Abstract
For several decades, p53 has been detected in cancer biopsies by virtue of its high protein expression level which is considered indicative of mutation. Surprisingly, however, mouse genetic studies revealed that mutant p53 is inherently labile, similar to its wild type (wt) counterpart. Consistently, in response to stress conditions, both wt and mutant p53 accumulate in cells. While wt p53 returns to basal level following recovery from stress, mutant p53 remains stable. In part, this can be explained in mutant p53-expressing cells by the lack of an auto-regulatory loop with Mdm2 and other negative regulators, which are pivotal for wt p53 regulation. Further, additional protective mechanisms are acquired by mutant p53, largely mediated by the co-chaperones and their paralogs, the stress-induced heat shock proteins. Consequently, mutant p53 is accumulated in cancer cells in response to chronic stress and this accumulation is critical for its oncogenic gain of functions (GOF). Building on the extensive knowledge regarding wt p53, the regulation of mutant p53 is unraveling. In this review, we describe the current understanding on the major levels at which mutant p53 is regulated. These include the regulation of p53 protein levels by microRNA and by enzymes controlling p53 proteasomal degradation.
Keywords: mutant p53, Mdm2, miRNA, proteasomal degradation, cancer
Introduction
Wild type (wt) p53 is a tumor suppressor, which plays a key role in the cellular stress response. Abrogating p53 function is a key event in human cancer, leading to deregulated cell cycle, genomic instability, resistance to stress signals, and ultimately leading to cancer development (1, 2). Dysfunction of p53 occurs in half the cases of cancers by direct mutations in the gene, whereas in the remainder, p53 becomes dysfunctional through a variety of regulatory breakdowns. Mutant p53 fails to emulate the transcriptional program executed by wt p53 to provide a robust response to stress.
Most p53 mutations are missense (hotspot mutations – R175, G245, R248, R249, R273, R282) and occur at its DNA-binding domain, which accounts for the improper DNA engagement and disruption of transcriptional activity. P53 mutants also gain new oncogenic functions including resistance to chemotherapies, enhanced cell growth, metabolism, and invasion [reviewed in Ref. (3, 4)].
Surprisingly, mutant p53, like its wt counterpart, is inherently labile (5–7). Sustained degradation of wt p53 in healthy cells protects them against potent cell growth inhibition, while stress provokes p53 accumulation and activation (8). Similarly, mutant p53 accumulates in response to stress (7). Thus, both wt and mutant p53 need to accumulate in order to execute their respective functions: wt p53 suppresses cancer while mutant p53 promotes cancer through its GOFs. It is therefore of great clinical importance to understand how wt and mutant p53 are regulated if we are to tailor treatments according to p53 status. That is, either reactivating wt p53 expression and function or counteracting mutant p53. In this review, we will outline the major levels at which mutant p53 is regulated and discuss the major players. As most of the regulation appears to occur post-transcriptionally [reviewed in Ref. (9)], this will form the major focus of this review.
Regulation of Mutant p53 by microRNA
MicroRNAs (miRNA) are the best-characterized members of the non-coding RNAs (ncRNAs) family. Typically these are 18–24 nucleotide (nt) RNA molecules that are not translated into proteins, and target messenger RNA (mRNA) species, through engagement of as few as six complementary nucleotides (10). Canonically, mature miRNAs bind mRNA 3′-untranslated-regions (3′-UTRs) and promote either target degradation or translational inhibition. Through engagement of coding regions (11), 5′-UTR (12), and open reading frames (ORF) (13), miRNAs can also regulate translation. The targeting flexibility of miRNAs allows them to affect multiple targets that are pertinent to both tumor suppression and oncogenesis: gene expression, protein regulation, homeostasis, and diseases.
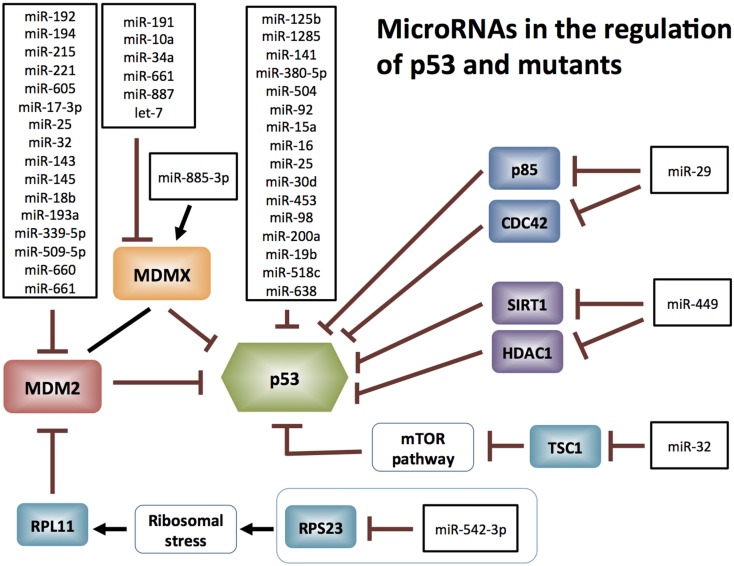
The expression of both wt and mutant p53 are subject to miRNA regulation directly. MiRNAs targeting p53 mRNA are incapable of discriminating between its wt and mutant mRNAs, unless an miRNA directly targets a mutated site. MiR-125b was the first miRNA demonstrated to bind p53 3′-UTR mRNA causing down-regulation of p53 protein and a consequent reduction in its activity, in human neuroblastoma cells and primary human lung fibroblasts (14). Additional p53-directed miRNAs have been identified both experimentally and from in silico analyses (Figure 1).
Figure 1.
MicroRNAs targeting p53: miR-125b, miR-504, miR-1285, miR-92, miR-141, miR-380-5p, miR-15a, miR-16, miR-25, miR-30d, miR-200a [reviewed in Ref. (88)], miR-453 (89), miR-98 (89), miR-19b (90), miR-518c (91), and miR-638 (91). MicroRNAs targeting MDM2: miR-192, miR-194, miR-215, miR-221, miR-605, miR-17-3p, miR-193a, miR-25, miR-32, miR-143, miR-145, miR-18b, miR-661 [reviewed in Ref. (15)], miR-339-5p (92, 93), miR-660 (94), and miR-509-5p (95). MicroRNAs targeting MDM4: miR-191, miR-10a, miR-885-3p, miR-34a, miR-661 [reviewed in Ref. (15)], miR-887 (96), and let-7 (11).
MicroRNAs also regulate p53 protein stability indirectly, by targeting its key regulators. For instance, the E3 ubiquitin ligase Mdm2, which is the major negative regulator of p53 [reviewed in Ref. (8, 15)] is extensively targeted by miRNAs for degradation. As a consequence of Mdm2 targeting, p53 (wt or mutant) is released from Mdm2-mediated ubiquitination and subsequent proteasomal degradation (Figure 1). Mdm4 (also known as MdmX), which is an Mdm2-related protein, is another key inhibitor of p53 transcriptional activity (16, 17) and is also targeted by miRNAs (Figure 1). Although miRNAs predominantly degrade mRNAs, an exceptional instance is miR-885-3p, which engages the 5′-UTR of Mdm4 mRNA and elevates Mdm4 protein levels (12).
Apart from Mdm2 and Mdm4, miRNAs also stabilize p53 through other regulatory pathways (Figure 1). MiR-29 activates p53 by targeting p85-alpha and CDC42 (18), miR-449 targets SIRT1 and HDAC1 (19), and miR-32 targets TSC1 and activates mTOR in human glioblastoma multiforme (20), all of which lead to the stabilization of p53. Recent study by Wang et al. demonstrated that miR 542-3p directly targets RPS23, resulting in subsequent RPL11 up-regulation, inhibiting Mdm2 and ultimately reducing proteasomal degradation of p53 (21).
Significantly for cancer, interaction of miRNAs with wt and mutant p53 is not unidirectional, and expression levels and biogenesis of miRNAs are affected by p53. Suzuki et al. demonstrated that wt p53 enhances post-transcriptional maturation of miR-16-1, miR-143, and miR-145 in response to DNA damage, while mutant p53 attenuates miRNA processing (22). Muller et al. further demonstrated that mutant p53 modulates miRNA processing, through direct inhibition of TAp63-mediated transcriptional activation of Dicer, and also through a TAp63-independent manner (23). Apart from global modulation of miRNA biogenesis, mutant p53 also affects expression of miRNAs, principally by downregulating tumor-suppressive miRNAs – miR-130b in endometrial cancer (24), miR-27a in breast cancer cells (MDA-MB-468) (25), miR-223 in breast and colon cells (26), let-7i in breast cancer and DLD1 cells (colorectal cancer) (27), and miR-205 (28), and elevating oncogenic miRNAs: miR-128-2 (29) and miR-155 in breast cancer cells (30) to mediate its oncogenic functions.
These studies collectively suggest that intertwined regulation of miRNAs, wt, and mutant p53 is vital to cancer. Given the importance of ncRNA in the regulation of wt and mutant p53, ncRNA represents feasible therapeutic targets for the development of new approach targeting mutant p53 in cancer.
Regulation of Mutant p53 Protein Stability
Overall, wt p53 is predominantly regulated at the protein stability level, under normal and stress conditions. Extensive study has defined that p53 stability is dictated by a variety of stabilizing post-translational modifications (PTM), while its degradation is largely the consequence of ubiquitination, executed by several E3 ligases [reviewed in Ref. (31, 32)]. Despite a drastic difference between the stability of mutant versus wt p53 in cancer cells, the majority of the regulatory pathways of p53 are shared between wt and mutant p53. However, a number of key differences promote the chronic stabilization and activation of mutant p53, which drive its oncogenic GOF. Understanding the regulation of mutant p53 has direct clinical implications. In this section, we will cover the major levels of mutant p53 regulation, with a focus on the degradation of mutant p53.
Regulation of Mutant p53 Degradation
p53 stability is tightly controlled by ubiquitin E3 ligases, which together with the enzymatic activities of E1 and E2, and in certain cases also E4 ligases coordinate the efficient degradation of proteins through the 26S proteasome machinery (33). The temporal and spatial modulation of the degradation of p53 is achieved by PTMs described below. In this section, we will outline the major E3 ligases that have been shown to control mutant p53 stability. A paradigm shift in our understanding of mutant p53 stability was demonstrated in the studies of mutant p53 knock-in mice (5, 34). These papers showed for the first time that mutant p53 is inherently labile in vivo.
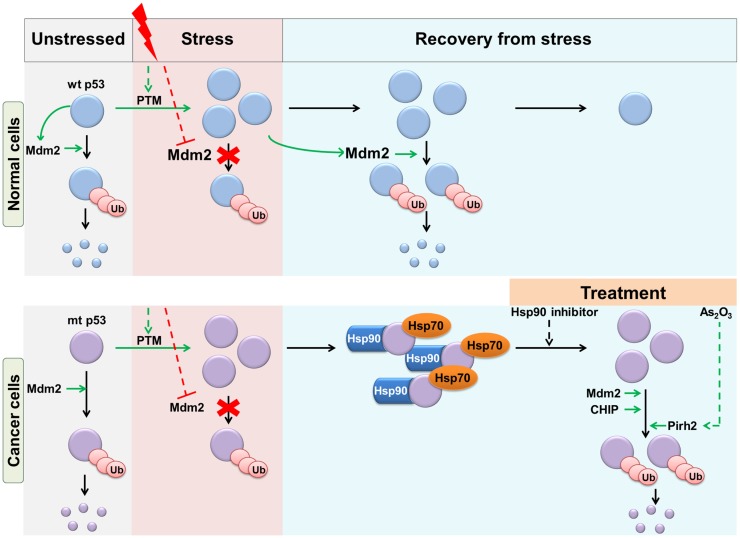
In the case of wt p53, the key physiological E3 ligase is Mdm2, which maintains p53 at low levels under basal conditions, and during recovery from stress (35, 36). Similarly, Mdm2 maintains the low basal levels of mutant p53 in vivo (7). In contrast to wt p53, mutant p53 does not form a feedback loop with Mdm2, as it is incapable of inducing Mdm2 transcription (37). Therefore, following stress-induced stabilization of wt and mutant p53, only wt p53 recovers to basal levels under the influence of Mdm2 (Figure 2). This can be corrected by enforced expression of Mdm2, which is able to efficiently degrade of mutant p53 (35). Mdm2 interacts with multiple domains of p53, which allows it to bind even to conformational p53 mutants, which are missense p53 mutations that either locally or globally disrupt the structure of p53, as distinct from “DNA contact mutants” (38, 39). The overall efficiency of mutant p53 ubiquitination, however, is reduced compared with that of wt p53 (40). The ubiquitination of mutant p53 is also enhanced by the activity of other E3 ligases: CHIP and Cop1 (40). Although ubiquitination of p53 by Mdm2 is enhanced by ubiquitin-interacting protein, hHR23a, the consequent ubiquitinated p53 accumulates but is not degraded (41). In a series of key in vivo studies, Terzian et al. (7) and Suh et al. (20) demonstrated that stress conditions (including oxidative stress, DNA damage) and oncogenic stress (such as the loss of p16) promote stabilization of mutant p53 and contribute to tumorigenesis. Subsequent studies have shown that multiple oncogenic effects can stabilize mutant p53 in vivo and drive its oncogenic functions. Interestingly, at least in the case of PML loss, the impact on mutant p53 GOF is gender-specific (42).
Figure 2.
Regulation of wt and mutant p53 stability in response to stress. Wt and mutant p53 are maintained at basal levels by Mdm2-mediated degradation. Upon stress stimulus, events such as post-translational modifications (PTM) result in stabilization and accumulation of p53. During recovery from stress, wt p53 returns to basal levels as a result of negative feedback through Mdm2. However, mutant p53 accumulates due to its failure to transactivate Mdm2. Mutant p53 is also protected from Mdm2 and CHIP mediated ubiquitination by chaperone proteins. This can be overcome by treatment with Hsp90 inhibitors. Treatment of cells with As2O3 induces the E3 ligase Pirh2, which ubiquitinates mutant p53.
Degradation of mutant p53 has also been described to occur via alternative forms of autophagy. The first is: “Macroautophagy,” which is triggered in response to starvation to recycle cellular contents through the lysosomes. A specific form of starvation is glucose restriction, which increases mutant p53 deacetylation, and sends it to degrade through the autophagic machinery. This degradation is Mdm2-dependent (43), but does not involve the proteasome (43, 44). A second form is: selective “chaperone-mediated autophagy” (CMA), in which specific cytosolic proteins are engaged by heat shock proteins (HSPs) and targeted to lysosomes. CMA is a normal cellular process that becomes more active in response to nutrient deprivation (43–46). Although mechanisms like autophagy seem to be less specific than proteasomal degradation, it has been observed that ubiquitinated proteins are targeted for lysosomal degradation and could play a major role in regulating mutant p53 (47).
CHIP is a chaperone-dependent E3 ligase able to ubiquitinate misfolded proteins, a process which is assisted by the Hsp70/90 chaperone machinery. Hsp90-bound substrates are protected from ubiquitination, whereas Hsp70-bound substrates are ubiquitinated by CHIP (48). Interestingly, Hsp70 is found to partially inhibit Mdm2-mediated degradation of mutant p53. This apparent discrepancy in Hsp70 activity with respect to the two E3 ligases is not completely understood. In contrast, Hsp90 protects mutant p53 from both CHIP and Mdm2-mediated degradation (49, 50). This is because mutant p53, unlike wt p53, forms a stable complex with Hsp90 (51, 52). Inhibition of Hsp90 by 17AAG or siRNA against HSF1 (a major transcription factor for Hsp) enhances the ubiquitination and degradation of mutant p53 (Figure 2) (53, 54). Indeed, it was observed that inhibition of Hsp90 reduced the viability of mutant p53-expressing cancer cells of breast (MBA-MB-468, MDA-MB-231, T47D), prostate (DU145), and colon (SW480) (49). Similarly, inhibition of HDAC6, a positive regulator of Hsp90, destabilizes mutant p53 and is preferentially toxic to mutant p53-expressing cancer cell lines (54, 55). Hsp90 directly, or indirectly via its transcriptional activator HSF1, is upregulated in many cancer types, which may contribute to mutant p53 stabilization (56). Mutant p53 exists in a positive forward loop with HSF1. Mutant p53 enhances HSF1 recruitment to DNA, thereby increasing the levels of HSPs, which further stabilize mutant p53 (57, 58). Indeed, treatment with Hsp90 inhibitor, 17DMAG (a derivative of 17AAG), can greatly reduce lymphoma formation and is known to improve survival in mice with mutant p53 (59). Lastly, RING domain containing E3 ligase, Pirh2, which directly ubiquitinates wt p53 (60), also interacts with and promotes the ubiquitination of mutant p53 (61, 62). Since Pirh2 is a p53 target gene, this negative feedback regulatory loop is interrupted by mutations in p53 (60). Treatment of mutant p53-expressing cells with Arsenic trioxide can induce Pirh2-mediated proteasomal degradation of mutant p53 (Figure 2) (62). The role of other E3 ligases, including ARF-BP1, which regulate wt p53, have been shown not to regulate mutant p53 [(40) and reviewed in Ref. (8)].
Countering the E3 ligases are the deubiquitinating enzymes (DUBs), which cleave ubiquitin from proteins. Unlike E3 ligases, the role of DUBs in controlling mutant p53 stability is poorly explored. USP10 deubiquitinates and stabilizes both wt and mutant p53 (63). Inhibition of USP10 by the protein spautin-1 reduces mutant p53 levels under glucose-restricted conditions (46). The USP7 DUB has a complex interplay with both wt p53 and Mdm2, and it deubiquitinates the p53 activator, Tip60 (64). To date, no correlation between USP7 and p53 status has been identified in cancers (65, 66). In wt p53-expressing cell lines, inhibition of USP7 stabilizes p53 and promotes apoptosis. But in at least one mutant p53-expressing cell line, the inhibitor had no effect (67). ABRO1 is able to deubiquitinate wt p53 and stabilize it by facilitating its interaction with USP7. However, there is no information pertaining to its ability to deubiquitinate mutant p53. Overexpression of ABRO1 in HT29 (colorectal adenocarcinoma) and BT474 (breast cancer) cells (both expressing mutant p53) results in increased cell growth, which can be correlated with mutant p53 stability (68). Another DUB, UCHL1, stabilizes wt and mutant p53 levels in breast cancer cell lines and affects cell viability by a mechanism remaining to be explored (69). USP29 can also deubiquitinate p53 in response to oxidative stress (70).
Although much of the focus in the field has been devoted to the 26S-mediated proteasomal degradation of p53 during post-stress recovery, the 20S proteasome has been identified as the destination of unmodified p53 that is inherently unstable, unless protected by the NADH quinone oxidoreductase 1 (NQO1). Specifically, inhibition of NQO1 (for example by dicoumarol) promotes p53 degradation through the 20S proteasome in an Mdm2-independent manner. Interestingly, mutant p53 interacts strongly with NQO1, rendering it resistant to NQO1 inhibitors [reviewed in Ref. (71)]. Pertinently, NQO1 is elevated in many cancers, which may contribute to stabilization of mutant p53 in these cases [reviewed in Ref. (72)].
Free ribosomal proteins are known to regulate the Mdm2/MdmX-p53 axis and activate wt p53, thereby inhibiting tumor proliferation [reviewed in Ref. (73)]. For example, RPS27 is repressed by wt but not mutant p53, and increased expression of RPS27 stabilizes mutant p53 protein, thereby forming a feed forward loop in cancer (74). On the other hand, RPL26 not only binds to the 5′-UTR of p53 mRNA and enhances translation but also interacts with Mdm2 and protects p53 from degradation (75, 76).
Post-Translational Modifications of Mutant p53
The regulation of wt p53 degradation is modulated by PTMs. Wt p53 is extensively modified post-translationally in response to stress conditions, which lead to the stabilization and/or activation of p53 [reviewed in Ref. (31)]. Critically, in most of the tested cases, the PTMs of p53 are non-discriminatory between wt and mutant proteins (7, 31, 77). Some of these modifications contribute to mutant p53 stability by shielding it from degradation by Mdm2 (Figure 2). Specifically, phosphorylation of p53 on serine 20 and threonine 18 in response to DNA damage protects it from Mdm2 and leads to its activation and stabilization (77–79). These phosphorylations are also induced on mutant p53 in response to stress (49, 77). Mutant p53 also escapes from Mdm2 by constitutive phosphorylation by ERK (80). Similarly, activation of SIRT1, which deacetylates mutant p53, can reduce mutant p53 levels in triple-negative breast cancer cell lines, revealing the role of acetylation in stability of the mutant protein (81, 82).
In addition to mutant p53 itself, modifications of Mdm2/MdmX contribute to the protection of mutant p53 from these key inhibitors. In response to DNA damage, Mdm2 is phosphorylated by ATM and c-Abl, which compromises the ability of Mdm2 to degrade p53 (83, 84). ATM-mediated phosphorylation contributes also through the impaired oligomerization of Mdm2 (85). Similarly, MdmX, the key inhibitor of p53 is phosphorylated by ATM and c-Abl, which impairs its capacity to inhibit p53 (86, 87). To what extent these key phosphorylations affect mutant p53 is yet to be demonstrated. The indiscriminate modifications of wt versus mutant p53 in response to stress can contribute to mutant p53 accumulation and activation.
Concluding Remarks
While wt and mutant p53 have distinct and opposing effects on cancer cells, many aspects of their regulation are shared. The majority of the positive and negative regulators of wt p53 that have been tested have a similar regulatory effect on mutant p53. Critically, however, the tightly controlled myriad of positive and negative auto-regulatory loops, which govern wt p53 levels, is uncoupled in the context of mutant p53. In addition, mutant p53 is recognized as a misfolded protein by the heat shock protein chaperons. Together, these contribute to the protection of mutant p53 from the well-coordinated recovery from stress conditions. This results in the chronic accumulation of active mutant p53, which exerts its gain of functions. It is therefore of prime importance to screen patients for p53 mutations prior to treatments, which are known to activate and stabilize p53. The identification of mechanisms that protect mutant p53, as shown by the chaperon HSP proteins, identifies novel approaches to expose mutant p53 to its negative regulators and drive its destruction. Future studies identifying the unique protectors of mutant p53 are a rational approach to define novel approaches to target mutant p53 in cancer cells.
Author Contributions
RV contributed to writing and editing the paper and prepared a figure. KT contributed to writing and prepared a figure. PJM contributed to writing. SH and YH contributed to writing and editing the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The work in the authors’ lab is supported by NHMRC project grants (1049179 and 1063389), NHMRC Fellowship to YH (9628426), and by a grant from CCV (1085154), by the Victoria India Doctoral Scholarship to RV, and a CTx PhD Top Up Scholarship to KT.
References
- 1.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature (2000) 408(6810):307–10. 10.1038/35042675 [DOI] [PubMed] [Google Scholar]
- 2.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat (2007) 28(6):622–9. 10.1002/humu.20495 [DOI] [PubMed] [Google Scholar]
- 3.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev (2012) 26(12):1268–86. 10.1101/gad.190678.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell (2014) 25(3):304–17. 10.1016/j.ccr.2014.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell (2004) 119(6):861–72. 10.1016/j.cell.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 6.Olivier M, Goldgar DE, Sodha N, Ohgaki H, Kleihues P, Hainaut P, et al. Li-Fraumeni and related syndromes: correlation between tumor type, family structure, and TP53 genotype. Cancer Res (2003) 63(20):6643–50. [PubMed] [Google Scholar]
- 7.Terzian T, Suh Y-A, Iwakuma T, Post SM, Neumann M, Lang GA, et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev (2008) 22(10):1337–44. 10.1101/gad.1662908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hock AK, Vousden KH. The role of ubiquitin modification in the regulation of p53. Biochim Biophys Acta (2014) 1843(1):137–49. 10.1016/j.bbamcr.2013.05.022 [DOI] [PubMed] [Google Scholar]
- 9.Saldana-Meyer R, Recillas-Targa F. Transcriptional and epigenetic regulation of the p53 tumor suppressor gene. Epigenetics (2011) 6(9):1068–77. 10.4161/epi.6.9.16683 [DOI] [PubMed] [Google Scholar]
- 10.Ellwanger DC, Buttner FA, Mewes HW, Stumpflen V. The sufficient minimal set of miRNA seed types. Bioinformatics (2011) 27(10):1346–50. 10.1093/bioinformatics/btr149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie C, Chen W, Zhang M, Cai Q, Xu W, Li X, et al. MDM4 regulation by the let-7 miRNA family in the DNA damage response of glioma cells. FEBS Lett (2015) 589(15):1958–65. 10.1016/j.febslet.2015.05.030 [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Chuang AY, Ratovitski EA. Phospho-DeltaNp63alpha/miR-885-3p axis in tumor cell life and cell death upon cisplatin exposure. Cell Cycle (2011) 10(22):3938–47. 10.4161/cc.10.22.18107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandke P, Wyatt N, Fraser J, Bates B, Berberich SJ, Markey MP. MicroRNA-34a modulates MDM4 expression via a target site in the open reading frame. PLoS One (2012) 7(8):e42034. 10.1371/journal.pone.0042034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev (2009) 23(7):862–76. 10.1101/gad.1767609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman Y, Pilpel Y, Oren M. microRNAs and Alu elements in the p53-Mdm2-Mdm4 regulatory network. J Mol Cell Biol (2014) 6(3):192–7. 10.1093/jmcb/mju020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J (1996) 15(19):5349–57. [PMC free article] [PubMed] [Google Scholar]
- 17.Shvarts A, Bazuine M, Dekker P, Ramos YF, Steegenga WT, Merckx G, et al. Isolation and identification of the human homolog of a new p53-binding protein, Mdmx. Genomics (1997) 43(1):34–42. 10.1006/geno.1997.4775 [DOI] [PubMed] [Google Scholar]
- 18.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol (2009) 16(1):23–9. 10.1038/nsmb.1533 [DOI] [PubMed] [Google Scholar]
- 19.Bou Kheir T, Futoma-Kazmierczak E, Jacobsen A, Krogh A, Bardram L, Hother C, et al. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol Cancer (2011) 10:29. 10.1186/1476-4598-10-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh SS, Yoo JY, Nuovo GJ, Jeon YJ, Kim S, Lee TJ, et al. MicroRNAs/TP53 feedback circuitry in glioblastoma multiforme. Proc Natl Acad Sci U S A (2012) 109(14):5316–21. 10.1073/pnas.1202465109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Huang JW, Castella M, Huntsman DG, Taniguchi T. p53 is positively regulated by miR-542-3p. Cancer Res (2014) 74(12):3218–27. 10.1158/0008-5472.CAN-13-1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature (2009) 460(7254):529–33. 10.1038/nature08199 [DOI] [PubMed] [Google Scholar]
- 23.Muller PA, Trinidad AG, Caswell PT, Norman JC, Vousden KH. Mutant p53 regulates dicer through p63-dependent and -independent mechanisms to promote an invasive phenotype. J Biol Chem (2014) 289(1):122–32. 10.1074/jbc.M113.502138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong P, Karaayvaz M, Jia N, Kaneuchi M, Hamada J, Watari H, et al. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene (2013) 32(27):3286–95. 10.1038/onc.2012.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Cheng B, Miao L, Mei Y, Wu M. Mutant p53-R273H gains new function in sustained activation of EGFR signaling via suppressing miR-27a expression. Cell Death Dis (2013) 4:e574. 10.1038/cddis.2013.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masciarelli S, Fontemaggi G, Di Agostino S, Donzelli S, Carcarino E, Strano S, et al. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene (2014) 33(12):1601–8. 10.1038/onc.2013.106 [DOI] [PubMed] [Google Scholar]
- 27.Subramanian M, Francis P, Bilke S, Li XL, Hara T, Lu X, et al. A mutant p53/let-7i-axis-regulated gene network drives cell migration, invasion and metastasis. Oncogene (2015) 34(9):1094–104. 10.1038/onc.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, et al. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A (2012) 109(38):15312–7. 10.1073/pnas.1110977109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donzelli S, Fontemaggi G, Fazi F, Di Agostino S, Padula F, Biagioni F, et al. MicroRNA-128-2 targets the transcriptional repressor E2F5 enhancing mutant p53 gain of function. Cell Death Differ (2012) 19(6):1038–48. 10.1038/cdd.2011.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neilsen PM, Noll JE, Mattiske S, Bracken CP, Gregory PA, Schulz RB, et al. Mutant p53 drives invasion in breast tumors through up-regulation of miR-155. Oncogene (2013) 32(24):2992–3000. 10.1038/onc.2012.305 [DOI] [PubMed] [Google Scholar]
- 31.Meek DW, Anderson CW. Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb Perspect Biol (2009) 1(6):a000950. 10.1101/cshperspect.a000950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruse JP, Gu W. Modes of p53 regulation. Cell (2009) 137(4):609–22. 10.1016/j.cell.2009.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem (1998) 67:425–79. 10.1146/annurev.biochem.67.1.425 [DOI] [PubMed] [Google Scholar]
- 34.MacPherson D, Kim J, Kim T, Rhee BK, Van Oostrom CT, DiTullio RA, et al. Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. EMBO J (2004) 23(18):3689–99. 10.1038/sj.emboj.7600363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature (1997) 387(6630):296–9. 10.1038/387296a0 [DOI] [PubMed] [Google Scholar]
- 36.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature (1997) 387(6630):299–303. 10.1038/387299a0 [DOI] [PubMed] [Google Scholar]
- 37.Midgley CA, Lane DP. p53 protein stability in tumour cells is not determined by mutation but is dependent on Mdm2 binding. Oncogene (1997) 15(10):1179–89. 10.1038/sj.onc.1201459 [DOI] [PubMed] [Google Scholar]
- 38.Shimizu H, Burch LR, Smith AJ, Dornan D, Wallace M, Ball KL, et al. The conformationally flexible S9-S10 linker region in the core domain of p53 contains a novel MDM2 binding site whose mutation increases ubiquitination of p53 in vivo. J Biol Chem (2002) 277(32):28446–58. 10.1074/jbc.M202296200 [DOI] [PubMed] [Google Scholar]
- 39.Wallace M, Worrall E, Pettersson S, Hupp TR, Ball KL. Dual-site regulation of MDM2 E3-ubiquitin ligase activity. Mol Cell (2006) 23(2):251–63. 10.1016/j.molcel.2006.05.029 [DOI] [PubMed] [Google Scholar]
- 40.Lukashchuk N, Vousden KH. Ubiquitination and degradation of mutant p53. Mol Cell Biol (2007) 27(23):8284–95. 10.1128/MCB.00050-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brignone C, Bradley KE, Kisselev AF, Grossman SR. A post-ubiquitination role for MDM2 and hHR23A in the p53 degradation pathway. Oncogene (2004) 23(23):4121–9. 10.1038/sj.onc.1207540 [DOI] [PubMed] [Google Scholar]
- 42.Haupt S, Mitchell C, Corneille V, Shortt J, Fox S, Pandolfi PP, et al. Loss of PML cooperates with mutant p53 to drive more aggressive cancers in a gender-dependent manner. Cell Cycle (2013) 12(11):1722–31. 10.4161/cc.24805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morselli E, Tasdemir E, Maiuri MC, Galluzzi L, Kepp O, Criollo A, et al. Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle (2008) 7(19):3056–61. 10.4161/cc.7.19.6751 [DOI] [PubMed] [Google Scholar]
- 44.Tasdemir E, Chiara Maiuri M, Morselli E, Criollo A, D’Amelio M, Djavaheri-Mergny M, et al. A dual role of p53 in the control of autophagy. Autophagy (2008) 4(6):810–4. 10.4161/auto.6486 [DOI] [PubMed] [Google Scholar]
- 45.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol (2010) 22(2):124–31. 10.1016/j.ceb.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vakifahmetoglu-Norberg H, Kim M, Xia HG, Iwanicki MP, Ofengeim D, Coloff JL, et al. Chaperone-mediated autophagy degrades mutant p53. Genes Dev (2013) 27(15):1718–30. 10.1101/gad.220897.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev (2002) 82(2):373–428. 10.1152/physrev.00027.2001 [DOI] [PubMed] [Google Scholar]
- 48.Pratt WB, Morishima Y, Peng HM, Osawa Y. Proposal for a role of the Hsp90/Hsp70-based chaperone machinery in making triage decisions when proteins undergo oxidative and toxic damage. Exp Biol Med (Maywood) (2010) 235(3):278–89. 10.1258/ebm.2009.009250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li D, Marchenko ND, Schulz R, Fischer V, Velasco-Hernandez T, Talos F, et al. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol Cancer Res (2011) 9(5):577–88. 10.1158/1541-7786.MCR-10-0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiech M, Olszewski MB, Tracz-Gaszewska Z, Wawrzynow B, Zylicz M, Zylicz A. Molecular mechanism of mutant p53 stabilization: the role of HSP70 and MDM2. PLoS One (2012) 7(12):e51426. 10.1371/journal.pone.0051426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blagosklonny MV, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci U S A (1996) 93(16):8379–83. 10.1073/pnas.93.16.8379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitesell L, Sutphin PD, Pulcini EJ, Martinez JD, Cook PH. The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol (1998) 18(3):1517–24. 10.1128/MCB.18.3.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller P, Hrstka R, Coomber D, Lane DP, Vojtesek B. Chaperone-dependent stabilization and degradation of p53 mutants. Oncogene (2008) 27(24):3371–83. 10.1038/sj.onc.1211010 [DOI] [PubMed] [Google Scholar]
- 54.Li D, Marchenko ND, Moll UM. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell Death Differ (2011) 18(12):1904–13. 10.1038/cdd.2011.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell (2005) 18(5):601–7. 10.1016/j.molcel.2005.04.021 [DOI] [PubMed] [Google Scholar]
- 56.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci (2006) 31(3):164–72. 10.1016/j.tibs.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 57.Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J (1999) 18(21):5943–52. 10.1093/emboj/18.21.5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li D, Yallowitz A, Ozog L, Marchenko N. A gain-of-function mutant p53-HSF1 feed forward circuit governs adaptation of cancer cells to proteotoxic stress. Cell Death Dis (2014) 5:e1194. 10.1038/cddis.2014.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alexandrova EM, Yallowitz AR, Li D, Xu S, Schulz R, Proia DA, et al. Improving survival by exploiting tumour dependence on stabilized mutant p53 for treatment. Nature (2015) 523(7560):352–6. 10.1038/nature14430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, et al. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell (2003) 112(6):779–91. 10.1016/S0092-8674(03)00193-4 [DOI] [PubMed] [Google Scholar]
- 61.Sheng Y, Laister RC, Lemak A, Wu B, Tai E, Duan S, et al. Molecular basis of Pirh2-mediated p53 ubiquitylation. Nat Struct Mol Biol (2008) 15(12):1334–42. 10.1038/nsmb.1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan W, Jung YS, Zhang Y, Chen X. Arsenic trioxide reactivates proteasome-dependent degradation of mutant p53 protein in cancer cells in part via enhanced expression of Pirh2 E3 ligase. PLoS One (2014) 9(8):e103497. 10.1371/journal.pone.0103497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell (2010) 140(3):384–96. 10.1016/j.cell.2009.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dar A, Shibata E, Dutta A. Deubiquitination of Tip60 by USP7 determines the activity of the p53-dependent apoptotic pathway. Mol Cell Biol (2013) 33(16):3309–20. 10.1128/MCB.00358-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masuya D, Huang C, Liu D, Nakashima T, Yokomise H, Ueno M, et al. The HAUSP gene plays an important role in non-small cell lung carcinogenesis through p53-dependent pathways. J Pathol (2006) 208(5):724–32. 10.1002/path.1931 [DOI] [PubMed] [Google Scholar]
- 66.Mori S, Ito G, Usami N, Yoshioka H, Ueda Y, Kodama Y, et al. p53 apoptotic pathway molecules are frequently and simultaneously altered in nonsmall cell lung carcinoma. Cancer (2004) 100(8):1673–82. 10.1002/cncr.20164 [DOI] [PubMed] [Google Scholar]
- 67.Fan YH, Cheng J, Vasudevan SA, Dou J, Zhang H, Patel RH, et al. USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell Death Dis (2013) 4:e867. 10.1038/cddis.2013.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, Cao M, Dong J, Li C, Xu W, Zhan Y, et al. ABRO1 suppresses tumourigenesis and regulates the DNA damage response by stabilizing p53. Nat Commun (2014) 5:5059. 10.1038/ncomms6059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiang T, Li L, Yin X, Yuan C, Tan C, Su X, et al. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer. PLoS One (2012) 7(1):e29783. 10.1371/journal.pone.0029783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J, Chung HJ, Vogt M, Jin Y, Malide D, He L, et al. JTV1 co-activates FBP to induce USP29 transcription and stabilize p53 in response to oxidative stress. EMBO J (2011) 30(5):846–58. 10.1038/emboj.2011.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsvetkov P, Reuven N, Shaul Y. Ubiquitin-independent p53 proteasomal degradation. Cell Death Differ (2010) 17(1):103–8. 10.1038/cdd.2009.67 [DOI] [PubMed] [Google Scholar]
- 72.Belinsky M, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev (1993) 12(2):103–17. 10.1007/BF00689804 [DOI] [PubMed] [Google Scholar]
- 73.Zhou X, Liao WJ, Liao JM, Liao P, Lu H. Ribosomal proteins: functions beyond the ribosome. J Mol Cell Biol (2015) 7(2):92–104. 10.1093/jmcb/mjv014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiong X, Zhao Y, He H, Sun Y. Ribosomal protein S27-like and S27 interplay with p53-MDM2 axis as a target, a substrate and a regulator. Oncogene (2011) 30(15):1798–811. 10.1038/onc.2010.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell (2005) 123(1):49–63. 10.1016/j.cell.2005.07.034 [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Wang J, Yuan Y, Zhang W, Guan W, Wu Z, et al. Negative regulation of HDM2 to attenuate p53 degradation by ribosomal protein L26. Nucleic Acids Res (2010) 38(19):6544–54. 10.1093/nar/gkq536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alsheich-Bartok O, Haupt S, Alkalay-Snir I, Saito S, Appella E, Haupt Y. PML enhances the regulation of p53 by CK1 in response to DNA damage. Oncogene (2008) 27(26):3653–61. 10.1038/sj.onc.1211036 [DOI] [PubMed] [Google Scholar]
- 78.Unger T, Juven-Gershon T, Moallem E, Berger M, Sionov RV, Lozano G. Critical role for Ser20 of human p53 in the negative regulation of p53 by Mdm2. EMBO J (1999) 18(7):1805–14. 10.1093/emboj/18.7.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci U S A (1999) 96(24):13777–82. 10.1073/pnas.96.24.13777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Melnikova VO, Santamaria AB, Bolshakov SV, Ananthaswamy HN. Mutant p53 is constitutively phosphorylated at serine 15 in UV-induced mouse skin tumors: involvement of ERK1/2 MAP kinase. Oncogene (2003) 22(38):5958–66. 10.1038/sj.onc.1206595 [DOI] [PubMed] [Google Scholar]
- 81.Yi YW, Kang HJ, Kim HJ, Kong Y, Brown ML, Bae I. Targeting mutant p53 by a SIRT1 activator YK-3-237 inhibits the proliferation of triple-negative breast cancer cells. Oncotarget (2013) 4(7):984–94. 10.18632/oncotarget.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang ZY, Hong D, Nam SH, Kim JM, Paik YH, Joh JW, et al. SIRT1 regulates oncogenesis via a mutant p53-dependent pathway in hepatocellular carcinoma. J Hepatol (2015) 62(1):121–30. 10.1016/j.jhep.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 83.Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D. Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc Natl Acad Sci U S A (1999) 96(26):14973–7. 10.1073/pnas.96.26.14973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goldberg Z, Sionov RV, Berger M, Zwang Y, Perets R, Van Etten RA, et al. Tyrosine phosphorylation of Mdm2 by c-Abl: implications for p53 regulation. EMBO J (2002) 21(14):3715–27. 10.1093/emboj/cdf384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng Q, Chen L, Li Z, Lane WS, Chen J. ATM activates p53 by regulating MDM2 oligomerization and E3 processivity. EMBO J (2009) 28(24):3857–67. 10.1038/emboj.2009.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pereg Y, Shkedy D, de Graaf P, Meulmeester E, Edelson-Averbukh M, Salek M, et al. Phosphorylation of Hdmx mediates its Hdm2-and ATM-dependent degradation in response to DNA damage. Proc Natl Acad Sci U S A (2005) 102(14):5056–61. 10.1073/pnas.0408595102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zuckerman V, Lenos K, Popowicz GM, Silberman I, Grossman T, Marine J-C, et al. c-Abl phosphorylates Hdmx and regulates its interaction with p53. J Biol Chem (2009) 284(6):4031–9. 10.1074/jbc.M809211200 [DOI] [PubMed] [Google Scholar]
- 88.Deng Q, Becker L, Ma X, Zhong X, Young K, Ramos K, et al. The dichotomy of p53 regulation by noncoding RNAs. J Mol Cell Biol (2014) 6(3):198–205. 10.1093/jmcb/mju017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang S, Zhang C, Li Y, Wang P, Yue Z, Xie S. miR-98 regulates cisplatin-induced A549 cell death by inhibiting TP53 pathway. Biomed Pharmacother (2011) 65(6):436–42. 10.1016/j.biopha.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 90.Fan Y, Yin S, Hao Y, Yang J, Zhang H, Sun C, et al. miR-19b promotes tumor growth and metastasis via targeting TP53. RNA (2014) 20(6):765–72. 10.1261/rna.043026.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tay Y, Tan SM, Karreth FA, Lieberman J, Pandolfi PP. Characterization of dual PTEN and p53-targeting microRNAs identifies microRNA-638/Dnm2 as a two-hit oncogenic locus. Cell Rep (2014) 8(3):714–22. 10.1016/j.celrep.2014.06.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang C, Liu J, Wang X, Wu R, Lin M, Laddha SV, et al. MicroRNA-339-5p inhibits colorectal tumorigenesis through regulation of the MDM2/p53 signaling. Oncotarget (2014) 5(19):9106–17. 10.18632/oncotarget.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jansson MD, Damas ND, Lees M, Jacobsen A, Lund AH. miR-339-5p regulates the p53 tumor-suppressor pathway by targeting MDM2. Oncogene (2015) 34(15):1908–18. 10.1038/onc.2014.130 [DOI] [PubMed] [Google Scholar]
- 94.Fortunato O, Boeri M, Moro M, Verri C, Mensah M, Conte D, et al. Mir-660 is downregulated in lung cancer patients and its replacement inhibits lung tumorigenesis by targeting MDM2-p53 interaction. Cell Death Dis (2014) 5:e1564. 10.1038/cddis.2014.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ren ZJ, Nong XY, Lv YR, Sun HH, An PP, Wang F, et al. Mir-509-5p joins the Mdm2/p53 feedback loop and regulates cancer cell growth. Cell Death Dis (2014) 5:e1387. 10.1038/cddis.2014.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stegeman S, Moya L, Selth LA, Spurdle AB, Clements JA, Batra J. A genetic variant of MDM4 influences regulation by multiple microRNAs in prostate cancer. Endocr Relat Cancer (2015) 22(2):265–76. 10.1530/ERC-15-0013 [DOI] [PubMed] [Google Scholar]