Abstract
Background: During pregnancy, the balanced dominance of the T helper17 response shifts to a Th2 response that is characterised by the production of IL-10, following the completion of the implantation process. Transforming growth factor-β (TGF-β) expression is associated with the completion of trophoblast invasion and placental growth. This study assessed the effect of malaria infection on the levels of IL-17, IL-10, and TGF-β in the plasma of pregnant mice with malaria.
Methods: Seventeen pregnant BALB/C mice were divided into two groups: mice infected with Plasmodium berghei (treatment group) and uninfected mice (control group). The mice were sacrificed on day 18 post-mating. Parasitemia was measured by Giemsa staining. The levels of IL-17, IL-10, and TGF-β were measured by ELISA.
Results: Using independent t test, the IL-17 levels in the treatment group were higher than those in the control group (= = 0.040). The IL-10 levels in the treatment group were lower than those in the control group (= = 0.00). There was no significant difference in the TGF-β levels (= = 0.055) between two groups. However, using SEM analysis the degree of parasitemia decreased the plasma TGF-β levels (tcount = 5.148; ≥ ttable = 1.96). SEM analysis showed that a high degree of parasitemia increased the IL-17 levels and decreased the IL-10 and TGF-β levels.
Conclusion: Malaria infection during pregnancy interferes with the systemic balance by increasing the IL-17 levels and decreasing the IL-10 and TGF-β levels.
Keywords: Plasmodium berghei, pregnancy, parasitemia, interleukin 17, interleukin 10, transforming growth factor-β
Introduction
The specific and non-specific activation of the immune response during malaria infection is influenced by the presence of the parasite and its product glycosylphosphatidylinositol (GPI) (1). Non-specific immune responses involve the clearance of the parasite by phagocytes and can lead to intracellular destruction by inflammatory cell mobilization (2). The non-specific immune system works quickly and is often necessary Original Articleto stimulate the specific immune system (3). Erythrocytes that are infected with malaria tend to be more sequestered in the vital tissues rather than circulating in the peripheral blood, which results in a systemic parasitic existence that does not always correlate with the systemic immune response.
The Th1 cytokine response, which consists of TNF-α, IFN-γ, IL-1, and IL-6, is required for the activation of immune cells against malaria infection (4,5). The presence of IL-17 in plasma as a result of the systemic inflammatory response occurs primarily during the early or acute phase of malaria infection (6). Higher Th1 and Th17 cytokine responses stimulate lymphocytes to produce Th2 cytokines such as IL-10, which has an immuno-regulator effect and can suppress Th1 dominance (7,8). The increased expression of Th2 cytokines is commonly associated with increases in regulatory T cell cytokines, such as transforming growth factor β (TGF-β). This environment has been associated with the lack of efficient control of Plasmodium falciparum parasitemia (9).
During normal pregnancy TGF-β, IL-6, and IL-1 induce Th17 to produce IL-17. This condition is necessary to initiate the angiogenesis process that is required for implantation (8). Once the process is completed, implantation dominance shifts the balance of Th1/Th17 responses to Th2 responses that are characterised by the production of IL-4, IL-9, and IL-10 (10,11). This shift ensures the growth of trophoblast cells in the decidua and placenta, resulting in normal foetal life.
Primigravida women who live in endemic areas represent the population that is at the greatest risk for placental malaria. Their placental infections are more frequent and severe and are characterised by higher parasitemia and stronger inflammation compared with multigravida women (12). The aim of this study was to investigate the effect of malaria infection on the balance of the systemic response immune (including Th-1, Th-2, and T regulator responses) in mice that were pregnant for the first time. We assessed the effect of malaria infection on the levels of IL-17, IL-10, and TGF-β in plasma from pregnant mice that were infected with malaria parasites.
Material and Methods
Research design
This in vivo experimental laboratory study was conducted by comparing data obtained from two groups of pregnant BALB/c strain mice. The treatment group mice were infected with Plasmodium berghei on the ninth day post-mating, and uninfected mice served as the control group. All mice on treatment and control groups were sacrificed on day 18 post-mating. This study was conducted at the Laboratory of Parasitology and Biomedical Laboratory, Faculty of Medicine, Universitas Brawijaya Malang, Indonesia.
Research samples
The pregnant mice used in this study weighed 20-30 g and were healthy 13- to 15-week-old female BALB/c mice. All of the mice were single pair mated within one night after the synchronisation of oestrus using the principle of Leeboot, Pheromone, and Whitten effects (13). The mice were evaluated daily (specifically their body weights and pregnancy symptoms).
Plasmodium berghei ANKA strain inoculation and examination of the degree of parasitemia
The Plasmodium berghei ANKA strain was obtained from the Laboratory of Parasitology Faculty of Medicine at the Universitas Brawijaya. Inoculation was accomplished by an intra-peritoneal injection of up to 1 × 106 Plasmodium berghei ANKA strain (first passage) per mL of blood on the ninth day post-mating. To observe the degree of parasitemia, 10 mL of blood was isolated from the end of the tail of the mice and dripped onto a glass slide. The droplets were smeared as a thin smear and dried. Then, the smear was fixed with absolute methanol until it was evenly distributed and dried. The slides were stained with Giemsa solution (a mixture of Giemsa stain (Merck, HX612241) and Giemsa buffer (Bioanalitika, Indonesia) at a ratio of 1:9 for 30 min, rinsed with water and dried. The degree of parasitemia was determined by examining a blood smear under a microscope at a magnification of 1000×. The percentage of parasitemia was computed based on the number of erythrocytes infected with malaria parasites per 1000 erythrocytes. The assessment of blood smears to measure the degree of parasitemia was performed daily from day 10 to day 18 post-mating when the mice were sacrificed.
Isolation of plasma and determination of IL-17, IL-10, and TGF-β levels in plasma
Blood was collected, transferred to a Vacutainer tube and centrifuged at 1000× g for 10 min at 4 °C to isolate the supernatant/plasma. The plasma levels of IL-17 were measured using the Quantikine ELISA kit (enzyme-linked immunosorbent assay; R&D Systems, catalog # M1700). The plasma levels of IL-10 were measured using the Mouse IL-10 ELISA Kit (abcam, catalog #ab108870). The plasma levels of TGF-β were measured using the Quantikine ELISA kit (Enzyme Immuno Assay) (R&D Systems, catalog #MB100B).
For all experiments, fifty μL of the assay diluents was added to wells that were coated with the primary antibody. Fifty microlitres each of the standard, control, and samples were added per well. The samples were mixed by mixing the plate frame for one minute, covered with an adhesive strip and incubated for two hours at room temperature. Each sample was aspirated, and the plate was then washed 4–5 times by filling each well with wash buffer (400 μL) and spraying it with a dispenser. After the last wash, the remaining wash buffer was removed by tapping the plate on a clean paper towel. Then, 100 μL of secondary antibody conjugated with biotin was added to each well. The plate was covered with a new adhesive strip and incubated for two hours at room temperature. The washing process was repeated three times. Then, 100 μL of substrate solution was added to each well and incubated for 30 min at room temperature, protected from light. Finally, 100 μL of stop solution was added to each well. The plate was read within 30 min using an ELISA reader at a wavelength of 450 nm.
Data analysis
Data analysis was performed using an independent t test to determine differences in the plasma IL-17, IL-10, and TGF-β levels between the treatment group and the control group. The Structural Equation Modelling (SEM) method in the Smart Partial Least Square (PLS) software was used to test the cause-and-effect relationship of the degree of parasitemia and the variables. This study set the level of significance at p-value of < 0.050; thus, a significant SEM result was obtained with a count value of t ≥ 1.96.
In this study, we used non-parametric SEM analysis because there were fewer than 100 samples. To develop a theory-based model to analyse the causal relationship between the dependent and independent variables, we constructed diagram paths for a causal relationship and converted the path diagrams into a structural model and measurement model. Using an input matrix and estimation models, we could observe the following: the paths that had a dominant causal effect compared with other lines and the paths where the independent variables had a greater effect on the dependent variables compared with other variables.
Ethical considerations
Ethical clearance was provided by the committees of research of the Faculty of Medicine Universitas Brawijaya (No.104/EC/KEPK-S2/03/2013). All animals were treated well during this study. The surgical process used the anaesthetic method and thus did not harm the animals.
Results
A total of 17 mice became pregnant and were thus, eligible for this study. Of those, 9 mice were placed in the infected/treatment group, and 8 mice were placed in the uninfected/control group.
The degree of parasitemia
The degree of parasitemia in mice infected with Plasmodium berghei was followed every day until day 18 post-mating or day 9 post-infection. The mean parasitemia on the day of surgery was 41.93% (SD 21.55). The degree of parasitemia in the treatment group from days 1 to 9 post-infection was examined by two observers and is shown in Figure 1.
Figure 1:
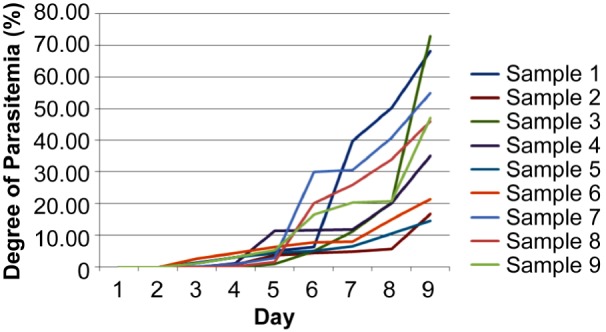
The degree of parasitemia in the treatment group from day-1 to 9 post- infection (mean (SD).
The morphology of Plasmodium berghei-infected erythrocytes from pregnant mice on day 18 post-mating or day 9 post-infection is shown in Figure 2.
Figure 2:
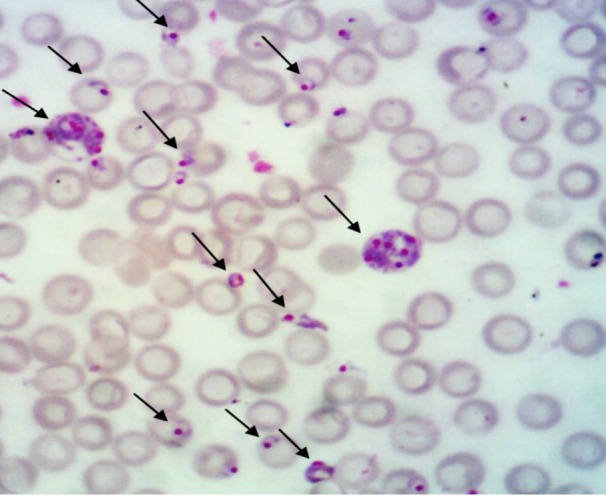
The erythrocytes infected with Plasmodium berghei ANKA strain. Arrows indicate infected erythrocytes on day 18 post-mating or day 9 post-infection of Plasmodium berghei infected pregnant mice.
Plasma IL-17 Levels
The levels of plasma IL-17 of the treatment and control groups were visualised using a box-plot diagram shown in Figure 3.
Figure 3:
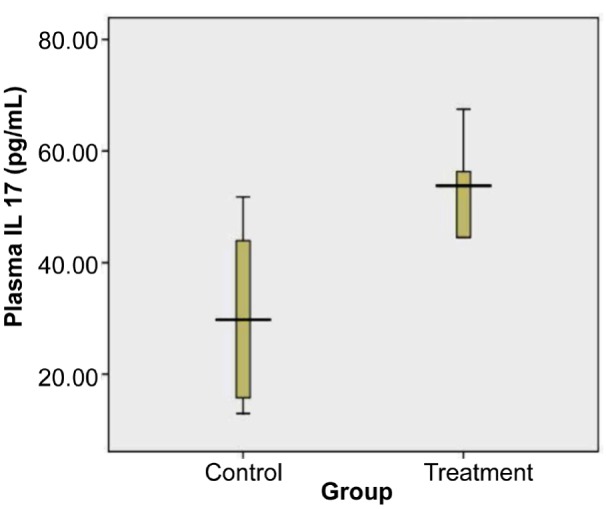
Levels of Plasma IL-17 of control and treatment group on day 18. Plasma IL-17 of treated group was significantly higher than in the control group (= = 0.040, independent t test). Total samples were 17. The mean (SD) of control group was 30.45 (SD 1.43) (n = 8) and treatment group was 52.13 (SD 14.96) (n = 9).
Plasma IL-10 Levels
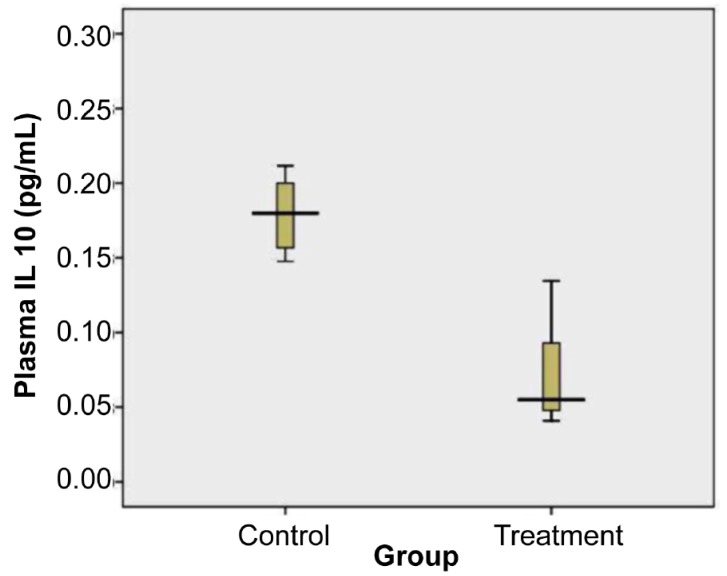
The levels of plasma IL-10 from the treatment and control groups were visualised using the box-plot diagram shown in Figure 4.
Figure 4:
Levels of Plasma IL-10 of control and treatment group on day 18. Plasma IL-10 of treated group was significantly lower than in the control group (= = 0.000, independent t test). Total samples were 17. The mean (SD) of control group was 0.18 (SD 0.02) (n = 8) and treatment group was 0.07 (SD 0.03) (n = 9).
Plasma TGF-β Levels
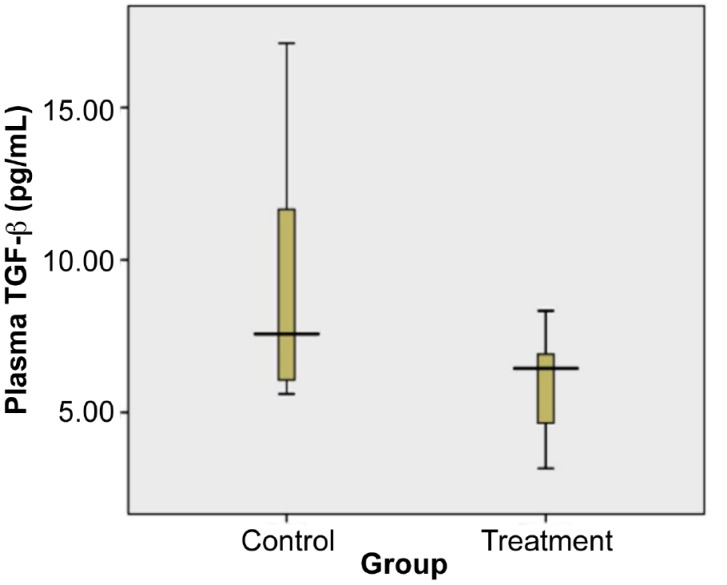
The levels of plasma TGF-β from the treatment and control groups were visualised using the box-plot diagram shown in Figure 5.
Figure 5:
Levels of Plasma TGF-β of control and treatment group on day 18. Plasma TGF-β of treated group was significantly lower than in the control group (= = 0.05, independent t test). A total samples were 17. The mean (SD) of control group was 9.69 (SD 4.43) (n = 8) and treatment group was 5.99 (SD 1.85) (n = 9).
Data analysis and calculations were performed using Non-Parametric Structural Equation Modelling (SEM); the results are presented in Figure 6.
Figure 6:
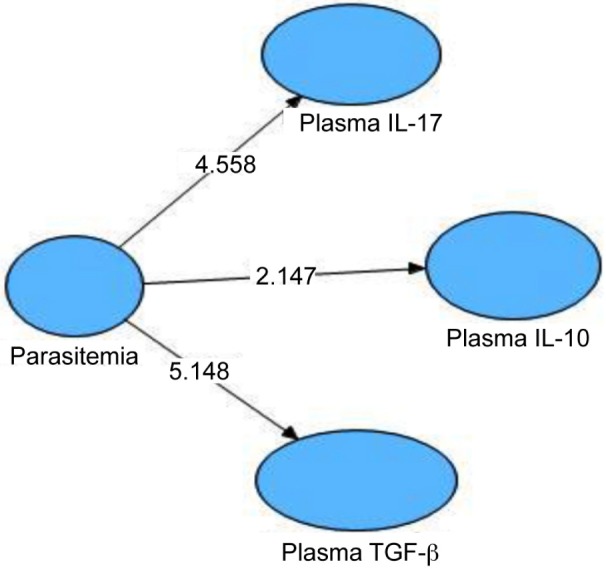
Structural Equation Modeling (SEM) for Determining Significance of Relationship between the Degree of Parasitemia, Plasma IL-17, IL-10, and TGF-β.
The hypothesis model of the relationship between the degrees of parasitemia and the IL-17, IL-10, and TGF-β levels in plasma was illustrated using Value Path Coefficients and R2, as shown in Figure 7.
Statistical analysis showed that the degree of parasitemia increased the plasma IL-17 levels (tcount = 4.558; ≥ ttable = 1.96; path coefficient = 0.094; R2= 0.009). The degree of parasitemia decreased the plasma IL-10 levels (tcount = 2.147; ≥ ttable = 1.96; path coefficients = –0.054; R2 = 0.003). The degree of parasitemia decreased the plasma TGF-β levels (tcount = 5.148; ≥ ttable = 1.96; path coefficients = -0.257; R2 = 0.066). Thus. the increase in plasma IL-17 levels, the decrease in plasma IL-10 levels and the decrease in plasma TGF-β levels caused by the increase in the degree of parasitemia.
Discussion
An evolving model of pregnancy-associated immune changes suggests that the hormonal environment associated with pregnancy contributes to the local suppression of cell-mediated immunity at the maternal-foetal interface by mediating a systemic change toward Th2 dominance. The local Th1-Th2 shift demonstrated in pregnant patients influences the systemic maternal immune response during pregnancy (14). Cytokines such as TNF-α and IL-1 are increased in a wide range of systemic inflammatory diseases, including malaria. These pro-inflammatory cytokines are most closely investigated in malaria patients and usually act as homeostatic agents, but they can cause pathology if they are produced excessively (15). Moreover, TGF-β (9) and IL-6 (5) have been detected in humans who have been infected with malaria parasites. TGF-β, IL-6, and IL-1 are cytokines that can induce the differentiation of naive T cells into Th17 cells that produce IL-17 (16).
In this study, we found a relationship between the high degree of parasitemia and high levels of IL-17 using SEM analysis, with a plasma tcount = 4.558, ≥ ttable = 1.96, and path coefficients = 0.094. IL-17 production is associated with a very high occurrence of chronic inflammation and immunopathological conditions (16). Anti-inflammatory cytokines such as IL-10 are secreted to reduce the inflammatory response during placental malaria. Interleukin 10 (IL-10) is a key cytokine that is involved in both protection and immuno-pathology during malaria infection. High levels of IL-10 were observed to be beneficial during malaria episodes by reducing the inflammatory response, but they were also detrimental due to their reduction of the cellular anti-parasitic immune response (17). IL-10 is an anti-inflammatory cytokine that acts by blocking the production of the inflammatory cytokines secreted by monocytes or macrophages, such as IL-6, TNF-α and IL-l (18). Interleukin-10 has previously been found to be increased during malaria episodes in both pregnant and non-pregnant individuals (7).
In contrast, high IL-10 levels were reported to be associated with a decreased ability to eliminate malaria parasitemia in children in Tanzania (19). In this study, there was a relationship between the high degree of parasitemia and the decreased plasma IL-10 levels (tcount = 2.147 ≥ ttable = 1.96; path coefficients = -0.054). High IL-10 levels have become the hallmark of African children with malaria anaemia and high levels of parasitemia (20).
The research conducted by Kabyemela et al 2008 (21) showed that the levels of IL-10 increased during placental malaria when the parasites bound to Chondroitin sulphate Acid (CSA) in the placenta and thus caused an inflammatory response. This response was associated with a high degree of parasitemia (22). Inflammatory cells play an important role in the clearance of the Plasmodium falciparum parasite through the phagocytosis of infected red blood cells. However, inflammatory cells can also lead to functional impairment of the placental villi, thereby disrupting the foeto-maternal exchange and causing low birth weight (23). Pregnancy loss can occur following the high systemic production of pro-inflammatory cytokines (i.e., IFN-gamma and IL-1 beta), splenic production of TNF, and high levels of soluble TNF receptor II (24). The high systemic production of IL-10, although it is protective against TNF-induced excessive weight loss and anaemia in mice (25), is apparently inadequate to block the deleterious, embryo-toxic effects of these pro-inflammatory cytokines.
In this study, there was a relationship between the high degree of parasitemia and decreased levels of TGF-β in plasma (tcount = 5.148 ≥ ttable = 1.96; path coefficients = –0.257). Plasma TGF-β appeared to be induced at a lower level compared with the control group. This is consistent with the findings of Abrams et al (26), who compared the levels of TGF-β in the cord blood of pregnant women infected with malaria with those of pregnant women without malaria infection and found a decrease in TGF-β levels in pregnant women infected with malaria.
TGF-β is one of the immunomodulator cytokines and plays an important role in limiting the effects of malaria pathology (27). Dodoo et al (28) showed that the production of TGF-β was associated with a reduction in the risk of fever. Furthermore, the regulation of pro-inflammatory cytokines by TGF-β may provide protection against the incidence of malaria pathology. In severe falciparum malaria (including cerebral malaria), increased production of TGF-β is associated with anti-inflammatory effects through the inhibition of or reduction of pro-inflammatory cytokines such as IL-8 and IL-12 during the initial phase of immune activation (27).
A study conducted by Perkins et al (29), also reported that the decreased level of TGF-β in Gabonese children was significantly associated with the incidence of severe malaria. Based on the results described above, we propose that decreased levels of TGF-β might be associated with the severity of malaria.
Conclusion
Malaria infection in pregnancy with high parasitemia may have a negative impact(s) on pregnancy due to the increase in Th1 cytokine levels and the decrease in the Th2/T regulator cytokine levels that function to maintain and save the pregnancy. We concluded that malaria infection during pregnancy increased levels of IL-17 and decrease levels of IL-10 and TGF-β.
Figure 7:
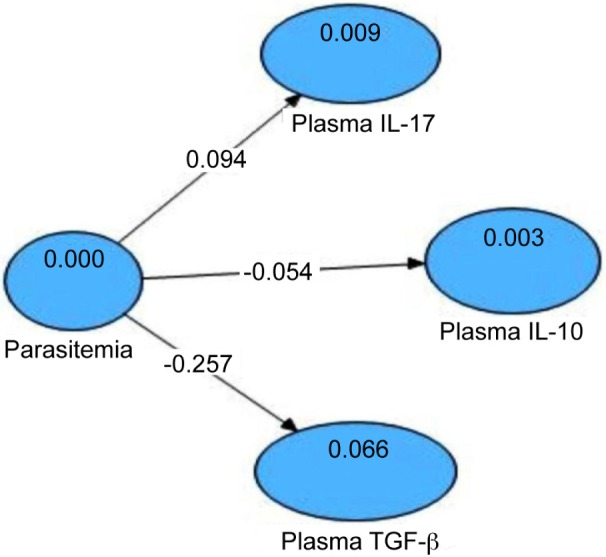
Model Hypothesis Double Paradigm with Three Dependent Variables. The Relationship between the Degree Parasitemia, Plasma IL-17, IL-10, and TGF-β, illustrated by Value Path Coefficients.
Acknowledgments
We express our appreciation to Heni Endrawati, SSi, the staff of the Parasitology Laboratory Faculty of Medicine Universitas Brawijaya for the animal housing, Bunga Prihadina, S.Si from Biomedical Laboratory Faculty of Medicine Universitas Brawijaya for her good assistance in ELISA assay.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funds
This research was funded by The Ministry of Education and Culture Republic of Indonesia and Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia.
Authors’ Contributions
Conception and design: LEF, TWS, ZR
Analysis and interpretation of the data: BS, LEF, ZR
Drafting of the article: SDS, ZR
Critical revision of the article for the important intellectual content: LEF, TWS
Final approval of the article and obtaining of funding: BS, LEF, ZR
Provision of study materials and collection and assembly of data: LEF, SDS, ZR
References
- 1.Perkins DJ, Were T, Davenport GC, Kempalah P, Hitner JB, Ong’echa JM. Severe malaria anemia: innate immunity and pathogenesis. Int J Biol Sci. 2011;7(9):1427–1442. doi: 10.7150/ijbs.7.1427. doi: 10.7150/ijbs.7.1427 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsakonas KA, Tongren JE, Riley EM. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin Exp Immunol. 2003;133(2):145–152. doi: 10.1046/j.1365-2249.2003.02174.x. doi: 10.1046/j.1365-2249.2003.02174.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbas AK, Lichtman AH. Cellular and Molecular Immunology. 2012 [Internet] Available from: http://www.studentconsult.com/content/default.cfm . [Google Scholar]
- 4.Hassan DA, Elhussin AM, Abdulhadi NH. Cytokines and their role in modulating the severity of Plasmodium falciparum malaria. Khartoum Medical Journal. 2010;3(1):373–376. [Google Scholar]
- 5.Abrams ET, Brown H, Chensue SW, Turner GDH, Tadesse E, Lema VM, et al. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J Immunol. 2003;170(5):2759–2764. doi: 10.4049/jimmunol.170.5.2759. doi: 10.4049/jimmunol.170.5.2759 . [DOI] [PubMed] [Google Scholar]
- 6.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10(4):427–436. doi: 10.1038/ni.1717. doi: 10.1038/ni.1717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suguitan AL, Leke RGF, Fouda G, Zhou A, Thuita L, Metenou S, et al. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. JID. 2003;188(7):1074–1082. doi: 10.1086/378500. doi: 10.1086/378500 . [DOI] [PubMed] [Google Scholar]
- 8.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-Cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–610. doi: 10.1111/j.1600-0897.2010.00852.x. doi: 10.1111/j.1600-0897.2010.00852.x . [DOI] [PubMed] [Google Scholar]
- 9.Walther M, Tongren JE, Andrews L, Korbel D, King E, Flether H, et al. Upregulation of TGF-β, FOXP3, and CD4+ CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23(3):287–296. doi: 10.1016/j.immuni.2005.08.006. doi: http://dx.doi.org/10.1016/j.immuni.2005.08.006 . [DOI] [PubMed] [Google Scholar]
- 10.Peck A, Mellins ED. Plasticity of T-Cell phenotype and function: the T helper type 17. Immunology. 2010;129(2):147–153. doi: 10.1111/j.1365-2567.2009.03189.x. doi: 10.1111/j.1365-2567.2009.03189.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crome SQ, Wang AY, Levings MK. Translational mini -review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol. 2010;159(2):109–119. doi: 10.1111/j.1365-2249.2009.04037.x. doi: 10.1111/j.1365-2249.2009.04037.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy PE. Plasmodium in the placenta: parasites, parity, protection, prevention and possibly preeclampsia. Parasitology. 2007;134(13):1877–1881. doi: 10.1017/S0031182007000170. doi: http://dx.doi.org/10.1017/S0031182007000170 . [DOI] [PubMed] [Google Scholar]
- 13.Sardjono TW. UNAIR. 2005. Effect of Toxoplasma infection on pregnancy outcome through interferon-gamma (IFN-γ), the activity of caspase 3 and apoptosis of placental cells. Library. [Google Scholar]
- 14.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging Infections and Pregnancy. Emerging Infectious Diseases. 2006;12(11):1638–1643. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malaria Journal. 2006;5(85) doi: 10.1186/1475-2875-5-85. doi: 10.1186/1475-2875-5-85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miossec P, Korn T, Kuchroo VJ. Mechanisms of disease interleukin-17 and type IL17 helper T cells. Review article. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. doi: 10.1056/NEJMra0707449 . [DOI] [PubMed] [Google Scholar]
- 17.Niikura M, Inoue SI, Kobayashi F. Role of Interleukin-10 in malaria: focusing on coinfection with lethal and non-lethal murine malaria parasites. Journal of Biomedicine & Biotechnology. 2011. 2011;8 doi: 10.1155/2011/383962. doi: 10.1155/2011/383962 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Waal Malefyt R, Abrams J, Bennett B, Figdor CG, De Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugosson E, Montgomery SM, Premji Z. Higher IL-10 levels are associated with less effective clearance of Plasmodium falciparum parasites. Impact of Malaria. Centers for Disease Control and Prevention. 2012. 1600 Clifton Rd. Atlanta, GA 30333, USA 800- CDCINFO (800-232-4636) TTY. (888):232–6348. [Google Scholar]
- 20.Ouma C, Davenport GC, Were T, Otieno MF, Hittner JB, Vulule JM, et al. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum Genet. 2008;124(5):515–524. doi: 10.1007/s00439-008-0578-5. doi: 10.1007/s00439-008-0578-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabyemela ER, Muehlenbachs A, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Maternal peripheral blood level of IL-10 as a marker for inflammatory placental malaria. Mal J. 2008;7:26. doi: 10.1186/1475-2875-7-26. doi: 10.1186/1475-2875-7-26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perkins DJ, Hittner JB, Mwaikambo ED, Granger DL, Weinberg JB, Anstey NM. Impaired systemic production of prostaglandin E2 in children with cerebral malaria. J Infect Dis. 2005;191(9):1548–1557. doi: 10.1086/429332. doi: 10.1086/429332 . [DOI] [PubMed] [Google Scholar]
- 23.Menendez C, Ordi J, Ismail MR, Venture PJ, Aponte JJ, Kahigwa EF, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181(5):1740–1745. doi: 10.1086/315449. doi: 10.1086/315449 . [DOI] [PubMed] [Google Scholar]
- 24.Poovassery J, Moore JM. Malaria-induced murine pregnancy failure: association with robust peripheral and placental cytokine responses. Infect Immun. 2009;77(11):4998–5006. doi: 10.1128/IAI.00617-09. doi: 10.1128/IAI.00617-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Sanni LA, Omer F, Riley E, Langhorne J. Pathology of Plasmodium chabaudi chabaudi infection and mortality in interleukin-10-deficient mice are ameliorated by anti-tumor necrosis factor alpha and exacerbated by anti-transforming growth factor beta antibodies. Infect Immun. 2003;71(9):4850–4856. doi: 10.1128/IAI.71.9.4850-4856.2003. doi: 10.1128/IAI.71.9.4850-4856.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abrams ET, Kwick JJ, Mwapasa V, Kamwendo DD, Tadesse E, Lema VM, et al. Malaria during pregnancy and foetal aematological status in Blantyre. Malawi Malar J. 2005;4:39. doi: 10.1186/1475-2875-4-39. doi: 10.1186/1475-2875-4-39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omer FM, Kurtzhals JAL, Riley EM. Maintaining the immunological balance in parasitic infection: A role for TGF-β. Parasitol Today. 2000;16(1):18–23. doi: 10.1016/s0169-4758(99)01562-8. doi: 10.1016/S0169-4758(99)01562-8 . [DOI] [PubMed] [Google Scholar]
- 28.Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum Malaria. J Infect Dis. 2002;185(7):971–979. doi: 10.1086/339408. doi: 10.1086/339408 . [DOI] [PubMed] [Google Scholar]
- 29.Perkins DJ, Weinberg JB, Kremsner PG. Reduced interleukin-12 and Transforming Growth Factor-β1 in severe childhood malaria: Relationship of cytokine balance with disease severity. J Infect Dis. 2000;182(3):988–992. doi: 10.1086/315762. doi: 10.1086/315762 . [DOI] [PubMed] [Google Scholar]