Abstract
Vanilloids, high temperature, and low pH activate the transient receptor potential vanilloid type 1 (TRPV1) receptor. In spinal dorsal root ganglia, co-activation of one of these gating sites on TRPV1 sensitized receptor gating by other modes. Here in rat brainstem slices, we examined glutamate synaptic transmission in nucleus of the solitary tract (NTS) neurons where most cranial primary afferents express TRPV1, but TRPV1 sensitization is unknown. Electrical shocks to the solitary tract (ST) evoked EPSCs (ST-EPSCs). Activation of TRPV1 with capsaicin (100 nM) increased spontaneous EPSCs (sEPSCs) but inhibited ST-EPSCs. High concentrations of the ultra-potent vanilloid resiniferatoxin (RTX, 1 nM) similarly increased sEPSC rates but blocked ST-EPSCs. Lowering the RTX concentration to 150 pM modestly increased the frequency of the sEPSCs without causing failures in the evoked ST-EPSCs. The sEPSC rate increased with raising bath temperature to 36°C. Such thermal responses were larger in 150 pM RTX, while the ST-EPSCs remained unaffected. Vanilloid sensitization of thermal responses persisted in TTX but was blocked by the TRPV1 antagonist capsazepine. Our results demonstrate that multimodal activation of TRPV1 facilitates sEPSC responses in more than the arithmetic sum of the two activators, i.e. co-activation sensitizes TRPV1 control of spontaneous glutamate release. Since action potential evoked glutamate release is unaltered, the work provides evidence for cooperativity in gating TRPV1 plus a remarkable separation of calcium mechanisms governing the independent vesicle pools responsible for spontaneous and evoked release at primary afferents in the NTS.
Keywords: nucleus of the solitary tract, resiniferatoxin, TRPV1
1. Introduction
The transient receptor protein vanilloid type 1 (TRPV1) is commonly associated with transduction of potentially damaging noxious stimuli at nociceptive primary afferents (Tominaga et al., 1998). TRPV1 possesses two particularly interesting features: tripartite gating and high calcium permeability. Three specific attributes associated with damaged tissue separately gate TRPV1 to open: low pH, vanilloid ligands, and/or heat (>42°C), and this triple-gating property of TRPV1 makes it a multimodal integrator (Kaszas et al., 2012). As a cation permeable ion channel, TRPV1 activation raises intracellular calcium and causes depolarization (Caterina et al., 1997). Thus, the dense expression of TRPV1 within the terminal fields of cranial visceral afferents (Tominaga et al., 1998) in the nucleus of the solitary tract (NTS) raises the prospect of a unique trigger of afferent neurotransmission.
Early neurotransmission work discovered that presynaptic action potentials triggered a rise in the calcium levels responsible for evoked release of neurotransmitter and that neurotransmission consisted of the release of quantal vesicles of transmitter (Fatt & Katz, 1952; del Castillo & Katz, 1954). Even in the absence of action potentials, however, quantal events occur spontaneously suggesting low rates of spontaneous vesicle fusion in most neurons. Mounting evidence now suggests that spontaneous events may arise from a separate pool of vesicles triggered by mechanisms distinct from action potential evoked neurotransmitter release (Kavalali, 2014). Examples of independent spontaneous release exist throughout the brain with widespread variations in spontaneous synaptic activity and regional characteristics in areas such as the cortex (Sara et al., 2005; Hofmann et al., 2011), cerebellum (Glitsch, 2006), and brainstem (Peters et al., 2010; Largent-Milnes et al., 2014).
Solitary tract (ST) afferent transmission at NTS neurons is sensitive to vanilloid activation of TRPV1 at a majority of neurons (Doyle et al., 2002). TRPV1 located at unmyelinated, C-fiber ST afferent synaptic terminals acts as a unique calcium source to trigger glutamate vesicle release registered as spontaneous EPSCs (sEPSCs). Surprisingly, physiological temperatures vigorously activate TRPV1 to trigger spontaneous glutamate release only from TRPV1-expressing ST afferents, whereas cooling steeply suppresses spontaneous but not evoked synaptic responses (Peters et al., 2010; Shoudai et al., 2010; Fawley et al., 2015). This ongoing drive of TRPV1 at 37°C contrasts with the canonical noxious heat thresholds of other primary sensory afferents. Here, we tested whether vanilloid stimulation cooperatively sensitizes thermal activation of TRPV1 in ST afferent transmission as it does in the spinal cord (Matta & Ahern, 2011). Low levels of TRPV1 activation with the ultra-potent vanilloid agonist resiniferatoxin (RTX) (Szallasi & Blumberg, 1999) augmented sEPSC rates without reducing evoked ST-EPSC amplitudes and substantially sensitized responses to thermal changes near 37°C. TRPV1 activation primarily slowed the conduction of evoked transmission and was opposite to the enhanced spontaneous release. Thus, temperature and vanilloid binding cooperatively gate TRPV1 to augment spontaneous release of glutamate without affecting action potential triggered EPSCs. Our findings highlight the independence of the two release mechanisms controlling the spontaneous pool and the evoked pool of vesicles, which rely on separate and independent calcium sources for release. Together, the multimodal activation of TRPV1 plus its coupling to release from the spontaneous glutamate pool imbues C-fiber afferent central terminals with a capacity to respond to a wide dynamic range of inputs as a unique afferent signaling mechanism.
2. Methods
2.1. Ethical Approval
All animal procedures were approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University and conformed to animal welfare guidelines issued by the National Institutes of Health publication Guide for the Care and use of Laboratory Animals.
2.2. Slice preparation
Brain slices containing NTS were prepared from adult (>130 g, n = 51) male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), fed ad libitum, as previously described (Doyle & Andresen, 2001). Briefly, rats were deeply anesthetized with 3% isoflurane and the brainstem removed and placed into ice-cold ACSF. The brainstem was tilted in order to cut a horizontal slice (250 µm) containing 1–3 mm of the ST in the same plane as the NTS. While in the ice-cold ACSF, slices were cut using a sapphire blade (Delaware Diamond Knives, Wilmington, DE) mounted on a vibrating microtome (VT1000 S; Leica Microsystems, Bannockburn, IL), and immediately submerged in a recording chamber containing ACSF. The ACSF consisted of, in mM,: 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 10 glucose, and 2 CaCl2, bubbled with 95% O2–5% CO2. The slice was perfused continuously (1.6–2.0 ml/min) at a bath temperature of 32°C controlled by an in-line heating system (TC2BIP with HPRE2HF and TH-10Km bath probe; Cell MicroControls, Norfolk, VA). This cooler than physiological base temperature maximized recording time periods compared to 37°C.
2.3. Patch-clamp recording
Under infrared illumination, neurons were visualized using 40 nM bandpass limited, differential interference contrast microscopy (Doyle et al., 2004) on a fixed-stage Axioskop 2 FS Plus (Zeiss, Thornwood, NJ) with a digital camera (SPOT Pursuit USB Camera; SPOT Imaging Solutions, Sterling Heights, MI). Patch pipettes (2.5–4.0 MΩ) were filled with an internal solution composed of, in mM,: 6 NaCl, 4 NaOH, 130 K-gluconate, 11 EGTA, 1 CaCl2, 10 HEPES, 1 MgCl2, 2 Na2ATP, and 0.2 Na2GTP (pH 7.3). Using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA), neurons were voltage clamped at −60 mV without liquid junction potential correction. Data were sampled at 20 kHz and filtered at 10 kHz (Digidata 1321A analog-to-digital converter using Clampex 9; Molecular Devices). Repeated −5 mV (200ms) hyperpolarizing steps were applied throughout each experiment to monitor access resistance and input resistance. The ACSF included the GABAA antagonist gabazine (SR-95531; 3 µM) to isolate EPSCs in all experiments. Drugs were bath-applied and purchased from either Tocris (R&D Systems, Minneapolis, MN) or Sigma-Aldrich (St. Louis, MO).
2.4. Afferent activation and identification
After submerging and securing the slice in the recording chamber, a concentric bipolar stimulating electrode was placed onto the ST at least 1 mm rostral from medial NTS. Following acquisition of a stable patch recording, bursts of five shocks (100 µs duration at 50 Hz) tested for ST evoked synaptic responses. Variations in stimulation intensity allowed for the determination of the threshold for obtaining a solitary tract evoked EPSC (ST-EPSC). Gradual increases in intensity created an ST-EPSC recruitment profile and generally, a minimal intensity above threshold was chosen for experiments. To verify the neuron as second-order, the ST burst of shocks was applied once every 6 s and the latency of the ST-EPSC was measured as the time from the stimulus artifact to the onset of the ST-EPSC. Using at least 50 iterations, the synaptic jitter was measured as the standard deviation of the latency. Evoked ST-EPSCs with a jitter <200 µs were classified as monosynaptic (Doyle & Andresen, 2001; Andresen & Peters, 2008). After collecting data for the jitter measurements, the protocol switched to stimulating the ST once every 10 s for the thermal and pharmacological tests. Classifying second-order neurons as receiving TRPV1+ ST afferents generally relied on the following criteria: high control (resting) frequency of sEPSCs in the unstimulated state, increased sEPSC frequencies in the 1 s following suprathreshold ST stimulation compared to the 1 s prior to stimulation (asynchronous sEPSCs) (Peters et al., 2010), and increases in sEPSC rate to increases in temperature. Neurons were verified as TRPV1+ using either capsaicin (CAP, 100 nM) or RTX (150 pM or 1 nM).
2.5. Data analysis and statistics
Analysis was performed using O-phys (see Hofmann et al., 2011; Gainesville, FL) and Origin 8.6 (OriginLab; Northampton, MA) except for latency measurements (Clampfit 9; Molecular Devices). For ST evoked synaptic responses, average values for ST-EPSC amplitude and latency were calculated using the final 3 min before drug application (i.e. 18 trials) and again for a 3 min period beginning 7 min after drug application. A two-sample t-test was performed within each individual neuron and a paired t-test was used for summary of group data for both ST-evoked amplitude and latency (OriginLab). From these same records, corresponding information of spontaneous transmission was assessed for sEPSC event frequencies and amplitudes collected from each test sweep for the 5 s prior to ST stimulation. Given that sEPSCs represent nonparametric observations, the distributions of values within individual neurons were assessed using a Kolmogorov-Smirnov (K-S) test. K-S tests determined the significance of any changes in sEPSC interevent time or amplitude distributions between the 2 min prior to RTX and after 8 minutes of exposure. Temperature changes were accomplished using the in-line heating system through commands generated by a Master-9 (A.M.P.I.; Jerusalem, Israel). These ramp and hold protocols incrementally increased the bath temperature from 32 to 36°C in a period of 3 min and then maintained temperature at 36°C for an additional 3 min before returning to 32°C. The thermal response in each condition was measured as the frequency over the last 2 min at 36°C minus the frequency over the 2 min prior to the temperature ramp (Δ Hz). Our previous studies suggest that the conduction of ST shocks to the triggering of synaptic responses at the ST terminal is independent from spontaneous release and that synaptic failures (absence of triggered ST-EPSCs) represents this process uniquely (Hofmann et al., 2014). We measured the change in failure rate of the ST-EPSC (missing events at times predicted following ST shock) as the 3 min at 36°C minus the 3 min prior to initiation of the temperature ramp and compared them between Control and RTX. Group data for summary purposes were compared using t-tests (OriginLab).
3. Results
3.1. Capsaicin triggers spontaneous release but blocks evoked release
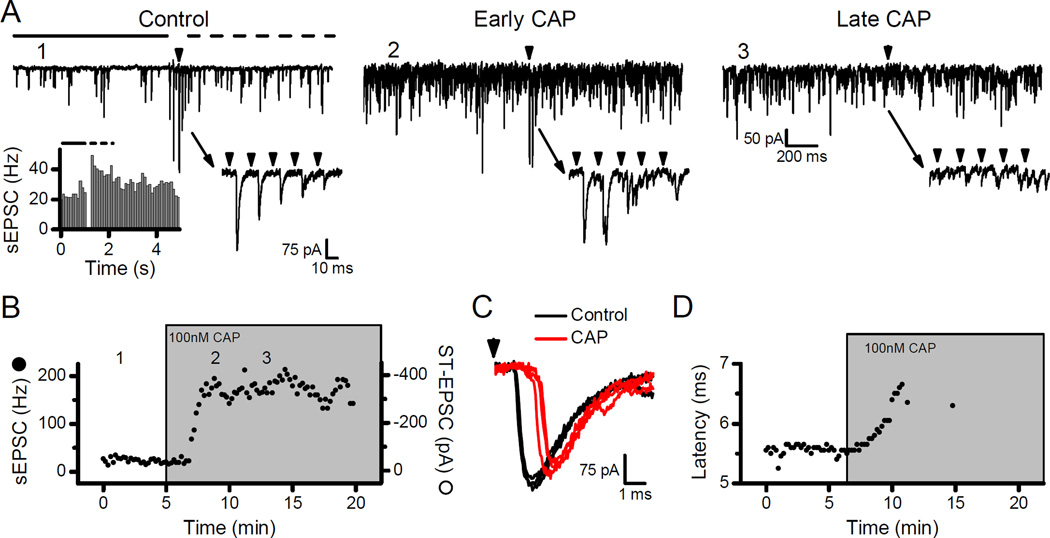
High rates of sEPSCs in NTS neurons often signify connections to ST afferents with unmyelinated, C-fiber axons and TRPV1 expression (Andresen et al., 2012). In such neurons (Fig. 1A, Control), suprathreshold shocks to the ST elicit ST-EPSCs with minimal latency variation that is diagnostic for a direct, monosynaptic primary afferent connection and a stimulus burst of ST shocks evoked frequency dependent depression (Fig. 1A, Control, lower right inset). The burst of shocks to the ST triggered asynchronous release – a transient increase in the frequency of sEPSCs trailing the evoked ST-EPSCs (Fig. 1A, Control, lower left inset). The combination of high sEPSC rates and asynchronous release suggested that the neuron received TRPV1+ inputs (Peters et al., 2010). In such neurons, application of CAP (100 nM) rapidly increased the sEPSC rate without altering the amplitude of evoked ST-EPSCs (Fig. 1A, Early CAP, and Fig. 1B). However, with prolonged exposure, CAP blocked the evoked ST-EPSCs while the rate of sEPSCs remained elevated (Fig. 1A, Late CAP, and Fig. 1B). Note that the ST-EPSC latency progressively increased without substantial declines in evoked amplitude prior to ST-EPSC failure (Fig. 1C and D) – a finding consistent with depolarization block and a sequence identical to conduction block of ST-EPSCs by local anesthetic action (Hofmann et al., 2014). Thus, TRPV1 activation with CAP has two distinct actions with opposite effects on glutamate release – a pronounced increase in spontaneous release, which precedes a delayed arrival of evoked ST-EPSCs and then full block of evoked release (Fig. 1B). Such observations are consistent with TRPV1 actions on two separate neurotransmitter vesicle pools – one pool responsible for spontaneous events whose release probability is enhanced by TRPV1 activation and a second pool responsible for evoked synaptic transmission whose release is blocked following TRPV1 activation.
Figure 1.
Capsaicin (CAP) increased the frequency of sEPSCs but inhibited action potential evoked ST-EPSCs from TRPV1+ afferents. In all examples, the arrowheads represent shocks to the ST. A) Control: Stimulation of the ST with a 5-pulse train (50 Hz) produced frequency dependent depression of the ST-EPSC amplitude (lower right inset). Immediately following stimulation the sEPSC frequency transiently increased (top panel, period marked with dashed line) compared to the pre-stimulation (top panel, solid line). This transient increase, termed asynchronous release, indicated a neuron likely receiving TRPV1+ afferents (left inset, 25 trials combined). Numeral labels (e.g. 1) indicate the timing of these original traces in the diary plot of B. Early CAP: 100 nM CAP dramatically elevated the rate of sEPSCs but ST shocks still evoked ST-EPSCs (lower right inset). Late CAP: After 7–10 minutes of exposure to CAP, the sEPSC rate remained elevated but ST shocks failed to evoke ST-EPSCs (lower right inset). B) Diary plot displays the time course of sEPSC frequency (solid circles) and ST-EPSC amplitude (open circles) changes to application of CAP. The sEPSC rate rapidly increased after 2–3 minutes of CAP perfusion but ST-EPSC failures appeared after 5–10 minutes and were often preceded by nearly full amplitude ST-EPSCs. C) Nearly synchronous with the onset of elevated sEPSC rates in CAP (gray or red) the latency of the ST-EPSC began to increase (Control, black). D) Diary plot of the ST-EPSC latency values before failures occurred in the presence of CAP.
3.2. 36°C gates high rates of TRPV1-operated sEPSCs
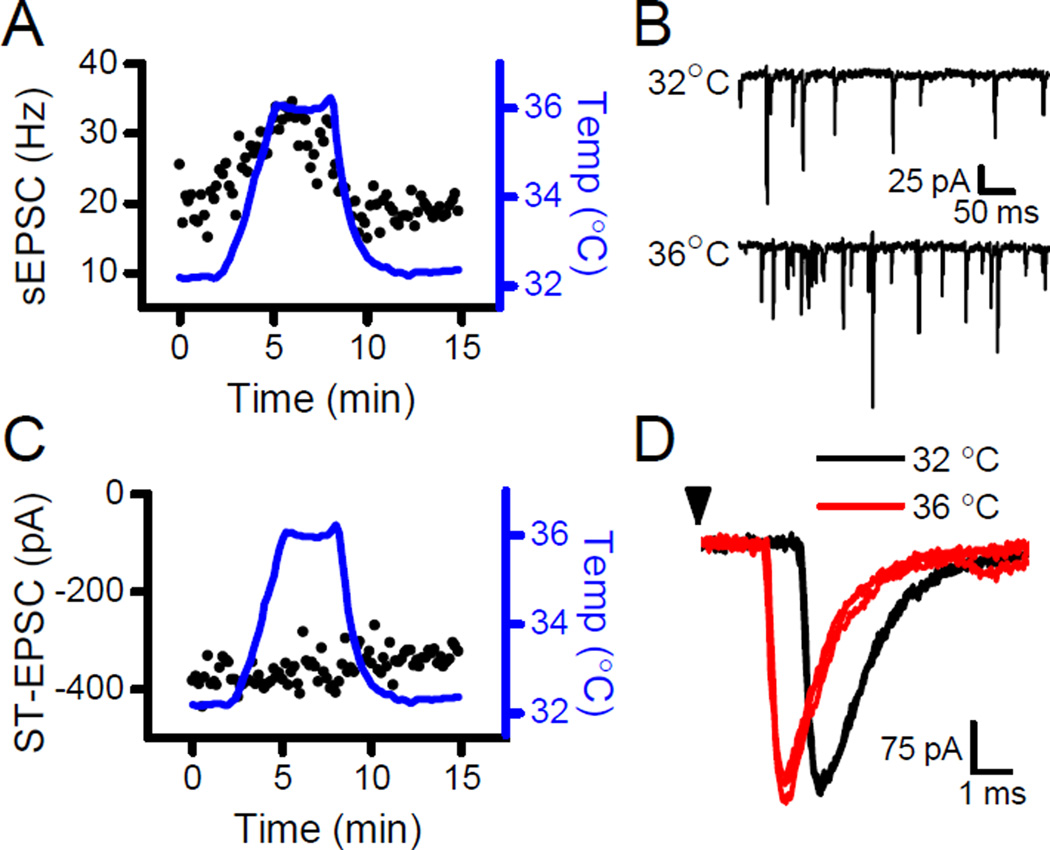
Vanilloids are often considered definitive and specific activators of TRPV1. In second order NTS neurons, small increments in bath temperature near 36°C rapidly and reversibly alter the sEPSC rate only in those neurons receiving TRPV1+ afferents (Shoudai et al., 2010; Fawley et al., 2015). Increasing the temperature by 4°C nearly doubled the frequency of sEPSCs (Fig. 2A and B), but the amplitudes of the evoked ST-EPSCs remained constant in the same neuron (Fig. 2C and D). The arrival time (evoked latency) shortened with warming suggesting a thermal acceleration of conduction velocity – a consistent action on ST-EPSCs for TRPV1+ and TRPV1- afferents (Fawley et al., 2015). Thus, normal brain temperatures foster enhanced glutamate release rates from the TRPV1-operated pool without affecting the evoked pool release probability providing additional evidence for two separate vesicle pools at primary afferent terminals within the NTS.
Figure 2.
Bath temperature sets the sEPSC rate without altering ST-EPSC amplitudes. A) Thermal steps from 32°C to 36°C reversibly increased sEPSC frequency. B) Original traces show a near doubling of sEPSC frequency in this neuron by increasing the temperature from 32°C to 36°C. C) In the same neuron, the same thermal steps did not affect the amplitude of the ST-EPSCs. D) Original traces show that this thermal step reversibly decreased the latency of the ST-EPSC with no changes to the amplitude (32°C, black; 36°C, gray or red). Arrowhead represents stimulation of the ST.
3.3. RTX acts similarly to CAP
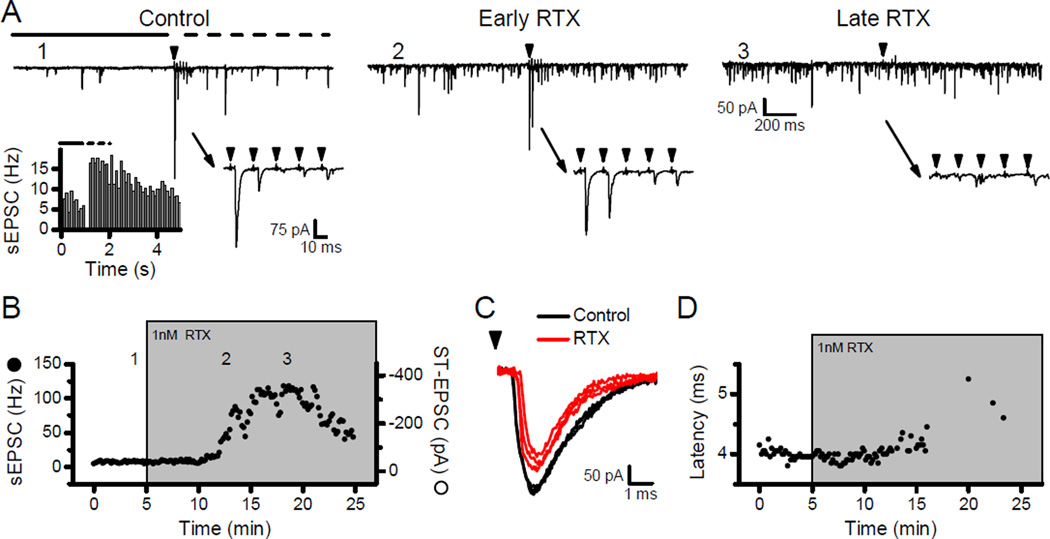
High concentrations of CAP can have nonspecific actions on neurons (Szallasi & Blumberg, 1999; Reynolds et al., 2006; Browning et al., 2013). To avoid those effects, we elected to use RTX and tested the characteristics of RTX alterations to ST transmission. In neurons with high resting sEPSC rates and clear asynchronous release following ST stimulation (Fig. 3A, Control), 1 nM RTX rapidly increased the frequency of sEPSCs while the evoked ST-EPSCs persisted (Fig. 3A, Early RTX, and Fig. 3B). With prolonged exposure to RTX, the ST-EPSCs failed while the sEPSC rate remained elevated (Fig. 3A, Late RTX, and Fig. 3B) – a sequence of responses similar to 100 nM CAP (Fig. 1). The increase in the ST-EPSC latency prior to failure also indicates that RTX, similar to CAP, likely depolarizes ST fibers resulting in retardation and then block of action potential conduction (Fig. 3C and D). Thus, at high concentrations, RTX produced qualitatively similar overall actions to CAP on synaptic transmission at TRPV1+ second order NTS neurons.
Figure 3.
Resiniferatoxin (RTX) increased the sEPSC frequency but led to block of the evoked ST-EPSC. In all examples, the arrowheads represent shocks to the ST. A) Control: The 5-pulse burst (50 Hz) of ST shocks evoked frequency dependent depression of the ST-EPSC (lower right inset). The transient increase in sEPSC frequency following stimulation (top panel, dashed line) compared to pre-stimulation (top panel, solid line) suggests this neuron has asynchronous release (lower left inset, 30 trials) and likely receives TRPV1+ ST afferents. Numeral labels (e.g. 1) indicate the timing of these original traces in the diary plot of B. Early RTX: Application of 1 nM RTX substantially increased the frequency of sEPSCs (top panel) but the ST-EPSC amplitudes remained unaffected (lower right inset). Late RTX: After 15 minutes of exposure to RTX, the rate of sEPSCs remained elevated (top panel) but ST-EPSCs were blocked (lower right inset). B) Diary plot displays the time course of sEPSC frequency (solid circles) and ST-EPSC amplitudes (open circles) with application of RTX. In 1 nM RTX, the sEPSC frequency increased several minutes before the ST-EPSCs began to fail, similar to the effects of CAP (Fig. 1). C) RTX (gray or red) increased the latency of the ST-EPSC compared to Control (black). D) Diary plot shows a RTX induced increase in ST-EPSC latencies before failures occurred.
3.4. Low RTX augments spontaneous but not evoked transmission
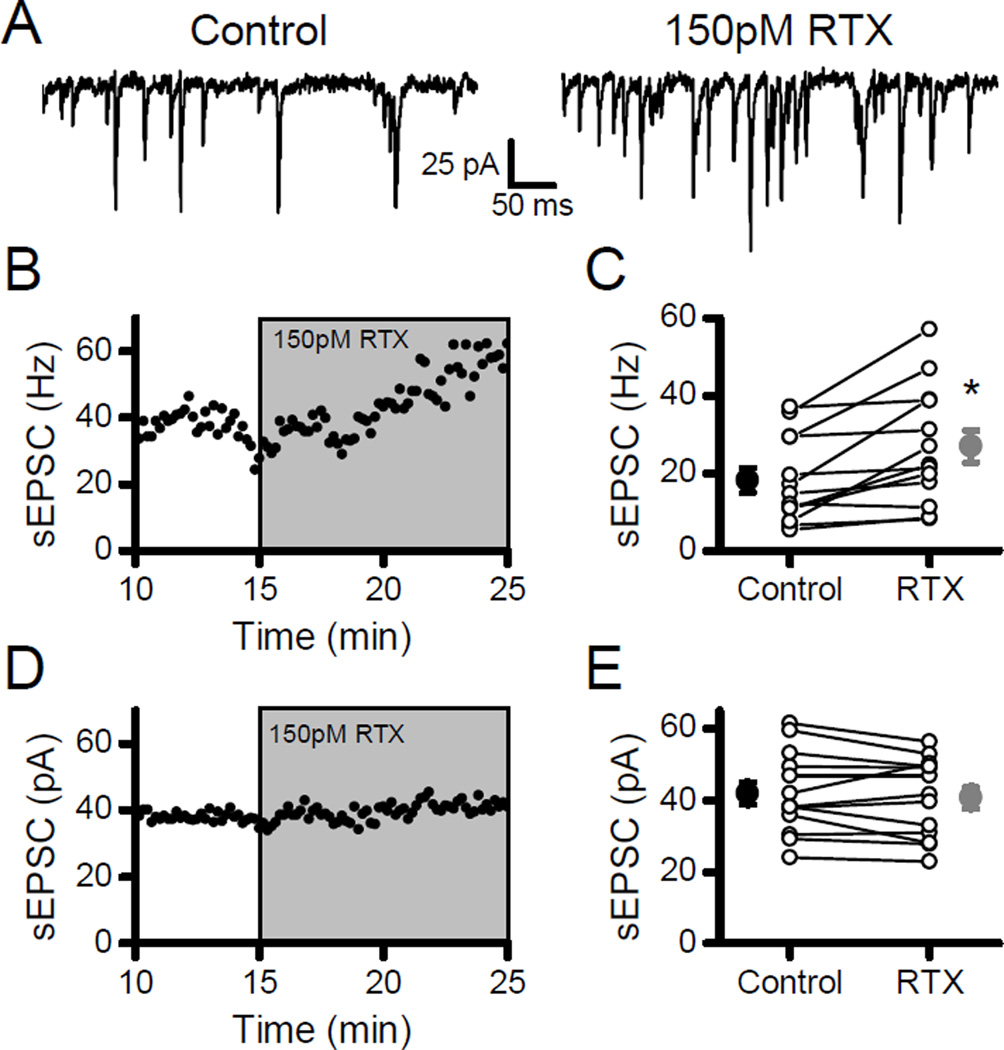
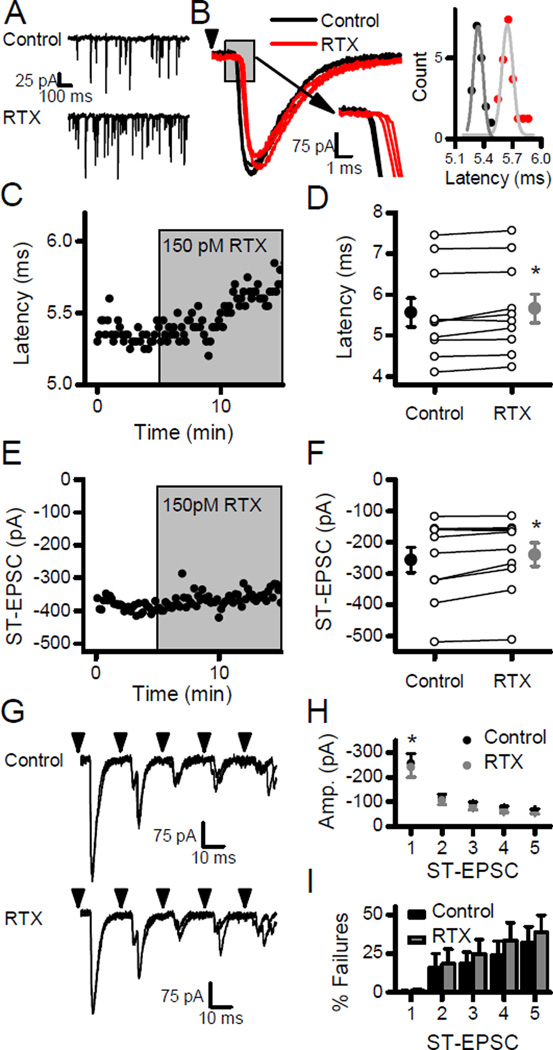
In order to test for the potential of cooperative interactions between vanilloid and temperature activation of TRPV1, modest activation of TRPV1 was accomplished using low concentrations of RTX. At 150 pM (30% of Kd at TRPV1, (Szallasi & Blumberg, 1999)), RTX consistently increased the frequency of sEPSCs by almost 50% (Fig. 4; n =13, p < 0.01, paired t-test) without changing the sEPSC event amplitudes (Fig. 4; n = 13, p = 0.31, paired t-test). Neurons in which low RTX increased sEPSC rates (Fig. 5A) and showed small but significant increases in the latencies of evoked ST-EPSCs (Fig. 5; n = 10, p = 0.02, paired t-test) and slight decreases in the amplitude of the evoked ST-EPSCs (Fig. 5; n = 10, p =0.03, paired t-test). In contrast to 1 nM RTX, failures of the evoked ST-EPSC were never observed with 150 pM RTX. Together the results suggest that the added calcium influx through TRPV1, which drives spontaneous release, did not facilitate evoked release. As an alternative gauge of the probability of evoked release, the variance of the evoked ST-EPSCs was unchanged (n = 10, p = 0.31, paired t-test). RTX also did not alter either frequency dependent depression (Fig. 5G and H) or the failure rates (Fig. 5G and I) for evoked ST-EPSCs during bursts of ST stimulation. Thus, a low concentration of RTX predominantly facilitated spontaneous glutamate release while subtly reducing ST conduction and evoked release. The modest level of TRPV1 activation from 150 pM RTX was suitable for investigating cooperative gating of TRPV1 in the face of unfailing evoked transmission.
Figure 4.
Low concentrations of RTX increased spontaneous release. A) Application of 150 pM RTX increased the frequency of sEPSCs. B) Diary plot demonstrates the gradual increase in sEPSC rate with RTX. C) On average (n = 13), RTX significantly increased sEPSC frequency in 11/13 neurons (K-S tests, p < 0.05) and overall changed mean frequency (large filled symbols) by almost 50% (Control, black; RTX, gray). D) In contrast, diary plot shows consistent sEPSC amplitudes in RTX. E) Overall, summary plot (n = 13) indicates no effect of RTX on mean sEPSC amplitude (large filled symbols; Control, black; RTX, gray). Asterisks represent p < 0.05.
Figure 5.
Low concentrations of RTX failed to block evoked ST-EPSCs despite augmenting sEPSC rates. Arrowheads represent stimulation of the ST. A) RTX (150 pM) increased the frequency of sEPSCs. B) In the same neuron, RTX (gray or red) slightly increased the latency of the ST-EPSC with no change in the amplitudes in this neuron (Control, black). The inset to the right depicts the distribution of all latency values measured in Control (black) and RTX (gray or red) for this neuron with each set fit to a Gaussian curve (Control, gray; RTX, light gray). C) Diary plot displays the increase in the latency of the ST-EPSCs during RTX. D) On average (n = 10), the mean latency of ST-EPSCs (large filled symbols) increased modestly in RTX. E) Diary plot of the representative example of panel A shows that the amplitude of the ST-EPSC did not change in 150 pM RTX and no failures were evident. F) On average (n = 10), RTX slightly but significantly reduced the mean amplitudes of the ST-EPSCs. G-I) RTX reduced only the amplitudes of the first EPSC in the burst of shocks to the ST but produced no increase in failures rates. Asterisks represent p < 0.05.
3.5. Low RTX sensitizes thermal responsiveness of spontaneous release
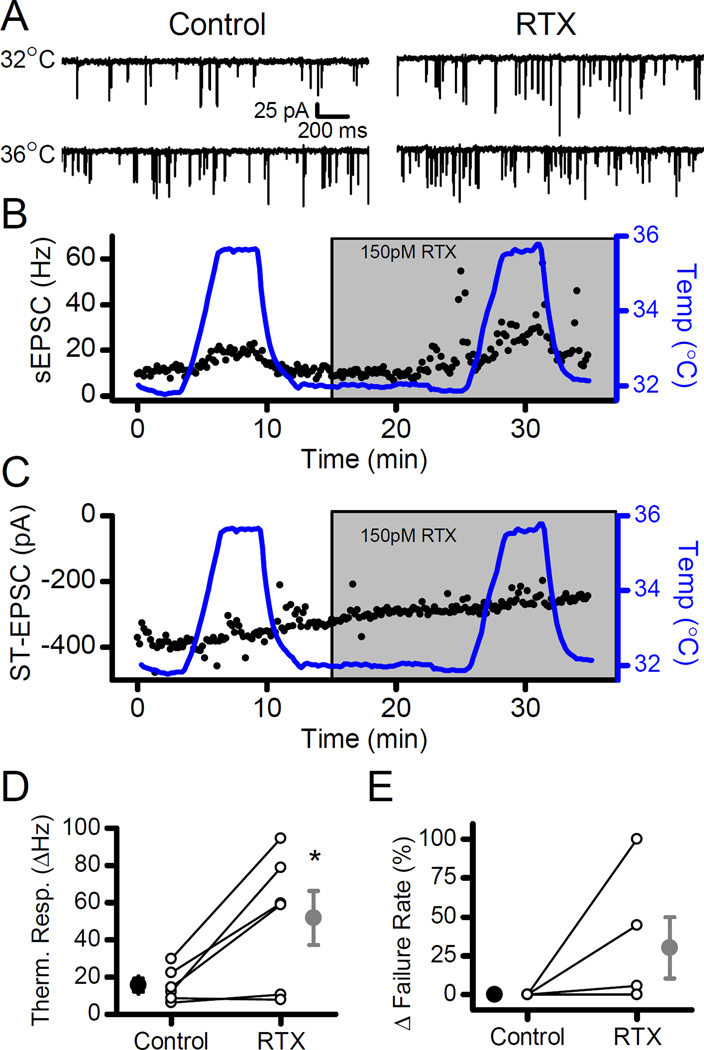
In spinal dorsal root ganglion neurons, the multiple gating mechanisms of TRPV1 provide cooperative gating in producing activated currents (Matta & Ahern, 2011). Given the much lower thermal threshold of TRPV1 coupled to glutamate release in ST afferents, we investigated whether cooperative interactions existed in glutamate release from cranial visceral afferents. Temperature challenges were performed on TRPV1+ NTS neurons in the absence and presence of 150 pM RTX. In individual neurons, RTX increased the frequency of sEPSCs at constant temperature (Fig. 6A). The response to thermal jumps from 32°C to 36°C increased substantially when repeated in the presence of 150 pM RTX in the same neurons (Fig. 6A). On average, the 4°C temperature elevation in the presence of RTX tripled the thermal response of sEPSC rates (Fig. 6D; n = 6, p = 0.03, paired t-test), but in the same neurons the evoked ST-EPSC amplitude and failure rate did not change (Fig. 6C and E; respectively, amplitudes, n = 4, p = 0.17; failure rates, n = 5, p = 0.19; paired t-test). Note that in two individual cases, failures increased significantly at 36°C with RTX (Fig. 6E). Overall, RTX sensitized thermally gated sEPSCs without affecting the ST evoked release process in the same neurons.
Figure 6.
Low-level vanilloid activation of TRPV1 sensitized the sEPSC rate increases to small thermal changes. A) Raising the temperature from 32°C to 36°C elevated the frequency of sEPSCs. RTX (150 pM) boosted the rate of sEPSCs at 32°C but dramatically augmented the frequency increase of sEPSCs in response to the thermal step to 36°C. B) Diary plot displays, first, the increased sEPSC frequency with RTX, followed by the elevated sEPSC frequency at 36°C with RTX. C) In the same neuron, RTX failed to alter the amplitudes of ST-EPSCs at 32°C or 36°C. D) On average (n = 6), the mean of the thermal response of sEPSC frequency (Δ frequency, large filled symbols) increased in RTX (gray) compared to Control (black). E) Overall, failure rates did not significantly change with a temperature challenge between RTX (gray) and Control (black). In two individual neurons, the failure rate of the ST-EPSC increased substantially at 36°C in the presence of RTX. All asterisks represent p < 0.05.
3.6. TRPV1 cooperativity does not rely on voltage dependent channels
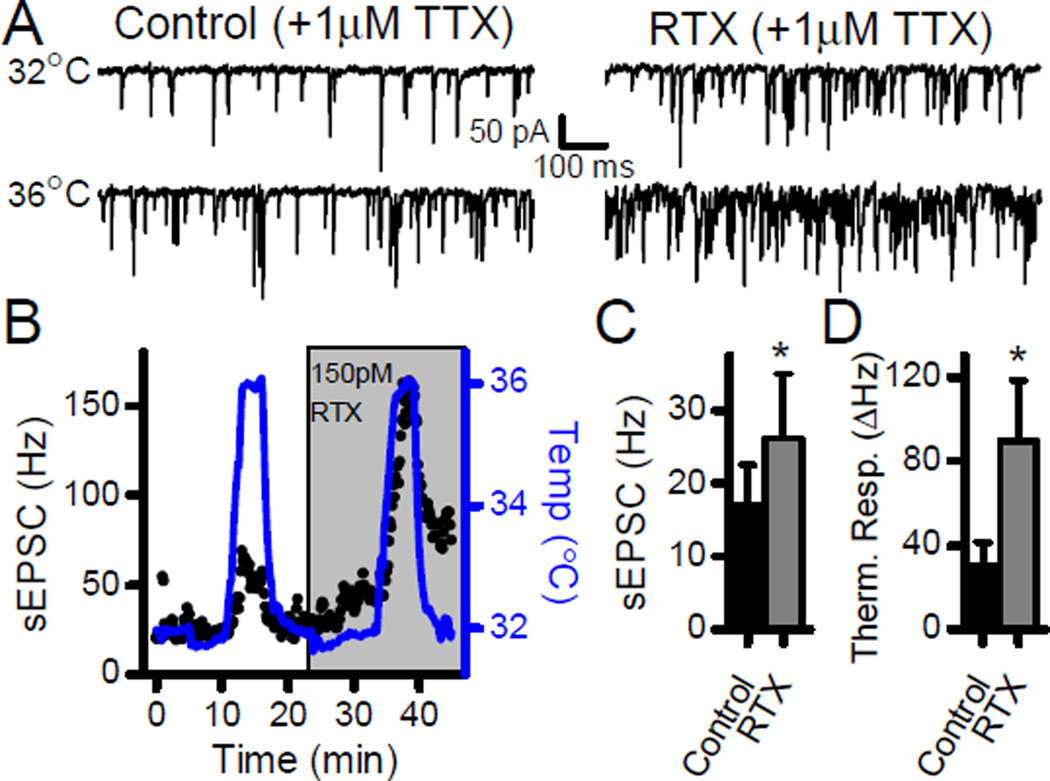
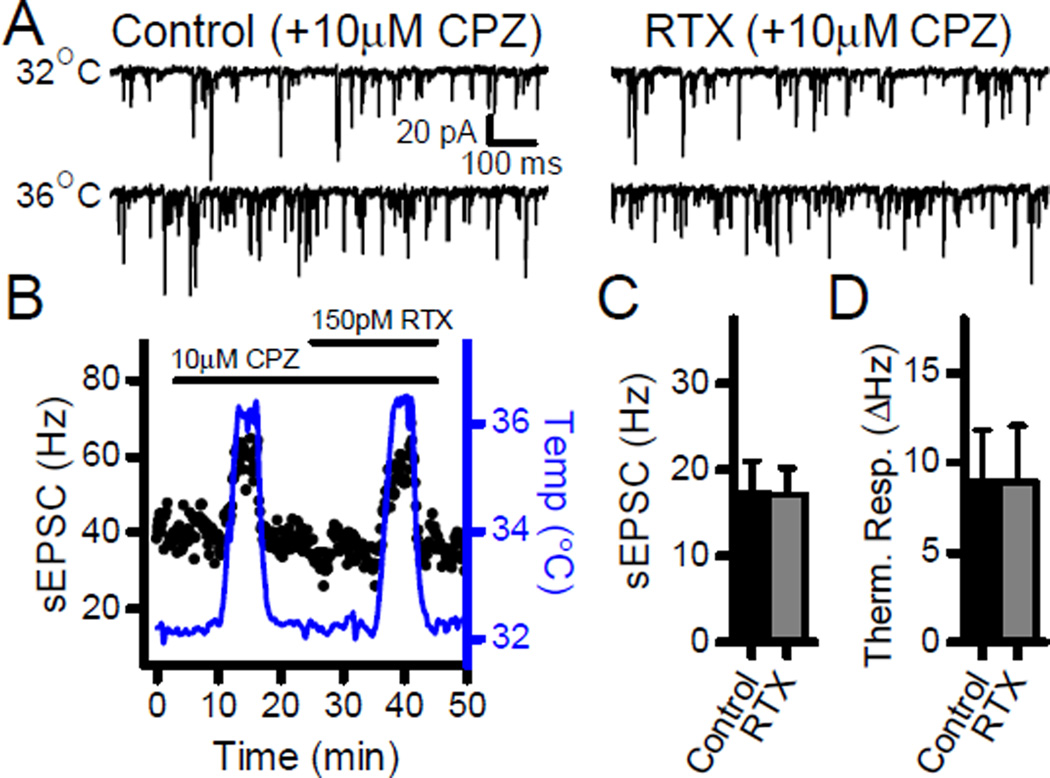
TRPV1 activation increases terminal calcium and causes terminal depolarization. To control for contributions from voltage dependent and/or conducted components of the response, we performed experiments in TTX (1 µM). Prior to TTX application, potential TRPV1+ second-order neurons were selected which displayed high basal and asynchronous sEPSC rates (data not shown). In TTX, 150 pM RTX significantly increased the sEPSC frequency at 32°C (Fig. 7; n = 5, p = 0.05, paired t-test), and tripled the sEPSC thermal response to a temperature challenge to 36°C (Fig. 7; n= 5, p = 0.05, paired t-test). Thus, TRPV1 activation facilitates responses to thermal challenges in the absence of network connections or local voltage activated processes. RTX is a very selective TRPV1 agonist and the TRPV1 antagonist capsazepine (CPZ, 10 µM) blocked the effects of RTX on sEPSC rates at 32°C (Fig. 8; n = 7, p = 0.91, paired t-test) as well as the RTX enhancement of the sEPSC thermal response (Fig. 8; n = 7, p = 0.99, paired t-test). Note that the competitive vanilloid antagonist CPZ blocked the effects of RTX on sEPSC rates at 32°C as well as the RTX-induced facilitation of the response to thermal challenges. CPZ did not block the temperature response itself in control conditions as thermal gating depends on a region of TRPV1 that is structurally independent of the vanilloid binding site (Grandl et al., 2010). Interestingly, CPZ also prevented the RTX mediated increase in latency of the ST-EPSC (n = 5, p = 0.08, paired t-test) indicating that each of these RTX actions depended on binding to the vanilloid site within TRPV1.
Figure 7.
RTX sensitization of thermally responsive sEPSCs does not require voltage dependent or conducted network activity. A) The sodium channel blocker TTX (1 µM) did not alter the RTX increases in sEPSC frequency at 32°C or at 36°C. B) Diary plot of the representative neuron demonstrates the dramatically elevated response to temperature in the presence of RTX. C) On average (n = 7), the summary plot demonstrates that RTX increased the frequency of sEPScs in the presence of TTX. D) Summary plot shows the increased thermal response of sEPSC rates to thermal challenges in the presence of RTX (n = 5). All asterisks represent p < 0.05.
Figure 8.
The effects of RTX on spontaneous release required TRPV1. A–B) The TRPV1 antagonist capsazepine (CPZ, 10 µM) prevented the increase in sEPSC frequency with RTX. In addition, it also blocked the RTX-induced sensitization of thermally responsive sEPSCs at 36°C. C) On average (n = 7), the summary shows that CPZ blocked the increase in sEPSCs caused by RTX. D) Summary displays that CPZ prevented the thermal sensitization induced by RTX (n = 7).
4. Discussion
Multimodal gating of TRPV1 (heat, H+, and vanilloids) is a molecular feature of this calcium permeable ion channel and these multiple activators provide the keys to TRPV1’s wide dynamic range of integrative function (Tominaga et al., 1998; Blackshaw, 2014). Our studies focused on TRPV1 coupling to synaptic transmission in primary sensory afferents. In this system, TRPV1 activity translates into the rate of sEPSCs and has surprisingly few effects on evoked glutamate release from the same ST inputs. We investigated potential interactions of two key TRPV1 gating triggers – heat and vanilloid binding – at cranial afferents within the NTS. High levels of TRPV1 activation, with CAP or RTX, acted similarly on glutamate transmission by increasing the sEPSC frequency but blocked the action potential evoked ST-EPSCs. These opposite actions, measured concurrently within neurons, suggest key differences in the mechanisms governing spontaneous and evoked glutamate release. In contrast to high concentrations of vanilloids, modest activation of TRPV1 with either low RTX concentrations or thermal activation increased sEPSC rates without altering the evoked ST-EPSCs. In the presence of modest TRPV1 activation with RTX, thermal steps yielded substantially greater sEPSC rate increases and these responses persisted in TTX. Thus, vanilloid binding amplified the responsiveness of TRPV1 to thermal steps – a result consistent with cooperative gating at TRPV1 in the control of spontaneous glutamate release without promoting evoked glutamate synaptic transmission.
4.1. Similar evoked ST transmission in myelinated and unmyelinated ST afferents
TRPV1 expression distinguishes unmyelinated C-fiber afferents from the myelinated A-fibers in NTS transmission (Doyle et al., 2002; Jin et al., 2004). All aspects of ST action potential evoked glutamate release are indistinguishable between TRPV1+ and TRPV1- inputs and a key aspect of ST evoked transmission is conduction to the synaptic terminals (Andresen & Peters, 2008). The local anesthetic QX-314 slowed conduction, observed as an increase in latency, and ultimately caused the failure of ST-EPSCs similarly in both TRPV1+ and TRPV1- afferents (Hofmann et al., 2014). Voltage dependent ionic currents, such as Na+ currents, presumably underlie the sensitivity of the axon conduction step of ST transmission to temperature and local anesthetics, irrespective of TRPV1 expression. In contrast, sEPSCs in neurons receiving TRPV1+ afferents are not altered by local anesthetics demonstrating independence from the voltage-dependent processes of the spontaneous release mechanism (Hofmann et al., 2014). These characteristics support the absolute dependence of evoked release on voltage-dependent processes including voltage activated calcium channels (VACCs) and reinforce the conclusion that spontaneous release depends on a calcium source separate from voltage dependent ion channels tied to evoked ST-EPSCs – a major distinction for the two forms of glutamate release.
4.2. Vanilloids induce afferent terminal depolarization
Calcium influx is clearly required for glutamate release but the source for sEPSC transmission depends strongly on influx through TRPV1 (Peters et al., 2010; Shoudai et al., 2010). TRPV1 essentially acts as a calcium channel due to the high permeability of calcium, but it also passes some sodium ions contributing to the depolarizing actions of TRPV1 activation (Caterina et al., 1997). In rat dorsal root ganglion neurons, activation of TRPV1 increased the intracellular sodium concentration and decreased the sodium current (Onizuka et al., 2011). The influx of cations through TRPV1 depolarizes neurons but the magnitude depends on the agonist concentration (Chung et al., 2008). In our experiments, CAP or RTX application increased the latency of the ST-EPSC prior to eventual failure of evoked transmission. These vanilloid actions are consistent with an effect on the voltage-dependent process of action potential conduction (Hofmann et al., 2014). In contrast, lowering the concentration of RTX, while still increasing sEPSC frequencies, only slightly increased the latencies of evoked ST-EPSCs and did not cause any failures of the ST-EPSCs. Thus, sustained depolarization by strong activation of TRPV1 likely causes substantial inactivation of voltage activated ion channels that delay and then block action potential conduction, whereas the minor delays and amplitude reductions during low-level activation of TRPV1 are consistent with more limited depolarization and thus reduced inactivation of voltage activated channels.
4.3. Functional separation of spontaneous and evoked transmission
The exocytosis of glutamate vesicles requires external calcium entry, although different proteins may govern spontaneous and evoked release (Pang & Sudhof, 2010; Kavalali, 2014). In the NTS, evoked release depends on calcium through VACCs (Mendelowitz et al., 1995). Yet, blocking VACCs fails to prevent TRPV1-mediated increases in calcium that augment spontaneous transmission (Jin et al., 2004; Peters et al., 2010; Shoudai et al., 2010; Fawley et al., 2011). Surprisingly, the calcium entering through TRPV1 did not increase the amplitudes of evoked ST-EPSCs irrespective of the mechanism for TRPV1 activation. Instead, strong vanilloid TRPV1 activation (high concentrations of CAP or RTX) induced sudden failures in ST-EPSCs, while TRPV1 activated by temperature or low concentrations of RTX did not facilitate evoked release. Variance-mean analysis of ST-EPSCs using calcium concentration indicates a parabolic relationship between ST-EPSC amplitude and amplitude variance as a measure of changes in release probability (Bailey et al., 2006; Andresen & Peters, 2008). In the present experiments, increasing calcium influx through TRPV1 did not alter the evoked ST-EPSC variance. In addition, calcium through TRPV1 did not alter the amplitude or failure rate of subsequent ST-EPSCs to bursts of stimuli, which provide additional measures of calcium effects on synaptic transmission (Zucker & Regehr, 2002). Taken together, our results suggest that the calcium entering through TRPV1 does not have access to the same spaces as calcium entering through VACCs. Thus, these observations are consistent with TRPV1 calcium interacting with different vesicles from those underlying evoked release and this independence may represent vesicle populations in microdomains governing each type of release at TRPV1+ afferents (Eggermann et al., 2012). This idea of physical or functional separation is consistent with CB1 inhibition of the ST-EPSCs (likely an action at VACCs) without altering the sEPSC frequency within the same neuron (Fawley et al., 2014) – a differential GPCR regulation of evoked release separate from spontaneous release. Whether the key molecular constituents governing spontaneous and evoked release differ substantially in the NTS remains unknown (Kavalali, 2014).
4.4. Cross-modal interactions in TRPV1+ ST presynaptic terminals
The molecular structure of TRPV1 makes it possible for integration of more than one stimulus and this is both a major physiological attribute to its behavior in vivo as well as a major difficulty for targeted drug development (Szallasi et al., 2007). Mutagenesis investigations identified unique and non-overlapping domains responsible for gating TRPV1 including the capsaicin-recognition region of TRPV1 (Grandl et al., 2010; Nilius & Szallasi, 2014). Much of this TRPV1 work is based on expression systems, isolated primary afferent neurons, and neurotransmission within the spinal dorsal horn (Szolcsanyi & Sandor, 2012). In NTS, the strong activation of TRPV1 at physiological temperature and its attendant high sEPSC rates are distinctions of TRPV1 operation in the cranial visceral afferent terminals making this mechanism effectively autonomous – i.e. not requiring sensory discharge. For example, dorsal root TRPV1 requires noxious temperatures (e.g. >42°C) to reach threshold for activation (Caterina et al., 1997) compared to the removal of high sEPSC rates by cooling a few degrees below physiological levels in TRPV1+ second order NTS neurons (Shoudai et al., 2010). TRPV1 antagonists can have differential profiles for interrupting TRPV1 gating by heat, pH or vanilloid (Wong & Gavva, 2009), but differences across tissues, especially compared expression systems, are poorly understood. Antagonists designed to block heat activation of cloned TRPV1 at 42°C do not alter the thermal release of glutamate in NTS second order neurons and might reflect unknown splice variants or other factors (unpublished observations). Dorsal root TRPV1 exhibits pronounced cross-modal facilitation and GPCR cross-sensitization (Matta & Ahern, 2011). In the NTS, 150 pM RTX dramatically increased the thermal sensitivity so that the changes in sEPSC frequency were much greater in response to the same 4°C temperature change. Consistent with these results, the endocannabinoid N-arachidonyldopamine also increased the sEPSC response to temperature elevation (Fawley et al., 2014). Taken together, the sensitization of thermal glutamate release in response to endocannabinoid or vanilloid application provides evidence for cooperative gating within TRPV1 at ST afferent terminals. The diversity of potential endogenous activators for TRPV1 expands the potential for sensitization of thermal gating at TRPV1 (Morales-Lazaro et al., 2013; Premkumar & Abooj, 2013). Indirect phosphorylation signals as well as direct binding by bioactive lipid metabolites also alter the temperature gating of TRPV1 providing additional potential mechanisms for TRPV1 sensitization (Vellani et al., 2001; Hu et al., 2002; Cao et al., 2013).
The presence of highly sensitized and active TRPV1 receptors on 75–80% of all of the ST afferents in caudal NTS raises the prospect of a wide range of potential interactions during pathophysiological circumstances. Evidence of TRPV1 within NTS acting on the neonatal laryngeal chemoreflex (LCR) suggests a role in sudden infant death syndrome (Kinney & Thach, 2009). The LCR triggers an airway-protective reflex by inducing prolonged apnea, swallowing, and coughing (Curran et al., 2005). A focal 2–2.5°C temperature increase to the NTS sub region facilitated the reflex apnea, and this sensitization could be ameliorated by microinjection of 5’iodoresiniferatoxin, an RTX-based TRPV1 antagonist (Curran et al., 2005; Xia et al., 2007; Xia et al., 2011). Given the ability of TRPV1 to transduce inflammatory signals, TRPV1 in ST afferents offers a potential mechanistic frame work for the well-established clinical risk factors for sudden infant death syndrome including hyperthermia and infection (Leiter & Bohm, 2007; Kinney & Thach, 2009). Thus, the predominance of highly active TRPV1 expressing ST terminals susceptible to TRPV1 sensitization may contribute to other pathological states such as cough (Materazzi et al., 2009) and obesity (Marshall et al., 2013).
Highlights.
Spontaneous and evoked release draw from separate pools of vesicles in NTS
Vanilloid activation of TRPV1 triggers spontaneous release but blocks evoked release
Low levels of vanilloids or thermal elevations only trigger spontaneous release
Co-activation of TRPV1 by vanilloids causes sensitization to thermal changes
TRPV1 sensitization only affects spontaneous release and not evoked release
Acknowledgements
This work was supported by grants from the National Institutes of Health, R01-HL-105703 (MCA) and F32 HL112419 (MEH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the NIH.
Abbreviations
- CAP
capsaicin
- CPZ
capsazepine
- NTS
nucleus of the solitary tract
- RTX
resiniferatoxin
- sEPSC
spontaneous EPSC
- ST
solitary tract
- ST-EPSC
solitary tract evoked EPSC
- TRPV1
transient receptor potential vanilloid type 1
- VACC
voltage activated calcium channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None.
References
- Andresen MC, Hofmann ME, Fawley JA. Invited Review: The un-silent majority - TRPV1 drives "spontaneous" transmission of unmyelinated primary afferents within cardiorespiratory NTS. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1207–R1216. doi: 10.1152/ajpregu.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen MC, Peters JH. Comparison of baroreceptive to other afferent synaptic transmission to the solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2008;295:H2032–H2042. doi: 10.1152/ajpheart.00568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Jin Y-H, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci. 2006;26:6131–6142. doi: 10.1523/JNEUROSCI.5176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA. Transient receptor potential cation channels in visceral sensory pathways. Br J Pharmacol. 2014;171:2528–2536. doi: 10.1111/bph.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Babic T, Holmes GM, Swartz E, Travagli RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J Physiol. 2013;591:1563–1580. doi: 10.1113/jphysiol.2012.246827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. NatNeurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- Curran AK, Xia L, Leiter JC, Bartlett D., Jr Elevated body temperature enhances the laryngeal chemoreflex in decerebrate piglets. J ApplPhysiol. 2005;98:780–786. doi: 10.1152/japplphysiol.00906.2004. [DOI] [PubMed] [Google Scholar]
- del Castillo J, Katz B. Quantal components of the end-plate potential. The Journal of Physiology. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic transmission in brain stem neurons in vitro. J Neurophysiol. 2001;85:2213–2223. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin Y-H, Andresen MC. Vanilloid receptors presynaptically modulate visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci. 2002;22:8222–8229. doi: 10.1523/JNEUROSCI.22-18-08222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin Y-H, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. JNeurosciMethods. 2004;37:37–48. doi: 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci. 2012;13:7–21. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Fawley JA, Hofmann ME, Andresen MC. Cannabinoid 1 and Transient Receptor Potential Vanilloid 1 receptors discretely modulate evoked glutamate separately from spontaneous glutamate transmission. J Neurosci. 2014;34:8324–8332. doi: 10.1523/JNEUROSCI.0315-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawley JA, Hofmann ME, Largent-Milnes TM, Andresen MC. Temperature differentially facilitates spontaneous but not evoked glutamate release from cranial visceral primary afferents. PLoS One. 2015;10:e0127764. doi: 10.1371/journal.pone.0127764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawley JA, Peters JH, Andresen MC. GABAB-mediated inhibition of multiple modes of glutamate release in the nucleus of the solitary tract. J Neurophysiol. 2011;106:1833–1840. doi: 10.1152/jn.00476.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M. Selective inhibition of spontaneous but not Ca2+ -dependent release machinery by presynaptic group II mGluRs in rat cerebellar slices. J Neurophysiol. 2006;96:86–96. doi: 10.1152/jn.01282.2005. [DOI] [PubMed] [Google Scholar]
- Grandl J, Kim SE, Uzzell V, Bursulaya B, Petrus M, Bandell M, Patapoutian A. Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat Neurosci. 2010;13:708–714. doi: 10.1038/nn.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann ME, Bhatia C, Frazier CJ. Cannabinoid receptor agonists potentiate action potential-independent release of GABA in the dentate gyrus through a CB1 receptor-independent mechanism. J Physiol. 2011;589:3801–3821. doi: 10.1113/jphysiol.2011.211482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann ME, Largent-Milnes TM, Fawley JA, Andresen MC. External QX-314 inhibits evoked cranial primary afferent synaptic transmission independent of TRPV1. J Neurophysiol. 2014;112:2697–2706. doi: 10.1152/jn.00316.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Bhave G, Gereau RW. Prostaglandin and protein kinase a-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. JNeurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals. J Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaszas K, Keller JM, Coddou C, Mishra SK, Hoon MA, Stojilkovic S, Jacobson KA, Iadarola MJ. Small molecule positive allosteric modulation of TRPV1 activation by vanilloids and acidic pH. J Pharmacol Exp Ther. 2012;340:152–160. doi: 10.1124/jpet.111.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2014;16:5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. NEnglJ Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largent-Milnes TM, Hegarty DM, Aicher SA, Andresen MC. Physiological temperatures drive glutamate release onto trigeminal superficial dorsal horn neurons. J Neurophysiol. 2014;111:2222–2231. doi: 10.1152/jn.00912.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter JC, Bohm I. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome. RespirPhysiol Neurobiol. 2007;159:127–138. doi: 10.1016/j.resp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Marshall NJ, Liang L, Bodkin J, Dessapt-Baradez C, Nandi M, Collot-Teixeira S, Smillie SJ, Lalgi K, Fernandes ES, Gnudi L, Brain SD. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension. 2013;61:246–252. doi: 10.1161/HYPERTENSIONAHA.112.201434. [DOI] [PubMed] [Google Scholar]
- Materazzi S, Nassini R, Gatti R, Trevisani M, Geppetti P. Cough sensors. II. Transient receptor potential membrane receptors on cough sensors. Handb Exp Pharmacol. 2009:49–61. doi: 10.1007/978-3-540-79842-2_3. [DOI] [PubMed] [Google Scholar]
- Matta JA, Ahern GP. TRPV1 and synaptic transmission. Curr Pharm Biotechnol. 2011;12:95–101. doi: 10.2174/138920111793937925. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Yang M, Reynolds PJ, Andresen MC. Heterogeneous functional expression of calcium channels at sensory and synaptic regions in nodose neurons. J Neurophysiol. 1995;73:872–875. doi: 10.1152/jn.1995.73.2.872. [DOI] [PubMed] [Google Scholar]
- Morales-Lazaro SL, Simon SA, Rosenbaum T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1) J Physiol. 2013;591:3109–3121. doi: 10.1113/jphysiol.2013.251751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Szallasi A. Transient Receptor Potential channels as drug targets: From the science of basic research to the art of medicine. In. Pharmacol Rev. 2014:676–814. doi: 10.1124/pr.113.008268. Copyright (c) 2014 by The American Society for Pharmacology and Experimental Therapeutics. [DOI] [PubMed] [Google Scholar]
- Onizuka S, Yonaha T, Tamura R, Hosokawa N, Kawasaki Y, Kashiwada M, Shirasaka T, Tsuneyoshi I. Capsaicin indirectly suppresses voltage-gated Na+ currents through TRPV1 in rat dorsal root ganglion neurons. Anesth Analg. 2011;112:703–709. doi: 10.1213/ANE.0b013e318204ea5b. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Sudhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron. 2010;65:657–669. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Abooj M. TRP channels and analgesia. Life Sci. 2013;92:415–424. doi: 10.1016/j.lfs.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds PJ, Fan W, Andresen MC. Capsaicin- resistant arterial baroreceptors. J NegatResults Biomed. 2006;5:6. doi: 10.1186/1477-5751-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci. 2010;30:14470–14475. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. PharmacolRev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. NatRevDrug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J, Sandor Z. Multisteric TRPV1 nocisensor: a target for analgesics. Trends Pharmacol Sci. 2012;33:646–655. doi: 10.1016/j.tips.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain ResRev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Xia L, Bartlett D, Jr, Leiter JC. TRPV1 channels in the nucleus of the solitary tract mediate thermal prolongation of the LCR in decerebrate piglets. Respir Physiol Neurobiol. 2011;176:21–31. doi: 10.1016/j.resp.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Damon TA, Leiter JC, Bartlett D., Jr Focal warming in the nucleus of the solitary tract prolongs the laryngeal chemoreflex in decerebrate piglets. J ApplPhysiol. 2007;102:54–62. doi: 10.1152/japplphysiol.00720.2006. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. AnnuRevPhysiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]