Abstract
To survive winter, many perennial plants become endodormant, a state of suspended growth maintained even in favorable growing environments. To understand vegetative bud endodormancy, we collected paradormant, endodormant, and ecodormant axillary buds from Populus trees growing under natural conditions. Of 44,441 Populus gene models analyzed using NimbleGen microarrays, we found that 1,362 (3.1%) were differentially expressed among the three dormancy states, and 429 (1.0%) were differentially expressed during only one of the two dormancy transitions (FDR p-value < 0.05). Of all differentially expressed genes, 69% were down-regulated from paradormancy to endodormancy, which was expected given the lower metabolic activity associated with endodormancy. Dormancy transitions were accompanied by changes in genes associated with DNA methylation (via RNA-directed DNA methylation) and histone modifications (via Polycomb Repressive Complex 2), confirming and extending knowledge of chromatin modifications as major features of dormancy transitions. Among the chromatin-associated genes, two genes similar to SPT (SUPPRESSOR OF TY) were strongly up-regulated during endodormancy. Transcription factor genes and gene sets that were atypically up-regulated during endodormancy include a gene that seems to encode a trihelix transcription factor and genes associated with proteins involved in responses to ethylene, cold, and other abiotic stresses. These latter transcription factors include ETHYLENE INSENSITIVE 3 (EIN3), ETHYLENE-RESPONSIVE ELEMENT BINDING PROTEIN (EBP), ETHYLENE RESPONSE FACTOR (ERF), ZINC FINGER PROTEIN 10 (ZAT10), ZAT12, and WRKY DNA-binding domain proteins. Analyses of phytohormone-associated genes suggest important changes in responses to ethylene, auxin, and brassinosteroids occur during endodormancy. We found weaker evidence for changes in genes associated with salicylic acid and jasmonic acid, and little evidence for important changes in genes associated with gibberellins, abscisic acid, and cytokinin. We identified 315 upstream sequence motifs associated with eight patterns of gene expression, including novel motifs and motifs associated with the circadian clock and responses to photoperiod, cold, dehydration, and ABA. Analogies between flowering and endodormancy suggest important roles for genes similar to SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL), DORMANCY ASSOCIATED MADS-BOX (DAM), and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1).
Keywords: chromatin, ecodormancy, endodormancy, gene expression, paradormancy, phytohormone, transcription factor, QTL
Introduction
Dormancy, the temporary suspension of growth (Lang et al., 1987), is a regulated process that controls plant growth, development, and architecture. Lang et al. (1987) subdivided dormancy processes into three types: paradormancy, endodormancy, and ecodormancy. Paradormancy denotes the state in which meristem growth (e.g., in buds or seeds) is inhibited by signals from other plant organs. For example, the shoot apex can inhibit the outgrowth of axillary buds by exerting apical dominance—but this state of paradormancy is released and outgrowth of the axillary buds occurs if the apex is removed. Endodormancy denotes the state in which meristem growth is inhibited by signals within the meristem itself. In plants adapted to cold climates, vegetative buds typically become endodormant in the fall and early winter, and prolonged periods of chilling (i.e., temperatures slightly above freezing) are needed before growth can resume, even under favorable environmental conditions. Even after the release of endodormancy, plants may remain ecodormant because of harsh environmental conditions such as cold or drought that are not conducive to cell division and elongation.
The regulated induction and release of bud endodormancy is critical for the survival and long-term growth of perennial plants in temperate, arid, and semiarid climates. Adaptation to local climatic conditions is generally achieved by natural selection in native populations and by artificial selection in forestry and agricultural populations. However, the matching of plant populations and local climatic cycles may become decoupled with rapid climate change. The induction and release of endodormancy are temporally associated with other changes that confer tolerance to cold and other abiotic stresses. Improved understanding of dormancy-associated gene expression may allow us to manipulate plant populations to speed climatic adaptation, and thus mitigate the adverse effects of climate change on forest and agricultural ecosystems.
Environmental and hormonal signals, including short days (SD), cold, ethylene, gibberellin (GA), and abscisic acid (ABA) play direct roles in growth cessation and bud set (Li et al., 2003; Mølmann et al., 2005; Ruonala et al., 2006). In many trees and other perennial plants, SD and low night temperatures in the fall act synergistically to induce growth cessation, vegetative bud set or shoot-tip abscission, and the first stage of cold acclimation (Howe et al., 1999; Mølmann et al., 2005; Ruttink et al., 2007; Rohde et al., 2011a). In some species (e.g., apple pear, Populus sp.), cold temperatures alone can induce growth cessation and endodormancy (Mølmann et al., 2005; Heide, 2008; Rohde et al., 2011a). In the model herbaceous perennial plant, leafy spurge (Euphorbia esula), cold night temperatures and long days appear to be most effective for inducing endodormancy (Horvath et al., 2010). Although cold can induce endodormancy in some species, extended chilling temperatures release vegetative bud endodormancy in nearly every temperate perennial species examined (Arora et al., 2003). After the release of endodormancy via chilling, warm temperatures in the spring promote cold deacclimation, vegetative bud flush, and the resumption of elongation growth. The quantitative genetics of bud set and bud flush have been well studied, and many quantitative trait loci (QTL) have been identified (Frewen et al., 2000; reviewed in Howe et al., 2003; Jermstad et al., 2003; Scotti-Saintagne et al., 2004; Pelgas et al., 2011; Rohde et al., 2011b).
The phytochrome photoreceptors and components of the circadian clock regulate short-day-induced dormancy in Populus and other perennial plants (Howe et al., 1996; Olsen et al., 1997; Ibanez et al., 2010; Kozarewa et al., 2010). In Populus, short-day signals induce growth cessation via a regulatory module consisting of poplar homologs of CONSTANS (CO) and FLOWERING LOCUS T (FT) in Arabidopsis (Bohlenius et al., 2006). Ultimately, SD signals lead to changes in poplar cell proliferation via the Like-APETALA 1 (LAP1) gene product, which acts on the AINTEGUMENTA-like 1 transcription factor, which is related to a regulator of cell proliferation in Arabidopsis (Azeez et al., 2014). In Populus, FT2 was also induced by chilling, which subsequently led to the induction of 1,3-β-glucanases, reopening of signal conduits, and release of endodormancy (Rinne et al., 2011). The authors hypothesized that the reopened conduits enabled movement of FT2 and CENTRORADIALIS 1 (CENL1) to locations where they promoted bud flush and shoot elongation (Rinne et al., 2011). The expression of other genes that regulate cold acclimation and other endodormancy-associated processes are induced by SD. Transcription factors such as C-REPEAT/DRE BINDING FACTOR 2/DEHYDRATION RESPONSE ELEMENT-BINDING PROTEIN (CBF/DREB) have been implicated in cold acclimation and endodormancy (Doğramaci et al., 2010). For example, overexpression of a CBF gene in apple resulted in the ability to induce endodormancy with SDs (Wisniewski et al., 2011).
Many of the same environmental and hormonal signals that regulate dormancy also regulate cold acclimation and flowering. Thus, it is not surprising that the flowering genes FT2 and CENL1 also seem to regulate endodormancy (Bohlenius et al., 2006; Ruonala et al., 2008; Hsu et al., 2011; Rinne et al., 2011). Likewise, proteins suspected of regulating FT2, such as those encoded by DORMANCY ASSOCIATED MADS-BOX (DAM) genes, have also been implicated in endodormancy regulation (Bielenberg et al., 2008; Horvath et al., 2010; Sasaki et al., 2011; Yamane et al., 2011). Chromatin remodeling processes associated with vernalization may also regulate bud endodormancy in perennials (Horvath et al., 2003), perhaps by modifying the promoters of DAM genes (Horvath et al., 2010; Leida et al., 2012). Indeed, chromatin remodeling seems to accompany changes in Populus dormancy states (Vining et al., 2012).
Microarray analysis in Populus and several other species have identified common signaling processes associated with endodormancy induction and release (Mazzitelli et al., 2007; Ruttink et al., 2007; Halaly et al., 2008; Horvath et al., 2008; Mathiason et al., 2009; Walton et al., 2009; Campbell et al., 2010; Doğramaci et al., 2010; Karlberg et al., 2010). In addition to flowering genes, genes involved in environmental and phytohormone signaling [e.g., photoperiod, cold, oxidative stress, ethylene, auxin, ABA, and jasmonic acid (JA)], chromatin remodeling, and circadian responses are often differentially expressed during the induction and release of endodormancy. However, only a modest number of genes (<15,000) have been assayed in most previous studies, making it difficult to compare differential expression among gene family members. Furthermore, there are few reports in which endodormancy induction and release were compared under natural conditions in the same study.
We used analyses of gene expression to infer physiological processes and cis-acting motifs associated with the induction and release of endodormancy in Populus. We collected vegetative axillary buds from the end of summer through early spring, and then used a NimbleGen genome-scale microarray to measure global changes in gene expression among dormancy states. Our primary objectives were to: (1) identify which individual genes, biological processes, molecular functions, and regulatory pathways were differentially expressed among dormancy states, (2) classify the differentially expressed genes into contrasting gene expression patterns, and (3) identify cis-acting elements associated with each gene expression group. We used this approach and previous observations on dormancy physiology and genomics to better understand the processes regulating endodormancy induction and release in Populus trees. We report extensive transcriptome remodeling that both confirm and contradict physiological pathway expectations from the published literature.
Materials and Methods
Plant Material
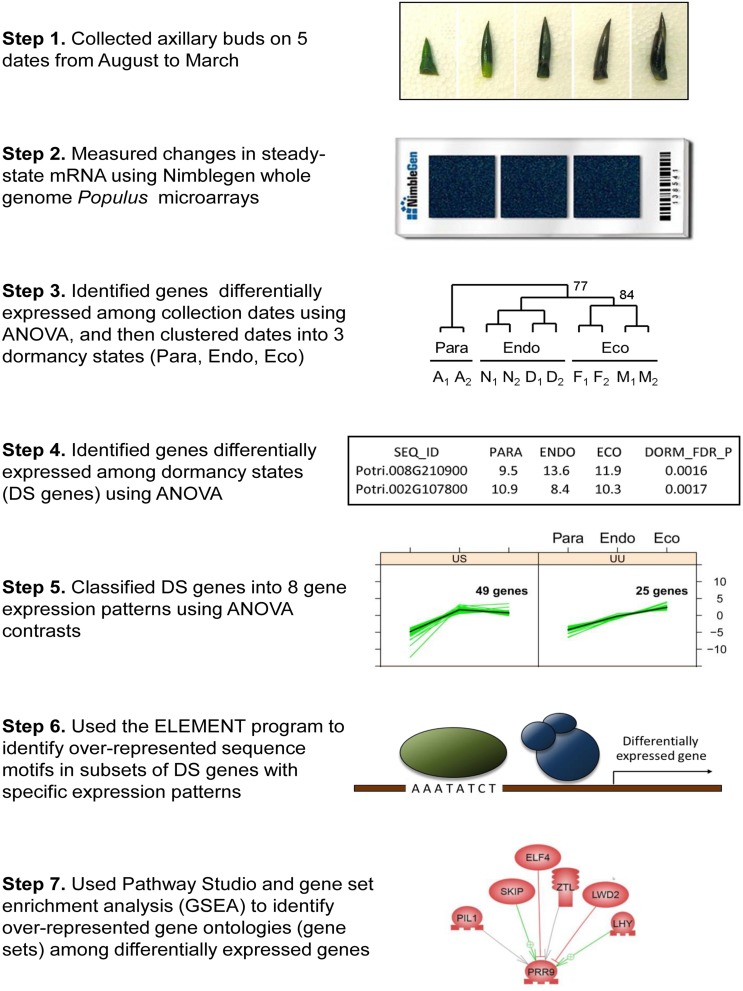
We collected axillary buds from the main stem of two, rapidly growing, 3-year-old Populus trichocarpa trees (clone Nisqually-1) growing on a field site in Corvallis, OR, USA on five dates between August 2005 and March 2006 (Step 1, Figure 1). Average temperatures and precipitation over the collection period are shown in Supplementary Figure S1. Separate RNA isolations were performed on a pooled sample of five buds from each of two trees on each date, resulting in two biological replicates that were used for array hybridizations. The buds were dissected in the field using sterile scalpel blades, immediately frozen in liquid nitrogen, and then subsequently stored at -80°C until they were used for RNA isolation. A few buds collected at the same time were fixed in FAA, dehydrated, and then embedded in wax for sectioning (WAX-IT Histology Services, Vancouver, BC, Canada). De-waxed stem sections were stained with Toluidine Blue-O (Jensen, 1962) and photographed.
FIGURE 1.
Flow diagram showing the steps used to analyze dormancy related gene expression in Populus. Step 1 shows representative axillary buds collected in August, November, December, February, and March (left to right). Step 2 shows the NimbleGen gene expression microarray used to measure relative gene expression. Step 3 shows results of clustering five collection time-points into three dormancy states based on the expression of differentially expressed genes. The dormancy states are paradormant (Para), endodormant (Endo), and ecodormant (Eco). Step 4 shows a section of Supplementary Data File 1, which includes results of analyses of variance (ANOVA). Step 5 shows genes that were classified into two of eight gene expression patterns. Step 6 shows a transcription factor binding to an upstream DNA sequence motif (Evening Element). Step 7 shows a representative regulatory network generated by the Pathway Studio program.
RNA Isolation
RNA was isolated using a Qiagen RNeasy kit according to the manufacturer’s protocol, including a DNase I treatment to remove contaminating genomic DNA (Qiagen, Valencia, CA, USA). The A260/A280 ratios of RNA samples used for hybridizations ranged from 1.8 to 2.0. The absence of contaminating genomic DNA and the integrity of RNA samples were examined by an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). The RNA Integrity Numbers (RIN; Mueller et al., 2004) of the RNA samples ranged from 8.5 to 10.0, indicating that high-quality RNA was used for the microarray hybridizations.
Microarray Analysis
We measured gene expression using a microarray designed to target all predicted genes in the P. trichocarpa nuclear and organellar genomes plus a set of divergent aspen transcripts (Step 2, Figure 1). The microarray, which was manufactured by Roche NimbleGen1, was originally designed to target 55,794 nuclear, 59 mitochondrial, 71 chloroplast, and 49 miRNA gene models based on version 1.1 of the P. trichocarpa genome sequence (v1.1; Tuskan et al., 2006), plus 9,995 unigenes derived from aspen ESTs (Sterky et al., 2004). With a few exceptions, each original gene model was represented by two copies of three different 60-mer isothermal probes. Original microarray information is archived in the NCBI Gene Expression Omnibus (GEO) database as accession numbers GPL2699 and GPL7424. To analyze the microarray data using the latest gene models, we used BLASTN (Altschul et al., 1990) to reassign the 194,260 Populus probe sequences from the original microarray to the transcript sequences from the Populus v3.0 genome assembly (file Ptrichocarpa_210_transcript.fa released as part of Phytozome v9.02). We assigned each array probe to one v3.0 gene model, omitting the filtering of low-complexity probes and using a 75% nucleotide identity cutoff. By reassigning the microarray probes to Populus v3.0 transcripts, we were able to measure the expression of 35,048 out of 41,335 v3.0 primary transcripts. For probes that could not be assigned to v3.0 transcripts, we retained the original gene model assignment. These 9,393 v1.1 transcripts were also included in the analyses described below.
Biotin-labeled cRNA was produced using the Ambion MessageAmpTM II aRNA amplification kit according to the manufacturer’s instructions (Van Gelder et al., 1990; Life Technologies, 2011), and then sent to NimbleGen for fragmentation, hybridization, and detection. Briefly, total RNA (∼1500 ng) from each sample was reversed transcribed using an oligo(dT) primer with a T7 promoter. After second-strand synthesis, the cDNA was used as a template for synthesizing biotin-labeled antisense RNA (cRNA) using in vitro transcription with T7 RNA polymerase. For each sample, ∼20 μg cRNA was sent to NimbleGen for fragmentation, hybridization, and detection as described by Kaushik et al. (2005). After hybridization and washing, the arrays were stained with a streptavidin-Cy3 conjugate, and then scanned with a GenePix 4000B microarray scanner.
The complete microarray dataset was deposited in the GEO database3 as accession GPL20616. We created new NimbleGen design files (ndf and ngd files) based on the probe reassignments described above, and then normalized the data using NimbleScan v2.6. Microarray data were log2 transformed, background corrected, and then normalized across all arrays using the Robust Multiple-array Average (RMA; Irizarry et al., 2003).
Characterization of Bud Dormancy States and Tests of Differential Expression
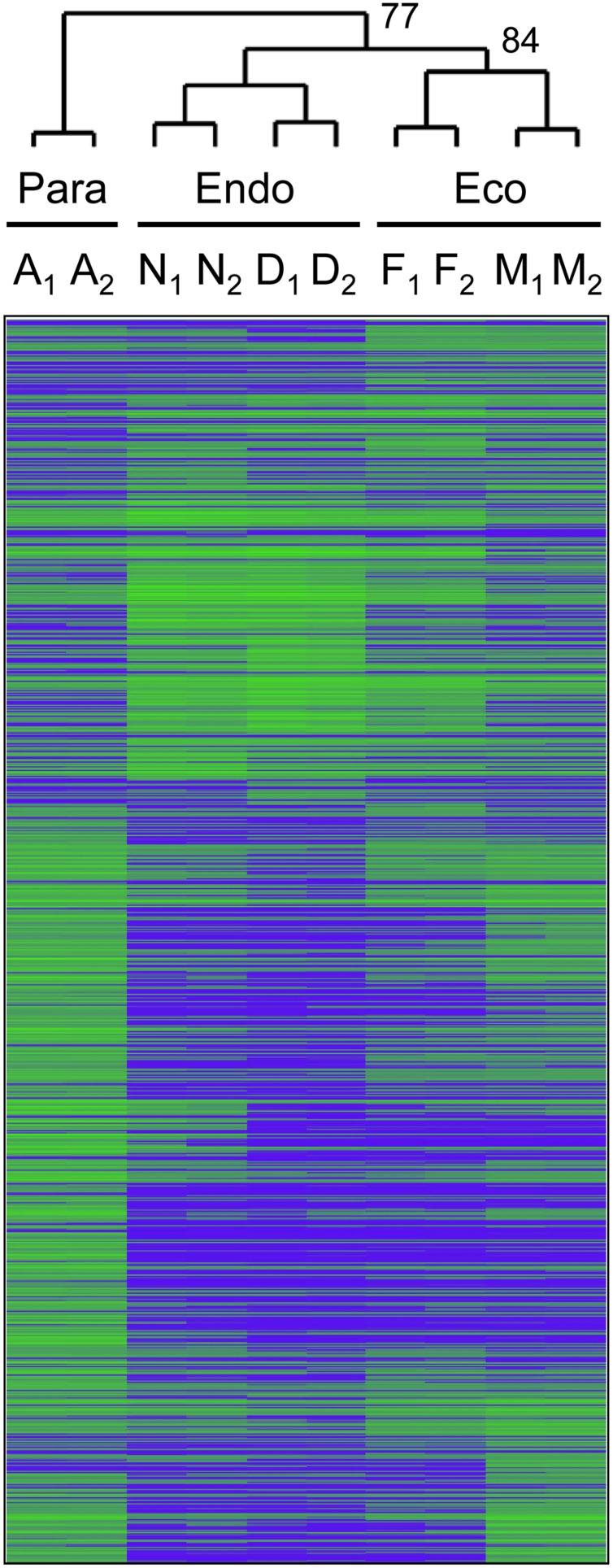
We assigned a dormancy state to each collection date using ANOVA and cluster analysis in SAS v9.3 (Statistical Analysis System, Cary, NC, USA). First, we used ANOVA and a false discovery rate (FDR) p-value < 0.05 to identify genes that were differentially expressed among the collection dates. We then used UPGMA and Neighbor-Joining hierarchical clustering to group the collection dates into dormancy states. The UPGMA analysis clustered the collection dates into three distinct clusters: August, November/December, and February/March, which we refer to as paradormant (Para), endodormant (Endo), and ecodormant (Eco) (Step 3, Figure 1; see Results), respectively. In the Neighbor-Joining analysis, the February samples clustered with November and December (rather than with March), but this was only weakly supported (i.e., as compared to the UPGMA alternative). Because the UPGMA assignments were judged to be more biologically accurate (i.e., based on morphological observations and past research on Populus dormancy), we used the UPGMA groupings for further analyses. We regrouped the samples based on the UPGMA cluster analysis, and then conducted a second ANOVA on the entire dataset to determine which genes were differentially expressed among the assigned dormancy states (treatments; Step 4, Figure 1). All subsequent references to ‘regulated’ or ‘differentially expressed’ genes refer to the set of 1,362 genes that were differentially expressed among dormancy states at an FDR p-value < 0.05.
Gene Expression Patterns and Sequence Motifs
A priori, we defined eight potential patterns of gene expression that could occur during two dormancy transitions: Para/Endo and Endo/Eco. For each transition, gene expression may either be up-regulated (U), stay the same (S), or be down-regulated (D), which results in eight possible patterns for two transitions when only the differentially expressed genes are tested (i.e., S/S patterns are not possible). That is, 8 patterns = (3 possible changes for the Para/Endo transition × 3 possible changes for the Endo/Eco transition) – 1 pattern (S/S). For each gene, we determined the p-value for each of the eight models using the CONTRAST option of SAS Proc ANOVA, and then used the treatment means and the model p-values to assign the gene expression pattern (Step 5, Figure 1). Because we were interested in clustering the genes based on directional changes in gene expression (not differences in mean expression), we first normalized the data using the ANOVA mean square error for each gene.
We used the ELEMENT program (Mockler et al., 2007) to identify sequence motifs that were overrepresented in each gene expression group (Step 6, Figure 1). These analyses were conducted using 2 kb of upstream sequence relative to the Populus v3.0 transcription start site. Motifs were associated with a particular gene expression pattern when the average number of motifs per sequence (MOTIF_MN_HITS) was significant at an FDR p-value < 0.05 for only one of the gene expression groups. We inferred potential functions of the motifs using the SIGNALSCAN program and the database of Plant Cis-acting Regulatory DNA Elements (PLACE4; Higo et al., 1999), and by comparing the motifs to motifs in the PlantCare database5) and published literature. We then ranked the motifs based on the number of sequences that contained one or more copies of the motif (SEQ_HIT_P), and identified the top 50 motifs.
Identification of Key Differentially Expressed Genes
We focused attention on genes encoding transcription factors, and genes associated with chromatin, phytohormone responses, or dormancy-related QTL. For each analysis (subset of genes), we classified the genes into four groups: up- or down-regulated from paradormancy to endodormancy, and up- or down-regulated from endodormancy to ecodormancy. Within each group, we ranked genes by FDR p-value, and then focused on the top 15 genes in each group if they had an FDR p-value < 0.05.
Chromatin-associated genes were identified using the Arabidopsis thaliana chromatin database (ChromDB; Gendler et al., 2008) and by including genes that had “chromatin” or “histone” in the TAIR10 functional annotation (defline) of the Populus v3.0 annotation file or in any of the “full name” aliases listed in the TAIR10 gene aliases text file6 Transcription factor genes were identified using the P. trichocarpa and A. thaliana Plant Transcription Factor Databases v3.0 (TFDB; Jin et al., 20147). Because phytohormones are involved in very large signaling networks with substantial cross-talk, we defined phytohormone-associated genes as those having direct roles affecting hormone responses via their influence on hormone metabolism (biosynthesis or inactivation), transport, or signaling. This definition encompassed genes that link hormone receptors to primary downstream transcription factors, but excluded genes that regulate hormone metabolic genes, secondary transcription factors, and other downstream response genes. Genes located within dormancy-related QTL were identified by mapping the gene models shown in Supplementary Table S4 of Rohde et al. (2011b) to the Populus v3.0 gene models using the gene model aliases shown in Supplementary Data File 1. Some gene models noted by Rohde et al. (2011b) were excluded if they no longer mapped to the same general region of the Populus v3.0 genome, and some new v3.0 gene models were added if they were contiguous to the genes previously described by Rohde et al. (2011b). The genes belonging to each of these subsets are identified in Supplementary Data File 1.
Identification of Differentially Expressed Gene Sets using Gene Set Enrichment Analysis (GSEA)
We used gene set enrichment analysis (GSEA) to identify gene sets that were overrepresented among the differentially expressed genes (Step 7, Figure 1). GSEA is a statistical approach for determining whether sets of genes defined a priori (e.g., genes with a common Gene Ontology term) preferentially occur toward the top or bottom of a ranked list of genes (Subramanian et al., 2005). Using the FDR p-values, we ranked all genes according to their changes in expression between (1) paradormancy versus endodormancy and (2) endodormancy vs. ecodormancy. For each dormancy transition, we conducted two analyses. First, we analyzed the data considering whether the genes were up-regulated or down-regulated. That is, we subtracted the FDR p-value from 1, and then multiplied the result by -1 if the gene was down-regulated. GSEA was then used to identify gene sets that were preferentially located near the top or bottom of this list. Second, we analyzed the data ignoring the direction of change (i.e., no -1 multiplier was used), and then used GSEA to identify genes that were preferentially located near the top of this list. This second analysis was used to identify gene sets whose members contribute to the same biological response via opposite changes in gene expression.
We implemented GSEA using the java application GSEA v2.0.13 (Broad Institute, Cambridge, MA, USA8) and 1000 bootstrap replications. We used default parameters, except for setting the minimum and maximum gene set sizes to 5 and 500. Gene sets were considered statistically significant at an FDR p-value of 0.10. We analyzed three datasets: two Gene Ontology (GO) datasets (biological process and molecular function GO categories9; Gene Ontology Consortium, 2012) and one Pathway Studio (PS) dataset (Pathway Studio v8, Elsevier). For the GO gene sets, Populus genes were assigned using the GO terms or Arabidopsis gene assignments downloaded from the Phytozome web site (Supplementary Data File 1; Phytozome v9.010). For the Pathway Studio dataset, we combined seven types of pathways. The combined dataset included sets of genes that encode (1) expression targets, (2) miRNA targets, (3) protein modification targets, (4) proteins regulating disease, (5) proteins regulating cell processes, and (6) binding partners and (7) neighbors of key proteins and biological processes.
Results
Assignment of Bud Dormancy States
We collected axillary buds on five dates between August and March, and then assigned these samples to three dormancy states or treatments based on cluster analysis of gene expression data. Of a total of 44,441 gene models represented on the microarray, 1,206 gene models (36 v1.1 and 1170 v3.0 transcripts) were differentially expressed among months (FDR p-value < 0.05; Supplementary Data File 1). Clustering of these differentially expressed genes produced three well-supported groups consisting of samples collected in (1) August, shortly after terminal bud set, (2) November and December, and (3) February and March (Figure 2). We classified the first group (August samples) as paradormant (Para), the November and December samples as endodormant (Endo) and the February and March samples as ecodormant (Eco). Morphological observations of bud development (Supplementary Figure S2), and past dormancy research on P. trichocarpa and other cottonwoods (see Discussion) support the assignment of these dormancy states.
FIGURE 2.
UPGMA cluster analysis was used to group the 10 samples into three dormancy states: paradormant (Para), endodormant (Endo), and ecodormant (Eco). Clustering was based on the relative expression of 1,206 genes that were differentially expressed among five collection dates in August (samples A1 and A2), November (N1 and N2), December (D1 and D2), February (F1 and F2), and March (M1 and M2). Green indicates high relative gene expression and blue indicates low relative gene expression. Bootstrap values (1000 replicates) were 100% for all branch points, except those labeled as 77 and 84%.
Major Transcriptome Changes Occur during Endodormancy Induction and Release
Of the 44,441 gene models analyzed, 1,362 genes (v1.1 = 43; v3.0 = 1,319) were differentially expressed among dormancy states (FDR p-value < 0.05; Supplementary Data File 1). Based on analyses of individual dormancy transitions, however, 1,523 genes (v1.1 = 25; v3.0 = 1,498) were differentially expressed between paradormancy and ecodormancy, and the majority of these (n = 1,168) were down-regulated. Only 293 transcripts (v1.1 = 13; v3.0 = 280) were differentially expressed between endodormancy and ecodormancy, and the majority of these (n = 193) were up-regulated. A total of 190 genes (v1.1 = 4; v3.0 = 186) were differentially expressed during both transitions. Of all the v3.0 differentially expressed genes discussed above, only 3.2% were novel—i.e., had no Arabidopsis match (Supplementary Data File 1). We found no evidence that transcripts associated with the mitochondrial, chloroplast, or miRNA gene models were differentially expressed. However, results for the organelle gene models are difficult to interpret because total RNA was reversed transcribed using an oligo(dT) primer.
Gene Set Enrichment Analysis (GSEA)
In addition to single-gene analyses, we used GSEA to help identify differentially expressed genes sets, which are groups of genes that share a common biological function, chromosomal location, or regulation (Subramanian et al., 2005). Compared to single-gene analyses, GSEA has the potential to identify biologically relevant genes, even when the results of single gene analyses are not statistically significant, and may yield insights that are not obvious from reviewing long lists of statistically significant genes. In particular, GSEA can be valuable for identifying important regulatory pathways. Significant gene sets identified using the GO-term, Pathway Studio, and phytohormone analyses are presented in Supplementary Tables S1–S8, and overrepresented ontologies of particular interest are described below.
Differential Expression of Chromatin-associated Genes
Overview
Among the Populus v3.0 genes on the array, 727 were identified as being chromatin-associated, 21 of which were differentially expressed between adjacent dormancy states (Supplementary Data File 1). During the transition from paradormancy to endodormancy, 19 genes were down-regulated and 2 were up-regulated. During the transition from endodormancy to ecodormancy, no genes were down-regulated, and 4 were up-regulated. Four genes were differentially expressed during both dormancy transitions. Changes in expression for the top genes (ranked on FDR p-value) are shown in Figure 3.
FIGURE 3.
Relative expression of differentially expressed chromatin-associated genes. Chromatin-associated genes were identified using the Arabidopsis thaliana chromatin database (ChromDB; Gendler et al., 2008). Genes were classified into four groups: up-regulated or down-regulated from paradormancy to endodormancy (A,B), or up-regulated or down-regulated from endodormancy to ecodormancy (C). Within each group, we ranked genes by FDR p-value, and then displayed the top 15 genes for each group if they had a FDR p-value < 0.05. Only four genes were differentially expressed between endodormancy and ecodormancy, and all of these were up-regulated (C). Gene expression values are the means for each month normalized to a mean of zero and a standard deviation equal to the ANOVA RMSE from the analysis of gene expression differences among months. ChromDB is the ChromDB identifier, Populus gene is the P. trichocarpa gene-locus name, and AGI and TAIR10 symbols are the Arabidopsis gene identifiers and gene symbols from the P. trichocarpa v3.0 annotations (http://www.phytozome.net/).
Chromatin-associated Gene Sets
Three chromatin-associated Pathway Studio gene sets were expressed at lower levels during endodormancy—with significant changes in expression at each of the two dormancy transitions. These gene sets were ‘Neighbors of RDR6’ (RNA-DEPENDENT RNA POLYMERASE 6), ‘Regulators of cytosine methylation,’ and ‘Regulators of maintenance of DNA methylation’ (Supplementary Tables S5 and S6). Sixteen other gene sets were differentially expressed during one of the two dormancy transitions, 13 of which were down-regulated from paradormancy to endodormancy. These Pathway Studio gene sets were ‘Neighbors of SWN’ (SWINGER), ‘Expression targets of RDR6,’ ‘Neighbors of DCL1’ (DICER-LIKE 1), ‘Neighbors of CMT3’ (CHROMOMETHYLASE3), ‘Regulators of DNA methylation,’ ‘Neighbors of HDA6’ (HISTONE DEACETYLASE A6), ‘Neighbors of histone,’ ‘Binding partners of FIE’ (FERTILIZATION INDEPENDENT ENDOSPERM), ‘Expression targets of DET1’ (DEETIOLATED 1), ‘Regulators of chromatin remodeling,’ ‘Neighbors of PRMT11’ (PROTEIN ARGININE METHYLTRANSFERASE 11), ‘Neighbors of AGO7’ (ARGONAUT7), ‘Neighbors of FIE,’ ‘Neighbors of HD1’ (HISTONE DEACETYLASE 1), ‘Neighbors of polycomb complex,’ and the GO molecular function gene set, ‘Histone-lysine n-methyltransferase activity.’ Finally, five additional Pathway Studio gene sets were differentially expressed, from paradormancy to endodormancy, but with no common pattern of expression among the gene set members (‘Up- or down-regulated’ gene sets in Supplementary Tables S5 and S6). These gene sets were ‘Binding partners of DDB1A’ (DNA DAMAGE-BINDING PROTEIN 1A), ‘Neighbors of DCL2’ (DICER-LIKE 2), ‘Regulators of histone methylation,’ ‘Binding partners of JAZ10’ (JASMONATE ZIM-DOMAIN PROTEIN 10), and ‘Neighbors of PKL’ (PICKLE).
Chromatin-associated Genes
Of the genes shown in Figure 3, four genes were down-regulated from paradormancy to endodormancy and then up-regulated from endodormancy to ecodormancy. One of these genes (Potri.004G087500; HMGA3) is similar to a gene that encodes a HIGH MOBILITY GROUP A (HMGA) protein in Arabidopsis. The second gene (Potri.008G155400; RHEL1) is similar to Arabidopsis SILENCING DEFECTIVE 3 (SDE3). The third gene (Potri.014G189400; DNG5) is similar to genes that encode DNA glycosylases involved in gene silencing and chromatin remodeling. The last gene (Potri.T029800; ABHF4) is similar to a gene that encodes an alpha/beta-hydrolase in Arabidopsis. Two genes had atypical patterns of expression—being strongly up-regulated from paradormancy to endodormancy, and then down-regulated from endodormancy to ecodormancy. The first gene (Potri.004G162500; GTA2), which is listed as encoding a GLOBAL TRANSCRIPTION FACTOR GROUP A2 (GTA2) protein in Figure 3, is similar to Arabidopsis SPT5-2, an ortholog of yeast SUPPRESSOR OF TY 5 (Durr et al., 2014). The second gene (Potri.001G034300; GTB1), which is listed as encoding a GLOBAL TRANSCRIPTION FACTOR GROUP B1 (GTB1) protein, is similar to the SPT6-like (SPT6L) gene from Arabidopsis (Li et al., 2010; Gu et al., 2012). Other distinct chromatin-associated genes were also differentially expressed (see ChromDB classifications PATPA1, EPB1, FLT4, NFA5, and CHR42 in Figure 3). These genes encode proteins that may be involved in histone ubiquitination and methylation, chromatin assembly or disassembly, histone and DNA binding, and chromatin remodeling.
Differential Expression of Transcription Factor Genes
Overview
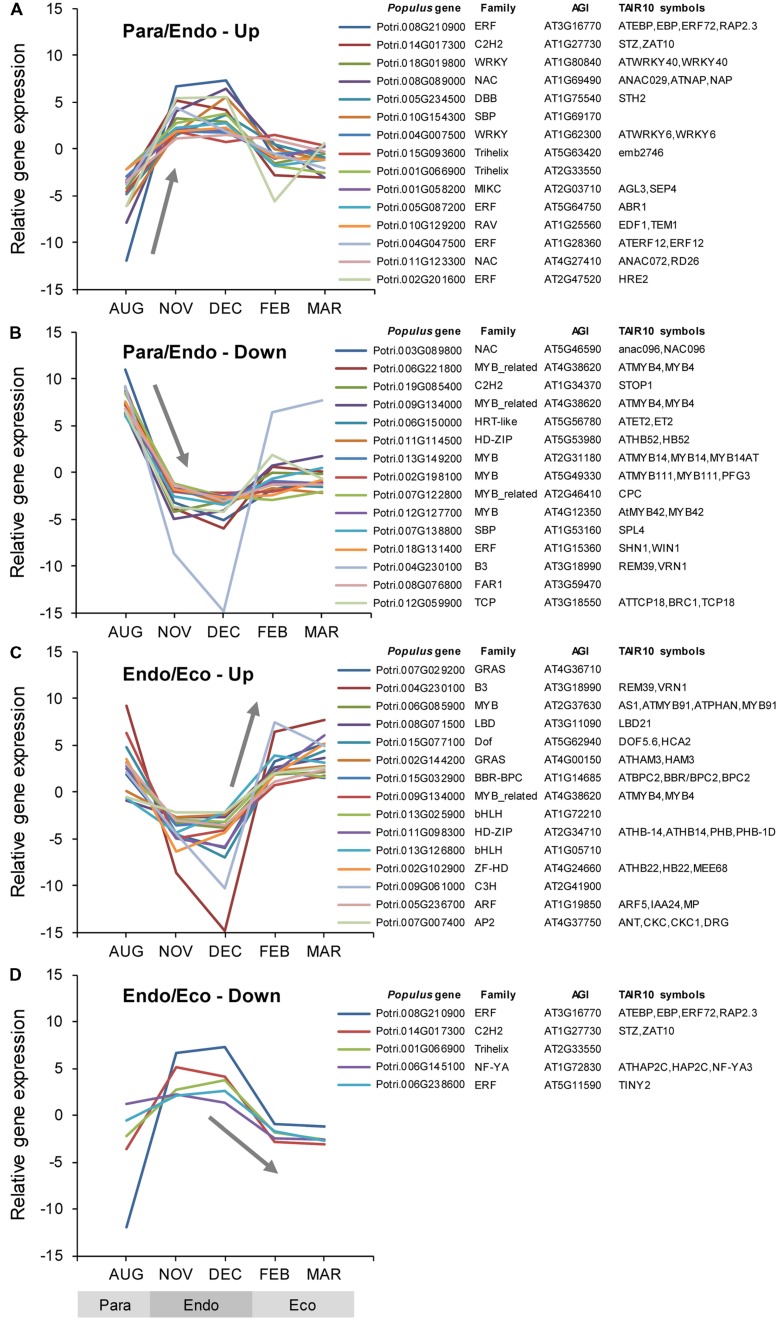
Among the Populus v3.0 genes on the array, 2,469 were identified as transcription factors, 117 of which were differentially expressed between adjacent dormancy states (Supplementary Data File 1). During the transition from paradormancy to endodormancy, 89 genes were down-regulated and 19 were up-regulated. During the transition from endodormancy to ecodormancy, 5 genes were down-regulated and 19 were up-regulated. Fifteen genes were differentially expressed during both dormancy transitions. Changes in expression for the top genes (ranked on FDR p-value) are shown in Figure 4.
FIGURE 4.
Relative expression of differentially expressed transcription factor genes. Transcription factor genes were identified using the P. trichocarpa and A. thaliana Plant Transcription Factor Databases v3.0 (TFDB; Jin et al., 2014; http://planttfdb.cbi.pku.edu.cn/). Genes were classified into four groups: up-regulated or down-regulated from paradormancy to endodormancy (A,B), or up-regulated or down-regulated from endodormancy to ecodormancy (C,D). Within each group, we ranked genes by FDR p-value, and then displayed the top 15 genes for each group if they had a FDR p-value < 0.05. Gene expression values are the means for each month normalized to a mean of zero and a standard deviation equal to the ANOVA RMSE from the analysis of gene expression differences among months. Family is the TFDB family designation for the corresponding Arabidopsis gene, Populus gene is the P. trichocarpa gene-locus name, and AGI and TAIR10 symbols are the Arabidopsis gene identifiers and gene symbols from the P. trichocarpa v3.0 annotations (http://www.phytozome.net/).
Transcription Factor Gene Sets
Nine transcription factor gene sets were differentially expressed during both dormancy transitions. Four were expressed at higher levels during endodormancy: ‘Neighbors of EIN3’ (ETHYLENE INSENSITIVE 3), ‘Expression targets of EIN3,’ ‘Neighbors of RHL41’ (RESPONSIVE TO HIGH LIGHT 41), and ‘Expression targets of WRKY” (Supplementary Tables S5 and S6). The other five gene sets were expressed at lower levels during endodormancy: ‘Neighbors of JLO’ (JAGGED LATERAL ORGAN), ‘Neighbors of SEU’ (SEUSS), ‘Neighbors of RPL’ (REPLUMLESS), ‘Neighbors of ARF2’ (AUXIN RESPONSE FACTOR 2), and ‘Neighbors of BASIC-HELIX-LOOP-HELIX PROTEIN.’
Transcription Factor Genes
Of the genes shown in Figure 4, five were differentially expressed during both dormancy transitions. Two genes were down-regulated from paradormancy to endodormancy and then up-regulated from endodormancy to ecodormancy. One of these genes (Potri.009G134000) is similar to a gene that encodes MYB DOMAIN PROTEIN 4 (MYB4) in Arabidopsis. The second gene (Potri.004G230100) is similar to Arabidopsis VERNALIZATION1 (VRN1). Three other genes had atypical patterns of expression—being strongly up-regulated from paradormancy to endodormancy, and then down-regulated from endodormancy to ecodormancy. Potri.008G210900 is similar to a gene that encodes an ETHYLENE-RESPONSIVE ELEMENT BINDING PROTEIN (EBP), Potri.014G017300 is similar to the SALT TOLERANCE ZINC FINGER (STZ) gene, and Potri.001G066900 is similar to an Arabidopsis gene that encodes a trihelix transcription factor.
Differential Expression of Phytohormone-associated Genes
Overview
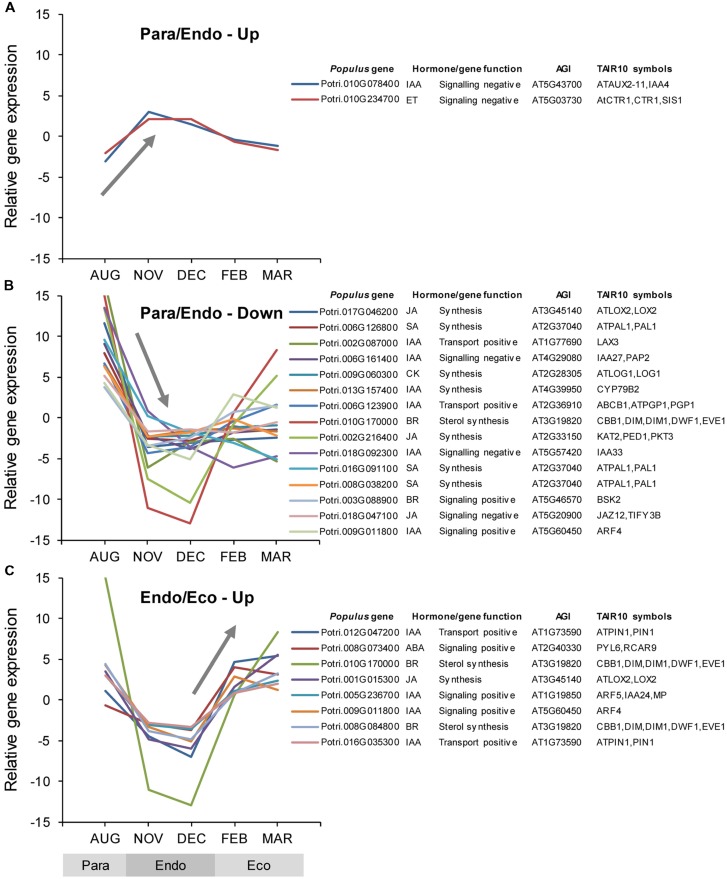
Among the Populus v3.0 genes on the array, 718 genes were identified as being putatively involved in hormone synthesis, catabolism, signaling, or transport. Of these, 41 were differentially expressed between adjacent dormancy states (Supplementary Data File 1). During the transition from paradormancy to endodormancy, 38 genes were down-regulated and only 2 were up-regulated. No genes were down-regulated from endodormancy to ecodormancy, but 8 genes were up-regulated. Seven genes were differentially expressed during both dormancy transitions. Changes in expression for the top genes (ranked on FDR p-value) are shown in Figure 5.
FIGURE 5.
Relative expression of differentially expressed phytohormone-associated genes. Genes were classified into four groups: up-regulated or down-regulated from paradormancy to endodormancy (A,B), or up-regulated or down-regulated from endodormancy to ecodormancy (C). Within each group, we ranked genes by FDR p-value, and then displayed the top 15 genes for each group if they had a FDR p-value < 0.05. All genes differentially expressed between endodormancy and ecodormancy were up-regulated (C). Gene expression values are the means for each month normalized to a mean of zero and a standard deviation equal to the ANOVA RMSE from the analysis of gene expression differences among months. Abbreviations for phytohormones: ABA, abscisic acid; BR, brassinosteroid; CK, cytokinin; ET, ethylene; GA, gibberellin; IAA, indole-3-butyric acid; JA, jasmonic acid, and SA, salicylic acid. Populus gene is the P. trichocarpa gene-locus name and AGI and TAIR10 symbols are the Arabidopsis gene identifiers and gene symbols from the P. trichocarpa v3.0 annotations (http://www.phytozome.net/).
Auxin-associated Gene Expression
The auxin-associated gene set was mostly down-regulated from paradormancy to endodormancy, and then up-regulated thereafter (Supplementary Tables S7 and S8). Changes in Pathway Studio gene sets provide additional support for the importance of auxin associated genes. One auxin-associated gene set (‘Neighbors of ARF6,’ AUXIN RESPONSE FACTOR 6) was up-regulated from paradormancy to endodormancy, but three other key gene sets, ‘Binding partners of ARF1,’ ‘Neighbors of ARF2,’ and ‘Binding partners of TIR1’ (TRANSPORT INHIBITOR RESPONSE 1), were down-regulated (Supplementary Tables S5 and S6). Two gene sets associated with ARF2 were subsequently up-regulated from endodormancy to ecodormancy.
Ethylene-associated Gene Expression
The ethylene gene set was up-regulated from paradormancy to endodormancy, and then down-regulated from endodormancy to ecodormancy (Supplementary Tables S7 and S8). More specifically, one of only two phytohormone genes that were significantly up-regulated from paradormancy to endodormancy is similar to a gene that encodes the CTR1 (CONSTITUTIVE TRIPLE RESPONSE 1) protein, which is a negative regulator of the ethylene response pathway in Arabidopsis. Changes in other genes that participate in ethylene responses were described above (see Differential Expression of Transcription Factor Genes).
GA-associated Gene Expression
Gibberellin-associated genes were generally up-regulated from paradormancy to endodormancy, but did not change from endodormancy to ecodormancy (Supplementary Tables S7 and S8). We then focused attention on genes encoding GA-20 oxidases and GA-2-oxidases because of their potential involvement in endodormancy. We identified genes encoding GA-20-oxidases and GA-2-oxidases based on similarities to Arabidopsis genes and the information presented in Gou et al. (2011), but none was differentially expressed. In fact, no individual GA-related genes were differentially expressed.
ABA-associated Gene Expression
The ABA-associated gene set did not change among dormancy states (Supplementary Tables S7 and S8), but our analyses of individual ABA genes identified four genes that were differentially expressed between one or more dormancy states. One gene (Potri.008G0734000), similar to a gene that encodes a PYL (PYRABACTIN RESISTANCE-LIKE) ABA receptor, was down-regulated slightly from paradormancy to endodormancy, and then significantly up-regulated from endodormancy to ecodormancy (Figure 5). Three other genes were significantly down-regulated from paradormancy to endodormancy, including genes that likely encode proteins involved in ABA transport (Potri.001G175700), ABA biosynthesis (Potri.003G176300), and positive regulation of ABA signaling (Potri.013G112500). The Pathway Studio gene set, ‘Neighbors of ABF2’ (ABA RESPONSIVE ELEMENTS-BINDING FACTOR 2), was significantly down-regulated from paradormancy to endodormancy. In contrast, the gene set, ‘Neighbors of ABF3,’ was down-regulated from endodormancy to ecodormancy.
Brassinosteroid-associated Gene Expression
The brassinosteroid (BR) gene set showed nearly the same expression pattern as the auxin gene set—general down-regulation from paradormancy to endodormancy, and then up-regulation from endodormancy to ecodormancy (but at a FDR p-value of 0.115). In addition, the Pathway Studio gene set, ‘Binding partners of BES1’ (BRI1-EMS SUPRESSOR 1), was down-regulated from paradormancy to endodormancy. Analyses of individual genes identified three BR-related genes (Potri.010G170000, Potri.008G084800, and Potri.003G088900) that showed the same general pattern of gene expression (Figure 5). The first two genes are similar to Arabidopsis CABBAGE1 (CBB1) and the third is similar to BRASSINOSTEROID-SIGNALING KINASE 2 (BSK2).
Salicylic-acid-associated Gene Expression
Among the phytohormone gene sets, the SA-associated gene set had the strongest evidence for differential expression (Supplementary Tables S7 and S8). Four of the seven SA-associated genes that were differentially expressed were similar to Arabidopsis PAL1 (PHENYLALANINE AMMONIA-LYASE 1), and all of these were down-regulated from paradormancy to endodormancy.
Jasmonic-acid-associated Gene Expression
Although the JA-associated gene set was not differentially expressed, the Pathway Studio gene sets, ‘Neighbors of JA,’ ‘Neighbors of MEJA,’ ‘Expression targets of COI1’ (CORONATINE INSENSITIVE 1), and ‘Neighbors of COI1’ were all down-regulated from paradormancy to endodormancy. Furthermore, we saw this same pattern of expression for all six JA-associated genes that were differentially expressed, the top three of which are shown in Figure 5. Five of these six genes are associated with JA synthesis (Supplementary Data File 1) and one is associated with negative JA signaling. This latter gene (Potri.018G047100) is similar to Arabidopsis JASMONATE ZIM-DOMAIN 12 (JAZ12). Another Pathway Studio gene set, ‘Binding partners of JAZ10,’ was differentially regulated, but with no consistent pattern among the gene set members.
Cytokinin-associated Gene Expression
Although the hormone gene set was not differentially expressed, the Pathway Studio gene set, ‘Neighbors of cytokinin,’ and three individual genes (Potri.009G060300, Potri.010G027100, and Potri.016G044100) were all down-regulated from paradormancy to endodormancy.
Differential Expression of Genes Associated with Bud Set QTL
Among the Populus v3.0 genes on the array, 2,181 were identified as being associated with bud set QTL. These genes covered genomic regions ranging from 1.9 Mbp for QTL LG13, to 7.3 Mbp for QTL LG3. A total of 103 genes were differentially expressed using two different criteria. Seventy of these genes were differentially expressed among the three dormancy states (F-test), whereas 94 were differentially expressed between either of the two adjacent dormancy states (Supplementary Data File 1). During the transition from paradormancy to endodormancy, 67 genes were down-regulated and 19 were up-regulated. Six genes were down-regulated from endodormancy to ecodormancy, and 15 genes were up-regulated. Fourteen genes were differentially expressed during both dormancy transitions. Changes in expression for the top genes (ranked on FDR p-value) are shown in Figure 6. Differentially expressed genes were well distributed among the six QTL (described below).
FIGURE 6.
Relative expression of differentially expressed genes located near dormancy-related QTL. Genes were classified into four groups: up-regulated or down-regulated from paradormancy to endodormancy (A,B), or up-regulated or down-regulated from endodormancy to ecodormancy (C,D). Within each group, we ranked genes by FDR p-value, and then displayed the top 15 genes for each group if they had a FDR p-value < 0.05. Gene expression values are the means for each month normalized to a mean of zero and a standard deviation equal to the ANOVA RMSE from the analysis of gene expression differences among months. QTL is the QTL designation from Rohde et al. (2011b), Populus gene is the P. trichocarpa gene-locus name, and AGI and TAIR10 symbols are the Arabidopsis gene identifiers and gene symbols from the P. trichocarpa v3.0 annotations (http://www.phytozome.net/).
Quantitative trait loci LG3 and LG5 each mapped near 16 differentially expressed genes (i.e., genes differentially expressed among the three dormancy states or between adjacent dormancy states), but none had any obvious regulatory role in bud set. In contrast, several potential regulatory genes were found among the 19 differentially expressed genes located near QTL LG6. These include genes that seem to encode a chromatin-associated DCL protein (Potri.006G188800) and a JAZ protein involved in JA signaling (Potri.006G217200; discussed above). Among the 19 differentially expressed genes located near QTL LG8a, are genes that seem to encode proteins involved in chromatin remodeling (Potri.008G073500), positive ABA signaling (Potri.008G073400), BR synthesis (Potri.008G084800), responses to far-red light (Potri.008G076800), organization of lateral organ boundaries (Potri.008G071500), and a NAC-domain transcription factor associated with leaf senescence (Potri.008G089000). QTL LG8b maps near 24 differentially expressed genes, including two chromatin-associated genes (Potri.008G155400 and Potri.008G136100), an Aux/IAA gene (Potri.008G172400), and a gene similar to MITOTIC ARREST-DEFICIENT 2 (Potri.008G179600). Nine differentially expressed genes mapped near QTL LG13, including a gene (Potri.013G011400) that seems to encode a plant homeodomain (PHD) finger family protein that was up-regulated during endodormancy, and a gene (Potri.013G025900) that may encode a bHLH transcription factor that was strongly down-regulated and mapped near the center of the QTL.
Patterns of Gene Expression and Regulatory Motifs
With three dormancy states, eight different patterns of gene expression are possible (Figure 7). We used ANOVA to identify genes that were differentially expressed among dormancy states (FDR p-value < 0.05), and then used contrast statements to assign each gene to a specific pattern of gene expression. The numbers of genes assigned to each expression pattern ranged from 25 to 470 (Figure 7); the gene expression pattern assigned to each gene is available in Supplementary Data File 1.
FIGURE 7.
Expression patterns of 1,362 genes that were differentially expressed among dormancy states. Genes were classified into eight gene expression patterns associated with two dormancy transitions: paradormancy to endodormancy and endodormancy to ecodormancy. For each transition, gene expression was either up-regulated (U), the same (S), or down-regulated (D). Solid green lines represent the mean normalized expression for each gene, and the solid black lines represent the mean expression of all genes shown in the panel (i.e., gene expression group).
We subsequently tested whether genes sharing a common pattern of gene expression have common upstream regulatory motifs. For each gene expression group, we analyzed 2 kb of upstream sequence, identifying 315 unique, overrepresented sequence motifs (Supplementary Data File 2). We found only a few unique motifs for four expression patterns (SU and US = 2; SD and UU = 3). Larger numbers of motifs were found for two expression patterns (DD = 16 and DU = 64). The largest numbers of motifs were found for patterns UD (n = 103) and DS (n = 122). The putative functions of the top 50 overrepresented motifs are shown in Table 1.
Table 1.
Top 50 upstream sequence motifs overrepresented in eight gene expression pattern-groups.
| Motif no. | Pattern | Sequence motif | Seq p-value | Motif p-value | Place sites and other motifs | Proposed functions |
|---|---|---|---|---|---|---|
| 208 | UD | AAATATCT | 5.82E-09 | 1.63E-14 | GATABOX, ROOTMOTIFTAPOX1, EVENINGELEMENTLIKE∗ | Cold, light, and circadian responses |
| 214 | UD | GCCGAC | 1.51E-08 | 4.92E-08 | LTRECOREATCOR15, DRECRTCOREAT, CBFHV | Cold, dehydration responses |
| 143 | DU | AAAAATCA | 2.26E-08 | 1.50E-07 | ARR1AT | Cytokinin response |
| 211 | UD | ACGTGTCC | 7.87E-08 | 1.59E-08 | ACGTABREMOTIFA2OSEM, ABRELATERD1, ACGTATERD1, GADOWNAT | ABA, GA, and light responses |
| 221 | UD | ATGTCGG | 2.88E-07 | 8.44E-07 | LTRECOREATCOR15 | Cold, dehydration responses |
| 213 | UD | CCGACA | 3.45E-07 | 1.73E-08 | LTRECOREATCOR15 | Cold, dehydration responses |
| 223 | UD | GCCGACA | 2.08E-06 | 1.13E-06 | LTRECOREATCOR15, DRECRTCOREAT, CBFHV | Cold, dehydration responses |
| 219 | UD | GTCGGCA | 2.20E-06 | 5.39E-07 | LTRECOREATCOR15, DRECRTCOREAT, CBFHV | Cold, dehydration responses |
| 41 | DS | GAAAAATA | 2.26E-06 | 1.91E-06 | GT1CONSENSUS, GT1GMSCAM4 | Stress and light responses |
| 212 | UD | CCGAC | 2.52E-06 | 1.62E-08 | LTRECOREATCOR15 | Cold, dehydration responses |
| 147 | DU | GTTTTTTA | 3.61E-06 | 2.20E-06 | ||
| 61 | DS | TATAATAA | 4.18E-06 | 1.51E-05 | ||
| 229 | UD | ATGTCGGC | 5.88E-06 | 8.31E-06 | LTRECOREATCOR15, DRECRTCOREAT, CBFHV | Cold, dehydration responses |
| 42 | DS | TATAATA | 6.52E-06 | 1.95E-06 | ||
| 224 | UD | AGCCGCC | 6.66E-06 | 2.77E-06 | AGCBOXNPGLB, GCCCORE | Ethylene and other responses |
| 311 | US | GGTGAAC | 1.05E-05 | 1.58E-06 | GTGANTG10 | Pollen expression |
| 55 | DS | AATTATTA | 1.69E-05 | 9.17E-06 | POLASIG3 | Polyadenylation-like motif |
| 210 | UD | AATATCT | 2.13E-05 | 1.12E-09 | GATABOX, ROOTMOTIFTAPOX1, EVENINGELEMENTLIKE∗ | Cold, light, and circadian responses |
| 264 | UD | GCCGCC | 2.24E-05 | 1.03E-04 | GCCCORE | Ethylene and other responses |
| 297 | UD | CCGTC | 2.45E-05 | 1.12E-03 | ||
| 142 | DU | AAAATAAC | 2.52E-05 | 8.32E-08 | TATABOX5 | TATA-box motif |
| 69 | DS | AAGTTTAT | 2.74E-05 | 2.56E-05 | ||
| 44 | DS | AATTATAT | 3.01E-05 | 3.37E-06 | ||
| 161 | DU | AGTAAAAA | 3.13E-05 | 1.97E-05 | CACTFTPPCA1 | Widely distributed cis-acting element |
| 245 | UD | CATGTCGG | 3.70E-05 | 5.14E-05 | LTRECOREATCOR15 | Cold, dehydration responses |
| 118 | DS | GGTAAAA | 3.96E-05 | 3.21E-04 | GT1CONSENSUS | Stress and light responses |
| 53 | DS | GTATTTTA | 4.51E-05 | 7.81E-06 | ||
| 231 | UD | GTCGGCAA | 4.55E-05 | 1.84E-05 | LTRECOREATCOR15, DRECRTCOREAT, CBFHV | Cold, dehydration responses |
| 230 | UD | AAGCCGCC | 4.56E-05 | 1.26E-05 | AGCBOXNPGLB, GCCCORE | Ethylene and other responses |
| 244 | UD | CCGACAC | 5.00E-05 | 5.06E-05 | LTRECOREATCOR15 | Cold, dehydration responses |
| 153 | DU | AATCATGG | 5.62E-05 | 7.96E-06 | ARR1AT | Cytokinin response |
| 261 | UD | CGAGGATA | 5.69E-05 | 9.10E-05 | GATABOX, MYBST1 | Light and MYB responses |
| 63 | DS | CTAGTCGC | 6.79E-05 | 1.95E-05 | ||
| 265 | UD | ACCGT | 7.03E-05 | 1.13E-04 | ||
| 258 | UD | CACGCCA | 7.81E-05 | 7.29E-05 | ||
| 21 | DS | ATATAAT | 8.23E-05 | 9.13E-10 | ||
| 207 | SU | CGTAC | 8.24E-05 | 8.29E-04 | CURECORECR | SBP response |
| 144 | DU | AAATATTT | 8.25E-05 | 1.61E-07 | ROOTMOTIFTAPOX1 | Starch degradation gene expression |
| 64 | DS | AAATAATA | 8.63E-05 | 1.98E-05 | POLASIG3, TATABOX5 | Polyadenylation-like and TATA motifs |
| 12 | DD | AACGAC | 8.76E-05 | 1.96E-04 | Auxin response | |
| 105 | DS | GTTAAAAA | 9.57E-05 | 1.58E-04 | ||
| 263 | UD | GCCGCCC | 9.64E-05 | 9.74E-05 | GCCCORE | Ethylene and other responses |
| 96 | DS | ACCGCACG | 1.03E-04 | 1.33E-04 | ||
| 108 | DS | AAACTTTA | 1.16E-04 | 1.68E-04 | DOFCOREZM, NTBBF1ARROLB, TAAAGSTKST1 | Auxin and Dof responses |
| 243 | UD | AGGACACG | 1.18E-04 | 5.01E-05 | ||
| 155 | DU | TAATAAAA | 1.20E-04 | 1.01E-05 | POLASIG1 | Polyadenylation-like motif |
| 250 | UD | ACGTGTC | 1.23E-04 | 6.25E-05 | ACGTABREMOTIFA2OSEM, ABRELATERD1, ACGTATERD1, GADOWNAT | ABA, GA, and light responses |
| 59 | DS | ATAAAAAT | 1.29E-04 | 1.12E-05 | SEF4MOTIFGM7S | Widely distributed cis-acting element |
| 114 | DS | CCGCACG | 1.30E-04 | 2.33E-04 | ||
| 181 | DU | ACGTGAT | 1.34E-04 | 1.79E-04 | GTGANTG10, ABRELATERD1, ACGTATERD1, RHERPATEXPA7 | ABA, cytokinin, and light responses |
The motif number can be used to find additional motif information in Supplementary Data File 1. ‘Pattern’ is the gene expression pattern, where the first letter indicates the direction of change from paradormancy to endodormancy (U = up-regulated, D = down-regulated, S = same or no change), and the second letter indicates the direction of change from endodormancy to ecodormancy. For example, a pattern of ‘DS’ indicates that the gene was down-regulated from paradormancy to endodormancy, and then did not change from endodormancy to ecodormancy. ‘Seq p-value’ is the random probability of finding the observed number of genes with at least one sequence motif. ‘Motif p-value’ is the random probability of finding the observed mean number of motifs per sequence. PLACE sites are the names of related motifs based on a search of the Database of Plant Cis-acting Regulatory DNA Elements (http://www.dna.affrc.go.jp/PLACE/index.html). Non-PLACE site names are designated with an asterisk.
Discussion
Rationale for the Classification of Dormancy Treatments
We classified the monthly time points into three dormancy treatments based on previous research on P. trichocarpa and other cottonwoods, and by grouping the monthly samples based on patterns of gene expression. The first sample was classified as ‘paradormant’ because black cottonwood shoots were still elongating on August 1. Furthermore, in eastern cottonwood, endodormancy is not evident until 2–3 weeks after SD-induced bud set, and does not peak until about 7 weeks after bud set (Howe et al., 1999). The induction of endodormancy seems to progress in a similar fashion in black cottonwood (Frewen et al., 2000; Chen et al., 2002). The November and December samples were classified as ‘endodormant.’ As noted above, SD-induced endodormancy peaked about 7 weeks after SD-induced bud set in eastern cottonwood, and was readily measurable for the next 4 weeks (Howe et al., 1999). This would place peak endodormancy somewhere between our November 1 and December 1 collection dates. Information on the release of endodormancy is also available from experiments on an F2 population of hybrids between black cottonwood and eastern cottonwood (Chen et al., 2002). Based on these data and other research on balsam poplar (Farmer and Reinholt, 1986), we classified the February and March samples as ‘ecodormant.’
Differential Expression among Dormancy States
During the transition from paradormancy to endodormancy, most of the differentially expressed genes (n = 913; 67%) were down-regulated. In contrast, during the transition from endodormancy to ecodormancy, the two largest groups of genes (n = 513 and 519; 38% each) were those that were either up-regulated or did not change. These patterns are consistent with the lower cell division and metabolic activity that occurs during endodormancy. Below, we focus on genes that had opposite changes in expression between the two dormancy transitions (n = 604; 44%). That is, genes that were clearly expressed at either higher or lower levels during endodormancy compared to the other two dormancy states. Finally, the smaller number of genes whose expression was atypically higher during endodormancy were of particular interest (n = 225; 17%).
Differential Expression of Chromatin-associated Genes
Overview
Large-scale changes in chromatin are associated with plant developmental changes and responses to the environment. These include (1) covalent modifications to histones or DNA and (2) non-covalent remodeling of chromatin, including changes in nucleosome position or stability, and substitution of one histone type for another (Gentry and Hennig, 2014). Several transcriptomic, physiological, and genetic studies have implicated chromatin modifications and remodeling in the regulation of endodormancy (Ruttink et al., 2007; Horvath et al., 2008, 2010; Karlberg et al., 2010). We found additional support for this link, identifying differentially expressed gene sets associated with ‘histone,’ ‘histone methylation,’ ‘chromatin remodeling,’ ‘DNA methylation,’ ‘cytosine methylation,’ and ‘maintenance of DNA methylation.’ The expression of other gene sets and individual genes are discussed in more detail below.
RNA-directed DNA Methylation (RdDM)
We observed many changes in genes and gene sets associated with transcriptional gene silencing (TGS) via RNA-directed DNA methylation (RdDM). RdDM is a gene silencing process that is regulated by the methylation and demethylation of DNA at target loci. In general, RNA-DEPENDENT RNA POLYMERASE (RDR) copies single-stranded transcripts into double-stranded RNAs (dsRNAs) that are then processed by DICER-like (DCL) proteins into short interfering RNAs (siRNAs). These siRNAs subsequently associate with ARGONAUT (AGO) proteins to form RNA-induced silencing complexes (RISCs) that mediate DNA methylation and TGS (Matzke and Mosher, 2014). In Arabidopsis, the ‘canonical’ RdDM pathway involves RDR2, DCL3, and AGO4 (Matzke and Mosher, 2014). Gene silencing is complicated because post-transcriptional gene silencing (PTGS) involves similar machinery, and the two processes (TGS and PTGS) interact (Matzke and Mosher, 2014). The genes typically associated with PTGS are RDR6, DCL2, DCL4, and AGO1 (Matzke and Mosher, 2014). After TGS is established, DNA methylation and gene silencing can be maintained by CMT3 (Matzke and Mosher, 2014). Differentially expressed genes and gene sets included genes that seem to encode RDR (RDR1, RDR6), DCL (DCL1, DCL2), ARGONAUT (AGO4, AGO7), and CHROMOMETHYLASE 3 (CMT3). Because of the complexity and similarities of the TGS and PTGS pathways, we did not attempt to link particular Populus genes and gene sets to each of these two gene silencing pathways (i.e., TGS versus PTGS). Endodormancy related changes in RdDM pathways have been observed in other perennial species. For example, a gene similar to Arabidopsis AGO4 and a gene that may be functionally similar to DCL4 were also down-regulated during endodormancy in leafy spurge (Horvath et al., 2008).
Histone Modifications
An assortment of gene sets involved in histone modifications were differentially expressed, including neighbors of ‘histone’ and ‘polycomb complex.’ In Arabidopsis, the Polycomb Repressive Complex 2 (PRC2) participates in stable gene silencing. PRC2 methylates histone H3, resulting in the repression of gene expression (Kim et al., 2012). For example, PRC2 controls flowering via histone methylation of FT chromatin (Jiang et al., 2008) and represses FLOWERING LOCUS C (FLC) during vernalization (De Lucia et al., 2008). Other differentially expressed gene sets included neighbors of ‘SWN,’ ‘FIE,’ and ‘PKL.’ In Arabidopsis, SWN and FIE encode core components of PRC2 (Deng et al., 2013), whereas PKL encodes a DNA-binding helicase which seems to associate with PRC2 target loci to enhance histone modification (Zhang et al., 2012). Interestingly, aspen genes similar to FIE and PKL were up-regulated during SD-induced bud set (Ruttink et al., 2007). Although genes that presumably encode ‘neighbors’ of FIE and PKL were differentially expressed in our study, the genes themselves were not. That is, we did not see differential expression of two PKL-like genes (Potri.006G262200 and Potri.018G021100) and one FIE-like gene (Potri.001G417300) in our study.
‘Binding partners of DDB1A’ was another differentially expressed gene set that seems to be associated with gene silencing via PRC2. In Arabidopsis, DDB1A is a component of the CULLIN 4 (CUL4)/DDB1 ubiquitin ligase complex that functions in a wide array of plant processes, including flowering, photomorphogenesis, and parental imprinting (Hou et al., 2014). The CUL4/DDB1 complex seems to interact with histone tails to repress the transcription of genes involved in photomorphogenesis (Benvenuto et al., 2002), and an association between CUL4/DDB1A and PRC2 seems to regulate flowering time in Arabidopsis (Dumbliauskas et al., 2011; Pazhouhandeh et al., 2011). The differential expression of a ‘DET1’ gene set provides a specific link to endodormancy. The DET1 protein interacts with CONSTITUTIVE PHOTOMORPHOGENIC 10 (COP10) and the CUL4/DDB1 complex to regulate responses to light and temperature (Delker et al., 2014).
Gene activation and silencing also involve histone acetylation and deacetylation. In general, histone acetylation is associated with gene activation, whereas deacetylation is associated with gene silencing. Two differentially expressed gene sets were associated with histone deacetylases—HDA6 and HDA19 (also known as HD1). HDA6 is a histone deacetylase that has been identified as a component of the Arabidopsis RdDM machinery (To et al., 2011a). In particular, deacetylation of histone H3 seems to be important for the subsequent methylation of histone H3 described above (To et al., 2011a). In Arabidopsis, HDA6 is involved in the regulation of flowering, senescence, leaf development, the circadian clock, and responses to salt stress, ABA, and JA (Wu et al., 2008b; Chen et al., 2010; To et al., 2011b; Liu et al., 2014), whereas HDA19 regulates seed maturation and flower development (Liu et al., 2014). Other differentially expressed genes and gene sets are connected to these histone deacetylases. For example, JAZ proteins recruit HDA6 to inhibit JA signaling (Zhu et al., 2011).
One of the strongly differentially expressed genes (Potri.014G189400; DNG5) is a putative homolog of the vertebrate gene MBD4 (METHYL-CPG-BINDING DOMAIN 4; Ramiro-Merina et al., 2013). MBD proteins may recruit histone deacetylases such as HDA6—thereby acting as the ‘bridges’ between DNA methylation and histone deacetylation (Liu et al., 2012).
Finally, two SPT-like genes had atypical patterns of expression—being strongly up-regulated from paradormancy to endodormancy, and then down-regulated from endodormancy to ecodormancy. Arabidopsis SPT5-2 is part of the SPT4/SPT5 transcript elongation factor that seems to link transcription elongation, histone modification, and chromatin remodeling in yeast and Arabidopsis (Hartzog and Fu, 2013; Durr et al., 2014). Furthermore, an Arabidopsis SPT5 homolog (KTF1/RDM3/SPT5-like) has been linked to AGO4-mediated gene silencing (Karlowski et al., 2010; Hartzog and Fu, 2013). A Populus gene similar to Arabidopsis SPT6L was also atypically expressed at higher levels during endodormancy—and Arabidopsis SPT6L seems to interact with AGO proteins to regulate embryo development (Gu et al., 2012). Thus, it is curious that a gene similar to AGO4 (Potri.001G219700) was down-regulated during endodormancy in our study and in other plants (Horvath et al., 2008).
Other Chromatin-associated Genes
Three other genes were clearly expressed at lower levels during endodormancy. The first gene, Potri.004G087500, is similar to Arabidopsis HMGA. HMGA proteins interact with A/T-rich DNA, altering the chromatin structure and transcription of their target genes (Reeves, 2010). The second gene, Potri.008G155400, is similar to Arabidopsis SILENCING DEFECTIVE 3 (SDE3), which is more clearly associated with PTGS. The third gene, Potri.T029800, is similar to an Arabidopsis gene (ALPHA/BETA HYDROLASE F4; ABHF4) that is associated with the alpha/beta-hydrolase superfamily of proteins with unknown function.
Roles of Chromatin-associated Genes in Endodormancy Induction and Release
Dormancy transitions were accompanied by changes in multiple genes associated with DNA methylation (e.g., via RdDM) and histone modifications (e.g., via PRC2). However, although we expected to see increased expression of TGS components during endodormancy—the opposite was true—most chromatin-associated genes and gene sets were expressed at lower levels during endodormancy. One explanation is that reduced gene silencing may activate genes that positively induce and maintain endodormancy. DAM genes, for example, which seem to participate in the induction and maintenance of endodormancy, show reduced histone methylation (Horvath et al., 2008; Leida et al., 2012). Second, reduced expression of chromatin-associated genes may simply reflect the lower cell division and metabolic activity that occurs during endodormancy. Most of the other non-chromatin-associated genes were also expressed at lower levels during endodormancy. Third, subtle changes in dormancy-specific TGS may have been swamped by other processes. We compared paradormant buds (not actively growing meristems) to endodormant buds, and only subtle differences in the complement of active and silenced genes may exist between these two dormant states. Finally, the transition from paradormancy to endodormancy may involve a transient increase in gene silencing activity—an increase that we missed with our 1–2 month sampling interval.
Differential Expression of Transcription Factor Genes
Ethylene-associated Transcription Factors
We found substantial evidence for the differential expression of genes associated with ethylene responses, including transcription factors. Gene sets associated with EIN3 and EIL1 (EIN3-LIKE 1) were expressed at higher levels during endodormancy. The EIN3/EIL1 transcription factors act downstream of the signaling protein EIN2 to positively regulate the ethylene response pathway, including leaf senescence (Kim et al., 2014). The induction of ethylene responses during endodormancy is also supported by the differential expression of gene sets associated with the ETHYLENE-RESPONSIVE ELEMENT BINDING PROTEIN (EREBP), EIN2 membrane protein, EIN4 ethylene receptor—as well as individual genes that encode ETHYLENE RESPONSE FACTOR (ERF) proteins, which belong to the APETALA 2 (AP2)/EREBP family of transcription factors. Five of the six ERFs described in Figure 4 seemed to be expressed at higher levels during endodormancy. Although the details differ, ERF genes have also been implicated in bud dormancy in hybrid aspen and Japanese apricot (Rohde et al., 2007; Zhong et al., 2013). Kim et al. (2014) proposed that AtNAP and other ‘senescence-associated’ NAC transcription factors act downstream of EIN2, and genes that seem to encode AtNAP and other NAC proteins were differentially expressed (Figure 4). Gene sets associated with EIN2, an ethylene signaling component, and EIN4, an ethylene receptor, were also expressed at higher levels during endodormancy. Changes in ethylene-associated transcription factors and other genes support broader physiological evidence that ethylene has an important functional role in bud dormancy (Ruonala et al., 2006; Rohde et al., 2007). Finally, our results suggest that JA interacts with ethylene to regulate bud dormancy. This is in agreement with indications of JA signaling during dormancy transitions observed in leafy spurge and Japanese apricot (Horvath et al., 2008; Zhong et al., 2013). In Arabidopsis, EIN3/EIL is a ‘key integration node’ that integrates signaling by ET and JA (Zhu et al., 2011), and gene sets associated with CORONATINE INSENSITIVE 1 (COI1) and JASMONATE ZIM-DOMAIN 10 (JAZ10) were differentially expressed. COI1 is a JA receptor and JAZ10 is a transcriptional repressor (Zhu et al., 2011). Furthermore, HDA6, a histone deacetylase involved in gene silencing (discussed above), interacts with JAZ proteins and COI1 to repress EIN3/EIL1-mediated transcription and JA signaling (Zhu et al., 2011).
WRKY DNA-binding Domain Transcription Factors
WRKY transcription factors, which contain the WRKY DNA-binding domain, have been described as ‘major hubs’ in abiotic stress signaling (Tripathi et al., 2014). Several genes associated with WRKY transcription factors were differentially expressed. These include gene sets associated with Arabidopsis WRKY, WRKY33, and WRKY70 (Supplementary Tables S5 and S6), and individual genes similar to Arabidopsis WRKY5, WRKY6, WRKY27, WRKY40, and WRKY33 (Figure 4; Supplementary Data File 1). There seem to be about 100 WRKY genes in P. trichocarpa, many of which are induced by SA, JA, cold, drought, salinity, or wounding (He et al., 2012; Jiang et al., 2014). Thus, the WRKY transcription factors provide one potential link between dormancy-associated gene expression and the phytohormones JA and SA (discussed below).
Cold-responsive Transcription Factors
Another gene set that was strongly associated with endodormancy was ‘Neighbors of RHL41’ (ZINC FINGER PROTEIN 12, ZAT12). ZAT12 is one of the transcription factors induced very quickly after exposure to cold temperatures (Vogel et al., 2005; Park et al., 2015). Other genes encoding ‘first wave’ transcription factors were differentially expressed in our study as well (e.g., WRKY33 and ZAT10/STZ; Figure 4), but others were not, including genes encoding the C-REPEAT BINDING FACTORS (CBFs). Induction of ZAT12 leads to the induction of some cold-responsive (COR) genes, and the repression of others; and overexpression of ZAT12 leads to enhanced freezing tolerance (Vogel et al., 2005). A gene that seems to encode a ZAT10/STZ transcription factor was expressed at higher levels during endodormancy (Figure 4). In Arabidopsis, the expression of ZAT10/STZ is regulated by cold, drought, and salt; and overexpression of ZAT10/STZ enhances drought tolerance (Sakamoto et al., 2004). Although CBF genes are clearly involved in acclimation to cold and drought (Thomashow, 2010), CBF-like genes were not differentially expressed in our study. Nonetheless, CBF binding motifs were significantly overrepresented in the promoters of genes that were up-regulated during endodormancy (discussed below). In a similar study of aspen, none of the four CBF-like genes was up-regulated during 5 weeks of SDs (Karlberg et al., 2010). Although another CBF-like gene was up-regulated for a short time, it was then down-regulated to almost the original level during the endodormant period. In our study, we may have missed transient increases in CBF gene expression because of the 1–2-month sampling interval we used.
Dormancy-associated Transcription Factors
Genes encoding other transcription factors were expected to be differentially expressed. For example, DORMANCY ASSOCIATED MADS-BOX (DAM) genes are putative transcription factors found in perennial plants that have been directly linked to vegetative endodormancy. They are similar to two genes, SHORT VEGETATIVE PHASE (SVP) and AGAMOUS-LIKE 24 (AGL24), that encode transcription factors regulating flowering time in Arabidopsis. In peach (Prunus persica), a deletion of DAM genes resulted in trees that were unable to become endodormant, and DAM expression is enhanced during endodormancy in several perennial species (Horvath et al., 2010; Jiménez et al., 2010). In our study, several DAM-like (SVP-like) genes were differentially expressed (Potri.005G155700, Potri.017G044500, and Potri.002G105600), but they were down-regulated during endodormancy, unlike the DAM genes in leafy spurge and peach (Horvath et al., 2010; Jiménez et al., 2010). A different DAM-like gene (Potri.007G010800) was up-regulated during the induction of endodormancy in hybrid aspen (Ruttink et al., 2007), and strongly down-regulated in early flushing trees that were overexpressing EARLY BUD-BREAK 1 (EBB1; Yordanov et al., 2014). However, this gene was not differentially expressed in our study. Finally, one gene (Potri.001G328400) was highly up-regulated in our December and February samples—but these differences were not significant among months or dormancy states (FDR p-value = 0.07 to 0.11). This gene encodes an unusual truncated transcript, which is reminiscent of the truncated splice variant of the endodormancy-induced DAM transcript in leafy spurge (Horvath et al., 2013). Overall, these disparate results provide limited insight into the connections between endodormancy and DAM-like genes in Populus.
Other Transcription Factors
Other gene sets associated with transcription factors were strongly down-regulated during endodormancy. JLO, SEU, RPL, and ARF2 seem to have various roles in auxin signaling, including organization of the shoot apical meristem and organ development (Franks et al., 2006; Sluis and Hake, 2015). This suggests they could be involved in the formation or development of new leaf primordia. If so, their patterns of expression (i.e., lower expression during endodormancy) are consistent with the cessation of primordia initiation and development that occurs during dormancy induction.
Genes that seem to encode MYB transcription factors were also common among the genes that were down-regulated from paradormancy to endodormancy (Figure 4). Given that the MYB family is very large, and endodormancy is associated with a general reduction in metabolic activity, the significance of these changes is uncertain. Nonetheless, MYB14 represses the CBF regulon in Arabidopsis, (Chen et al., 2013), but we did not see differential expression of the Populus CBF/DREB genes in our study (discussed above).
Finally, genes encoding other flowering-associated transcription factors were also differentially expressed, including SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1). These and other flowering-associated genes are discussed in more detail below.
Differential Expression of Phytohormone-associated Genes
Auxin-associated Gene Expression
Auxin-associated genes were generally down-regulated during endodormancy, with most changes expected to lead to a reduction in auxin signaling. This, and other more specific changes in gene expression, suggests that auxin signaling undergoes important changes during dormancy transitions. The first step in auxin signaling involves an interaction between auxin, an auxin receptor such as TIR1, and AUXIN/INDOLE 3-ACETIC ACID (Aux/IAA) proteins. This interaction ultimately leads to the degradation of the Aux/IAA proteins, which normally repress ARF transcription factors. The reduction in Aux/IAA leads to enhanced transcription of auxin-inducible genes by ARF and downstream auxin responses (Korasick et al., 2014). In our study, genes that encode binding partners of TIR1 were down-regulated from paradormancy to endodormancy, and a gene that was strongly up-regulated (Potri.010G078400) is similar to the Arabidopsis gene that encodes IAA4. This latter change, in particular, is consistent with a reduction in auxin signaling and auxin responses during endodormancy. Furthermore, gene sets associated with three ARF transcription factors were differentially expressed between dormancy states. In Arabidopsis, ARFs are also negatively regulated by miRNAs, including miR160 (Rhoades et al., 2002; Paponov et al., 2009). In our study, the gene set associated with miR160A was significantly down-regulated from paradormancy to endodormancy (Supplementary Table S5). Likewise, genes that encode neighbors and targets of miR393A and miR393B were differentially expressed between paradormancy to endodormancy. This miRNA seems to negatively regulate the gene encoding TIR1 (Liu et al., 2009). Corresponding changes in auxin and auxin-associated gene expression have been found in other species. In silver birch, auxin declined during SD-induced endodormancy (Li et al., 2003), and auxin-associated genes were down-regulated during endodormancy in the cambial meristem of Populus (Baba et al., 2011) and the buds of leafy spurge (Horvath et al., 2008). Our results concur, and because of its atypical pattern of expression, point to a particularly important role for the IAA4-like gene (Potri.010G078400).
Down-regulation of auxin transport also seems to occur during endodormancy. For example, genes similar to two Arabidopsis genes involved in auxin transport were down-regulated from paradormancy to endodormancy. The first gene (Potri.002G087000) is similar to a gene that encodes the auxin influx carrier, LAX3 (LIKE AUX3). The second (Potri.006G123900) is similar to a gene that encodes an ATP-BINDING CASSETTE (ABC) transporter that regulates basipetal auxin transport. Consistent with these changes, two other genes (Potri.012G047200 and Potri.016G035300) were up-regulated from endodormancy to ecodormancy, both of which are similar to the Arabidopsis PIN1 (PIN-FORMED 1), which encodes a putative auxin efflux transporter (Sluis and Hake, 2015).
Finally, changes in the expression of genes associated with the synthesis of phenylpropanoids and flavonoids may affect auxin responses. As discussed below, these genes were mostly down-regulated during endodormancy, which could enhance auxin transport, but also destabilize auxin levels by increasing auxin oxidation (Brown et al., 2001; Buer and Muday, 2004; Peer et al., 2013).
Ethylene-associated Gene Expression
The ethylene-associated gene set was up-regulated during endodormancy, which was opposite from what we observed for auxin and BR. However, this gene set included a mix of genes with positive and negative effects on ethylene signaling. For example, genes that seem to encode negative (CTR1) and positive (ERF5) regulators of ethylene signaling were both up-regulated during endodormancy. Although this seems counterintuitive, genes similar to CRT1 and ERF are induced by exogenous ethylene, demonstrating some degree of coordinate regulation (Vahala et al., 2013; Zou et al., 2014). Other related gene sets were up-regulated during endodormancy, including those associated with the ethylene receptor EIN4 and the transcription factors EIN2 and EIN3. Up-regulation was also observed for multiple genes that seem to encode ERF transcription factors, at least some of which have been specifically associated with responses to ethylene. Ethylene has been implicated in bud endodormancy, perhaps in concert with ABA. Ruttink et al. (2007), for example, concluded that ethylene and ABA act sequentially during SD-induced bud dormancy in Populus. Although they emphasized the transient nature of increases in ethylene-associated gene expression, we observed up-regulation of multiple ethylene-associated genes over a two-month period under natural conditions. Interacting roles for ethylene and ABA during endodormancy induction and release have also been reported in birch and grape, which is consistent with our results (Ruonala et al., 2006; Ophir et al., 2009; Zheng et al., 2015).
GA- and ABA-associated Gene Expression
Perhaps the most consistent associations between bud endodormancy and phytohormones are the opposing changes in GA and ABA—GA levels tend to decline and ABA levels increase early in the induction of endodormancy (reviewed in Olsen, 2010). For example, SD-induced down-regulation of GA-20-oxidase reduces the levels of active GAs in Salix and Populus (reviewed in Olsen, 2010), and up-regulation of GA-2-oxidases may lead to GA inactivation (Zawaski and Busov, 2014). However, we did not observe differential expression of any genes that seem to encode GA-20-oxidase or GA-2-oxidase, and the changes in other genes associated with GA and ABA were contrary to expectations. It is possible that transient changes in GA- and ABA-related gene expression were missed due to our monthly sampling scheme. Most previous analyses focused on the early stages of dormancy induction, often focusing on changes in response to SDs. Our results suggest that GA and ABA have (at most) modest roles in the maintenance of endodormancy per se. For example, Populus trees genetically engineered to underexpress or overexpress the ABA INSENSITIVE 3 (ABI3) transcription factor still became endodormant under SDs (Ruttink et al., 2007), and other studies suggest that ABA is primarily involved in the formation of the dormant bud, not in the maintenance of endodormancy (reviewed in Olsen, 2010).
Brassinosteroid-associated Gene Expression
Brassinosteroid has non-redundant roles as a “potent” growth-promoting phytohormone (Schröder et al., 2014). Although clear involvement in bud endodormancy has not been previously established, changes in the expression BR-related genes point in this direction. First, the BR hormone-associated gene set, ‘Binding partners of BES1,’ was down-regulated during endodormancy. BES1 is a BR-regulated transcription factor, and low BES1 signaling helps ensure stem cell quiescence in plants (Vilarrasa-Blasi et al., 2014). Second, three genes expected to enhance BR signaling were all down-regulated during endodormancy (i.e., compared to paradormancy or ecodormancy). Two of these genes are similar to an Arabidopsis gene (CBB1) that encodes a sterol reductase involved in the early steps of BR synthesis (Choe et al., 1999). The third gene is similar to BRASSINOSTEROID SIGNALING KINASE 2 (BSK2), which belongs to a family of genes that encode positive regulators of BR signaling (Sreeramulu et al., 2013). Thus, it appears that down-regulation of BR biosynthetic and signaling genes may help maintain the reduced cell division associated with endodormancy. Because of crosstalk between BR and SA signaling (Divi et al., 2010), there may be a link between the changes in BR-associated genes described above, and the changes in SA associated genes described below.
Salicylic-acid-associated Gene Expression
During the transition from paradormancy to endodormancy, we found clear evidence for changes in the expression SA-associated genes. Genes with the strongest patterns of differential expression include three genes similar to Arabidopsis PAL1, which encodes a key enzyme in SA biosynthesis. All three genes were down-regulated from paradormancy to endodormancy. Although connections between SA and bud endodormancy have not been widely reported, SA promoted endodormancy release in grape (i.e., as measured by H2O2 production; Or et al., 2000). Nonetheless, changes in SA-associated genes may be more closely related to general changes in the phenylpropanoid pathway, rather than endodormancy per se—such as changes in gene sets associated with anthocyanin and flavonoid biosynthesis (Nugroho et al., 2002). Although genes associated with proanthocyanin production have been associated with seed dormancy (Debeaujon et al., 2000), corresponding gene sets were mostly down-regulated in our study, including gene sets associated with GLABRA 3, ENHANCER OF GLABRA 3, TRANSPARENT TESTA 2 (TT2), TT8, TRANSPARENT TESTA GLABRA 1 (TTG1), and CAPRICE (Supplementary Tables S5 and S6). Although SA may contribute to endodormancy, the changes in phenylpropanoid and SA gene expression may have no direct role in endodormancy.
Jasmonic-acid-associated Gene Expression
We found consistent evidence for down-regulation of many JA-associated genes and gene sets from paradormancy to endodormancy, including genes involved in JA synthesis and signaling. Because enhanced JA signaling is associated with responses to stress and decreased growth, many of these changes contradict the expected roles that JA might have during endodormancy. Given that these changes coincide with broader reductions in gene expression during endodormancy, their relevance is uncertain. Nonetheless, because JAZ proteins repress JA signaling, the down-regulation of a gene similar to JAZ12 (Potri.018G047100) could lead to increased JA-repressed growth during endodormancy. Finally, because JA regulates anthocyanin accumulation through COI, down-regulation of JA signaling could contribute to the reduction in in phenylpropanoid and SA-associated gene expression described above. Additional efforts are needed to identify the mechanisms underlying the seemingly contradictory responses in JA signaling and synthesis.
Cytokinin-associated Gene Expression
We found limited evidence to suggest that changes in cytokinin (CK) gene expression are important during endodormancy induction and release. Although one gene set, ‘Neighbors of cytokinin,’ and three individual genes were all down-regulated from paradormancy to endodormancy, these changes mirror the broader reductions in gene expression that occurred during endodormancy. Therefore, their relevance is uncertain. On balance, however, these changes are consistent with the hypothesis that cytokinin acts as an antagonist of auxin-mediated apical dominance by promoting the outgrowth of paradormant buds (Mueller and Leyser, 2011).
Flowering Genes and Processes are Associated with Vegetative Bud Dormancy
We previously presented a general model for endodormancy involving FT-based regulatory networks analogous to the networks that regulate flowering (reviewed by Horvath, 2009). Consistent with this model, our current results suggest an important role for genes similar to SPL in Populus. In Populus, FT2 inhibits growth cessation and bud set. Thus, down-regulation of FT2 seems to be an important early step in the induction of endodormancy (Bohlenius et al., 2006; Ruonala et al., 2008; Hsu et al., 2011). In Arabidopsis, photoperiodic regulation of FT involves a network of miR156, SPL proteins, miR172, and a set of AP2-like transcription factors, including AP2, TOE1-3 (TARGET OF EARLY ACTIVATION TAGGED 1-3), SCHLAFMÜTZE (SMZ), and SCHNARCHZAPFEN (SNZ; Aukerman and Sakai, 2003; Schmid et al., 2003; Jung et al., 2007). A model has emerged in which SPLs positively regulate miR172, which normally represses AP2, TOEs, SMZ, and SNZ (Wellmer and Riechmann, 2010). Thus, up-regulation of SPLs represses these AP2-like transcription factors, leading to an increase in FT expression and the promotion of flowering. In our study, eight SPL-like genes were down-regulated from paradormancy to endodormancy. By analogy to the flowering pathway described above, down-regulation of SPL genes should lead to down-regulation of miR172, up-regulation of genes similar to AP2, TOE, SMZ, and SNZ, repression of FT2, and ultimately, growth cessation, bud set, and endodormancy. However, despite the consistent down-regulation of SPL-like genes in our study, two other observations contradict this simple model. First, three DAM-like (SVP-like) genes were unexpectedly down-regulated in our study, which is opposite of what has been seen in other perennial plants (discussed above). Furthermore, because SVP negatively regulates miR172 in Arabidopsis (Cho et al., 2012), down-regulation of DAM/SVP genes in Populus is ultimately expected to increase, rather than decrease, the expression of FT2. However, SVP is not uniformly up-regulated during flower development. In Arabidopsis, SVP is repressed in early flower development to prevent flower reversion, and in late flower development to allow the activation of SEPALATA3 (Lee and Lee, 2010). Therefore, our results may indicate that the timing of DAM/SVP expression is important in Populus as well. Second, FT2 itself was not differentially expressed. However, this was not surprising because FT2 expression decreases dramatically after only a few SD (Resman et al., 2010). Therefore, the longer-term changes in SPL gene expression that we observed may help keep the expression of FT2 and other flowering-related genes at already low levels, rather than being the direct, early cause of FT2 down-regulation.
Like FT, SOC1 (also known as AGL20) is considered a major integrator of flowering signals in Arabidopsis. In our study, two SOC1-like genes and the gene set “Binding partners of AGL20” were down-regulated from paradormancy to endodormancy, and similar results were observed in other studies of Populus (Ruttink et al., 2007) and leafy spurge (Horvath et al., 2008; data not shown). SOC1 expression generally promotes flowering in Arabidopsis. More specifically, studies in Arabidopsis (Tao et al., 2012) show that SOC1 directly down-regulates AP2, TOE1, and SMZ, which are downstream targets of SPLs and miR172 (described above). Finally, allelic variation in a SOC1 homolog has been linked to dormancy in apricot (Trainin et al., 2013). Given the functional and regulatory similarities between DAM and FLC, a repressor of SOC1, a similar mechanism involving SOC1-like genes might regulate vegetative bud endodormancy.
Despite the potential connections between endodormancy and flowering-like genes, the genes described above have been implicated in diverse processes, including endodormancy induction (e.g., photoperiodism), endodormancy release (e.g., chilling), cold acclimation, flowering, and fruit development (Preston and Sandve, 2013). For example, in Arabidopsis, there seems to be a feedback loop between cold responses and flowering-time that involves interactions between CBF, SOC1, and FLC (Seo et al., 2009). Therefore, it will be challenging to dissect the functional significance of dormancy-associated changes in expression, particularly for genes that serve as key regulatory hubs.
Differential Expression of Genes Associated with Bud Set QTL
Rohde et al. (2011b) identified six robust QTL associated with various components of bud set, and then compared the QTL locations to the locations of differentially expressed genes (Ruttink et al., 2007). We conducted a similar analysis using a larger number of genes and updated gene models (Populus v3.0). Although hundreds of genes were associated with each QTL, the number of differentially expressed genes ranged from 9 to 24. We then examined relationships between the QTL and the various classes of genes described above (chromatin-associated, transcription factor, and hormone-associated) to identify promising QTL candidates.
We found 14 differentially expressed genes that are reasonable QTL candidates based on their differential expression and putative functions. These include four genes that seem to be involved in chromatin remodeling (e.g., DCL4). Other differentially expressed genes seem to be involved in BR synthesis (CBB1) or phytohormone signaling via ABA (PYL), JA (JAZ12), or auxin (IAA13). Additional genes are more generally associated with responses to far-red light (FAR-RED IMPAIRED RESPONSIVE 1), organization of lateral organ boundaries (LOB DOMAIN-CONTAINING PROTEIN 21), and mitotic arrest (MAD2). Finally, we also found genes that seem to encode a NAC domain transcription factor associated with leaf senescence, and other PHD and bHLH transcription factors that are not as well characterized. In addition to these candidates, a few other differentially expressed genes with unknown functions should be considered.
Overall, these results suggest that differential expression can be used to reduce a large number of positional candidate genes (>2,000) to a much smaller set of plausible QTL candidate genes. Nonetheless, it would still be challenging to investigate all of these candidates in detail using functional genomics. For example, the genes underlying these QTL may not be differentially expressed, or the changes in expression may be transient. Furthermore, transgenic functional approaches often cause major, poorly timed perturbations that may lead to responses that do not accurately reflect natural gene functions. A combination of approaches including fine-scale mapping, association genetics, analyses of gene expression, and subtle gene perturbations will be needed to understand the roles of the numerous genes that appear to regulate dormancy transitions.
Upstream Sequence Motifs are Associated with Specific Patterns of Gene Expression
Motifs associated with photoperiodic responses and circadian patterns of gene expression were highly enriched in some gene expression pattern groups. For example, two of the top-ranked motifs match binding sites for two central transcription factors that regulate the circadian clock, LATE ELONGATED HYPOCOTYL (LHY) and CIRCADIAN CLOCK ASSOCIATED 1 (CCA1). The first motif matches the EVENING ELEMENT-LIKE (EEL) motif (AATATCT). The EEL and the EVENING ELEMENT itself (EE, AAAATATCT) are important regulators of circadian clock and cold-responsive genes (Mikkelsen and Thomashow, 2009). The EEL motif was our top-ranked motif, being significantly overrepresented in genes that were up-regulated during endodormancy. As summarized by Hsu and Harmer (2014), most clock components regulate the transcription of genes that contain EE, or are regulated by other clock components through EE in their own promoters. The second motif matches a binding site (AAAAATCA) that is found in the target genes of CCA1 (Maxwell et al., 2003), being enriched in genes that were down-regulated during endodormancy.
Other motifs previously associated with circadian patterns of gene expression were also found, including G-box (CACGTG), I-box core (GATAA), PIF-binding E-box (PBE-box; CACATG), and the CAB2 DET1-ASSOCIATED FACTOR 1 binding site motif (CDA-1; CAAAA). Both the G-box and PBE-box elements are binding sites for PIF3, a transcription factor that interacts with phytochromes A and B (Zhang et al., 2013). Furthermore, the HORMONE UP AT DAWN (HUD) element, which has the same sequence as the PBE-box, is overrepresented in the promoters of phytohormone genes (Michael et al., 2008). We also found many motifs that matched the circadian elements described in Table 1 of Smieszek et al. (2014), only some of which were identified using the PLACE database. Photoperiodic and circadian regulation of endodormancy has been well documented (Howe et al., 1996; Rohde and Bhalerao, 2007; Horvath et al., 2010), and was also highlighted by our GSEA. Gene sets associated with two components of the circadian clock, ZEITLUPE (ZTL) and LIGHT-REGULATED WD 2 (LWD2), were up-regulated during endodormancy. ZTL is a blue light photoreceptor that regulates photoperiodic responses (Kim et al., 2007), and LWD2 encodes a WD (tryptophan and aspartate) protein that contributes to clock function (Wu et al., 2008a).
A second class of overrepresented motifs was broadly associated with responses to cold, dehydration, and ABA. The most prominent of these is the RCCGAC motif, which is the core of the C-repeat (CRT) element, also known as the dehydration responsive element (DRE). The CRT/DRE element is the binding site for CBFs (C-REPEAT BINDING FACTORS), some of the most important transcription factors involved in cold-induced gene expression (Benedict et al., 2006). CBFs regulate gene expression in response to cold and dehydration. Other transcription factors, such as the ABA RESPONSIVE ELEMENT BINDING PROTEIN (AREB), regulate gene expression in response to cold and dehydration in an ABA-dependent manner. These transcription factors bind to ABA responsive elements (ABRE; ACGT core), ABRE-like elements (ABREL; AGCTG), and G-box elements (Mikkelsen and Thomashow, 2009). We found many enriched motifs that contain these core sequences.
We found a number of dehydrin-like genes that were up-regulated from paradormancy to endodormancy, and these genes provide good models for how different combinations of cis-regulatory elements can lead to different patterns of gene expression. For example, Zolotarov and Stromvik (2015) identified 14 conserved motifs in 350 dehydrin promoters from 51 plant genomes, many of which were similar to motifs that were overrepresented in our differentially expressed genes, as well as in genes that have been associated with responses to cold, dehydration, ABA, and light.
We also found motifs associated with other phytohormones, including ethylene, auxin, SA, and JA. Among the top-ranked motifs, we found four with the GCC-box core motif (GCCGCC), which serves as a binding site for ethylene-responsive genes (Ohme-Takagi and Shinshi, 1995). These motifs were highly enriched in genes that were up-regulated from paradormancy to endodormancy. This coincides with our GSEA results indicating that ethylene is an important regulator of endodormancy. We also found five motifs that matched two auxin responsive elements (NTBBF1ARROLB and TGA-element; Table 1), and all five motifs were enriched in genes that were down-regulated from paradormancy to endodormancy. Again, this supports our GSEA results indicating that auxin related genes were mostly down-regulated from paradormancy to endodormancy. We found a large number of motifs that are associated with responses to cytokinin, but their significance is unclear; we saw no strong trends in cytokinin-related gene expression. The longest matching PLACE motif was CPBCSPOR (TATTAG), which exhibits cytokinin-enhanced protein binding, but the other two PLACE motifs, ARR1AT (NGATT) and RHERPATEXPA7 (KCACGW), are much less specific. We also found a small number of motifs that have been non-specifically associated with JA and SA.
In sum, our analyses of promoter motifs showed clear associations between patterns of endodormancy-related gene expression and two broad classes of genes—those associated the circadian clock and photoperiodic responses, and those associated with phytohormone-mediated responses to cold and dehydration. An understanding of the finer details of gene regulation are complicated by the fact that many of the consensus motifs are short and widely distributed among plant promoters involved in responses to light, biotic and abiotic stresses, and phytohormones. Furthermore, about 45% of the enriched motifs had no assigned functions, suggesting that more work is needed to understand the functions of these motifs and their potential roles in the regulation endodormancy-associated processes. Further insights could be gained by analyses that focus on understanding how the numbers, distributions, and combinations of motifs are associated with genes known to have specific patterns of gene expression across Populus species.
Conclusion
Our work highlights both the conserved nature and the extraordinary complexity of transcriptome changes associated with vegetative dormancy. For example, we confirmed and elaborated upon earlier evidence from studies of chromatin remodeling. We found multiple genes associated with DNA methylation (e.g., via RdDM) and histone modifications (e.g., via PRC2) that were differentially expressed during the induction and release of endodormancy. We identified 19 chromatin-associated genes that were down-regulated during endodormancy, and two genes that were strongly and atypically up-regulated. These latter two genes are similar to Arabidopsis SPT5-2 and SPT6L, which encode proteins described as ‘global’ transcription factors. We also identified links to genes that regulate the onset of flowering, pointing to potentially important roles for genes similar to SPL, DAM/SVP, and SOC1. Differential expression of SPL genes corroborates earlier observations and implicates miRNA-associated regulatory pathways in the repression of FT2 during endodormancy.
A number of surprises emerged from our analyses of phytohormone-related genes. Changes in genes encoding GA-20-oxidase and GA-2-oxidase were not observed, and changes in genes associated with ABA were contrary to expectations. Although we may have missed transient changes associated with short-day-induced bud set, these results suggest that these phytohormones have relatively narrow windows of action. In contrast, we saw clearer evidence for changes in the expression of genes associated with ethylene, auxin, BR, SA, and JA. For example, genes and gene sets that were atypically up-regulated during endodormancy included those associated with responses to ethylene (EIN3, EBP, ERFs), and a gene similar to Arabidopsis IAA4. However, genes associated with auxin, BR, SA, and JA were mostly down-regulated during endodormancy. Because of the general down-regulation of metabolic activity and gene expression during endodormancy, the biological significance of these changes warrants further study.
Other genes that were atypically up-regulated during endodormancy included those encoding transcription factors associated with responses to cold and other abiotic stresses (ZAT10/STZ, ZAT12/RHL41, WRKY), and a gene that seems to encode a trihelix transcription factor. The down-regulation of other transcription factor genes was consistent with changes known to accompany endodormancy. These include genes with various roles in auxin signaling, organization of the shoot apical meristem, and organ development.
We identified many novel and previously identified promoter motifs that appear to regulate these dormancy-associated changes in gene expression. The most common motifs were those associated with the circadian clock and others associated with responses to photoperiod, cold, dehydration, and ABA. Among the most common motifs were the EVENING ELEMENT-LIKE motif, a binding site found in genes targeted by CCA1, CBF-binding sites, and various ABA responsive elements.
Finally, we found many differentially expressed genes that were located near bud set QTL, some of which are clear candidates for having functional roles in the induction of endodormancy. These latter genes are potential targets for basic research and for manipulating dormancy-associated processes using molecular breeding and transgenic approaches. Additional gene expression, fine-scale mapping, functional, and population genetic studies should help elucidate the roles of the many genes and biological processes we identified.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Scott Kolpak and Kori Ault for help with tables and figures.
Footnotes
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00989
Average monthly temperature and precipitation in Corvallis, OR, USA during the sample collection period.
Bud morphology and histology during the sample collection period.
References
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215 403–410. 10.1016/s0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- Arora R., Rowland L. J., Tanino K. (2003). Induction and release of bud dormancy in woody perennials: a science comes of age. HortScience 38 911–921. [Google Scholar]
- Aukerman M. J., Sakai H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15 2730–2741. 10.1105/Tpc.016238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeez A., Miskolczi P., Tylewicz S., Bhalerao R. P. (2014). A tree ortholog of APETALA1 mediates photoperiodic control of seasonal growth. Curr. Biol. 24 717–724. 10.1016/j.cub.2014.02.037 [DOI] [PubMed] [Google Scholar]
- Baba K., Karlberg A., Schmidt J., Schrader J., Hvidsten T. R., Bako L., et al. (2011). Activity-dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proc. Natl. Acad. Sci. U.S.A. 108 3418–3423. 10.1073/pnas.1011506108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C., Skinner J. S., Meng R., Chang Y. J., Bhalerao R., Huner N. P. A., et al. (2006). The CBF1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell Environ. 29 1259–1272. 10.1111/j.1365-3040.2006.01505.x [DOI] [PubMed] [Google Scholar]
- Benvenuto G., Formiggini F., Laflamme P., Malakhov M., Bowler C. (2002). The photomorphogenesis regulator DET1 binds the amino-terminal tail of histone H2B in a nucleosome context. Curr. Biol. 12 1529–1534. 10.1016/S0960-9822(02)01105-3 [DOI] [PubMed] [Google Scholar]
- Bielenberg D. G., Wang Y., Li Z. G., Zhebentyayeva T., Fan S. H., Reighard G. L., et al. (2008). Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genet. Genomes 4 495–507. 10.1007/s11295-007-0126-9 [DOI] [Google Scholar]
- Bohlenius H., Huang T., Charbonnel-Campaa L., Brunner A. M., Jansson S., Strauss S. H., et al. (2006). CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312 1040–1043. 10.1126/science.1126038 [DOI] [PubMed] [Google Scholar]
- Brown D. E., Rashotte A. M., Murphy A. S., Normanly J., Tague B. W., Peer W. A., et al. (2001). Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 126 524–535. 10.1104/Pp.126.2.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer C. S., Muday G. K. (2004). The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16 1191–1205. 10.1105/Tpc.020313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Gleichsner A., Alsbury R., Horvath D., Suttle J. (2010). The sprout inhibitors chlorpropham and 1,4-dimethylnaphthalene elicit different transcriptional profiles and do not suppress growth through a prolongation of the dormant state. Plant Mol. Biol. 73 181–189. 10.1007/s11103-010-9607-6 [DOI] [PubMed] [Google Scholar]
- Chen L. T., Luo M., Wang Y. Y., Wu K. (2010). Involvement of Arabidopsis histone deacetylase HDA6 in ABA and salt stress response. J. Exp. Bot. 61 3345–3353. 10.1093/jxb/erq154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. H. H., Howe G. T., Bradshaw H. D., Jr. (2002). Molecular genetic analysis of dormancy-related traits in poplars. Weed Sci. 50 232–240. 10.2307/4046369 [DOI] [Google Scholar]
- Chen Y., Chen Z. L., Kang J. Q., Kang D. M., Gu H. Y., Qin G. J. (2013). AtMYB14 regulates cold tolerance in Arabidopsis. Plant Mol. Biol. Rep. 31 87–97. 10.1007/s11105-012-0481-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. J., Kim J. J., Lee J. H., Kim W., Jung J. H., Park C. M., et al. (2012). SHORT VEGETATIVE PHASE (SVP) protein negatively regulates miR172 transcription via direct binding to the pri-miR172a promoter in Arabidopsis. FEBS Lett. 586 2332–2337. 10.1016/j.febslet.2012.05.035 [DOI] [PubMed] [Google Scholar]
- Choe S., Dilkes B. P., Gregory B. D., Ross A. S., Yuan H., Noguchi T., et al. (1999). The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 119 897–907. 10.1104/pp.119.3.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I., Leon-Kloosterziel K. M., Koornneef M. (2000). Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 122 403–413. 10.1104/Pp.122.2.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delker C., Sonntag L., James G. V., Janitza P., Ibanez C., Ziermann H., et al. (2014). The DET1-COP1-HY5 pathway constitutes a multipurpose signaling module regulating plant photomorphogenesis and thermomorphogenesis. Cell Rep. 9 1983–1989. 10.1016/j.celrep.2014.11.043 [DOI] [PubMed] [Google Scholar]
- De Lucia F., Crevillen P., Jones A. M. E., Greb T., Dean C. (2008). A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc. Natl. Acad. Sci. U.S.A. 105 16831–16836. 10.1073/pnas.0808687105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. W., Buzas D. M., Ying H., Robertson M., Taylor J., Peacock W. J., et al. (2013). Arabidopsis polycomb repressive complex 2 binding sites contain putative GAGA factor binding motifs within coding regions of genes. BMC Genomics 14:593 10.1186/1471-2164-14-593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divi U. K., Rahman T., Krishna P. (2010). Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 10:151 10.1186/1471-2229-10-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doğramaci M., Horvath D., Chao W., Foley M., Christoffers M., Anderson J. (2010). Low temperatures impact dormancy status, flowering competence, and transcript profiles in crown buds of leafy spurge. Plant Mol. Biol. 73 207–226. 10.1007/s11103-010-9621-8 [DOI] [PubMed] [Google Scholar]
- Dumbliauskas E., Lechner E., Jaciubek M., Berr A., Pazhouhandeh M., Alioua M., et al. (2011). The Arabidopsis CUL4-DDB1 complex interacts with MSI1 and is required to maintain MEDEA parental imprinting. EMBO J. 30 731–743. 10.1038/emboj.2010.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr J., Lolas I. B., Sorensen B. B., Schubert V., Houben A., Melzer M., et al. (2014). The transcript elongation factor SPT4/SPT5 is involved in auxin-related gene expression in Arabidopsis. Nucleic Acids Res. 42 4332–4347. 10.1093/Nar/Gku096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer R. E., Reinholt R. W. (1986). Genetic-variation in dormancy relations of balsam poplar along a latitudinal transect in Northwestern Ontario. Silvae Genet. 35 38–42. [Google Scholar]
- Franks R. G., Liu Z. C., Fischer R. L. (2006). SEUSS and LEUNIG regulate cell proliferation, vascular development and organ polarity in Arabidopsis petals. Planta 224 801–811. 10.1007/s00425-006-0264-6 [DOI] [PubMed] [Google Scholar]
- Frewen B. E., Chen T. H. H., Howe G. T., Davis J., Rohde A., Boerjan W., et al. (2000). Quantitative trait loci and candidate gene mapping of bud set and bud flush in Populus. Genetics 154 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler K., Paulsen T., Napoli C. (2008). ChromDB: the chromatin database. Nucleic Acids Res. 36 D298–D302. 10.1093/nar/gkm768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene Ontology Consortium (2012). The Gene Ontology: enhancements for 2011. Nucleic Acids Res. 40 D559–D564. 10.1093/nar/gkr1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry M., Hennig L. (2014). Remodelling chromatin to shape development of plants. Exp. Cell Res. 321 40–46. 10.1016/j.yexcr.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Gou J. Q., Ma C., Kadmiel M., Gai Y., Strauss S., Jiang X. N., et al. (2011). Tissue-specific expression of Populus C-19 GA 2-oxidases differentially regulate above– and below-ground biomass growth through control of bioactive GA concentrations. New Phytol. 192 626–639. 10.1111/j.1469-8137.2011.03837.x [DOI] [PubMed] [Google Scholar]
- Gu X. L., Wang H., Huang H., Cui X. F. (2012). SPT6L encoding a putative WG/GW-repeat protein regulates apical-basal polarity of embryo in Arabidopsis. Mol. Plant 5 249–259. 10.1093/mp/ssr073 [DOI] [PubMed] [Google Scholar]
- Halaly T., Pang X. Q., Batikoff T., Crane O., Keren A., Venkateswari J., et al. (2008). Similar mechanisms might be triggered by alternative external stimuli that induce dormancy release in grape buds. Planta 228 79–88. 10.1007/s00425-008-0720-6 [DOI] [PubMed] [Google Scholar]
- Hartzog G. A., Fu J. H. (2013). The Spt4-Spt5 complex: a multi-faceted regulator of transcription elongation. Biochim. Biophys. Acta 1829 105–115. 10.1016/j.bbagrm.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H. S., Dong Q., Shao Y. H., Jiang H. Y., Zhu S. W., Cheng B. J., et al. (2012). Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 31 1199–1217. 10.1007/s00299-012-1241-0 [DOI] [PubMed] [Google Scholar]
- Heide O. M. (2008). Interaction of photoperiod and temperature in the control of growth and dormancy of Prunus species. Sci. Hortic. 115 309–314. 10.1016/j.scienta.2007.10.005 [DOI] [Google Scholar]
- Higo K., Ugawa Y., Iwamoto M., Korenaga T. (1999). Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 27 297–300. 10.1093/nar/27.1.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath D. (2009). Common mechanisms regulate flowering and dormancy. Plant Sci. 177 523–531. 10.1016/j.plantsci.2009.09.002 [DOI] [Google Scholar]
- Horvath D. P., Anderson J. V., Chao W. S., Foley M. E. (2003). Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci. 8 534–540. 10.1016/j.tplants.2003.09.013 [DOI] [PubMed] [Google Scholar]
- Horvath D. P., Chao W. S., Suttle J. C., Thimmapuram J., Anderson J. V. (2008). Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). BMC Genomics 9:536 10.1186/1471-2164-9-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath D. P., Kudrna D., Talag J., Anderson J. V., Chao W. S., Wing R., et al. (2013). BAC library development and clone characterization for dormancy-responsive DREB4A, DAM, and FT from leafy spurge (Euphorbia esula) identifies differential splicing and conserved promoter motifs. Weed Sci. 61 303–309. 10.1614/Ws-D-12-00175.1 [DOI] [Google Scholar]
- Horvath D. P., Sung S., Kim D., Chao W., Anderson J. (2010). Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant Mol. Biol. 73 169–179. 10.1007/s11103-009-9596-5 [DOI] [PubMed] [Google Scholar]
- Hou P., Ren P. F., Zeng D. E., Yu G. R., Tang W., Zheng X., et al. (2014). Differential expression patterns and a novel interaction factor of Damaged DNA Binding Protein 1A (DDB1A) and DDB1B in Arabidopsis thaliana. J. Plant Biol. 57 239–244. 10.1007/s12374-014-0221-z [DOI] [Google Scholar]
- Howe G. T., Aitken S. N., Neale D. B., Jermstad K. D., Wheeler N. C., Chen T. H. H. (2003). From genotype to phenotype: unraveling the complexities of cold adaptation in forest trees. Can. J. Bot. 81 1247–1266. 10.1139/B03-141 [DOI] [Google Scholar]
- Howe G. T., Davis J., Jeknic Z., Chen T. H. H., Frewen B., Bradshaw H. D., et al. (1999). Physiological and genetic approaches to studying endodormancy-related traits in Populus. HortScience 34 1174–1184. [Google Scholar]
- Howe G. T., Gardner G., Hackett W. P., Furnier G. R. (1996). Phytochrome control of short-day-induced bud set in black cottonwood. Physiol. Plant. 97 95–103. 10.1034/j.1399-3054.1996.970115.x [DOI] [Google Scholar]
- Hsu C. Y., Adams J. P., Kim H. J., No K., Ma C. P., Strauss S. H., et al. (2011). FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc. Natl. Acad. Sci. U.S.A. 108 10756–10761. 10.1073/pnas.1104713108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. Y., Harmer S. L. (2014). Wheels within wheels: the plant circadian system. Trends Plant Sci. 19 240–249. 10.1016/j.tplants.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C., Kozarewa I., Johansson M., Ogren E., Rohde A., Eriksson M. E. (2010). Circadian clock components regulate entry and affect exit of seasonal dormancy as well as winter hardiness in Populus trees. Plant Physiol. 153 1823–1833. 10.1104/pp.110.158220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry R. A., Hobbs B., Collin F., Beazer-Barclay Y. D., Antonellis K. J., Scherf U., et al. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4 249–264. 10.1093/biostatistics/4.2.249 [DOI] [PubMed] [Google Scholar]
- Jensen W. A. (1962). Botanical Histochemistry: Principles and Practice. San Francisco, CA: W. H. Freeman. [Google Scholar]
- Jermstad K. D., Bassoni D. L., Jech K. S., Ritchie G. A., Wheeler N. C., Neale D. B. (2003). Mapping of quantitative trait loci controlling adaptive traits in coastal Douglas fir. III. Quantitative trait loci-by-environment interactions. Genetics 165 1489–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Wang Y., Wang Y., He Y. (2008). Repression of FLOWERING LOCUS C and FLOWERING LOCUS T by the Arabidopsis polycomb repressive complex 2 components. PLoS ONE 3:e3404 10.1371/journal.pone.0003404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. Z., Duan Y. J., Yin J., Ye S. L., Zhu J. R., Zhang F. Q., et al. (2014). Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. J. Exp. Bot. 65 6629–6644. 10.1093/Jxb/Eru381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez S., Li Z. G., Reighard G. L., Bielenberg D. G. (2010). Identification of genes associated with growth cessation and bud dormancy entrance using a dormancy-incapable tree mutant. BMC Plant Biol. 10:25 10.1186/1471-2229-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Zhang H., Kong L., Gao G., Luo J. (2014). PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 42 D1182–D1187. 10.1093/nar/gkt1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J. H., Seo Y. H., Seo P. J., Reyes J. L., Yun J., Chua N. H., et al. (2007). The GIGANTEA-regulated MicroRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 19 2736–2748. 10.1105/tpc.107.054528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlberg A., Englund M., Petterle A., Molnar G., Sjodin A., Bako L., et al. (2010). Analysis of global changes in gene expression during activity-dormancy cycle in hybrid aspen apex. Plant Biotechnol. 27 1–16. 10.5511/plantbiotechnology.27.1 [DOI] [Google Scholar]
- Karlowski W. M., Zielezinski A., Carrere J., Pontier D., Lagrange T., Cooke R. (2010). Genome-wide computational identification of WG/GW Argonaute-binding proteins in Arabidopsis. Nucleic Acids Res. 38 4231–4245. 10.1093/Nar/Gkq162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik N., Fear D., Richards S. C. M., McDermott C. R., Nuwaysir E. F., Kellam P., et al. (2005). Gene expression in peripheral blood mononuclear cells from patients with chronic fatigue syndrome. J. Clin. Pathol. 58 826–832. 10.1136/jcp.2005.025718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Hong S. H., Kim Y. W., Lee I. H., Jun J. H., Phee B. K., et al. (2014). Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. J. Exp. Bot. 65 4023–4036. 10.1093/Jxb/Eru112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Lee J., Eshed-Williams L., Zilberman D., Sung Z. R. (2012). EMF1 and PRC2 cooperate to repress key regulators of Arabidopsis development. PLoS Genet. 8:e1002512 10.1371/journal.pgen.1002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. Y., Fujiwara S., Suh S. S., Kim J., Kim Y., Han L. Q., et al. (2007). ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449 356–360. 10.1038/Nature06132 [DOI] [PubMed] [Google Scholar]
- Korasick D. A., Westfall C. S., Lee S. G., Nanao M. H., Dumas R., Hagen G., et al. (2014). Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc. Natl. Acad. Sci. U.S.A. 111 5427–5432. 10.1073/pnas.1400074111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarewa I., Ibanez C., Johansson M., Ogren E., Mozley D., Nylander E., et al. (2010). Alteration of PHYA expression change circadian rhythms and timing of bud set in Populus. Plant Mol. Biol. 73 143–156. 10.1007/s11103-010-9619-2 [DOI] [PubMed] [Google Scholar]
- Lang G. A., Early J. D., Martin G. C., Darnell R. L. (1987). Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. HortScience 22 371–377. [Google Scholar]
- Lee J., Lee I. (2010). Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 61 2247–2254. 10.1093/jxb/erq098 [DOI] [PubMed] [Google Scholar]
- Leida C., Conesa A., Llácer G., Badenes M. L., Ríos G. (2012). Histone modifications and expression of DAM6 gene in peach are modulated during bud dormancy release in a cultivar-dependent manner. New Phytol. 193 67–80. 10.1111/j.1469-8137.2011.03863.x [DOI] [PubMed] [Google Scholar]
- Li C., Junttila O., Ernstsen A., Heino P., Palva E. T. (2003). Photoperiodic control of growth, cold acclimation and dormancy development in silver birch (Betula pendula) ecotypes. Physiol. Plant. 117 206–212. 10.1034/j.1399-3054.2003.00002.x [DOI] [Google Scholar]
- Li L., Ye H. X., Guo H. Q., Yin Y. H. (2010). Arabidopsis IWS1 interacts with transcription factor BES1 and is involved in plant steroid hormone brassinosteroid regulated gene expression. Proc. Natl. Acad. Sci. U.S.A. 107 3918–3923. 10.1073/pnas.0909198107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Life Technologies (2011). MessageAmp II aRNA Amplification Kit, Part Number 1751M. Carlsbad, CA: Life Technologies Corporation, 38. [Google Scholar]
- Liu D. M., Song Y., Chen Z. X., Yu D. Q. (2009). Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol. Plant. 136 223–236. 10.1111/j.1399-3054.2009.01229.x [DOI] [PubMed] [Google Scholar]
- Liu X., Yang S., Zhao M., Luo M., Yu C. W., Chen C. Y., et al. (2014). Transcriptional repression by histone deacetylases in plants. Mol. Plant 7 764–772. 10.1093/mp/ssu033 [DOI] [PubMed] [Google Scholar]
- Liu X., Yu C. W., Duan J., Luo M., Wang K., Tian G., et al. (2012). HDA6 directly interacts with DNA methyltransferase MET1 and maintains transposable element silencing in Arabidopsis. Plant Physiol. 158 119–129. 10.1104/pp.111.184275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiason K., He D., Grimplet J., Venkateswari J., Galbraith D., Or E., et al. (2009). Transcript profiling in Vitis riparia during chilling requirement fulfillment reveals coordination of gene expression patterns with optimized bud break. Funct. Integr. Genomics 9 81–96. 10.1007/s10142-008-0090-y [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Mosher R. A. (2014). RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat. Rev. Genet. 15 394–408. 10.1038/Nrg3683 [DOI] [PubMed] [Google Scholar]
- Maxwell B. B., Andersson C. R., Poole D. S., Kay S. A., Chory J. (2003). HY5, Circadian Clock-Associated 1, and a cis-element, DET1 dark response element, mediate DET1 regulation of Chlorophyll a/b-Binding Protein 2 expression. Plant Physiol. 133 1565–1577. 10.1104/pp.103.025114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzitelli L., Hancock R. D., Haupt S., Walker P. G., Pont S. D. A., McNicol J., et al. (2007). Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J. Exp. Bot. 58 1035–1045. 10.1093/Jxb/Erl266 [DOI] [PubMed] [Google Scholar]
- Michael T. P., Breton G., Hazen S. P., Priest H., Mockler T. C., Kay S. A., et al. (2008). A morning-specific phytohormone gene expression program underlying rhythmic plant growth. PLoS Biol. 6:e225 10.1371/journal.pbio.0060225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M. D., Thomashow M. F. (2009). A role for circadian evening elements in cold-regulated gene expression in Arabidopsis. Plant J. 60 328–339. 10.1111/j.1365-313X.2009.03957.x [DOI] [PubMed] [Google Scholar]
- Mockler T. C., Michael T. P., Priest H. D., Shen R., Sullivan C. M., Givan S. A., et al. (2007). The DIURNAL project: DIURNAL and circadian expression profiling, model-based pattern matching, and promoter analysis. Cold Spring Harb. Symp. Quant. Biol. 72 353–363. 10.1101/sqb.2007.72.006 [DOI] [PubMed] [Google Scholar]
- Mølmann J. A., Asante D. K. A., Jensen J. B., Krane M. N., Ernstsen A., Junttila O., et al. (2005). Low night temperature and inhibition of gibberellin biosynthesis override phytochrome action and induce bud set and cold acclimation, but not dormancy in PHYA overexpressors and wild-type of hybrid aspen. Plant Cell Environ. 28 1579–1588. 10.1111/j.1365-3040.2005.01395.x [DOI] [Google Scholar]
- Mueller D., Leyser O. (2011). Auxin, cytokinin and the control of shoot branching. Ann. Bot. 107 1203–1212. 10.1093/Aob/Mcr069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller O., Lightfoot S., Schröder A. (2004). RNA Integrity Number (RIN) Standardization of RNA quality control. Application Note [Online]. Available at: http://www.chem.agilent.com/Library/applications/5989-1165EN.pdf [Google Scholar]
- Nugroho L. H., Verberne M. C., Verpoorte R. (2002). Activities of enzymes involved in the phenylpropanoid pathway in constitutively salicylic acid-producing tobacco plants. Plant Physiol. Biochem. 40 755–760. 10.1016/S0981-9428(02)01437-7 [DOI] [Google Scholar]
- Ohme-Takagi M., Shinshi H. (1995). Ethylene-inducible DNA-binding proteins that interact with an ethylene-responsive element. Plant Cell 7 173–182. 10.2307/3869993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. E. (2010). Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant Mol. Biol. 73 37–47. 10.1007/s11103-010-9620-9 [DOI] [PubMed] [Google Scholar]
- Olsen J. E., Junttila O., Nilsen J., Eriksson M. E., Martinussen I., Olsson O., et al. (1997). Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J. 12 1339–1350. 10.1046/j.1365-313x.1997.12061339.x [DOI] [Google Scholar]
- Ophir R., Pang X. Q., Halaly T., Venkateswari J., Lavee S., Galbraith D., et al. (2009). Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene-ABA interplay and cell enlargement. Plant Mol. Biol. 71 403–423. 10.1007/s11103-009-9531-9 [DOI] [PubMed] [Google Scholar]
- Or E., Vilozny I., Eyal Y., Ogrodovitch A. (2000). The transduction of the signal for grape bud dormancy breaking induced by hydrogen cyanamide may involve the SNF-like protein kinase GDBRPK. Plant Mol. Biol. 43 483–494. 10.1023/A:1006450516982 [DOI] [PubMed] [Google Scholar]
- Paponov I. A., Teale W., Lang D., Paponov M., Reski R., Rensing S. A., et al. (2009). The evolution of nuclear auxin signalling. BMC Evol. Biol. 9:126 10.1186/1471-2148-9-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lee C. M., Doherty C. J., Gilmour S. J., Kim Y., Thomashow M. F. (2015). Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 82 193–207. 10.1111/Tpj.12796 [DOI] [PubMed] [Google Scholar]
- Pazhouhandeh M., Molinier J., Berr A., Genschik P. (2011). MSI4/FVE interacts with CUL4-DDB1 and a PRC2-like complex to control epigenetic regulation of flowering time in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 108 3430–3435. 10.1073/pnas.1018242108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer W. A., Cheng Y., Murphy A. S. (2013). Evidence of oxidative attenuation of auxin signalling. J. Exp. Bot. 64 2629–2639. 10.1093/Jxb/Ert152 [DOI] [PubMed] [Google Scholar]
- Pelgas B., Bousquet J., Meirmans P. G., Ritland K., Isabel N. (2011). QTL mapping in white spruce: gene maps and genomic regions underlying adaptive traits across pedigrees, years and environments. BMC Genomics 12:145 10.1186/1471-2164-12-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J. C., Sandve S. R. (2013). Adaptation to seasonality and the winter freeze. Front. Plant Sci. 4:167 10.3389/Fpls.2013.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro-Merina A., Ariza R. R., Roldan-Arjona T. (2013). Molecular characterization of a putative plant homolog of MBD4 DNA glycosylase. DNA Repair 12 890–898. 10.1016/j.dnarep.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Reeves R. (2010). Nuclear functions of the HMG proteins. Biochim. Biophys. Acta 1799 3–14. 10.1016/j.bbagrm.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resman L., Howe G., Jonsen D., Englund M., Druart N., Schrader J., et al. (2010). Components acting downstream of short day perception regulate differential cessation of cambial activity and associated responses in early and late clones of hybrid poplar. Plant Physiol. 154 1294–1303. 10.1104/pp.110.163907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M. W., Reinhart B. J., Lim L. P., Burge C. B., Bartel B., Bartel D. P. (2002). Prediction of plant microRNA targets. Cell 110 513–520. 10.1016/S0092-8674(02)00863-2 [DOI] [PubMed] [Google Scholar]
- Rinne P. L. H., Welling A., Vahala J., Ripel L., Ruonala R., Kangasjärvi J., et al. (2011). Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-β-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell 23 130–146. 10.1105/tpc.110.081307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde A., Bastien C., Boerjan W. (2011a). Temperature signals contribute to the timing of photoperiodic growth cessation and bud set in poplar. Tree Physiol. 31 472–482. 10.1093/treephys/tpr038 [DOI] [PubMed] [Google Scholar]
- Rohde A., Storme V., Jorge V., Gaudet M., Vitacolonna N., Fabbrini F., et al. (2011b). Bud set in poplar – genetic dissection of a complex trait in natural and hybrid populations. New Phytol. 189 106–121. 10.1111/j.1469-8137.2010.03469.x [DOI] [PubMed] [Google Scholar]
- Rohde A., Bhalerao R. P. (2007). Plant dormancy in the perennial context. Trends Plant Sci. 12 217–223. 10.1016/j.tplants.2007.03.012 [DOI] [PubMed] [Google Scholar]
- Rohde A., Ruttink T., Hostyn V., Sterck L., Van Driessche K., Boerjan W. (2007). Gene expression during the induction, maintenance, and release of dormancy in apical buds of poplar. J. Exp. Bot. 58 4047–4060. 10.1093/Jxb/Erm261 [DOI] [PubMed] [Google Scholar]
- Ruonala R., Rinne P. L. H., Baghour M., Moritz T., Tuominen H., Kangasjarvi J. (2006). Transitions in the functioning of the shoot apical meristem in birch (Betula pendula) involve ethylene. Plant J. 46 628–640. 10.1111/j.1365-313X.2006.02722.x [DOI] [PubMed] [Google Scholar]
- Ruonala R., Rinne P. L. H., Kangasjarvi J., van der Schoot C. (2008). CENL1 expression in the rib meristem affects stem elongation and the transition to dormancy in Populus. Plant Cell 20 59–74. 10.1105/tpc.107.056721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttink T., Arend M., Morreel K., Storme V., Rombauts S., Fromm J., et al. (2007). A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 19 2370–2390. 10.1105/tpc.107.052811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Maruyama K., Sakuma Y., Meshi T., Iwabuchi M., Shinozaki K., et al. (2004). Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 136 2734–2746. 10.1104/pp.104.046599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki R., Yamane H., Ooka T., Jotatsu H., Kitamura Y., Akagi T., et al. (2011). Functional and expressional analyses of PmDAM genes associated with endodormancy in Japanese apricot. Plant Physiol. 157 485–497. 10.1104/pp.111.181982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M., Uhlenhaut N. H., Godard F., Demar M., Bressan R., Weigel D., et al. (2003). Dissection of floral induction pathways using global expression analysis. Development 130 6001–6012. 10.1242/dev.00842 [DOI] [PubMed] [Google Scholar]
- Schröder F., Lisso J., Obata T., Erban A., Maximova E., Giavalisco P., et al. (2014). Consequences of induced brassinosteroid deficiency in Arabidopsis leaves. BMC Plant Biol. 14:309 10.1186/S12870-014-0309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti-Saintagne C., Bodenes C., Barreneche T., Bertocchi E., Plomion C., Kremer A. (2004). Detection of quantitative trait loci controlling bud burst and height growth in Quercus robur L. Theor. Appl. Genet. 109 1648–1659. 10.1007/s00122-004-1789-3 [DOI] [PubMed] [Google Scholar]
- Seo E., Lee H., Jeon J., Park H., Kim J., Noh Y. S., et al. (2009). Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator FLC. Plant Cell 21 3185–3197. 10.1105/tpc.108.063883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluis A., Hake S. (2015). Organogenesis in plants: initiation and elaboration of leaves. Trends Genet. 31 300–306. 10.1016/j.tig.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Smieszek S. P., Yang H. X., Paccanaro A., Devlin P. F. (2014). Progressive promoter element combinations classify conserved orthogonal plant circadian gene expression modules. J. R. Soc. Interface 11 1–11. 10.1098/Rsif.2014.0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreeramulu S., Mostizky Y., Sunitha S., Shani E., Nahum H., Salomon D., et al. (2013). BSKs are partially redundant positive regulators of brassinosteroid signaling in Arabidopsis. Plant J. 74 905–919. 10.1111/Tpj.12175 [DOI] [PubMed] [Google Scholar]
- Sterky F., Bhalerao R. R., Unneberg P., Segerman B., Nilsson P., Brunner A. M., et al. (2004). A Populus EST resource for plant functional genomics. Proc. Natl. Acad. Sci. U.S.A. 101 13951–13956. 10.1073/pnas.0401641101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102 15545–15550. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z., Shen L., Liu C., Liu L., Yan Y., Yu H. (2012). Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis. Plant J. 70 549–561. 10.1111/j.1365-313X.2012.04919.x [DOI] [PubMed] [Google Scholar]
- Thomashow M. F. (2010). Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol. 154 571–577. 10.1104/pp.110.161794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To T. K., Kim J. M., Matsui A., Kurihara Y., Morosawa T., Ishida J., et al. (2011a). Arabidopsis HDA6 regulates locus-directed heterochromatin silencing in cooperation with MET1. PLoS Genet. 7:e1002055 10.1371/journal.pgen.1002055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To T. K., Nakaminami K., Kim J. M., Morosawa T., Ishida J., Tanaka M., et al. (2011b). Arabidopsis HDA6 is required for freezing tolerance. Biochem. Biophys. Res. Commun. 406 414–419. 10.1016/j.bbrc.2011.02.058 [DOI] [PubMed] [Google Scholar]
- Trainin T., Bar-Ya’akov I., Holland D. (2013). ParSOC1, a MADS-box gene closely related to Arabidopsis AGL20/SOC1, is expressed in apricot leaves in a diurnal manner and is linked with chilling requirements for dormancy break. Tree Genet. Genomes 9 753–766. 10.1007/s11295-012-0590-8 [DOI] [Google Scholar]
- Tripathi P., Rabara R. C., Rushton P. J. (2014). A systems biology perspective on the role of WRKY transcription factors in drought responses in plants. Planta 239 255–266. 10.1007/s00425-013-1985-y [DOI] [PubMed] [Google Scholar]
- Tuskan G. A., DiFazio S., Jansson S., Bohlmann J., Grigoriev I., Hellsten U., et al. (2006). The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313 1596–1604. 10.1126/science.1128691 [DOI] [PubMed] [Google Scholar]
- Vahala J., Felten J., Love J., Gorzsas A., Gerber L., Lamminmaki A., et al. (2013). A genome-wide screen for ethylene-induced ethylene response factors (ERFs) in hybrid aspen stem identifies ERF genes that modify stem growth and wood properties. New Phytol. 200 511–522. 10.1111/Nph.12386 [DOI] [PubMed] [Google Scholar]
- Van Gelder R. N., von Zastrow M. E., Yool A., Dement W. C., Barchas J. D., Eberwine J. H. (1990). Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc. Natl. Acad. Sci. U.S.A. 87 1663–1667. 10.1073/pnas.87.5.1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarrasa-Blasi J., Gonzalez-Garcia M. P., Frigola D., Fabregas N., Alexiou K. G., Lopez-Bigas N., et al. (2014). Regulation of plant stem cell quiescence by a brassinosteroid signaling module. Dev. Cell 30 36–47. 10.1016/j.devcel.2014.05.020 [DOI] [PubMed] [Google Scholar]
- Vining K. J., Pomraning K. R., Wilhelm L. J., Priest H. D., Pellegrini M., Mockler T. C., et al. (2012). Dynamic DNA cytosine methylation in the Populus trichocarpa genome: tissue-level variation and relationship to gene expression. BMC Genomics 13:27 10.1186/1471-2164-13-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. T., Zarka D. G., Van Buskirk H. A., Fowler S. G., Thomashow M. F. (2005). Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 41 195–211. 10.1111/j.1365-313X.2004.02288.x [DOI] [PubMed] [Google Scholar]
- Walton E. F., Wu R.-M., Richardson A. C., Davy M., Hellens R. P., Thodey K., et al. (2009). A rapid transcriptional activation is induced by the dormancy-breaking chemical hydrogen cyanamide in kiwifruit (Actinidia deliciosa) buds. J. Exp. Bot. 60 3835–3848. 10.1093/jxb/erp231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer F., Riechmann J. L. (2010). Gene networks controlling the initiation of flower development. Trends Genet. 26 519–527. 10.1016/j.tig.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Wisniewski M., Norelli J., Bassett C., Artlip T., Macarisin D. (2011). Ectopic expression of a novel peach (Prunus persica) CBF transcription factor in apple (Malus x domestica) results in short-day induced dormancy and increased cold hardiness. Planta 233 971–983. 10.1007/s00425-011-1358-3 [DOI] [PubMed] [Google Scholar]
- Wu J. F., Wang Y., Wu S. H. (2008a). Two new clock proteins, LWD1 and LWD2, regulate Arabidopsis photoperiodic flowering. Plant Physiol. 148 948–959. 10.1104/pp.108.124917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Zhang L., Zhou C., Yu C. W., Chaikam V. (2008b). HDA6 is required for jasmonate response, senescence and flowering in Arabidopsis. J. Exp. Bot. 59 225–234. 10.1093/jxb/erm300 [DOI] [PubMed] [Google Scholar]
- Yamane H., Ooka T., Jotatsu H., Hosaka Y., Sasaki R., Tao R. (2011). Expressional regulation of PpDAM5 and PpDAM6, peach (Prunus persica) dormancy-associated MADS-box genes, by low temperature and dormancy-breaking reagent treatment. J. Exp. Bot. 62 3481–3488. 10.1093/jxb/err028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanov Y. S., Ma C., Strauss S. H., Busov V. B. (2014). EARLY BUD-BREAK 1 (EBB1) is a regulator of release from seasonal dormancy in poplar trees. Proc. Natl. Acad. Sci. U.S.A. 111 10001–10006. 10.1073/pnas.1405621111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawaski C., Busov V. B. (2014). Roles of gibberellin catabolism and signaling in growth and physiological response to drought and short-day photoperiods in Populus trees. PLoS ONE 9:e86217 10.1371/journal.pone.0086217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Bishop B., Ringenberg W., Muir W. M., Ogas J. (2012). The CHD3 remodeler PICKLE associates with genes enriched for trimethylation of histone H3 lysine 27. Plant Physiol. 159 418–432. 10.1104/pp.112.194878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Mayba O., Pfeiffer A., Shi H., Tepperman J. M., Speed T. P., et al. (2013). A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet. 9:e1003244 10.1371/journal.pgen.1003244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C. L., Halaly T., Acheampong A. K., Takebayashi Y., Jikumaru Y., Kamiya Y., et al. (2015). Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 66 1527–1542. 10.1093/Jxb/Eru519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W. J., Gao Z. H., Zhuang W. B., Shi T., Zhang Z., Ni Z. J. (2013). Genome-wide expression profiles of seasonal bud dormancy at four critical stages in Japanese apricot. Plant Mol. Biol. 83 247–264. 10.1007/s11103-013-0086-4 [DOI] [PubMed] [Google Scholar]
- Zhu Z., An F., Feng Y., Li P., Xue L., Mu A., et al. (2011). Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 108 12539–12544. 10.1073/pnas.1103959108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotarov Y., Stromvik M. (2015). De novo regulatory motif discovery identifies significant motifs in promoters of five classes of plant dehydrin genes. PLoS ONE 10:e0129016 10.1371/journal.pone.0129016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Zhang L., Rao S., Zhu X. Y., Ye L. L., Chen W. X., et al. (2014). The relationship between the expression of ethylene-related genes and papaya fruit ripening disorder caused by chilling injury. PLoS ONE 9:e116002 10.1371/journal.pone.0116002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Average monthly temperature and precipitation in Corvallis, OR, USA during the sample collection period.
Bud morphology and histology during the sample collection period.