Abstract
Neuropathological studies of human traumatic brain injury (TBI) cases have described amyloid plaques acutely after a single severe TBI, and tau pathology after repeat mild TBI (mTBI). This has helped drive the hypothesis that a single moderate to severe TBI increases the risk of developing late-onset Alzheimer’s disease (AD), while mTBI increases the risk of developing chronic traumatic encephalopathy (CTE). In this review we critically assess this position—examining epidemiological and case-control human studies, neuropathological evidence, and preclinical studies.
Epidemiological studies emphasize that TBI is associated with the increased risk of developing multiple types of dementia, not just AD-type dementia, and that TBI can also trigger other neurodegenerative conditions such as Parkinson’s disease. Further, human post-mortem studies on either single TBI and repeat mTBI can show combinations of amyloid, tau, TDP-43, and Lewy body pathology indicating that the neuropathology of TBI is best described as a ‘polypathology’. Preclinical studies confirm that multiple proteins associated with the development of neurodegenerative disease accumulate in the brain after TBI.
The chronic sequelae of both single TBI and repeat mTBI share common neuropathological features and clinical symptoms of classically defined neurodegenerative disorders. However, while the spectrum of chronic cognitive and neurobehavioral disorders that occur following repeat mTBI are viewed as the symptoms of CTE, the spectrum of chronic cognitive and neurobehavioral symptoms that occur after a single TBI is considered to represent distinct neurodegenerative diseases such as AD. These data support the suggestion that the multiple manifestations of TBI-induced neurodegenerative disorders be classified together as traumatic encephalopathy or trauma-induced neurodegeneration, regardless of the nature or frequency of the precipitating TBI.
Keywords: Traumatic brain injury (TBI), chronic traumatic encephalopathy (CTE), Alzheimer’s disease (AD), amyloid (Aβ), tau, tauopathy, dementia
Introduction
Traumatic brain injury (TBI) increases the likelihood of developing dementia later in life, including Alzheimer’s disease (AD). Current theories about what drives the development of dementia after TBI are largely based on observations of AD-associated amyloid and tau pathologies in the brain after injury, as the presence of these hallmark pathologies provides a potential pathological link. However, these pathologies are only found in a subset of patients after TBI and there is little clinical or preclinical evidence supporting a direct link between these pathological changes, particularly when observed acutely after TBI, and development of dementia later in life.
In this review we will first summarize epidemiological studies of dementia after TBI to emphasize the current understanding that TBI is associated with increased risk of developing multiple types of dementia, not just AD, and highlight potential factors that may increase an individual’s risk of dementia after TBI. Second, we will critically examine previous studies of amyloid and tau pathologies in the brain after TBI in humans and animals in order to similarly identify factors that may explain why these neurodegenerative pathologies are observed in only a subset of patients after injury. As it remains unclear how neuropathological findings of TBI relate to the reported increased risk of dementia and AD, we will speculate on the potential relevance of these pathologies in the brain after TBI and whether there are similarities between factors influencing pathology and dementia development after injury. Finally, we will highlight the emerging hypothesis that while neuropathological features and clinical symptoms following TBI may overlap those observed in classically defined neurodegenerative disorders, these may be signs of TBI-induced neurodegeneration, or traumatic encephalopathy, as opposed to the development of a specific neurodegenerative disease.
TBI as a risk factor for dementia
While the detrimental effects of repeat mild TBI (mTBI) in sports has been known since the 1920’s (Martland, 1928), the association between single TBI and dementia is still an active topic of research. Up to 4 million TBIs occur annually in the United States (Langlois et al., 2006), with the vast majority being mTBI. The acute and chronic symptoms of head trauma have been historically documented. In 1927 the existence of chronic postconcussion symptoms in a cohort of over 100 clinical cases was termed “traumatic encephalitis” (Osnato and Giliberti, 1927). The “punch-drunk” symptoms of clumsiness, ataxia and disorientation found in professional boxers were described shortly thereafter in 1928 (Martland, 1928). While most cases described by Martland were mild, the severe cases were described with what we would now call parkinsonism-type symptoms and dementia (Martland, 1928). The term “dementia pugilistica” was first used in 1937 to describe these stereotypical symptoms in boxers (Millspaugh, 1937), and “chronic post-traumatic encephalopathy” has been in use since the late 1950s (McCown, 1959). Chronic traumatic encephalopathy (CTE) is now used by the medical field to describe the neuropathological changes that occur as a result of repeat concussive or subconcussive blows to the head as this diseased has since been described in non-boxers.
To date, there has been approximately 160 pathological descriptions of CTE in the brains of boxers, athletes, soldiers and civilians with a history of repeat mTBI (reviewed in (Smith et al., 2013)). While this work has helped described the neuropathology of CTE, many questions still remain and the clinical presentation of CTE has yet to be fully determined. The patient history of those with pathologically confirmed CTE show that they can present with a multi-faceted clinical and pathological presentation with aspects of Alzheimer’s disease (AD), frontotemporal dementia (FTD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) (McKee et al., 2013).
The question of whether a single moderate-to-severe TBI triggers the development of late-onset dementia remains somewhat controversial. AD accounts for 60–80% of all dementias and the focus of most studies on TBI and dementia risk have focused specifically on the development of AD after injury. Several retrospective and prospective trials have been conducted, and a significant number report that there is no effect of TBI on the development of AD (Shalat et al., 1987; Katzman et al., 1989; Broe et al., 1990; Fratiglioni et al., 1993; Tsolaki et al., 1997). Conversely, there are many reports that find a positive interaction between brain trauma and AD, with reported relative risk varying from two (O'Meara et al., 1997) to fourteen (Rasmusson et al., 1995). To date, two in-depth meta-analyses have been conducted (Mortimer et al., 1991; Fleminger et al., 2003). The first analyzed 11 case-controlled studies (Mortimer et al., 1991), the second analyzed 15 case-controlled studies (Fleminger et al., 2003). Consistent from these studies was the finding that TBI increased the risk of developing AD by 58–82% and that males, but not females, were at increased risk of developing AD after TBI (Mortimer et al., 1991; Fleminger et al., 2003). More focused studies on gender disparities following TBI are required as it remains unclear why females have a reduced risk of developing AD after TBI, especially given their general increased risk of AD compared to males (Bachman et al., 1992).
Brain injury has also been shown to lead to the development of non-AD dementias. A study of male World War II Navy and Marine veterans, 548 with a medically confirmed record of TBI and 1228 with non-head injury type wounds or infection, assessed patients for AD and other dementias 50 years after their injuries and found that both moderate and severe TBI significantly increased the risk of developing either late-onset AD or non-AD dementia. (Plassman et al., 2000). The increased risk of AD or non-AD dementia was identical after TBI, clearly showing that a single moderate/severe TBI earlier in life can increase the risk of multiple types of late-onset dementia, and not just AD (Plassman et al., 2000).
This view of TBI as a risk factor for multiple types of dementia is supported in another retrospective cohort study (Gardner et al., 2014). Gardner and colleagues examined the association between TBI and the development of dementia in 164,661 patients over the age of 55 years-old with a history of TBI or non-brain trauma in the prior 5–7 years. Their search criteria of the California State Inpatient Databases and State Emergency Department Databases included diagnoses of AD, Pick’s disease or FTD and the authors demonstrate that moderate to severe TBI can increase the risk of dementia for all patients 55 and older with a minimum hazard ratio of 1.3 (Gardner et al., 2014). Similarly, the view that TBI can increase specific non-AD dementias is enhanced by studies that focus specifically on FTD risk after TBI. A retrospective case-control study of 80 FTD patients and 124 controls demonstrated that TBI increases the risk of developing FTD with an odds ratio of 3.3 (Rosso et al., 2003). A larger retrospective case-control study of 845 veterans found an association between TBI and FTD with an odds ratio of 4.4 (Kalkonde et al., 2012) – however it should be noted that this same study did not find an association between TBI and AD. Finally, a large retrospective cohort study of 147,510 patients in the Taiwanese Longitudinal Health Insurance Database found that those patients with a fractured skull and intracranial injury had a 4.13 times greater risk of developing FTD in the four years following TBI compared to non-brain injured controls (Wang et al., 2015). This was especially prominent in those under the age of 65, who had at least a 6-fold greater risk of developing FTD compared to age matched controls (Wang et al., 2015).
Potential factors influencing dementia risk after TBI
The risk of dementia following TBI is altered by several external and internal factors including injury severity, survival time, patient age at time of injury, genotype, and cognitive reserve. The World War II Navy and Marine veteran study found an injury severity-dependent association of TBI with dementia. While no risk was associated with mTBI occurring 50 years prior to injury, moderate TBI increased the risk of AD or other dementias by two-fold and severe TBI increased risk by four-fold (Plassman et al., 2000). Due to the length of time between the recorded injury and dementia onset in the Plassman study, it may be important to striate the patient population when examining the risk of dementia using the patient age at time of injury as a variable. Indeed, data shows that the risk of AD increases as the time between the last brain injury event and the onset of disease symptoms diminishes (Graves et al., 1990). This is confirmed in a meta-analysis showing that when TBI occurs more than 10 years prior to disease onset the relative risk of AD is 1.63, but when the TBI occurs within 10 years of disease onset the relative risk increases to 5.33 (Mortimer et al., 1991). This age-discrimination effect is also apparent with mTBI, as Gardner and colleagues report that mTBI did not increase the risk of dementia in patients aged under the age of 65, however there was a 20% increase in the risk of dementia for mTBI patients aged 65 and older (Gardner et al., 2014).
Independent internal risk factors for neurodegenerative disease can also combine with TBI to increase the overall risk of developing dementia. The APOE4 gene alone increases the risk of developing AD (Corder et al., 1993) and the synergistic interaction of TBI and APOE4 together increases the risk of developing AD above predicted levels (Mayeux et al., 1995), however this remains controversial (O'Meara et al., 1997) and more study in this area is required. A second internal modifier of dementia risk after TBI involves the concept of cognitive reserve. The protective effect of cognitive reserve is seen in the increased risk of AD in those with low levels of education (Caamano-Isorna et al., 2006) and cognitive reserve may also predict long-term outcome after TBI. In a 30-year follow up of Vietnam veterans with a history of penetrating injury, a lower pre-injury intelligence was predictive of chronic cognitive decline after trauma (Raymont et al., 2008). This is an interesting area that requires more exploration.
While TBI is associated with the development of dementia, TBI is also associated with the risk of developing neurodegenerative diseases outside the umbrella of dementia. A meta-analysis of 22 case studies revealed that TBI increased the risk of PD by 57% (Jafari et al., 2013). This is confirmed in a retrospective cohort study of 165,799 patients aged over 55 with a history of TBI 5–7 years prior to disease onset that demonstrated a 44% increase in PD risk after TBI (Gardner et al., 2015). As this retrospective cohort is essentially the same group used to look for dementia risk (Gardner et al., 2014), we can determine that TBI is a risk factor for a diverse group of neurodegenerative diseases.
In light of the emerging evidence it is apparent that TBI should be viewed as a risk factor for multiple types of neurodegenerative disease. The question becomes whether we should view TBI as a trigger for individual neurodegenerative disease, or if we should view the chronic clinical symptoms of TBI as an umbrella disease with multiple possible manifestations.
Pathological protein accumulation after TBI – seeds of a disease or evidence of cerebral dysfunction?
A common factor between different neurodegenerative disorders is abnormal aggregation, misfolding and/or accumulation of proteins in the brain. Amyloid-beta (Aβ) plaques and p-tau tangles are the hallmark proteinopathies of AD (Hardy and Selkoe, 2002), α-synuclein accumulates in PD (Polymeropoulos et al., 1997), and transactive response DNA-binding protein 43 kDa (TDP-43) accumulates in FTD and ALS (Neumann et al., 2006). While the role of these proteins in the initiation of disease is still under debate, it is clear that this abnormal accumulation is indicative of abnormal cellular processes and cerebral dysfunction. A unifying feature of acute and chronic pathology after TBI or repeat mTBI is the abnormal accumulation of pathological proteins related to neurodegenerative disease. Acutely TBI brains can present with Aβ, p-tau, and α-synuclein pathology (Uryu et al., 2007). Chronically TBI brains can present with Aβ, p-tau, TDP-43 and α-synuclein pathology (Johnson et al., 2012; McKee et al., 2013). In this review we will focus on Aβ and tau pathologies after TBI.
Aβ pathology after TBI
Aβ accumulation and deposition into plaque after TBI has been widely studied in both humans and animal models, providing insight into the common but complex neurodegenerative pathways that can be triggered after TBI. The presence of Aβ plaques in the brain acutely after severe TBI was first reported in a small post-mortem study by Roberts et al. in 1991 (Roberts et al., 1991). Analysis of 16 TBI cases with survival of 6–18 days post-injury found deposition of diffuse Aβ-positive plaques in 38% of injured brains. While TBI cases ranging in age from 10–63 years-old were analyzed, 4 of the 6 plaque-positive cases were older than 50 years old (Roberts et al., 1991), an age range at which plaques can occur in the normally aging population (Braak and Braak, 1997). This study was repeated with a much larger cohort of TBI cases in 1994 (Roberts et al., 1994). In this analysis of 152 post-mortem TBI brains from people aged 8 weeks to 85 years old and surviving 4h-2.5 years after injury, a total of 46 cases (30%) were positive for Aβ deposition. When teased apart, there was a 20% incidence of amyloid plaque in the brains aged less than 50, and 60% of the TBI brains aged 51–60, compared to 0% of controls. Both control and TBI brains above the age of 60 displayed amyloid deposits, but the TBI brains always had a higher incidence. These data indicate that those above the age of 50 years-old may be more susceptible to amyloid deposition after TBI (Roberts et al., 1994).
While these post-mortem studies demonstrate the incidence of plaque formation following TBI, a small study on surgically resected tissue from living patients demonstrated just how fast this plaque deposition occurs (Ikonomovic et al., 2004). 18 living TBI patients suffering from severe TBI were examined, and Aβ plaque deposition was found in cortical tissue from 6 patients (33%). Three of these six patients were in their 30’s, again indicating that TBI can reduce the age at which amyloid accumulation is normally observed. Further, with the time between injury and surgery ranging from only 2–12h post-TBI, this study demonstrates just how rapidly Aβ can accumulate and aggregate after TBI (Ikonomovic et al., 2004).
Aβ pathology has also been detected in long-term survivors of TBI. Post-mortem analysis of brains from patients surviving 1–47 years after a single TBI again identified plaques in approximately 30% of long-term survivors (Johnson et al., 2012). While Aβ plaques were also found in 30% of control cases, a comparison of plaque density and Thioflavin-S staining revealed a trend towards greater plaque density in the brains of long-term TBI survivors and the appearance of more fibrillar, Thioflavin-S positive Aβ plaques (Johnson et al., 2012).
These neuropathological studies are invaluable to our understanding of Aβ deposition in the brain after TBI and have provided the scientific basis for current studies investigating Aβ as a potential biomarker for brain trauma. However, a major limitation of neuropathological studies is that they can only provide a snapshot of what is occurring in the brain. The development of amyloid-imaging tracers for positron emission tomography (PET) which have had success in imaging amyloid in AD patients may provide the type of temporal resolution that has been previously impossible in TBI patients. Comparison of an Aβ-binding agent called Pittsburgh compound B (PiB) (Wu et al., 2005) in 15 moderate and severe TBI patients and 11 controls revealed increased PiB-binding in the cortical gray matter and striatum of TBI patients when imaged less than 1 year post-injury (Hong et al., 2014). Future longitudinal Aβ-imaging in TBI patients will be able to address questions about the fate of TBI-induced Aβ plaque—including whether acute plaques are cleared from the brain, and whether TBI patients are more susceptible to early or more aggressive amyloid deposition as they age.
While only approximately 30% of severe TBI brains present with acute amyloid plaques, the study of cortical tissue resected from 18 living TBI patients found increased intracellular staining of Aβ in 80% cases (Ikonomovic et al., 2004), suggesting that intracellular accumulation of non-plaque species of Aβ is more common than plaque deposition after TBI. This is also true for accumulation of Aβ in axons after TBI, which occurs in a majority of injured brain samples, regardless of the presence or absence of plaque (Smith et al., 2003b; Chen et al., 2009). Prefibrillar, but aggregating, amyloid species have been detected in TBI patients, with high molecular weight Aβ oligomers detected in the cerebrospinal fluid of a subgroup of TBI patients within 72 hours of injury (Gatson et al., 2013). Together, these data suggest that TBI causes increased accumulation of Aβ peptides in the brain, but aggregation and deposition only occurs in a subset of patients.
One potential source of abnormal Aβ accumulation after TBI is increased production in the traumatized axon (Uryu et al., 2007; Chen et al., 2009). APP accumulates along the lengths of damaged axons in areas of diffuse axonal injury in humans up to 3 years post-injury, and co-localizes with β-secretase (BACE1) and the presenilin subunit (PS1) of γ-secretase, proteins essential for cleavage of APP into Aβ (Chen et al., 2009). Comparison of antibodies recognizing Aβ40 or Aβ42 found that the species accumulating in the axonal bulbs of injured brains is Aβ42 (Uryu et al., 2007). Aβ accumulation after injury has also been reproduced in animal models of TBI by several groups (Chen et al., 2004; Conte et al., 2004; Abrahamson et al., 2006; Abrahamson et al., 2009; Loane et al., 2009; Laskowitz et al., 2010; Tran et al., 2011a; Washington et al., 2013) and these studies have proven useful for investigating the source of increased Aβ after TBI. Levels of the proteins involved in Aβ production (BACE1, PS1 and APP) are increased in the injured cortex after TBI, and follow a timecourse similar to that of Aβ (Loane et al., 2009; Washington et al., 2014). Preclinical studies confirm that damaged axons are a hotspot of amyloid production after rotational injury in swine (Chen et al., 2004), and cortical impact in mice (Tran et al., 2011d; Washington et al., 2014), and targeting the APP secretase enzymes can prevent the increase in Aβ after TBI (Loane et al., 2009; Winston et al., 2013) indicating that increased production of Aβ after TBI is the driver of acute amyloid accumulation after injury.
Potential factors influencing Aβ pathology after TBI
Given that human and preclinical evidence points to increased production of Aβ after TBI, it is somewhat confusing that amyloid plaques are not observed in the majority of human TBI post-mortem brains (70%) (Roberts et al., 1991; Roberts et al., 1994; Ikonomovic et al., 2004). This suggests that there are factors influencing Aβ accumulation in the brain after TBI. Two potential factors mentioned earlier are age at time of injury and length of survival, as the incidence of Aβ plaques is higher after acute severe TBI in older patients (Roberts et al., 1994), and longer survival appears to lead to greater extent and more mature Aβ pathology (Johnson et al., 2012). Another potential factor is genetics. Polymorphisms in the gene for neprilysin, an enzymatic protein associated with degradation of Aβ in the brain (Iwata et al., 2000) have been shown to effect Aβ plaque deposition acutely after TBI, with extended GT repeats in the neprilysin allele associated with increased risk of Aβ plaques, and a separate polymorphism associated with decreased risk of plaques after TBI (Johnson et al., 2009). In long-term survivors of TBI, neprilysin accumulates in axons and in the same axonal bulbs as APP and Aβ - indicating that it may be important for the local clearance of TBI-induced Aβ (Chen et al., 2009). Furthermore, levels of neprilysin are reduced in patients with traumatic encephalopathy compared to non-demented controls (Kokjohn et al., 2013).
A second protein known to be involved in Aβ clearance from the brain is apolipoprotein E. Polymorphisms in this proteins gene results in three common alleles: APOE2, APOE3 and APOE4, with the APOE4 allele associated with impaired Aβ clearance from the brain (Deane et al., 2008; Jiang et al., 2008). Retrospective analysis of cases from the Roberts et al 1994 study found increased incidence of Aβ plaque deposition acutely after TBI in APOE4 carriers (Nicoll et al., 1995). This occurred in an APOE4 allele dose-dependent manner, so that while plaques were found in only 10% of those with no APOE4 allele (5 of 50 cases), Aβ plaques were found in 35% of those heterozygous for APOE4 (12 of 34 cases) and 100% of those homozygous for APOE4 (6 of 6 cases) (Nicoll et al., 1995). Similarly, in mice expressing human APOE isoforms there is increased amyloid deposition, including fibrillar Aβ deposits in PDAPP/APOE4 mice 3 months after TBI compared to PDAPP/APOE3 mice (Hartman et al., 2002), and greater intracellular Aβ accumulation occurs in PDAPP/APOE4 from 1 week to 12 weeks post-injury compared to PDAPP/APOE3 mice (Laskowitz et al., 2010). Together these data demonstrate that polymorphisms in two genes associated with Aβ clearance may alter removal of TBI-induced Aβ and predispose certain individuals to greater Aβ accumulation and aggregation in the brain acutely after injury.
Lastly, it is important to note that there are similarities and differences between the plaques described in the brain after TBI and in AD. Similar to the composition of plaques in AD, Aβ42 has been identified as the primary Aβ species in amyloid deposits in TBI brains (Gentleman et al., 1997; Ikonomovic et al., 2004). However, the plaques found in the brain acutely after TBI are more diffuse than the dense-cored neuritic plaque, or senile plaque, found in advanced-staged AD (Roberts et al., 1991; Roberts et al., 1994; Ikonomovic et al., 2004) – indicating that the plaques deposited after TBI are a rapidly and recently formed aggregate. While neuritic plaques have been found in a few TBI brains, these were restricted to the oldest cases in the study of acute single TBI (Roberts et al., 1994) and Thioflavin-S staining for beta-sheet structure in aggregated plaques was found in only one of 18 acute cases (Ikonomovic et al., 2004). Again, this may be a factor of time, either from increased age at injury or increased survival time, as more fibrillar, Thioflavin-S positive plaques were seen following long-term survival after TBI (Johnson et al., 2012).
Tau pathology after TBI
Tau pathology has also been studied after acute and chronic survival following single TBI. Evidence of tau phosphorylation at the advanced Ser396/Ser404 epitope is seen in axons and white matter of excised TBI brain tissue within 24h of injury, but somatodendritic p-tau staining is rare (Ikonomovic et al., 2004), suggesting that hyperphosphorylation, but not tangle formation, occurs acutely after TBI. Sporadic cases of p-tau immunoreactivity have been reported in acute postmortem TBI brains, but only in 11% of cases (Uryu et al., 2007). Tau-positive glia also occur in up to 20% of severe TBI post-mortem brains (Smith et al., 2003a; Uryu et al., 2007). More recently the appearance of tau pathology has been examined in 39 severe TBI brains with survival times of 1–47 years after injury, and compared to 47 control brains (Johnson et al., 2012). In TBI brains aged below 60 years-old, 34% of TBI brains presented with tau pathology compared to only 9% of controls. The distribution of tau pathology in TBI brains was different to that seen in control brains with abnormal tau staining in the sulcal depths and superficial layers of the cortex (Johnson et al., 2012). Tau pathology in TBI brains was widespread and could be observed in the cingulate gyrus, superior frontal gyrus, and insular cortex, but pathology in control brains was limited to the entorhinal cortex and hippocampus (Johnson et al., 2012).
Most reports of tau pathology after TBI have been in repeat mTBI brains with CTE. Suggesting that repeat exposure may be a potential factor influencing development of tau pathology after TBI. While the majority of historical reports have focused on boxers (Constantinidis and Tissot, 1967; Corsellis et al., 1973; Allsop et al., 1990; Dale et al., 1991; Tokuda et al., 1991; Hof et al., 1992; Geddes et al., 1996; Geddes et al., 1999; Schmidt et al., 2001; Areza-Fegyveres et al., 2007; McKee et al., 2009; Saing et al., 2012; McKee et al., 2013; McKee et al., 2015), in the last 5 years there have been multiple cases of tau pathology in players of impact sports (McKee et al., 2009; Omalu et al., 2011; Goldstein et al., 2012; McKee et al., 2013). Recent studies of post-mortem brains of former military personnel with a history of blast- and military-related concussion have also revealed tau pathology (Goldstein et al., 2012), suggesting that a variety of injury types can drive tau pathology. While CTE is viewed as primarily a tauopathy, the evidence for polypathology in CTE is highlighted by a recent report where 52% of 114 neuropathologically confirmed CTE brains demonstrated concomitant Aβ plaque deposition (Stein et al., 2015). TDP-43 and α-synuclein have also been identified in CTE brains (McKee et al., 2013).
As occurs with Aβ, there are similarities and differences between the tau pathology observed after TBI compared to AD. The tau-positive somatodendritic inclusions seen in CTE are morphologically similar to those found in AD, however there is much more astrocytic tau in CTE compared to AD. Another distinct difference is the distribution of neocortical tangles. As seen in long term survivors of a single TBI (Johnson et al., 2012), tau immunoreactivity in CTE characteristically presents with preferential deposition in layers II and III of the cortex compared to preferential deposition in layers V and VI in AD (Hof et al., 1992). However, there are still many unanswered questions about tau pathology after TBI—we know little about the biochemical profiles of the tau that accumulates after injury, such as the inclusion type (paired helical filaments, straight, or ribbon filaments), the primary phosphorylation sites, or the roles of different tau isoforms. To date, there are only 2 studies of CTE in the literature that examine the ratio of 4 repeat (4R) to 3 repeat (3R) tau. In the first case, biochemical analysis reveal that both 3R and 4R tau are hyperphosphorylated in brain extracts from two boxers (Schmidt et al., 2001). In the second case, immunohistological staining demonstrates both 4R and 3R staining in a human CTE case, with 4R tau thought to be predominant in astrocytic tau inclusions (McKee et al., 2013). No biochemical analysis has occurred on the chronic tau pathology observed in single TBI brain.
A major obstacle to gaining a better understanding of tau pathology after TBI is the lack of an animal model of repeat mTBI that recapitulates the tau pathology observed in CTE. The acute accumulation of p-tau in more severe TBI models is similar to that described for TBI-induced Aβ, with an acute increase of abnormal p-tau observed in in areas of axonal injury. Following cortical impact in the 3xTg AD model mouse (harboring a human P301L tau mutation), the accumulation of p-tau in multiple brain regions was punctate and primarily axonal (Tran et al., 2011a), and was also reportedly increased in the somatodendritic compartments of the contralateral CA1 neuronal cells (Tran et al., 2011a). Other groups have also demonstrated that endogenous mouse tau can be acutely phosphorylated at multiple sites following blast (Goldstein et al., 2012) and closed head injury (Laskowitz et al., 2010; Namjoshi et al., 2014). However, driving chronic accumulation of p-tau, even in tau transgenic mice, has proven to be difficult. A study of repeat mTBI using mice overexpressing the shortest tau isoform (T44 mice), mice were exposed to four mTBI/day, once a week for four weeks (16 impacts over 4 weeks), with a recovery time of 9 months and only one mouse was found to have accelerated tau deposition (Yoshiyama et al., 2005). A similar study using aged (18-month) human tau mice (hTau mice, which express all 6 tau isoforms) exposed to 5 mTBIs over a 9-day period and had accelerated tau pathology 3 weeks after injury compared to sham or single mTBI hTau mice (Ojo et al., 2013). In general, the mechanisms of action driving this accelerated tau pathology remains unclear and recapitulating the pathology of repeat mTBI in animals remains challenging. It is unclear if an brain injury has to be above a certain velocity, in a specific brain region, or if a certain number of impacts are required to begin the destructive cascade that results in chronic tau accumulation. Other practical issues such as skin deflection, tissue necrosis, and repeat anesthesia further complicate the development of repeat mTBI models.
Demonstrating polypathology after TBI
While there are human studies that examine discrete neurodegenerative pathology after TBI, and descriptive or smaller studies looking at multiple pathologies in individual cases, the field is lacking a systematic analysis of multiple pathologies in individual cases in a large TBI and control population. Such a comprehensive study could help identify factors that lead to different neuropathological phenotypes after TBI, and help explain the “polypathology” that has been reported in acute and chronic TBI brains (Newell et al., 1999; Johnson et al., 2011; Johnson et al., 2012; McKee et al., 2013; Smith et al., 2013; McKee et al., 2015). Such widespread studies may provide a more accurate sense of the actual incidence of neurodegenerative pathology after TBI. Current studies looking for a single pathology report the incidence of amyloid and tau at approximately 30%, however the incidence of neurodegenerative pathology in the brain after TBI could be considerably higher if all the possible disease-related proteins are studied in the same controlled cohorts.
Animal studies would also benefit from investigating the presence of multiple pathologies simultaneously in individual mice. This could help determine whether different pathologies follow different timecourses after injury, and whether a particular pathology is more associated with injury than others. For example the studies by Tran et al., investigated both Aβ and tau pathologies after cortical impact injury in the 3xTg-AD model mouse (Tran et al., 2011a, Tran et al., 2011b). An example of acute Aβ and p-tau polypathology after TBI in 3xTg-AD mice is shown in Figure 1. Future studies can begin incorporating the factors that appear to influence the phenotype of neurodegenerative pathology after TBI, including injury severity, age at injury, length of survival, and APOE genotype and look for mixed pathology.
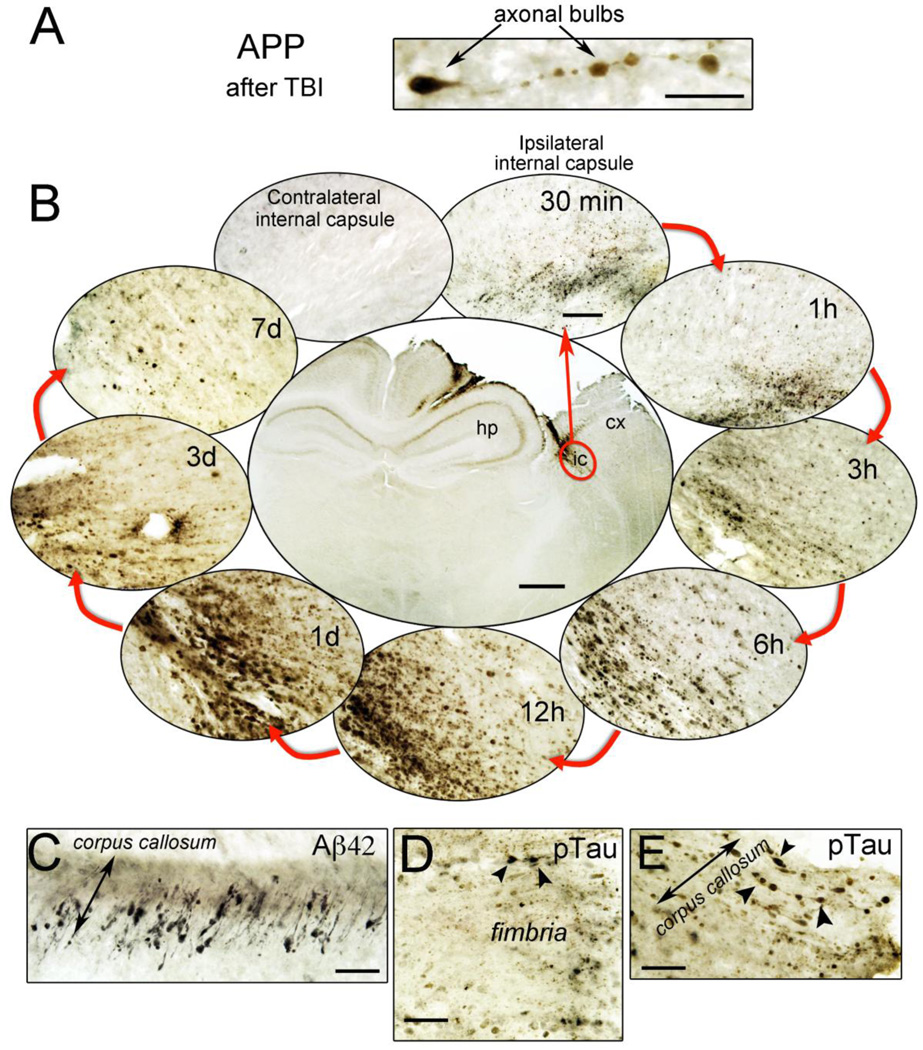
Figure 1. Acute polypathology after experimental TBI in AD transgenic mice.
3xTg-AD mice received a unilateral controlled cortical impact injury. A. Axon injury after TBI is characterized by APP-positive axonal bulbs. This abnormal APP staining after TBI reflects damage to the microtubule network and accumulation of proteins conveyed by axonal transport. B. The timecourse of axonal APP accumulation occurring in the internal capsule of C57/Bl6 mice after cortical impact. APP accumulation can be seen within 30 minutes of TBI, and is still visible 7d post-injury. C. Aβ42 positive staining occurs in the white matter tracts of 3xTg-AD mice 24h after TBI. D and E. Intraxonal accumulation of AT8-positive phosphorylated tau (ptau) occurs in the fimbria and corpus callosum of 3xTg mice 24h post-injury. Scale bar for A, C, D, E = 20 µm, and for B = 500 µm (central image) and 50 µm (surrounding images). cx = cortex, hp = hippocampus.
Does disease pathology after TBI relate to dementia risk?
Similar to the case in other neurodegenerative diseases we are forced to ask what does the presence of neurodegenerative disease pathology in a subset of patients after TBI mean? Is it related to dementia risk? Does it drive further neurodegeneration or accelerate brain aging? Indeed, the appearance of neuritic Aβ plaques in CTE brains is associated with advanced CTE disease staging and dementia (Stein et al., 2015), suggesting that this amyloid may accelerate or drive disease state following repeat mTBI. Looking ahead at potential influencing factors of dementia risk after TBI and the appearance of pathology does seem to be the largest associating factor. Other factors include age at the time of injury, duration of survival, repeated exposure or increased severity of injury, and APOE4 genotype. The establishment of large, case- controlled cohorts of TBI brains will be essential for teasing apart the impact of these factors on dementia onset after injury.
Conclusion
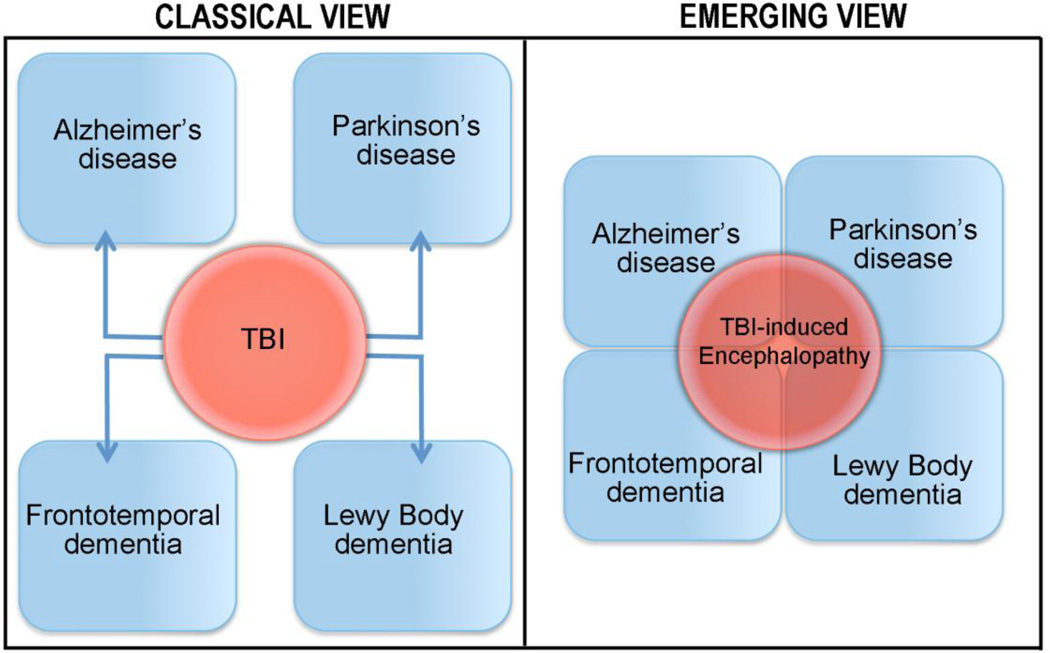
As the TBI field continues to grow, a clearer picture is emerging of the array of neuropathological changes and clinical symptoms that can occur. The chronic sequelae of both single TBI and repeat mTBI are as heterogeneous and personalized to the individual patient as other aspects of brain trauma, however they share common neuropathological features and clinical symptoms of classically defined neurodegenerative disorders. While the spectrum of chronic cognitive and neurobehavioral disorders that occur following repeat mTBI are viewed as the symptoms of CTE, the spectrum of chronic cognitive and neurobehavioral symptoms that occur after a single TBI is considered to represent distinct neurodegenerative diseases such as AD. These data support the suggestion that the multiple manifestations of TBI-induced neurodegenerative disorders be classified together as traumatic encephalopathy or trauma-induced neurodegeneration, regardless of the nature or frequency of the precipitating TBI (Figure 2).
Figure 2.
The traditional view of the chronic effects of TBI classifies brain injury as a risk factor for the precipitation of individual neurodegenerative diseases. The emerging view supported in this review is that TBI-induced neurodegenerative disease, or traumatic encephalopathy, is a spectrum disorder that shares clinical and neuropathological hallmarks with other neurodegenerative disorders.
Acknowledgements
This work was supported by grant number R01 NS067417 from the National Institute for Neurological Disorders and Stroke (MPB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- Abrahamson EE, Ikonomovic MD, Dixon CE, Dekosky ST. Simvastatin therapy prevents brain trauma-induced increases in beta-amyloid peptide levels. Ann Neurol. 2009;66:407–414. doi: 10.1002/ana.21731. [DOI] [PubMed] [Google Scholar]
- Abrahamson EE, Ikonomovic MD, Ciallella JR, Hope CE, Paljug WR, Isanski BA, Flood DG, Clark RS, DeKosky ST. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: implications for clinical outcome. Exp Neurol. 2006;197:437–450. doi: 10.1016/j.expneurol.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Allsop D, Haga S, Bruton C, Ishii T, Roberts GW. Neurofibrillary tangles in some cases of dementia pugilistica share antigens with amyloid beta-protein of Alzheimer's disease. Am J Pathol. 1990;136:255–260. [PMC free article] [PubMed] [Google Scholar]
- Areza-Fegyveres R, Rosemberg S, Castro RM, Porto CS, Bahia VS, Caramelli P, Nitrini R. Dementia pugilistica with clinical features of Alzheimer's disease. Arq Neuropsiquiatr. 2007;65:830–833. doi: 10.1590/s0004-282x2007000500019. [DOI] [PubMed] [Google Scholar]
- Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, D'Agostino RB, White LR. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer's disease. Neurobiology of aging. 1997;18:S85–S88. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- Broe GA, Henderson AS, Creasey H, McCusker E, Korten AE, Jorm AF, Longley W, Anthony JC. A case-control study of Alzheimer's disease in Australia. Neurology. 1990;40:1698–1707. doi: 10.1212/wnl.40.11.1698. [DOI] [PubMed] [Google Scholar]
- Caamano-Isorna F, Corral M, Montes-Martinez A, Takkouche B. Education and dementia: a meta-analytic study. Neuroepidemiology. 2006;26:226–232. doi: 10.1159/000093378. [DOI] [PubMed] [Google Scholar]
- Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathology. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Siman R, Iwata A, Meaney DF, Trojanowski JQ, Smith DH. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 2004;165:357–371. doi: 10.1016/s0002-9440(10)63303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis J, Tissot R. Generalized Alzheimer's neurofibrillary lesions without senile plaques. (Presentation of one anatomo-clinical case) Schweiz Arch Neurol Neurochir Psychiatr. 1967;100:117–130. [PubMed] [Google Scholar]
- Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, Trojanowski JQ, Lee VM, McIntosh TK, Pratico D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 2004;90:758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3:270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- Dale GE, Leigh PN, Luthert P, Anderton BH, Roberts GW. Neurofibrillary tangles in dementia pugilistica are ubiquitinated. J Neurol Neurosurg Psychiatry. 1991;54:116–118. doi: 10.1136/jnnp.54.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for late-onset Alzheimer's disease: a population-based, case-control study. Ann Neurol. 1993;33:258–266. doi: 10.1002/ana.410330306. [DOI] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol. 2015 doi: 10.1002/ana.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatson JW, Warren V, Abdelfattah K, Wolf S, Hynan LS, Moore C, Diaz-Arrastia R, Minei JP, Madden C, Wigginton JG. Detection of beta-amyloid oligomers as a predictor of neurological outcome after brain injury. J Neurosurg. 2013;118:1336–1342. doi: 10.3171/2013.2.JNS121771. [DOI] [PubMed] [Google Scholar]
- Geddes JF, Vowles GH, Robinson SF, Sutcliffe JC. Neurofibrillary tangles, but not Alzheimer-type pathology, in a young boxer. Neuropathol Appl Neurobiol. 1996;22:12–16. [PubMed] [Google Scholar]
- Geddes JF, Vowles GH, Nicoll JA, Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- Gentleman SM, Greenberg BD, Savage MJ, Noori M, Newman SJ, Roberts GW, Griffin WS, Graham DI. A beta 42 is the predominant form of amyloid beta-protein in the brains of short-term survivors of head injury. Neuroreport. 1997;8:1519–1522. doi: 10.1097/00001756-199704140-00039. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AB, White E, Koepsell TD, Reifler BV, van Belle G, Larson EB, Raskind M. The association between head trauma and Alzheimer's disease. Am J Epidemiol. 1990;131:491–501. doi: 10.1093/oxfordjournals.aje.a115523. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Laurer H, Longhi L, Bales KR, Paul SM, McIntosh TK, Holtzman DM. Apolipoprotein E4 influences amyloid deposition but not cell loss after traumatic brain injury in a mouse model of Alzheimer's disease. J Neurosci. 2002;22:10083–10087. doi: 10.1523/JNEUROSCI.22-23-10083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Bouras C, Buee L, Delacourte A, Perl DP, Morrison JH. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer's disease cases. Acta Neuropathol. 1992;85:23–30. doi: 10.1007/BF00304630. [DOI] [PubMed] [Google Scholar]
- Hong YT, Veenith T, Dewar D, Outtrim JG, Mani V, Williams C, Pimlott S, Hutchinson PJ, Tavares A, Canales R, Mathis CA, Klunk WE, Aigbirhio FI, Coles JP, Baron JC, Pickard JD, Fryer TD, Stewart W, Menon DK. Amyloid imaging with carbon 11-labeled Pittsburgh compound B for traumatic brain injury. JAMA Neurol. 2014;71:23–31. doi: 10.1001/jamaneurol.2013.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, Clark RS, Marion DW, Wisniewski SR, Dekosky ST. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord. 2013;28:1222–1229. doi: 10.1002/mds.25458. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathology. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Trojanowski JQ, Smith DH. Acute and chronically increased immunoreactivity to phosphorylation-independent but not pathological TDP-43 after a single traumatic brain injury in humans. Acta Neuropathol. 2011;122:715–726. doi: 10.1007/s00401-011-0909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Graham DI, Stewart JE, Praestgaard AH, Smith DH. A neprilysin polymorphism and amyloid-beta plaques after traumatic brain injury. Journal of neurotrauma. 2009;26:1197–1202. doi: 10.1089/neu.2008.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkonde YV, Jawaid A, Qureshi SU, Shirani P, Wheaton M, Pinto-Patarroyo GP, Schulz PE. Medical and environmental risk factors associated with frontotemporal dementia: a case-control study in a veteran population. Alzheimers Dement. 2012;8:204–210. doi: 10.1016/j.jalz.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Katzman R, Aronson M, Fuld P, Kawas C, Brown T, Morgenstern H, Frishman W, Gidez L, Eder H, Ooi WL. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol. 1989;25:317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- Kokjohn TA, Maarouf CL, Daugs ID, Hunter JM, Whiteside CM, Malek-Ahmadi M, Rodriguez E, Kalback W, Jacobson SA, Sabbagh MN, Beach TG, Roher AE. Neurochemical profile of dementia pugilistica. Journal of neurotrauma. 2013;30:981–997. doi: 10.1089/neu.2012.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Song P, Wang H, Mace B, Sullivan PM, Vitek MP, Dawson HN. Traumatic brain injury exacerbates neurodegenerative pathology: improvement with an apolipoprotein E-based therapeutic. Journal of neurotrauma. 2010;27:1983–1995. doi: 10.1089/neu.2010.1396. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Pocivavsek A, Moussa CE, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, Burns MP. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martland HS. Punch Drunk. Journal of the American Medical Association. 1928;91:1103–1107. [Google Scholar]
- Mayeux R, Ottman R, Maestre G, Ngai C, Tang MX, Ginsberg H, Chun M, Tycko B, Shelanski M. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer's disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- McCown IA. Boxing injuries. The American Journal of Surgery. 1959;98:509–516. [Google Scholar]
- McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–364. doi: 10.1111/bpa.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millspaugh HS. Dementia pugilistica. United States Medical Bulliten. 1937;35:297–303. [Google Scholar]
- Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA, Shalat SL, Soininen H, Hofman A. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- Namjoshi DR, Cheng WH, McInnes KA, Martens KM, Carr M, Wilkinson A, Fan J, Robert J, Hayat A, Cripton PA, Wellington CL. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): a novel, surgery-free model of traumatic brain injury. Molecular neurodegeneration. 2014;9:55. doi: 10.1186/1750-1326-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Newell KL, Boyer P, Gomez-Tortosa E, Hobbs W, Hedley-Whyte ET, Vonsattel JP, Hyman BT. Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J Neuropathol Exp Neurol. 1999;58:1263–1268. doi: 10.1097/00005072-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Roberts GW, Graham DI. Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med. 1995;1:135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- O'Meara ES, Kukull WA, Sheppard L, Bowen JD, McCormick WC, Teri L, Pfanschmidt M, Thompson JD, Schellenberg GD, Larson EB. Head injury and risk of Alzheimer's disease by apolipoprotein E genotype. Am J Epidemiol. 1997;146:373–384. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, Fitzsimmons R. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69:173–183. doi: 10.1227/NEU.0b013e318212bc7b. discussion 183. [DOI] [PubMed] [Google Scholar]
- Osnato M, Giliberti V. Postconcussion Neurosis - Traumatic Encephalitis: a conception of postconcussion phenomena. Arch Neurol Psychiatry. 1927;18:181–214. [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rasmusson DX, Brandt J, Martin DB, Folstein MF. Head injury as a risk factor in Alzheimer's disease. Brain Inj. 1995;9:213–219. doi: 10.3109/02699059509008194. [DOI] [PubMed] [Google Scholar]
- Raymont V, Greathouse A, Reding K, Lipsky R, Salazar A, Grafman J. Demographic, structural and genetic predictors of late cognitive decline after penetrating head injury. Brain. 2008;131:543–558. doi: 10.1093/brain/awm300. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Graham DI. beta A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1994;57:419–425. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SM, Landweer EJ, Houterman M, Donker Kaat L, van Duijn CM, van Swieten JC. Medical and environmental risk factors for sporadic frontotemporal dementia: a retrospective case-control study. J Neurol Neurosurg Psychiatry. 2003;74:1574–1576. doi: 10.1136/jnnp.74.11.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saing T, Dick M, Nelson PT, Kim RC, Cribbs DH, Head E. Frontal cortex neuropathology in dementia pugilistica. Journal of neurotrauma. 2012;29:1054–1070. doi: 10.1089/neu.2011.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ML, Zhukareva V, Newell KL, Lee VM, Trojanowski JQ. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer's disease. Acta Neuropathol. 2001;101:518–524. doi: 10.1007/s004010000330. [DOI] [PubMed] [Google Scholar]
- Shalat SL, Seltzer B, Pidcock C, Baker EL., Jr Risk factors for Alzheimer's disease: a case-control study. Neurology. 1987;37:1630–1633. doi: 10.1212/wnl.37.10.1630. [DOI] [PubMed] [Google Scholar]
- Smith C, Graham DI, Murray LS, Nicoll JA. Tau immunohistochemistry in acute brain injury. Neuropathol Appl Neurobiol. 2003a;29:496–502. doi: 10.1046/j.1365-2990.2003.00488.x. [DOI] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Iwata A, Graham DI. Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg. 2003b;98:1072–1077. doi: 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- Stein TD, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015 doi: 10.1007/s00401-015-1435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda T, Ikeda S, Yanagisawa N, Ihara Y, Glenner GG. Re-examination of ex-boxers' brains using immunohistochemistry with antibodies to amyloid beta-protein and tau protein. Acta Neuropathol. 1991;82:280–285. doi: 10.1007/BF00308813. [DOI] [PubMed] [Google Scholar]
- Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011a;31:9513–9525. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HT, Sanchez L, Esparza TJ, Brody DL. Distinct temporal and anatomical distributions of amyloid-beta and tau abnormalities following controlled cortical impact in transgenic mice. PLoS One. 2011d;6:e25475. doi: 10.1371/journal.pone.0025475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolaki M, Fountoulakis K, Chantzi E, Kazis A. Risk factors for clinically diagnosed Alzheimer's disease: a case-control study of a Greek population. Int Psychogeriatr. 1997;9:327–341. doi: 10.1017/s104161029700447x. [DOI] [PubMed] [Google Scholar]
- Uryu K, Chen XH, Martinez D, Browne KD, Johnson VE, Graham DI, Lee VM, Trojanowski JQ, Smith DH. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HK, Lee YC, Huang CY, Liliang PC, Lu K, Chen HJ, Li YC, Tsai KJ. Traumatic brain injury causes frontotemporal dementia and TDP-43 proteolysis. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Washington PM, Morffy N, Parsadanian M, Zapple D, Burns MP. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer's disease mouse model. Journal of neurotrauma. 2013 doi: 10.1089/neu.2013.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington PM, Morffy N, Parsadanian M, Zapple DN, Burns MP. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer's disease mouse model. J Neurotrauma. 2014;31:125–134. doi: 10.1089/neu.2013.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston CN, Chellappa D, Wilkins T, Barton DJ, Washington PM, Loane DJ, Zapple DN, Burns MP. Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation. Journal of neurotrauma. 2013;30:1966–1972. doi: 10.1089/neu.2013.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Pike VW, Wang Y. Amyloid imaging: from benchtop to bedside. Curr Top Dev Biol. 2005;70:171–213. doi: 10.1016/S0070-2153(05)70008-9. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Uryu K, Higuchi M, Longhi L, Hoover R, Fujimoto S, McIntosh T, Lee VM, Trojanowski JQ. Enhanced neurofibrillary tangle formation, cerebral atrophy, and cognitive deficits induced by repetitive mild brain injury in a transgenic tauopathy mouse model. J Neurotrauma. 2005;22:1134–1141. doi: 10.1089/neu.2005.22.1134. [DOI] [PubMed] [Google Scholar]