Abstract
Purpose
The aim of the present study was to analyze the lipid profile of follicular fluid from patients with endometriosis and endometrioma who underwent in vitro fertilization treatment (IVF).
Methods
The control group (n = 10) was composed of women with tubal factor or minimal male factor infertility who had positive pregnancy outcomes after IVF. The endometriosis group consisted of women with endometriosis diagnosed by videolaparoscopy (n = 10), and from the same patients, the endometriomas fluids were collected, which composed the endometrioma group (n = 10). From the follicular fluid and endometriomas, lipids were extracted by the Bligh and Dyer method, and the samples were analyzed by tandem mass spectrometry.
Results
We observed phosphatidylglycerol phosphate, phosphatidylcholine, phosphatidylserine, and phosphatidylnositol bisphosphate in the control group. In the endometriosis group, sphingolipids and phosphatidylcholines were more abundant, while in the endometrioma group, sphingolipids and phosphatidylcholines with different m/z from the endometriosis group were found in high abundance.
Conclusion
This analysis demonstrated that there is a differential representation of these lipids according to their respective groups. In addition, the lipids found are involved in important mechanisms related to endometriosis progress in the ovary. Thus, the metabolomic approach for the study of lipids may be helpful in potential biomarker discovery.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-015-0592-1) contains supplementary material, which is available to authorized users.
Keywords: Endometriosis; Endometrioma; IVF; Follicular fluid; Metabolomics; Mass spectrometry, lipids
Introduction
Endometriosis is a chronic gynecological and inflammatory disease affecting approximately 10 % of women of reproductive age [1]. This disease is characterized by the presence of endometrial tissue outside the uterine cavity, which can cause pelvic pain, dysmenorrhea, and infertility [2].
An important and severe form of endometriosis is endometrioma, occurring in approximately 17 to 44 % of women affected by the disease. Endometrioma presents as a cyst of non-neoplastic cells on the surface of the ovary [3]. The impact of endometriomas on fertility in women with endometriosis is unclear. The gold standard for endometrioma excision and treatment is through videolaparoscopy; however, this treatment has been shown to cause follicle loss and a consequent reduction of the ovarian reserve [4, 5].
In addition, endometriosis may have an impact on folliculogenesis, resulting in poor oocyte quality, low fertilization rates, and poor embryo quality [6]. In order to evaluate this impact, follicular fluid (FF) analyses may give valuable insight regarding potential biomarkers of endometriosis and of oocyte competence, given that FF provides the microenvironment for the oocyte development [7].
Although extensive research has been performed to identify potential biomarkers to diagnose endometriosis, the diagnostic importance of the majority of the biomarkers identified by several “omics” approaches, such as genomics, transcriptomics, and proteomics, remains unclear. Moreover, studies based on biomarker discovery have not been consistent in proposing a single and/or panel of biomarkers with clinical application [8].
Current studies have focused on metabolomic approaches to find biomarkers for endometriosis, using electrospray tandem mass spectrometry (ESI-MS/MS) and nuclear magnetic resonance (NMR) in blood plasma. The results from both techniques indicate that metabolomics is a powerful diagnostic tool for the identification of molecules related to the disease [9, 10].
For this purpose, metabolomic profiling is a powerful and reliable tool for total metabolite identification in a biological system under a particular physiological condition [11]. In the present study, the metabolomic profile of follicular fluid and endometrioma from women with endometriosis was analyzed, with an aim of identifying a panel of potential biomarkers related to ovarian endometriosis evolution. The ESI-MS/MS experiment allowed us to elucidate lipid subclasses of potential biomarkers through an experiment of molecule fragmentation that determined the polar portion of the lipid structures identified in the MS/MS spectra [12]. By furthering our understanding of this disease through metabolomic methods, the development of better treatment plans and pregnancy outcomes for these patients may be achieved.
Materials and methods
The study was approved by the Institutional Ethics Review Board from the Sao Paulo Federal University. Written informed consent was provided by each patient prior to the study beginning.
Study design and sample processing
This study included follicular fluid samples collected from patients who underwent in vitro fertilization treatment (IVF) at the Human Reproduction Department at Sao Paulo Hospital. All patients received a similar hormonal ovarian stimulation protocol for IVF, which was performed as follows.
Initially, exogenous gonadotrophins (225 IU FSH/day) were administered starting on cycle day 2. When the leading follicle reached 13 mm in diameter, a GnRH antagonist analog (0.25 mg/day; cetrorelix, Cetrotide, Merck Serono) was used to suppress endogenous luteinizing hormone (LH) release until the day of human chorionic gonadotropin (hCG) administration. When the leading follicle reached 17 mm in diameter, a total dose of 250 mg of hCG was administered. Ultrasound-guided transvaginal oocyte retrieval was performed 35 h after hCG administration. Finally, FF was obtained from the group of follicles present in each ovary for all patients.
The inclusion criterion for all groups was a maximum age of 35 years, and the exclusion criteria were as follows: patients with a history of polycystic ovary syndrome, cancer, premature ovarian failure, or other gynecologic factors leading to infertility.
The tubal patients group (n = 10) was composed of patients who had tubal factor as an infertility factor or no evidence of female infertility and were treated mainly due to a male infertility factor (at least 3 million sperms per milliliter and over 5 % strict morphology) [13]. The endometriosis patients group was composed of women with endometriosis grade III or IV with the presence of endometriomas (n = 10). From this study group, FF and endometriomas were obtained from both ovaries of the same patient and divided into two groups, considering endometrioma as a separated sample (endometriosis, n = 10 FF; and endometrioma, n = 10 endometriomas).
The FF aspirated was centrifuged at 800 ×g for 10 min to remove erythrocytes and leukocytes. The supernatant was collected and maintained frozen at −20 °C until processing.
Lipids analysis—ESI-MS
Lipids were extracted from the FF according to the Bligh and Dyer method, with modifications [14]. First, volumes of 50 μL of FF, 50 μL of water, 125 μL of chloroform (Synth, Diadema, SP, Brazil), and 250 μL of methanol (Synth, Diadema, SP, Brasil) were placed in a 2-mL microtube. This mixture was vortexed for 30 s. Next, the polar and apolar phases were separated by the addition of 100 μL of water and 125 μL of chloroform. The lower phase containing the lipids were recovered by pipetting and transferred to a 0.5-mL microtube that was stored at −20 °C until analysis. For the ESI-MS experiment, samples were dissolved in 300 μL of methanol with 0.1 % formic acid.
ESI(+)-MS/MS
Each sample was analyzed in triplicate, and this assay was performed in the positive mode using a hybrid mass spectrometer quadrupole time-of-flight (Q-TOF) from Micromass (Manchester, UK). The conditions of the experiment were as follows: source temperature of 100 °C, desolvation temperature of 100 °C, capillary voltage of 3 kV, and cone voltage of approximately 35 V. The samples were injected by an infusion pump (Harvard Apparatus) at a flow rate of 10 μL min−1. Data was acquired in the m/z 700–1200 range. MS/MS was performed by the collision induced dissociation (CID) for lipid ions with a significant difference among the groups, using argon as the collision gas. The collision energy ranged from 4 to 50 V.
Statistical analysis
The MassLynx 4.1 software (Waters, Manchester, UK) was used for pre-processing the mass spectra obtained by ESI-MS. Each spectrum was processed to remove the background, for smoothing and for centralization of ions. The spectra were processed to remove isotopes considering a maximum load of +1. The list of ions was transferred to an Excel spreadsheet, and relative abundances from 0.0 to over 50 % of the samples from each group were removed.
Pareto scaling normalized the relative abundances. Statistical processing was performed using MetaboAnalyst 2.0 (http://www.metaboanalyst.ca) software. The principal component analysis (PCA), which detects intrinsic clusters based on the lipid profile, and partial least squares discriminant analysis (PLS-DA), which maximizes the group discrimination, were applied to the data set, and an alpha of 5 % was adopted. The R2 values (representing the variation within the data set explained by the model components) and Q2 (power projection model) found by cross-validation were used to determine the quality of the models built by PLS-DA.
In order to validate the robustness of the model, a receiver operating characteristic (ROC) curve analysis was performed considering the group comparison two by two.
After the construction of the PLS-DA models, the variable importance in the projection (VIP) was used to identify 15 ions that had greater discrimination between the groups in the component with the highest power projection. Of the 15 ions differentially expressed in each group, fragmentation analyses were performed by MS/MS individually, aiming to identify lipid subclasses. For ions that had intensities that were too low for detection, it was not possible to identify the lipid by MS/MS, but these lipids were identified via the human metabolome database (HMDB) databank search (http://www.hmdb.ca/spectra/ms/search), assuming a maximum error of 50 ppm.
Results
Clinical data
Clinical data analysis demonstrated that no differences were observed between the groups, considering the IVF treatment (Table 1).
Table 1.
Clinical characteristics from patients included in the study
| Tubal patients Mean; SD |
Endometriosis Mean; SD |
p < 0.05 (95 % CI) | |
|---|---|---|---|
| Age | 34.30; 3.33 | 32.30; 2.67 | 0.156 |
| BMI | 24.06; 2.03 | 23.30; 2.57 | 0.521 |
| LH (mIU/mL) | 3.27; 1.65 | 6.23; 4.35 | 0.600 |
| FSH (mIU/mL) | 5.76; 2.28 | 6.83; 3.03 | 0.384 |
| Endometrial thickness (mm) | 10.08; 2.05 | 11.54; 1,42 | 0.800 |
| Follicles (n) | 15; 8.26 | 17.30; 10.41 | 0.591 |
| Oocyte retrieval rate | 0.59; 0.19 | 0.57; 0.29 | 0.906 |
| MII (n) | 6; 4.08 | 8; 6.32 | 0.412 |
| Degenerated oocytes (n) | 2.33; 1.53 | 2; 1.41 | 0.572 |
| Intracytoplasmic issues | 0.32; 0.29 | 0.31; 0.24 | 0.954 |
| Extracytoplasmic issues | 0.50; 0.27 | 0.52; 0.20 | 0.837 |
| Inseminated oocytes (n) | 5.80; 4.18 | 7.40; 5.97 | 0.496 |
| Fertilization rate | 4.70; 2.83 | 5.80; 5.14 | 0.162 |
| Embryo day 3 (n) | 4.50; 2.84 | 5.70; 4.90 | 0.511 |
| Transferred embryos (n) | 2.30; 0.48 | 1.78; 0.83 | 0.109 |
| Good embryos rate | 0.58; 0.12 | 0.50; 0.71 | 0.614 |
| Good embryos transferred (n) | 1.00; 1.04 | 1.00; 0.00 | 0.769 |
The variables were normalized by Z score for the Student’s t test comparison between the control and the study group, considering p < 0.05
CI confidence interval, BMI body mass index, LH luteinizing hormone, FSH follicle-stimulating hormone, n number, MII metaphase II
Lipids analysis by ESI-MS/MS
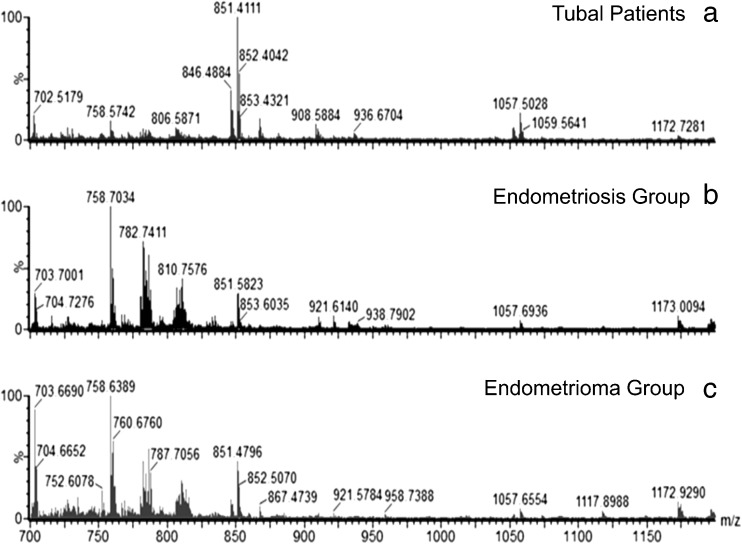
Characteristic spectra of each group were generated from the mass spectrometry analysis, as shown in Fig. 1. It is possible to visually observe differences in the relative intensities of the ions in the lipid profile between groups. The raw data was processed, and an average of 1244 ions were used for statistical analysis.
Fig. 1.
Typical ESI-MS data. a Tubal patients group. b Endometriosis. c Endometrioma. The y-axis shows relative abundances whereas the x-axis shows m/z values
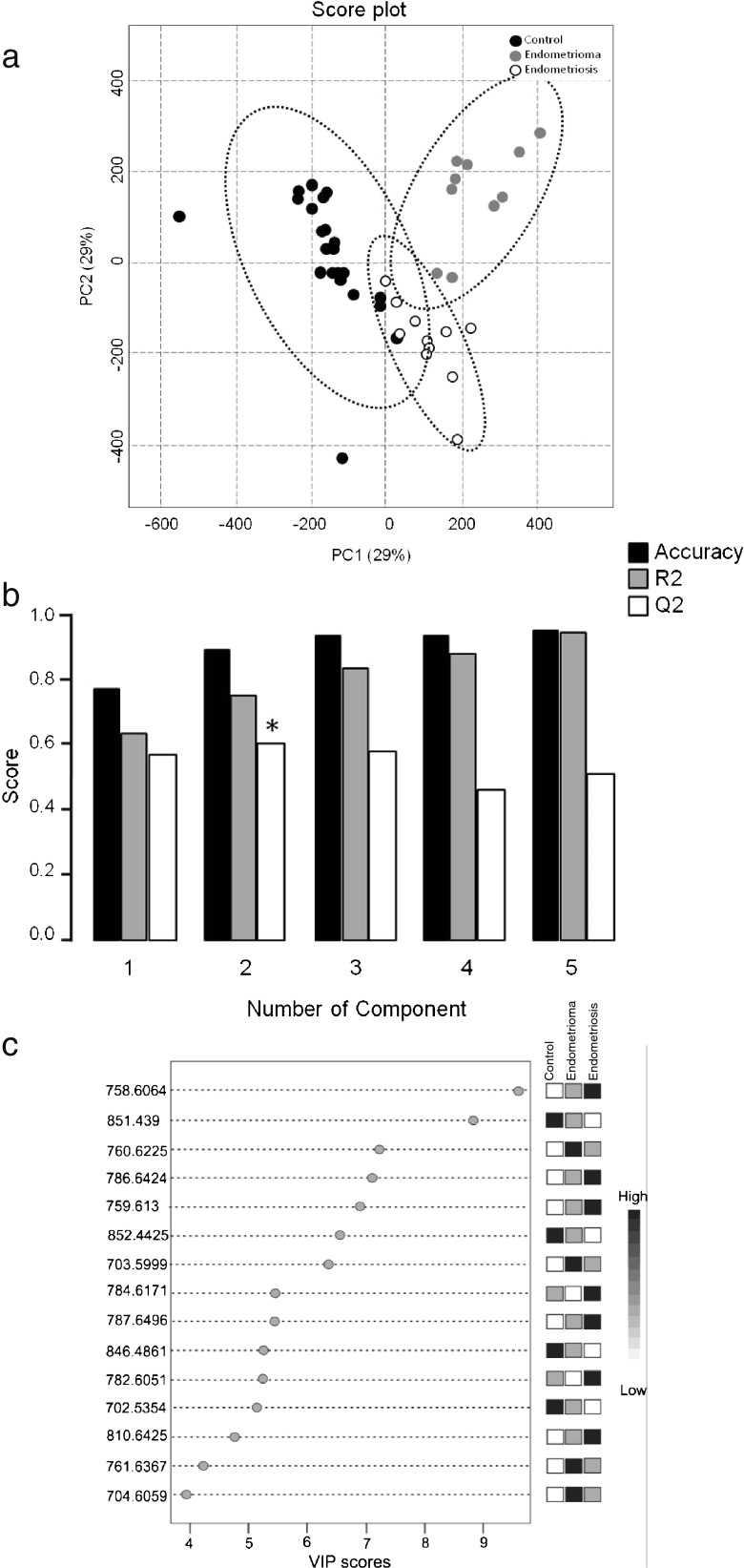
Principal component analysis is a non-supervised analysis that is used to combine several variables, both explanatory and unexplanatory, into a few principal components that help to explain the variability within the experiment and to demonstrate the tendency of group separation (Fig. 2a).
Fig. 2.
a PCA 2D score plot: the variance among the groups according to the principal components and without considering the group division. b Cross-validation chart. Black bars: accuracy of the model; grey bars: R 2 (variations); white bars: Q 2 (prediction of the model). Asterisk indicates the component with higher power of projection for the differentiation of the groups. c Ions with higher contribution for difference among the groups in component 2
These results are reinforced by the cross-validation performed by PLS-DA constructed from the PCA model, showing that the first five principal components contributed positively to the model (Q2 with positive values), indicating that the first two components were ideal for the model prediction (Q2 = 60 %). Furthermore, the ions explained 75.7 % of the variance (R2), and the model accuracy corresponds to 89.6 % (Fig. 2b).
For the lipid identification, PLS-DA generated a list of 15 ions corresponding to VIPs, which represent potential lipids involved in endometriosis (Fig. 2c). The MS/MS spectra show the ion structures in relation to the precursor ions and their possible fragments, in which the polar group of the molecule is observed (see supplementary figures).
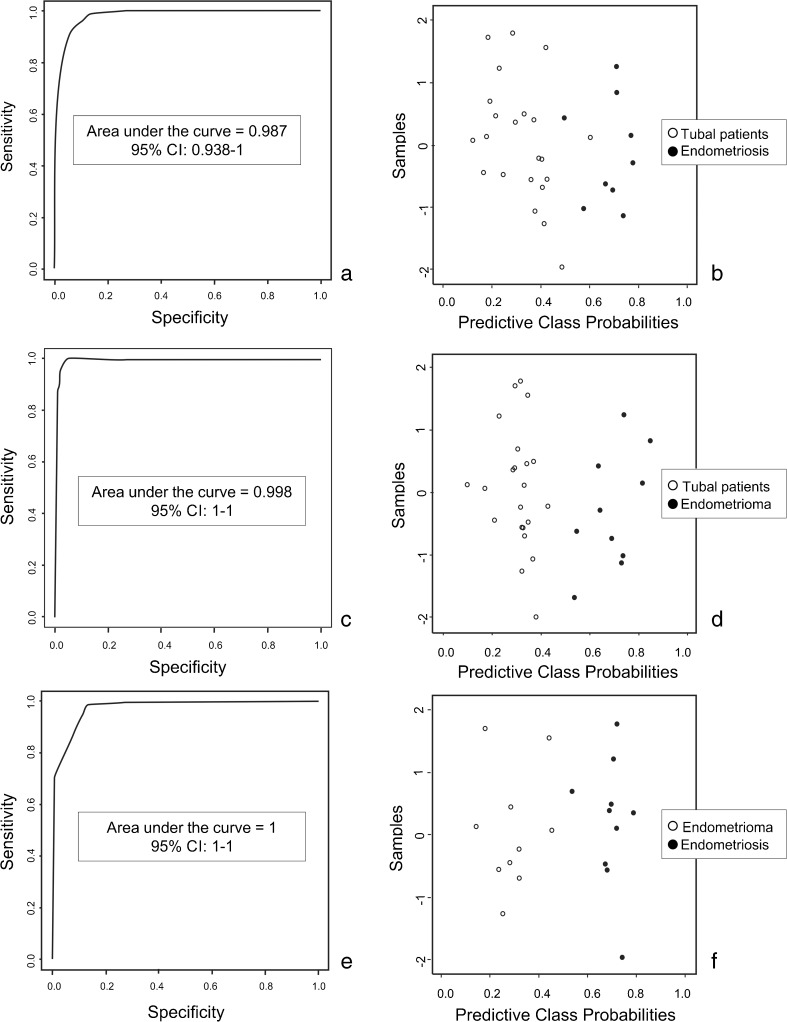
In addition to the cross-validation, the ROC curve based on the VIP scores was performed two by two. The comparison between controls and endometriosis demonstrated that the area under the curve was 0.98 (Fig. 3a) and the classification rate was 97 % (Fig. 3b). When control was compared to endometrioma group, the ROC curve validation showed an area under the curve of 0.99 (Fig. 3c) and a classification rate of 100 % (Fig. 3d). For the comparison between endometriosis and endometrioma group, the area under the curve was 1 (Fig. 3e) and the classification rate was 100 % (Fig. 3f).
Fig. 3.
ROC curve based on the VIP scores. a, b Comparison between control and endometriosis. c, d Comparison between control and endometrioma. e, f Comparison between endometriosis and endometrioma
In the tubal patients group, there were four lipids of high abundance: phosphatidylglycerol phosphate (PtdGro), phosphatidylcholine (ChoGpl), phosphatidylserine (PtdSer), and phosphatidylnositol bisphosphate (PtdIns-4,5-P2). In the endometriosis group, sphingolipids and ChoGpls were observed, while in the endometrioma group, sphingolipids and ChoGpls with different m/z from the endometriosis group were observed (Table 2).
Table 2.
Ions identified in each group
| Group | m/z | Subclass | Adduct | Formula |
|---|---|---|---|---|
| Control | 851,4390 | PtdGro | [M + H]+ | C42H76O13P2 |
| 852,4425 | – | – | – | |
| 702,5354 | ChoGpl | [M + H]+ | C38H72NO8P | |
| 846,4861 | PtdSer | [M + K]+ | C42H81NO11S | |
| 1057,5482 | PtdIns-4,5-P2 | [M + CH3OH + H]+ | C45H87O19P3 | |
| Endometriosis | 759,6130 | Sphingolipid | [M + H]+ | C43H87N2O6P |
| 787,6496 | Sphingolipid | [M + CH3OH]+ | C45H91N2O6P | |
| 784,6171 | ChoGpl | [M + H]+ | C44H82NO8P | |
| 782,6051 | ChoGpl | [M + H]+ | C44H80NO8P | |
| 810,6425 | ChoGpl | [M + H]+ | C46H84NO8P | |
| 786,6424 | Sphingolipid | [M + H]+ | C45H90N2O6P | |
| 758,6064 | ChoGpl | [M + H]+ | C42H80NO8P | |
| Endometrioma | 760,6225 | ChoGpl | [M + H]+ | C42H82NO8P |
| 761,6367 | Sphingolipid | [M + H]+ | C43H89N2O6P | |
| 703,5999 | Sphingolipid | [M + H]+ | C39H79N2O6P |
The ions were classified according to their lipid subclass, adduct, and molecular formula
PtdGro phosphatidylglycerol phosphate, ChoGpl phosphatidylcholine, PtdSer phosphatidylserine, PtdIns-4,5-P2 phosphatidylnositol bisphosphate
Discussion
Alterations in lipid patterns are common in several conditions and are the result of a response to disease, toxin exposure, and environmental or genetic modifications [15]. Furthermore, a comparison of the lipid profile from opposite healthy conditions can be used as a diagnostic tool and may support the development of new treatment strategies and disease monitoring.
In this study, the lipid fingerprinting analysis of follicular fluid of women with endometriosis and their endometrioma compared to their healthy counterpart with pregnancy outcomes was performed using ESI(+)-MS/MS, a robust and efficient analysis, with minimal sample preparation that allowed elucidating lipid subclasses by determining the polar portion of the lipid structures identified in the MS/MS spectra [12].
Several studies have identified biomarkers of endometriosis, and the biochemical function of these molecules has been related to oxidative stress and malignancy characteristics; however, these studies do acknowledge the need for further research [10, 11].
Two important classes of lipids, the phosphatidylcholines and sphingomyelines, have been suggested as possible biomarkers for endometriosis as these lipids are strongly related to apoptosis suppression [9]. However, the authors highlighted a need for biomarker validation, in order to determine if lipids can be used to discriminate between patients with endometriosis and their healthy counterparts with any sort of accuracy [9]. Similarly, in the present study, it was possible to observe a high representation of ChoGpl and sphingolipids, which might be related to the same processes, although an apoptosis analysis was not investigated.
In this study, we observed different phospholipids present in the tubal patients group that serve in a variety of cellular functions such as enzymatic regulation, transcription, signal transduction, secondary messengers, and transport [16]. Among the subclasses identified, these lipids are PtdGro, PtdSer, PtdIns-4,5-P2, and PtdCho (Table 2).
PtdGro phosphate is a precursor for phosphatidylglycerol synthesis, through a dephosphorylation reaction by PtdGro phosphatase enzyme (PGPP). The reduction of this lipid was related to an increase in oxidative stress in animal tissues [17]. Likewise, the presence of this lipid in the tubal patients group and its decrease in endometriosis and endometrioma groups may be associated with oxidative stress in follicular fluid of women with endometriosis, a process that has already been described in the blood plasma [11].
We observed a higher level of PtdSer in the tubal patients group. These PtdSer are abundant in the inner portion of the plasma membrane and assist with membrane fusion [18]. Cells undergoing apoptosis display PtdSer on their surface as a signal for phagocytosis. In contrast, viable cells maintain this lipid on the inner portion [19]; thus, this mechanism minimizes any inflammatory reaction, avoiding compromising healthy tissue structures [20].
The presence of this phospholipid confirms that apoptosis is a highly regulated physiological process of cell death, playing an important role in the homeostasis of different tissues in response to numerous stimuli. Moreover, this process is crucial for embryonic development, maturation of the immune system against viral infections, and response to cancer [21]. The presence of PtdSer in the tubal patients group may be associated with oocyte homeostasis, indicating that an adequate response to the induction of final follicular maturation consequently can be related to embryo quality and a proper fetal development [22].
PtdIns-4,5-P2 was described as a secondary messenger in the regulation of the corpus luteum. PtdIns-4,5-P2 signaling pathway activation begins with the hydrolysis of PtdIns-4,5-P2, generating inositol triphosphate (IP3) and diacylglycerol (DAG). DAG activates protein kinase C, and IP3 stimulates calcium release from intracellular stores [23]. These events are important in the beginning of a number of physiological responses, suggesting that PtdIns-4,5-P2 pathway may be involved in the luteal regression of various species, including primates [24]. The presence of PtdIns-4,5-P2 in the follicular fluid of the tubal patients group indicates that there may be better control of the luteinization process induced by administration of hCG stimulation protocols in controlled ovarian.
PtdIns-4,5-P2 can also play a crucial role in cell survival, since it mediates the trophic ligand actions in pre-implanted embryo of mammals [25]. Therefore, we hypothesize that the presence of PtdIns-4,5-P2 in the follicular microenvironment has an important role in oocyte quality and support of the first embryonic cleavages.
Another subclass described in the tubal patients group was ChoGpl, the most important subclass of the phospholipids category, which plays a role in the membrane structure and cell signaling [16]. One of the most important processes related to ChoGpl is cell proliferation. For example, increased ChoGpls were associated with cumulus cell expansion after IVF treatment in patients positive for pregnancy outcomes [26, 27]. Furthermore, it was suggested that the ovarian stimulation produced by inducing LH may lead to a higher production of ChoGpls in patients with positive pregnancy outcomes, indicating that this effect probably occurs in the follicular fluid of women included in this study.
For the endometriosis and endometrioma group, the main lipid subclasses were ChoGpl and sphingolipids. As in the tubal patients group, ChoGpl was higher in the endometriosis and endometrioma groups, and this lipid is involved in cell proliferation and the occurrence of various malignant tumors, including ovarian cancer [28, 29]. The increase in ChoGpl production serves as a substrate for phospholipase A2 (PA2) enzyme, which is often overexpressed in endometriotic lesions. Additionally, PA2 is responsible for the production of lysophosphatidic acid, a lipid involved in cell proliferation, cancer, and endometriosis [30]. As the ChoGpl alone is already considered a marker of high proliferation in malignant tissues, it can be postulated that this subclass may correlate as a potential biomarker for endometriosis, considering its specific m/z.
Sphingolipids are important both in signal transduction and in cell recognition [15]. Changes in the metabolism of these lipids have been described in various biological conditions, such as in cancer [31]. Some metabolites in the sphingolipid biochemical pathway, such as sphingosine and ceramide, induce apoptosis whereas the sphingosine-1-phosphate promotes cell survival in response to apoptotic stimuli [32].
Sphingomyelin, one of the most important lipids in this subclass, is related to the ongoing denervation and re-innervation process that occurs in endometriosis, suggesting that this process is also associated with suppression of apoptosis in patients with the disease [33]. Thus, the presence of sphingolipids in these groups may be related to an accumulation of sphingomyelin and a consequent decrease of ceramide, favoring cell proliferation [34].
In conclusion, multivariate analysis of the ESI-MS results provides an effective approach for the assessment of follicular fluid of women with endometriosis and ovarian endometrioma. Several of the lipids identified demonstrated differential expression among the groups and are involved in follicle development during IVF and embryo cleavage, as well as in apoptosis and cell proliferation. Furthermore, the results of this study have shown the effect of endometriosis in follicular fluid, suggesting substantial alterations in the ovary of women with the disease, although the study presents a small number of samples, indicating that the differences found represent an overview of possible alterations that must be confirmed in future studies. Further research of different types of samples, such as blood plasma, is required to confirm differences observed in the current study. Successful identification of biomarkers associated with endometriosis and endometrioma will have a significant impact on future diagnostics and therapy improvement.
Electronic supplementary material
(DOC 387 kb)
Acknowledgments
This work was supported by CNPq (National Council for Scientific and Technological Development), Brazil.
Conflict of interest
All authors declare that there are no conflicts of interest.
Footnotes
Capsule Lipid profiling of follicular fluid identifies catgories that may be involved in cell signaling during the progression of ovarian endometriosis.
Work conducted: Sao Paulo Federal University – Sao Paulo Hospital, Sao Paulo, Brazil
References
- 1.Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 3.Bektaş H, Bilsel Y, Sari YS, Ersöz F, Koç O, Deniz M, et al. Abdominal wall endometrioma: a 10-year experience and brief review of the literature. J Surg Res. 2010;164:77–81. doi: 10.1016/j.jss.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 4.Loo TC, Lin MY, Chen SH, Chung MT, Tang HH, Lin LY, et al. Endometrioma undergoing laparoscopic ovarian cystectomy: its influence on the outcome of in vitro fertilization and embryo transfer (IVF-ET) J Assist Reprod Genet. 2005;22:329–33. doi: 10.1007/s10815-005-5914-2. [DOI] [PubMed] [Google Scholar]
- 5.Hachisuga T, Kawarabayashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002;17:432–5. doi: 10.1093/humrep/17.2.432. [DOI] [PubMed] [Google Scholar]
- 6.Pellicer A, Albert C, Garrido N, Navarro J, Remohi J, Simon C. The pathophysiology of endometriosis-associated infertility: follicular environment and embryo quality. J Reprod Fertil Suppl. 2000;55:109–19. [PubMed] [Google Scholar]
- 7.Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50:225–32. doi: 10.1095/biolreprod50.2.225. [DOI] [PubMed] [Google Scholar]
- 8.May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16:651–74. doi: 10.1093/humupd/dmq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vouk K, Hevir N, Ribic-Pucelj M, Haarpaintner G, Scherb H, Osredkar J, et al. Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Human Reprod. 2012;27:2955–65. doi: 10.1093/humrep/des152. [DOI] [PubMed] [Google Scholar]
- 10.Dutta M, Joshi M, Srivastava S, Lodh I, Chakravartyc B, Chaudhury K. A metabonomics approach as a means for identification of potential biomarkers for early diagnosis of endometriosis. Mol Biosyst. 2012;30:3281–7. doi: 10.1039/c2mb25353d. [DOI] [PubMed] [Google Scholar]
- 11.Jana SK, Dutta M, Joshi M, Srivastava S, Chakravarty B, Chaudhury K. 1H NMR based targeted metabolite profiling for understanding the complex relationship connecting oxidative stress with endometriosis. Biomed Res Int. 2013;2013:329058. doi: 10.1155/2013/329058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Z, Thomas MJ. Phospholipid profiling by tandem mass spectrometry. J Chromatog B. 2009;877:2709–15. doi: 10.1016/j.jchromb.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23. [DOI] [PubMed]
- 14.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Khalil MB, Hou W, Zhou H, Elisma F, Swayne LA, Blanchard AP, et al. Lipidomics era: accomplishments and challenges. Mass Spectrom Rev. 2010;29:877–929. doi: 10.1002/mas.20294. [DOI] [PubMed] [Google Scholar]
- 16.Lykidis A. Comparative genomics and evolution of eukaryotic phospholipid biosynthesis. Prog Lipid Res. 2007;46:171–99. doi: 10.1016/j.plipres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki K, Kuge O, Chang SC, Heacock PN, Rho M, Suzuki K, et al. Isolation of a chinese hamster ovary (CHO) cDNA encoding phosphatidylglycerophosphate (PGP) synthase, expression of which corrects the mitochondrial abnormalities of a PGP synthase-defective mutant of CHOK1 cells. J Biol Chem. 1999;274:1828–34. doi: 10.1074/jbc.274.3.1828. [DOI] [PubMed] [Google Scholar]
- 18.Orsi NM, Gopichandran N, Leese HJ, Picton HM, Harris SE. Fluctuations in bovine ovarian follicular fluid composition throughout the oestrous cycle. Reproduction. 2005;129:219–28. doi: 10.1530/rep.1.00460. [DOI] [PubMed] [Google Scholar]
- 19.Fadok VA, Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–7. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 20.Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria. Trends Cell Biol. 1998;8:267–71. doi: 10.1016/S0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- 21.Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry(Mosc) 2005;70:231–9. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 2007;282:18357–64. doi: 10.1074/jbc.M700202200. [DOI] [PubMed] [Google Scholar]
- 23.Houmard BS, Guan Z, Stokes BT, Ottobre JS. The effects of gonadotropin on the phosphatidylinositol pathway in the primate corpus luteum. Mol Cel Endocrinol. 1994;104:113–20. doi: 10.1016/0303-7207(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 24.Rojas FJ, Moretti-Rojas I, Balmaceda JP, Asch RH. Regulation of gonadotropin-stimulable adenylyl cyclase of the primate corpus luteum. J Steroid Biochem. 1989;32:175–82. doi: 10.1016/0022-4731(89)90161-1. [DOI] [PubMed] [Google Scholar]
- 25.O’Neill C. Phosphatidylinositol 3-kinase signaling in mammalian preimplantation embryo development. Reproduction. 2008;136:147–56. doi: 10.1530/REP-08-0105. [DOI] [PubMed] [Google Scholar]
- 26.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597–601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montani DA, Cordeiro FB, Regiani T, Victorino AB, Pilau EJ, Gozzo FC, et al. The follicular microenviroment as a predictor of pregnancy: MALDI-TOF MS lipid profile in cumulus cells. J Assist Reprod Genet. 2012;29:1289–97. doi: 10.1007/s10815-012-9859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iorio E, Mezzanzanica D, Alberti P, Spadaro F, Ramoni C, D’Ascenzo S, et al. Alterations of choline phospholipid metabolism in ovarian tumor progression. Cancer Res. 2005;15:9369–76. doi: 10.1158/0008-5472.CAN-05-1146. [DOI] [PubMed] [Google Scholar]
- 29.Iorio E, Ricci A, Bagnoli M, Pisanu ME, Castellano G, Di Vito M, et al. Activation of phosphatidylcholine cycle enzymes in human epithelial ovarian cancer cells. Cancer Res. 2010;70:2126–35. doi: 10.1158/0008-5472.CAN-09-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy AA, Santanam N, Morales AJ, Parthasarathy S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T-lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab. 1998;83:2110–3. doi: 10.1210/jcem.83.6.4823. [DOI] [PubMed] [Google Scholar]
- 31.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 32.Cuvillier O. Sphingosine in apoptosis signaling. Biochim Biophys Acta. 2002;30:153–62. doi: 10.1016/S1388-1981(02)00336-0. [DOI] [PubMed] [Google Scholar]
- 33.Vance DE, Vance JE. Physiological consequences of disruption of mammalian phospholipid biosynthetic genes. J Lipid Res. 2009;50:132–7. doi: 10.1194/jlr.R800048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Separovic D, Hanada K, Maitah MY, Nagy B, Hang I, Tainsky MA, et al. Sphingomyelin synthase 1 suppresses ceramide production and apoptosis post-photodamage. Biochem Biophys Res Commun. 2007;358:196–202. doi: 10.1016/j.bbrc.2007.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 387 kb)