starting dosage was 1,000 mg/m2 once weekly. Other planned dose levels included 1,400 mg/m2 and 1,800 mg/m2. At least three patients were treated at each dosage level. Patients could receive up to six courses in the absence of disease progression. MK-0752 therapy could be extended for up to 13 additional courses in patients with stable disease after 6 courses. The MTD was defined as the highest dose at which six patients had been treated with no more than one patient experiencing a DLT and the next higher dose level was too toxic [22]. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (version 4.0). Hematologic DLT was defined as any grade 4 neutropenia or thrombocytopenia. Non-hematologic DLT was defined as any grade 3 or 4 toxicity with the specific exclusion of grade 3 nausea or vomiting < 5 days or responsive to antiemetics; grade 3 diarrhea responsive to antidiarrheal agents; grade 3 fever or infection < 5 days; grade 3 hypophosphatemia, hypokalemia, or hypomagnesemia responsive to oral supplementation; or grade 3 ALT/AST elevation that returns to grade ≤ 1 prior to the next scheduled dose.
Pretreatment evaluations included a history, physical examination, performance status, disease evaluation, blood counts, electrolytes, renal and liver function tests, and a pregnancy test for females of childbearing age. Blood counts were obtained weekly during course 1, every 2 weeks during course 2, and before each subsequent course. History, physical examination, and serum chemistries were obtained weekly in course 1 and before each subsequent course. Disease evaluations were obtained at baseline, after course 2, and after every other course thereafter. Tumor response was reported as previously described [21].
Pharmacokinetic Studies
Blood samples (2 mL) were collected in heparinized tubes before the MK-0752 dose and at 1, 2, 3, 6, 8 (±2), and 24 (±4), and 48 hours after dose 1, course 1. The plasma concentrations of MK-0752 were determined using a validated liquid chromatography tandem mass spectrometry method [23].
The concentration-time data for MK-0752 was analyzed using non-compartmental techniques. For each set of serial PK samples, the peak plasma concentration (Cmax) and time to Cmax (Tmax) were determined from the plasma concentration-time profile. The log-linear terminal slope (β) was defined by the last two measurable concentration-time data points in the serial sampling window. Terminal half-life (t1/2) was calculated as t1/2 = ln(2)/β. The area under the plasma concentration versus time curve from time zero to the last measurable sampling time point (AUC0-Tlast) was calculated using the linear-up/log-down trapezoidal rule.
Notch Receptor Expression and Signal Activity
Expression of NOTCH1 (antibody #3608, Cell Signaling Technology, Danvers, MA), HES1 (#5702, Millipore, Billerica, MA), and HES5 (#5708, Millipore) was analyzed in pretreatment formalin-fixed paraffin-embedded tumor sections by immunohistochemistry (IHC) as described previously [21]. Expression of the cleaved NICD (#4147, Cell Signaling), HES1, and HES5 proteins in PBMCs were quantified by Western blot analysis before, and 8 and 24 hours following, treatment with MK-0752. Changes in expression of NOTCH-cleaved forms and NOTCH pathway target genes were correlated with MK-0752 systemic exposure (e.g., Cmax and AUC).
Results
Patient Characteristics
Ten patients, all eligible, were enrolled. One patient was inevaluable for toxicity due to study withdrawal during course 1. The median number of courses was 2 (range 1–10). Table I summarizes the characteristics of the eligible patients. The sponsoring company prematurely withdrew support for this study, focusing on adult combination studies with MK-0752.
Table I.
Characteristics of eligible patients (n = 10)
| Characteristic | |
|---|---|
| Median Age | 8.8 years (3.1 to 19.2) |
| Male | 6 (60%) |
| Female | 4 (40%) |
| Diagnosis | |
| Brainstem glioma | 3 |
| Ependymoma | 2 |
| Anaplastic astrocytoma | 1 |
| Choroid plexus carcinoma | 2 |
| Medulloblastoma | 1 |
| Primitive neuroectodermal tumor | 1 |
| Prior Therapy | |
| Median (range) of prior | 2 (1 to 5) |
| chemotherapy regimens | |
| # with prior radiation | 10 |
| Courses of MK-0752 | |
| Median | 2 |
| Range | 1 to 10 |
Toxicities
Table II summarizes the observed DLTs. At 1,000 mg/m2, 1 of 6 patients had a DLT (grade 3 fatigue). No DLTs occurred at 1,400 mg/m2 (n=3). Table III summarizes all toxicities at least possibly attributable to MK-0752 in evaluable patients. Non-dose-limiting grade 3 toxicities included lymphopenia, neutropenia, and anemia. There were no grade 4 toxicities.
Table II.
Dose-limiting toxicity summary (course 1)
| MK-0752 | No. Entered | No. Evaluable | No. with DLT | Type of DLT(n) |
|---|---|---|---|---|
| 1,000 mg/m2 | 7 | 6 | 1 | Grade 3 Fatigue (1) |
| 1,400mg/m2 | 3 | 3 | 0 |
Table III.
All toxicities at least possibly attributed to therapy in evaluable patients (n=9)
| Toxicity | Maximum grade of toxicity across all cycles (total 29 cycles) | ||
|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |
| Leukopenia | 10 | 2 | |
| Vomiting | 11 | 2 | |
| Lymphopenia | 7 | 3 | |
| Thrombocytopenia | 7 | ||
| Anemia | 1 | 3 | 1 |
| Diarrhea | 4 | ||
| Neutropenia | 2 | 1 | 1 |
| Hypoalbuminemia | 2 | ||
| Hypophosphatemia | 2 | ||
| ALT increase | 2 | ||
| AST increase | 2 | ||
| Hypokalemia | 1 | 1 | |
| Hypomagenesemia | 2 | ||
| Fatigue | 1 | ||
| Hypermagnesemia | 1 | ||
| Anorexia | 1 | ||
| Dizziness | 1 | ||
| Allergic rhinitis | 1 | ||
| Hypotension | 1 | ||
| Dry skin | 1 | ||
| Increased Hemoglobin | 1 | ||
| Hyperglycemia | 1 | ||
| Nausea | 1 | ||
Responses
No objective responses were reported. Prolonged SD (≥ 3 courses) was observed in two patients at 1,000 mg/m2/week, one patient with myxopapillary ependymoma (10 courses) and one patient with CPC (6 courses).
Pharmacokinetics
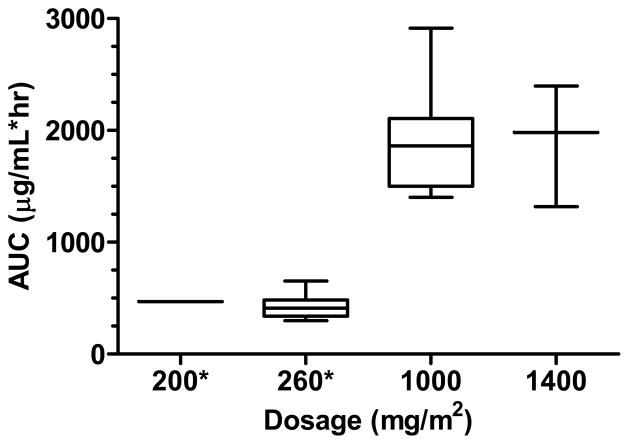
After dose 1, course 1 of MK-0752, PK studies were obtained in 7 patients receiving 1,000 mg/m2 and 3 patients receiving 1,400 mg/m2. The median Cmax and Tmax (range) of MK-0752 for patients treated at 1,000 mg/m2/week were 88.2 μg/mL (40.6 to 109 μg/mL) and 3.0 hours (1 to 8.6 hours), respectively. The median Cmax and Tmax (range) of MK-0752 for patients treated at 1,400 mg/m2/week were 60.3 μg/mL (59.2 to 91.9 μg/mL) and 8.1 hours (6.1 to 24.1 hours), respectively. As depicted in Fig. I, the median (range) AUC0-Tlast values for 1,000 and 1,400 mg/m2/week were 1860 μg/mL*hour (1,400 – 2914 μg/mL*hour) and 1981 μg/mL*hour (1319 – 2397 μg/mL*hour). The median (range) t1/2 observed in all 10 patients was 21.7 hours (9.8 – 42.2 hours).
Fig. I.
Box & whisker plot, 5th–95th percentile of MK-0752 AUC values by dosage from course 1, day 1 in the present study and our previously published results [21] (noted by an asterisk *)
NOTCH1 and HES Protein Expression
Pretrial tumor samples were available from 5 patients, one each with CPC, myxopapillary ependymoma, anaplastic astrocytoma, primitive neuroectodermal tumor (PNET), and medulloblastoma. NOTCH1 expression was detected in 3 of 5 tumors; the myxopapillary ependymoma and medulloblastoma were immunonegative. The PNET had the greatest level of expression detected by IHC. All tumors displayed nuclear expression of HES1, with the lowest and highest percentages of positive nuclei detected in ependymoma (10% of nuclei positive) and PNET (60–100% of nuclei positive), respectively. Expression of HES5 was also less predominant in ependymoma (30% of nuclei positive) and most pronounced in CPC and PNET (60–100% of nuclei positive). These data suggest broad expression of the NOTCH signal pathway in several types of pediatric brain tumors.
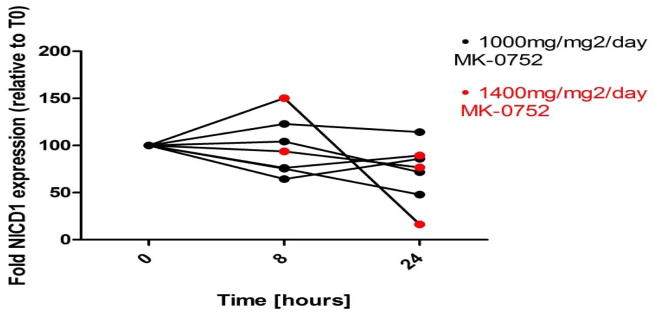
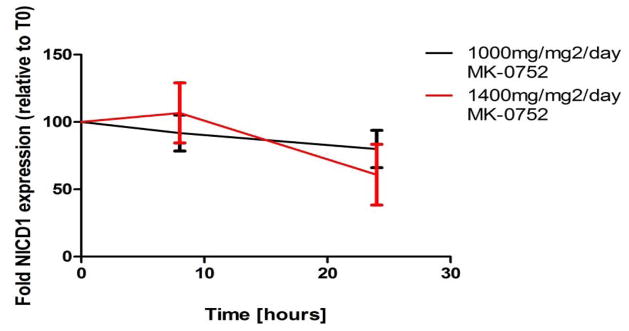
We used Western blotting to measure expression of NICD1 in PBMCs to determine whether MK-0752 blocked NOTCH1 cleavage in vivo. PBMCs were obtained before and at 8 and 24 hours post-MK-0752 in 7 evaluable patients (4 from 1,000 mg/m2 and 3 from 1,400 mg/m2) (Figure II). NICD1 was readily detected in all PBMC pellets. NICD1 expression was decreased in 5 of 7 (71%) patients at 8 hours post MK-0752. Two with higher levels of NICD1 at 8 hours post MK-0752 included one each from the 1,000 mg/m2 and 1,400 mg/m2 dose levels; NICD1 expression in the patient treated at 1,400 mg/m2 subsequently decreased below baseline at 24 hours. NICD1 expression was decreased from baseline in 6 of 7 patients at 24 hours post MK-0752, including all three treated at 1,400 mg/m2 (Figure II).
Fig. II.
Variability for NICD Expression in PBMCs of patients
Discussion
We report a novel dosing schedule of MK-0752 in 10 children with recurrent CNS tumors; the highest dose tested, 1,400 mg/m2, was biologically effective. Though the trial was not completed and an MTD not determined, MK-0752 was well-tolerated, with only one DLT (fatigue) at 1,000 mg/m2 and none at 1,400 mg/m2. Fatigue was seen less frequently than in adults with refractory solid tumors receiving a 3-days-on/4-days-off schedule of MK-0752 [19]. Unlike in adult studies [19, 20], dose-limiting diarrhea and GI toxicity were not observed in this study. As we speculated previously [21], the lower incidence of diarrhea in this study may be attributable to concurrent glucocorticoid therapy, which may protect against intestinal goblet cell metaplasia induced by inhibition of NOTCH signaling [24]. Weekly administration of MK-0752 was better tolerated than the 3-days-on/4-days-off schedule of MK-7052 in children, in which dose-limiting elevations of AST and ALT prevented dose escalation beyond 260 mg/m2 [21].
Though no objective responses were reported, 2 of 9 patients had prolonged SD (6 and 10 cycles), proportionally more than that seen with the 3-days-on/4-days-off schedule of MK-0752 in children in which 2 of 17 evaluable patients experienced prolonged SD (3 and 4 cycles) [21]. However, one patient with prolonged SD in this study had a diagnosis of myxopapillary ependymoma, the natural history of which is more consistent with longer time to recurrence and thus may not indicate MK-0752 effect. In contrast, better responses were seen in adult glioma patients, including one CR > 1 year in a patient with anaplastic astrocytoma (1,800 mg once weekly) and SD > 1 year in a patient with glioblastoma multiforme (4,200 mg once weekly) [19]. Prolonged SD (≥ 4 cycles) was seen in 11 other patients at doses ranging from 600 mg to 3,200 mg, including 9 additional patients with glioma, lending credence to the ability of MK-0752 to cross the blood-brain barrier and mitigate tumor growth. Objective responses and prolonged disease stabilization were also seen in adults with advanced breast cancer treated with MK-0752 (days 1–3) and docetaxel [20]. Of 30 evaluable patients, 9 and 11 exhibited PR and SD, respectively, though the authors acknowledge that responses could be secondary to docetaxel effect alone.
IHC analyses in our study confirmed high-level expression of NOTCH family genes in pediatric brain tumors. The highest baseline level of NOTCH expression was seen in PNET, compatible with relatively frequent amplification of the region containing NOTCH2 in embryonal brain tumors [14, 25]. We detected target inhibition of NICD1 at 24 hours post-MK-0752 treatment in PBMCs from 6 of 7 patients for whom sample was available, including all 3 patients at 1,400 mg/m2/week. In the adult phase I study, target inhibition was seen at weekly MK-0752 doses from 1,800 mg to 4,200 mg, corresponding to 1,040 mg/m2 to 2,400 mg/m2. Though we recommend a biologically effective dose of 1,400 mg/m2/week, it is possible that greater and more prolonged target inhibition may be observed at higher dose levels, which warrants further study. Data from healthy adults suggest once weekly dosing of MK-0752 decreases expression of NOTCH pathway genes for at least 96 hours [26]. In general, we found a dose-dependent decrease in NICD expression in PBMCs at 24 hours after the higher dose (1,400 mg) of MK-0752. Our longest pharmacodynamic time point of 24 hours limits our assessment of the true duration of target inhibition with weekly MK-0752, though our study is more informative than the adult phase I study, which only evaluated target inhibition in hair follicles collected 4–6 hours post-treatment [19].
MK-0752 exhibits linear pharmacokinetics based on a comparison of the median AUC0-Tlast for 1,000 and 1,400 mg/m2 and our prior results, which investigated 200 and 260 mg/m2 on the 3-days-on/4-days-off schedule (Figure I). The median t1/2 (21.7 hours) in this study is longer than 11.4 hours from our previously published results [21]. A phase I study in adults with advanced solid tumors reported PK values for weekly MK-0752 at numerous dose levels. The t1/2 of 21.7 hours observed in the present study is longer than 13.8 hours and 16.2 hours reported in adults given doses of 1,800 (~1,040 mg/m2) and 2,400 mg (~1,400 mg/m2), respectively. The geometric mean Cmax in children receiving 1,000 mg/m2 in the present study is 75.2 μg/mL, which is similar to 84.2 μg/mL reported in adults receiving 1,800 mg (~1,040 mg/m2), suggesting similar MK-0752 exposure when given weekly in children and adults [19].
Crosstalk between NOTCH and other important cancer signaling pathways provides an avenue by which rational combination of targeted agents may potentiate the effect of MK-0752 or other gamma-secretase inhibitors in development (e.g. RO4929097 and PF-03084014). NOTCH influences a number of signaling pathways in a context-dependent manner, including several that are strongly implicated in pediatric brain tumors, such as Wnt/TCF [27, 28], SHH [29], RAF/RAS/MEK/ERK [30], and PI3K/Akt/mTOR [31]; the latter holds the greatest potential for rapid clinical translation. Zhao et al. demonstrated strong positive regulation of NOTCH1 on Akt/mTOR pathways in glioblastoma cells in vitro [32]. RNA interference of NOTCH1 diminished phosphorylated Akt and suppressed downstream activation of mTOR, inhibiting glioblastoma cell growth and inducing caspase-mediated apoptosis. Jin et al. further exemplified the interplay of these pathways by evaluating the individual and combined effects of MRK003, a less potent preclinical analog of MK-0752, and Akt inhibitor MK-2206 in glioblastoma cell lines [33]. Combination treatment demonstrated synergistic inhibition of the migratory and invasive potential of glioblastoma and enhanced inhibition of Akt phosphorylation compared to monotherapy. An adult study investigating the combination of mTOR inhibition (Ridaforolimus) with MK-0752 is ongoing (NCT01295632). Multiple inhibitors of the Akt/mTOR pathway have been deemed tolerable and effective in children [34, 35], leaving open the possibility of investigating this combination in the pediatric population.
Overexpression of NOTCH receptors and targets has been associated with chemotherapy resistance in solid tumors [28, 36], suggesting utility for gamma secretase inhibition in combination with conventional chemotherapeutic agents. Though resistance is not common in newly diagnosed brain tumors, recurrent tumors are notoriously more resistant to standard therapy. McAuliffe et al. demonstrated Notch-mediated cisplatin resistance in an ovarian carcinoma xenograft model, which was overcome by siRNA NOTCH inhibition [37]. Groeneweg et al. reported synergy between MRK-003 (analog of MK-0752) and paclitaxel in a platinum-resistant ovarian carcinoma xenograft model [38]. Hiddingh et al. also demonstrated the ability of NOTCH inhibition to induce temozolomide sensitivity in glioblastoma in vitro and in vivo [39], work which led to an ongoing clinical trial of gamma-secretase inhibitor RO4929097 and temozolomide in adult patients with glioblastoma (NCT01119599).
This study demonstrates tolerability, favorable pharmacokinetics, and target inhibition of weekly MK-0752 in children with recurrent brain tumors at both dose levels studied. The study was prematurely closed due to industry support withdrawal; however, MK-0752 was well-tolerated at the highest dose level tested (1,400 mg/m2 weekly), and target inhibition in PBMCs was uniformly noted, suggesting that a biologically effective dose was achieved. In contrast to the lack of objective responses reported in this study, objective responses were reported in adults with gliomas receiving doses in a similar range once weekly, including several with aggressive phenotypes [19]. Preclinical work highlighting complex interactions between NOTCH and other cancer pathways suggests that gamma secretase inhibitors, such as MK-0752, in combination with targeted agents (e.g. mTOR/Akt inhibitors), or chemotherapeutic agents (e.g. temozolomide) could be explored in phase I trials.
Fig. III.
Mean data points for NICD expression in PBMCs
Acknowledgments
This study was funded in part by the National Institutes of Health Grant No. U01 CA81457 for the Pediatric Brain Tumor Consortium and by the American Lebanese Syrian Associated Charities.
Maryam Fouladi received research funding by Merck.
Footnotes
Compliance with Ethical Standards
Informed consent: Informed consent was obtained from all individual participants included in the study.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 2.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 3.Lu P, Bai X-C, Ma D, et al. Three-dimensional structure of human γ-secretase. Nature. 2014;512:166–170. doi: 10.1038/nature13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonon G, Modi S, Wu L, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, McGee J, Chen X, et al. Identification of druggable cancer driver genes amplified across TCGA datasets. PLoS ONE. 2014;9:e98293. doi: 10.1371/journal.pone.0098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan X, Mikolaenko I, Elhassan I, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 7.Hatakeyama J, Sakamoto S, Kageyama R. Hes1 and Hes5 regulate the development of the cranial and spinal nerve systems. Dev Neurosci. 2006;28:92–101. doi: 10.1159/000090756. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Hibbs MA, Gard AL, et al. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells. 2012;30:741–752. doi: 10.1002/stem.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuevas IC, Slocum AL, Jun P, et al. Meningioma transcript profiles reveal deregulated Notch signaling pathway. Cancer Res. 2005;65:5070–5075. doi: 10.1158/0008-5472.CAN-05-0240. [DOI] [PubMed] [Google Scholar]
- 10.Beschorner R, Waidelich J, Trautmann K, et al. Notch receptors in human choroid plexus tumors. Histol Histopathol. 2013;28:1055–1063. doi: 10.14670/HH-28.1055. [DOI] [PubMed] [Google Scholar]
- 11.Fiaschetti G, Schroeder C, Castelletti D, et al. NOTCH ligands JAG1 and JAG2 as critical pro-survival factors in childhood medulloblastoma. Acta Neuropathol Commun. 2014;2:39. doi: 10.1186/2051-5960-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristoffersen K, Villingshøj M, Poulsen HS, Stockhausen M-T. Level of Notch activation determines the effect on growth and stem cell-like features in glioblastoma multiforme neurosphere cultures. Cancer Biol Ther. 2013;14:625–637. doi: 10.4161/cbt.24595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Jones DTW, Jäger N, Kool M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natarajan S, Li Y, Miller EE, et al. Notch1-induced brain tumor models the sonic hedgehog subgroup of human medulloblastoma. Cancer Res. 2013;73:5381–5390. doi: 10.1158/0008-5472.CAN-13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 17.Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LoRusso P, Demuth TEH. Phase I study of the gamma secretase inhibitor MK-0752 in patients with metastatic breast and other advanced solid tumors. 100th Annual Meeting of the American Association for Cancer Research; Denver, CO. April 18–22, 2009; p. abstr 3605. [Google Scholar]
- 19.Krop I, Demuth T, Guthrie T, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30:2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- 20.Schott AF, Landis MD, Dontu G, et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res. 2013;19:1512–1524. doi: 10.1158/1078-0432.CCR-11-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fouladi M, Stewart CF, Olson J, et al. Phase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Clin Oncol. 2011;29:3529–3534. doi: 10.1200/JCO.2011.35.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skolnik JM, Barrett JS, Jayaraman B, et al. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26:190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 23.Bai F, Tagen M, Colotta C, et al. Determination of the gamma-secretase inhibitor MK-0752 in human plasma by online extraction and electrospray tandem mass spectrometry (HTLC-ESI-MS/MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2348–2352. doi: 10.1016/j.jchromb.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Real PJ, Tosello V, Palomero T, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackman SC, Podtelezhnikov A, Railkar RA. Notch pathway inhibition with MK-0752 leads to dose- and time-dependent transcriptional alterations in proliferation, PI3K, and Wnt pathway genes in plucked human hair follicles. Proc Am Assoc for Cancer Res. 51:abst 26. [Google Scholar]
- 27.Balint K, Xiao M, Pinnix CC, et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest. 2005;115:3166–3176. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang T, Holt CM, Xu C, et al. Notch3 activation modulates cell growth behaviour and cross-talk to Wnt/TCF signalling pathway. Cell Signal. 2007;19:2458–2467. doi: 10.1016/j.cellsig.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Brechbiel J, Miller-Moslin K, Adjei AA. Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat Rev. 2014;40:750–759. doi: 10.1016/j.ctrv.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Tremblay I, Paré E, Arsenault D, et al. The MEK/ERK pathway promotes NOTCH signalling in pancreatic cancer cells. PLoS ONE. 2013;8:e85502. doi: 10.1371/journal.pone.0085502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hales EC, Taub JW, Matherly LH. New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia. Cell Signal. 2014;26:149–161. doi: 10.1016/j.cellsig.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Zhao N, Guo Y, Zhang M, et al. Akt-mTOR signaling is involved in Notch-1-mediated glioma cell survival and proliferation. Oncol Rep. 2010;23:1443–1447. doi: 10.3892/or_00000782. [DOI] [PubMed] [Google Scholar]
- 33.Jin R, Nakada M, Teng L, et al. Combination therapy using Notch and Akt inhibitors is effective for suppressing invasion but not proliferation in glioma cells. Neurosci Lett. 2013;534:316–321. doi: 10.1016/j.neulet.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Fouladi M, Perentesis JP, Phillips CL, et al. A phase I trial of MK-2206 in children with refractory malignancies: a Children’s Oncology Group study. Pediatr Blood Cancer. 2014;61:1246–1251. doi: 10.1002/pbc.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fouladi M, Laningham F, Wu J, et al. Phase I study of everolimus in pediatric patients with refractory solid tumors. J Clin Oncol. 2007;25:4806–4812. doi: 10.1200/JCO.2007.11.4017. [DOI] [PubMed] [Google Scholar]
- 36.Gu F, Ma Y, Zhang Z, et al. Expression of Stat3 and Notch1 is associated with cisplatin resistance in head and neck squamous cell carcinoma. Oncol Rep. 2010;23:671–676. doi: 10.3892/or_00000683. [DOI] [PubMed] [Google Scholar]
- 37.McAuliffe SM, Morgan SL, Wyant GA, et al. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA. 2012;109:E2939–48. doi: 10.1073/pnas.1206400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groeneweg JW, DiGloria CM, Yuan J, et al. Inhibition of notch signaling in combination with Paclitaxel reduces platinum-resistant ovarian tumor growth. Front Oncol. 2014;4:171. doi: 10.3389/fonc.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiddingh L, Tannous BA, Teng J, et al. EFEMP1 induces γ-secretase/Notch-mediated temozolomide resistance in glioblastoma. Oncotarget. 2014;5:363–374. doi: 10.18632/oncotarget.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]