Abstract
Purpose
The aim of the study is to investigate the regulation of DNA repair genes by microRNAs (miRNAs). miRNAs are short non-coding RNAs that regulate transcriptional and post-transcriptional gene silencing. Several miRNAs that are expressed during preimplantation embryo development have been shown or are predicted to target genes that regulate cell cycle checkpoints and DNA repair in response to DNA damage.
Methods
This study compares the expression level of 20 miRNAs and 9 target transcripts involved in DNA repair. The statistical significance of differential miRNA expression between oocytes and blastocysts was determined by t test analysis using the GraphPad Prism v6 software. The possible regulatory roles of miRNAs on their target messenger RNAs (mRNAs) were analysed using a Pearson correlation test.
Results
This study shows for the first time that several miRNAs are expressed in human oocytes and blastocysts that target key genes involved in DNA repair and cell cycle checkpoints. Blastocysts exhibited statistically significant lower expression levels for the majority of miRNAs compared to oocytes (p < 0.05). Correlation analyses showed that there was both inverse and direct association between miRNAs and their target mRNAs.
Conclusions
miRNAs target many mRNAs including ones involved in DNA repair mechanisms. This study suggests that miRNAs and their target mRNAs involved in DNA repair are expressed in preimplantation embryos. Similar to the miRNAs expressed in adult tissues, these miRNAs seem to have regulatory roles on their target DNA repair mRNAs during preimplantation embryo development.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0585-0) contains supplementary material, which is available to authorized users.
Keywords: MicroRNA, Expression, DNA repair, Human oocyte and blastocyst, Preimplantation embryo development
Introduction
Coordination of the cell cycle and activation of DNA repair mechanisms upon DNA damage are crucial in the early developing embryo to preserve the genomic integrity. If the repair mechanisms are unable to repair the damage, apoptosis of an embryonic cell can be detrimental to the early developing embryo [1]. Therefore, correct activation of genes and proteins is critical for the survival of a preimplantation embryo.
The regulation of transcripts is controlled by complex mechanisms. Recent studies proposed that more than half of the human transcriptome is regulated by microRNAs (miRNAs) [2, 3]. Several miRNAs have been shown to regulate and be regulated by genes functioning at cell cycle checkpoints [4–10] and in different repair mechanisms: nucleotide excision repair [11], mismatch repair [12–15] and double-strand break repair [16–21] in cancer cells and cell lines.
MiRNAs are small non-coding RNAs of 17–25 nucleotides in length which either degrade or inhibit the expression of their target genes and therefore the function of these genes can be altered [22–29]. More recently studies suggested that these non-coding RNAs may stabilise the expression of their target messenger RNAs (mRNAs) [27, 30–36].
Expression of miRNAs has been observed in murine, bovine and human gametes and preimplantation embryos [22, 25, 37–40]. Similar expression profiles of miRNAs in mature mouse oocytes and in the early developing embryos were observed that may be due to the maternally inherited miRNAs in the early embryo [38]. Expression of miRNAs was also detected in the sperm, and about 20 % of these miRNAs are present within the nuclear or perinuclear part of the sperm that are transferred to the zygote [41]. However, these miRNAs are thought not to play a significant role since these miRNAs are already present in the oocytes [42]. Expression levels of these miRNAs vary during cleavage stage divisions, such that in murine embryos when the maternally inherited miRNAs are being degraded, a 60 % decrease in the miRNA expression was observed [38, 39]. In this study, we investigate the expression of specific miRNAs that are known to target mRNA transcripts associated with DNA repair in human oocytes and blastocysts to correlate the expression profiles of these mRNA and miRNAs and to elucidate the potential regulatory role and activity of the repair mechanisms in human oocytes and preimplantation embryos.
Materials and methods
This work was licensed by the Human Fertilisation and Embryology Authority (HFEA project reference: RO113), and ethical approval was granted by the National Research Ethics Service (NRES), Research Ethics Committee (REC reference number: 10/H0709/26).
Sample collection and processing
Immature oocytes and surplus cryopreserved blastocysts were collected from patients who had given an informed consent following in vitro fertilisation (IVF) treatment. The zona pellucida of oocytes and blastocysts was removed following brief exposure to acidified Tyrode’s solution (Medi-Cult, Surrey, UK). The samples were then washed in phosphate-buffered saline with 0.1 % polyvinyl alcohol solution (PBS/PVA; Sigma, USA) and 0.3 U/μl RNasin plus RNAse inhibitor (Promega, UK) to prevent RNA degradation. Single oocytes and single blastocysts were transferred in a minimum volume of PBS/PVA with RNasin solution to an empty 0.2-ml MicroAmp reaction tube.
Selection of mRNA and miRNA for analysis
An expression profile of DNA repair genes in human oocytes and blastocysts was previously established using microarrays [43, 44]. This study was used as a guideline to identify mRNAs that were differentially expressed in human oocytes relative to blastocysts. In order to deduce the activity of different DNA repair pathways, one sensor gene or a gene functioning at the earlier stages of the particular repair pathway and one gene functioning at later stages of the repair pathway were selected. In addition to this group of repair genes, the expression of one miRNA-processing gene and one housekeeping gene was analysed as control genes. miRNAs targeting the selected DNA repair genes were identified using miRNA databases (http://www.microrna.org/microrna/home.do, http://www.targetscan.org/ and http://mirdb.org/miRDB/) and previously published articles [22, 38, 40, 43, 45, 46]. A further literature search was conducted to select from these miRNAs that were shown to be expressed in human, murine and bovine embryos. Two miRNAs, namely hsa-miR-15a and hsa-miR-212, that were previously shown to be expressed in human oocytes and two miRNAs, namely hsa-let-7a and hsa-miR-21, that were reported to be expressed in human blastocysts were analysed as controls. Additional 16 miRNAs expressed in murine and bovine oocytes and blastocysts and embryonic stem cells were selected from the previous publications.
Oocytes and blastocysts were grouped according to morphological grade and maternal age. For each mRNA and miRNA, expression analyses were performed in six repeat samples (six individual oocytes and six individual blastocysts) to eliminate the sample variation with at least two replicates.
mRNA and miRNA expression
mRNA expression was analysed from 9 oocytes and 10 blastocysts using the TaqMan® Gene Expression Cells-to-CT™ Kit (Ambion, Life Technologies, UK). miRNA expression was analysed using the TaqMan® MicroRNA Cells-to-CT™ Kit (Ambion, Life Technologies, UK) in a further 22 oocytes and 23 blastocysts. Both mRNA and miRNA quantification involved three main steps: lysis, reverse transcription and real-time quantitative PCR (qPCR). Cell lysis was performed in 25 μl lysis solution with 0.25 μl DNase I at room temperature (RT) for 8 min. The lysis was stopped by addition of the 2.5 μl stop solution and incubation at RT for 2 min.
Reverse transcription and qPCR for both mRNA and miRNA were performed following the respective manufacturer’s protocol. For each reaction, negative controls in the absence of embryo lysates and cDNA products were performed. Overall, 10 mRNAs and 20 miRNAs were analysed. Real-time qPCR for each sample was carried out in duplicates, and the expression level of each mRNA or miRNA was determined in a minimum of 6 different oocytes and 6 different blastocysts. All the samples that were used for mRNA expression analysis were also tested for ACTB expression. Similarly, all samples that were used for miRNA expression analysis were tested for RNU48. ACTB and RNU48 were selected for endogenous reference genes since both were shown to be expressed at a constant level in human oocytes and preimplantation embryos.
The comparative ΔΔCq method was used to examine the expression levels of miRNAs and mRNAs [40]. Determination of Cq values was performed using the LightCycler Nano Software (Roche, UK), and ΔCq values were determined as follows after normalisation with the endogenous reference gene ACTB for mRNA and RNU48 for miRNA, respectively, for each oocyte and blastocyst sample:
The fold change was analysed using the relative quantification method (2−ΔΔCq)
Statistical analyses
All the statistical analyses were carried out using the GraphPad Prism v6 software. The level of expression of each mRNA and miRNA in oocytes relative to blastocysts was examined by applying an unpaired two-tailed Student’s t test with the Welch correction, respectively. The correlation between each miRNA and its target mRNA was investigated using the Pearson correlation test. An inverse correlation was defined as r = −1 and a direct correlation as r = +1 following Pearson correlation test. Similarly, a perfect correlation was defined as Pearson’s r2 coefficient equal to 1. For all the statistical analyses, p < 0.05 indicated a statistical significance.
Results
A total of 31 immature oocytes and 33 surplus cryopreserved blastocysts were collected from 20 women (maternal age 35 ± 5) and 16 couples (maternal age 38 ± 7), respectively. mRNA expression of nine transcripts involved in DNA repair and one involved in miRNA processing was investigated in a total of 9 oocytes and 10 blastocysts. Differentially expressed DNA repair genes in human oocytes and blastocysts were identified previously [43]. A literature search to identify miRNAs expressed in human oocytes and blastocysts was performed. This search was narrowed down on the miRNAs that were shown or predicted to regulate these differentially expressed DNA repair genes. From these mRNA and miRNA profiles, repair genes such as miRNAs targeting an important cell cycle checkpoint gene, sensor genes from each pathway and genes involved in different repair pathways were selected to be analysed in this study (Table 1, Fig. 1). miRNAs were selected to target more than one gene that is involved in different repair pathways to be able to have a general idea of the possible regulatory roles of miRNAs in DNA repair pathways. The expression of 20 miRNAs targeting these repair genes was analysed in 22 oocytes and 23 blastocysts, respectively. The expression level of each mRNA or miRNA was determined in a minimum of 6 different oocytes and 6 different blastocysts. Each analysis was carried out in duplicate.
Table 1.
List of miRNAs and their association with DNA repair
| Cell cycle checkpoint | Nucleotide excision repair | Base excision repair | Double-strand break repair | Mismatch repair | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RB1 | GTF2H2 | ERCC3 | PARP1 | DCLRE1A | PARP1 | BRCA1 | RAD50 | MSH2 | MSH3 | MSI |
| Let-7 | miR-23b | miR-192 | miR-7 | miR-15a | miR-7 | miR-7 | miR-15a | miR-21 | miR-7 | Let-7a |
| miR-7 | miR-101 | miR-31 | miR-16 | miR-31 | miR-145 | miR-16 | miR-145 | miR-21 | miR-31 | |
| miR-34c | miR-128 | miR-130 | miR-23b | miR-130 | miR-155 | miR-128 | miR-155 | miR-192 | miR-101 | |
| miR-101 | miR-181c | miR-182 | miR-128 | miR-182 | miR-182 | miR-155 | miR-192 | miR-145 | ||
| miR-128 | miR-192 | miR-192 | miR-145 | miR-192 | miR-196 | miR-194 | miR-155 | |||
| miR-181c | miR-212 | miR-196 | miR-155 | miR-196 | miR-210 | miR-212 | miR-181c | |||
| miR-192 | miR-210 | miR-212 | miR-210 | miR-212 | miR-192 | |||||
| miR-194 | miR-196b | |||||||||
| miR-212 | miR-212 | |||||||||
Genes involved in cell cycle checkpoint, nucleotide excision, base excision, double-strand break and mismatch repair pathways and the miRNAs targeting these genes are listed. These associations were either published previously [11, 47–50], or bioinformatics studies showed that these miRNAs target the mRNAs (http://www.targetscan.org/, http://www.microrna.org/microrna/home.do, http://mirdb.org/miRDB/)
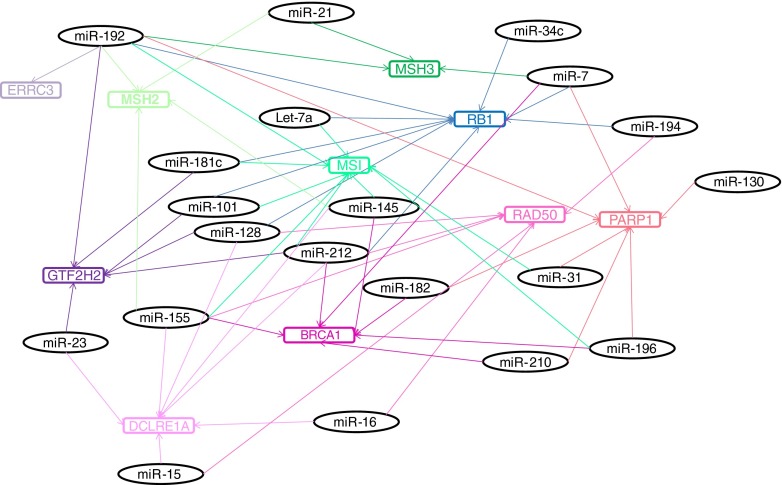
Fig. 1.
Schematic diagram of the complex association of miRNAs in DNA repair pathways. miRNAs that were shown or predicted to target genes involved in repair are shown in a target map. miRNAs that were associated with microsatellite instability (indicative of defective mismatch repair) are also shown in this diagram. miRNAs are colour coded according to their target mRNAs. Double-strand break repair genes (DCLRE1A, PARP1, BRCA1 and RAD50) are shown in pink, mismatch repair genes (MSH2 and MSH3) and microsatellite instability in green, nucleotide excision repair genes (ERCC3 and GTF2H2) in purple and checkpoint genes (RB1 and) in blue shades, respectively
mRNA and miRNA expression
Quantification for each mRNA and miRNA was performed after normalisation with endogenous control genes, ACTB and RNU48, respectively. All 10 mRNAs analysed (PARP1, BRCA1, RAD50, MSH2, MSH3, GTF2H2, ERCC3, DCLRE1A, RB1 and DICER) were expressed both in oocytes and blastocysts. Of the 20 miRNAs analysed, 11 (hsa-let-7a, hsa-miR-16, hsa-miR-21, hsa-miR-31, hsa-miR-101, hsa-miR-145, hsa-miR-182, hsa-miR-192, hsa-miR-194, hsa-miR-210 and hsa-miR-212) were detected in oocytes and 18 (hsa-let-7a, hsa-miR-7-2, hsa-miR-15a, hsa-miR-16, hsa-miR-21, hsa-miR-23b, hsa-miR-31, hsa-miR-34c, hsa-miR-101, hsa-miR-128, hsa-miR-130, hsa-miR-145, hsa-miR-155, hsa-miR-182, hsa-miR-192, hsa-miR-194, hsa-miR-210 and hsa-miR-212) in blastocysts, respectively. Two miRNAs analysed (hsa-miR-181c and hsa-miR-196) were neither detected in oocytes nor in blastocysts, whereas 7 were only detected in blastocysts (hsa-miR-7-2, hsa-miR-15a, hsa-miR-23, hsa-miR-34, hsa-miR-128, hsa-miR-130 and hsa-miR-155). All miRNAs detected in oocytes were also detected in blastocysts. The fold differences of these mRNAs and miRNAs between oocytes and blastocysts are listed in Supplementary Table 2.
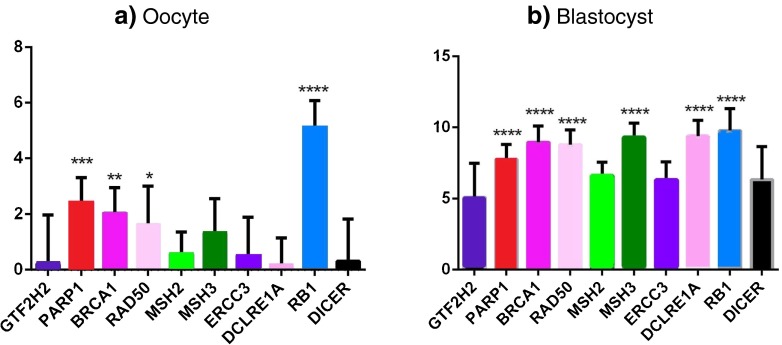
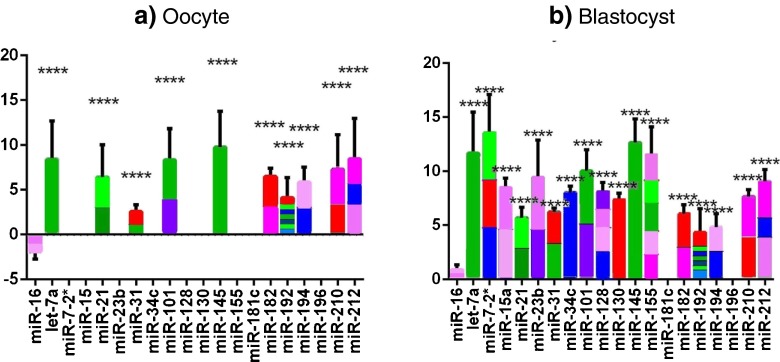
The expression levels of all mRNAs were significantly higher in oocytes compared to blastocysts (p < 0.05; Fig. 2). Six miRNAs (hsa-miR-31, hsa-miR-16, let-miR-7a, hsa-miR-145, hsa-miR-210 and hsa-miR-212) were expressed at significantly higher levels in oocytes compared to blastocysts (Fig. 3), whereas 5 miRNAs (hsa-miR-21, hsa-miR-101, hsa-miR-182, hsa-miR-192 and hsa-miR-194) were expressed at similar levels in oocytes and blastocysts.
Fig. 2.
mRNA expression levels quantified by real-time PCR after normalisation with the endogenous control, ACTB. Quantification of target mRNAs compared to GTF2H2 is shown to be expressed at the highest level in a oocytes and b blastocysts. ANOVA followed by Dunnett’s post test was applied to all the mRNAs normalised to ACTB, where ****p < 0.0001 indicates the most statistical significance; ***0.0001 < p < 0.001, **0.001 < p < 0.01 and *0.01 < p < 0.05 indicate the less statistical significance; and p ≥ 0.05 indicates no statistical significance. The x-axis represents all the miRNAs analysed in this study, and the y-axis represents the ΔCq values. GTF2H2 is shown in dark purple, PARP1 in red, BRCA1 in pink, RAD50 in light pink, MSH2 in light green, MSH3 in dark green, ERCC3 in purple, DCLRE1A in mid pink, RB1 in aqua and DICER1 in black shades, respectively
Fig. 3.
miRNA expression levels quantified by real-time PCR after normalisation with the endogenous control, RNU48. Quantification of target miRNAs compared to hsa-miR-16 in a oocytes and b blastocysts. ANOVA followed by Dunnett’s post test was applied to all miRNAs compared to hsa-miR-16. The x-axis represents all the miRNAs analysed in this study, and the y-axis represents the ΔCq values. In oocytes, the expression of hsa-miR-16 was at a higher expression level compared to the endogenous control RNU48 represented by a negative profile for this miRNA. miRNAs were colour coded according to their target genes as in Fig. 2, i.e. miRNAs targeting PARP1 is shown in red, BRCA1 in pink, RAD50 in light pink, DCLRE1A in mid pink, MSH2 in light green, MSH3 in dark green, ERCC3 in purple, GTF2H2 in dark purple and RB1 in aqua shades, respectively. ****p < 0.001, the difference in the level of expression of the miRNA from that of hsa-miR-16 is statistically significant
Correlation between the expression levels of mRNA and miRNA
Two miRNAs, hsa-miR-181c and hsa-miR-196, that were neither expressed in oocytes nor in blastocysts were excluded from the correlation analysis. Pearson correlation test showed that there was a trend for both negative and positive correlations between miRNAs and their target mRNAs. Inverse associations were observed in blastocysts (p < 0.05) for hsa-miR-23b and its target gene GTF2H2, hsa-miR-128 and DCLRE1A and hsa-miR-128 and RAD50 and direct association for hsa-miR-34c and RB1 that may indicate a possible stabilisation effect of miRNAs on their target mRNAs.
Discussion
The level of gene expression is affected by many factors that may influence the DNA repair capacity. In the recent years, one of the regulatory mechanisms of transcripts was suggested to be through miRNA control. Although many studies have shown that several repair gene transcripts are regulated or regulate several miRNAs in cancer cells and cell lines, no studies were performed to analyse the association between miRNAs and their target mRNAs involved in DNA repair in human oocytes and embryos. The aim of this study was to analyse this association by correlating the expression levels of a selection of miRNAs and their target mRNAs.
In this study, the expression of 10 mRNAs and 20 miRNAs was analysed in human oocytes and blastocysts. The expression of all the mRNAs tested and 11 miRNAs was detected in both oocytes and blastocysts. Higher mRNA expression levels were detected in oocytes relative to the blastocysts (Fig. 2). This is not surprising since the oocytes are required to be packed with mRNAs and passed on the early embryo to support itself until the embryonic genome activation and the maternal mRNAs are expected to be degraded post embryonic genome activation. Similar to the repair genes, a majority of the miRNAs showed a higher expression level in oocytes relative to blastocysts. It has been well established that miRNAs silence many genes for translational inhibition, cleavage, degradation or destabilisation [23–28]. Therefore, the higher expression of miRNAs in oocytes may be required to degrade the maternally inherited mRNAs in the early developing embryos as also reported in zebrafish [51, 52] and in rainbow trout [53].
More recently studies have proposed that miRNAs may stabilise their target mRNAs as well [27, 30–36]. This study further investigated the possibility of the stabilisation effect of mRNAs on their target mRNAs by analysing whether individual samples with higher miRNA expression levels might tend to have higher or consistent expression levels of their target mRNAs. Alternatively, a correlation in the other direction was questioned in order to understand the possible down-regulatory roles of miRNAs on their targets.
A trend for both direct and inverse relationships was observed between miRNAs and their target mRNAs. It is possible that these direct and inverse relationships are miRNA specific and each miRNA regulates each target differently. It may also depend on the embryonic development stage, or it is possible that these observations do not hold a biological significance. A direct association was observed between the expression of miRNAs and RB1, active at G1/S transition, in oocytes and blastocysts. This direct relationship was also observed in oocytes amongst the miRNAs and mismatch repair genes, MSH2 and MSH3, functioning at G2 cell cycle checkpoint. This association may be necessary to stabilise the target mRNAs in the early developing embryos to direct DNA repair until the embryonic genome activation. However both direct and indirect relationships were observed for the nucleotide excision repair genes as also reported previously for miR-192 and ERCC3 [11], double-strand break repair and interstrand cross-link repair genes. The down-regulatory effect of some miRNAs on BRCA1 and PARP1 was also reported previously [54, 55]. The inverse relationships between miRNAs and their target mRNAs functioning at the G1/S phase of cell cycle may impair the detection and repair capacity of DNA damage. This may be the reason for oocytes to continue the meiotic divisions in the presence of DNA damage as reported previously by Marangos and Carroll [56].
One of the main limitations of this study was the practical inability following the expression of miRNA and mRNA through different developmental stages. We have analysed the expression level of miRNAs and mRNAs in immature oocytes and at blastocyst stage of embryonic development. Previously published studies in humans have shown that 2 % of the miRNAs analysed showed different expression levels between GV and MII oocytes [40]. Similarly, bovine studies have shown that miRNA expression varies during preimplantation embryo development and embryonic genome activation [38, 39]. However, in our study due to the scarcity of human samples, we could only analyse the expression of a limited number of miRNA and mRNA in limited sample types. Although the investigation of the miRNA and the target mRNA expression within the same group of oocytes and blastocysts would provide a more applicable analysis, due to technical reasons and scarcity of the samples, the analysis was performed on a separate group of oocytes and blastocysts obtained from different women and couples. While use of oocytes and embryos from different women and couples is a weakness of our study, it could also be considered as strength since it eliminates any possible hierarchal conclusions. Furthermore, even though there may be some differential miRNA expression amongst different oocytes and embryos, the difference is not expected to be significant since none of the patients were diagnosed with complications, such as polycystic ovarian syndrome or endometriosis that may have caused differences in miRNA expression [57, 58]. With the improvements in the amplification techniques and lowering the bias introduced, future experiments may be performed on amplified human samples enabling expression studies on the same set of oocytes and blastocysts. The amplification has the benefit of analysis of many more miRNAs and mRNAs from the same set of oocytes and blastocysts. Further studies focusing on expression analysis of more miRNAs and target mRNAs will provide better understanding of the association between miRNAs and their target mRNAs. However, the expression analyses alone would still not be sufficient to prove a true regulation and functional studies are crucial for a definite conclusion.
Conclusions
Regulation of mRNAs involves complex mechanisms, amongst which included the action of miRNAs. It is well established that miRNAs target many mRNAs including ones involved in DNA repair mechanisms. This study showed that miRNAs and their target mRNAs involved in DNA repair are expressed in preimplantation embryos. Similar to the miRNAs expressed in different tissue types, the miRNAs expressed in embryos seem to have regulatory roles on their target DNA repair mRNAs during preimplantation embryo development.
Supplemental Table 1 shows the list of (a) mRNAs and (b) miRNAs, fold difference and log2 of fold change in blastocyst compared with oocyte. mRNAs and miRNAs and ΔCq values in oocytes and blastocysts are listed. Average normalised ΔCq values generated from six repeats with two replicates of each sample are shown. Standard deviation (SD) for each data point of ΔCq is shown in parenthesis. The greater Cq values reflect lower expression. MiRNAs, fold change difference and log2 of fold change in blastocyst compared with oocyte are listed. Fold change was calculated by relative quantification method (2ΔΔCq). The log2 scale transformation of the fold change facilitates the interpretation of the difference in expression between the two samples. The negative log2 values indicated increased expression of the gene in the blastocyst compared with the oocyte. N/A stands for not applicable.
Electronic supplementary material
(DOC 93 kb)
Footnotes
Capsule
In this study, we have shown that the miRNAs expressed in human oocytes and blastocysts are associated with their target genes that are involved in DNA repair.
This work was done in the UCL Centre for PGD, Institute for Women’s Health, University College London, UK.
References
- 1.Lukas J, Lukas C, Bartek J. Mammalian cell cycle checkpoints: signalling pathways and their organization in space and time. DNA Repair (Amst) 2004;3(8–9):997–1007. doi: 10.1016/j.dnarep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13(4):271–82. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, et al. miR-375 targets the p53 gene to regulate cellular response to ionizing radiation and etoposide in gastric cancer cells. DNA Repair (Amst) 2013;12(9):741–50. doi: 10.1016/j.dnarep.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Pok S, et al. Cyclin E facilitates dysplastic hepatocytes to bypass G1/S checkpoint in hepatocarcinogenesis. J Gastroenterol Hepatol. 2013;28(9):1545–54. doi: 10.1111/jgh.12216. [DOI] [PubMed] [Google Scholar]
- 6.Noto JM, Peek RM. The role of microRNAs in Helicobacter pylori pathogenesis and gastric carcinogenesis. Front Cell Infect Microbiol. 2012;1:21. doi: 10.3389/fcimb.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2009;17(2):215–220. doi: 10.1038/cdd.2009.69. [DOI] [PubMed] [Google Scholar]
- 8.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13(1):39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurado JO, et al. IL-17 and IFN-gamma expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol. 2012;91(6):991–1002. doi: 10.1189/jlb.1211619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massirer KB, et al. The miR-35-41 family of microRNAs regulates RNAi sensitivity in Caenorhabditis elegans. PLoS Genet. 2012;8(3) doi: 10.1371/journal.pgen.1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie QH, et al. MiR-192 inhibits nucleotide excision repair by targeting ERCC3 and ERCC4 in HepG2.2.15 cells. Biochem Biophys Res Commun. 2011;410(3):440–5. doi: 10.1016/j.bbrc.2011.05.153. [DOI] [PubMed] [Google Scholar]
- 12.Svrcek M, et al. Overexpression of microRNAs-155 and 21 targeting mismatch repair proteins in inflammatory bowel diseases. Carcinogenesis. 2013;34(4):828–34. doi: 10.1093/carcin/bgs408. [DOI] [PubMed] [Google Scholar]
- 13.Mao G, et al. Modulation of microRNA processing by mismatch repair protein MutLalpha. Cell Res. 2012;22(6):973–85. doi: 10.1038/cr.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He YQ, et al. Estradiol regulates miR-135b and mismatch repair gene expressions via estrogen receptor-beta in colorectal cells. Exp Mol Med. 2012;44(12):723–32. doi: 10.3858/emm.2012.44.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valeri N, et al. Modulation of mismatch repair and genomic stability by miR-155. Proc Natl Acad Sci. 2010;107(15):6982–6987. doi: 10.1073/pnas.1002472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman AE, et al. microRNA miR-196a-2 and breast cancer: a genetic and epigenetic association study and functional analysis. Cancer Res. 2009;69(14):5970–7. doi: 10.1158/0008-5472.CAN-09-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neijenhuis S, et al. Identification of miRNA modulators to PARP inhibitor response. DNA Repair (Amst) 2013;12(6):394–402. doi: 10.1016/j.dnarep.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan K, et al. MicroRNA-182-5p targets a network of genes involved in DNA repair. RNA. 2013;19(2):230–42. doi: 10.1261/rna.034926.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, et al. Genetic variation in a hsa-let-7 binding site in RAD52 is associated with breast cancer susceptibility. Carcinogenesis. 2013;34(3):689–93. doi: 10.1093/carcin/bgs373. [DOI] [PubMed] [Google Scholar]
- 20.Mansour WY, et al. Aberrant overexpression of miR-421 downregulates ATM and leads to a pronounced DSB repair defect and clinical hypersensitivity in SKX squamous cell carcinoma. Radiother Oncol. 2012;106(1):147–54. doi: 10.1016/j.radonc.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Wu CW, et al. MicroRNA-18a attenuates DNA damage repair through suppressing the expression of ataxia telangiectasia mutated in colorectal cancer. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCallie B, Schoolcraft WB, Katz-Jaffe MG. Aberration of blastocyst microRNA expression is associated with human infertility. Fertil Steril. 2010;93:2374–82. doi: 10.1016/j.fertnstert.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 23.Bagga S, et al. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122(4):553–63. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi K, et al. MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS One. 2008;3(3) doi: 10.1371/journal.pone.0001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tesfaye D, et al. Identification and expression profiling of microRNAs during bovine oocyte maturation using heterologous approach. Mol Reprod Dev. 2009;76(7):665–77. doi: 10.1002/mrd.21005. [DOI] [PubMed] [Google Scholar]
- 26.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 27.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5(5):396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 28.Miranda KC, et al. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–17. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Place RF, et al. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008;105(5):1608–13. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmena L, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146(3):353–8. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seitz H. Redefining microRNA targets. Curr Biol. 2009;19(10):870–3. doi: 10.1016/j.cub.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 32.Arvey A, et al. Target mRNA abundance dilutes microRNA and siRNA activity. Mol Syst Biol. 2010;6:363. doi: 10.1038/msb.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DY, et al. A 3′-untranslated region (3′UTR) induces organ adhesion by regulating miR-199a* functions. PLoS One. 2009;4(2) doi: 10.1371/journal.pone.0004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poliseno L, et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033–8. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franco-Zorrilla JM, et al. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet. 2007;39(8):1033–7. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- 36.Cazalla D, Yario T, Steitz JA. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science. 2010;328(5985):1563–6. doi: 10.1126/science.1187197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenbluth EM, et al. MicroRNA expression in the human blastocyst. Fertil Steril. 2012;99(3):855–861. doi: 10.1016/j.fertnstert.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Tang F, et al. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21(6):644–8. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, et al. Determination of microRNAs in mouse preimplantation embryos by microarray. Dev Dyn. 2008;237(9):2315–27. doi: 10.1002/dvdy.21666. [DOI] [PubMed] [Google Scholar]
- 40.Xu YW, et al. Differentially expressed microRNAs in human oocytes. J Assist Reprod Genet. 2011;28(6):559–66. doi: 10.1007/s10815-011-9590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, et al. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol. 2005;7(7):719–23. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amanai M, Brahmajosyula M, Perry AC. A restricted role for sperm-borne microRNAs in mammalian fertilization. Biol Reprod. 2006;75(6):877–84. doi: 10.1095/biolreprod.106.056499. [DOI] [PubMed] [Google Scholar]
- 43.Jaroudi S, et al. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum Reprod. 2009;24(10):2649–55. doi: 10.1093/humrep/dep224. [DOI] [PubMed] [Google Scholar]
- 44.Kakourou G, et al. Investigation of gene expression profiles before and after embryonic genome activation and assessment of functional pathways at the human metaphase II oocyte and blastocyst stage. Fertil Steril. 2012;99(3):803–814. doi: 10.1016/j.fertnstert.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 45.Yang GH, et al. MicroRNAs are involved in erythroid differentiation control. J Cell Biochem. 2009;107(3):548–56. doi: 10.1002/jcb.22156. [DOI] [PubMed] [Google Scholar]
- 46.Mondou E, et al. Analysis of microRNAs and their precursors in bovine early embryonic development. Mol Hum Reprod. 2012;18:425–34. doi: 10.1093/molehr/gas015. [DOI] [PubMed] [Google Scholar]
- 47.McPhee F, et al. Resistance analysis of the hepatitis C virus NS3 protease inhibitor asunaprevir. Antimicrob Agents Chemother. 2012;56:3670–81. doi: 10.1128/AAC.00308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valeria N, et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2) PNAS. 2010;107:21098–21103. doi: 10.1073/pnas.1015541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 50.Mueller AC, Sun D, Dutta A. The miR-99 family regulates the DNA damage response through its target SNF2H. Oncogene. 2013;32:1164–72. doi: 10.1038/onc.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen PY, et al. The developmental miRNA profiles of zebrafish as determined by small RNA cloning. Genes Dev. 2005;19(11):1288–93. doi: 10.1101/gad.1310605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giraldez AJ, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312(5770):75–9. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 53.Ramachandra RK, et al. Cloning and characterization of microRNAs from rainbow trout (Oncorhynchus mykiss): their expression during early embryonic development. BMC Dev Biol. 2008;8:41. doi: 10.1186/1471-213X-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moskwa P, et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol Cell. 2010;41(2):210–220. doi: 10.1016/j.molcel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Volinia S, et al. Breast cancer signatures for invasiveness and prognosis defined by deep sequencing of microRNA. Proc Natl Acad Sci U S A. 2012;109(8):3024–9. doi: 10.1073/pnas.1200010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol. 2012;22(11):989–94. doi: 10.1016/j.cub.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 57.Sorensen AE, et al. MicroRNAs related to polycystic ovary syndrome (PCOS) Genes (Basel) 2014;5(3):684–708. doi: 10.3390/genes5030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang L, Liu HY. Small RNA molecules in endometriosis: pathogenesis and therapeutic aspects. Eur J Obstet Gynecol Reprod Biol. 2014;183:83–8. doi: 10.1016/j.ejogrb.2014.10.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 93 kb)