Abstract
We evaluated the effects of the supplementation with L-glutamine and glutamine dipeptide (GDP) on biochemical and morphophysiological parameters in streptozotocin-diabetic rats. For this purpose, thirty animals were distributed into six groups treated orally (gavage) during thirty days: non diabetic rats (Control) + saline, diabetic + saline; Control + L-glutamine (248 mg/kg), Diabetic + L-glutamine (248 mg/kg), Control + GDP (400 mg/kg), Diabetic + GDP (400 mg/kg). Diabetes was induced by an intravenous injection of streptozotocin (60 mg/kg) and confirmed by fasting glucose ≥ 200 mg/dL. Physiological parameters, i.e., body mass, food intake, blood glucose, water intake, urine and faeces were evaluated during supplementation. After the period of supplementation, the animals were euthanized. The blood was collected for biochemical assays (fructosamine, transaminases, lipid profile, total protein, urea, ammonia). Moreover, the jejunum was excised and stored for morphophysiological assays (intestinal enzyme activity, intestinal wall morphology, crypt proliferative index, number of serotoninergic cells from the mucosa, and vipergic neurons from the submucosal tunica). The physiological parameters, protein metabolism and intestinal enzyme activity did not change with the supplementation with L-glutamine or GDP. In diabetic animals, transaminases and fructosamine improved with L-glutamine and GDP supplementations, while the lipid profile improved with L-glutamine. Furthermore, both forms of supplementation promoted changes in jejunal tunicas and wall morphometry of control and diabetic groups, but only L-glutamine promoted maintenance of serotoninergic cells and vipergic neurons populations. On the other hand, control animals showed changes that may indicate negative effects of L-glutamine. Thus, the supplementation with L-glutamine was more efficient for maintaining intestinal morphophysiology and the supplementation with GDP was more efficient to the organism as a whole. Thus, we can conclude that local differences in absorption and metabolism could explain the differences between the supplementation with L-glutamine or GDP.
Introduction
L-glutamine is considered a conditionally essential amino acid [1]. It is the precursor of peptides, proteins, neurotransmitters, nitrogenous bases and is used as an energy source by various organs such as the intestine. Other functions are assigned to this amino acid, such as maintenance of cell proliferation, immune function, acid-base balance and regulation of gene expression [2].
However, there are limitations of using L-glutamine as a supplement, such as low solubility in water and instability, especially during heat sterilization and prolonged storage. This led to the development of more stable synthetic forms, such as dipeptides with L-glutamine residues that are highly soluble in water and more resistant to thermal shock and prolonged storage [3].
In humans, approximately half of the oral L-glutamine is used by the enterocytes, generating a plasma increase around 50% of the amount supplied [4]. However, the intestinal absorption of L-glutamine in the form of glutamine dipeptide (GDP) is more effective than the free form [5].
Diabetes is a chronic disease characterized by decreased blood levels of L-glutamine [6]. Diabetes also affects the gastrointestinal tract, generating enzymatic, morphological and functional changes [7–9]. The hyperglycemia in human diabetes increases oxidative stress [10], which affects the enteric nervous system (ENS), composed of the submucous and myenteric ganglionated plexuses, which coordinate secretion, blood flow and motility of the digestive tract [9].
It is suggested that in diabetes the supplementation with L-glutamine can positively act reducing the oxidative stress and enteric neurodegeneration [10–13]. Moreover, considering that the intestinal absorption of peptides is increased in diabetes [7], supplementation with L-glutamine or GDP could influence the tissue response.
Thus, the purpose of this study was to evaluate the effects of the supplementation with L-glutamine or GDP on biochemical and morphophysiological parameters in streptozotocin-diabetic rats.
Materials and Methods
Drugs and chemicals
L-glutamine and GDP were obtained from Ajinomoto, Japan. Streptozotocin was purchased from Sigma-Aldrich, USA; glucometer and strips Optium Xceed were from Abbott, Brazil; while Thionembutal was purchased from Abbott Laboratories, USA. The blood laboratory kits were obtained from Gold Analisa Diagnostics Ltd., Brazil.
Anti-PCNA, anti-serotonin, secondary Alexa Fluor 488 antibodies, and Prolong Gold Antifade were purchased from Life Technologies, USA. Anti-VIP antibody was obtained from Bachem Americas, EUA. All other reagents were of the best quality available.
Animals and treatment
Thirty male Wistar rats (Rattus norvegicus, 50 days, 188,9±9,4 g), from the Central Animal House of the State University of Maringá, were kept under controlled temperature (23°C / 25°C) and 12 h light/dark cycles, receiving standard diet (Nuvital®—Nuvilab, Colombo, PR, Brazil) and water ad libitum. All experimental protocols were approved by the Ethics Commission on the Use of Animals (CEUA/UEM—137/2013).
The animals were distributed into 6 groups (n = 5) that received by gavage during 30 days: control group (C group) and diabetic group (D group) received saline; control glutamine group (CG group) and diabetic glutamine group (DG group) received L-glutamine (248 mg/kg); control GDP group (CGDP group) and diabetic GDP group (DGDP group) received GDP (400 mg/kg). During the 30 days of the experimental period, body mass, food intake, water ingestion, urine volume, blood glucose and faeces were evaluated.
Induction and confirmation of the diabetic state
The animals in groups D, DG and DGDP, after overnight fasting (14 h), received an intravenous injection of streptozotocin (60 mg/kg body mass) dissolved in citrate buffer, pH 4.5 (10 mM), to induce experimental type 1 diabetes. Animals with glycemia above 200 mg/dL (glucometer and strips) were considered diabetic [14]. The control animals (C, CG and CGDP) received an intravenous injection of citrate buffer.
Tissue collection and material processing
After thirty days of supplementation, the animals were intraperitoneally anesthetized (40 mg/kg body mass) with intraperitoneal thionembutal. Blood samples were collected by cardiac puncture and the serum was used to measure total protein, triglycerides, total cholesterol and fractions, aspartate aminotransferase (AST), alanine aminotransferase (ALT); fructosamine; ammonia and urea.
Vertical laparotomy was made for collection and measurement of the length of the small intestine (SI). Subsequently, jejunal samples were separated and washed in phosphate buffered saline (PBS, 0.1 M, pH 7.3), and fixed either (1) in liquid nitrogen for analysis of the specific activity of intestinal enzymes; or (2) in 4% paraformaldehyde for histological and immunohistochemical processing.
Analysis of intestinal enzymes
Samples of the jejunum were macerated, suspended in sodium phosphate buffer (50 mM, pH 6.5) and centrifuged under refrigeration. The supernatant was used to determine the levels of alkaline phosphatase [15], lipase [16], β-galactosidase [15] and maltase [17] in a spectrophotometer (Shimadzu UV-VIS—UV1800).
A unit of enzyme activity (U) was defined as the amount of enzyme that produced 1.0 μmol per mL of product per min under the assay conditions. The specific activity was expressed as U/g of jejunum (wet weight).
Processing and histological analysis
After 6 h of fixation in 4% paraformaldehyde, jejunum samples were dehydrated in increasing series of alcohols, cleared in xylene and embedded in paraffin. Then, 6 μm thick semiserial transversal sections were made with Leica RM 2145 microtome.
Morphometric analysis of the intestinal wall components
The histological sections were stained with hematoxylin and eosin (HE) for morphologic and morphometric analysis of jejunal wall components. The images were captured under a light microscope (Olympus BX41, Olympus America Inc., New York, USA) coupled to a high resolution camera (Olympus Q Color 3 Olympus America Inc., New York, USA) under a 10x objective to analyze the total wall thickness, mucosa and submucosa, and height of the villi. The crypt depth was measured under a 20x objective. One hundred measurements of each parameter were performed (10 points per section) per animal using the program Image Pro Plus 4.5 (Media Cybernetics, Maryland, USA).
Proliferating cells and serotoninergic indexes
Histological sections of the jejunum kept on slides with poly-L-lysine were deparaffinized, hydrated and treated with H2O2 (3%) in methanol for 10 min to eliminate endogenous peroxidase activity. After two 5 min washes with phosphate-buffered saline (PBS; pH 7.4; 0.1 M), the sections were blocked with a solution containing goat serum (10%) for 10 min. The tissues were then incubated with a solution containing anti-PCNA (proliferating cell nuclear antigen) (Cat# 18–0110, AB_86659) or rabbit anti-serotonin primary antibody (Cat# 18–0077, AB_86641) diluted 1:200 in PBS for 2 h. After two 5 min washes with PBS (pH 7.4; 0.1 M), the sections were incubated with biotinylated secondary antibody (broad spectrum kit solution) for 10 min, washed again, and treated with the streptavidin-peroxidase conjugate for 10 min. After further washes with PBS to remove excess enzyme conjugate, the immunohistochemical reaction was revealed by diaminobenzidine (DAB) in PBS and H2O2 for 15 min. After washing in distilled water, the sections were counterstained with hematoxylin, dehydrated in ethanol, diaphanized in xylene, and mounted under coverslips with Permount synthetic resin. All the procedures were performed at room temperature. The Histostain Plus Kit was used to perform this technique
To assess cell proliferation (PCNA), the slides were analyzed under light microscope, under 40x objective; 2500 crypt cells per animal were recorded to obtain the percentage of labeled to unlabeled cells (proliferation index). In the immunostained serotoninergic cells 2500 epithelial cells from longitudinally oriented villi and crypts were counted, to obtain the ratio of labeled cells. For both techniques, the index was calculated by the number of labeled cells*100 / total number of cells counted.
Labeling and quantification of immunoreactive VIP-neurons
After fixation in paraformaldehyde, jejunal samples were dissected under a stereomicroscope with transillumination to obtain whole-mounts from the submucosa. Subsequently the whole-mounts were washed (2 x 10 min) with PBS plus detergent Triton X-100 0.5% (T) and incubated in blocking solution containing 2% bovine serum albumin (BSA) and 10% donkey serum (1 h). Then, the tissues were incubated for 48 h with primary anti-VIP antibody (Cat# T-4245.0400, AB_518686) diluted (1:500) in a mother solution (PBS, 1% BSA and 10% donkey serum), washed in PBS+T (2 x 10 min), incubated for 2 h with secondary antibody (Alexa Fluor 488; Cat# A21206, AB_10049650) diluted in mother solution (1:500) and washed with PBS (2 x 10 min). Subsequently the whole-mounts were mounted in slides with Prolong Gold Antifade Reagents. All of the procedures were performed at room temperature.
The slides were examined under a fluorescence microscope (Olympus FSX100). The VIP-immunoreactive neurons found in 40 microscopic fields under 20x objective were counted, with a total analyzed area of 5.87 mm2 /animal.
Statistical analysis
The obtained data were subjected to the Kolmogorov-Smirnov normality test. Parametric data (body mass, food intake, blood glucose, intestinal length, blood biochemical analysis, intestinal enzyme activity, proliferative and serotoninergic cells number) were subjected to analysis of variance (ANOVA) followed by Tukey's post-test. For nonparametric data (morphometry of intestinal tunicas and quantification of VIP-immunoreactive neurons) the Kruskal-Wallis test followed by Dunns post-test was adopted. The results were presented as mean ± standard error (SEM). The statistical data were analyzed using GraphPad Prism program (GraphPad Software, version 5.0, USA) and considered significant at p<0.05.
Results
Physiological parameters
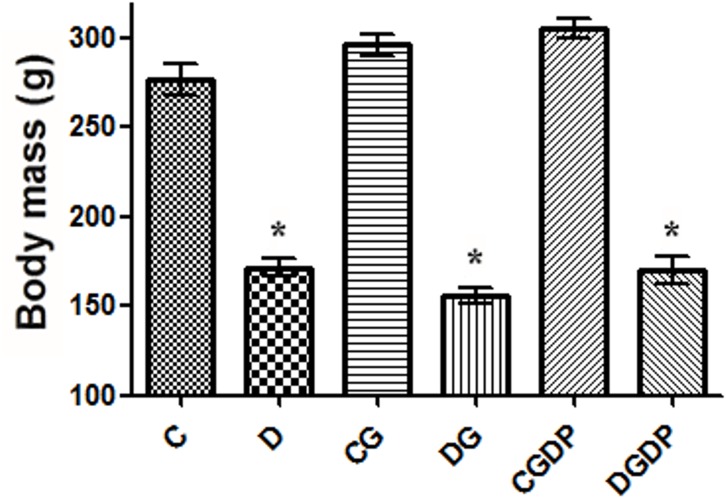
The streptozotocin-diabetic animals (D groups) showed the classic signs of the disease such as hyperglycemia (Table 1), lower body mass (Fig 1), and polyphagia (p<0.05) when compared to group C. The food intake was higher (p<0.05) in groups D (32.4±1.6 g/d) and DGDP (32.7±1.0 g/d) compared to the control group C (24.5±0.7 g/d), while that of the DG group was not significantly different (31.7±2.0 g/d). The CG (30.3±1.4 g/d) and CGDP (28.3±1.7 g/d) groups did not differ statistically from C (p>0.05). The supplementation with L-glutamine did not affect any of these parameters, but glutamine dipeptide (CGDP) promoted an increase (p<0.05) in body mass only relative to the control group C, while food intake and blood glucose levels (Table 1) were not affected (p>0.05). Also, polydipsia, polyuria, and constant diarrhea were observed in diabetic animals.
Table 1. Blood biochemical parameters after 30 days receiving saline (C and D groups), L-glutamine (G) or glutamine dipeptide (GDP) in non diabetic rats (C group), and diabetic rats (D).
The animais were distributed in six groups: C, D, CG, DG, CGDP, and DGDP.
| C | D | CG | DG | CGDP | DGDP | |
|---|---|---|---|---|---|---|
| Fasting glucose (mg/dL) | 76.6±3.0 | 385.8±10.2* | 67±2.5 | 417.0±31.3 | 82.0±2.9 | 394.6±20.7 |
| Fructosamine (mmol/L) | 0.88±0.02 | 1.55±0.08* | 0.62±0.04 | 1.62±0.07 | 0.72±0.04 | 1.18±0.05# |
| AST (U/L) | 41.15±2.7 | 205.40±36.1* | 64.57±6.9 | 104.40±8.27 | 69.21±10.1 | 135.90±13.2 |
| ALT (U/L) | 12.08±1.3 | 232.30±33.7* | 13.16±0.7 | 122.90±13.2# | 21.80±1.8 | 260.30±14.3 |
| Triglycerides (mg/dL) | 28.50±1.2 | 71.38±7.8* | 59.13±3.3 | 42.63±5.9# | 58.70±11.0 | 71.83±10.2 |
| Total cholesterol (mg/dL) | 62.37±4.2 | 44.41±1.3 | 73.32±5.3 | 61.13±5.3# | 86.35±1.3* | 45.72±2.9 |
| HDL cholesterol (mg/dL) | 43.63±1.7 | 23.63±1.2* | 38.63±1.5 | 38.13±3.8# | 43.60±2.3 | 28.90±2.1 |
| Total protein (mg/dL) | 5.93±0.1 | 5.43±0.2 | 5.39±0.08 | 5.77±0.2 | 6.42±0.1 | 5.67±0.3 |
| Ammonia (mg/dL) | 3.05±0.06 | 3.42±0.1 | 3.05±0.08 | 3.236±0.2 | 2.89±0.1 | 2.73±0.2 |
| Urea (mg/dL) | 24.21±0.7 | 66.91±1.8* | 30.69±1.5 | 58.97±1.8 | 30.86±2.7 | 66.87±3.2 |
Results expressed as mean±SEM (n = 5/group).
* p<0.05 vs. C group
# p<0.05 vs. D group
one-way ANOVA and Tukey post test.
Fig 1. Body mass after 30 days receiving saline (C and D groups), L-glutamine (G) or glutamine dipeptide (GDP) in non diabetic rats (C groups), and diabetic rats (D).
The animais were distributed in six groups: C, D, CG, DG, CGDP, and DGDP. Results presented as mean±SEM (n = 5/group). * p<0.05 compared to group C; one-way ANOVA and Tukey post test.
Table 1 shows the blood biochemical profile of the animals. Confirming diabetes, high levels of fructosamine were detected (p<0.05) in D, DG and DGDP groups compared with their respective controls and a reduction (p<0.05) for DGDP compared to the untreated group (D). Liver enzymes (AST and ALT), indicative of liver tissue damage, showed high levels (p<0.05) in groups D and DGDP compared with their respective controls. There was a positive effect of the supplementation with L-glutamine in decreasing the transaminases (D group vs. DG group). On the other hand, L-glutamine or GDP supplementation did not affect transaminase levels (p>0.05) of control-treated groups (CG and CGDP).
Triacylglycerol increased (p<0.05) in D group compared with C group. Only L-glutamine avoided (p<0.05) the increase of blood triacylglycerol of D group. There was a slight, increase (p>0.05) of triacylglycerol in the CG and CGDP groups in comparison with CS group.
Total cholesterol increased (p<0.05) in group CGDP when compared to group C, whereas HDL cholesterol decreased (p<0.05) in group D. Supplementation with L-glutamine in the diabetic group (DG) maintained total cholesterol and HDL within the normality range for C group, while supplementation with the GDP did not recover this parameter in the DGDP group.
Total proteins and ammonia did not show changes (p>0.05) among the groups, while urea was increased (p<0.05) in all diabetic groups. But, no effect of supplementation was observed (Table 1).
Intestinal morphophysiology
Intestinal enzyme activity
There was an increase (p<0.05) of alkaline phosphatase (AF) and lipase activity in D and DG groups (vs. C group). The DGDP group had a tendency of an increase (p>0.05) for these enzymes. The L-glutamine supplementation increased (p<0.05) the activities of β-galactosidase and maltase in DG group. Supplementation with GDP did not alter the enzymatic activities in both groups, except maltase (p<0.05) in diabetic animals DGDP group (Table 2).
Table 2. Enzymatic activity (U/g) of the jejunum after 30 days receiving saline (C and D groups), L-glutamine (G) or glutamine dipeptide (GDP) in non diabetic rats (C group), and diabetic rats (D).
The animais were distributed in six groups: C, D, CG, DG, CGDP, and DGDP.
| C | D | CG | DG | CGDP | DGDP | |
|---|---|---|---|---|---|---|
| Alkaline Phosphatase | 0.056±0.007 | 0.091±0.004* | 0.056±0.005 | 0.087±0.007 | 0.043±0.005 | 0.083±0.004 |
| β-galactosidase | 0.449±0.05 | 0.588±0.02 | 0.468±0.03 | 0.859±0.02# | 0.447±0.03 | 0.719±0.05 |
| Lipase | 1.245±0.10 | 1.603±0,04* | 1.126±0.07 | 1.592±0.11 | 0.947±0.08 | 1.313±0.08 |
| Maltase | 224.4±34.18 | 346.0±25.72 | 239.6±19.15 | 578.1±59.19# | 324.7±11.31 | 515.3±28.39# |
Results expressed as mean±SEM (n = 5/group).
* p<0.05 vs. C group
# p<0.05 vs. D group
one-way ANOVA and Tukey post test.
Intestinal morphometry
The intestinal morphology is presented in Table 3. D and DGDP groups had a greater length of the small intestine compared to C groups. A longer small intestine (p<0.05) was also observed in CG group.
Table 3. Small intestine length (cm) and jejunum morphometry (μm) after 30 days receiving saline (C and D groups), L-glutamine (G) or glutamine dipeptide (GDP) in non diabetic rats (C group), and diabetic rats (D).
The animais were distributed in six groups: C, D, CG, DG, CGDP, and DGDP.
| C | D | CG | DG | CGDP | DGDP | |
|---|---|---|---|---|---|---|
| Small intestine lenght | 92.0±2.5 | 121.8±1.4* | 104.8±1.8* | 87.0±2.2 | 91.0±0.9 | 120.8±1.3 |
| Total wall | 742.9±4.5 | 790.2±3.0* | 691.8±3.1* | 774.8±4.5 | 786.3±3.2* | 718.3±3.2# |
| Mucosa | 642.6±3.9 | 693.8±2.7* | 613.7±4.1* | 697.8±4.5 | 668.0±3.1* | 599.5±4.0# |
| Submucosa | 23.1±0.2 | 18.2±0.1* | 21.4±0.2* | 17.6±0.1 | 27.0±0.2* | 17.9±0.1 |
| Villus height | 460.4±2.6 | 463.1±1.9 | 407.9±2.3* | 474.7±3.5 | 439.2±2.6* | 405.1±2.7# |
| Crypt depth | 177.1±1.1 | 191.7±1.0* | 176.6±1.1 | 200.1±1.3# | 191.7±0.9* | 176.5±0.9# |
Results expressed as mean±SEM (n = 5/group).
* p<0.05 vs. C group
# p<0.05 vs. D group
one-way ANOVA and Tukey post test.
The histological organization of the intestine was preserved in all groups. However, there were differences in the intestinal morphometric parameters between the groups.
It was observed that the jejunum of diabetic animals had total wall, mucosa and crypts depth larger than the controls, but a thinner submucosa (p<0.05). There was a positive effect (p<0.05) with glutamine dipeptide supplementation (DGDP) leading to reduction of the total wall, mucosa, crypt depth and villus height.
By contrast, non-diabetic animals showed a trophic effect (p<0.05) in total length, thickness of the mucosa and submucosa and crypt depth when supplemented with GDP. However, L-glutamine caused a reduction (p<0.05) of all the intestinal wall parameters, except the crypt depth in the CG group.
PCNA and serotonin immunohistochemistry
Analysis of cell proliferation by PCNA labeling showed an increase of 26.6% (p<0.05) in the number of labeled cells in the crypts of D group vs. C group. L-glutamine supplementation showed no effect (p>0.05) on the CG and DG groups, as well as the C group supplemented with GDP (CGDP). There was an increase of 13.43% (p>0.05) in DGDP group relative to D (Fig 2B).
Fig 2. Immunostaining for PCNA and serotonin.
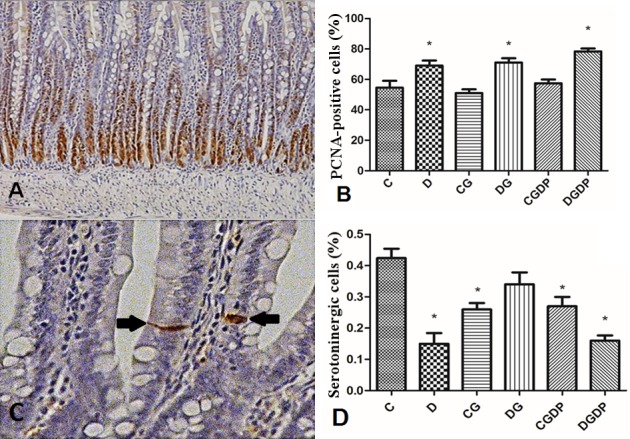
(A) Jejunal mucosa of rats immunomarked for proliferation cell nuclear antigen (PCNA) (200x magnification); (B) Percentage of cells immunostained for PCNA after 30 days of experimentation; (C) Detail of enteroendocrine serotoninergic cells of jejunal mucosa (arrow) (200x magnification); (D) Percentage of immunostained cells for serotonin after 30 days receiving saline (C and D groups), L-glutamine (G) or glutamine dipeptide (GDP) in non diabetic rats (C group), and diabetic rats (D). The animais were distributed in six groups: C, D, CG, DG, CGDP, and DGDP. Results presented as mean±SEM (n = 5/group). * p<0.05 compared to group C; one-way ANOVA and Tukey post test.
The immunostained serotoninergic cell index (Fig 2D) decreased by 64.62% (p<0.05) in group D compared with group C. L-glutamine (DG) prevented this reduction compared to the diabetic group (D), showing no difference (p>0.05) with the control group (C). The control group supplemented with GDP (CGDP) decreased by 36.32% (p<0.05) compared to C, while the diabetic supplemented group (DGDP) was not statistically different from group D.
Immunohistochemistry for submucous vipergic neurons
The count of VIP-immunoreactive neurons (VIP-IR) (Fig 3) showed a 15.05% (p<0.05) reduction of these cells in D group (vs. C group). The L-glutamine supplementation promoted maintenance of the neuronal numbers in the DG group (vs. D group), keeping close to the number of neurons in C group, while it did not cause difference in the CG group (vs. C group). The CGDP group had a reduction (p<0.05) of 14.78% in the number of neurons i(vs. C group), while DGDP group showed no differences in comparison with D group.
Fig 3. Submucous vipergic neurons.
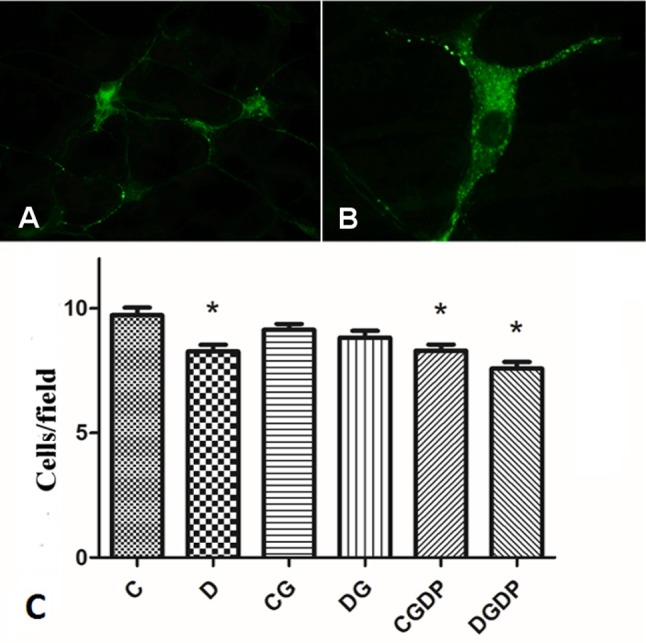
(A) Immunolabeling for vipergic ganglia of the jejunal submucous plexus of rats (200x magnification); (B) VIP-immunoreactive neuron (200x magnification); (C) Number of VIP-immunoreactive neurons in the jejunal submucous plexus of rats after 30 days receiving saline (C and D groups), L-glutamine (G) or glutamine dipeptide (GDP) in non diabetic rats (C group), and diabetic rats (D). The animais were distributed in six groups: C, D, CG, DG, CGDP, and DGDP. Mean values were obtained from the neuron count in 40 microscopic fields per animal, with a total area of 5.87 mm²/animal. Results presented as mean±SEM (n = 5/group). * p<0.05 compared to group C; Kruskal-Wallis test followed by Dunns post test.
Discussion
Physiological parameters
Experimental diabetes induced by streptozotocin was confirmed by the increased blood glucose and fructosamine (Table 1). The dose (60 mg/kg) was similar to those reported in the literature for rodents [18].
We observed not only hyperglycemia (Table 1) but also typical manifestations of the diabetic condition, such as hyperphagia, lower body mass [7, 19], polyuria, polydipsia, and diarrhea [20].
The L-glutamine amount of 248 mg/kg supplemented in this study was proportional to the specific amount of the amino acid in 400 mg/kg of glutamine dipeptide (GDP). The amount of GDP was similar to the maximum concentration used in humans that does not generate hyperammonemia [21].
Daily supplementation with L-glutamine by gavave during 30 days did not affect the physiopathological changes induced by streptozotocin (D group vs. DG group) and had no effect on the condition in the non-diabetic animals treated with L-glutamine (C group vs. CG group), as found by Tronchini et al. [19] and Tsai et al. [22]. Interestingly, in these studies [19, 22] they used the same period of supplementation but the animals received L-glutamine in the chow.
GDP behaves similarly to L-glutamine, i.e., in spite the fact that oral GDP did not show toxical effects [23], this dipeptide was not was able to improve the physiopathological changes induced by streptozotocin.
It is known that oral GDP performed better in terms of blood L-glutamine availability than oral L-glutamine [5]. This could be because the enterocytes utilize GDP at low rates [1, 2], which increases its availability for intestinal absorption. These differences between L-glutamine and GDP explain the fact that GDP is less effective for the gastrointestinal functions and more effective to functions that require plasma L-glutamine [5].
In agreement with the well-established increased protein catabolism due to the lack of insulin [24,25], streptozotocin-diabetic rats (D, DG and DGDP groups vs. C, CG and CGDP groups) showed high (P<0.05) blood levels of urea.
Lipid disorders in experimental diabetes are associated with insulin deficiency [26]. For example, insulin deficiency decreases the lipoprotein lipase activity increasing blood triacylglycerol [27]. In our study, triacylglycerol levels increased in D group, while the total cholesterol and HDL-cholesterol were reduced in D group if compared with C group. On the other hand, L-glutamine (DG group) increased HDL levels (DG group vs. D group). In agreement with these results, Badole et al. [28] reported hyperlipidemia and low HDL in a rat model of streptozotocin-induced type 2 diabetes plus nicotinamide; and an overall recovery in the lipid profile after treatment with L-glutamine.
The supplemented diabetic rats (DG group) showed an increased total cholesterol and HDL reaching the normal values observed in the non-diabetic rats (C group). However, this favorable effect of L-glutamine was not observed to GDP.
L-glutamine has beneficial effects in several diseases [29], but there are few studies about its supplementation in healthy individuals. Lowe et al. [30] showed that parenteral L-glutamine for seven days is well tolerated in healthy humans. In addition, in humans, Jian et al. [31] found no adverse effects after six days of supplementation with GDP. However, Schwimmer et al. [32] showed that L-glutamine is able to increase the absorption of triacylglycerol in the intestine of rats and this may be related to the elevation of blood lipids promoted by this amino acid [33]. Another possible justification for the effects of L-glutamine on the lipid profile could be the increased availability of energy substrates as consequence of the supplementation. However, GDP did not show these effects, in spite of its increased blood availability in comparison with L-glutamine [5].
Streptozotocin induces not only diabetes but also several additional physiopathological changes including hepatotoxicity [34–35]. However, this tissue damage was improved by supplementation with L-glutamine or GDP, as evidenced by the decreased levels of the enzymes AST and ALT (Table 1) in the DG or DGDP groups in comparison with D group. On the other side, the supplementation with L-glutamine or GDP, showed no significant effect on this enzymes in non-diabetic groups (C group vs. CG or CGDP groups).
Fructosamine, which reflects the average glycemic level over the preceding 2–3 weeks [36] was not influenced by L-glutamine. However, the supplementation with GDP was effective in reducing fructosamine levels.
Intestinal morphophysiology
As previously demonstrated [37] we also observed increased intestinal enzymatic activity in diabetes, corroborating the literature, being related to greater proliferation and DNA expression in diabetic rats [38], which are directly related to higher absorption rates by the diabetic intestine [39]. The enzymes lipase and alkaline phosphatase are involved in lipid absorption [40]. The high lipid absorption in diabetic subjects contributed to the high levels seen in the lipid profile in diabetic animals. Since the supplemented control animals did not have these enzymes increased, L-glutamine could influence other pathways of lipid absorption.
The intestinal alkaline phosphatase is also related to the reduction of bowel inflammation by acting on the detoxification of bacterial lipopolysaccharides [40], and the increase observed in this study may also be a response caused by diabetic inflammation.
The disaccharidase increase (β-galactosidase and maltase) in diabetes is related to increased absorption of carbohydrates [37], resulting from intestinal hyperplasia [41], which was also demonstrated in our study, although not significantly. The supplementations used in this study did not prevent the changes of proliferation and intestinal expression caused by diabetes [38], consequently not affecting enzyme activity.
We verified higher length of the small intestine (SI) in the diabetic group compared to the control, supporting the literature concerning this parameter [18] and the weight of the SI, similarly reported by other authors [8]. These changes have a linear relationship with blood glucose [42], and may be caused by the influence of increased advanced glycation end-products in the gastrointestinal tract [43]. L-glutamine increased this parameter in the control group, while preventing the increase in the diabetic group. Being widely used as structural and energy molecule by the intestine, L-glutamine might have caused an increase in the length of the SI by greater availability of substrates, in addition to the effects on the increased proliferation and decreased cell death [44]. L-glutamine prevented hypertrophy of SI of diabetic rats (DG) during the experimental period, confirming its beneficial effects in stressful situations [45]. The GDP did not alter the intestinal length in CGDP or DGDP group compared to their controls, possibly due to the lower effect on the SI related to the differential absorption of dipeptides [5].
Despite the large mass loss due to catabolic state, the intestinal wall of the diabetic animal paradoxically increased in thickness, and this effect was mainly due to the increase in the mucosa [14]. This trophic effect of diabetes is related to hyperglycemia, since insulin therapy showed tissue normalization [46]. In the initial phase of diabetes, the enterocytes has increased proliferation and inhibition of apoptosis, which are responsible for the observed hyperplasia [47]. Corroborating the literature, diabetes increased the thickness of the jejunal wall in this study due to hyperplasia of the mucosa. Supplementation with L-glutamine was not able to mitigate the effects of diabetes on the intestinal wall. However, GDP was able to relieve the hyperplasia, which may be related to the reduction of AGE's, based on our fructosamine results.
The involvement of the intrinsic innervation is significant for the maintenance and adaptation of the intestinal structural change to ensure homeostasis of this organ. See et al. [48] reported that the myenteric plexus regulates mucosal cell proliferation in an inhibitory manner. Loss of myenteric neurons [49] and enhanced cell body area [50] of submucous neurons are reported in the literature, and can be associated with reduction of the total number of neurons in diabetes. Tronchini et al. [19] showed that L-glutamine had no effect on neuronal loss in the myenteric plexus of diabetic rats. The oxidative stress resulting from hyperglycemia [10] is the leading cause of neuronal death in diabetes, and could be affecting the intestinal wall, causing hyperplasia. As L-glutamine is rapidly metabolized in the mucosa, it would not be effective for the layers more distant from the intestinal lumen, such as the muscle tunicas where the myenteric neurons are located. On other hand, GDP could have more contact with this layer, influencing neurons to alleviate hyperplasia, as shown in this study, and reducing wall thickness.
The supplementation of L-glutamine to control animals (CG) reduced the jejunal wall thickness, in contrast with the increased length of SI evaluated in this study. This effect may be due to the increase in glutaminase enzyme activity in the intestine, which may occur in the control group supplemented with L-glutamine [51]. As the control group has no lack of L-glutamine, glutaminase could lead to a large production of L-glutamate, which is related to neurotoxicity [52]. The affected neurons could be unregulating proliferative and motor processes in the intestine. Tronchini et al. [19] and Hermes-Uliana et al. [53] also detected plastic and morphometric neuronal changes, respectively, by means of supplementation with L-glutamine. Tronchini et al. [19] observed a moderate reduction in height of the villi in control animals supplemented with L-glutamine, but in the ileum of rats, not the jejunum.
The reduction of the submucosa thickness in diabetic groups can be attributed to protein glycation in this tunica, especially collagen [54], affecting the mechanical properties of the gut, and hence its functions [8]. Despite being able to reduce blood fructosamine, GDP and L-glutamine as well were not able to prevent glycation in the submucosa. The supplementation for extended periods may result in better responses concerning this aspect.
Our data show that the increase of the mucosa in the diabetic animals results from increased crypt depth, since the height of the villi did not change. In the literature, increase of the villi and crypts is common in this disease [8]. This effect is more evident in the more distal parts of the intestine affected by diabetes [14], explaining the lower impact found in our villi data, together with the time of exposure to diabetes, of 30 days. The supplementation of diabetic rats with L-glutamine did not affect the villi height neither was able to reverse the hyperplasia of the crypts. Supplementation with GDP in diabetic rats (DGDP) reduced villi height and crypt depth as well the height of the mucosa, indicating a higher rate of apoptosis and/or cell migration compared to untreated diabetic groups. This effect can be attributed to an influence on the altered myenteric neurons in the diabetic condition which is associated with control of proliferation, as mentioned above [48]. This would be a positive influence of GDP on the myenteric plexus, buffering the neuronal changes observed.
The PCNA labeling demonstrates the increased cell proliferation in the crypts of diabetic animals, which confirms the increase of its depth. Miller et al. [38] also showed that the crypts proliferation in the small intestine is increased in diabetes. A higher mitotic index was found in the gut epithelium of denervated animals, showing connection between damage to the nervous tissue and mucosal cell proliferation [48]. However, the absence of change in height of the villi shows that there was no lower rate of apoptosis. This could indicate increased turnover, i.e., migration of intestinal wall cells. The two forms of L-glutamine supplementation did not normalize proliferation. L-glutamine has a certain ability to promote cell proliferation and migration [44], so maybe it was not able to normalize these factors, already high in the jejunum.
The supplementation with L-glutamine [11, 44] or GDP [55] has shown effectiveness in the model of intestine injuryby acetic acid and chemotherapy, through increased cell proliferation and/or reduced apoptosis of enterocytes, thus aiding in recovery. The lack of L-glutamine by intestinal tissue seems to be more effective in the presence of tissue injury [56].
The changes caused by diabetes in serotonin produced by the endocrine cells of the intestinal mucosa are contradictory in the literature. Takahara et al. [57] detected increase in serotonin levels in the SI of diabetic rats induced by streptozotocin. By contrast, there are also reports of reduction in these levels in SI of diabetic animals induced by streptozotocin [58] and alloxan [59]. Our results show reduction of serotoninergic cell numbers in the jejunum in diabetic rats. Gastrointestinal problems resulting from diabetes may also be related to this reduction, since serotonine has a role in the regulation of peristalsis, influencing the enteric neurons [60]. These neurons also have the activity of its serotoninergic receptors altered in diabetes [57]. Cicin-Sain and Jernej [59] point out that this reduction is due to lower production of serotonin, caused by the reduction of its substrates/precursors in diabetes. It is also possible that this is an organ response to neuropathy, to offset the high motor activity caused by diabetes neuronal deregulation [61].
In our study, L-glutamine prevented the decrease of serotonininergic cell numbers, while GDP did not change this parameter, probably due to the increased availability of the free form to the intestine. The literature lacks information associating L-glutamine and serotonin in the gut of diabetic rats.
Our results show that the installation and maintenance of a diabetic profile for 30 days reduces the number of jejunal submucous plexus vipergic neurons. Adeghate et al. [62] obtained reduced numbers of vipergic neurons in the gastroduodenal region of diabetic rats after four weeks, while Hermes-Uliana et al. [53] detected increase of this subpopulation in the jejunum of diabetic rats after 120 days, which was considered as an adaptive response to promote neuronal protection. The imbalance of neurotransmitters caused by diabetic neuropathy is one of the causes of the typical gastrointestinal diabetic symptoms. This neuropathy is mainly a consequence of oxidative stress [9, 10], caused by hyperglycemia. Diabetic neuropathy, with reduction of cell body area and loss of neurons, has already been described in the myenteric [63] and submucous [13] plexuses.
There may also be a relationship between vipergic neuronal change and the reduced amount of serotonininergic cells discussed above. As part of the lost neuronal inhibitory function, there would be lower production of serotonin, a motility promoter, in response to the faster intestinal transit in an attempt to counterbalance the motor function.
The neuroprotection caused by L-glutamine in the diabetic group (DG) in this study has been reported in the literature for vipergic [12, 53] and general [64] populations. Supplementation with L-glutamine has proven effective in minimize oxidative stress [22, 28, 65, 66], increasing its availability to tissues under stress [67]. The GDP also has antioxidant potential [65], but its supplementation was not able to protect the submucous neurons. This effect was attributed to the low effect of GDP on enterocytes.
Conclusions
The supplementation with L-glutamine or GDP did not affect the typical physiopathological changes induced by streptozotocin administration. However, L-glutamine produced positive better responses than GDP on lipid fractions and transaminases levels. Moreover, with respect to jejunum morphophysiology in diabetic rats, it was preserved the number of enteroendocrine serotoninergic cells and vipergic neuronal subpopulation of the submucous plexus. On the other hand, the supplementation with GDP was effective in reducing fructosamine levels and intestinal mucosa hyperplasia in diabetic rats. Thus, the results open the possibility of using mixed supplementation (L-glutamine plus GDP) to improve the beneficial effects of each substance.
Supporting Information
(RAR)
Acknowledgments
The authors would like to acknowledge the technical support from Department of Morphological Sciences, Department of Physiological Sciences and Department of Biochemical.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES 40004015001M9 (http://www.capes.gov.br/) to CVDR SCSFA RBB RMP NCB VAFG MRMN. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R, Glutamine and glutamate–their central role in cell metabolism and function. Cell Biochem Funct. 2003;21: 1–9. [DOI] [PubMed] [Google Scholar]
- 2. Newsholme P, Abdulkader F, Rebelato E, Romanatto T, Pinheiro CEJ, Vitzel KF, et al. Amino acids and diabetes: implications for endocrine, metabolic and immune function. Frontiers in Bioscience, 2011; 16: 315–319. [DOI] [PubMed] [Google Scholar]
- 3. Fürst P, Pogan K, Stehle P, Glutamine dipeptides in clinical nutrition. Nutrition. 1997;13: 731–7. [DOI] [PubMed] [Google Scholar]
- 4. Bowtell JL, Gelly K, Jackman ML, Patel A, Simeoni M, Rennie MJ, Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. J Appl Physiol. 1999;86: 1770–7. [DOI] [PubMed] [Google Scholar]
- 5. Minguetti-Câmara VC, Marques ACR, Schiavon FPM, Vilela VR, Brusch ML, Bazotte RB, A comparison of the effects of oral glutamine dipeptide, glutamine, and alanine on blood amino acid availability in rats submitted to insulin-induced hypoglycemia. Nutrients 2014;6: 4520–30. 10.3390/nu6104520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menge BA, Schrader H, Ritter PR, Ellrichmann M, Uhl W, Schmidt WE, et al. Selective amino acid deficiency in patients with impaired glucose tolerance and type 2 diabetes. Regul Pept. 2010;160: 75–80. 10.1016/j.regpep.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 7. Lorenz-Meyer H, Thiel F, Menge H, Gottesbüren H, Riecken EO, Structural and functional studies on the transformation of the intestinal mucosa in rats with experimental diabetes. Res Exp Med (Berl). 1977;170: 89–99. [DOI] [PubMed] [Google Scholar]
- 8. Zhao J, Yang J, Gregersen H, Biomechanical and morphometric intestinal remodelling during experimental diabetes in rats. Diabetologia. 2003;46: 1688–97. [DOI] [PubMed] [Google Scholar]
- 9. Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23: 131–8. 10.1111/j.1365-2982.2010.01611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vincent AM, Russell JW, Low P, Feldman EL, Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25: 612–28. [DOI] [PubMed] [Google Scholar]
- 11. Owari M, Wasa M, Oue T, Nose S, Fukuzawa M, Glutamine prevents intestinal mucosal injury induced by cyclophosphamide in rats. Pediatr Surg Int. 2012;28: 299–303. 10.1007/s00383-011-3023-0 [DOI] [PubMed] [Google Scholar]
- 12. Alves EP, Alves AM, Pereira RV, de Miranda Neto MH, Zanoni JN, Immunohistochemical study of vasoactive intestinal peptide (VIP) enteric neurons in diabetic rats supplemented With L-glutamine. Nutr Neurosci. 2010;13: 43–51. 10.1179/147683010X12611460763841 [DOI] [PubMed] [Google Scholar]
- 13. Pereira RV, Tronchini EA, Tashima CM, Alves EP, Lima MM, Zanoni JN, L-glutamine supplementation prevents myenteric neuron loss and has gliatrophic effects in the ileum of diabetic rats. Dig Dis Sci. 2011;56: 3507–16. 10.1007/s10620-011-1806-8 [DOI] [PubMed] [Google Scholar]
- 14. Zoubi SA, Mayhew TM, Sparrow RA, The small intestine in experimental diabetes: cellular adaptation in crypts and villi at different longitudinal sites. Virchows Arch. 1995;426: 501–7. [DOI] [PubMed] [Google Scholar]
- 15. Nordström C, Dahlqvist A, Josefsson L, Quantitative determination of enzimes in differents parts of the villi and crypts of rat small intestine: comparison of alkaline phosphatase, dissacharidases and dipeptidases. J. J Histochem Cytochem. 1967;15: 713–21. [DOI] [PubMed] [Google Scholar]
- 16. Gupta N, Rathi P, Gupta R, Simplified para-nitrophenylpalmitate assay for lipases and esterases. Anal Biochem. 2002;311: 98–9. [DOI] [PubMed] [Google Scholar]
- 17. Bergmeyer HE, Bernet E, D-glucose determination with glucose oxidase and peroxidase In: Bergmeyer HU, Gawehn K ed. Methods of enzymatic analysis. 2nd ed. 1974; New York: Academic Press: 1205–15. [Google Scholar]
- 18. Izbéki F, Wittman T, Rosztóczy A, Linke N, Bódi N, Fekete E, et al. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res Clin Pract. 2008;80: 192–8. 10.1016/j.diabres.2007.12.013 [DOI] [PubMed] [Google Scholar]
- 19. Tronchini EA, Trevizan AR, Tashima CM, De Freitas P, Bazotte RB, Pereira MA, et al. Effect of l-glutamine on myenteric neuron and of the mucous of the ileum of diabetic rats. An Acad Bras Cienc. 2013;85: 1165–76. 10.1590/S0001-37652013005000052 [DOI] [PubMed] [Google Scholar]
- 20. Rodrigues ML, Motta ME, Mechanisms and factors associated with gastrointestinal symptoms in patients with diabetes mellitus. J Pediatr. 2012;88: 17–24. [DOI] [PubMed] [Google Scholar]
- 21. Ward E, Picton S, Reid U, Thomas D, Gardener C, Smith M, et al. Oral glutamine in paediatric oncology patients: a dose finding study. Eur J Clin Nutr. 2003;57: 31–6. [DOI] [PubMed] [Google Scholar]
- 22. Tsai PH, Liu JJ, Chiu WC, Pai MH, Yeh SL, Effects of dietary glutamine on adhesion molecule expression and oxidative stress in mice with streptozotocin-induced type 1 diabetes. Clin Nutr. 2011;30: 124–9. 10.1016/j.clnu.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 23. Oda S, Mullaney T, Bowles AJ, Durward R, Lynch B, Sugimura Y, Safety studies of l-alanyl-l-glutamine (l-AG). Regul Toxicol Pharmacol. 2008;50: 226–38. [DOI] [PubMed] [Google Scholar]
- 24. Hebert SL, Nair KS, Protein and energy metabolism in type 1 diabetes. Clin Nutr. 2010;29: 13–7. 10.1016/j.clnu.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gajdosík A, Gajdosíková A, Stefek M, Navarová J, Hozová R, Streptozotocin-induced experimental diabetes in male Wistar rats. Gen Physiol Biophys. 1999;18: 54–62. [PubMed] [Google Scholar]
- 26. Morel DW, Chisolm GM, Antioxidant treatment of diabetic rats inhibits lipoprotein oxidation and cytotoxicity. J Lipid Res. 1989;30: 1827–34. [PubMed] [Google Scholar]
- 27. Goldberg RB, Lipid disorders in diabetes. Diabetes Care. 1981;4: 561–72. [DOI] [PubMed] [Google Scholar]
- 28. Badole SL, Bagul PP, Mahamuni SP, Khose RD, Joshi AC, Jangam GB, et al. Oral L-glutamine increases active GLP-1 (7–36) amide secretion and improves glycemic control in stretpozotocin-nicotinamide induced diabetic rats. Chem Biol Interact. 2013;203: 530–41. 10.1016/j.cbi.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 29. Melis GC, ter Wengel N, Boelens PG, van Leeuwen PA, Glutamine: recent developments in research on the clinical significance of glutamine. Curr Opin Clin Nutr Metab Care. 2004;7: 59–70. [DOI] [PubMed] [Google Scholar]
- 30. Lowe DK, Benfell K, Smith RJ, Jacobs DO, Murawski B, Ziegler TR, et al. Safety of glutamine-enriched parenteral nutrient solutions in humans. Am J Clin Nutr. 1990;52: 1101–6. [DOI] [PubMed] [Google Scholar]
- 31. Jian ZM, Cao JD, Zhu XG, Zhao WX, Yu JC, Ma EL, et al. The impact of alanyl-glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: a randomized, double-blind, controlled study of 120 patients. JPEN J Parenter Enteral Nutr. 1999;23: S62–6. [DOI] [PubMed] [Google Scholar]
- 32. Schwimmer JB, Ee L, Zheng S, Tso P, Glutamine promotes triglyceride absorption in a dose-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2002;282: G317–23. [DOI] [PubMed] [Google Scholar]
- 33. Garlick PJ, Assessment of the safety of glutamine and other amino acids. J Nutr. 2001;131: 2556S–61S. [DOI] [PubMed] [Google Scholar]
- 34. Tomlinson KC, Gardiner SM, Hebden RA, Bennett T. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol Rev. 1992;44: 103–50. [PubMed] [Google Scholar]
- 35. Zarei A, Vaezi G, Malekirad AA, Abdollahi M. Effects of ethanol extract of Salvia hydrangea on hepatic and renal functions of streptozotocin-induced diabetic rats. Avicenna J Phytomed. 2015;5: 138–47. [PMC free article] [PubMed] [Google Scholar]
- 36. Nansseu JR, Fokom-Domgue J, Noubiap JJ, Balti EV, Sobngwi E, Kengne AP. Fructosamine measurement for diabetes mellitus diagnosis and monitoring: a systematic review and meta-analysis protocol. BMJ Open. 2015;5: e007689 10.1136/bmjopen-2015-007689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caspary WF, Rhein AM, Creutzfeldt W, Increase of intestinal brush border hydrolases in mucosa of streptozotocin-diabetic rats. Diabetologia. 1972;8: 412–4. [DOI] [PubMed] [Google Scholar]
- 38. Miller DL, Hanson W, Schedl HP, Osborne JW, Proliferation rate and transit time of mucosal cells in small intestine of the diabetic rat. Gastroenterology. 1977;73: 1326–32. [PubMed] [Google Scholar]
- 39. Nakabou Y, Ishikawa Y, Misake A, Hagihira H, Effect of food intake on intestinal absorption and mucosal hydrolases in alloxan diabetic rats. Metabolism. 1980;29: 181–5. [DOI] [PubMed] [Google Scholar]
- 40. Lallès JP, Intestinal alkaline phosphatase: novel functions and protective effects. Nutr. Rev. 2014;72: 82–94. 10.1111/nure.12082 [DOI] [PubMed] [Google Scholar]
- 41. Adachi T, Mori C, Sakurai K, Shihara N, Tsuda K, Yasuda K, Morphological changes and increased sucrase and isomaltase activity in small intestines of insulin-deficient and type 2 diabetic rats. Endocr J. 2003;50: 271–9. [DOI] [PubMed] [Google Scholar]
- 42. Sha H, Zhao JB, Zhang ZY, Zhou SP, Tong XL, Zhuang FY, et al. Effect of Kaiyu Qingwei Jianji on the morphometry and residual strain distribution of small intestine in experimental diabetic rats. World J Gastroenterol. 2006;12: 7149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu GF, Zhao JB, Zhen Z, Sha H, Chen PM, Li M, Effect of tangweian jianji on upper gastrointestinal remodeling in streptozotocin-induced diabetic rats. World J Gastroenterol. 2012;18: 4875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swaid F, Sukhotnik I, Matter I, Berkowitz D, Hadjittofi C, Pollak Y, et al. Dietary glutamine supplementation prevents mucosal injury and modulates intestinal epithelial restitution following acetic acid induced intestinal injury in rats. Nutr Metab. 2013;10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Labow BI, Souba WW, Glutamine. World J Surg. 2000;24: 1503–13. [DOI] [PubMed] [Google Scholar]
- 46. Jørgensen CS, Ahrensberg JM, Gregersen H, Flyvberg A, Tension-strain relations and morphometry of rat small intestine in experimental diabetes. Dig Dis Sci. 2001;46: 960–7. [DOI] [PubMed] [Google Scholar]
- 47. Noda T, Iwakiri R, Fujimoto K, Yoshida T, Utsumi H, Sakata H, et al. Suppression of apoptosis is responsible for increased thickness of intestinal mucosa in streptozotocin-induced diabetic rats. Metabolism. 2001;50: 259–64. [DOI] [PubMed] [Google Scholar]
- 48. See NA, Epstein ML, Dahl JL, Bass P, The myenteric plexus regulates cell growth in rat jejunum. J Auton Nerv Syst. 1990;31: 219–29. [DOI] [PubMed] [Google Scholar]
- 49. Tronchini EA, Trevizan AR, Tashima CM, Pereira RV, Zanoni JN, Supplementation with 0.1% and 2% vitamin E in diabetic rats: analysis of myenteric neurons immunostained for myosin-V and nNOS in the jejunum. Arq Gastroenterol. 2012;49: 284–90. [DOI] [PubMed] [Google Scholar]
- 50. Zanoni JN, Hernandes L, Bazotte RB, Miranda Neto MH, Terminal ileum submucous plexus: Study of the VIP-ergic neurons of diabetic rats treated with ascorbic acid. Arq Neuropsiquiatr. 2002;60: 32–37. [DOI] [PubMed] [Google Scholar]
- 51. Labow BI, Souba WW, Abcouwer SF, Mechanisms governing the expression of the enzymes of glutamine metabolism-glutaminase and glutamine synthetase. J Nutr. 2001;131: 2467S–74S. [DOI] [PubMed] [Google Scholar]
- 52. Sengul G, Coskun S, Cakir M, Coban MK, Saruhan F, Hacimuftuoglu A, Neuroprotective effect of ACE inhibitors in glutamate—induced neurotoxicity: rat neuron culture study. Turk Neurosurg. 2011;21: 367–71. 10.5137/1019-5149.JTN.4313-11.0 [DOI] [PubMed] [Google Scholar]
- 53. Hermes-Uliana C, Panizzon CP, Trevizan AR, Sehaber CC, Ramalho FV, Martins HA, et al. Is L-glutathione more effective than L-glutamine in preventing enteric diabetic neuropathy? Dig Dis Sci. 2014;59: 937–48. 10.1007/s10620-013-2993-2 [DOI] [PubMed] [Google Scholar]
- 54. Paul RG, Bailey AJ, Glycation of collagen: the basis of its central role in the late complications of ageing and diabetes. Int J Biochem Cell Biol. 1996;28: 1297–310. [DOI] [PubMed] [Google Scholar]
- 55. Satoh J, Tsujikawa T, Fujiyama Y, Banba T, Enteral alanyl-glutamine supplement promotes intestinal adaptation in rats. Int J Mol Med. 2003;12: 615–20. [PubMed] [Google Scholar]
- 56. Sukhotnik I, Khateeb K, Mogilner JG, Helou H, Lurie M, Coran AG, et al. Dietary glutamine supplementation prevents mucosal injury and modulates intestinal epithelial restitution following ischemia-reperfusion injury in the rat. Dig Dis Sci. 2007;52: 1497–504. [DOI] [PubMed] [Google Scholar]
- 57. Takahara H, Fujimura M, Taniguchi S, Hayashi N, Nakamura T, Fujimiya M, Changes in serotonin levels and 5-HT receptor activity in duodenum of streptozotocin-diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2001;281: G798–808. [DOI] [PubMed] [Google Scholar]
- 58. Portela-Gomes GM, Wilander E, Grimelius L, Bergström R, Ruhn G, The enterochromaffin cells in the mouse gastrointestinal tract after streptozotocin treatment. Pathol Res Pract. 1990;186: 260–4. [DOI] [PubMed] [Google Scholar]
- 59. Cićin-Sain L, Jernej B, Reduction of gastrointestinal serotonin in alloxan-diabetic rats: reversal by 5-hydroxytryptophan treatment. Behav Brain Res. 1996;73: 285–8. [DOI] [PubMed] [Google Scholar]
- 60. Grider JR, Foxx-Orenstein AE, Jin JG, 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115: 370–80. [DOI] [PubMed] [Google Scholar]
- 61. Schmidt RE, Dorsey DA, Beaudet LN, Frederick KE, Parvin CA, Plurad SB, et al. Non-obese diabetic mice rapidly develop dramatic sympathetic neuritic dystrophy: a new experimental model of diabetic autonomic neuropathy. Am J Pathol. 2003;163: 2077–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Adeghate E, Ponery AS, Sharma AK, El-Sharkawy T, Donáth T, Diabetes mellitus is associated with a decrease in vasoactive intestinal polypeptide content of gastrointestinal tract of rat. Arch Physiol Biochem. 2001;109: 246–51. [DOI] [PubMed] [Google Scholar]
- 63. da Silva GG, Zanoni JN, Buttow NC, Neuroprotective action of Ginkgo biloba on the enteric nervous system of diabetic rats. World J Gastroenterol. 2011;17: 898–905. 10.3748/wjg.v17.i7.898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zanoni JN, Tronchini EA, Moure SA, Souza ID, Effects of l-glutamine supplementation on the myenteric neurons from the duodenum and cecum of diabetic rats. Arq Gastroenterol. 2011;48: 66–71. [DOI] [PubMed] [Google Scholar]
- 65. Cruzat VF, Bittencourt A, Scomazzon SP, Leite JS, de Bittencourt PI Jr, Tirapegui J, Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia. Nutrition. 2014;30: 602–11. 10.1016/j.nut.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 66. Badole SL, Chaudhari SM, Bagul PP, Mahamuni SP, Khose RD, Joshi AC, et al. Effect of concomitant administration of l-glutamine and cycloart-23-ene-3b, 25-diol (B2) with sitagliptin in GLP-1 (7–36) amide secretion, biochemical and oxidative stress in streptozotocin—nicotinamide induced diabetic sprague dawley rats. PLoS One. 2013; 8(8):e72817 10.1371/journal.pone.0072817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li P, Yin YL, Li D, Kim SW, Wu G, Amino acids and immune function. Br J Nutr. 2007;98: 237–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RAR)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.