Abstract
Purpose
The study aims to describe the newborn health parameters of the 50 first children conceived after autologous oocyte vitrification in France.
Methods
The 50 children born after autologous oocyte vitrification/warming cycle (VAO children) have been retrospectively compared with 364 children conceived by micromanipulation using freshly recovered non-vitrified oocytes (ICSI children). Children included in the study were born between 2011 and 2015. Maternal characteristics (age, body mass index, smoking habits), obstetric outcomes (diabetes, hypertension, placenta previa, parity, mode of delivery), and perinatal outcome (twinning, sex, birth weight, macrosomia, birth defects) were analyzed. The generalized estimating equation for correlated data was performed to evaluate perinatal outcomes and caesarean section.
Results
No statistically significant difference was found between VAO children and ICSI children, even after adjusting confounding factors (low birth weigh odds ratio (OR) 0.8, 95 % confident interval (CI) 0.3–2.2, adjusted (AOR) 0.5, 95 % CI 0.2–1.7; large for gestational age OR 1.5, 95 % CI 0.3–7.0, AOR 1.6, 95 % CI 0.3–7.5; birth defects OR 0.4, 95 % CI 0.1–3.2, AOR 0.5, 95 % CI 0.1–3.7; caesarean section OR 1.8, 95 % CI 0.9–3.4, AOR 1.8, 95 % CI 0.9–3.7).
Conclusions
According to our results, newborn health parameters of children conceived in our center by micromanipulation using vitrified/warmed autologous oocytes seem not to be different from children born after micromanipulation on freshly recovered oocytes.
Keywords: Vitrification, Autologous oocytes, Children health, Retrospective study
Introduction
Since the birth of first baby conceived by in vitro fertilization (IVF) in 1978, the field of human IVF has considerably evolved including improvements in ovarian stimulation protocols, oocyte recovery (with the use of ultrasound), embryo culture, and cryobiology development. These advances enabled elective embryo transfer, reducing the number of embryo transferred and thus twinning rate while also providing better results and safer outcomes in terms of children’s health [1]. Improvement in cryobiology allowed the development of female gamete vitrification that has dramatically changed the habits of professionals. Many applications for this lately developed technique were subsequently proposed such as oocyte donation and female fertility preservation [2–7]. Furthermore, producing large number of embryos for transfer became obsolete and can be avoided thanks to oocyte vitrification, particularly in some countries where embryo freezing is not allowed [8]. Similarly, French authorities encourage practitioners to limit embryo freezing (Article 31 Law n° 2011–814 July 7, 2011). Therefore, since the introduction and the first evaluation of oocyte vitrification in France [9], it has been used in routine management of infertility and proposed to couples in cases where a large number of mature oocyte was collected, embryo transfer was contraindicated for medical reasons and when no or to few spermatozoa were available for intracytoplasmic sperm injection (ICSI) on the day of oocyte retrieval [9, 10].
In order to objectively evaluate the welfare of the children born after in vitro fertilization with micromanipulation (ICSI), we have constituted a historical cohort comprising 3200 children whose follow-up is performed in our center and still ongoing [11]. As recommended when a new technique has been introduced in a laboratory [12], we evaluated data collected from children born after ICSI using vitrified/warmed autologous oocytes. Finally, 3 years after the opening of the oocyte vitrification program in our center, we are able to report on the first 50 live births obtained in France after ICSI on vitrified/warmed autologous oocytes as compared to 364 children of our historical cohort.
Material and methods
Study design
This study is a retrospective analysis of a historical monocentric cohort of children born after ICSI. Written, informed consent was obtained for oocyte vitrification and the study was approved by the Institutional Review Board (April 3, 2008, reference number 208 R06).
Population
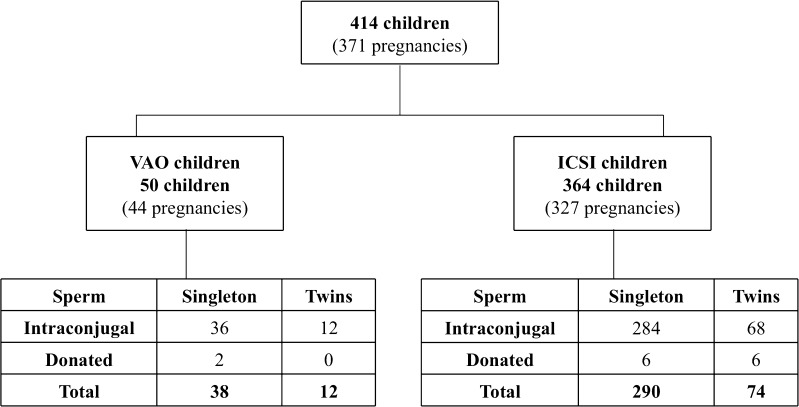
Singletons and twins born after ICSI on vitrified/warmed autologous oocytes (VAO children) and ICSI on freshly recovered non-vitrified autologous oocytes (ICSI children) issued from a historical cohort were selected for the present study [11] (Fig. 1). No pregnancies have been lost to follow-up since the beginning of the cohort in 1995.
Fig. 1.
Flowchart showing the distribution of the sample population. ICSI children, born after ICSI using freshly recovered non-vitrified oocytes, and VAO children, born after ICSI on vitrified/warmed exclusively autologous oocytes
Oocyte collection
In both groups, ICSI cycles were conducted according to standard procedures, ovarian stimulation was performed with conventional protocols based on pituitary control with GnRH agonist or antagonist, administration of u-FSH or r-FSH was used to induce ovulation, and oocyte recovery was performed approximately 36 h after triggering ovulation by hCG administration, under transvaginal ultrasound-guided aspiration.
Oocyte vitrification and warming
Oocytes were vitrified on Cryotop® device using Kitazato vitrification kit (BioPharma, France) with the following protocol. Briefly, equilibration solution (ES: 7.5 % ethylene glycol + 7.5 % dimethylsulfoxide [DMSO]) and vitrification solution (VS: 15 % ethylene glycol + 15 % DMSO + 0.5 M sucrose) were allowed to warm up to room temperature (20–27 °C). Oocytes were subjected to a gradient of ES during 15 min and then exposed to VS, placed on the Cryotop® strip in a minimum volume, and were directly immersed into liquid nitrogen within 1 min.
The number of oocytes to warm was decided on the basis of several criteria: fertilization rate of fresh sibling oocytes (when available), oocyte survival rate after warming, maternal age, attempt rank, and parity. Oocyte warming cycles were performed using the Kitazato warming kit following the manufacturer’s recommendations: The thawing solution (TS), 1.0 M sucrose in tissue culture media + 20 % synthetic serum substitute, was let overnight to warm up at 37 °C in a dish. The strip of the Cryotop® was quickly immersed into the TS. Oocytes were aspirated within the end of the capillary 1 min after immersion into TS. At room temperature, oocytes were transferred in the diluent solution: (0.5 M sucrose) for 3 min and washed twice in droplets of wash solution (5 and 1 min). Oocytes were incubated at 37 °C, CO2 6 %, for at least 2 h before micro-injection. Embryos obtained during oocyte warming cycles were transferred in the context of a spontaneous ovarian cycle (when women ovulated normally) or using hormone therapy (in other cases) for endometrium preparation. A maximum of two embryos were transferred.
Embryo culture
Embryos derived from ICSI on freshly recovered oocytes and vitrified/warmed ones were cultured under exactly the same conditions; in global medium (LifeGlobal®, France) microdrops covered with mineral oil under standard culture conditions. Although day 5 embryo transfer was privileged, depending on embryo quality, transfer on day 2 and day 3 was punctually offered. As oocyte warming cycles involved smaller numbers of oocyte than fresh ICSI cycles, day 2 or day 3 embryo transfer was most often performed in warming cycles. Poor-quality embryos were discarded. Pregnancies were assessed by blood hCG detection (>100 UI/L) followed by ultrasound assessment of fetal heartbeat.
VAO children
Vitrification of at least a part of autologous oocytes was proposed at day 0 to patients enrolled in an infertility treatment program, in three situations: (1) more than six mature oocytes were obtained cohort at pick up (68.3 %), (2) when embryo transfer was contraindicated for medical reasons (26.8 %), and (3) when very few or no spermatozoa was available for ICSI at day 0 (4.9 %). The 50 VAO children reported in the present study included 38 singletons and 12 twins (for a total of 44 pregnancies) who were conceived between 2012 and 2015 (Fig. 1).
ICSI children
ICSI children were conceived in the center from 1995 to 2015. The 364 ICSI children comprise 290 singleton and 74 twins conceived between 2011 and 2015 (327 pregnancies) (Fig. 1). These children served as a comparison group for VAO children health investigation.
Data collection
Data compiled for both groups of children, namely VAO and ICSI children, were collected by auto-questionnaire filled by parents. This information was validated from maternity hospitalization report and/or a copy of the personal child health record document delivered to every parent at child birth. Missing data correspond to information which could not be verified by a medical report.
Maternal characteristics and obstetric outcomes
Maternal characteristics were previously recorded in the database when preparing infertility assessment and the medical folder for infertility treatment: main cause of infertility (male factor infertility, tubal origin, idiopathic, other), day of embryo transfer (day 2 or 3 and blastocyst stage), number of embryos transferred, number of fetal sac, maternal age (years), mother’s body mass index (BMI) (kg/m2), smoking habit, and parity.
The obstetric outcomes were extracted from Medifirst Software (Montigny le Bretonneux, France), used in the center for recording medical, clinical, and biological data such as pregnancy outcomes (diabetes, high blood pressure, and placenta previa) and mode of delivery (vaginal deliveries and caesarean section).
Perinatal outcomes
Nine perinatal outcome measures were analyzed between two groups: gender, percentage of preterm birth (PTB) defined as birth <37 weeks of gestational age, very preterm birth (VPTB) defined as birth <32 weeks of gestational age; percentage of low birth weight (LBW) defined as a birth weight <2500 g; percentage of very low birth weight (VLBW) defined as a birth weight <1500 g; small for gestational age (SGA) and large for gestational age (LGA) were calculated using the Audipog Software, version 1.3 (Association des Utilisateurs de Dossiers Informatisés en Pédiatrie, Obstétrique et Gynécologie, Lyon, France). This statistical model estimates the expected birth weight taking into account gestational age, sex, and birth rank of the infant, maternal age, maternal height, and pre-pregnancy weight [13]. Macrosomia was defined as a birth weight >4000 g. Birth defects in the studied population of children were coded using the European Concerted Action on Congenital Anomalies and Twins (EUROCAT) classification [14]. The EUROCAT system is a European network of registries for the epidemiologic surveillance of congenital anomalies based on data collected in general population. It comprises 14 subgroups of anomalies, namely, nervous system, eye, ear, face and neck, congenital heart defects, respiratory, orofacial clefts, digestive system, abdominal wall defects, urinary, genital, limb, other anomalies/syndromes, and chromosomal. Other minor anomalies were excluded of EUROCAT list.
Statistical analysis
All variables were described by proportions N (%) and means (SD) with 95 % confidence intervals (95 % CI). Student’s t test was used to compare quantitative variables and Pearson chi-squared or Fisher’s exact test is used for qualitative variables. Risk differences in maternal characteristics, obstetric outcomes, and perinatal outcomes were reported as a crude odds ratio (ORs) in VAO children versus ICSI children (Table 1).
Table 1.
Baseline characteristics and risk factors
| Maternal characteristics and obstetric outcomes N = 371 pregnancies |
Vitrified oocytes N = 44 |
Fresh oocytes N = 327 |
p value | |
| Maternal Age (years) (m ± SDa) | 33.3 (±4.0) | 33.0 (±4.6) | 0.71 | |
| Maternal BMIb (kg/m2) (m ± SD) | 22.4 (±3.2) | 22.6 (±3.9) | 0.62 | |
| Smoking habit, N(%) (four missing data—fresh oocytes) | 5 (11.4) | 41 (12.7) | 0.80 | |
| Pregnancy outcome, N(%) (29 missing data—fresh oocytes) | Gestational diabetes | 3 (6.8) | 16 (5.4) | 0.72 |
| Pregnancy induced high blood pressure | 1 (2.3) | 10 (3.4) | 0.99 | |
| Placenta previa | 0 (0) | 7 (2.3) | 0.60 | |
| Parity, N(%) | Primipara | 39 (88.6) | 269 (82.3) | 0.29 |
| Higher order | 5 (11.4) | 58 (17.7) | ||
| Main cause of infertility, N(%) | Male factor infertility | 25 (56.8) | 171 (52.3) | 0.57 |
| Tubal origin | 4 (9.1) | 30 (9.2) | 0.99 | |
| Idiopathic | 10 (22.7) | 71 (21.7) | 0.88 | |
| Day of embryo transfer, N(%) | Day 2, 3 | 21 (47.7) | 77 (23.5) | 0.001 |
| Day 5 | 23 (52.3) | 250 (76.5) | ||
| Number of embryos transferred (m ± SD) | 1.8 (±0.4) | 1.4 (±0.5) | <0.001 | |
| Number of fetal sac (m ± SD) | 1.2 (±0.4) | 1.1 (±0.3) | 0.45 | |
| Mode of delivery, N(%) (five missing data—fresh oocytes ) | Vaginal deliveries | 25 (56.8) | 226 (70.2) | 0.07 |
| Caesarean section | 19 (43.2) | 96 (29.8) | ||
| Perinatal outcomes N(%) N = 414 children |
Vitrified oocytes N = 50 |
Fresh oocytes N = 364 |
p value | |
| Singleton | 38 (76.0) | 290 (79.7) | 0.55 | |
| Twins | 12 (24.0) | 74 (20.3) | ||
| Female | 26 (52.0) | 190 (52.2) | 0.98 | |
| PTBc (<37 WGAd) | 5 (10.0) | 55 (15.1) | 0.34 | |
| VPTBe (<32 WGA) | 1 (2.0) | 2 (0.5) | 0.32 | |
| LBWf (<2500 g) | 7 (14.0) | 62 (17.0) | 0.59 | |
| VLBWg (<1500 g) | 0 (0) | 5 (1.4) | 0.99 | |
| SGAh | 3 (6.0) | 30 (8.2) | 0.78 | |
| LGAi | 2 (4.0) | 10 (2.7) | 0.65 | |
| Macrosomia (>4000 g) | 4 (8.0) | 21 (5.8) | 0.53 | |
| Birth defects (42 missing data—fresh oocytes) | 1 (2.0) | 15 (4.7) | 0.71 | |
aStandard deviation
bBody mass Index
cPreterm birth
dWeeks of gestational age
eVery preterm birth
fLow birth weight
gVery low birth weight
hSmall for gestational age
iLarge for gestational age
To analyze perinatal outcomes and caesarean section according to technique (VAO versus non-vitrified autologous oocytes) and adjusted on other confounding factors, we performed a generalized estimating equation for correlated data, due to the multiple pregnancies. The perinatal outcomes were adjusted (AOR) on maternal age (years), mother’s BMI (kg/m2), parity (first or higher order), singleton, and twins. The caesarean section prevalence was evaluated with adjustment on maternal age (years), mother’s BMI (kg/m2), and singleton/twins (Table 2).
Table 2.
Adjusted odds ratio for mode of delivery and perinatal outcomes
| Crude OR [95 % CI] p value | Adjusted OR [95 % CI] p value | |
|---|---|---|
| Maternal characteristics and obstetric outcomes | ||
| Caesarean sectiona | 1.8 [0.9–3.4] 0.08 | 1.8 [0.9–3.7] 0.09 |
| Perinatal Outcomes | ||
| Femaleb | 1.0 [0.6–1.7] 0.98 | 1.0 [0.6–1.8] 0.99 |
| PTB (<37 weeks)b | 0.6 [0.2–2.2] 0.47 | 0.5 [0.1–1.7] 0.26 |
| LBW (<2500 g)b | 0.8 [0.3–2.2] 0.65 | 0.5 [0.2–1.7] 0.28 |
| SGAb | 0.7 [0.2–2.4] 0.58 | 0.7 [0.2–2.3] 0.53 |
| LGAc | 1.5 [0.3–7.0] 0.62 | 1.6 [0.3–7.5] 0.57 |
| Macrosomia (>4000 g)d | 1.4 [0.5–4.4] 0.54 | 1.6 [0.5–5.1] 0.41 |
| Birth defectsb | 0.4 [0.1–3.2] 0.40 | 0.5 [0.1–3.7] 0.46 |
aAdjusted OR (VAO children vs ICSI children) : maternal age (years), mother’s BMI (kg/m2), singleton/twins
bAdjusted OR (VAO children vs ICSI children): Singleton/twins, maternal age (years), mother’s BMI (kg/m2), parity (primipara or higher order)
c, dThe adjusted odds ratio does not include twins, because there were no cases of LGA and macrosomia in the twins studied in both populations studied
Statistical analyses were performed using Statistical Package for Social Sciences, version 1.7 (SPSS, IBM corporation, USA). A two-sided p value of <0.05 was considered statistically significant.
Results
Clinicobiological results
For informational purposes, we have calculated clinicobiological data for the whole population involved in ICSI program and the population who benefited oocyte warming cycles at the same period as the reported groups in our center. Fertilization rate is 62.5 and 67.2 % in fresh oocytes and vitrified warmed oocytes. A clinical pregnancy rate of 30.3 % was obtained after the transfer of embryo deriving from fresh oocytes and of 22.0 % after the transfer of embryo deriving from vitrified/warmed oocytes and 15.9 % of pregnancy obtained after the transfer of embryo deriving from fresh oocytes ended in a miscarriage and 17.0 % for vitrified warmed oocytes.
Maternal characteristic and obstetric outcomes
The baseline characteristics and risk factors are shown in Table 1. No statistically significant difference was found between the two studied groups, except for the proportion of day embryo transfer day 2–3/day 5 (p = 0.001) and the number of embryos transferred (p < 0.001). The mean age and mother’s BMI in the two groups were similar, approximately 33 years and 22.5 kg/m2, respectively. Mothers who smoked during pregnancy represented around 11 % in each group. Approximately 6 % of mothers presented gestational diabetes in VAO children. Women pregnant with ICSI children showed higher blood pressure (3.4 %) than pregnancies obtained after ICSI on warmed oocyte VAO children (2.3 %), but this difference was not significant (0.99). Primipara women represented more than 80 % of pregnancies in both groups. Caesarean sections were more frequent in VAO children (43.2 %) than in ICSI children (29.8 %), but the difference remains not significant (p = 0.07), even after adjusting the potential confounding factors (p = 0.09) (Table 2). In mothers of the VAO children, no case of placenta previa was found.
Perinatal outcomes
The perinatal outcomes are described in Table 1. No statistically significant difference was found between VAO children and ICSI children, even after adjusting confounding factors (Table 2). The percentage of singletons and twins is similar in the two groups. The singletons represented 79 % (88.3 % monofetal pregnancies) of the sample and twins 21 % (11.7 % twin pregnancies). The proportion of children born before 37 weeks of gestational age and having a birth defect was more substantial in ICSI children (15.1 and 4.7 %) when comparing to VAO children (10.0 and 2.0 %). The percentage of children with LBW and SGA was higher in ICSI children (17.0 and 8.2 %) than in VAO children (14.0 and 6.0 %), unlike the percentage of children with LGA and macrosomia that turned out to be more representative in VAO children (4.0 and 8.0 %) than in ICSI children (2.7 and 5.8 %).
Discussion
This is the first French report on children born after ICSI performed on exclusively autologous vitrified/warmed oocyte as compared to children conceived by ICSI realized on non-vitrified freshly recovered autologous oocytes. Maternal characteristics, obstetric outcomes, and perinatal outcome were evaluated for the purpose of the present study. The present data are consistent with recently published Spanish evaluation [15], since they also demonstrate the safety of oocyte vitrification regarding the health of children. Indeed, maternal characteristics, obstetric outcomes, and perinatal outcomes are similar in VAO children group when comparing with ICSI children group. The present evaluation points out only two significant differences among the parameters assessed between the two compared groups: more embryos are transferred with a lesser percentage of day 5 embryos transferred in the VAO group. These differences are not informative since they are the result of bias introduced by the study design.
In order to limit embryo cryopreservation as recommended by French authorities, oocyte warming cycles involved smaller number of oocyte as compared to fresh ICSI cycles. Consequently, as fewer embryos were available, our results show that day 2/day 3 embryo transfer was more frequently proposed in oocyte warming cycle. Although we adhere to single embryo transfer policy, the transfer of two embryos was favored by both couples and gynecologists during oocyte warming cycle to enhance the success chances. Oocyte warming cycles are more often conducted subsequently to pregnancy failure with fresh oocytes. Therefore, patients and gynecologists tend to ask for the transfer of two embryos rather than only one to enhance success chances during warming cycles. As a result of lower implantation rate after day 2/day 3 embryo transfer, twinning rate is not higher in VAO children even though more embryos are transferred in this group.
Pregnancy is known to be related to health complications such as diabetes, placenta previa, and high blood pressure. These diseases were studied in the IVF pregnancies, and in some cases their prevalence is more important that the prevalence observed in the general population [16–18]. Therefore, we evaluated whether VAO pregnancies were at higher risk for such pathologies. The present data show that pregnancy outcomes are comparable in VAO and ICSI groups. This observation is consistent with recent results showing that similarly to other pregnancy-related complications, hypertension incidence is similar in children born after ICSI using vitrified/warmed oocyte and naturally conceived children [15]. Interestingly, pregnant women belonging to the VAO group suffered less frequently from pregnancy-induced hypertension than those in the ICSI one. As in spontaneous conception, VAO pregnancies were obtained in the context of natural or hormonal replacement therapy ovarian cycle with no ovarian hyperstimulation. Supraphysiological hormone levels in IVF-ICSI may have cardiovascular effect [16]. The absence of ovarian hyperstimulation in the VAO group may account for the lower incidence of hypertension when comparing to ICSI pregnancies. Furthermore, ovarian hyperstimulation has been reported to adversely affect cardiovascular and metabolic profile of children conceived with assisted reproductive technologies [19–21]; this is an interesting aspect to be evaluated in VAO children.
The literature shows that children born after frozen embryo transfer (FET) display an increased risk to be LGA and macrosomic compared with singleton born after fresh embryos and naturally conceived ones [22–25]. The authors conclude that the absence of ovarian stimulation in FET cycles has a positive effect on perinatal outcome. Although our results are not significant, we observe similar tendency of LGA and macrosomia in VAO children while ICSI children are SGA and display LBW. A larger cohort of VAO children is required to clarify this issue. However, this observation reinforces the hypothesis that the absence of ovarian stimulation favors better clinical outcome.
Lower placenta previa prevalence was described in FET [23, 26, 27], which may suggest that freezing does not lead to placental abnormalities and that likely the absence of ovarian hyperstimulation supports a better implantation. Likewise, in our study, we did not found any case of placenta previa in the VAO group.
The limitation in this study is the small sample size in VAO children which considerably reduces the probability of highlighting significant difference, if any. Despite this limitation, the present study is the first publication dealing with children born after exclusively autologous oocytes vitrified in France. Moreover, it is an original report on perinatal outcomes whose data were reliable and accurate since they were collected and verified by a medical doctor of the team, in charge of children follow-up [11]. Overall, our preliminary data about perinatal outcomes after oocyte vitrification are promising and warmly encourage other centers to use autologous oocyte vitrification as a routine technique in infertility management. Indeed, this procedure helped to rescue sensible situations at risk of hyperstimulation syndrome and cases where no spermatozoon was available on the day of oocyte retrieval. Development of autologous oocyte vitrification especially in Europe may add new data in already existing registries which will enable large-scale reporting on newborn’s parameters [22, 28, 29].
The present study participates in the quality and safety control when new techniques become used at a large scale. Our results suggest that VAO children seem not to be at increased risk to develop perinatal trouble when comparing to children conceived with fresh oocytes.
Acknowledgments
The authors acknowledge the gynecologists for sending patients to the “Service de Médecine et Biologie de la Reproduction” at the Hospital Saint Joseph; the parents who sent the questionnaires and kept in touch with us; and laboratory staff for technical input.
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
No external funding was obtained for this study.
Conflict of interest
None declared.
Footnotes
Capsule
This is the first report on the health of children born after ICSI using vitrified/warmed autologous oocytes as compared to children conceived by ICSI on freshly recovered non-vitrified oocytes in France.
References
- 1.Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Blastocyst culture and transfer in clinical-assisted reproduction: a committee opinion. Fertil Steril. 2013:1;99:667–72. [DOI] [PubMed]
- 2.Ubaldi F, Anniballo R, Romano S, Baroni E, Albricci L, Colamaria S, et al. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum Reprod Oxf Engl. 2010;25:1199–205. doi: 10.1093/humrep/deq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RA, Wallace WHB. Fertility preservation in girls and young women. Clin Endocrinol (Oxf) 2011;75:409–19. doi: 10.1111/j.1365-2265.2011.04100.x. [DOI] [PubMed] [Google Scholar]
- 4.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2011;96:277–85. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Cobo A, Remohí J, Chang C-C, Nagy ZP. Oocyte cryopreservation for donor egg banking. Reprod Biomed Online. 2011;23:341–6. doi: 10.1016/j.rbmo.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Cobo A, Garrido N, Crespo J, José R, Pellicer A. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod Biomed Online. 2012;24:424–32. doi: 10.1016/j.rbmo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Cobo A, de los Santos MJ, Castellò D, Gámiz P, Campos P, Remohí J. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3,150 warming cycles. Fertil Steril. 2012;98:1138–46.e1. doi: 10.1016/j.fertnstert.2012.07.1107. [DOI] [PubMed] [Google Scholar]
- 8.Rienzi L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod Oxf Engl. 2012;27:1606–12. doi: 10.1093/humrep/des088. [DOI] [PubMed] [Google Scholar]
- 9.Boyer P, Montjean D, Tourame P, Gervoise-Boyer M. Oocyte vitrification in an ART laboratory. Gynécologie Obstétrique Fertil. 2013;41:551–3. doi: 10.1016/j.gyobfe.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Montjean D, Courageot J, Altié A, Amar-Hoffet A, Rossin B, Geoffroy-Siraudin C, et al. Normal live birth after vitrified/warmed oocytes intracytoplasmic sperm injection with immotile spermatozoa in a patient with Kartagener’s syndrome. Andrologia. 2014;1. [DOI] [PubMed]
- 11.Meddeb L, Boyer M, Pauly V, Tourame P, Rossin B, Pfister B, et al. Procedure used to follow-up a cohort of IVF children. Interests and limits of tools performed to longitudinal follow up for a monocentric cohort. Rev Dépidémiologie Santé Publique. 2011;59:97–105. doi: 10.1016/j.respe.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Harper J, Magli MC, Lundin K, Barratt CLR, Brison D. When and how should new technology be introduced into the IVF laboratory? Hum Reprod Oxf Engl. 2012;27:303–13. doi: 10.1093/humrep/der414. [DOI] [PubMed] [Google Scholar]
- 13.Mamelle N, Cochet V, Claris O. Definition of fetal growth restriction according to constitutional growth potential. Biol Neonate. 2001;80:277–85. doi: 10.1159/000047157. [DOI] [PubMed] [Google Scholar]
- 14.EUROCAT-Guide-1.4-Section-3.3.pdf [Internet]. [cited 2015 Jul 22]. Available from: http://www.eurocat-network.eu/content/EUROCAT-Guide-1.4-Section-3.3.pdf
- 15.Cobo A, Serra V, Garrido N, Olmo I, Pellicer A, Remohí J. Obstetric and perinatal outcome of babies born from vitrified oocytes. Fertil Steril. 2014;102:1006–15.e4. doi: 10.1016/j.fertnstert.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Westerlund E, Brandt L, Hovatta O, Wallén H, Ekbom A, Henriksson P. Incidence of hypertension, stroke, coronary heart disease, and diabetes in women who have delivered after in vitro fertilization: a population-based cohort study from Sweden. Fertil Steril. 2014;102:1096–102. doi: 10.1016/j.fertnstert.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur J Obstet Gynecol Reprod Biol. 2014;176:149–52. doi: 10.1016/j.ejogrb.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Takemura Y, Osuga Y, Fujimoto A, Oi N, Tsutsumi R, Koizumi M, et al. Increased risk of placenta previa is associated with endometriosis and tubal factor infertility in assisted reproductive technology pregnancy. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. 2013;29:113–5. doi: 10.3109/09513590.2012.706669. [DOI] [PubMed] [Google Scholar]
- 19.Gao Q, Pan H-T, Lin X-H, Zhang J-Y, Jiang Y, Tian S, et al. Altered protein expression profiles in umbilical veins: insights into vascular dysfunctions of the children born after in vitro fertilization. Biol Reprod. 2014;91:71. doi: 10.1095/biolreprod.114.120659. [DOI] [PubMed] [Google Scholar]
- 20.Seggers J, Haadsma ML, La Bastide-Van Gemert S, Heineman MJ, Middelburg KJ, Roseboom TJ, et al. Is ovarian hyperstimulation associated with higher blood pressure in 4-year-old IVF offspring? Part I: multivariable regression analysis. Hum Reprod Oxf Engl. 2014;29:502–9. doi: 10.1093/humrep/det396. [DOI] [PubMed] [Google Scholar]
- 21.Pontesilli M, Painter RC, Grooten IJ, van der Post JA, Mol BW, Vrijkotte TGM, et al. Subfertility and assisted reproduction techniques are associated with poorer cardiometabolic profiles in childhood. Reprod Biomed Online. 2015;30:258–67. doi: 10.1016/j.rbmo.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Pelkonen S, Koivunen R, Gissler M, Nuojua-Huttunen S, Suikkari A-M, Hydén-Granskog C, et al. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995–2006. Hum Reprod Oxf Engl. 2010;25:914–23. doi: 10.1093/humrep/dep477. [DOI] [PubMed] [Google Scholar]
- 23.Sazonova A, Källen K, Thurin-Kjellberg A, Wennerholm U-B, Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod Oxf Engl. 2012;27:1343–50. doi: 10.1093/humrep/des036. [DOI] [PubMed] [Google Scholar]
- 24.Kato O, Kawasaki N, Bodri D, Kuroda T, Kawachiya S, Kato K, et al. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2012;161:46–50. doi: 10.1016/j.ejogrb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Andersen AN. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod Oxf Engl. 2014;29:618–27. doi: 10.1093/humrep/det440. [DOI] [PubMed] [Google Scholar]
- 26.Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98:368–77.e1–9. doi: 10.1016/j.fertnstert.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Korosec S, Ban Frangez H, Verdenik I, Kladnik U, Kotar V, Virant-Klun I, et al. Singleton pregnancy outcomes after in vitro fertilization with fresh or frozen-thawed embryo transfer and incidence of placenta praevia. BioMed Res Int. 2014;2014:431797. doi: 10.1155/2014/431797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wennerholm U-B, Henningsen A-KA, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod Oxf Engl. 2013;28:2545–53. doi: 10.1093/humrep/det272. [DOI] [PubMed] [Google Scholar]
- 29.Henningsen A-KA, Pinborg A. Birth and perinatal outcomes and complications for babies conceived following ART. Semin Fetal Neonatal Med. 2014;19:234–8. doi: 10.1016/j.siny.2014.04.001. [DOI] [PubMed] [Google Scholar]